Abstract
We have developed a multiplex assay, based on multiplex ligation-dependent probe amplification (MLPA), that allows simultaneous detection of multiple drug resistance mutations and genotype-specific mutations at any location in the Mycobacterium tuberculosis genome. The assay was validated on a reference panel of well-characterized strains, and the results show that M. tuberculosis can be accurately characterized by our assay. Eighteen discriminatory markers identifying drug resistance (rpoB, katG, inhA, embB), members of the M. tuberculosis complex (16S rRNA, IS6110, TbD1), the principal genotypic group (katG, gyrA), and Haarlem and Beijing strains (ogt, mutT2, mutT4) were targeted. A sequence specificity of 100% was reached for 16 of the 18 selected genetic targets. In addition, a panel of 47 clinical M. tuberculosis isolates was tested by MLPA in order to determine the correlation between phenotypic drug resistance and MLPA and between spoligotyping and MLPA. Again, all mutations present in these isolates that were targeted by the 16 functional probes were identified. Resistance-associated mutations were detected by MLPA in 71% of the identified rifampin-resistant strains and in 80% of the phenotypically isoniazid-resistant strains. Furthermore, there was a perfect correlation between MLPA results and spoligotypes. When MLPA is used on confirmed M. tuberculosis clinical specimens, it can be a useful and informative instrument to aid in the detection of drug resistance, especially in laboratories where drug susceptibility testing is not common practice and where the rates of multidrug-resistant and extensively drug resistant tuberculosis are high. The flexibility and specificity of MLPA, along with the ability to simultaneously genotype and detect drug resistance mutations, make MLPA a promising tool for pathogen characterization.
Effective tuberculosis (TB) control requires firstly that patients be identified and placed on proper antituberculosis therapy and secondly that good epidemiological information be available for infection control. Early detection of drug resistance and the genotype would allow appropriate treatment of the patient and could thereby reduce the incidence of multidrug-resistant TB (MDR-TB) or extensively drug resistant TB (XDR-TB) and secondary cases. Mathematical models have suggested that each year, approximately 70% of prevalent infectious MDR-TB cases must be detected and treated, and 80% cured, in order to interrupt the transmission of MDR- and XDR-TB (11).
Sputum microscopy is widely used to confirm pulmonary TB disease, but unfortunately, microscopy provides no information on drug resistance, genetic background, or even the species of the mycobacterium detected. As a consequence, almost all new patients are initially placed on standard therapy with first-line drugs, leading to the further spread of drug-resistant strains in areas where primary MDR-TB infections are prevalent.
Methods that can identify the mycobacterial genotype or detect most resistance to the primary first-line antibiotics are available (18, 21, 24, 31, 42, 46). However, the phenotypic or genotypic characterization of every Mycobacterium tuberculosis isolate or even every sputum sample containing acid-fast bacilli (AFB) is currently time-consuming as well as costly and is practical only in countries with a low burden of TB.
Unlike many other bacterial pathogens, M. tuberculosis is a clonal organism with no evidence of horizontal gene transfer and with a low recombination rate (3, 34). In addition, the majority of drug resistance in M. tuberculosis is due to the acquisition of point mutations (23, 27, 30, 37, 40). These traits make M. tuberculosis especially suitable for characterization via single nucleotide polymorphism (SNP) analysis (2, 13, 16, 20).
Over the years, many important genetic markers for the genotype (e.g., IS6110, gyrA, katG, and TbD1), drug resistance (e.g., rpoB, katG, inhA, and embB), and possibly increased adaptive potential (e.g., mutT2, mutT4, and ogt) have been identified. The ability to determine all these aspects in one assay would be advantageous for transmission studies, molecular biology research, and the selection of optimal treatment regimens. Multiplex PCR enables simultaneous amplification of two or more genetic loci in one reaction. Unfortunately, multiplex PCRs are difficult to optimize, because separate primer pairs are required for every targeted locus, each requiring a different optimal combination of reagents.
Here we report the design, development, validation, and initial application of a multiplex assay based on multiplex ligation-dependent probe amplification (MLPA), allowing simultaneous genotyping and detection of drug resistance mutations for M. tuberculosis. MLPA is a simple and robust assay that allows multiplexed identification of multiple SNPs by the amplification of sequence-specific MLPA probes rather than target DNA (32). The specificity of the assay is ensured by an initial ligation step, and its sensitivity is ensured by PCR amplification. The sizes of the resulting MLPA products correspond to the targeted SNPs. The MLPA products can subsequently be identified by capillary electrophoresis.
Currently MLPA is widely used for many applications involving the screening of human DNA (12, 19), but to date there have been no reports of the use of MLPA for the characterization of bacterial genomes. This type of assay offers significant advantages over PCR-based methods in that multiple mutations distributed throughout the genome can be targeted in a single assay without compromising the sensitivity, specificity, or simplicity of the method. Therefore, MLPA will allow a more extensive characterization of cultured M. tuberculosis isolates than is currently practical.
In addition, the application of MLPA directly to AFB-positive clinical material would be of particular value in regions with a high or increasing incidence of primary drug resistance, allowing patients with MDR-TB to be identified and managed more effectively.
MATERIALS AND METHODS
Selection of strains/DNA targets.
A panel of 10 representative M. tuberculosis strains and 2 other members of the M. tuberculosis complex were used for validation of the MLPA probes (Table 1). This reference panel contains wild-type and mutant forms of all the targeted loci and was chosen from a library of laboratory-generated mutants (1) and clinical isolates from Brazil (33). The sequences targeted by MLPA probes were determined by dideoxy sequencing. In addition to the M. tuberculosis strains, DNA samples from three other bacterial species were included in the reference panel as negative controls: Escherichia coli (strain K-12), Staphylococcus aureus (strain 3347-1), and Mycobacterium malmoense (strain MOTT 1)
TABLE 1.
Sequences of targeted codons or base pairs of strains in the reference panel as determined by dideoxy sequencing or from publicly available databases
| Strain | Origin | Sequence of targeted codon or nucleotidea
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| embB 306 | katG 315 | inhA (−15) | 16S rRNA (208) | rpoB 176 | rpoB 531 | rpoB 526 | rpoB 522 | IS6110 (1288) | katG 463 | gyrA 95 | mutT2 58 | mutT4 48 | ogt 12 | ogt 15 | ogt 37 | TbD1 | ||
| MTB72 | M. tuberculosis laboratory strain | ATG | AGC | C | A | GTC | TCG | CAC | TCG | TCA | CGG | ACC | GGA | CGG | GGG | AGC | CGC | NP |
| MTB213 | M. tuberculosis laboratory strain | ATG | AGC | C | A | GTC | TCG | CAC | TCG | TCA | CTG | ACC | GGA | GGG | GGG | ACC | CTC | NP |
| MTB217 | M. tuberculosis laboratory strain | ATG | AGC | C | A | GTC | TCG | CAC | TCG | TCA | CTG | ACC | CGA | GGG | GGA | ACC | CGC | NP |
| R2 | M. tuberculosis laboratory strain | ATG | AGC | C | A | GTC | TCG | TAC | TCG | TCA | CGG | ACC | GGA | CGG | GGG | AGC | CGC | NP |
| R4 | M. tuberculosis laboratory strain | ATG | AGC | C | A | GTC | TCG | GAC | TCG | TCA | CGG | ACC | GGA | CGG | GGG | AGC | CGC | NP |
| R46 | M. tuberculosis laboratory strain | ATG | AGC | C | A | GTC | TTG | CAC | TCG | TCA | CGG | ACC | GGA | CGG | GGG | AGC | CGC | NP |
| RB14 | M. tuberculosis laboratory strain | ATG | AGC | C | A | TTC | TCG | CAC | TTG | TCA | CGG | ACC | GGA | CGG | GGG | AGC | CGC | NP |
| H37Rv | M. tuberculosis laboratory strain | ATG | AGC | C | A | GTC | TCG | CAC | TCG | TCA | CGG | AGC | GGA | CGG | GGG | ACC | CGC | NP |
| ES-3793 | M. tuberculosis clinical isolate, Brazil | ATA | ACC | C | A | GTC | TCG | GAC | TCG | TCA | CGG | ACC | GGA | CGG | GGG | AGC | CGC | NP |
| RS-353 | M. tuberculosis clinical isolate, Brazil | GTG | AGC | T | A | GTC | TCG | CAC | TCG | TCA | CGG | ACC | GGA | CGG | GGG | ACC | CGC | NP |
| M. bovis 13 | Laboratory strain | ATG | AGC | C | A | GTC | TCG | CAC | TCG | TCA | CTG | ACC | GGA | CGG | GGG | ACC | CGC | TAT |
| MOTT 1 | Laboratory strain | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| M. africanum 3 | Laboratory strain | ATG | ND | ND | A | ND | ND | ND | ND | TCA | ND | ND | ND | ND | ND | ND | ND | TAT |
| S. aureus 3347-1 | Laboratory strain | ND | ND | ND | ND | ND | ND | ND | ND | NP | ND | ND | ND | ND | ND | ND | ND | NP |
| E. coli K-12 | Laboratory strain | ND | ND | ND | ND | ND | ND | ND | ND | NP | ND | ND | ND | ND | ND | ND | ND | NP |
For inhA, IS6110, and the 16S rRNA gene, the number of the targeted nucleotide is given in parentheses. The numbering of M. tuberculosis reference strain H37Rv was used. Sequences that correlate exactly with the sequence of the probe and are thus expected to generate ligation products with MLPA are boldfaced. ND, not done; NP, sequence not present in the genome. MOTT, mycobacterium other than M. tuberculosis.
To validate MLPA as a tool for genotyping and determination of drug resistance, we also selected a panel of clinical isolates on the basis of their spoligotypes and/or drug resistance profiles (Table 2). All 47 strains selected were at least phenotypically resistant to isoniazid (INH).
TABLE 2.
Spoligotypes, nucleotide changes in drug resistance genes and ogt codon 15, and phenotypic drug resistance profiles as determined by drug susceptibility testing of selected clinical strains from Brazila
| Strain | INH resistance profile | Change in katG sequence | Change in inhA sequence | RIF resistance profile | Change in rpoB sequence | EMB resistance profile | Change in ogt codon 15 sequence | Spoligotypeb |
|---|---|---|---|---|---|---|---|---|
| 1 | R | — | — | — | — | — | ACC15AGC | Haarlem3 |
| 2 | R | — | — | — | — | — | wt | X2 |
| 3 | R | — | — | — | — | — | wt | LAM6 |
| 4 | R | — | — | — | — | — | ACC15AGC | Haarlem1 |
| 5 | R | — | — | — | — | — | ACC15AGC | Haarlem3 |
| 6 | R | — | — | — | — | — | ACC15AGC | Haarlem3 |
| 7 | R | — | — | — | — | — | wt | Beijing |
| 8 | R | — | — | — | — | — | wt | LAM9 |
| 9 | R | — | — | — | — | — | ACC15AGC | Haarlem3 |
| 10 | R | — | — | — | — | — | ACC15AGC | Haarlem3 |
| 11 | R | — | — | — | — | — | wt | S |
| 12 | R | — | — | — | — | — | ACC15AGC | Haarlem3 |
| 13 | R | — | — | — | — | — | ACC15AGC | Haarlem3 |
| 14 | R | CTG300CCG | C(−15)T | — | CAC526GAC | — | wt | LAM4 |
| 15 | R | wt | C(−15)T | — | TCG531TTG | — | wt | T1 |
| 16 | R | wt | C(−15)T | — | TCG531TTG | — | wt | T1 |
| 17 | R | AGC315ACC | wt | R | TCG531TGG | R | wt | LAM2 |
| 18 | R | AGC315ACC | wt | R | TCG531TTG | — | wt | LAM3 |
| 19 | R | wt | C(−15)T | S | wt | R | wt | T1 |
| 20 | R | wt | C(−15)T | S | wt | S | wt | LAM9 |
| 21 | R | AGC315ACC | wt | R | ACC525ACG, CAC526TCC, AAG527CAG | S | wt | LAM9 |
| 22 | R | wt | wt | S | wt | S | wt | LAM4 |
| 23 | R | wt | C(−15)T | S | wt | S | wt | LAM2 |
| 24 | R | AGC315ACC | C(−15)T | — | CAC526TCC | — | wt | T1 |
| 25 | R | AGC315ACC | C(−15)T | — | CAC526TAC | — | wt | LAM1 |
| 26 | R | AGC315ACC | wt | R | TCG531TTG | — | ACC15AGC | Haarlem1 |
| 27 | R | AGC315ACC | C(−15)T | R | CAC526TAC | S | wt | LAM4 |
| 28 | R | wt | wt | R | GAC516TAC | R | wt | U |
| 29 | R | AGC315ACC | wt | R | GAC516GTC | S | wt | LAM3/S |
| 30 | R | AGC315ACC | C(−15)T | — | CAC526TAC | — | — | — |
| 31 | R | AGC315ACC | wt | R | CAC526CTC | R | — | — |
| 32 | R | AGC315ACC | wt | R | TCG531TTG | R | — | — |
| 33 | R | AGC315ACC | wt | R | TCG531TTG | R | — | — |
| 34 | R | wt | wt | R | wt | R | — | — |
| 35 | R | AGC299GGC | wt | R | TCG531TTG | R | — | — |
| 36 | R | wt | wt | S | wt | R | — | — |
| 37 | R | wt | wt | — | ATG515ATT, GAC516TAC | — | — | — |
| 38 | R | wt | C(−15)T | R | TCG531TTG | S | — | — |
| 39 | R | wt | C(−15)T | — | TCG531TTG | — | — | — |
| 40 | R | wt | C(−15)T | — | TCG531TTG | — | — | — |
| 41 | R | AGC315ACC | wt | R | GTC176TTC | — | — | — |
| 42 | R | AGC315ACC | wt | R | GAC516GAG, TCG522TTG | S | — | — |
| 43 | R | wt | wt | R | TCG531TTG | R | — | — |
| 44 | R | wt | C(−15)T | R | TCG531TTG | R | — | — |
| 45 | R | AGC315ACC | wt | R | TCG531TTG | S | — | — |
| 46 | R | AGC315ACC | wt | R | CAC526GAC | S | — | — |
| 47 | R | AGC315ACC | wt | S | wt | R | — | — |
R, resistant; S, susceptible; —, not determined or data not available; wt, wild type.
See reference 6.
Strains 1 to 29 were selected for their specific spoligotypes and were used to determine the correlation between MLPA, the spoligotype, and the sequence of ogt codon 15 (a marker for the M. tuberculosis Haarlem genotype [26]), as an example of the performance of the genotype-specific probes included in the assay. Strains 14 to 47 were selected for their drug resistance profiles and were used to determine the correlation between MLPA and phenotypic drug resistance. Targeted drug resistance genes and ogt codon 15 were sequenced after the results of MLPA were known in order to check the proportion of SNPs correctly identified by MLPA.
DNA isolation and sequencing. (i) Preparation of DNA from cultured bacteria.
Mycobacteria were inoculated from pure colonies on Löwenstein-Jensen or Coletsos slopes and were grown for 10 to 14 days in Middlebrook 7H9 medium (Difco, BD, Sparks, MD) supplemented with OADC Enrichment (BBL, BD, Sparks, MD). From each culture 150 μl was taken and centrifuged at 5,000 × g for 3 min. Cells were lysed by replacing the supernatant with 150 μl Tris-EDTA buffer containing 1% Triton X-100 (BDH Laboratory Supplies, Poole, England) and heating at 95°C for 30 min. After lysis, cells were spun down at 5,000 × g for 3 min, and 130 μl of the supernatant was collected as a DNA sample.
To increase the specificity of the assay, 1 μl of 5-mg/ml DNase-free RNase (Roche Diagnostics GmbH, Mannheim, Germany) was added to each DNA sample at least 30 min prior to the MLPA assay.
(ii) Sequencing of targeted loci.
All genes targeted by MLPA probes were sequenced in selected strains from the collection to confirm the sequence specificity of the MLPA assay. The primers used to amplify and sequence these genes are described in Table 3. Cycle sequencing of PCR products was performed in both directions according to protocols published previously (1).
TABLE 3.
Primers used in this study for PCR and sequencing of the loci targeted by MLPA probes
| Primera | Sequence | Product size (bp) | Locus |
|---|---|---|---|
| katG-315 FW | 5′-CATGAACGACGTCGAAACAG-3′ | 233 | katG codon 315 |
| katG-315 RV | 5′-CGAGGAAACTGTTGTCCCAT-3′ | ||
| katG-463 FW | 5′-TCCCGTTGCGAGATACCTT-3′ | 300 | katG codon 463 |
| katG-463 RV | 5′-AGGGTGCGAATGACCTTG-3′ | ||
| embB-306 FW | 5′-CTCCTCCTCAGGCCGTTC-3′ | 293 | embB codon 306 |
| embB-306 RV | 5′-AGACTGGCGTCGCTGACAT-3′ | ||
| gyrA-95 FW | 5′-GGTGCTCTATGCAATGTTC-3′ | 236 | gyrA codon 95 |
| gyrA-95 RV | 5′-GGGCTTCGGTGTACCTCAT-3′ | ||
| inhA-15 FW | 5′-CGAAGTGTGCTGAGTCACACCG-3′ | 203 | inhA regulatory region −15 |
| inhA-15 RV | 5′-TCCGGTAACCAGGACTGAAC-3′ | ||
| ogt FW | 5′-GAAGATCGCATGATTCACTAC-3′ | 234 | ogt codons 12, 15, and 37 |
| ogt RV | 5′-GTCGGTTCCCCGGAGGTCAAG-3′ | ||
| mutT2 FW | 5′-GAACTTCCCGGCGGTAAGGTC-3′ | 149 | mutT2 codon 58 |
| mutT2 RV | 5′-AGCGTCGTCGTGCCGTTCAAC-3′ | ||
| mutT4 FW | 5′-GAATCACATGGACGCCCAACC-3′ | 132 | mutT4 codon 48 |
| mutT4 RV | 5′-AACCCTCCAGCCGATGTTTCG-3′ | ||
| rpoB 2F | 5′-CCCAGGACGTSGAGGCSATCAC-3′ | 537 | rpoB codons 522, 526, and 531 |
| rpoB 2R | 5′-GGCGSGGYGASACGTCCATGTA-3′ | ||
| rpoB 7F | 5′-CTTCTCCGGGTCGATGTCGTTG-3′ | 365 | rpoB codon 176 |
| rpoB 7R | 5′-CGCGCTTGTCGACGTCAAACTC-3′ |
FW, forward; RV, reverse.
Design of probes. (i) Selection of genetic markers or loci.
Eighteen discriminatory markers were selected, and MLPA probes were designed accordingly, providing information about drug resistance, principal genotypic group (PGG) (37), and (mycobacterial) species (Table 4).
TABLE 4.
Summary of the MLPA probes designed and used in this studya
| Probe | Length (bp) | Target-specific sequence | Target or information provided (reference) |
|---|---|---|---|
| embB-306 | 142 | GTCGGACGACGGCTACATCCTGGGCATggcccgagtcgccgaccacgccggctac | EMB resistance marker (38) |
| katG-315 | 160 | caccggaaccggtaaggacgcgatcaccaCCGGCATCGAGGTCGTATGGACGAACACCCC | INH resistance marker (27) |
| inhA-15 | 178 | CGATTTCGGCCCGGCCGCGGCGAGATgataggttgtcggggtgactgccacagcc | INH resistance marker (27) |
| 16S rRNA | 202 | CACGGGATGCATGTCTTGTGGTGGAAAgcgctttagcggtgtgggatgagcccgcggc | 16S rRNA gene, M. tuberculosis complex specific |
| rpoB-176 | 229 | cacgttcatcatcaacgggaccgagcgtgtggtgTTCAGCCAGCTGGTGCGGTCGCCC | RIF resistance marker (1) |
| rpoB-531 | 256 | GTTGACCCACAAGCGCCGACTGTTggcgctggggcccggcggtctgtcacgt | RIF resistance marker (27) |
| rpoB-526G | 265 | caacccgctgtcggggttgaccGACAAGCGCCGACTGTCGGCGCTGGGGCC | RIF resistance marker (27) |
| rpoB-526T | 274 | caacccgctgtcggggttgaccTACAAGCGCCGACTGTCGGCGCTGGGGCC | RIF resistance marker (27) |
| rpoB-522 | 283 | agccaattcatggaccagaacaacccgctgtTGGGGTTGACCCACAAGCGCCGAC | RIF resistance marker (1) |
| IS6110 | 301 | GTCGAACTCGAGGCTGCCTACTACGCTcaacgccagagaccagccgccggctgaggtctcagat | Insertion element IS6110, M. tuberculosis complex specific |
| katG-463 | 319 | GATTGCCAGCCTTAAGAGCCAGATCCGggcatcgggattgactgtctcacagctagtttcgacc | Genotype marker, specific for PGG 2 and 3 (37) |
| gyrA-95 | 328 | GCACGGCGACGCGTCGATCTACGACACcctggtgcgcatggcccagccctgg | Genotype marker, specific for PGG 1 and 2 (37) |
| mutT2-58 | 355 | CCCGAGAGCTCGCCGAAGAACTGCgactcgaggtcgccgacctcgcggtggg | Genotype marker, specific for Beijing 2 (26) |
| mutT4-48 | 364 | CGACCCCGGCAACGGCGAAGGggtcccggtcccgctcacctcgtcgcgggt | Genotype marker, specific for Beijing 1, 2, and 3 (26) |
| ogt-12 | 373 | CGCACCATCGATAGCCCCATCGGAccattaaccctggccgggcatggctcggtgttga | Genotype marker, specific for Beijing 2 (26) |
| ogt-15 | 382 | taccgcaccatcgatagccccatcgggccattaaGCCTGGCCGGGCATGGCTCGGTGTTGA | Genotype marker, specific for Haarlem (26) |
| ogt-37 | 391 | cctgcggatgctcgagcagacgtatgagccaagccTCACACACTGGACACCCGACCCC | Genotype marker, specific for Beijing 3 and 4 (26) |
| TbD1 | 418 | GCGGTCGCGGGATTCAGCGTCTATcggttgcacggcatcttcggctcgcacgaca | Absent in modern M. tuberculosis strains |
Probes are named after the gene and specific codon or region that they target. Target-specific sequences of probes include sequences targeted by the M13-derived probe (lowercase letters) and the synthetic probe (capital letters), as well as the SNP/ligation site sequence (boldface). Sequences for the 16S rRNA gene, IS6110, and TbD1 were derived from the M. tuberculosis reference strain H37Rv.
Drug resistance markers (targeted by probes rpoB-522, rpoB-526G, rpoB-526T, rpoB-531, rpoB-176, inhA-15, katG-315, and embB-306) were chosen on the basis of their in vivo prevalence and the importance of the drug to which they confer resistance. It is estimated that with the selected markers, in a typical collection of clinical isolates, 70 to 85% of rifampin (RIF) resistance, 65 to 80% of INH resistance, and 45 to 65% of ethambutol (EMB) resistance would be identified (23, 28, 30, 38, 40). All drug resistance probes target the drug resistance-conferring mutation, with the exception of probe embB-306. This probe targets the wild-type sequence, since many different base pair changes can occur in this codon (27, 38).
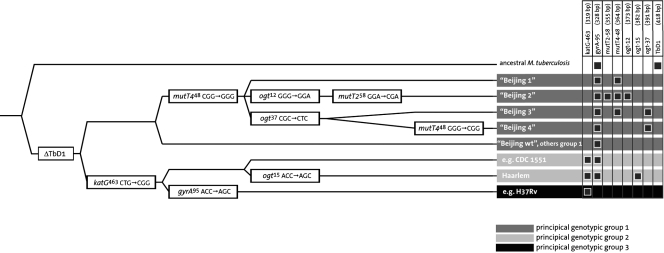
Genotypic markers (gyrA codon 95, katG codon 463) were chosen because of their abilities to discriminate between the three PGGs (10, 37) and to identify putative virulent strains, such as Haarlem (ogt codon 15) and the various Beijing lineages (mutT2 codon 58, mutT4 codon 48, ogt codon 12, ogt codon 37) (26) (Fig. 1).
FIG. 1.
Divergence of modern M. tuberculosis strains into the three PGGs, based on characteristic SNPs and the loss of TbD1. The SNPs shown in this diagram were selected for inclusion in the M. tuberculosis-specific MLPA assay described in this study. The diagram gives the expected MLPA products for each subgroup. The subdivision of Beijing strains is not officially recognized or correlated to the main genotyping methods but is done here simply to illustrate the different subtypes that can be distinguished on the basis of the mutations in these genes. The data are abstracted from references 26 and 37.
We also included discriminatory regions (specific to members of the M. tuberculosis complex) of the 16S rRNA gene and IS6110, an insertion element used for restriction fragment length polymorphism typing of M. tuberculosis (42). Probes targeting these regions are included both as internal controls and as determinants of mycobacterial species.
Finally, a probe targeting TbD1, a region that is absent in the genome of “modern” M. tuberculosis strains but present in all other members of the M. tuberculosis complex (3, 5), was included.
(ii) Preparation of probes.
MLPA probes targeting selected regions or markers were manufactured by MRC-Holland (Amsterdam, The Netherlands) according to previously published protocols (32). Details of the MLPA probes used in this study can be found in Table 4.
The sizes of the probes were selected in such a way that MLPA products are clustered according to characteristic (e.g., rifampin resistance or genotyping).
DST.
Drug susceptibility testing (DST) was performed using the proportion method on Löwenstein-Jensen medium, according to the standard procedures; final drug concentrations in the medium were 0.2 μg/ml for INH, 40.0 μg/ml for RIF, and 2.0 μg/ml for EMB (8). The antibiotics were obtained in pure powder form (Sigma-Aldrich Chemie BV, Zwijndrecht, The Netherlands). Stock solutions were prepared at 10 mg/ml by dissolving INH and EMB in sterile distilled water and RIF in methanol. The stock solutions were stored at −20°C until use.
Spoligotyping.
Mycobacterium tuberculosis strains were spoligotyped by using a commercial kit (Isogen Bioscience BV, Maarssen, The Netherlands) and following the method of Kamerbeek et al. (21). The results were recorded both in a 43-digit binary format representing the 43 spacers and as an octal code, as previously described (6, 9). The person who performed the spoligotyping was blinded to the results of DST. The spoligotyping patterns were compared with an updated SpolDB4 international spoligotype database of the Pasteur Institute of Guadeloupe (the initial version is available at http://www.pasteur-guadeloupe.fr:8081/SITVITDemo).
MLPA analysis.
MLPA was performed according to the standard protocol developed by MRC-Holland with minor modifications (32). Briefly, DNA samples were treated with RNase, and 2 to 3 μl of each extract was used for analysis. The melting of the template DNA, prior to addition of the probe mix, was extended to 10 min at 98°C. The DNA of mycobacterial species has a high GC content; extension of the melting time increased the general signal strength and thereby the sensitivity of the MLPA assay. This extension was also described earlier for methylation-specific MLPA, due to the high GC content of CpG-islands (25).
After the sample DNA was denatured, it was hybridized to MLPA probes overnight (±16 h) at 60°C in 8-μl reaction mixtures, followed by ligation of the probes, performed in 40-μl reaction mixtures at 54°C for 15 min and then at 98°C for 5 min to inactivate the ligase. During ligation, only probes that are completely hybridized to the target DNA are ligated; if the target DNA differs at the ligation site, the probes should not be ligated. Subsequently, an aliquot (10 μl) of the ligated products was amplified by PCR (35 cycles of 30 s at 95°C, 30 s at 60°C, and 60 s at 72°C, followed by a final 20 min at 72°C), for which only 1 primer pair was needed, since all probes carry the same primer sequence. Only the ligated probes are amplified in this step, thus selecting for either the mutant or the wild type to be identified.
Each mutation is represented by a probe of a different length, so the fragments can easily be separated and identified by electrophoresis (32). For this study, the amplified products were analyzed by capillary electrophoresis (one of the MLPA primers is labeled with 6-carboxyfluorescein).
All MLPA reagents were manufactured and supplied by MRC-Holland (Amsterdam, The Netherlands).
RESULTS
Being the first to adapt MLPA for the screening of bacterial DNA, we encountered some problems that either were not observed or were negligible in experiments with human DNA.
The initial results were not encouraging, with a lack of specificity mainly for probes targeting the 16S rRNA gene and RIF resistance markers. These observations suggested that RNA present in the crude DNA extracts was either interfering with probe-target hybridization, leading to false-negative results, or binding incorrectly to the probes, leading to false-positive results. After RNA digestion of the DNA samples, both the sequence specificity and the sensitivity of the assay increased significantly. Further validation of the assay was therefore performed on RNase-treated samples.
Validation of the multiplex assay on reference strains.
The performance of the M. tuberculosis-specific MLPA assay was tested on a panel of representative strains chosen on the basis of their specific sequences targeted by MLPA probes, using all 18 probes (Tables 1 and 4).
With each of the 10 selected M. tuberculosis strains, we observed 100% sequence specificity for all probes except embB-306 and mutT4-48 (Table 5). The mutT4-48 probe appeared to be nonfunctional (see below). The embB-306 probe was unusual in the panel in that it targets the wild-type sequence. Eight of the 10 strains had a wild-type codon 306 in embB, and MLPA correctly resulted in a ligated and amplified product. Strains RS-353 and ES-3793 each carry a mutation in codon 306, but only in strain RS-353 was the embB-306 product absent. The specific mutation present in ES-3793 (G→A) did not prevent the ligation of this probe, although this resistance-conferring mutation was situated at the ligation site (the T or the G in codon 306). In strain RS-353 the first nucleotide was mutated (A→G); this mutation should not have affected ligation, but it did.
TABLE 5.
Results of MLPA for strains from the reference panela
| Strain | MLPA result obtained with probe:
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| embB-306 | katG-315 | inhA-15 | 16S rRNA | rpoB-176 | rpoB-531 | rpoB-526G | rpoB-526T | rpoB-522 | IS6110 | katG-463 | gyrA-95 | mutT2-58 | mutT4-48 | ogt-12 | ogt-15 | ogt-37 | TbD1 | |
| MTB72 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||||
| MTB213 | ▪ | ▪ | ▪ | ▪ | − | ▪ | ||||||||||||
| MTB217 | ▪ | ▪ | ▪ | ▪ | ▪ | − | ▪ | |||||||||||
| R2 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | |||||||||||
| R4 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | |||||||||||
| R46 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | |||||||||||
| RB14 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||
| H37Rv | ▪ | ▪ | ▪ | ▪ | ||||||||||||||
| ES-3793 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||
| RS-353 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||||
| M. bovis 13 | ▪ | ▪ | ▪ | ▪ | ▪ | |||||||||||||
| MOTT 1 | ▪ | |||||||||||||||||
| M. africanum 1 | ▪ | ▪ | ▪ | ▪ | ▪ | |||||||||||||
| S. aureus 3347-1 | ||||||||||||||||||
| E. coli K-12 | ||||||||||||||||||
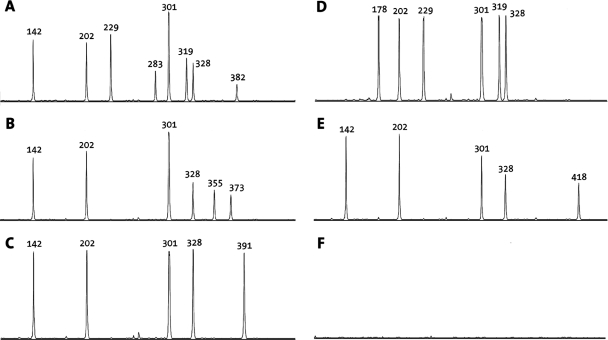
See Fig. 2 for an example of analysis by capillary electrophoresis. ▪, a product was obtained for the targeted SNP or region; −, no product was obtained, although one was expected based on the sequence. Where there is no symbol, no product was detected.
For some of the probes, the signal strength was initially below the detection limit (data not shown). Increasing the probe concentration resolved this issue for probes rpoB-526G, rpoB-526T, IS6110, and ogt-15 but not for probe mutT4-48. Both MTB213 and MTB217 carry the targeted mutation but failed to generate a specific signal for probe mutT4-48 (Table 5 and Fig. 2).
FIG. 2.
Example of MLPA performed on reference samples as analyzed by capillary electrophoresis (compare Table 5). Peaks represent the presence of targeted SNPs or regions in the DNA. The lengths of the resulting products (in base pairs) are given above the peaks. See Table 4 for detailed information on MLPA probes. (A) RB14; (B) MTB217; (C) MTB213; (D) RS-353; (E) M. bovis 13; (F) E. coli K-12.
The probes 16S rRNA, IS6110, embB-306, TbD1, and gyrA-95 all target wild-type M. tuberculosis sequences and were chosen to discriminate between the different bacterial species. The TbD1 region is absent in modern M. tuberculosis strains but present in all other members of the M. tuberculosis complex, represented in the selected panel by Mycobacterium africanum and Mycobacterium bovis. As expected, the TbD1 probe was specific for M. bovis and M. africanum (Table 5; Fig. 2), whereas the gyrA-95, IS6110, and 16S rRNA probes were ligated not only with M. tuberculosis but also with M. bovis and M. africanum. For strain MOTT 1, only embB-306 resulted in a product (Table 5).
For the two strains included in the reference panel that were not related to M. tuberculosis, S. aureus and E. coli, we did not detect any MLPA products (Table 5; Fig. 2).
Validation of the probe mixture on clinical isolates. (i) Genotype-specific mutations.
We selected 47 clinical isolates from Brazil to validate MLPA for purposes of genotyping and drug resistance determination (Table 2). Spoligotype profiles were available for 29 strains of this panel (strains 1 to 29), which were used to determine the performance of the current MLPA with regard to genotyping. This selection included 9 strains with a Haarlem spoligotype, 12 with a LAM (Latin-American/Mediterranean) spoligotype, and 8 with miscellaneous spoligotypes (Table 2). A specific marker for the Haarlem genotype, ogt codon 15 (26), was included in the MLPA assay, and the sequence of this codon was determined for all 29 strains to confirm the results obtained for probe ogt-15. For the various Beijing genotypes, there were also specific markers included in the set of MLPA targets (Fig. 1) (26), but since Beijing strains are not prevalent in Brazil, only one strain was present in this collection for this experiment; it was correctly identified by the MLPA.
The 29 spoligotyped isolates were tested using the MLPA probe mixture containing all 18 probes (Table 4). Only the nine isolates with a Haarlem spoligotype were positive for the typical ogt codon 15 mutation by MLPA (Table 6). These results were confirmed by sequencing of ogt (Table 2).
TABLE 6.
Results of MLPA for drug-resistant clinical M. tuberculosis isolates from Brazila
| Strain | MLPA result obtained with probe:
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| embB-306 | katG-315 | inhA-15 | 16S rRNA | rpoB-176 | rpoB-531 | rpoB-526G | rpoB-526T | rpoB-522 | IS6110 | katG-463 | gyrA-95 | mutT2-58 | ogt-12 | ogt-15 | ogt-37 | TbD1 | |
| 1 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||
| 2 | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||||
| 3 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | |||||||||||
| 4 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||
| 5 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||
| 6 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | |||||||||||
| 7 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||
| 8 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||
| 9 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||
| 10 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | |||||||||
| 11 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | |||||||||||
| 12 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||
| 13 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||
| 14 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||
| 15 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | |||||||||||
| 16 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | |||||||||||
| 17 | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||||
| 18 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||
| 19 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | |||||||||||
| 20 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | |||||||||||
| 21 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | |||||||||||
| 22 | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||||
| 23 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | |||||||||||
| 24 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||
| 25 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||
| 26 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | |||||||||
| 27 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | |||||||||
| 28 | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||||
| 29 | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||||
| 30 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | |||||||||
| 31 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | |||||||||||
| 32 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||
| 33 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | |||||||||||
| 34 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | |||||||||||
| 35 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||
| 36 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | |||||||||||
| 37 | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||||
| 38 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||
| 39 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||
| 40 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||
| 41 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||
| 42 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||
| 43 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | |||||||||||
| 44 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||
| 45 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||
| 46 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ||||||||||
| 47 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | |||||||||||
▪, a product was obtained for the targeted SNP or region. Where there is no symbol, no product was detected. Genotyping data and data on phenotypic and genotypic drug resistance are given in Table 2.
The single Beijing strain present in the panel (strain 7) was negative for the katG codon 463 mutation but positive for gyrA codon 95, which is indeed typical for PGG 1 (Fig. 1) (37). In addition, strain 7 was also positive for mutT2 codon 58 and ogt codon 12, a pattern that is characteristic for Beijing 2.
(ii) Drug resistance mutations.
To validate MLPA for the detection of (primary) drug resistance in M. tuberculosis, we performed the MLPA assay on 34 clinical strains from Brazil with various known phenotypic or genotypic resistance profiles for RIF, INH, and EMB (Table 2, strains 14 to 47). The same probe mixture as that for the reference panel was used. The sequences of the drug resistance markers targeted by the assay were determined in order to confirm the results obtained by MLPA.
Of the 34 strains that were selected, 28 either were resistant to RIF, as determined by DST, or carried a mutation in rpoB that is known to confer resistance to RIF (Table 2). Among these 28 strains, 20 carried mutations targeted by MLPA probes, and indeed all these mutations (20/20) were identified by MLPA (Table 6).
All 34 strains were resistant to INH, as determined by DST or sequencing (Table 2). Of these 34 strains, 19 carried a resistance-conferring mutation in katG, 17 of which were katG S315T, the SNP targeted by MLPA. The second most prevalent INH resistance-conferring mutation (14, 23), inhA C(−15)T, occurred in 14 of the 34 resistant strains. MLPA correctly identified all (17/17) of the katG 315 mutants and all (14/14) of the inhA(−15) mutants (Table 6).
Unfortunately, phenotypic resistance data for EMB were not available for this collection of strains (Table 2), and the embB gene was not sequenced. Therefore, the sequence specificity of probe emb-306 could not be determined for this panel of strains.
For 4/34 strains, probe embB-306 did not result in a product, whereas for the other 30 strains, a product was observed (Table 6). The experiments with MLPA on the reference strains (Table 5) showed that not all mutations can be detected with the current probe, but that at least mutations of the A in codon 306 resulted in the absence of a product for probe embB-306. This, in combination with the results obtained and described here, suggests that the four strains not resulting in an embB-306 product carry a mutation in codon 306.
DISCUSSION
Although with the provision of DOTS (directly observed treatment, short course) the incidence of TB is stabilizing and in some countries even declining, drug-resistant TB is rapidly emerging in a significant number of areas (45, 44, 47). Under standard treatment regimens it is often not possible to identify primary drug-resistant cases, and these regimens are therefore unsuitable for the control of drug-resistant strains. Improved TB control thus relies on improvement of TB detection and early detection of drug-resistant TB, preferably with rapid and accurate screening tools.
Mycobacterial typing methods have been developed for epidemiological purposes (21, 39, 42), and molecular methods that can detect most drug resistance to the primary first-line drugs are available (18, 31, 46), since the majority of clinical drug resistance is due to a limited range of diagnostic point mutations (23, 30, 35). Currently, mutations in mycobacterial genes are generally identified by PCR and subsequent analysis, typically by reverse hybridization to macroarrays (24), melting point analysis, or sequencing. These methods, although highly effective for certain applications, generally target clustered SNPs and do not lend themselves to significant multiplexing; i.e., the identification of mutations widely dispersed in the bacterial genome.
Because M. tuberculosis is a clonal organism, point mutations accumulate and are preserved in the different lineages (5, 34). A range of genotype-specific point mutations have been identified and provide excellent markers (3, 37), some of which may even have implications for the virulence or adaptability of specific lineages (26). Identification of MDR and mycobacterial genotype by multiplexed mutation detection could benefit current control measures and prevent a further spread of the epidemic.
Unfortunately, the countries with high burdens of TB and MDR-TB usually have limited resources, leaving little or no possibility of implementing modern methods. Especially in these areas, there is a need for robust, rapid, and cost-effective new methods for the detection of MDR-TB and of the nosocomial spread of the infection.
Standard culturing of clinical samples could allow for a more complete diagnosis and drug resistance testing if the detection and screening methods following culture are rapid and affordable. MLPA is such a method; the turn-around time is less than 24 h. It is estimated that the screening of one isolate for as many as 40 loci will cost less than 10 euros.
The most suitable place for implementation of the MLPA assay would be the central laboratories in these high-burden areas, where it could be used to rapidly screen confirmed clinical specimens, allowing the spread of drug resistance and prevalent genotypes to be monitored.
We have shown that with MLPA it is possible to rapidly and accurately screen M. tuberculosis DNA samples from culture. The assay reached 100% sequence specificity for all other probes except mutT4-48 and embB-306, making it a promising tool for the multiplexed detection of both dispersed and clustered SNPs in bacterial genomes.
With M. tuberculosis-specific MLPA, we were able to accurately identify members of the M. tuberculosis complex, classify isolates into the three PGGs, and identify the major Haarlem and Beijing types and subtypes. All of the drug-resistant strains carrying mutations targeted by MLPA were detected, accounting for 71% (20/28) of the sequenced RIF-resistant strains and 82% (27/34) of the sequenced INH-resistant strains. In a previous study (43), 90% of MDR-TB cases were identified by merely targeting rpoB codon 531, rpoB codon 526, and katG codon 315, three codons that are included in the assay. Specific mutations in these codons are generally the dominant mechanisms of MDR in many regions, but their prevalence, and thus the percentage of MDR identified with these codons, may differ considerably between regions (4, 7, 22, 29, 36). This may be partially a consequence of the genetic background of prevalent strains (14, 17).
Moreover, MLPA allowed us to characterize fully, in one assay, strains for which the drug susceptibility profile or the genotype had not been determined previously. For strains 1 to 13 (Table 2), only the spoligotype and the sequence of ogt codon 15 were known, but MLPA results revealed that additional mutations were present in embB codon 306 for at least 3 strains, in katG codon 315 for 11 strains, in rpoB codon 531 for 5 strains, and in rpoB codon 526 for 1 strain (Table 6). In the panel where spoligotyping data was missing (Table 2, strains 30 to 47), another three Haarlem strains were identified by MLPA (Table 6). This indicates the versatility of MLPA.
An additional advantage of MLPA over other molecular tools is that the complete assay is performed in a liquid system. This means that the dynamic equilibrium is toward that of accurate hybridization (and ligation), reducing the incidence of false-positive or false-negative results. Since the probe mixes can be prepared in batches and assayed for performance using well-characterized strains, the problems of dropout are significantly less than with reverse hybridization systems, where certain probes may be damaged or missing from individual strips. In addition, the composition of probes (and thus targeted loci) can be adapted to the prevalence of certain mutations in the local population, simply by addition or removal of probes from the master mix.
We believe that besides the analysis of DNA derived from culture, it is also feasible to perform MLPA on DNA directly derived from sputum samples. Initial experiments with diluted DNA samples suggest that the sensitivity of MLPA is comparable to that of PCR. However, with MLPA, information on drug resistance and the genotype can be obtained simultaneously. The optimal use of MLPA would be to characterize mycobacterial DNA derived from AFB-positive sputa or from cultures, complementing current diagnostic methods by allowing combined epidemiology and identification of MDR strains.
MLPA products can also be analyzed by gel electrophoresis; the resolution on an agarose gel allows 15 to 20 loci to be accurately targeted. In our assay, MLPA products are clustered according to characteristic for this reason. Unfortunately, the longer probes, such as IS6110, katG-463, and gyrA-95, differ by only a few base pairs in length, making it difficult to confidently distinguish them on a gel (data not shown). For this study we set out to validate the performance of the probes, and we therefore chose to perform the analysis by capillary electrophoresis, since the resolution is better and the products can be easily separated and accurately identified (Fig. 2). However, the use of capillary electrophoresis increases the cost and handling time to complete analysis, making it less suitable for lower-income countries. Allocating a different size to some of the probes (e.g., IS6110 and katG-463) would be a simple and efficient way to allow accurate identification of these products on an agarose gel.
When capillary electrophoresis is available for analysis, as many as 40 loci can be targeted simultaneously (32). The addition of more probes (and thus more targets) allows for a more detailed screening of M. tuberculosis, enabling the identification of an extended spectrum of drug resistance mutations or more precise genotyping (3, 10, 13, 20). This could be useful for several applications in molecular biology and epidemiology, involving mutation detection, in areas where research is being carried out and adequate resources are available.
MLPA, first described in 2002, is currently in use for the diagnosis of numerous human genetic disorders (12, 19). To our knowledge, we are the first to have adapted MLPA for the characterization of bacterial DNA.
The predictive value of M. tuberculosis-specific MLPA depends on which and how many markers are chosen. For optimal use of MLPA as a tool for multiplexed mutation detection, knowledge of the distribution and frequency of locally prevalent genotypes and drug resistance mutations is essential. For this proof-of-principle study, MLPA probes that target the most prevalent and discriminative mutations were chosen. Depending on the application, a wider range of mutations can be targeted, resulting in more detailed characterization of strains (15, 16, 41). We have shown that, conceptually, MLPA is highly sequence specific and has the potential to be a rapid, flexible, and robust tool useful for the early detection and typing of MDR-TB in a single assay.
Acknowledgments
We thank Jan Schouten and Anders Nygren from MRC-Holland (Amsterdam, The Netherlands) for generosity and helpful discussions and Kristin Kremer (RIVM, Bilthoven, The Netherlands) for critical reading of the manuscript and for providing us with strain H37Rv.
This work was partly funded by the EU 6th Framework Programme TBAdapt.
Footnotes
Published ahead of print on 12 December 2007.
REFERENCES
- 1.Anthony, R. M., A. R. Schuitema, I. L. Bergval, T. J. Brown, L. Oskam, and P. R. Klatser. 2005. Acquisition of rifabutin resistance by a rifampicin resistant mutant of Mycobacterium tuberculosis involves an unusual spectrum of mutations and elevated frequency. Ann. Clin. Microbiol. Antimicrob. 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold, C., L. Westland, G. Mowat, A. Underwood, J. Magee, and S. Gharbia. 2005. Single-nucleotide polymorphism-based differentiation and drug resistance detection in Mycobacterium tuberculosis from isolates or directly from sputum. Clin. Microbiol. Infect. 11122-130. [DOI] [PubMed] [Google Scholar]
- 3.Baker, L., T. Brown, M. C. Maiden, and F. Drobniewski. 2004. Silent nucleotide polymorphisms and a phylogeny for Mycobacterium tuberculosis. Emerg. Infect. Dis. 101568-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bártfai, Z., A. Somoskövi, C. Ködmön, N. Szabó, E. Puskás, L. Kosztolányi, E. Faragó, J. Mester, L. M. Parsons, and M. Salfinger. 2001. Molecular characterization of rifampin-resistant isolates of Mycobacterium tuberculosis from Hungary by DNA sequencing and the line probe assay. J. Clin. Microbiol. 393736-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brosch, R., S. V. Gordon, M. Marmiesse, P. Brodin, C. Buchrieser, K. Eiglmeier, T. Garnier, C. Gutierrez, G. Hewinson, K. Kremer, L. M. Parsons, A. S. Pym, S. Samper, D. van Soolingen, and S. T. Cole. 2002. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc. Natl. Acad. Sci. USA 993684-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brudey, K., J. R. Driscoll, L. Rigouts, W. M. Prodinger, A. Gori, S. A. Al-Hajoj, C. Allix, L. Aristimuño, J. Arora, V. Baumanis, L. Binder, P. Cafrune, A. Cataldi, S. Cheong, R. Diel, C. Ellermeier, J. T. Evans, M. Fauville-Dufaux, S. Ferdinand, D. Garcia de Viedma, C. Garzelli, L. Gazzola, H. M. Gomes, M. C. Guttierez, P. M. Hawkey, P. D. van Helden, G. V. Kadival, B. N. Kreiswirth, K. Kremer, M. Kubin, S. P. Kulkarni, B. Liens, T. Lillebaek, M. L. Ho, C. Martin, I. Mokrousov, O. Narvskaïa, Y. F. Ngeow, L. Naumann, S. Niemann, I. Parwati, Z. Rahim, V. Rasolofo-Razanamparany, T. Rasolonavalona, M. L. Rossetti, S. Rüsch-Gerdes, A. Sajduda, S. Samper, I. G. Shemyakin, U. B. Singh, A. Somoskövi, R. A. Skuce, D. van Soolingen, E. M. Streicher, P. N. Suffys, E. Tortoli, T. Tracevska, V. Vincent, T. C. Victor, R. M. Warren, S. F. Yap, K. Zaman, F. Portaels, N. Rastogi, and C. Sola. 2006. Mycobacterium tuberculosis complex genetic diversity: mining the fourth international spoligotyping database (SpolDB4) for classification, population genetics and epidemiology. BMC Microbiol. 623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavusoglu, C., S. Hilmioglu, S. Guneri, and A. Bilgic. 2002. Characterization of rpoB mutations in rifampin-resistant clinical isolates of Mycobacterium tuberculosis from Turkey by DNA sequencing and line probe assay. J. Clin. Microbiol. 404435-4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins, C. H., J. M. Grange, and M. D. Yates. 1997. Tuberculosis bacteriology: organization and practice, 2nd ed. Butterworth-Heinemann, Oxford, United Kingdom.
- 9.Dale, J. W., D. Brittain, A. A. Cataldi, D. Cousins, J. T. Crawford, J. Driscoll, H. Heersma, T. Lillebaek, T. Quitugua, N. Rastogi, R. A. Skuce, C. Sola, D. van Soolingen, and V. Vincent. 2001. Spacer oligonucleotide typing of bacteria of the Mycobacterium tuberculosis complex: recommendations for standardised nomenclature. Int. J. Tuberc. Lung Dis. 5216-219. [PubMed] [Google Scholar]
- 10.Donoghue, H. D., M. Spigelman, C. L. Greenblatt, G. Lev-Maor, G. K. Bar-Gal, C. Matheson, K. Vernon, A. G. Nerlich, and A. R. Zink. 2004. Tuberculosis: from prehistory to Robert Koch, as revealed by ancient DNA. Lancet Infect. Dis. 4584-592. [DOI] [PubMed] [Google Scholar]
- 11.Dye, C., and B. G. Williams. 2000. Criteria for the control of drug-resistant tuberculosis. Proc. Natl. Acad. Sci. USA 978180-8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erlandson, A., L. Samuelsson, B. Hagberg, M. Kyllerman, M. Vujic, and J. Wahlström. 2003. Multiplex ligation-dependent probe amplification (MLPA) detects large deletions in the MECP2 gene of Swedish Rett syndrome patients. Genet. Test. 7329-332. [DOI] [PubMed] [Google Scholar]
- 13.Filliol, I., A. S. Motiwala, M. Cavatore, W. Qi, M. H. Hazbón, M. Bobadilla del Valle, J. Fyfe, L. García-García, N. Rastogi, C. Sola, T. Zozio, M. I. Guerrero, C. I. León, J. Crabtree, S. Angiuoli, K. D. Eisenach, R. Durmaz, M. L. Joloba, A. Rendón, J. Sifuentes-Osornio, A. Ponce de León, M. D. Cave, R. Fleischmann, T. S. Whittam, and D. Alland. 2006. Global phylogeny of Mycobacterium tuberculosis based on single nucleotide polymorphism (SNP) analysis: insights into tuberculosis evolution, phylogenetic accuracy of other DNA fingerprinting systems, and recommendations for a minimal standard SNP set. J. Bacteriol. 188759-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gagneux, S., M. V. Burgos, K. DeRiemer, A. Encisco, S. Muñoz, P. C. Hopewell, P. M. Small, and A. S. Pym. 2006. Impact of bacterial genetics on the transmission of isoniazid-resistant Mycobacterium tuberculosis. PLoS Pathog. 2e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goh, K. S., E. Legrand, C. Sola, and N. Rastogi. 2001. Rapid differentiation of “Mycobacterium canettii” from other Mycobacterium tuberculosis complex organisms by PCR-restriction analysis of the hsp65 gene. J. Clin. Microbiol. 393705-3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutacker, M. M., B. Mathema, H. Soini, E. Shashkina, B. N. Kreiswirth, E. A. Graviss, and J. M. Musser. 2006. Single-nucleotide polymorphism-based population genetic analysis of Mycobacterium tuberculosis strains from 4 geographic sites. J. Infect. Dis. 193121-128. [DOI] [PubMed] [Google Scholar]
- 17.Hillemann, D., T. Kubica, S. Rüsch-Gerdes, and S. Niemann. 2005. Disequilibrium in distribution of resistance mutations among Mycobacterium tuberculosis Beijing and non-Beijing strains isolated from patients in Germany. Antimicrob. Agents Chemother. 491229-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hillemann, D., M. Weizenegger, T. Kubica, E. Richter, and S. Niemann. 2005. Use of the Genotype MTBDR assay for rapid detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis complex isolates. J. Clin. Microbiol. 433699-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hogervorst, F. B., P. M. Nederlof, J. J. Gille, C. J. McElgunn, M. Grippeling, R. Pruntel, R. Regnerus, T. van Welsem, R. van Spaendonk, F. H. Menko, I. Kluijt, C. Dommering, S. Verhoef, J. P. Schouten, L. J. van't Veer, and G. Pals. 2003. Large genomic deletions and duplications in the BRCA1 gene identified by a novel quantitative method. Cancer Res. 631449-1453. [PubMed] [Google Scholar]
- 20.Huard, R. C., M. Fabre, P. de Haas, L. C. Lazzarini, D. van Soolingen, D. Cousins, and J. L. Ho. 2006. Novel genetic polymorphisms that further delineate the phylogeny of the Mycobacterium tuberculosis complex. J. Bacteriol. 1884271-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamerbeek, J., L. Schouls, A. Kolk, M. van Agterveld, D. van Soolingen, S. Kuijper, A. Bunschoten, H. Molhuizen, R. Shaw, M. Goyal, and J. van Embden. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mani, C., N. Selvakumar, S. Narayanan, and P. R. Narayanan. 2001. Mutations in the rpoB gene of multidrug-resistant Mycobacterium tuberculosis clinical isolates from India. J. Clin. Microbiol. 392987-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Musser, M. 1995. Antimicrobial agent resistance in mycobacteria: molecular genetic insights. Clin. Microbiol. Rev. 8496-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nikolayevsky, V., T. Brown, Y. Balabanova, M. Ruddy, I. Fedorin, and F. Drobniewski. 2004. Detection of mutations associated with isoniazid and rifampin resistance in Mycobacterium tuberculosis isolates from Samara Region, Russian Federation. J. Clin. Microbiol. 424498-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nygren, A. O., N. Ameziane, H. M. Duarte, R. N. Vijzelaar, Q. Waisfisz, C. J. Hess, J. P. Schouten, and A. Errami. 2005. Methylation-specific MLPA (MS-MLPA): simultaneous detection of CpG methylation and copy number changes of up to 40 sequences. Nucleic Acids Res. 33e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rad, M. E., P. Bifani, C. Martin, K. Kremer, S. Samper, J. Rauzier, B. Kreiswirth, J. Blazquez, M. Jouan, D. van Soolingen, and B. Gicquel. 2003. Mutations in putative mutator genes of Mycobacterium tuberculosis strains of the W-Beijing family. Emerg. Infect. Dis. 9838-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramaswamy, S., and J. M. Musser. 1998. Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis: 1998 update. Tuber. Lung Dis. 793-29. [DOI] [PubMed] [Google Scholar]
- 28.Ramaswamy, S. V., R. Reich, S. J. Dou, L. Jasperse, X. Pan, A. Wanger, T. Quitugua, and E. A. Graviss. 2003. Single nucleotide polymorphisms in genes associated with isoniazid resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 471241-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rinder, H., P. Dobner, K. Feldmann, M. Rifai, G. Bretzel, S. Rüsch-Gerdes, and T. Löscher. 1997. Disequilibria in the distribution of rpoB alleles in rifampicin-resistant M. tuberculosis isolates from Germany and Sierra Leone. Microb. Drug Resist. 3195-197. [DOI] [PubMed] [Google Scholar]
- 30.Riska, P. F., W. R. Jacobs, and D. Alland. 2000. Molecular determinants of drug resistance in tuberculosis. Int. J. Tuberc. Lung Dis. 4(2 Suppl. 1)S4-S10. [PubMed]
- 31.Rossau, R., H. Traore, H. De Beenhouwer, W. Mijs, G. Jannes, P. De Rijk, and F. Portaels. 1997. Evaluation of the INNO-LiPA Rif. TB assay, a reverse hybridization assay for the simultaneous detection of Mycobacterium tuberculosis complex and its resistance to rifampin. Antimicrob. Agents Chemother. 412093-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schouten, J. P., C. J. McElgunn, R. Waaijer, D. Zwijnenburg, F. Diepvens, and G. Pals. 2002. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res. 30e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silva, M. S., S. G. Senna, M. O. Ribeiro, A. R. Valim, M. A. Telles, A. Kritski, G. P. Morlock, R. C. Cooksey, A. Zaha, and M. L. Rossetti. 2003. Mutations in katG, inhA, and ahpC genes of Brazilian isoniazid-resistant isolates of Mycobacterium tuberculosis. J. Clin. Microbiol. 414471-4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith, N. H., J. Dale, J. Inwald, S. Palmer, S. V. Gordon, R. G. Hewinson, and J. M. Smith. 2003. The population structure of Mycobacterium bovis in Great Britain: clonal expansion. Proc. Natl. Acad. Sci. USA 10015271-15275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Somoskövi, A., L. M. Parsons, and M. Salfinger. 2001. The molecular basis of resistance to isoniazid, rifampin, and pyrazinamide in Mycobacterium tuberculosis. Respir. Res. 2164-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spindola de Miranda, S., A. Kritski, I. Filliol, C. Mabilat, G. Panteix, and E. Drouet. 2001. Mutations in the rpoB gene of rifampicin-resistant Mycobacterium tuberculosis strains isolated in Brazil and France. Mem. Inst. Oswaldo Cruz 96247-250. [DOI] [PubMed] [Google Scholar]
- 37.Sreevatsan, S., X. Pan, K. E. Stockbauer, N. D. Connell, B. N. Kreiswirth, T. S. Whittam, and J. M. Musser. 1997. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc. Natl. Acad. Sci. USA 949869-9874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sreevatsan, S., K. E. Stockbauer, X. Pan, B. N. Kreiswirth, S. L. Moghazeh, W. R. Jacobs, A. Telenti, and J. M. Musser. 1997. Ethambutol resistance in Mycobacterium tuberculosis: critical role of embB mutations. Antimicrob. Agents Chemother. 411677-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Supply, P., E. Mazars, S. Lesjean, V. Vincent, B. Gicquel, and C. Locht. 2000. Variable human minisatellite-like regions in the Mycobacterium tuberculosis genome. Mol. Microbiol. 36762-771. [DOI] [PubMed] [Google Scholar]
- 40.Telenti, A. 1997. Genetics of drug resistance in tuberculosis. Clin. Chest Med. 1855-64. [DOI] [PubMed] [Google Scholar]
- 41.van den Braak, N., G. Simons, R. Gorkink, M. Reijans, K. Eadie, K. Kremers, D. van Soolingen, P. Savelkoul, H. Verbrugh, and A. van Belkum. 2004. A new high-throughput AFLP approach for identification of new genetic polymorphism in the genome of the clonal microorganism Mycobacterium tuberculosis. J. Microbiol. Methods 5649-62. [DOI] [PubMed] [Google Scholar]
- 42.van Embden, J. D. A., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Gicquel, P. W. M. Hermans, C. Martin, R. McAdam, T. M. Shinnick, and P. M. Small. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Rie, A., R. Warren, I. Mshanga, A. M. Jordaan, G. D. van der Spuy, M. Richardson, J. Simpson, R. P. Gie, D. A. Enarson, N. Beyers, P. D. van Helden, and T. C. Victor. 2001. Analysis for a limited number of gene codons can predict drug resistance of Mycobacterium tuberculosis in a high-incidence community. J. Clin. Microbiol. 39636-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.World Health Organization. 2004. Anti-tuberculosis drug resistance in the world: report no. 3. Document WHO/HTM/TB/2004.343. World Health Organization, Geneva, Switzerland. http://www.who.int/tb/publications/who_htm_tb_2004_343/en/.
- 45.World Health Organization. 2007. Global tuberculosis control: surveillance, planning, financing. WHO Report 2007. Document WHO/HTM/TB/2007.376. World Health Organization, Geneva, Switzerland. http://www.who.int/tb/publications/global_report/2007/en/index.html.
- 46.Zhao, J. R., Y. J. Bai, Y. Wang, Q. H. Zhang, M. Luo, and X. J. Yan. 2005. Development of a pyrosequencing approach for rapid screening of rifampin, isoniazid and ethambutol-resistant Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 9328-332. [PubMed] [Google Scholar]
- 47.Zignol, M., M. S. Hosseini, A. Wright, C. L. Weezenbeek, P. Nunn, C. J. Watt, B. G. Williams, and C. Dye. 2006. Global incidence of multidrug-resistant tuberculosis. J. Infect. Dis. 194479-485. [DOI] [PubMed] [Google Scholar]