Abstract
Streptococcus pneumoniae is bactericidal to Staphylococcus aureus in vitro. To determine whether this in vitro effect accounts for the inverse relation between S. pneumoniae and S. aureus colonization reported in previous epidemiologic studies, we compared S. pneumoniae and S. aureus strains from cocolonized children to those from noncocolonized children. Cocolonizing pneumococci were less bactericidal and cocolonizing staphylococci less susceptible to this effect; however, the magnitude of the effect was small. Thus, in vitro killing is not the major determinant of the pattern of cocolonization.
Streptococcus pneumoniae and Staphylococcus aureus are common causes of community-acquired infections and account for significant morbidity and mortality worldwide (3-6). Nasal S. aureus colonization and nasopharyngeal S. pneumoniae colonization serve as the major source for person-to-person spread and as the source for endogenous invasive disease (1, 15).
Several epidemiologic studies have demonstrated an inverse relationship between S. aureus and S. pneumoniae carriage in children (2, 7, 10, 14). These findings have raised concerns that widespread use of pneumococcal conjugate vaccines may lead to an increased incidence of S. aureus morbidity (13). We recently studied the direct in vitro effect of S. pneumoniae on S. aureus, using a few laboratory and clinical strains and their variants. We have reported that S. pneumoniae is bactericidal to S. aureus and that the bactericidal factor is hydrogen peroxide (H2O2) (11). We observed that different streptococcal species exhibit a variable bactericidal activity and that different staphylococcal species exhibit variable susceptibility to this effect.
The aim of this study was to assess whether variability in bactericidal activity of S. pneumoniae strains and/or variability in susceptibility of S. aureus strains to this effect could predict the pattern of S. aureus and S. pneumoniae cocolonization. All strains tested in this study were collected in our previously reported epidemiologic study that assessed the association between nasopharyngeal carriage of S. pneumoniae and nasal carriage of S. aureus (10). In the original study, nasopharyngeal and nasal swabs were separately obtained from children aged 40 months or younger, on a single occasion. The study population was not vaccinated against S. pneumoniae, as the pneumococcal conjugate vaccine was not registered in Israel at the time of the study. Nasopharyngeal swabs were streaked onto tryptic soy agar plates supplemented with 5% sheep blood and 5 μg/ml gentamicin. Colonies that were alpha-hemolytic and susceptible to optochin were identified as S. pneumoniae. Nasal swabs were streaked on tryptic soy agar plates supplemented with 5% sheep blood. S. aureus was identified by morphology, beta-hemolysis, catalase, DNase, and coagulase. All isolated strains were stored at −80°C.
For the present study, we randomly picked 27 S. pneumoniae strains isolated from cocolonized children and 30 strains isolated from children colonized only with S. pneumoniae (10). We compared the mean bactericidal effects of the two groups of strains.
To quantify the bactericidal effect, we followed a method described previously (11). Briefly, all strains were initially grown to late logarithmic phase (optical density, 0.4 to 0.8), stored at −80°C, and thawed on the day of the experiment. The bactericidal effect of each pneumococcal strain was tested on S. aureus strain Newman (NCTC 8178). Serial twofold dilutions of the staphylococcal strain (beginning with 1 × 108 CFU/ml) were mixed with serial twofold dilutions of the streptococcal strain (beginning with 4 × 107 CFU/ml) in a final volume of 100 μl. Cultures were incubated at 37°C in 5% CO2. After 6 h of incubation, approximately 2 μl of each culture was plated using a replica plater (Sigma-Aldrich) on selective media, i.e., tryptic soy agar supplemented with 5% sheep blood and 8 μg/ml gentamicin for S. pneumoniae and mannitol-salt agar for S. aureus. The log10 of the maximum inoculum of this strain that was “killed” (reduced below the detection limit of 50 CFU/well) by 106 CFU of streptococci at 6 h is reported as the IK6.
Similarly, to assess whether S. aureus strains carried by cocolonized children were less susceptible to the pneumococcal bactericidal effect, we compared strains from cocolonized children to strains from children colonized only with S. aureus, using the same protocol as described above. In this experiment, the reference S. pneumoniae strain used was TIGR4 (12), against which all S. aureus strains were tested. During the experiments the investigator was blinded to the colonization status of the strains tested (cocolonized or not).
Since we observed between-day variation in IK6 results for the same strain, we adjusted for this variation in both the S. aureus and the S. pneumoniae comparisons by using a mixed-effects model in which “day” was incorporated as a random-effect variable.
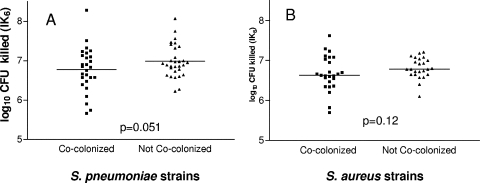
Streptococcus pneumoniae strains from children cocolonized with S. aureus (n = 27) showed a slightly lower mean bactericidal effect (IK6 = 6.72 ± 0.12) than those from noncocolonized children (n = 30) (IK6 = 6.98 ± 0.09) (P = 0.051). S. aureus strains from children cocolonized with S. pneumoniae strains (n = 25) were slightly more resistant to the bactericidal effect (IK6 = 6.70 ± 0.09) than those from noncocolonized children (n = 24) (IK6 = 6.81 ± 0.08) (P = 0.12). Thus, in both cases the difference was small and the effect did not reach statistical significance. Ninety-five percent confidence intervals for the difference in IK6 (cocolonizers versus single colonizers) were −0.34 to 0.08 for S. pneumoniae and −0.20 to 0.01 for S. aureus, suggesting at most a modest difference of about two- to threefold killing in 6 h between the two groups (Fig. 1A and B).
FIG. 1.
(A) S. pneumoniae bactericidal effect and (B) S. aureus susceptibility to the S. pneumoniae effect, as measured by IK6. Strains from cocolonized children (squares) are compared to strains from noncocolonized children (triangles). The reference S. aureus strain used for panel A is S. aureus Newman. The reference S. pneumoniae strain used for panel B is TIGR4.
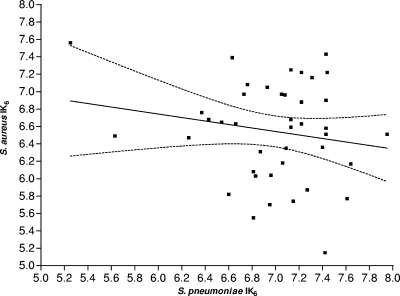
We next hypothesized that the strains carried by cocolonized children should include either a highly resistant S. aureus strain or a less bactericidal S. pneumoniae strain, or possibly both. To examine this hypothesis, we tested 41 pairs of S. aureus and S. pneumoniae strains that were simultaneously isolated from cocolonized children and assessed whether there was a negative correlation between the susceptibility of a cocolonizing S. aureus strain and the bactericidal effect of the cocolonizing S. pneumoniae strain from the same subject. To adjust for daily variation, in this experiment we included a specific strain that was examined every day, and the results were standardized according to its daily variation. Although a trend toward a negative correlation (consistent with our hypothesis) was observed, the correlation was low and nonsignificant (Spearman ρ = −0.184; P = 0.249) (Fig. 2).
FIG. 2.
Correlation between the S. pneumoniae IK6 and the S. aureus IK6 of pairs of cocolonizing bacteria. The dashed lines show the 95% confidence interval of the fitted correlation line.
Our study suggests that results of the in vitro evaluation of the bactericidal effect of S. pneumoniae and susceptibility of S. aureus are not major predictors of the epidemiologic pattern of cocolonization. While in all experiments we did observe a trend that was consistent with our hypothesis (i.e., strains from cocolonized children were less bactericidal [for S. pneumoniae] or less susceptible [for S. aureus]), this trend was nearly statistically significant only for the S. pneumoniae strains, and the effect was small in all cases. A larger study would have perhaps yielded narrower confidence intervals and thus defined this modest effect as statistically significant, but since the 95% confidence intervals defining the difference are already narrow relative to the variability in each population, we suggest that variability in the in vitro bactericidal effect probably has at most a minor role in determining the pattern of cocolonization. Our findings are also consistent with those of Melles et al., who failed to find an association between bacterial genotypes and the pattern of cocolonization (8).
The discrepancy between the strong in vitro interference (11) and the minor differences observed between cocolonizing and noncocolonizing strains has several potential explanations. First, we might have misclassified “cocolonizing strains” due to our original epidemiologic study design, which assessed point prevalence. Because we could not assess the dynamics of colonization, we have no way of knowing whether one of the strains found as a cocolonizer on the day of sampling might have been about to be cleared from that host or, on the other hand, whether a strain found without the other species might have been found cocolonizing a day later. As a result, strains with the ability to cocolonize may have been detected as noncocolonizers on the particular day of sampling, while strains that tended to inhibit or be inhibited by the other species may have been transiently cocolonizing on the day of sampling. Thus, the point prevalence design may have resulted in misclassification of the ability of any given strain to cocolonize, tending to bias findings toward the null. Second, the strong in vitro effect of H2O2 may have only a subtle role in in vivo interference, and host immune factors may play a more significant role in vivo. A recent report by McNalley et al. (7) which shows that the negative association between S. pneumoniae and S. aureus carriage observed in children not infected with human immunodeficiency virus does not exist in human immunodeficiency virus-positive children suggests that the association between S. pneumoniae and S. aureus requires an intact host immune response. The fact that S. aureus and S. pneumoniae often colonize different parts of the upper respiratory tract (1, 9) may also be suggestive of a host immune response mechanism rather than a direct pathogen interference mechanism.
Although our study suggests that the variation in H2O2 production does not explain the pattern of cocolonization, it does not exclude the possibility that H2O2 may be necessary for the interference to occur, whether through a direct bacterial interference mechanism or through an indirect host response mechanism. Even if H2O2 was directly responsible for this interference, the degree of variation in production (S. pneumoniae) and susceptibility (S. aureus) might be too limited to be a major determinant of the pattern of cocolonization.
Both mechanistic and epidemiologic studies are required to further elucidate the mechanism of in vivo interference between S. aureus and S. pneumoniae and to determine the implications of the wide use of pneumococcal conjugate vaccine for S. aureus colonization and infections.
Acknowledgments
We thank Claudette M. Thompson and Krzysztof Trzcinski for their thoughtful advice and suggestions.
This study was supported by National Institutes of Health grant RO1 AI048935 supporting G.R.-Y. and M.L. and grant RO1 AI066013 supporting R.M.
Footnotes
Published ahead of print on 26 November 2007.
REFERENCES
- 1.Bogaert, D., R. De Groot, and P. W. Hermans. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 4144-154. [DOI] [PubMed] [Google Scholar]
- 2.Bogaert, D., A. van Belkum, M. Sluijter, A. Luijendijk, R. de Groot, H. C. Rumke, H. A. Verbrugh, and P. W. Hermans. 2004. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet 3631871-1872. [DOI] [PubMed] [Google Scholar]
- 3.Bridy-Pappas, A. E., M. B. Margolis, K. J. Center, and D. J. Isaacman. 2005. Streptococcus pneumoniae: description of the pathogen, disease epidemiology, treatment, and prevention. Pharmacotherapy 251193-1212. [DOI] [PubMed] [Google Scholar]
- 4.Cohen, P. R. 2007. Community-acquired methicillin-resistant Staphylococcus aureus skin infections: a review of epidemiology, clinical features, management, and prevention. Int. J. Dermatol. 461-11. [DOI] [PubMed] [Google Scholar]
- 5.Cunha, B. A. 2006. Antimicrobial therapy of multidrug-resistant Streptococcus pneumoniae, vancomycin-resistant enterococci, and methicillin-resistant Staphylococcus aureus. Med. Clin. N. Am. 901165-1182. [DOI] [PubMed] [Google Scholar]
- 6.Grundmann, H., M. Aires-de-Sousa, J. Boyce, and E. Tiemersma. 2006. Emergence and resurgence of methicillin-resistant Staphylococcus aureus as a public-health threat. Lancet 368874-885. [DOI] [PubMed] [Google Scholar]
- 7.McNally, L. M., P. M. Jeena, K. Gajee, A. W. Sturm, A. M. Tomkins, H. M. Coovadia, and D. Goldblatt. 2006. Lack of association between the nasopharyngeal carriage of Streptococcus pneumoniae and Staphylococcus aureus in HIV-1-infected South African children. J. Infect. Dis. 194385-390. [DOI] [PubMed] [Google Scholar]
- 8.Melles, D. C., D. Bogaert, R. F. Gorkink, J. K. Peeters, M. J. Moorhouse, A. Ott, W. B. van Leeuwen, G. Simons, H. A. Verbrugh, P. W. Hermans, and A. van Belkum. 2007. Nasopharyngeal co-colonization with Staphylococcus aureus and Streptococcus pneumoniae in children is bacterial genotype independent. Microbiology 153686-692. [DOI] [PubMed] [Google Scholar]
- 9.Mertz, D., R. Frei, B. Jaussi, A. Tietz, C. Stebler, U. Fluckiger, and A. F. Widmer. 2007. Throat swabs are necessary to reliably detect carriers of Staphylococcus aureus. Clin. Infect. Dis. 45475-477. [DOI] [PubMed] [Google Scholar]
- 10.Regev-Yochay, G., R. Dagan, M. Raz, Y. Carmeli, B. Shainberg, E. Derazne, G. Rahav, and E. Rubinstein. 2004. Association between carriage of Streptococcus pneumoniae and Staphylococcus aureus in children. JAMA 292716-720. [DOI] [PubMed] [Google Scholar]
- 11.Regev-Yochay, G., K. Trzcinski, C. M. Thompson, R. Malley, and M. Lipsitch. 2006. Interference between Streptococcus pneumoniae and Staphylococcus aureus: in vitro hydrogen peroxide-mediated killing by Streptococcus pneumoniae. J. Bacteriol. 1884996-5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293498-506. [DOI] [PubMed] [Google Scholar]
- 13.Veenhoven, R., D. Bogaert, C. Uiterwaal, C. Brouwer, H. Kiezebrink, J. Bruin, E. Ijzerman, P. Hermans, R. de Groot, B. Zegers, W. Kuis, G. Rijkers, A. Schilder, and E. Sanders. 2003. Effect of conjugate pneumococcal vaccine followed by polysaccharide pneumococcal vaccine on recurrent acute otitis media: a randomised study. Lancet 3612189-2195. [DOI] [PubMed] [Google Scholar]
- 14.Watson, K., K. Carville, J. Bowman, P. Jacoby, T. V. Riley, A. J. Leach, and D. Lehmann. 2006. Upper respiratory tract bacterial carriage in Aboriginal and non-Aboriginal children in a semi-arid area of Western Australia. Pediatr. Infect. Dis. J. 25782-790. [DOI] [PubMed] [Google Scholar]
- 15.Wertheim, H. F., D. C. Melles, M. C. Vos, W. van Leeuwen, A. van Belkum, H. A. Verbrugh, and J. L. Nouwen. 2005. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 5751-762. [DOI] [PubMed] [Google Scholar]