Abstract
PfPK7 is an orphan protein kinase of Plasmodium falciparum with maximal homology to MEK3/6 and to fungal protein kinase A proteins in its C-terminal and N-terminal regions, respectively. We showed previously that recombinant PfPK7 is active on various substrates but is unable to phosphorylate the Plasmodium falciparum mitogen-activated protein kinase homologues, suggesting that it is not a MEK functional homologue. Using a reverse genetics approach to investigate the function of this enzyme in live parasites, we now show that PfPK7− parasite clones display phenotypes at two stages of their life cycle: first, a decrease in the rate of asexual growth in erythrocytes associated with a lower number of daughter merozoites generated per schizont, and second, a dramatic reduction in the ability to produce oocysts in the mosquito vector. A normal asexual growth rate and the ability to produce oocysts are restored if a functional copy of the PfPK7 gene is reintroduced into the PfPK7− parasites. Hence, PfPK7 is involved in a pathway that regulates parasite proliferation and development.
Malaria parasites still impose a huge burden on the developing world, with 40% of the world's population at risk and an estimated 350 million to 500 million clinical cases annually. This translates into 1 million to 3 million deaths each year, mostly among young children in sub-Saharan Africa. In view of the growing concern caused by the emergence and spread of drug resistance in Plasmodium falciparum, the species responsible for the vast majority of lethal malaria cases, the development of novel control agents is an urgent task (18). Research into novel drug targets has been immensely facilitated by the availability of genomic sequence databases (14).
Malaria pathogenesis is caused by the asexual multiplication of parasites in erythrocytes. After invading a red blood cell, the infecting merozoite develops into a so-called ring form, which grows to form a trophozoite, in which DNA synthesis is initiated around 30 h postinvasion. Several rounds of genome replication and nuclear mitoses lead to the formation of multinucleated schizonts, which eventually (48 h postinvasion) rupture and release 16 to 32 new merozoites. Some merozoites, after invading the host's red blood cells, arrest their cell cycle and differentiate into male or female gametocytes. These sexual cells do not contribute to pathology but are required for transmission to the mosquito vector (for a review, see reference 26). Upon ingestion by the insect, they complete their development to gametes; for male gametocytes, this involves three rounds of division leading to eight flagellated gametes, a process known as exflagellation. Fertilization and subsequent meiosis lead to the development of a motile ookinete, which crosses the midgut epithelium and establishes itself as an oocyst at the basal lamina of the epithelium. Sporogony, the asexual generation of several thousand sporozoites, occurs within the oocyst. Sporozoites invade the insect's salivary glands, which are primed to infect a new human host during a subsequent blood meal taken by the mosquito. Injected sporozoites first establish an infection inside a hepatocyte, which results in the generation of several thousand merozoites (exoerythrocytic schizogony) able to infect erythrocytes.
Mitogen-activated protein kinase (MAPK) pathways play crucial roles in the control of eukaryotic cell proliferation in response to various extra- or intracellular stimuli (for a review, see reference 20). Despite the presence of two MAPK-encoding genes, Pfmap-1 (6, 12, 15) and Pfmap-2 (7), the P. falciparum genome does not possess orthologues of the MAPK kinase (also called MEK, for MAP/ERK kinase) family (1, 28), raising the question of the mode of activation of Pfmap-1 and Pfmap-2. PfPK7 (PlasmoDB database identifier PFB0605w) is the P. falciparum enzyme with the highest level of homology to MEKs (8). However, although the C-terminal lobe of PfPK7 displays maximal homology to the MEK family, its N-terminal lobe is more closely related to that of fungal cyclic AMP-dependent kinases. We previously showed that despite possessing protein kinase activity in vitro on a variety of substrates, PfPK7 was not able to phosphorylate the two plasmodial MAPK homologues and was therefore unlikely to be a MEK functional homologue (8).
Here we present a reverse genetics study in which parasite clones with a disrupted PfPK7 gene were generated to address the question of the function of the atypical kinase in the P. falciparum life cycle. This revealed an important role for the enzyme in the control of parasite proliferation and development.
MATERIALS AND METHODS
Vector construction and genotyping of PfPK7− clones.
The PfPK7 knockout plasmid (pCAM-PK7) was generated by inserting an amplicon corresponding to the central region of the catalytic domains of the enzyme into the pCAM-BSD vector (25), which contains a cassette conferring resistance to blasticidin. A fragment spanning nucleotides 111 to 1315 (numbered according to the PlasmoDB entry) was obtained using forward (GGGGGGATCCATAACATAATTATATTATTATATATGT) and reverse (GCGGCCGCAGGGGGCATGAATTCATAGGTACC) primers carrying BamHI and NotI sites (underlined), respectively, and inserted into pCAM-BSD.
The complementation plasmid pCHD-PK7 was obtained by the insertion of a cDNA encoding the entire PfPK7 coding region (8) into the pHGB vector (27), thus placing PfPK7 expression under the control of the PfHsp86 promoter present in the vector. The resulting plasmid was used as a donor vector in a recombination reaction with the pCHD plasmid, which contains a cassette conferring resistance to the antifolate drug WR99210. After recombination, the final complementation pCHD-PK7 plasmid carries a resistance cassette and contains the PfPK7 cDNA under the control of the PfHsp86 promoter.
Parasite culture and transfection.
Asexual stages of the 3D7 clone of Plasmodium falciparum were grown as described previously (7), using 0.5% Albumax I instead of human serum (23). Transfection was carried out by the electroporation of ring stage parasites with 60 μg of plasmid DNA, as previously described (25). Blasticidin was added to a final concentration of 2.5 μg/ml 48 h posttransfection to select for transformed parasites. Blasticidin-resistant parasites appeared after 3 to 4 weeks of culture, and their genotype was evaluated by PCR to verify that integration had occurred at the target locus (see Results). The parasites were then cloned by limiting dilution in 96-well plates (0.25 to 1 parasite/well) (24), and clones were selected by genotype screening using PCR (see Fig. 1).
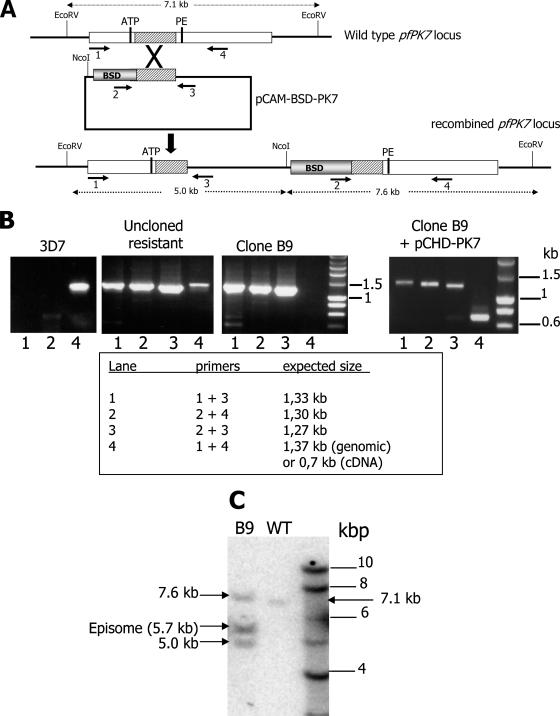
FIG. 1.
Gene disruption and genotyping. (A) Disruption of the PfPK7 locus. Primers used for the detection of integration events are indicated by numbered arrows (see Materials and Methods for the primer sequences). ATP, the glycine triad in subdomain I of the catalytic domain that is required for the proper orientation of the ATP molecule; PE, the proline-glutamate dipeptide in subdomain VIII in which the E residue is required for the structural stability of the enzyme; BSD, blasticidin resistance cassette. (B) PCR analysis of the PfPK7 locus. Genomic DNA from wild-type 3D7, from the uncloned blasticidin-resistant population, from the PfPK7− clone B9, and from B9 parasites complemented with pCHD-PK7 was isolated and subjected to PCR analysis. The following primer pairs were used (see panel A): primers 1 and 3 to detect integration at the 5′ end of the insert (lane 1); primers 2 and 4 to detect integration at the 3′ end of the insert (lane 2); primers 2 and 3 to detect the episome (lane 3); and primers 1 and 4 to detect the wild-type locus (lane 4). The expected sizes of the amplicons are indicated on the right. (C) Southern blot analysis of the PfPK7 locus in 3D7 and PfPK7− (clone B9) parasites. Genomic DNA was cleaved with EcoRV and NcoI, transferred, and probed with a PfPK7 probe (see Materials and Methods). The sizes of the bands are indicated on the right (see panel A for their identities). WT, wild type.
For the complementation of the PfPK7− clone B9, blasticidin-resistant B9 parasites were retransfected with the pCHD-PK7 plasmid. Forty-eight hours postelectroporation, dual selection (2.5 μg/ml blasticidin plus 5 nM WR99210) was applied. Parasites resistant to both compounds appeared after 4 to 5 weeks and were used (without cloning) for genotype verification and phenotype characterization.
Genotype characterization. (i) PCR.
For the detection of integration at the 5′ and 3′ flanks of the insert, the wild-type gene, and the episomes, various primer combinations were used (see Fig. 1 for primer numbers) to amplify PCR products from genomic DNA (see below for the DNA extraction protocol). The sequences for the primers are as follows: primer 1, GGAGGGGGATCCATGAAGGATATTTTATCAAT; primer 2, ATTTATTAAACTGCAGCCC; primer 3, AAGCTGGAGCTCCACCGC; and primer 4, ACCCAAACTCCATATATCCACCTTTGC. Primers 1 and 4 correspond to PfPK7 sequences, and primers 2 and 3 correspond to pCAM-BSD vector sequences flanking the insertion site.
(ii) Southern blotting.
Genomic DNA isolated from 3D7 and from PfPK7− (clone B9) parasites was obtained as follows: a pellet of parasites obtained by saponin lysis was treated with 150 μg/ml proteinase K and 2% sodium dodecyl sulfate at 55°C for 2 h. The DNA was precipitated with ethanol and 0.3 M sodium acetate after phenol-chloroform-isoamyl alcohol (25:24:1) extraction. Five micrograms of DNA was then digested with EcoRV and NcoI, fractionated on a 0.8% agarose gel, and transferred to a Hybond N+ membrane, according to the manufacturer's procedures (Amersham). The PfPK7 amplicon (the same one that had been inserted into pCAM-BSD [see above]) was used as a fluorescein-labeled probe, and chemoluminescence detection was performed by following the manufacturer's protocol (Amersham).
Western blotting.
Parasite pellets were obtained from asynchronous cultures (6 to 8% parasitemia) of either wild-type or mutant parasites by saponin lysis. The pellets were lysed by boiling them for 3 min in Laemmli buffer. After centrifugation in a microcentrifuge, the supernatants were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Western blotting was carried out as previously described, using antibodies against PfPK7 (8) and Pfcyc-4 (17). Detection was performed using the ECL chemiluminescence system by following the manufacturer's protocol (Amersham).
Mosquito infections.
Gametocytes of each parasite line were grown in vitro and fed to Anopheles gambiae mosquitoes through membrane feeders as described previously (3), using medium containing 10% human serum instead of Albumax. Mosquitoes were dissected 10 days postinfection for microscopic analysis of oocyst infection of the midgut. DNA was extracted from oocyst-positive midguts by use of previously published methods (21). Oocyst prevalences between parasite clones were compared using χ2 tests or Fisher's exact test as appropriate. Oocyst densities between parasite clones were compared by χ2 comparisons of oocyst distributions fitted to negative binomials by use of the Genmod procedure with the SAS v.8e for Windows statistical package (2).
RESULTS
Disruption of the PfPK7 gene.
Our strategy for knocking out Plasmodium genes encoding protein kinases relies on a single crossover homologous recombination (9). A plasmid based on the pCAM-BSD vector (25), containing an insert corresponding to the central region of the PfPK7 catalytic domain next to a cassette conferring resistance to blasticidin, was transferred by electroporation into asexual parasites of the 3D7 clone of P. falciparum. Homologous recombination at the PfPK7 locus was expected to generate a pseudo-diploid configuration, with both truncated copies lacking essential regions of the catalytic domain (Fig. 1A). The parasite is haploid, and PfPK7 is a single-copy gene, which makes only one round of drug selection necessary to obtain a null mutant. PCR examination of the genotypes of blasticidin-resistant parasite populations at the PfPK7 locus, using various primers to distinguish (i) the wild-type locus, (ii) the 5′ integration event, (iii), the 3′ integration event, and (iv) the episomal form of the transfected plasmid, demonstrated that the populations did include parasites where integration had occurred (Fig. 1B, “uncloned resistant” panel). Clones were obtained by dilution and screened by PCR for a disrupted PfPK7 locus. Two clones, B9 and C4, were selected for further characterization. PCR analysis of clone B9 (Fig. 1B) showed that (i) these parasites had lost the wild-type gene (lane 4), (ii) amplicons with expected sizes for 5′ and 3′ integration events were recovered (lanes 1 and 2), and (iii) the episome was still present in the cloned parasites (lane 3) (this band might also arise from integrated plasmid concatemers). The same results were obtained with clone C4 (data not shown). To independently ascertain integration at the proper locus, we performed Southern blotting of DNA from wild-type 3D7 and clone B9 by using a PfPK7 probe, which gave the expected bands (Fig. 1C; see Fig. 1A for predicted fragment sizes). The expected bands were also detected during blotting performed with genomic DNA from clone C4 (data not shown). Taken together, the PCR and Southern blot analyses show that the PfPK7 locus has been disrupted in clone B9. We next verified by Western blot analysis whether this disruption resulted in the disappearance of the PfPK7 protein. As shown in Fig. 2A (left panel), the PfPK7 protein was undetectable in both the B9 and the C4 clones (lanes 2 and 3), although it was readily detected in the 3D7 wild-type parasite (lane 1). Antibodies against an unrelated protein (the Pfcyc-4 cyclin [17]) used as a loading control yielded similar signals in all lanes.
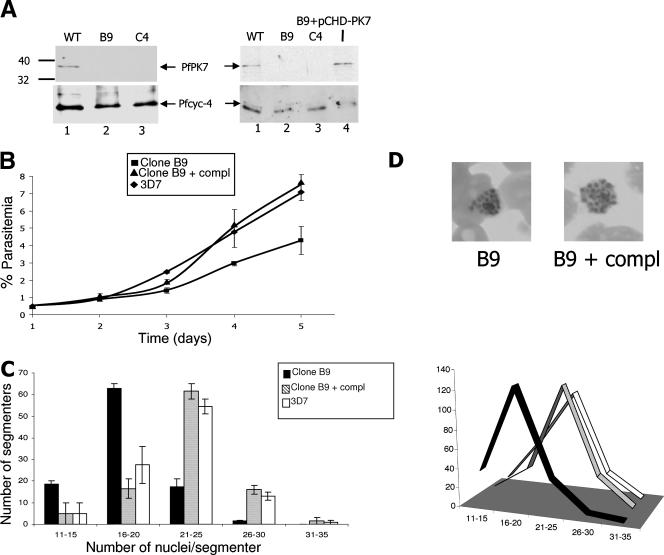
FIG. 2.
Phenotype of PfPK7− parasites during the erythrocyte asexual cycle. (A) Western blot analysis. Top panels, anti-PfPK7 antibodies were used; bottom panels, anti-Pfcyc-4 antibodies (loading control) were used. Two experiments are depicted, using two PfPK7− clones (B9 and C4). The right panel also shows the reexpression of PfPK7 in clone B9 complemented with a plasmid driving the expression of PfPK7 cDNA. The sizes of the comigrating markers are indicated in kDa on the left. WT, wild type. (B) Growth rate of the PfPK7− B9 clone compared to that of wild-type 3D7 and of the B9 clone complemented with pCHD-PK7. Parasitemia was measured for three independent cultures over 5 days. Ten different fields from Giemsa-stained smears were counted for each time point, and the mean parasitemia was determined. Standard errors of the means are indicated by error bars. (C) Numbers of nuclei per segmenter in populations (200 segmenters) of the PfPK7− clone, wild-type 3D7, and the B9 clone complemented with pCHD-PK7. The data are from one experiment, for which 100 segmenters were examined independently by two different observers. Standard errors of the means are indicated by error bars. The panel at the right provides a more visual depiction of the differences between the clones. This experiment was repeated twice with similar results. (D) Giemsa-stained schizonts from the B9 clone and from B9 parasites complemented with pCHD-PK7.
Complementation of the knockout parasites with a plasmid expressing PfPK7 cDNA.
In order to be able to formally assign any phenotype of the PfPK7− parasites (see below) to the absence of the PfPK7 enzyme, we generated “complemented B9” parasites that expressed PfPK7 from an episome. This was achieved by transfecting (PfPK7−) B9 parasites with plasmid pCHD-PK7, in which a cDNA of the full-length PfPK7 coding region was placed under the control of the Hsp86 promoter present in the pCHD vector (27), which is selectable using the antifolate drug WR99210. Parasites resistant to both blasticidin and WR99210 were obtained, and PCR analysis confirmed that the parasites contained the shorter (intronless) PfPK7 cDNA, while the genomic locus was still disrupted (Fig. 1B, right panel, lane 4). The restoration of PfPK7 protein expression in the complemented parasites to levels similar to those observed in wild-type 3D7 was evidenced by Western blot analysis (Fig. 2A, right panel; compare lanes 1 and 4).
Asexual growth rate is decreased in PfPK7− parasites.
That we obtained parasite clones unable to express PfPK7 demonstrates that this enzyme is not essential for erythrocytic schizogony. However, monitoring the progression of parasitemia of the PfPK7− B9 clone in parallel with that of wild-type 3D7 allowed us to detect a defect in the kinase knockout parasites: the growth rate of the latter was significantly lower (by a factor of approximately 2) than that of 3D7 (Fig. 2B). This was also observed with clone C4 (not shown). That this lower rate was indeed due to the absence of PfPK7 in the mutated clone was ascertained by complementation: clone B9 parasites in which PfPK7 expression was restored from the pCHD-PK7 episome displayed a growth rate similar to that of the wild-type parasites (Fig. 2B).
The decrease in the parasite growth rate might be caused by the lengthening of one or more phases of the asexual cycle, by a lower number of merozoites generated per schizont, or by decreased infectivity of daughter merozoites. To address this question, we examined the distribution of segmenters (mature schizonts) with a given number of nuclei in B9 and wild-type 3D7 parasites. While there was no obvious difference in the morphologies of schizonts and segmenters from the two lines (see Fig. 2D), we found that the average number of nuclear bodies per segmenter was shifted toward lower values in PfPK7− (B9) parasites than in wild-type 3D7 parasites. Like the decrease in growth rate, this phenotype was rescued by complementation with pCHD-PK7 (Fig. 2C).
PfPK7− parasites do not complete sporogony in the mosquito vector.
We next tested the ability of the PfPK7− parasites to undergo the sexual cycle. The PfPK7− clones B9 and C4 were able to undergo gametocytogenesis, male gamete production (exflagellation), and female gametogenesis (qualitative observations; data not shown). Mature gametocytes from wild-type 3D7 and PfPK7− clones B9 and C4 were fed to Anopheles gambiae mosquitoes through membrane feeders. Ten days postfeeding, the mosquito midguts were isolated and examined for the presence of oocysts (Table 1). The wild-type parasites were able to establish an infection and produce oocysts in 21/24 mosquitoes, giving a prevalence of 88% (Table 1, experiment 1). In contrast, the number of oocysts produced by PfPK7− parasites appeared to be dramatically reduced, to zero in this experiment (0/25 mosquitoes infected with C4 and 0/4 with B9; 0% prevalence) and to almost zero in a subsequent experiment (Table 1, experiment 2; 1/30 mosquitoes infected with B9, giving a 3.3% prevalence; see below). It is well established that prolonged cultivation of asexual stages of malaria parasites can result in the loss of infectivity for the mosquito. Therefore, in order to verify that the defect in oocyst formation was due to the absence of PfPK7 in the mutant clones rather than to the loss of infectivity caused by prolonged cultivation, the feeding experiment was repeated with the addition of (i) mock-transfected wild-type 3D7, which had been in continuous culture for the same length of time as the B9 clone, and (ii) B9 parasites complemented with pCHD-PK7 (Table 1, experiment 2). The (long-cultured) mock-transfected line displayed reduced infectivity compared with that of fresh 3D7, as expected. Most importantly, complementation of the B9 clone with pCHD-PK7 restored the ability of the clone to produce oocysts to levels statistically indistinguishable from those obtained with the mock-transfected line (P values of 0.79 for prevalence and 0.22 for oocyst intensity) (see Table 1, experiment 2; for a statistical analysis of the data, see Table 2). It follows that the PfPK7 enzyme is required for mature oocyst formation.
TABLE 1.
Oocyst infections in Anopheles gambiae mosquitoes at day 10 postfeeding
| Expt and parasite | No. of mosquitoes infected/total no. dissected | Prevalence of infection (%) | No. of oocysts in infected mosquitoes |
|---|---|---|---|
| Expt 1 | |||
| 3D7 PfPK7− clone B9 | 0/4 | 0 | 0 |
| 3D7 PfPK7− clone C4 | 0/25 | 0 | 0 |
| 3D7 wild type | 21/24 | 88 | 1-9 |
| Expt 2 | |||
| 3D7 wild type | 27/29 | 93 | 1-45 |
| 3D7 mock-transfected control | 12/30 | 40 | 1-3 |
| 3D7 PfPK7− clone B9 | 1/30 | 3 | 4 |
| 3D7 PfPK7− clone B9 + pCHD-PK7 | 11/30 | 37 | 1-5 |
TABLE 2.
Statistical comparisons of oocyst prevalence and intensity
| Expt and parasites compared |
P value
|
|
|---|---|---|
| Oocyst prevalence | Oocyst intensity | |
| Expt 1 | ||
| 3D7 wild type vs 3D7 PfPK7− clone B9 | 0.0002 | NDa |
| 3D7 wild type vs 3D7 PfPK7− clone C4 | <0.0001 | ND |
| Expt 2 | ||
| 3D7 wild type vs 3D7 mock-transfected | <0.0001b | <0.0001b |
| 3D7 mock-transfected vs 3D7 PfPK7− clone B9 | 0.0006b | 0.0037b |
| 3D7 mock-transfected vs 3D7 PfPK7− clone B9 + pCHD-PK7 | 0.79 | 0.22 |
| 3D7 PfPK7− clone B9 vs 3D7 PfPK7− clone B9 + pCHD-PK7 | 0.0012b | 0.0012b |
ND, not done; the distribution does not fit a negative binomial adequately (all negative in one group).
The P value indicates a significant difference.
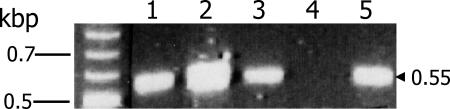
Although no oocysts were observed, parasite material was detected by PCR amplification of the Pfmdr1 gene from the midguts of mosquitoes infected by B9 parasites 10 days after infection, as was the case with two positive controls: wild-type parasites (not shown) and clone H4, a PfPK7+ blasticidin-resistant clone lacking another kinase (Pfmap-1) and able to generate oocysts as efficiently as the wild type (9) (Fig. 3). This suggests that the parasites were able to cross the midgut epithelium, as no parasite material survives in the midgut lumen for this length of time (L. Ranford-Cartwright, unpublished observation; see Discussion).
FIG. 3.
Evidence that PfPK7− parasites cross the mosquito midgut. Parasite DNA from the midguts of mosquitoes infected with clone B9 (lanes 1 and 2; two different mosquitoes), clone C4 (lane 3), and H4, a PfPK7+ clone that produces wild-type levels of oocysts (lane 5), was amplified 10 days postfeeding. Lane 4, negative control (midgut from an uninfected mosquito). Nested-PCR amplification of the Pfmdr1 gene was performed as described previously (10); the expected amplicon size in kb is indicated on the right. Wild-type 3D7 gave a signal similar to that yielded by the H4 clone (lane 5; not shown).
DISCUSSION
Parasites lacking PfPK7 multiply more slowly than wild-type parasites. It may seem counterintuitive that such impaired parasites can be selected from the transfected population, as one would predict that they should be outgrown in the uncloned transformed population by parasites carrying the episome but retaining a wild-type PfPK7 locus. Our tentative explanation is as follows: before integration into the PfPK7 locus has occurred, the episome may not be segregated evenly through the entire progeny of a given schizont; indeed, a single copy of the episome per schizont may be sufficient to confer resistance to blasticidin. It follows that only a fraction of the merozoite progeny may harbor the selectable marker, causing a low growth rate (which is what we typically observe in the early stages post-transfection/selection). However, once the construct is integrated into the target locus, the progeny in its entirety is blasticidin resistant and may therefore compete successfully with parasites carrying the episome but no integrated copy of the construct, even if the associated phenotype is a multiplication rate that is lower than that of the wild-type parasite.
The slow-asexual-growth phenotype of PfPK7− parasites (Fig. 2B) is clearly associated with a lower number of nuclear bodies observed both in mixed schizonts (not shown) and in segmenter (Fig. 2C) populations. This demonstrates that merozoite release is not dependent on the completion of the biogenesis of a fixed number of merozoites. Although we did not detect any striking differences in the length of the various phases of the asexual cycle or in invasion efficiency by using standard methods (examination of Giemsa-stained slides throughout the cycle), it is difficult to determine whether the smaller number of daughter merozoites per schizont in B9 parasites is sufficient to quantitatively explain the low growth rate. Indeed, the level of resolution of the standard methods we used might be too low to allow the detection of subtle differences in these parameters. In this respect, a recent comparative study of the asexual-cycle parameters of P. falciparum clones Dd2 and Hb3 reported that (i) the parasite growth rate of Dd2 is approximately twice as high as that of Hb3 and (ii) Dd2 segmenters contain on average more nuclei than Hb3 segmenters (22). These results are strikingly similar to the difference documented here between parasite clones that express PfPK7 and those that do not. In addition, Reilly et al. (22) also detected subtle differences in the lengths of the asexual cycle (Dd2 completing its cycle marginally more quickly than Hb3) and in the invasion capabilities of the merozoites from the two clones. Could this also be the case with the PfPK7− parasites? Work is in progress to investigate these parameters in more detail. The authors of the Dd2/Hb3 study (22) mention cyclins and cyclin-dependent kinases (classical regulators of the eukaryotic cell cycle) as potential mediators of the phenotype and propose to conduct a genetic study of a Dd2 × Hb3 cross (from which progeny clones are already available) to detect the linkage disequilibrium associated with the growth rate phenotype and thereby identify implicated genes. Although it is possible (and even likely) that this is mediated through multigene regulation, our data suggest that PfPK7 is a strong candidate. Polymorphism data from PlasmoDB do not include any striking difference in the PfPK7 coding regions from 3D7, Hb3, and Dd2, but this is not conclusive with respect to parameters such as spatiotemporal expression and activity levels of the enzyme. It would certainly be of interest to compare PK7 expression and activity levels in Dd2 and Hb3 and to monitor the effect of the overexpression of the gene in Hb3 on growth rate and merozoite production.
The molecular basis for the slow growth of the PfPK7− parasites remains unexplained. Ongoing comparative transcriptome and proteome analyses, as well as the identification of PfPK7 substrates and interactors, are likely to shed some light on this issue. In the first instance, it will be of interest to determine whether any item in the growing list of putative Plasmodium cell cycle regulators available as recombinant proteins (e.g., cyclins or cyclin-dependent kinases) act as PfPK7 substrates in vitro.
Interestingly, other P. falciparum genes whose disruption has been shown to cause slow growth are two genes encoding aspartic proteases of the plasmepsin family; the longer parasite doubling time observed in the plasmepsin knockout parasites was associated with a decreased level of progeny (16) (as described here for the PfPK7 knockout parasites) rather than to a lengthening of the asexual cycle (as was shown, for example, for Plasmodium berghei clones lacking the translation elongation factor eeF1A [13]). The similarity in phenotypes might reflect a functional link between PfPK7 and the plasmepsins, an intriguing possibility; such functional links between proteases and protein kinases have indeed been documented (for an example, see reference 19).
With respect to the block in the sporogonic cycle displayed by the PfPK7− parasites, the B9 clone was able to generate gametocytes and to undergo male and female gametogenesis, as determined by qualitative observations (data not shown). Thus, the block that causes the inability of PfPK7− parasites to form oocysts most likely occurs between gametogenesis and oocyst maturation. Positive PCR detection of parasite material from mosquitoes 10 days postfeeding (Fig. 3) suggests (but does not formally demonstrate) that ookinetes are able to escape the midgut environment. Two studies support this proposition. First, human DNA (which is more abundant than parasite DNA!) is not amplifiable from blood-fed mosquitoes from 100 h postfeeding (4), presumably a consequence of the excretion of the remains of the blood meal bolus from the mosquito, which occurs around 2 days after the blood feeding, and of the degradation of the material remaining in the stomach. Second, DNA from the rodent parasite Plasmodium yoelii in Aedes mosquitoes (where parasites do not invade the midgut epithelium and where there is no sporogonic development) is not detectable by PCR amplification after 5 to 6 days postinfection (11). This suggests that the developmental block in the PfPK7− parasites occurs after the formation of ookinetes and the invasion of the midgut wall but before the appearance of mature oocysts. To determine whether the absence of mature oocysts 10 days postfeeding was due to slow development, we examined mosquito midguts 16 days postfeeding, but no oocysts were observed even after this prolonged time (data not shown). Hence, the absence of mature oocysts in mosquitoes infected with PfPK7− parasites appears to be a consequence of the inability of the parasite to undergo ookinete-oocyst transition or subsequent early oocyst development. This may, in turn, be a consequence of a cell cycle defect: impairment of the intense cell division that characterizes sporogony may result in the parasite's inability to establish mature oocysts (i.e., that are detectable by light microscopy examination). Thus, the phenotype in sporogony might share a similar molecular basis with that in erythrocytic schizogony, and PfPK7 may have similar roles in promoting asexual cell division in the two cases. Further work is in progress to validate (or disprove) this hypothesis.
As indicated in Table 1, 1 of the 30 mosquitoes infected with the B9 clone was oocyst positive. This may be due either to the partial penetrance of the phenotype or to the sporadic reversion to the wild-type locus by the recombination of the sequences that are duplicated in the pseudo-diploid configuration. Such low-level reversion has actually been recently documented in oocysts obtained from a clone in which the gametocyte-specific gene Pfg377 is interrupted by a single crossover recombination and which shows a strong oocyst-deficient phenotype (5). We were unable to determine whether this was the explanation for the B9 oocyst-infected mosquito described in Table 1.
PfPK7 is an “orphan” protein kinase, with no orthologues in mammalian cells or other eukaryotes. Therefore, its function cannot be inferred or predicted from that of homologues in other systems. The present study provides the first step toward understanding the physiological role of this atypical protein kinase. On the basis of the data presented here, we can predict that a PfPK7-specific chemical inhibitor would presumably decrease the virulence of the parasite and act as a transmission-blocking agent, making PfPK7 a potentially interesting target.
Acknowledgments
We are grateful to D. Fidock (Columbia University, NY), who provided the pCAM-BSD plasmid as well as very useful advice on how to use it; G. McFadden (University of Melbourne) for providing the pHGB vector system; and D. Jacobus (Princeton) for WR99210. We thank R. Tewari and O. Billker (Imperial College London, United Kingdom) for fruitful discussions, L. Reininger for comments on the manuscript, Liz Peat and Dorothy Armstrong for maintenance of the Anopheles mosquito colonies, Jonathan Mwangi for help with the mosquito blood meal analysis, and J. Chevalier (Scientific Department, French Embassy, London, United Kingdom) for continuing interest and support.
This work was supported by the European Commission (SIGMAL and ANTIMAL projects), by the French Ministère de la Défense (Délégation Générale pour l'Armement [DGA]), and, in its early phase, by the UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases (TDR). The laboratory of C. Doerig also benefits from support from the Novartis Institute for Tropical Diseases (NITD) and from the Scientific Department of the French Embassy in London, United Kingdom.
Footnotes
Published ahead of print on 14 December 2007.
REFERENCES
- 1.Anamika, N. Srinivasan, and A. Krupa. 2005. A genomic perspective of protein kinases in Plasmodium falciparum. Proteins 58180-189. [DOI] [PubMed] [Google Scholar]
- 2.Bell, A. S., and L. C. Ranford-Cartwright. 2004. A real-time PCR assay for quantifying Plasmodium falciparum infections in the mosquito vector. Int. J. Parasitol. 34795-802. [DOI] [PubMed] [Google Scholar]
- 3.Carter, R., L. Ranford-Cartwright, and P. Alano. 1993. The culture and preparation of gametocytes of Plasmodium falciparum for immunochemical, molecular, and mosquito infectivity studies. Methods Mol. Biol. 2167-88. [DOI] [PubMed] [Google Scholar]
- 4.Coulson, R. M., C. F. Curtis, P. D. Ready, N. Hill, and D. F. Smith. 1990. Amplification and analysis of human DNA present in mosquito bloodmeals. Med. Vet. Entomol. 4357-366. [DOI] [PubMed] [Google Scholar]
- 5.de Koning-Ward, T., A. Oliveri, L. Bertuccini, A. Hood, F. Silvestrini, C. Charvalias, P. Berzosa-Diaz, G. Camarda, T. McElwain, T. Papenfuss, J. Healer, L. Baldassari, B. Crabb, P. Alano, and L. Ranford-Cartwright. 2008. The role of osmiophilic bodies and Pfg377 expression in female gametocyte emergence and mosquito infectivity in the human malaria parasite Plasmodium falciparum. Mol. Microbiol. 67278-290. [DOI] [PubMed] [Google Scholar]
- 6.Doerig, C. M., D. Parzy, G. Langsley, P. Horrocks, R. Carter, and C. D. Doerig. 1996. A MAP kinase homologue from the human malaria parasite, Plasmodium falciparum. Gene 1771-6. [DOI] [PubMed] [Google Scholar]
- 7.Dorin, D., P. Alano, I. Boccaccio, L. Ciceron, C. M. Doerig, R. Sulpice, D. Parzy, and C. Doerig. 1999. An atypical mitogen-activated protein kinase (MAPK) homologue expressed in gametocytes of the human malaria parasite Plasmodium falciparum. Identification of a MAPK signature. J. Biol. Chem. 27429912-29920. [DOI] [PubMed] [Google Scholar]
- 8.Dorin, D., J. P. Semblat, P. Poullet, P. Alano, J. P. Goldring, C. Whittle, S. Patterson, D. Chakrabarti, and C. Doerig. 2005. PfPK7, an atypical MEK-related protein kinase, reflects the absence of typical three-component MAP kinase pathways in the human malaria parasite Plasmodium falciparum. Mol. Microbiol. 55184-196. [DOI] [PubMed] [Google Scholar]
- 9.Dorin-Semblat, D., N. Quashie, J. Halbert, A. Sicard, C. Doerig, E. Peat, L. Ranford-Cartwright, and C. Doerig. 2007. Functional characterization of both MAP kinases of the human malaria parasite Plasmodium falciparum by reverse genetics. Mol. Microbiol. 651170-1180. [DOI] [PubMed] [Google Scholar]
- 10.Duraisingh, M. T., L. V. von Seidlein, A. Jepson, P. Jones, I. Sambou, M. Pinder, and D. C. Warhurst. 2000. Linkage disequilibrium between two chromosomally distinct loci associated with increased resistance to chloroquine in Plasmodium falciparum. Parasitology 1211-7. [DOI] [PubMed] [Google Scholar]
- 11.Fabian, M. M., H. Toma, T. Arakawa, and Y. Sato. 2004. Malaria parasite developmental analyses by the nested polymerase chain reaction method: an implication for the evaluation of mosquito infection rates in epidemiological studies. Southeast Asian J. Trop. Med. Public Health 35820-827. [PubMed] [Google Scholar]
- 12.Graeser, R., P. Kury, R. M. Franklin, and B. Kappes. 1997. Characterization of a mitogen-activated protein (MAP) kinase from Plasmodium falciparum. Mol. Microbiol. 23151-159. [DOI] [PubMed] [Google Scholar]
- 13.Janse, C. J., A. Haghparast, M. A. Speranca, J. Ramesar, H. Kroeze, H. A. del Portillo, and A. P. Waters. 2003. Malaria parasites lacking eef1a have a normal S/M phase yet grow more slowly due to a longer G1 phase. Mol. Microbiol. 501539-1551. [DOI] [PubMed] [Google Scholar]
- 14.Kissinger, J. C., B. P. Brunk, J. Crabtree, M. J. Fraunholz, B. Gajria, A. J. Milgram, D. S. Pearson, J. Schug, A. Bahl, S. J. Diskin, H. Ginsburg, G. R. Grant, D. Gupta, P. Labo, L. Li, M. D. Mailman, S. K. McWeeney, P. Whetzel, C. J. Stoeckert, and D. S. Roos. 2002. The Plasmodium genome database. Nature 419490-492. [DOI] [PubMed] [Google Scholar]
- 15.Lin, D. T., N. D. Goldman, and C. Syin. 1996. Stage-specific expression of a Plasmodium falciparum protein related to the eukaryotic mitogen-activated protein kinases. Mol. Biochem. Parasitol. 7867-77. [DOI] [PubMed] [Google Scholar]
- 16.Liu, J., I. Y. Gluzman, M. E. Drew, and D. E. Goldberg. 2005. The role of Plasmodium falciparum food vacuole plasmepsins. J. Biol. Chem. 2801432-1437. [DOI] [PubMed] [Google Scholar]
- 17.Merckx, A., K. Le Roch, M. P. Nivez, D. Dorin, P. Alano, G. J. Gutierrez, A. R. Nebreda, D. Goldring, C. Whittle, S. Patterson, D. Chakrabarti, and C. Doerig. 2003. Identification and initial characterization of three novel cyclin-related proteins of the human malaria parasite Plasmodium falciparum. J. Biol. Chem. 27839839-39850. [DOI] [PubMed] [Google Scholar]
- 18.Olliaro, P. 2005. Drug resistance hampers our capacity to roll back malaria. Clin. Infect. Dis. 41(Suppl. 4)S247-S257. [DOI] [PubMed] [Google Scholar]
- 19.Plun-Favreau, H., K. Klupsch, N. Moisoi, S. Gandhi, S. Kjaer, D. Frith, K. Harvey, E. Deas, R. J. Harvey, N. McDonald, N. W. Wood, L. M. Martins, and J. Downward. 2007. The mitochondrial protease HtrA2 is regulated by Parkinson's disease-associated kinase PINK1. Nat. Cell Biol. 91243-1252. [DOI] [PubMed] [Google Scholar]
- 20.Raman, M., and M. H. Cobb. 2003. MAP kinase modules: many roads home. Curr. Biol. 13R886-R888. [DOI] [PubMed] [Google Scholar]
- 21.Ranford-Cartwright, L. C., P. Balfe, R. Carter, and D. Walliker. 1993. Frequency of cross-fertilization in the human malaria parasite Plasmodium falciparum. Parasitology 10711-18. [DOI] [PubMed] [Google Scholar]
- 22.Reilly, H. B., H. Wang, J. A. Steuter, A. M. Marx, and M. T. Ferdig. 2007. Quantitative dissection of clone-specific growth rates in cultured malaria parasites. Int. J. Parasitol. 371599-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ringwald, P., F. S. Meche, J. Bickii, and L. K. Basco. 1999. In vitro culture and drug sensitivity assay of Plasmodium falciparum with nonserum substitute and acute-phase sera. J. Clin. Microbiol. 37700-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosario, V. 1981. Cloning of naturally occurring mixed infections of malaria parasites. Science 2121037-1038. [DOI] [PubMed] [Google Scholar]
- 25.Sidhu, A. B., S. G. Valderramos, and D. A. Fidock. 2005. pfmdr1 mutations contribute to quinine resistance and enhance mefloquine and artemisinin sensitivity in Plasmodium falciparum. Mol. Microbiol. 57913-926. [DOI] [PubMed] [Google Scholar]
- 26.Sinden, R. E., G. A. Butcher, O. Billker, and S. L. Fleck. 1996. Regulation of infectivity of Plasmodium to the mosquito vector. Adv. Parasitol. 3853-117. [DOI] [PubMed] [Google Scholar]
- 27.Tonkin, C. J., G. G. van Dooren, T. P. Spurck, N. S. Struck, R. T. Good, E. Handman, A. F. Cowman, and G. I. McFadden. 2004. Localization of organellar proteins in Plasmodium falciparum using a novel set of transfection vectors and a new immunofluorescence fixation method. Mol. Biochem. Parasitol. 13713-21. [DOI] [PubMed] [Google Scholar]
- 28.Ward, P., L. Equinet, J. Packer, and C. Doerig. 2004. Protein kinases of the human malaria parasite Plasmodium falciparum: the kinome of a divergent eukaryote. BMC Genomics 579. [DOI] [PMC free article] [PubMed] [Google Scholar]