Abstract
Sampangine, a plant-derived alkaloid found in the Annonaceae family, exhibits strong inhibitory activity against the opportunistic fungal pathogens Candida albicans, Cryptococcus neoformans, and Aspergillus fumigatus. In the present study, transcriptional profiling experiments coupled with analyses of mutants were performed in an effort to elucidate its mechanism of action. Using Saccharomyces cerevisiae as a model organism, we show that sampangine produces a transcriptional response indicative of hypoxia, altering the expression of genes known to respond to low-oxygen conditions. Several additional lines of evidence obtained suggest that these responses could involve effects on heme. First, the hem1Δ mutant lacking the first enzyme in the heme biosynthetic pathway showed increased sensitivity to sampangine, and exogenously supplied hemin partially rescued the inhibitory activity of sampangine in wild-type cells. In addition, heterozygous mutants with deletions in genes involved in five out of eight steps in the heme biosynthetic pathway showed increased susceptibility to sampangine. Furthermore, spectral analyses of pyridine extracts indicated significant accumulation of free porphyrins in sampangine-treated cells. Transcriptional profiling experiments were also performed with C. albicans to investigate the response of a pathogenic fungal species to sampangine. Taking into account the known differences in the physiological responses of C. albicans and S. cerevisiae to low oxygen, significant correlations were observed between the two transcription profiles, suggestive of heme-related defects. Our results indicate that the antifungal activity of the plant alkaloid sampangine is due, at least in part, to perturbations in the biosynthesis or metabolism of heme.
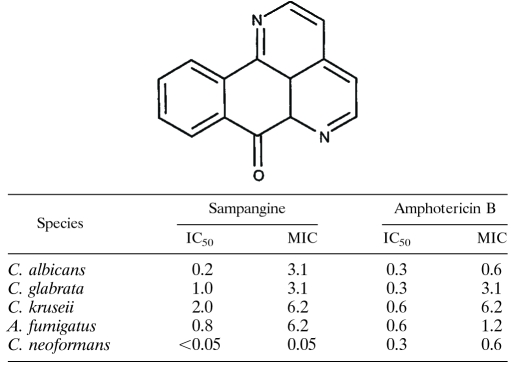
The azaoxoaporphine alkaloid sampangine (Table 1) belongs to the aporphine family of alkaloids, which have been reported to show antibacterial, antifungal, antiviral, antiparasitic, and antitumor activities (e.g., 11, 12, 23, 27, 28, 49, 63). The production of sampangine is associated with species within the plant family Annonaceae (custard-apple family), including Duguetia hadrantha and Cananga odorata (51, 57, 60). Like other aporphine alkaloids, sampangine is of particular interest as a potential therapeutic agent, as it exhibits strong inhibitory activity against the human fungal pathogens Candida albicans, Cryptococcus neoformans, and Aspergillus fumigatus (51, 57). It also displays activity against the bacterium Mycobacterium tuberculosis and the malaria parasite Plasmodium falciparum and has activity against human cancer cells, including malignant melanoma cells and HL-60 leukemia cells (32, 33, 51, 57).
TABLE 1.
Structure of sampangine and the in vitro antifungal activity of sampangine compared to that of amphotericin Ba
Broth microdilution assays were performed according to CLSI guidelines (52, 53). The media used were RPMI for A. fumigatus and Candida spp. and Sabouraud dextrose for C. neoformans. The temperature of incubation was 37°C for Candida spp. and 30°C for C. neoformans and A. fumigatus. Numbers are in micrograms/milliliter and are average values from duplicate experiments. The strains used were C. albicans ATCC 90028, C. glabrata ATCC 90030, C. kruseii ATCC 6258, A. fumigatus ATCC 90906, and C. neoformans ATCC 90113.
For both antimicrobial and antitumor properties, little is known concerning sampangine's molecular mechanism of action. Kluza et al. (33) have reported that sampangine induces apoptosis in human HL-60 leukemia cells via the generation of reactive oxygen species. This has been attributed to the iminoquinone moiety in its structure (Table 1) that is also present in the closely related marine alkaloid ascididemin, which was similarly reported to generate reactive oxygen species, leading to DNA damage in tumor cells (48). However, transcriptional profiling experiments conducted with ascididemin in M. tuberculosis indicated that the profile generated by this compound clustered with the profiles of known iron-scavenging agents, and subsequent experiments confirmed that ascididemin inhibited M. tuberculosis growth through iron depletion (9). In the case of ascididemin, oxidative stress and DNA damage are therefore likely to represent secondary effects of the inhibitor caused by depletion of cellular iron. Thus, whether the oxidative stress associated with the antitumor properties of sampangine reflects its primary mechanism of action remains to be determined.
Further characterization of the biological activities of sampangine will be useful not only in facilitating its pharmacological development but also in understanding its importance in the producing plant species, where it is likely to play a role in chemical defense. In the present study, we have conducted transcriptional profiling experiments followed by analyses of mutants in an effort to gain insight into its mechanism of action. Using Saccharomyces cerevisiae as a model, we show that sampangine produces a transcriptional response suggestive of hypoxia, causing significant changes in the expression of genes known to respond to low-oxygen conditions. Interestingly, as previously observed with human HL-60 leukemia cell lines (33), sampangine also induced an oxidative stress response in S. cerevisiae cells. Further experiments revealed that these effects are likely due to an alteration in heme metabolism. Results obtained using the important human fungal pathogen C. albicans were consistent with those obtained using S. cerevisiae when known differences in the physiological responses of these two fungal species to low-oxygen conditions were taken into account. Collectively, our results suggest that heme plays an integral role in the antifungal activity of sampangine.
MATERIALS AND METHODS
Strains and media.
S. cerevisiae and C. albicans strains used in this study are listed in Table 2. Synthetic dextrose (SD) medium, containing 0.67% yeast nitrogen base without amino acids and 2% dextrose, was used to grow wild-type S. cerevisiae S288C and C. albicans SC5314 strains. The medium was buffered with 0.165 M 3-[N-morpholino]propanesulfonic acid (MOPS), and the pH was adjusted with NaOH to 7.0 for S. cerevisiae and to 4.5 for C. albicans to maintain yeast morphology. Synthetic complete (SC) medium, consisting of SD medium supplemented with complete supplement mixture (Qbiogene, Inc., Carlsbad, CA) was used for growing S. cerevisiae deletion mutants obtained from Open Biosystems (Huntsville, AL). For C. albicans, all mutant strains and their isogenic wild-type strains were grown in YPD medium (1% yeast extract, 2% peptone, 2% dextrose) supplemented with uridine (YPD + Uri medium) at 80 μg/ml. The hem1Δ strains of S. cerevisiae (yMH339) and C. albicans (KRC1) were maintained in YPD medium or YPD + Uri medium supplemented with 200 μg/ml (S. cerevisiae) or 50 μg/ml (C. albicans) of δ-aminolevulinate (ALA; YPD + ALA or YPD + Uri + ALA).
TABLE 2.
S. cerevisiae and C. albicans strains used in this study
| Strain | Description | Source or reference |
|---|---|---|
| S. cerevisiae strains | ||
| S288C | MATα SUC2 mal mel gal2 CUP1 flo1 flo8-1 | ATCC |
| JK93dαa | MATα his4 HMLa leu2-3,112 rme1 trp1 ura3-52 | J. Heitman |
| BY4743 | MATa/α his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lys2Δ0 | Open Biosystems |
| BY4741 | MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Open Biosystems |
| yMH339b | MATaura3Δ0 his3Δ200 leu2Δ0 lys2-128 HAP1 hem1Δ::kanMX | Unpublished data |
| HEM1/hem1Δ | HEM1/hem1Δ in BY4743 | Open Biosystems |
| HEM2/hem2Δ | HEM2/hem2Δ in BY4743 | Open Biosystems |
| HEM3/hem3Δ | HEM3/hem3Δ in BY4743 | Open Biosystems |
| HEM4/hem4Δ | HEM4/hem4Δ in BY4743 | Open Biosystems |
| HEM12/hem12Δ | HEM12/hem12Δ in BY4743 | Open Biosystems |
| HEM13/hem13Δ | HEM13/hem13Δ in BY4743 | Open Biosystems |
| HEM14/hem14Δ | HEM14/hem14Δ in BY4743 | Open Biosystems |
| HEM15/hem15Δ | HEM15/hem15Δ in BY4743 | Open Biosystems |
| yap1Δ/yap1Δ | yap1Δ/yap1Δ in BY4743 | Open Biosystems |
| sod2Δ/sod2Δ | sod2Δ/sod2Δ in BY4743 | Open Biosystems |
| cin5Δ/cin5Δ | cin5Δ/cin5Δ in BY4743 | Open Biosystems |
| TXSc001 | pRS426 in JK93dα | This study |
| TXSc025 | pRS426-CIN5 in JK93dα | This study |
| C. albicans strains | ||
| SC5314a | URA3/URA3 | 21 |
| BWP17a | ura3::λimm434/ura3::λimm 434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG | 74 |
| KRC1c | hem1Δ::URA3/hem1Δ::ARG4 in BWP17 | 5 |
Received from Joseph Heitman (Duke University).
Received from Mark Hickman (Harvard University).
Received from Martin Bard (Indiana University).
All reagents, including ketoconazole, ergosterol, hemin, ALA, and dimethyl sulfoxide (DMSO), were obtained from Sigma (St. Louis, MO). Stock solutions of ketoconazole and sampangine were made in DMSO. Ergosterol stock solution was prepared in Tween 80/ethanol (1:1 [vol/vol]). A 6.5 mg/ml stock solution of hemin was prepared by dissolving in 0.1 M NaOH at 37°C, followed by the addition of 1 M TrisHCl, pH 7.5, to a final concentration of 0.1 M and an adjustment of the final pH to 7.0 with HCl.
IC50 determinations for microarray experiments.
In all microarray experiments, S. cerevisiae strain S288C and C. albicans strain SC5314 were used. All experiments were conducted at 30°C for S. cerevisiae and 37°C for C. albicans. For 50% inhibitory concentration (IC50) determinations in small-scale cultures, broth microdilution assays were performed according to Clinical and Laboratory Standards Institute (CLSI, formerly NCCLS) protocols (52), with the modification that the inoculum size was 2 × 106 CFU/ml. This cell density, which is ∼200 times greater than the standard protocol, was used in order to mimic microarray culture conditions.
For determination of IC50 values in large-scale cultures, an overnight culture (started from a single colony) in late exponential phase was used to inoculate 50 ml of SD medium to an optical density at 600 nm (OD600) of 0.1 (cell count of ∼2 × 106 CFU/ml). Multiple cultures were started for each drug in order to test four to five different drug concentrations. The cultures were incubated in an environmental shaker (200 rpm) and allowed to recover from stationary phase until an OD600 of 0.2 was reached. Sampangine was then added at two- to fourfold serial dilutions into each culture. Two rounds of experiments were conducted, with a broad range of sampangine concentrations tested in the first round and a narrow range tested in duplicate experiments in the second round. The cultures were grown to late exponential phase (17 h for S. cerevisiae and 9 h for C. albicans), and a final OD600 was measured using an Ultrospec 2000 spectrophotometer (Amersham Biosciences, Piscataway, NJ). The IC50 values were determined to be 1.17 μg/ml for S. cerevisiae and 6.13 μg/ml for C. albicans (data not shown).
Cell culture and drug exposure for microarray experiments.
A single colony of S. cerevisiae or C. albicans was inoculated into 25 ml of SD medium and grown overnight in an environmental shaker (200 rpm) until late exponential phase. The culture was used to inoculate 50 ml of SD medium to an OD600 of 0.1. Six independent 50-ml cultures were grown for each experiment, three for treated and three for untreated samples. The cultures were allowed to recover from stationary phase until an OD600 of 0.2 was reached. Sampangine was then added to each culture at a concentration equivalent to the IC50 value (1.17 μg/ml for S. cerevisiae and 6.13 μg/ml for C. albicans). Control cultures were simultaneously treated with 0.25% dimethyl sulfoxide (DMSO). The cultures were allowed to grow until an OD600 of 0.5 was reached (∼3 h for S. cerevisiae and ∼1.5 h for C. albicans). Cells were harvested by centrifugation, flash frozen in liquid nitrogen, and stored at −80°C.
RNA preparation.
Total RNA was isolated using a Qiagen RNeasy Midi kit (Qiagen, Valencia, CA) according to the manufacturer's instructions, with the following modifications: frozen cells were ground into a powder with a mortar and pestle in liquid nitrogen to facilitate cell disruption. The cell powder was resuspended in 4 ml of lysis buffer provided in the kit and homogenized for 2 min at 30-s intervals at 25,000 rpm using a PT3100 polytron (Brinkmann Instruments, Westbury, NY). The RNA concentration and purity were determined spectrophotometrically by measuring the absorbance at 230, 260, 280, and 320 nm. The purity and integrity of the RNA were confirmed by agarose gel electrophoresis.
Microarray analysis: sample preparation, hybridization, and data analysis.
For S. cerevisiae, target preparation was performed according to the GeneChip expression analysis protocol (Affymetrix, Santa Clara, CA). A One-Cycle target labeling and control reagent kit (Affymetrix) was used for first-strand and second-strand cDNA synthesis, biotin-labeled cRNA synthesis, cRNA fragmentation, and hybridization. Ten micrograms of total RNA was used for first-strand cDNA synthesis. The quantity of cRNA generated was determined spectrophotometrically, and the quality of the cRNA and fragmented cRNA was analyzed on an agarose gel. Fifteen micrograms of fragmented cRNA was hybridized to a GeneChip yeast genome S98 array (Affymetrix) in a GeneChip hybridization oven 640. Washing and staining with streptavidin phycoerythrin were performed using a GeneChip fluidics station 450, and scanning was performed using a GeneChip scanner 3000. Image analysis, scaling, and probe set-level data analysis were performed using the default parameters of GeneChip Operating Software version 1.1 (GCOS; Affymetrix). The report file generated by the software was used to assess the quality of each experiment, to ensure that the scaling factor was similar across the arrays, and to ensure that the values obtained from housekeeping controls as well as the spike-in controls were within the recommended limits.
For C. albicans, target preparation was performed according to the aminoallyl labeling protocol of The Institute for Genomic Research (http://www.tigr.com). First-strand synthesis was performed with 15 μg of total RNA. The cDNA was coupled with Cy3 dye or Cy5 dye, and the labeled target was quantitated by using a spectrophotometer. Equal amounts (∼500 ng of cDNA [each]) of the Cy3- and Cy5-labeled target samples were hybridized to C. albicans glass arrays. The arrays consisted of ∼6,400 70-mer oligonucleotides (Qiagen) that were printed on glass slides (Microarrays, Inc., Nashville, TN). Arrays were hybridized and washed according to the protocol outlined in the Corning UltraGAPS coated slides instruction manual. Arrays were scanned using a ScanArray 5000 scanner (PerkinElmer, Wellesley, MA), and image analysis was performed using QuantArray version 3.0 software (PerkinElmer). The quality of each experiment was assessed using the Scatter Plot feature of QuantArray (Cy5 signal intensity plotted against Cy3 signal intensity of all spots) to ensure that the majority of spots were evenly distributed close to the 1:1 identity line.
Differentially expressed genes were identified using BRB Array Tools version 3.2.3 software developed by Richard Simon and Amy Peng Lam (http://linus.nci.nih.gov/BRB-ArrayTools.html). For S. cerevisiae, data from CHP files generated with GCOS software (background-corrected and normalized signal values after probe set-level data analysis) were uploaded into BRB Array Tools. For C. albicans, data generated using QuantArray software (background-corrected signal intensities) were uploaded and were normalized using the Lowess algorithm available in BRB Array Tools. For both data sets, signal values were log transformed and filtered using the default parameters available to remove probe sets or spots with very low signal values, missing signal values, and similar signal values across all the arrays in a given experiment. Differentially expressed genes were identified by performing a two-class comparison between untreated and treated classes using the default statistical parameters available in BRB Array Tools, and genes with a P value of ≤0.001 were considered to be significant. Gene annotations were obtained from the Saccharomyces Genome Database (SGD) or the Candida Genome Database (CGD). The Gene Ontology (GO) Term Finder and GO Slim Mapper tools in SGD or CGD was used to distribute the genes into GO-based categories, and overrepresented GO terms were identified for each data set.
Sensitivity assays with S. cerevisiae and C. albicans strains.
Broth microdilution assays were performed according to CLSI guidelines (52). An overnight culture started from a single colony was diluted in the appropriate medium after comparison to the 0.5 McFarland standard to afford a final inoculum of ∼1 × 104 CFU/ml. After dilution, 190 μl of the inoculum was added to a 96-well flat-bottom microplate (Corning, Inc., Corning, NY) containing 10 μl of sampangine at various concentrations. Sampangine was prepared as a stock solution of 400 μg/ml in DMSO, which was twofold serially diluted in DMSO, followed by a fivefold dilution in the appropriate medium. The starting concentration of sampangine in the final assay was 4 μg/ml. The microplates were read at 600 nm prior to and after incubation using a BioTek Powerwave XS microplate reader (BioTek Instruments, Inc., Winooski, VT). For S. cerevisiae, the temperature of incubation was 30°C, and the time of incubation was 48 h. For C. albicans, the temperature of incubation was 37°C, and the time of incubation was 24 h.
To test whether ergosterol or hemin could alter the sensitivity to sampangine, broth microdilution assays were performed as described above except that 180 μl of the inoculum was added to a microplate containing 10 μl of sampangine and 10 μl of either ergosterol or hemin. The concentrations of ergosterol and hemin used were 10 μg/ml and 32.5 μg/ml, respectively. The growth of S. cerevisiae and C. albicans cells was not inhibited at these concentrations, as determined by pilot checkerboard assays.
To determine the sensitivities of various mutant strains to sampangine, agar-based drop test assays were performed. Overnight cultures at an OD600 of 3.0 were used to prepare 1:5 serial dilutions in the appropriate media, and they were spotted in 3-μl amounts onto agar plates. Plates were incubated for 2 to 3 days at the required temperature. For testing the S. cerevisiae deletion mutants obtained from Open Biosystems (Table 2), overnight cultures from single colonies of the mutants and the wild-type strain BY4743 were grown in SC broth (plus 200 μg/ml G418 for the mutants only), diluted to an OD600 of 3.0, and used in drop test assays on SC agar plates containing DMSO or 1.25 μg/ml or 2.0 μg/ml of sampangine.
For testing the hem1Δ mutant of S. cerevisiae, overnight cultures of hem1Δ mutant and the parent strain (BY4741) were grown in YPD + ALA broth (200 μg/ml) and YPD broth, respectively. The cultures were washed three times in sterile distilled water, and cells from the mutant strain were resuspended in YPD + ALA broth (8 μg/ml), while cells from the parent strain were resuspended in YPD broth. The cells were allowed to grow with shaking for ∼5 h. The cells were diluted to an OD600 of 3.0 and used in drop test assays on YPD + ALA (8 μg/ml) agar plates containing either DMSO or 1.25 μg/ml of sampangine. Plates were incubated for 2 days at 30°C. The hem1Δ/Δ strain of C. albicans was tested in a similar manner, except the medium used was YPD + Uri, the concentration of ALA used for overnight cultures was 50 μg/ml, and the concentration of sampangine used was 5 μg/ml.
Pyridine spectrum analysis.
Pyridine spectral analysis was performed according to the methods described by Falk (16). Overnight cultures of S. cerevisiae or C. albicans grown in SD broth were used to inoculate fresh cultures at an OD600 of 0.1. After one doubling, sampangine was added to each culture at a concentration equivalent to the IC50 value (1.17 μg/ml for S. cerevisiae and 6.13 μg/ml for C. albicans). Control cultures were simultaneously treated with 0.25% DMSO. The cultures were allowed to grow for ∼4 doublings. Equal numbers of cells from the corresponding treated and untreated cultures were harvested by centrifugation and washed once with sterile distilled water. The cell pellets were resuspended in alkaline pyridine (pyridine:0.1N NaOH, 2:1 [vol/vol]), and incubated for 10 min in the dark. Cell lysates were cleared by centrifugation and analyzed spectrophotometrically. Two independent experiments were performed with independently grown cultures.
Detection of carbonylated proteins.
An OxyBlot protein oxidation detection kit from Millipore (Billerica, MA) was used to detect carbonylated proteins. The kit provides reagents for the immunodetection of carbonyl groups introduced into proteins that have undergone oxidative modification. Carbonylated proteins were successfully detected with the kit in a trial experiment performed with S. cerevisiae cells that were exposed to 1 mM H2O2 in early log phase for 3 h (data not shown). To detect oxidative stress due to sampangine, an overnight culture of S. cerevisiae was grown in SD broth (MOPS buffered; pH 7.0) and used to inoculate fresh medium at an OD600 of 0.1. After one doubling, either sampangine (1.17 μg/ml) or DMSO (0.25%) was added to the cultures. The cells were allowed to grow and were harvested by centrifugation at one doubling (2.5 h) and two doublings (5 h) after treatment. Five-milliliter aliquots of cells were washed two times with sterile distilled water, and the pellets were stored at −80°C. The experimental conditions, including media, temperature, aeration, and concentration of sampangine, were identical to the conditions used in the transcript profiling experiments. Two independent experiments were performed with independently grown cultures.
Protein extracts were prepared by treating the cell pellets with 50 μl of Y-PER reagent (Pierce Biotechnology, Inc., Rockford, IL) containing 1% (vol/vol) beta-mercaptoethanol and 10 mM phenylmethanesulfonyl fluoride, according to the manufacturer's instructions. The protein concentration was determined using the Bradford assay. Twenty micrograms of protein from each extract was derivatized with 2,4-dinitrophenylhydrazine (DNPH) in the presence of 6% sodium dodecyl sulfate (SDS), according to the instructions in the OxyBlot kit manual. Identical negative control reactions were performed for each extract to generate nonderivatized samples. The samples were separated by SDS-polyacrylamide gel electrophoresis followed by Western blotting. The blotted membrane was incubated with primary antibody (1:145 dilution), specific to the DNP moiety, followed by incubation with horseradish peroxidase-conjugated secondary antibody (1:275 dilution). Chemiluminescent detection was performed with reagents from an ECL Western blotting system (Amersham Biosciences). To ensure equal loading and intact proteins, a polyacrylamide gel was run with an aliquot of the derivatized and nonderivatized samples and stained with GelCode Blue stain reagent (Pierce Biotechnology).
Multicopy library screening.
S. cerevisiae strain JK93dα was transformed with a multicopy genomic DNA library received from J. Heitman (Duke University), which was constructed in the 2μ vector pRS426. Transformants were selected on SC-Ura (SC minus Ura) agar plates. A total of ∼ 1 × 106 colonies were obtained and pooled in SC-Ura broth. Glycerol stocks (15% [vol/vol] final glycerol concentration) were prepared from aliquots of the pooled material and stored at −80°C. The preparation of glycerol stocks from pooled transformants eliminates the need to perform a high-efficiency yeast transformation for each screening. To screen the library against sampangine, a glycerol stock was thawed on ice, diluted to a density of 1 × 106 cells/ml, and plated on 10 SC-Ura agar plates (150 by 15 mm in size) with 0.3 ml of diluted cells per plate. After 2 days of incubation at 30°C, the transformants were pooled and diluted to a density of 1 × 106 cells/ml, and 0.3 ml of cells was plated onto each of 10 SC-Ura agar plates (150 by 15 mm in size) containing 4 μg/ml sampangine. At this concentration of sampangine, yeast cells carrying vector alone (strain TXSc001) plated at a similar cell density showed no growth up to 5 days. A total of 50 transformants that survived on sampangine plates were obtained after 5 days of incubation at 30°C. Plasmid DNA was isolated from the transformants using a QIAPrep Spin MiniPrep kit (Qiagen) with the modification that cell lysis was performed with P1 buffer in the presence of acid-washed glass beads (Sigma) in a bead mill (Retsch, Inc., Newtown, PA). The plasmids were transformed into Escherichia coli DH5α cells, amplified, and purified using standard protocols. The plasmid DNA was digested to check for the presence of inserts and to identify duplicate clones. Twelve clones had no inserts or showed indications of missing restriction sites and rearrangements. Of the remaining 38 clones, 14 different restriction patterns were obtained upon digestion with EcoRI. Clones representing each pattern were partially sequenced using T7 and T3 primers, and the genes present in them were identified using SGD. All 14 clones contained the CIN5 gene present in genomic fragments of various lengths. Clones carrying only the full-length CIN5 open reading frame were retransformed into JK93dα cells, and their resistance to sampangine was confirmed in drop test agar assays, as described above. One of the confirmed S. cerevisiae strains carrying pRS426-CIN5 was designated TXSc025.
Profiling data accession number.
The transcriptional profiling data described in this article have been deposited in NCBI's Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO series accession no. GSE10104.
RESULTS
Transcriptome response to sampangine in S. cerevisiae.
Sampangine displays potent antifungal activity, comparable to that of the widely used drug amphotericin B, against the fungal pathogens C. albicans, Candida glabrata, Candida kruseii, C. neoformans, and A. fumigatus (Table 1). In order to understand the cellular effects of sampangine in fungal cells, we made use of S. cerevisiae, an established model organism that has been extensively used for elucidating the molecular targets of antifungal and therapeutic compounds (reviewed in references 56 and 64). A transcriptional profiling study was conducted using S. cerevisiae cells that were treated with a concentration of sampangine that resulted in 50% growth inhibition (1.17 μg/ml). Genes that were significantly differentially expressed between the treated and untreated cells (P value, ≤0.001) were identified as described in Materials and Methods. A total of 204 genes showed expression changes in response to sampangine, with 129 genes showing increased expression and 75 genes showing decreased expression. Data for a few selected genes are shown in Fig. 1 to highlight the overall biological response to sampangine.
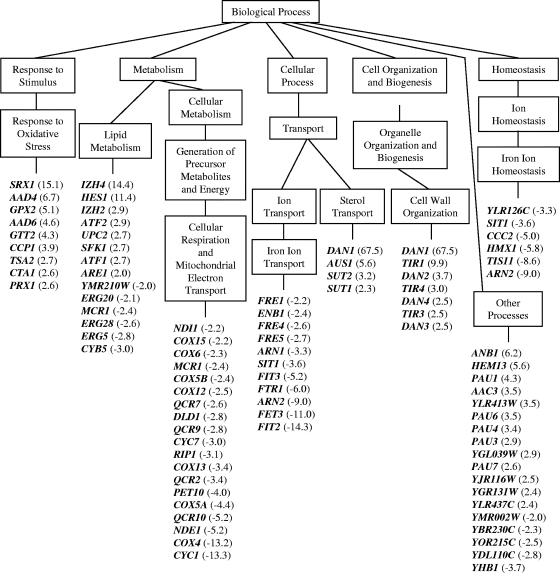
FIG. 1.
Distribution of genes responding to sampangine in S. cerevisiae. Data are shown for a subset of genes that were significantly induced or repressed (P value, ≤0.001) in three biological replicate samples. Data were organized into various biological processes using GO Term Finder and GO Slim Mapper tools in SGD. GO terms shown are those that were considered significantly overrepresented by the analyses. The terms “cell wall organization” and “other processes” were included separately to highlight additional genes that are relevant to the overall biological response to sampangine. For simplicity, not all “child terms” within a “parent term” are shown. Numbers in parentheses represent average change in gene expression. Positive values indicate induction, and negative values indicate repression. A complete list of all significant genes can be found in Table S1 in the supplemental material.
The global transcriptional response to sampangine revealed several similarities to that shown by S. cerevisiae cells grown under anaerobic conditions in previous studies (8, 36, 40, 41, 58, 65, 67). In these studies, the most consistent gene expression responses to anaerobiosis appeared to be the downregulation of genes required for mitochondrial respiration and energy metabolism regulated by the transcription factors Hap1p and Hap2/3/4/5p, the upregulation of genes required for sterol homeostasis and cell wall function under the regulation of the transcription factor Upc2p, and the upregulation of genes regulated by the transcription factor Rox1p. All of these transcription factors are known to regulate gene expression in response to altered heme and/or cellular oxygen levels. Many of the genes responding to sampangine are also regulated by these transcription factors (Table 3).
TABLE 3.
Distribution of sampangine-responding genes in S. cerevisiae based on their potential regulation by known transcription factors
| Regulator | Target genes | Biological process | References |
|---|---|---|---|
| Hap1p, Hap2/3/4/5p | COX4, COX5A, COX6, COX12, COX13, COX15, QCR2, QCR7, QCR9, QCR10, CYC1, DLD1, RIP1, PET10, NDE1, NDI1 | Respiration and electron transport | 35, 66,a68, 78, 80 |
| Upc2p | DAN1, DAN2, DAN4, TIR1, TIR3, TIR4 | Cell wall organization | 1, 66,a73 |
| DAN1, AUS1 | Sterol transport | ||
| HES1, UPC2 | Lipid metabolism | ||
| PAU1, PAU3, PAU4, PAU6, PAU7 | Other processes | ||
| Rox1p | ATF1, ARE1 | Lipid metabolism | 1, 35, 36, 66,a68 |
| DAN1, TIR1, TIR3 | Cell wall organization | ||
| ANB1, HEM13, AAC3 | Other processes | ||
| Aft1p | FRE1, FRE4, FRE5, FET3, FTR1, FIT2, FIT3, ARN1, ARN2, ENB1, SIT1 | Iron ion transport | 30, 62, 66,a76, 77 |
| ARN2, SIT1, TIS11, HMX1, CCC2, YLR126C | Iron ion homeostasis |
Describes the YEASTRACT database, which is a curated repository of more than 27,800 regulatory associations between transcription factors and target genes in S. cerevisiae. Target genes listed above fall into the category of “documented association” in the database, based on experimental evidence of at least one of the following: (i) change in expression of target gene due to a deletion (or mutation) in the gene encoding the transcription factor and (ii) binding of the transcription factor to the promoter region of the target gene.
Several genes that were repressed by sampangine treatment are regulated by the heme-dependent transcription factors Hap1p and/or Hap2/3/4/5p (Fig. 1 and Table 3). The majority of these genes are activated by these transcription factors under aerobic growth conditions (reviewed in references 35, 78, and 80), and repressed under anaerobic conditions (8, 36, 40, 41, 58, 65, 67). The downregulation of these “aerobic” genes during anaerobiosis is consistent with the fact that many of them encode cytochromes, cytochrome-related oxidoreductases, and NADH-dependent dehydrogenases that play important roles in mitochondrial electron transport and respiration. Thus, sampangine treatment appears to block the Hap1p- and Hap2/3/4/5p-dependent expression of genes normally induced under the aerobic conditions employed in the present work. It should be noted that the HAP1 gene in the S. cerevisiae S288C strain used in this study is interrupted by a Ty1 element in the 3′ end of its open reading frame (data not shown), resulting in a hypomorphic allele that is partially functional under anaerobic conditions, thus retaining some activity in this strain (19).
Sampangine treatment also resulted in the induction of several genes known to be regulated by the transcription factor Upc2p (Fig. 1 and Table 3). All of these genes were previously shown to be induced under anaerobic conditions in various microarray studies with S. cerevisiae (8, 36, 40, 41, 58, 65, 67). Interestingly, two of the Upc2p-induced genes listed in Table 3, AUS1 and DAN1, are involved in the uptake of extracellular sterol (73). Two additional sampangine-induced genes not known to be regulatory targets of Upc2p, SUT1, and SUT2 (listed under “Sterol Transport” in Fig. 1), are also involved in sterol uptake (2, 54). The gene SUT1 was also reported to be upregulated under low-oxygen conditions by both Tai et al. (65) and Ter Linde et al. (67). An increased expression level of sterol uptake genes in S. cerevisiae under anaerobic conditions is associated with the phenomenon of “aerobic sterol exclusion” (45). Under aerobic conditions, S. cerevisiae cells fulfill their sterol requirements via de novo synthesis of ergosterol, while under anaerobic conditions, sterol biosynthesis is compromised and the cells are dependent upon sterol uptake for survival. Interestingly, sterol uptake can occur aerobically in heme-deficient mutants (e.g., 22, 43), indicating a close relationship between oxygen, heme, and sterol homeostasis in S. cerevisiae.
Rox1p is a heme-dependent repressor of anaerobic genes (reviewed in references 35, 78, and 80), and several Rox1p-regulated anaerobic genes were induced in the presence of sampangine (Fig. 1 and Table 3). All of these genes have also been previously reported to be induced under anaerobic conditions in S. cerevisiae (8, 36, 40, 41, 58, 65, 67). The genes ARE1 (sterol metabolism; 71), HEM13 (heme biosynthesis; 31), and ATF1 (synthesis of unsaturated fatty acids; 17) all encode proteins that play important roles in heme-dependent pathways.
Treatment with sampangine resulted in the downregulation of numerous genes involved in iron transport and homeostasis (Fig. 1 and Table 3) that are known to be regulated by the iron-sensing transcription factor Aft1p (reviewed in reference 30). Interestingly, Lai et al. (40) have reported the downregulation of genes required for iron homeostasis (FRE1, UTR1, FTR1, and FET3) in S. cerevisiae in response to anaerobiosis. Crisp et al. (13) have also shown that the inhibition of heme biosynthesis results in decreased transcription of iron uptake genes in S. cerevisiae. This interplay between iron, heme, and oxygen can be attributed to the fact that many iron-containing proteins play important roles in oxygen-dependent reactions involving heme, such as heme biosynthesis, sterol metabolism, lipid desaturation, and mitochondrial respiration (reviewed in reference 30).
Overall, the transcriptional response seen for S. cerevisiae cells following exposure to sampangine in the present work is highly reminiscent of that observed for S. cerevisiae cells grown under conditions of reduced oxygen availability. One notable distinction is that sampangine treatment resulted in the induction of genes involved in responses to oxidative stress, which are typically observed to be downregulated under low-oxygen conditions in S. cerevisiae (e.g., 41). Oxidative stress-related genes induced by sampangine included SRX1, AAD4, GPX2, AAD6, GTT2, CCP1, TSA2, CTA1, and PRX1 (Fig. 1). These genes encode various peroxidases, catalases, and oxidoreductases that play a role in defense responses to oxidative stress. However, this result is consistent with a previous report documenting the production of reactive oxygen species in human cancer cells following exposure to sampangine (33). The oxidative stress response to sampangine is discussed in more detail below (see Discussion).
Role of heme in the activity of sampangine against S. cerevisiae.
Anaerobic responses in S. cerevisiae are regulated at the level of gene expression, and heme plays a pivotal role in this regulation (reviewed in reference 35). When oxygen levels decrease, heme synthesis declines, and transcription factors such as Hap1p, Hap2/3/4/5p, Rox1p, and Upc2p are directly affected, resulting in the induction of anaerobic genes and the downregulation of aerobic genes. Given the important role played by heme in the regulation of oxygen-responsive genes, we were therefore also interested in determining whether the inhibitory effects of sampangine involved perturbations in heme metabolism.
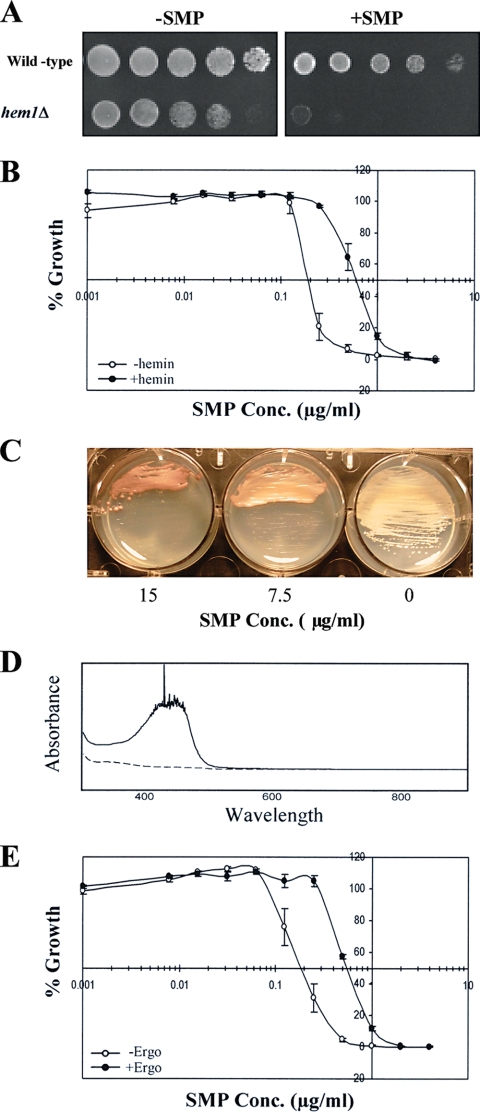
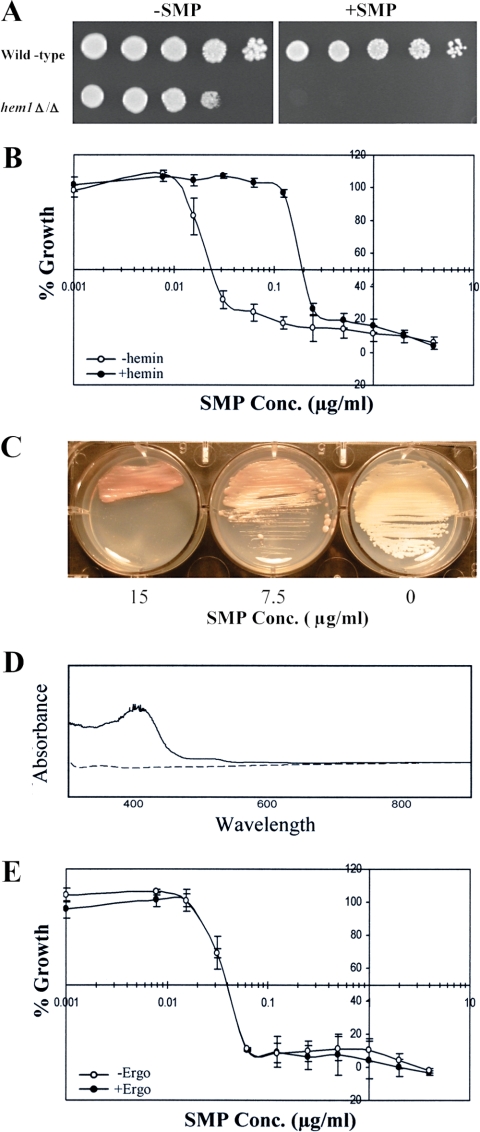
To examine the potential involvement of heme, we first tested the susceptibility to sampangine of a hem1Δ mutant of S. cerevisiae, which has a deletion in the gene encoding the enzyme ALA synthase that catalyzes the first step in the heme biosynthetic pathway (70). The hem1Δ mutant showed dramatically increased sensitivity to sampangine compared to the corresponding wild-type strain (Fig. 2A). Since this mutant requires ALA for survival, we included ALA in the medium; however, in order test the effect of heme deficiency on sampangine sensitivity, a low concentration of ALA was used (8 μg/ml). We also tested whether the presence of exogenous heme would alter the sensitivity to sampangine in wild-type S. cerevisiae cells. The presence of exogenously supplied heme in the form of hemin increased the IC50 for sampangine from 0.2 μg/ml to 0.6 μg/ml and the MIC from 0.5 μg/ml to 2.0 μg/ml (Fig. 2B).
FIG. 2.
Role of heme in the activity of sampangine in S. cerevisiae. (A) Dilutions (fivefold) of wild-type (BY4741) and hem1Δ (yMH339) strains grown as described in Materials and Methods were inoculated onto YPD + ALA (8 μg/ml) agar medium and incubated for 2 days at 30°C. −SMP, medium containing solvent (DMSO); +SMP, medium containing sampangine at 1.25 μg/ml. (B) Results shown are from broth microdilution assays performed in triplicate using S. cerevisiae strain S288C in the presence of various concentrations (Conc.) of sampangine with 32.5 μg/ml hemin (+hemin) or without hemin (−hemin). Percent growth is shown as the mean ± standard error of the mean (SEM). (C) S. cerevisiae S288C colonies grown on YPD agar were streaked on SD agar medium containing various concentrations of sampangine and incubated for 3 days at 30°C. The 0 represents medium containing solvent (DMSO). (D) Absorption spectra of alkaline pyridine extracts prepared from S. cerevisiae S288C cells treated with sampangine (solid line) or with DMSO solvent (dashed line). (E) Results are shown from broth microdilution assays performed in triplicate with S. cerevisiae strain S288C in the presence of various concentrations of sampangine with 10 μg/ml ergosterol (+Ergo) or without ergosterol (−Ergo). Percent growth is shown as the mean ± SEM.
Intriguingly, we also observed that wild-type S. cerevisiae cells grown on agar plates in the presence of high concentrations of sampangine accumulated a red pigmentation after 3 days of growth (Fig. 2C). This result is suggestive of a possible disruption in heme biosynthesis, resulting in the accumulation of heme biosynthetic precursors, such as porphyrins, in the cells. For example, a similar phenotype has been observed for the pop1 mutants of Saccharomycopsis lipolytica, which produce red-pigmented colonies due to the accumulation of protoporphyrin IX, the immediate precursor of heme in the heme biosynthetic pathway (7). In addition, mutants of S. cerevisiae with partial defects in heme biosynthesis also accumulate porphyrins and exhibit red fluorescence under UV light (34, 69). To further investigate the nature of the pigment accumulating in S. cerevisiae cells exposed to sampangine, we compared the absorption spectra of alkaline pyridine extracts prepared from wild-type cells treated with sampangine to those treated with DMSO solvent alone. Compared with solvent-treated cells, the sampangine-treated cells exhibited increased absorbance, with a maximum at approximately 400 nm (Fig. 2D), characteristic of the Soret peak of free porphyrins (16). These results suggest that sampangine exposure in S. cerevisiae might result in the accumulation of heme biosynthetic intermediates, arising either through the inhibition of a heme biosynthetic enzyme or through the inhibition of secondary processes that regulate the pathway.
An additional indicator of potential heme involvement is the observation that S. cerevisiae cells grown under aerobic conditions become dependent upon exogenous sterol for survival under conditions of heme deficiency (e.g., see references 22 and 43). Therefore, we determined whether the presence of exogenous sterol would alter the sensitivity of S. cerevisiae to sampangine. The IC50 of sampangine increased from 0.2 μg/ml to 0.55 μg/ml, and the MIC increased from 0.5 to 2.0 μg/ml, in the presence of ergosterol (Fig. 2E), indicating that ergosterol reduced the susceptibility of S. cerevisiae cells to sampangine. This result indicates that, just like heme biosynthetic mutants, wild-type S. cerevisiae cells treated with sampangine have the ability to import exogenous sterol under aerobic conditions.
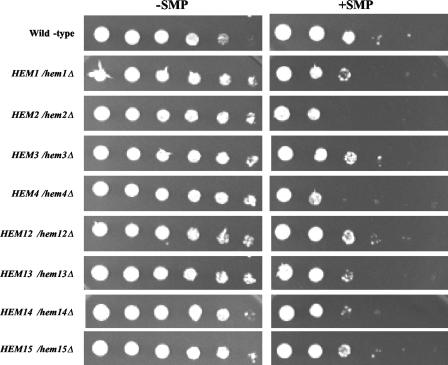
To further explore the effect of sampangine on heme metabolism, heterozygous mutants of S. cerevisiae carrying deletions in genes involved in each step of the heme biosynthetic pathway were also analyzed. This haploinsufficiency approach has been successfully used in several studies to determine the molecular targets of various clinically relevant drugs (e.g., see references 4, 20, and 46). As can be seen in Fig. 3, mutants with heterozygous deletions in the HEM2 and HEM4 genes, involved in the second and fourth steps in the heme biosynthetic pathway, showed a distinct increase in susceptibility to sampangine compared to the wild-type strain, and smaller but clearly discernible increases in sensitivity were also observed for mutants with heterozygous deletions in the HEM1, HEM13, and HEM14 genes. These results provide further evidence that the inhibitory activity of sampangine against S. cerevisiae is likely associated with effects on heme biosynthesis or heme-requiring pathways.
FIG. 3.
Effect of sampangine on the growth of heterozygous mutants harboring deletions in heme biosynthetic genes. Wild-type (BY4743), HEM1/hem1Δ, HEM2/hem2Δ, HEM3/hem3Δ, HEM4/hem4Δ, HEM12/hem12Δ, HEM13/hem13Δ, HEM14/hem14Δ, and HEM15/hem15Δ strains were grown as described in Materials and Methods. Dilutions (fivefold) were prepared from each culture, inoculated onto SC agar medium, and incubated for 3 days at 30°C. −SMP, medium containing solvent (DMSO); +SMP, medium containing sampangine at 2.0 μg/ml.
Sampangine induces oxidative stress in S. cerevisiae.
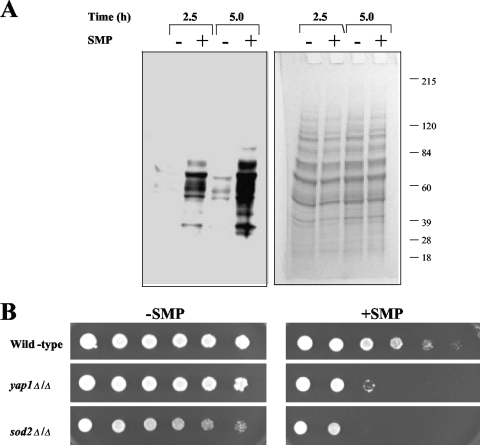
As mentioned above, in addition to the induction of anaerobic genes, sampangine treatment also resulted in the upregulation of a variety of genes associated with responses to oxidative stress. The latter observation is consistent with previous reports documenting the occurrence of oxidative stress in human cancer cells following exposure to this compound (33). To confirm if similar effects occur in S. cerevisiae cells, we monitored protein carbonylation levels after exposure to sampangine. Oxidative stress results in the formation of carbonyl groups on proteins due to the oxidation of specific amino acids, which can be detected by derivatization to DNP-hydrazone followed by Western analysis using a DNP-specific antibody (42). Using the same experimental conditions as were used in the transcriptional profiling experiments, S. cerevisiae cells were exposed to sampangine (1.17 μg/ml) for one doubling (2.5 h) and two doublings (5 h). As a control, cells were also treated with DMSO solvent alone. As can be seen in Fig. 4A, sampangine-treated cells contained increased levels of carbonylated protein compared with control treatments, demonstrating the occurrence of oxidative stress. In addition, the signals were stronger at 5 h, indicating that the amount of protein carbonylation in response to sampangine increased over time.
FIG. 4.
Role of oxidative stress in the activity of sampangine in S. cerevisiae. (A) S. cerevisiae S288C cells in early exponential phase were grown in the presence or absence of sampangine (SMP) for the indicated amount of time. Cells were harvested, protein extracts were prepared, and 20 μg of protein from each extract was derivatized with DNPH. The derivatized proteins were separated by SDS-polyacrylamide gel electrophoresis, blotted to polyvinylidene difluoride membrane, and detected with anti-DNP antibody. A stained protein gel on which aliquots of the same samples were separated is shown on the right. (B) Dilutions (fivefold) of wild-type (BY4743), yap1Δ/Δ, and sod2Δ/Δ strains grown as described in Materials and Methods were inoculated onto SC agar medium and incubated for 2 days at 30°C. −SMP, medium containing solvent (DMSO); +SMP, medium containing sampangine at 1.25 μg/ml.
We also made use of S. cerevisiae mutants carrying deletions in genes playing critical roles in cellular responses to oxidative stress, which exhibit hypersensitivity to chemical agents promoting the formation of reactive oxygen species. Mutants with deletions in YAP1, which encodes a transcription factor required for oxidative stress tolerance, as well as SOD2 encoding superoxide dismutase, showed increased sensitivity to sampangine compared to the corresponding wild-type parent strain (Fig. 4B). Taken together, the results shown in Fig. 4 indicate that sampangine exposure induces oxidative stress in S. cerevisiae cells, as previously observed for human cancer cells; thus, the generation of oxidative stress potentially represents a common consequence of exposure to this compound in diverse biological systems.
Overexpression of CIN5 confers resistance to sampangine in S. cerevisiae.
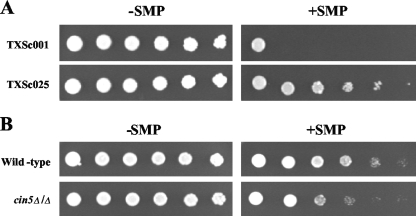
To further explore the cellular responses to sampangine in S. cerevisiae, an overexpression screening was performed using a genomic DNA library constructed in the 2μ vector pRS426. Thirty-eight insert-containing sampangine-resistant transformants were obtained from a primary screening of approximately one million library clones, and all resistant clones were subsequently identified as containing the CIN5 gene within their genomic inserts. One representative clone, designated TXSc025, exhibiting reduced sensitivity to sampangine relative to the empty vector-containing clone, is shown in Fig. 5A. The corresponding deletion mutant of CIN5 was similarly compared to wild-type S. cerevisiae cells, and it exhibited mild hypersensitivity to the compound (Fig. 5B).
FIG. 5.
Effect of CIN5 overexpression and deletion on susceptibility to sampangine. (A) JK93dα cells containing vector pRS426 (TXSc001) and a CIN5-overexpressing plasmid (TXSc025) were inoculated onto SC-Ura agar medium and incubated for 5 days at 30°C. (B) Dilutions (fivefold) of wild-type (BY4743) and cin5Δ/Δ strains were inoculated onto SC agar medium and incubated for 2 days at 30°C. −SMP, medium containing solvent (DMSO); +SMP, medium containing sampangine at 4 μg/ml (A) and 1.25 μg/ml (B).
Cin5p, also known as Yap4p, belongs to the Yap1p family of transcription factors and has been shown to be induced under various stress conditions, such as osmotic, heat, metal, and oxidative stresses (55). The overexpression of Cin5p has also been shown to confer resistance to DNA-damaging agents, such as cisplatin and methyl methanesulfonate (18), and to the antimalarial drug quinidine (14); however, the specific mechanism by which CIN5 overexpression confers resistance to various toxins is unclear at present. Interestingly, CIN5 was itself found to be upregulated in response to sampangine (see Table S1 in the supplemental material), suggesting that this transcription factor could also be required for long-term adaptation to the effects of heme deficiency that are implicated in sampangine's activity in the present study.
Transcriptome response to sampangine in C. albicans.
The most frequently encountered Candida species in opportunistic fungal infections of immunocompromised hosts is C. albicans, also considered to be the fourth-most-common cause of hospital-acquired bloodstream infections, with a mortality rate of approximately 40% (50, 75). While S. cerevisiae is an excellent model organism, it is nonpathogenic and does not provide information on possible fungal pathogenesis-related responses to sampangine, which could prove useful for its potential pharmacological development. Furthermore, sampangine displays potent activity against C. albicans (Table 1); therefore, transcriptional profiling studies were pursued for this organism as well.
As described above for the S. cerevisiae transcriptional profiling studies, experiments were conducted using C. albicans cells treated with a concentration of sampangine that results in 50% growth inhibition (6.13 μg/ml). Genes that were significantly differentially expressed between the treated and untreated cells (P value, ≤0.001) were identified as described in Materials and Methods. A total of 191 genes showed expression changes in response to sampangine, with 157 genes showing increased expression and 34 genes showing decreased expression. Data for a few selected genes are shown in Fig. 6 to highlight the overall biological response to sampangine.
FIG. 6.
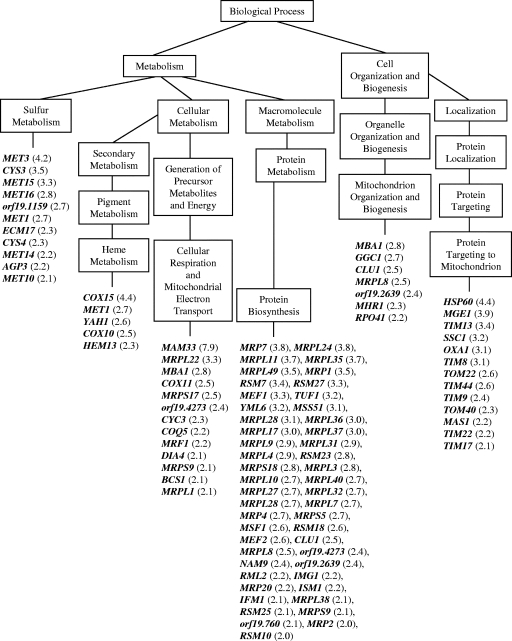
Distribution of genes responding to sampangine in C. albicans. Data are shown for a subset of genes that were significantly induced (P value, ≤0.001) in three biological replicate samples. Data were organized into various biological processes using the GO Term Finder tool in CGD. GO terms shown are those that were considered significantly overrepresented by the analysis. For simplicity, not all “child terms” within a “parent term” are shown. Numbers in parentheses represent average changes in gene expression. Gene names shown represent S. cerevisiae homologs. The systematic (orf19) name from CGD is used for genes with no known S. cerevisiae homologs. A complete list of all significant genes can be found in Table S2 in the supplemental material.
The majority of genes upregulated by sampangine in C. albicans are involved in mitochondrial functions related to sulfur metabolism, heme metabolism, respiration, electron transport, mitochondrial protein synthesis, mitochondrial organization and biogenesis, and protein targeting to the mitochondrion (Fig. 6). While these results suggest fundamental differences in the overall transcriptome responses of S. cerevisiae and C. albicans to sampangine (as discussed further below), striking similarities were also observed. First, genes involved in ergosterol biosynthesis were downregulated in both organisms: ERG5, ERG20, ERG28, and CYB5 in S. cerevisiae (Fig. 1) and ERG6 and ERG25 in C. albicans (see Table S2 in the supplemental material). Second, as seen for S. cerevisiae, several genes involved in iron homeostasis were downregulated in C. albicans; these included FTH1, RBT5, SIT1, and HMX1 (see Table S2 in the supplemental material). Third, the heme biosynthetic gene HEM13 was upregulated in both organisms (Fig. 1 and 6). A few additional genes involved in heme metabolism were upregulated in C. albicans (COX10, COX15, YAH1, MET1), homologs of which in S. cerevisiae are known to be involved in heme A and siroheme biosynthesis (6, 25). Furthermore, the induction of several additional genes in C. albicans could potentially be accounted for by perturbations in heme metabolism. For example, the induction of genes involved in sulfur metabolism could be related to the fact that some of the early steps in methionine biosynthesis are catalyzed by heme-containing enzymes, such as those encoded by MET10 and ECM17 (Fig. 6) (47). A few additional heme-related genes that were induced include genes that are involved in the import of cytochrome precursors into the mitochondria (e.g., TOM22 and TOM40, listed under “Protein Targeting to Mitochondrion” in Fig. 6) (72) and in the attachment of heme to cytochrome c (e.g., CYC3, listed under “Respiration and Electron Transport” in Fig. 6) (15). Thus, significant parallels appear to exist between the transcriptome responses to sampangine in C. albicans and in S. cerevisiae, suggesting that sampangine exposure could similarly affect heme-related pathways in the two organisms.
Role of heme in the activity of sampangine against C. albicans.
As described above for S. cerevisiae, additional experiments were performed to further examine the possibility that heme-related effects were also involved in sampangine's activity against C. albicans. First, as seen for S. cerevisiae (Fig. 2A), a hem1Δ/Δ mutant of C. albicans showed increased sensitivity to sampangine (Fig. 7A). As in the S. cerevisiae experiment, ALA was added to the medium at a low concentration (8 μg/ml) in order to test the effect of heme deficiency on sampangine sensitivity. Second, we tested whether the presence of exogenous heme would alter the sensitivity to sampangine in C. albicans cells. The presence of exogenously supplied heme in the form of hemin increased the IC50 of sampangine from 0.025 μg/ml to 0.2 μg/ml (Fig. 7B). Third, as observed for S. cerevisiae (Fig. 2C), when C. albicans cells were grown on agar plates in the presence of high concentrations of sampangine for 3 days, the cells accumulated a reddish pigmentation, suggesting that there was an accumulation of porphyrin intermediates (Fig. 7C). This accumulation was further confirmed by a comparative analysis of pyridine spectra from sampangine-treated and untreated C. albicans cells. Sampangine-treated cells revealed an absorption peak at ∼400 nm, indicative of free porphyrins (Fig. 7D).
FIG. 7.
Role of heme in the activity of sampangine in C. albicans. (A) Dilutions (fivefold) of wild-type (BWP17) and hem1Δ/Δ (KRC1) strains grown as described in Materials and Methods were inoculated onto YPD + Uri + ALA (8 μg/ml) agar medium and incubated for 2 days at 30°C. −SMP, medium containing solvent (DMSO); +SMP, medium containing sampangine at 5 μg/ml. (B) Results shown are from broth microdilution assays performed in triplicate on C. albicans strain SC5314 in the presence of various concentrations (Conc.) of sampangine with 32.5 μg/ml hemin (+hemin) or without hemin (−hemin). Percent growth is shown as the mean ± SEM. (C) C. albicans strain SC5314 colonies grown on YPD agar were streaked on SD agar medium containing various concentrations (Conc.) of sampangine and incubated for 3 days at 30°C. The 0 represents medium containing solvent (DMSO). (D) Absorption spectra of alkaline pyridine extracts prepared from C. albicans strain SC5314 cells treated with sampangine (solid line) or with DMSO solvent (dashed line). (E) Results are shown from broth microdilution assays performed in triplicate on C. albicans strain SC5314 in the presence of various concentrations (Conc.) of sampangine with 10 μg/ml ergosterol (+Ergo) or without ergosterol (−Ergo). Percent growth is shown as the mean ± SEM.
We also tested the effect of ergosterol on the susceptibility of C. albicans to sampangine. In contrast to S. cerevisiae (Fig. 2E), ergosterol did not alter the sensitivity of C. albicans cells to sampangine (Fig. 7E). This result is in agreement with the fact that heme mutants of S. cerevisiae rely on exogenous sterol for growth (22, 43), while a hem1Δ mutant of C. albicans cannot grow on exogenously supplied sterol (5).
DISCUSSION
Using a combination of genomic and genetic approaches, we have shown that heme plays an important role in the antifungal activity of the plant alkaloid sampangine. This conclusion is based on several lines of evidence obtained from the present work. (i) The transcriptome response of S. cerevisiae to sampangine is indicative of a hypoxia-like response, which is known to be mediated by heme through the transcription factors Hap1p, Hap2/3/4/5p, Rox1p, and Upc2p. (ii) Ergosterol reduced the susceptibility of S. cerevisiae to sampangine, suggesting that exposure to sampangine mimics heme deficiency, a condition which allows the uptake of exogenous sterol. (iii) A hem1Δ mutant of both S. cerevisiae and C. albicans exhibited increased sensitivity to sampangine, and exogenous heme provided as hemin reduced the sensitivity to sampangine in both organisms. (iv) Upon prolonged exposure to high concentrations of sampangine, both organisms produced red pigmentation which can be attributed to increased levels of heme biosynthetic precursors (free porphyrins) in the cells, based on spectrophotometric analysis of alkaline pyridine extracts. (v) S. cerevisiae mutants with heterozygous deletions in genes involved in five out of eight steps in the heme biosynthetic pathway showed increased sensitivity to sampangine.
While our results strongly suggest that the antifungal activity of sampangine most likely involves a disruption in heme metabolism or function, the exact mechanism behind this effect is unclear at this time. One possibility is that sampangine directly inhibits the activity of one of the enzymes in the heme biosynthetic pathway. However, it is unlikely that sampangine inhibits the enzyme ALA synthase (the first committed step in heme biosynthesis), given that exogenously supplied ALA had no apparent effect on sensitivity to sampangine (data not shown). In addition, the production of red pigmentation in the presence of sampangine, indicating the accumulation of porphyrin intermediates, would be consistent with pathway inhibition occurring at a later step.
Sampangine could also interfere with heme metabolism through indirect mechanisms, such as causing a reduction in available iron or the misdirection of biosynthetic intermediates, since heme biosynthesis in S. cerevisiae occurs as a multistep pathway spatially separated between the cytosol and the mitochondria (37). Heme biosynthesis is tightly coupled to iron availability due to the requirement of iron by the enzyme ferrochelatase, which catalyzes the final step in the heme biosynthetic pathway (38). Two mitochondrial iron transporters, Mrs3p and Mrs4p, have been shown to play an important role in the rapid transport of iron into the mitochondria for heme biosynthesis in S. cerevisiae (79). Interestingly, MRS4 is induced by sampangine treatment in S. cerevisiae cells (see Table S1 in the supplemental material), which could indicate a response to decreased mitochondrial iron levels. As mentioned above, another possibility is that sampangine could be involved in the misdirection of heme biosynthetic intermediates. In S. cerevisiae, most of the porphyrin intermediates are synthesized in the cytosol, and the final two steps leading to the synthesis of heme occur in the mitochondria. Thus, if sampangine were to, for example, interfere with the transport of porphyrin intermediates into the mitochondria, it would cause a reduction in the synthesis of heme. ATP-binding cassette-type transporters involved in mitochondrial porphyrin transport have been identified for mammalian systems (reviewed in reference 24), although the corresponding transporters in S. cerevisiae have yet to be identified.
From the results obtained for S. cerevisiae, it is evident that sampangine induces oxidative stress. This conclusion is supported by the following observations: (i) the induction of oxidative stress-responsive genes, (ii) the increased sensitivity of deletion mutants of YAP1 and SOD2 to sampangine, and (iii) the increased levels of protein carbonylation in the presence of sampangine. These results are consistent with previous reports indicating that exposure to sampangine, as well as to the structurally related marine alkaloid ascididemin, induces the formation of reactive oxygen species in cancer cell lines (33, 48). While the present data do not point to a specific mechanism for how sampangine could cause oxidative stress, one possibility would be directly related to the iminoquinone moiety within its structure (Table 1), which could participate in redox cycling reactions leading to the generation of reactive oxygen species within cells (48). Alternatively, oxidative stress could occur as a by-product of heme deficiency through the resultant accumulation of porphyrins, which are known to be potent generators of singlet oxygen in the presence of light (26). Heme depletion could also result in the generation of superoxide radicals through a mechanism involving a decrease in the activity of complex IV, the heme A-containing terminal complex in the electron transport chain, resulting in the leakage of electrons to molecular oxygen. Such a mechanism has been demonstrated with human fibroblasts (reviewed in reference 3). Further experimentation will be required to determine whether oxidative stress and perturbations in heme metabolism represent distinct or interrelated mechanisms in the inhibitory activity of sampangine.
As previously discussed, significant parallels were observed between the transcriptional responses in S. cerevisiae and C. albicans to sampangine treatment, although in S. cerevisiae, the global response was highly reminiscent of hypoxia-induced gene expression changes, which was not apparent in the case of C. albicans. This disparity is perhaps not unexpected given the very different strategies employed by these two organisms in responding to alterations in cellular oxygen levels. While S. cerevisiae responds to hypoxia by altering respiration, sterol transport, and cell wall biogenesis (reviewed in reference 35), C. albicans responds to hypoxia primarily by inducing glycolysis and the expression of hypha-specific genes (61). Setiadi et al. (61) have suggested that transcription factors required for regulating the hypoxic response in S. cerevisiae are either missing or have acquired divergent functions in C. albicans. For example, no apparent homolog of HAP1 exists in C. albicans, and the RFG1 gene of C. albicans (homolog of ROX1) does not participate in the regulation of hypoxic genes but instead functions in the regulation of filamentous growth (29). Based on these considerations, the differences seen in the genomic profiling of sampangine exposure between these species are not surprising, given the possibility that heme-related pathways could be altered by this compound. A clearer answer could likely be obtained from transcription profiling studies using comparable heme-deficient mutants from both organisms.
Given its essential role in numerous cellular processes, heme biosynthesis or heme signaling could serve as a highly effective target for inhibiting fungal growth and could contribute to a chemical defense strategy against fungal pathogens in aporphinoid-producing plant species of the Annonaceae family (10, 27, 28, 39, 44, 51, 57, 60). However, due to the importance of heme in all eukaryotes, a therapeutic drug that targets heme metabolism could most likely lack specificity for the fungal pathogen. Yet it is possible that specificity could be achieved if heme metabolism is under different regulatory controls between fungal and mammalian cells. For example, heme deficiency leads to mitochondrial iron accumulation in mammalian erythroid cells (59), whereas this is not the case in yeast (13). Further studies will be required to determine the utility of heme-inhibiting compounds like sampangine as therapeutic antifungal agents. Irrespective of these potential limitations, such compounds could serve as important pharmacological tools in investigating the consequences of heme deficiency and the relationships between heme, oxygen, sterol, and iron regulation in fungal cells.
This study lays the groundwork for future studies to determine the precise mechanism of action of sampangine. While our data strongly suggest an important role for heme in its antifungal activity, further analysis will be required to confirm that sampangine causes heme depletion and to determine the mechanism involved in this effect.
Supplementary Material
Acknowledgments
This work was supported in part by a grant from the Public Health Service, National Institute of Allergy and Infectious Diseases grant no. R01 AI27094, and USDA Agricultural Research Service Specific Cooperative Agreement no. 58-6408-2-0009.
We are grateful to Joseph Heitman (Duke University), Martin Bard (Indiana University), and Mark Hickman (Harvard University) for providing strains and for helpful discussions. We also thank Mark Hickman for providing primer sequences for HAP1 genotype determination.
Footnotes
Published ahead of print on 21 December 2007.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Abramova, N. E., B. D. Cohen, O. Sertil, R. Kapoor, K. J. Davies, and C. V. Lowry. 2001. Regulatory mechanisms controlling expression of the DAN/TIR mannoprotein genes during anaerobic remodeling of the cell wall in Saccharomyces cerevisiae. Genetics 1571169-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alimardani, P., M. Regnacq, C. Moreau-Vauzelle, T. Ferreira, T. Rossignol, B. Blondin, and T. Berges. 2004. SUT1-promoted sterol uptake involves the ABC transporter Aus1 and the mannoprotein Dan1 whose synergistic action is sufficient for this process. Biochem. J. 381195-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atamna, H., P. B. Walter, and B. N. Ames. 2002. The role of heme and iron-sulfur clusters in mitochondrial biogenesis, maintenance, and decay with age. Arch. Biochem. Biophys. 397345-353. [DOI] [PubMed] [Google Scholar]
- 4.Baetz, K., L. McHardy, K. Gable, T. Tarling, D. Rebérioux, J. Bryan, R. J. Andersen, T. Dunn, P. Hieter, and M. Roberge. 2004. Yeast genome-wide drug-induced haploinsufficiency screen to determine drug mode of action. Proc. Natl. Acad. Sci. USA 1014525-4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bard, M., A. M. Sturm, C. A. Pierson, S. Brown, K. M. Rogers, S. Nabinger, J. Eckstein, R. Barbuch, N. D. Lees, S. A. Howell, and K. C. Hazen. 2005. Sterol uptake in Candida glabrata: rescue of sterol auxotrophic strains. Diagn. Microbiol. Infect. Dis. 52285-293. [DOI] [PubMed] [Google Scholar]
- 6.Barros, M. H., and A. Tzagoloff. 2002. Regulation of the heme A biosynthetic pathway in Saccharomyces cerevisiae. FEBS Lett. 516119-123. [DOI] [PubMed] [Google Scholar]
- 7.Bassel, J., P. Hambright, R. Mortimer, and A. J. Bearden. 1975. Mutant of the yeast Saccharomycopsis lipolytica that accumulates and excretes protoporphyrin IX. J. Bacteriol. 123118-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becerra, M., L. J. Lombardia-Ferreira, N. C. Hauser, J. D. Hoheisel, B. Tizon, and M. E. Cerdan. 2002. The yeast transcriptome in aerobic and hypoxic conditions: effects of hap1, rox1, rox3 and srb10 deletions. Mol. Microbiol. 43545-555. [DOI] [PubMed] [Google Scholar]
- 9.Boshoff, H. I., T. G. Myers, B. R. Copp, M. R. McNeil, M. A. Wilson, and C. E. Barry III. 2004. The transcriptional responses of Mycobacterium tuberculosis to inhibitors of metabolism: novel insights into drug mechanisms of action. J. Biol. Chem. 27940174-40184. [DOI] [PubMed] [Google Scholar]
- 10.Bracher, F. 1994. Polycyclic aromatic alkaloids 10. Annonaceous alkaloids with antimycotic activity. Arch. Pharm. 327371-375. [DOI] [PubMed] [Google Scholar]
- 11.Camacho, M. R., G. C. Kirby, D. C. Warhurst, S. L. Croft, and J. D. Phillipson. 2000. Oxoaporphine alkaloids and quinones from Stephania dinklagei and evaluation of their antiprotozoal activities. Planta Med. 66478-480. [DOI] [PubMed] [Google Scholar]
- 12.Clark, A. M., E. S. Watson, M. K. Ashfaq, and C. D. Hufford. 1987. In vivo efficacy of antifungal oxoaporphine alkaloids in experimental disseminated candidiasis. Pharm. Res. 4495-498. [DOI] [PubMed] [Google Scholar]
- 13.Crisp, R. J., A. Pollington, C. Galea, S. Jaron, Y. Yamaguchi-Iwai, and J. Kaplan. 2003. Inhibition of heme biosynthesis prevents transcription of iron uptake genes in yeast. J. Biol. Chem. 27845499-45506. [DOI] [PubMed] [Google Scholar]
- 14.Delling, U., M. Raymond, and E. Schurr. 1998. Identification of Saccharomyces cerevisiae genes conferring resistance to quinoline ring-containing antimalarial drugs. Antimicrob. Agents Chemother. 421034-10341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dumont, M. E., T. S. Cardillo, M. K. Hayes, and F. Sherman. 1991. Role of cytochrome c heme lyase in mitochondrial import and accumulation of cytochrome c in Saccharomyces cerevisiae. Mol. Cell. Biol. 115487-5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falk, J. E. 1964. Porphyrins and metalloporphyrins. Elsevier Publishing Co., New York, NY.
- 17.Fujiwara, D., H. Yoshimoto, H. Sone, S. Harashima, and Y. Tamai. 1998. Transcriptional co-regulation of Saccharomyces cerevisiae alcohol acetyltransferase gene, ATF1 and Δ-9 fatty acid desaturase gene, OLE1 by unsaturated fatty acids. Yeast 14711-721. [DOI] [PubMed] [Google Scholar]
- 18.Furuchi, T., H. Ishikawa, N. Miura, M. Ishizuka, K. Kajiya, S. Kuge, and A. Naganuma. 2001. Two nuclear proteins, Cin5 and Ydr259c, confer resistance to cisplatin in Saccharomyces cerevisiae. Mol. Pharmacol. 59470-474. [DOI] [PubMed] [Google Scholar]
- 19.Gaisne, M., A. M. Bécam, J. Verdière, and C. J. Herbert. 1999. A ‘natural’ mutation in Saccharomyces cerevisiae strains derived from S288c affects the complex regulatory gene HAP1 (CYP1). Curr. Genet. 36195-200. [DOI] [PubMed] [Google Scholar]
- 20.Giaever, G., P. Flaherty, J. Kumm, M. Proctor, C. Nislow, D. F. Jaramillo, A. M. Chu, M. I. Jordan, A. P. Arkin, and R. W. Davis. 2004. Chemogenomic profiling: identifying the functional interactions of small molecules in yeast. Proc. Natl. Acad. Sci. USA 101793-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillum, A. M., E. Y. Tsay, and D. R. Kirsch. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 198179-182. [DOI] [PubMed] [Google Scholar]
- 22.Gollub, E. G., K. P. Liu, J. Dayan, M. Adlersberg, and D. B. Sprinson. 1977. Yeast mutants deficient in heme biosynthesis and a heme mutant additionally blocked in cyclization of 2,3-oxidosqualene. J. Biol. Chem. 2522846-2854. [PubMed] [Google Scholar]
- 23.Guinaudeau, H., M. Lebœuf, and A. Cavé. 1994. Aporphinoid alkaloids, V. J. Nat. Prod. 571033-1135. [Google Scholar]
- 24.Hamza, I. 2006. Intracellular trafficking of porphyrins. ACS Chem. Biol. 1627-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen, J., M. Muldbjerg, H. Cherest, and Y. Surdin-Kerjan. 1997. Siroheme biosynthesis in Saccharomyces cerevisiae requires the products of both the MET1 and MET8 genes. FEBS Lett. 40120-24. [DOI] [PubMed] [Google Scholar]
- 26.Hopf, F. R., and D. G. Whitten. 1978. In D. Dolphin (ed.), The porphyrins, vol. 2. Academic Press, New York, NY.
- 27.Hufford, C. D., A. S. Sharma, and B. O. Oguntimein. 1980. Antibacterial and antifungal activity of liriodenine and related oxoaporphine alkaloids. J. Pharm. Sci. 691180-1183. [DOI] [PubMed] [Google Scholar]
- 28.Hufford, C. D., S. Liu, A. M. Clark, and B. O. Oguntimein. 1987. Anticandidal activity of eupolauridine and onychine, alkaloids from Cleistopholis patens. J. Nat. Prod. 50961-964. [DOI] [PubMed] [Google Scholar]
- 29.Kadosh, D., and A. D. Johnson. 2001. Rfg1, a protein related to the Saccharomyces cerevisiae hypoxic regulator Rox1, controls filamentous growth and virulence in Candida albicans. Mol. Cell. Biol. 212496-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan, J., D. McVey Ward, R. J. Crisp, and C. C. Philpott. 2006. Iron-dependent metabolic remodeling in S. cerevisiae. Biochim. Biophys. Acta 1763646-651. [DOI] [PubMed] [Google Scholar]
- 31.Keng, T. 1992. HAP1 and ROX1 form a regulatory pathway in the repression of HEM13 transcription in Saccharomyces cerevisiae. Mol. Cell. Biol. 122616-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kluza, J., A. M. Clark, and C. Bailly. 2003. Apoptosis induced by the alkaloid sampangine in HL-60 leukemia cells: correlation between the effects on the cell cycle progression and changes of mitochondrial potential. Ann. N. Y. Acad. Sci. 1010331-334. [DOI] [PubMed] [Google Scholar]
- 33.Kluza, J., R. Mazinghien, K. Degardin, A. Lansiaux, and C. Bailly. 2005. Induction of apoptosis by the plant alkaloid sampangine in human HL-60 leukemia cells is mediated by reactive oxygen species. Eur. J. Pharmacol. 52532-40. [DOI] [PubMed] [Google Scholar]
- 34.Kurlandzka, A., and J. R. Rytka. 1985. Mutants of Saccharomyces cerevisiae partially defective in the last steps of the haem biosynthetic pathway: isolation and genetical characterization. J. Gen. Microbiol. 1312909-2918. [DOI] [PubMed] [Google Scholar]
- 35.Kwast, K. E., P. V. Burke, and R. O. Poyton. 1998. Oxygen sensing and the transcriptional regulation of oxygen-responsive genes in yeast. J. Exp. Biol. 2011177-1195. [DOI] [PubMed] [Google Scholar]
- 36.Kwast, K. E., L. C. Lai, N. Menda, D. T. James III, S. Aref, and P. V. Burke. 2002. Genomic analyses of anaerobically induced genes in Saccharomyces cerevisiae: functional roles of Rox1 and other factors in mediating the anoxic response. J. Bacteriol. 184250-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Labbe-Bois, R., and P. Labbe. 1990. Tetrapyrrole and heme biosynthesis in the yeast Saccharomyces cerevisiae, p. 235-285. In H. Dailey (ed.), Biosynthesis of heme and chlorophylls. Green, New York, NY.
- 38.Labbe-Bois, R. 1990. The ferrochelatase from Saccharomyces cerevisiae. Sequence, disruption, and expression of its structural gene HEM15. J. Biol. Chem. 26572787283. [PubMed] [Google Scholar]
- 39.Lago, J. H., M. H. Chaves, M. C. Ayres, D. G. Agripino, and M. C. Young. 2007. Evaluation of antifungal and DNA-damaging activities of alkaloids from branches of Porcelia macrocarpa. Planta Med. 73292-295. [DOI] [PubMed] [Google Scholar]
- 40.Lai, L. C., A. L. Kosorukoff, P. V. Burke, and K. E. Kwast. 2005. Dynamical remodeling of the transcriptome during short-term anaerobiosis in Saccharomyces cerevisiae: differential response and role of Msn2 and/or Msn4 and other factors in galactose and glucose media. Mol. Cell. Biol. 254075-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lai, L. C., A. L. Kosorukoff, P. V. Burke, and K. E. Kwast. 2006. Metabolic-state-dependent remodeling of the transcriptome in response to anoxia and subsequent reoxygenation in Saccharomyces cerevisiae. Eukaryot. Cell 51468-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levine, R. L., J. A. Williams, E. R. Stadtman, and E. Shacter. 1994. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 233346-357. [DOI] [PubMed] [Google Scholar]
- 43.Lewis, T. A., F. R. Taylor, and L. W. Parks. 1985. Involvement of heme biosynthesis in control of sterol uptake by Saccharomyces cerevisiae. J. Bacteriol. 163199-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu, S. C., B. Oguntimein, C. D. Hufford, and A. M. Clark. 1990. 3-Methoxysampangine, a novel antifungal copyrine alkaloid from Cleistopholis patens. Antimicrob. Agents Chemother. 34529-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lorenz, R. T., and L. W. Parks. 1991. Involvement of heme components in sterol metabolism of Saccharomyces cerevisiae. Lipids 26598-603. [DOI] [PubMed] [Google Scholar]
- 46.Lum, P. Y., C. D. Armour, S. B. Stepaniants, G. Cavet, M. K. Wolf, J. S. Butler, J. C. Hinshaw, P. Garnier, G. D. Prestwich, A. Leonardson, P. Garrett-Engele, C. M. Rush, M. Bard, G. Schimmack, J. W. Phillips, C. J. Roberts, and D. D. Shoemaker. 2004. Discovering modes of action for therapeutic compounds using a genome-wide screen of yeast heterozygotes. Cell 116121-137. [DOI] [PubMed] [Google Scholar]
- 47.Masselot, M., H. and De Robichon-Szulmajster. 1975. Methionine biosynthesis in Saccharomyces cerevisiae. I. Genetical analysis of auxotrophic mutants. Mol. Gen. Genet. 139121-132. [DOI] [PubMed] [Google Scholar]
- 48.Matsumoto, S. S., J. Biggs, B. R. Copp, J. A. Holden, and L. R. Barrows. 2003. Mechanism of ascididemin-induced cytotoxicity. Chem. Res. Toxicol. 16113-122. [DOI] [PubMed] [Google Scholar]
- 49.Montanha, J. A., M. Amoros, J. Boustie, and L. Girre. 1995. Anti-herpes virus activity of aporphine alkaloids. Planta Med. 61419-424. [DOI] [PubMed] [Google Scholar]
- 50.Morgan, J. 2005. Global trends in candidemia: review of reports from 1995-2005. Curr. Infect. Dis. Rep. 7429-439. [DOI] [PubMed] [Google Scholar]
- 51.Muhammad, I., D. C. Dunbar, S. Takamatsu, L. A. Walker, and A. M. Clark. 2001. Antimalarial, cytotoxic, and antifungal alkaloids from Duguetia hadrantha. J. Nat. Prod. 64559-562. [DOI] [PubMed] [Google Scholar]
- 52.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A2. NCCLS, Wayne, PA.
- 53.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M38-A. NCCLS, Wayne, PA.
- 54.Ness, F., S. Bourot, M. Regnacq, R. Spagnoli, T. Berges, and F. Karst. 2001. SUT1 is a putative Zn[II]2Cys6-transcription factor whose upregulation enhances both sterol uptake and synthesis in aerobically growing Saccharomyces cerevisiae cells. Eur. J. Biochem. 2681585-1595. [PubMed] [Google Scholar]
- 55.Nevitt, T., J. Pereira, and C. Rodrigues-Pousada. 2004. YAP4 gene expression is induced in response to several forms of stress in Saccharomyces cerevisiae. Yeast 211365-1374. [DOI] [PubMed] [Google Scholar]
- 56.Parsons, A. B., R. Geyer, T. R. Hughes, and C. Boone. 2003. Yeast genomics and proteomics in drug discovery and target validation. Prog. Cell Cycle Res. 5159-166. [PubMed] [Google Scholar]
- 57.Peterson, J. R., J. K. Zjawiony, S. Liu, C. D. Hufford, A. M. Clark, and R. D. Rogers. 1992. Copyrine alkaloids: synthesis, spectroscopic characterization, and antimycotic/antimycobacterial activity of A- and B-ring-functionalized sampangines. J. Med. Chem. 354069-4077. [DOI] [PubMed] [Google Scholar]
- 58.Piper, M. D., P. Daran-Lapujade, C. Bro, B. Regenberg, S. Knudsen, J. Nielsen, and J. T. Pronk. 2002. Reproducibility of oligonucleotide microarray transcriptome analyses. An interlaboratory comparison using chemostat cultures of Saccharomyces cerevisiae. J. Biol. Chem. 27737001-37008. [DOI] [PubMed] [Google Scholar]
- 59.Ponka, P. 1997. Tissue-specific regulation of iron metabolism and heme synthesis: distinct control mechanisms in erythroid cells. Blood 891-25. [PubMed] [Google Scholar]
- 60.Rao, J. U. M., G. S. Giri, T. Hanumaiah, and K. V. J. Rao. 1986. Sampangine, a new alkaloid from Cananga odorata. J. Nat. Prod. 49346-347. [Google Scholar]
- 61.Setiadi, E. R., T. Doedt, F. Cottier, C. Noffz, and J. F. Ernst. 2006. Transcriptional response of Candida albicans to hypoxia: linkage of oxygen sensing and Efg1p-regulatory networks. J. Mol. Biol. 361399-411. [DOI] [PubMed] [Google Scholar]
- 62.Shakoury-Elizeh, M., J. Tiedeman, J. Rashford, T. Ferea, J. Demeter, E. Garcia, R. Rolfes, P. O. Brown, D. Botstein, and C. C. Philpott. 2004. Transcriptional remodeling in response to iron deprivation in Saccharomyces cerevisiae. Mol. Biol. Cell 151233-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stevigny, C., C. Bailly, and J. Quetin-Leclercq. 2005. Cytotoxic and antitumor potentialities of aporphinoid alkaloids. Curr. Med. Chem. Anticancer Agents 5173-182. [DOI] [PubMed] [Google Scholar]
- 64.Sturgeon, C. M., D. Kemmer, H. J. Anderson, and M. Roberge. 2006. Yeast as a tool to uncover the cellular targets of drugs. Biotechnol. J. 1289-298. [DOI] [PubMed] [Google Scholar]
- 65.Tai, S. L., V. M. Boer, P. Daran-Lapujade, M. C. Walsh, J. H. de Winde, J. M. Daran, and J. T. Pronk. 2005. Two-dimensional transcriptome analysis in chemostat cultures. Combinatorial effects of oxygen availability and macronutrient limitation in Saccharomyces cerevisiae. J. Biol. Chem. 280437-447. [DOI] [PubMed] [Google Scholar]
- 66.Teixeira, M. C., P. Monteiro, P. Jain, S. Tenreiro, A. R. Fernandes, N. P. Mira, M. Alenquer, A. T. Freitas, A. L. Oliveira, and I. Sá-Correia. 2006. The YEASTRACT database: a tool for the analysis of transcription regulatory associations in Saccharomyces cerevisiae. Nucleic Acids Res. 34D446-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ter Linde, J. J., H. Liang, R. W. Davis, H. Y. Steensma, J. P. van Dijken, and J. T. Pronk. 1999. Genome-wide transcriptional analysis of aerobic and anaerobic chemostat cultures of Saccharomyces cerevisiae. J. Bacteriol. 1817409-7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ter Linde, J. J., and H. Y. Steensma. 2002. A microarray-assisted screen for potential Hap1 and Rox1 target genes in Saccharomyces cerevisiae. Yeast 19825-840. [DOI] [PubMed] [Google Scholar]
- 69.Urban-Grimal, D., and R. Labbe-Bois. 1981. Genetic and biochemical characterization of mutants of Saccharomyces cerevisiae blocked in six different steps of heme biosynthesis. Mol. Gen. Genet. 18385-92. [DOI] [PubMed] [Google Scholar]
- 70.Urban-Grimal, D., C. Volland, T. Garnier, P. Dehoux, and R. Labbe-Bois. 1986. The nucleotide sequence of the HEM1 gene and evidence for a precursor form of the mitochondrial 5-aminolevulinate synthase in Saccharomyces cerevisiae. Eur. J. Biochem. 156511-519. [DOI] [PubMed] [Google Scholar]
- 71.Valachovic, M., V. Klobucnikova, P. Griac, and I. Hapala. 2002. Heme-regulated expression of two yeast acyl-CoA:sterol acyltransferases is involved in the specific response of sterol esterification to anaerobiosis. FEMS Microbiol. Lett. 206121-125. [DOI] [PubMed] [Google Scholar]
- 72.Wiedemann, N., V. Kozjak, T. Prinz, M. T. Ryan, C. Meisinger, N. Pfanner, and K. N. Truscott. 2003. Biogenesis of yeast mitochondrial cytochrome c: a unique relationship to the TOM machinery. J. Mol. Biol. 327465-474. [DOI] [PubMed] [Google Scholar]
- 73.Wilcox, L. J., D. A. Balderes, B. Wharton, A. H. Tinkelenberg, G. Rao, and S. L. Sturley. 2002. Transcriptional profiling identifies two members of the ATP-binding cassette transporter superfamily required for sterol uptake in yeast. J. Biol. Chem. 27732466-32472. [DOI] [PubMed] [Google Scholar]
- 74.Wilson, R. B., D. Davis, and A. P. Mitchell. 1999. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J. Bacteriol. 1811868-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wisplinghoff, H., T. Bischoff, S. M. Tallent, H. Seifert, R. P. Wenzel, and M. B. Edmond. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39309-317. [DOI] [PubMed] [Google Scholar]
- 76.Yamaguchi-Iwai, Y., A. Dancis, and R. D. Klausner. 1995. AFT1: a mediator of iron regulated transcriptional control in Saccharomyces cerevisiae. EMBO J. 141231-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamaguchi-Iwai, Y., R. Stearman, A. Dancis, and R. D. Klausner. 1996. Iron-regulated DNA binding by the AFT1 protein controls the iron regulon in yeast. EMBO J. 153377-3384. [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang, L., and A. Hach. 1999. Molecular mechanism of heme signaling in yeast: the transcriptional activator Hap1 serves as the key mediator. Cell. Mol. Life Sci. 56415-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang, Y., E. R. Lyver, S. A. Knight, E. Lesuisse, and A. Dancis. 2005. Frataxin and mitochondrial carrier proteins, Mrs3p and Mrs4p, cooperate in providing iron for heme synthesis. J. Biol. Chem. 28019794-19807. [DOI] [PubMed] [Google Scholar]
- 80.Zitomer, R. S., and C. V. Lowry. 1992. Regulation of gene expression by oxygen in Saccharomyces cerevisiae. Microbiol. Rev. 561-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.