Abstract
Procyclic Trypanosoma brucei cells were synchronized with 0.2 mM hydroxyurea. The cells did not arrest at the G1/S boundary but proceeded through one round of replication and arrested near the end of S phase. The mitochondrial genome (kinetoplast DNA network) replicated, forming two progeny networks, but the repair of minicircle gaps was inhibited.
Trypanosoma brucei, the sleeping sickness parasite, has a unique mitochondrial genome known as kinetoplast DNA (kDNA). kDNA has several thousand minicircles (1 kb) and a few dozen maxicircles (23 kb) which are topologically interlocked in a giant network (reviewed in references 9 and 16). For studies on kDNA, we needed to synchronize T. brucei cultures. However, there were no published procedures. Hydroxyurea (HU) is used to synchronize other cell types (3); it inhibits ribonucleotide reductase (including T. brucei's [5]), an enzyme involved in deoxynucleoside triphosphate (dNTP) synthesis, by targeting its tyrosyl free radical (8, 14). The rationale for HU-mediated synchronization is that dNTP depletion prevents DNA replication, therefore stopping the cell cycle at the start of S phase, or, if already undergoing replication, within S phase.
HU at various concentrations synchronizes other kinetoplastids, such as Leishmania tarentolae (2.5 mM) (17), Crithidia fasciculata (2.5 mM) (2, 13), Leishmania major (5 mM) (20), Trypanosoma cruzi (20 mM) (4), and Leishmania infantum (5 mM) (18). In previous studies of T. brucei bloodstream forms, 0.1 mM HU did not affect DNA replication and could not synchronize the cells but 0.25 mM HU was toxic (12). For T. brucei procyclics, growth was inhibited by 0.67 mM HU, but DNA synthesis was not (1). These studies suggested that T. brucei cannot be synchronized by HU.
We have reinvestigated whether HU can synchronize T. brucei procyclics. We cultured cells (strain 29-13, from George Cross) at 27°C in semidefined medium (SDM-79) containing 10% fetal bovine serum, 15 μg/ml G418 (Sigma), and 50 μg/ml hygromycin B (Roche) (19). We incubated a 10-ml culture (2.5 × 106 cells/ml) in medium containing 0.2 mM HU (12 h, 27°C). We then removed the HU by centrifugation (1,200 × g, 10 min), followed by washing the cells twice with medium (10 ml, room temperature). We then continued culturing the cells without HU (12 h, 27°C). To assess synchrony, we fixed cells every 2 h, stained them with propidium diiodide, and conducted flow cytometry (7).
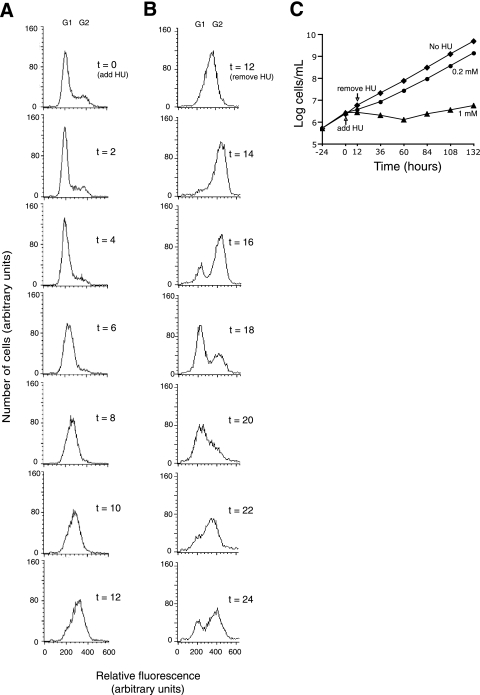
Since 0.2 mM HU only partially inhibited DNA synthesis, synchronization developed in an unexpected manner. Figure 1A (0 h) shows the results of flow cytometry of an asynchronous culture before the addition of HU. Some cells are in G1, and others, with apparently double the DNA content, are in G2. S-phase cells are in between. Within the first 4 h of HU treatment (0 h to 4 h), the cells become concentrated in the G1 and early S phases. Then, they progress slowly through S, developing synchrony and reaching late S phase at 12 h. Figure 1B shows that synchrony persists after the removal of HU (panel B shows the results of a different experiment from that whose results are shown in Panel A but which followed an identical HU treatment). During the first 4 h, the peak traverses through S and G2 (Fig. 1B, 14 h and 16 h) and the G1 peak gradually reappears (16 h and 18 h), indicating the start of the next cycle. At 20 and 22 h, the cells again traverse through S phase, and by 24 h they begin accumulating in G2 phase for the second time. In later stages of the experiment, the peak broadens (compare 22 h with 12 h), indicating gradual loss of synchrony. The growth curve (Fig. 1C) is consistent with the flow cytometry results. Without HU, the cells multiply with a doubling time of 12 h. On the addition of 0.2 mM HU (0 h), the doubling time declines >4-fold. Then, following the removal of HU (12 h) and a lag of ∼6 h, growth resumes with a doubling time comparable to that of untreated cells. HU at 0.4 mM also induced synchrony but at 0.1 mM did not. HU at 1 mM was toxic (Fig. 1C).
FIG. 1.
HU Synchronization of procyclic T. brucei. (A) Cells were treated with 0.2 mM HU for 12 h (0 h to 12 h), stained with propidium diiodide, and analyzed by flow cytometry. (B) Cells were treated the same as described for panel A, but the results are from the period after HU washout (12 h to 24 h). Panels A and B are from different but identical experiments. (C) Growth of T. brucei in medium supplemented with 0.2 mM HU (•), 1 mM HU (▴), or no HU (⧫). Extrapolation of the growth curve, after washout of 0.2 mM HU, indicated a lag of ∼6 h before the resumption of normal growth. Values on the y axis were calculated from measured values of cells/ml multiplied by the dilution factor.
Since our interest is kinetoplast replication, we evaluated the kDNA status during synchronization with 0.2 mM HU (the same conditions used for the experiment whose results are shown in Fig. 1). kDNA synthesis involves the release of covalently closed minicircles from the network for replication as free minicircles. The progeny-free minicircles, containing gaps, migrate to the antipodal sites (two protein assemblies flanking the kDNA disk, ∼180° apart). Within these sites, most, but not all, minicircle gaps are repaired. Then the progeny minicircles, still containing one or more gaps, are linked to the network periphery. When the minicircle copy number has doubled, the network splits in two and all gaps are repaired.
To evaluate kDNA replication, we stained HU-treated cells with 4′,6′-diamidino-2-phenylindole (DAPI) to determine the number of nuclei (1N or 2N) and kinetoplasts (1K or 2K). We also labeled the cells with terminal deoxynucleotidyl transferase (TdT) and a fluorescent dNTP (11). TdT labels 3′-hydroxyls in gaps in newly replicated network minicircles, as well as in free minicircle replication intermediates concentrated in the antipodal sites. There are several types of labeling patterns representing different stages of replication (Fig. 2A; see legend for description). Figure 2B and C show the kinetics of the appearance of each pattern. At the time of the addition of HU (Fig. 2B, 0 h), the TdT-positive trypanosomes, which are undergoing kDNA replication, constitute 25% of the cells (typical of asynchronous cultures) (11). By 6 h of HU treatment (0 h to 6 h), nearly all the cells become TdT positive. Inspection of the TdT-labeling pattern showed an accumulation of “early”-labeled cells (peaking at 3 h), followed by “late”-labeled cells (peaking at 6 h). Then, during the last 6 h of HU treatment (6 h to 12 h), the kinetoplast divides, forming 1N2K cells which remain TdT positive (“post”-replicative stage). Thus, the kDNA status during HU washout (12 h) is explained by one round of kDNA synthesis and division during the 12-h HU treatment. However, most minicircle gaps remain unrepaired.
FIG. 2.
Kinetics of kDNA replication in the presence of HU and after HU washout. The TdT-labeling pattern reveals the extent of replication. TdT-positive (TdT+) cells are undergoing replication, whereas TdT-negative (TdT-) cells include those that have not initiated replication and those that have completed postreplication gap repair. Based on the results of previous studies (10), kinetoplasts with labeling in antipodal sites and network poles are defined as “early” replicative stage, those with most or all of the network uniformly labeled are in “late” stages, and those with two TdT-positive networks are in the “post”-replicative stage. Cells were incubated with 0.2 mM HU for 12 h (0 h to 12 h), washed, and further incubated without HU (12 h to 24 h). Samples were examined by fluorescence microscopy after DAPI staining and TdT labeling. About 100 cells were categorized at each time point. (A) Examples of the different stages of kDNA replication. Cells are categorized by their TdT status and by the number of nuclei and kinetoplasts. Scale bar, 5 μm. (B) Kinetics of the appearance of the different stages during HU treatment. (C) The results of a different experiment, after HU washout, are shown.
Are the cells arrested at this point or would replication progress further with a longer HU incubation? In experiments not shown, an extra 6 h of incubation in 0.2 mM HU (total, 18 h) caused only 3% conversion of 1N2K to 2N2K. A low level of minicircle gap filling converted 35% of the TdT-positive cells to TdT negative. After HU removal, the 12- and 18-h HU-treated samples behaved similarly, indicating that the effects of extra HU treatment were reversible. Thus, kDNA replication and segregation must occur in 0.2 mM HU, with most cells arresting at the 1N2K/TdT-positive stage. A marked inhibition in gap filling blocked further progress.
As for the nucleus, we are sure that replication was nearly complete, because of the results of flow cytometry (Fig. 1A and B; 12 h) and because DAPI staining showed that most nuclei had grown in size and brightness (Fig. 2A; compare nuclei in no. 1 and no. 4).
After HU washout, there was a progressive appearance of other forms (Fig. 2C). At 14 h, most of the cells are still 1N2K but they are predominantly TdT negative, indicating efficient gap repair after HU removal. Nuclear division then occurs (2N2K cells are abundant at 16 and 18 h), followed by cytokinesis (1N1K cells peak at 18 and 20 h). A second round of kDNA replication is indicated by the abundance of TdT-positive cells at 18 and 20 h. As expected, the appearance of TdT-positive cells with “early” labeling precedes that of cells with “late” labeling.
Why does 0.2 mM HU synchronize cells? This concentration allows low-level nuclear DNA synthesis and slow progression through S phase; it may take 12 h to traverse S phase compared to the usual 3 h (Fig. 1A). If cells outside S phase move unretarded through G2 and/or G1 phase, they catch up with the cells traversing S phase, stopping near the end of S phase. Thus, during the slow passage through S phase, the cells gradually become synchronous, and they remain synchronous when DNA synthesis is fully restored by HU washout.
But why does HU arrest cells prior to nuclear division? One possibility could involve a nuclear DNA polymerase that functions after replication or late in S phase, assisting the repair of DNA damage prior to nuclear division, thus providing a cell cycle checkpoint. The Km for dNTPs of this repair polymerase might be higher than the Km of replicative nuclear polymerases. Therefore, upon HU treatment, the dNTP level in the nucleus might fall below the level required to sustain repair, but the replicative polymerases, with a lower Km, could operate at near-maximum velocity.
Similarly, the effect on kinetoplast replication could be related to the Kms of different mitochondrial polymerases for dNTPs. If the Km of the DNA polymerase β-PAK (the enzyme thought to fill gaps in network minicircles) (15), is higher than the Kms of kDNA replicative polymerases (6), its activity could be selectively reduced if HU depleted the dNTP level below its Km.
Further studies are needed to clarify the mechanism of cell cycle arrest near the end of nuclear replication and of the toxicity of higher HU concentrations. Despite these uncertainties, this new procedure should be useful as a tool for studying trypanosome biology.
Acknowledgments
This work was supported by National Institutes of Health grant AI-058613.
We thank Barbara Sollner-Webb, Michele Klingbeil, and members of our lab for helpful suggestions. We also thank Gokben Yildirir for technical assistance.
Footnotes
Published ahead of print on 14 December 2007.
REFERENCES
- 1.Brun, R. 1980. Hydroxyurea: effect on growth, structure, and [3H]thymidine uptake of Trypanosoma brucei procyclic culture forms. J. Protozool. 27122-128. [DOI] [PubMed] [Google Scholar]
- 2.Cosgrove, W. B., M. J. Skeen, and S. L. Hajduk. 1979. Effects of hydroxyurea on Crithidia fasciculata. J. Protozool. 26643-648. [DOI] [PubMed] [Google Scholar]
- 3.Davis, P. K., A. Ho, and S. F. Dowdy. 2001. Biological methods for cell-cycle synchronization of mammalian cells. BioTechniques 301322-1331. [DOI] [PubMed] [Google Scholar]
- 4.Galanti, N., J. A. Dvorak, J. Grenet, and J. P. McDaniel. 1994. Hydroxyurea-induced synchrony of DNA replication in the Kinetoplastida. Exp. Cell Res. 214225-230. [DOI] [PubMed] [Google Scholar]
- 5.Hofer, A., P. P. Schmidt, A. Graslund, and L. Thelander. 1997. Cloning and characterization of the R1 and R2 subunits of ribonucleotide reductase from Trypanosoma brucei. Proc. Natl. Acad. Sci. USA 946959-6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klingbeil, M. M., S. A. Motyka, and P. T. Englund. 2002. Multiple mitochondrial DNA polymerases in Trypanosoma brucei. Mol. Cell 10175-186. [DOI] [PubMed] [Google Scholar]
- 7.Kulikowicz, T., and T. A. Shapiro. 2006. Distinct genes encode type II topoisomerases for the nucleus and mitochondrion in the protozoan parasite Trypanosoma brucei. J. Biol. Chem. 2813048-3056. [DOI] [PubMed] [Google Scholar]
- 8.Liermann, B., G. Lassmann, and P. Langen. 1990. Quenching of tyrosine radicals of M2 subunit from ribonucleotide reductase in tumor cells by different antitumor agents: an EPR study. Free Radic. Biol. Med. 91-4. [DOI] [PubMed] [Google Scholar]
- 9.Liu, B., Y. Liu, S. A. Motyka, E. E. Agbo, and P. T. Englund. 2005. Fellowship of the rings: the replication of kinetoplast DNA. Trends Parasitol. 21363-369. [DOI] [PubMed] [Google Scholar]
- 10.Liu, Y., and P. T. Englund. 2007. The rotational dynamics of kinetoplast DNA replication. Mol. Microbiol. 64676-690. [DOI] [PubMed] [Google Scholar]
- 11.Liu, Y., S. A. Motyka, and P. T. Englund. 2005. Effects of RNA interference of Trypanosoma brucei structure-specific endonuclease-I on kinetoplast DNA replication. J. Biol. Chem. 28035513-35520. [DOI] [PubMed] [Google Scholar]
- 12.Mutomba, M. C., and C. C. Wang. 1996. Effects of aphidicolin and hydroxyurea on the cell cycle and differentiation of Trypanosoma brucei bloodstream forms. Mol. Biochem. Parasitol. 8089-102. [DOI] [PubMed] [Google Scholar]
- 13.Pasion, S. G., G. W. Brown, L. M. Brown, and D. S. Ray. 1994. Periodic expression of nuclear and mitochondrial DNA replication genes during the trypanosomatid cell cycle. J. Cell Sci. 1073515-3520. [DOI] [PubMed] [Google Scholar]
- 14.Reichard, P., and A. Ehrenberg. 1983. Ribonucleotide reductase-a radical enzyme. Science 221514-519. [DOI] [PubMed] [Google Scholar]
- 15.Saxowsky, T. T., G. Choudhary, M. M. Klingbeil, and P. T. Englund. 2003. Trypanosoma brucei has two distinct mitochondrial DNA polymerase beta enzymes. J. Biol. Chem. 27849095-49101. [DOI] [PubMed] [Google Scholar]
- 16.Shlomai, J. 2004. The structure and replication of kinetoplast DNA. Curr. Mol. Med. 4623-647. [DOI] [PubMed] [Google Scholar]
- 17.Simpson, L., and P. Braly. 1970. Synchronization of Leishmania tarentolae by hydroxyurea. J. Protozool. 17511-517. [DOI] [PubMed] [Google Scholar]
- 18.Soto, M., L. Quijada, C. Alonso, and J. M. Requena. 2000. Histone synthesis in Leishmania infantum is tightly linked to DNA replication by a translational control. Biochem. J. 34699-105. [PMC free article] [PubMed] [Google Scholar]
- 19.Wang, Z., J. C. Morris, M. E. Drew, and P. T. Englund. 2000. Inhibition of Trypanosoma brucei gene expression by RNA interference using an integratable vector with opposing T7 promoters. J. Biol. Chem. 27540174-40179. [DOI] [PubMed] [Google Scholar]
- 20.Zick, A., I. Onn, R. Bezalel, H. Margalit, and J. Shlomai. 2005. Assigning functions to genes: identification of S-phase expressed genes in Leishmania major based on posttranscriptional control elements. Nucleic Acids Res. 334235-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]