Abstract
Lethocerus indirect flight muscle has two isoforms of troponin C, TnC-F1 and F2, which are unusual in having only a single C-terminal calcium binding site (site IV, isoform F1) or one C-terminal and one N-terminal site (sites IV and II, isoform F2). We show here that thin filaments assembled from rabbit actin and Lethocerus tropomyosin (Tm) and troponin (Tn) regulate the binding of rabbit myosin to rabbit actin in much the same way as the mammalian regulatory proteins. The removal of calcium reduces the rate constant for S1 binding to regulated actin about threefold, independent of which TmTn is used. This is consistent with calcium removal causing the TmTn to occupy the B or blocked state to about 70% of the total. The mid point pCa for the switch differed for TnC-F1 and F2 (pCa 6.9 and 6.0, respectively) consistent with the reported calcium affinities for the two TnCs. Equilibrium titration of S1 binding to regulated actin filaments confirms calcium regulated binding of S1 to actin and shows that in the absence of calcium the three actin filaments (TnC-F1, TnC-F2 and mammalian control) are almost indistinguishable in terms of occupancy of the B and C states of the filament. In the presence of calcium TnC-F2 is very similar to the control with ∼80% of the filament in the C-state and 10-15% in the fully on M-State while TnC-F1 has almost 50% in each of the C and M states. This higher occupancy of the M-state for TnC-F1, which occurs above pCa 6.9, is consistent with this isoform being involved in the calcium activation of stretch activation. However, it leaves unanswered how a C-terminal calcium binding site of TnC can activate the thin filament.
Keywords: troponin C isoforms, tropomyosin, thin filament, calcium, stretch activation
Introduction
Insect flight needs special adaptations in the power producing flight muscles. The muscles must be relatively large to produce the power needed for flight, activation and deactivation must be rapid, and there must be an adequate energy supply. Many insects have asynchronous flight muscle in which contractions are not synchronous with impulses from the nerve. The muscles are activated by periodic stretches at constant low concentrations of Ca2+, and oscillatory contraction results from resonant changes in the shape of the thorax produced by opposing sets of indirect flight muscles (IFMs).1 Since individual contractions are not triggered by changes in Ca2+ concentration, there is little sarcoplasmic reticulum, and the muscle volume is largely occupied by myofibrils and mitochondria.2 Small insects with high wingbeat frequencies, such as Drosophila (frequency 200 Hz), have asynchronous muscle, as well as some larger insects with lower wingbeat frequencies. The giant waterbug Lethocerus with a wingbeat frequency of 30 Hz has asynchronous flight muscle and the IFM has been used to study the structure and mechanics of this type of muscle.
IFM can be activated by a second mechanism, which is not capable of producing flight. Single twitches are produced by nerve impulses and these fuse to give a tetanus at high frequencies of stimulation;3,4 fibres are activated isometrically and there is no resonant oscillation of the thorax. This type of activation is used to warm up the muscles before flight in bees and large insects.4 In isolated Lethocerus IFM fibres, the Ca2+ concentration needed to prime the fibres for stretch-activated contraction is lower than that needed for isometric contraction.5,6 Two recent studies on isolated Lethocerus fibres have shown that stretch-activated and isometric tension are complementary: as the Ca2+ concentration is increased, stretch-activated tension rises to a maximum, and decreases as the fibres produce more isometric tension.7,8
The two types of contraction are regulated by the tropomyosin-troponin (TmTn) complex on thin filaments.7 Thin filament regulatory proteins in IFM differ from those in vertebrate skeletal and cardiac muscle. In Lethocerus IFM, tropomyosin is a 70 kDa dimer like tropomyosin in vertebrate skeletal muscle; TnT has a negatively charged extension at the C terminus that is not present in vertebrate TnT; and the inhibitory component (TnH) is a fusion protein of TnI and a C-terminal extension rich in proline, alanine and glutamic acid.9,10 There are two isoforms of TnC in IFM: F1 which binds a single Ca2+ with high affinity at site IV in the C-terminal lobe, and F2 which binds two Ca2+, one with low affinity at site II in the N-terminal lobe, and one with higher affinity at site IV. Most of the TnC in IFM is the F1 isoform (the ratio of F1:F2 is 7:1; K. Leonard and B.B., unpublished results). By exchanging the TnC in isolated IFM fibres with F1 or F2, we have found that a troponin complex with F1 produces stretch-activated tension and a complex with F2 produces Ca2+-activated isometric tension.7 F2 is likely to regulate the thin filament by reversible Ca2+ binding to site II, in the same way as vertebrate TnC; but it is not known how F1 responds to stretch.
In the work presented here we examine the in vitro properties of the two forms of isolated TnC. Actin thin filaments were assembled from pyrene labelled rabbit actin (pyr-actin) and tissue purifed Lethocerus Tm, TnT and TnH (the insect equivalent of TnI) together with either of the two forms of Lethocerus TnC expressed in Escherichia coli. We show here that the Lethocerus proteins regulate the binding of rabbit S1 to actin in much the same way as the mammalian TmTn complex using the in vitro assays we have developed. Both TnC isoforms, in conjunction with the other Tm and Tn components limit the binding of S1 to actin but with different calcium sensitivities.
Results
Figure 1 shows a typical kinetic experiment to define the fraction of actinTmTn filament in the B-state. In Figure 1(a) the control assay is shown using the well-characterised rabbit protein system. In this assay 2.5 μM pyr-actin that had been pre-incubated with 1.0 μM rabbit TmTn was rapidly mixed with 0.25 μM rabbit S1 in the presence or absence of calcium. The observed transients were well described by a single exponential and the best-fit line is superimposed in each case. The amplitudes were almost identical with the kobs values of 3 s-1 and 0.85 s-1 in the presence and absence of calcium, respectively. The data thus show the typical 3.5-fold reduction in kobs on removal of calcium consistent with our published data.17,19 If KT is assumed to be small then k-Ca/k+Ca=KB/(1 + KB)=0.28 and therefore KB=0.38.
Figure 1.
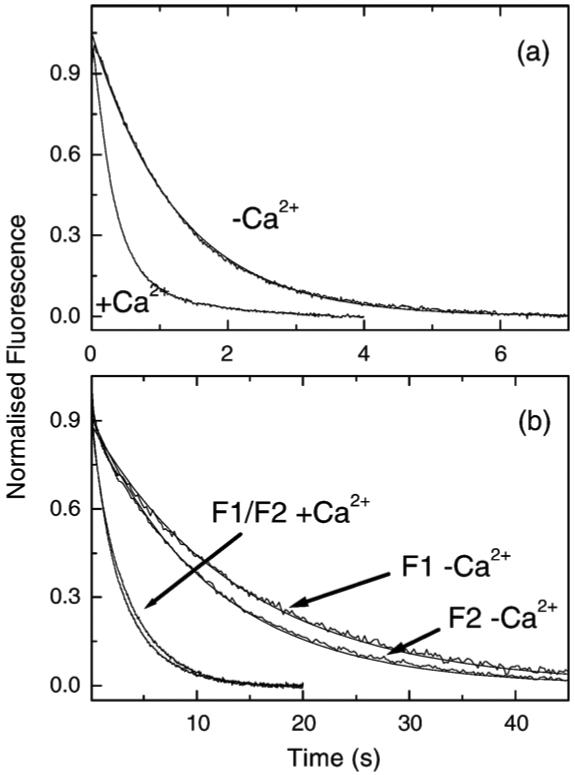
Influence of Lethocerus TmTn on the binding of rabbit S1 to actin. The fluorescence transients observed when 2.5 μM pyr-actin pre-incubated with 1 μM rabbit or Lethocerus TmTn complex was mixed with 0.25 μM rabbit S1. The best-fit single exponential fit is superimposed. (a) Control assay of rabbit S1 binding to rabbit actin with rabbit TmTn. The values of kobs were 3 s-1 and 0.85 s-1 for + and - Ca2+, respectively. (b) Assay for rabbit S1 binding to rabbit actin with Lethocerus TmTn containing TnC-F1 or F2. The values of kobs were 0.34 s-1 and 0.08 s-1 for F1 and 0.39 s-1 and 0.1 s-1 for F2 with and without Ca2+, respectively. Experimental conditions: 140 mM KCl, 5 mM MgCl2, 1 mM DTT, 20 mM Mops (pH 7.0) at 20 °C, with either 2 mM CaCl2 or 2 mM EGTA.
The experiment was repeated using the same concentrations of proteins but using rabbit actin that had been pre-incubated with 1 μM of Lethocerus TmTnTH together with 1 μM Lethocerus TnC isoform F1 or F2 and the results are shown in Figure 1(b). In all cases a single exponential transient was observed but the kobs values were reduced by about tenfold compared to the rabbit TmTn controls. The values of kobs were 0.34 s-1 and 0.08 s-1 for F1 and 0.39 s-1 and 0.1 s-1 for F2 with and without Ca2+, respectively. These results were independent of using increased Tm or Tn concentrations showing that the system was saturated with the regulatory proteins. Increasing actin concentrations increased the kobs values proportionally, indicating that the reaction was pseudo-first-order in actin concentration. For some preparations of the insect proteins a biphasic reaction was observed with the fast phase being approximately ten times faster than the values shown here but with an amplitude of less than 10% of the total. This indicated that the actin was not saturated with TmTn and the fast phase could be reduced or eliminated by increasing the concentration of TmTn. Note that the ratio of k-Ca/k+Ca was similar for both F1 and F2 (0.24, 0.26) and similar but slightly smaller than that of the rabbit control experiment (0.28).
The simplest explanation of the observed data is that the presence of the Lethocerus regulatory proteins reduces the second-order rate constant for myosin binding by a factor of 10, compared to the rabbit TmTn. To test this interpretation the experiment was repeated (for the +calcium case) with excess S1 binding to 0.25 μM actinTmTn. The kobs values were similar to those for excess actin, confirming that the slower kobs is due to a change in the apparent second-order rate constant not the availability of the actin sites. The reduction in kobs for the excess actin case, on removal of calcium is then due to a similar occupancy of the blocked states as for rabbit proteins. We will assume that this is the correct interpretation and return to this again in the Discussion. Since we do not yet have a value of KT we can only make an estimate of KB by assuming that KT is <1. In this case KB would be 0.31 and 0.34 for F1 and F2, respectively.
Having defined the extent of the calcium-induced switch to the blocked state we examined the calcium sensitivity of the occupancy of the blocked state. This can be done simply by repeating the measurements of Figure 1 over a range of calcium concentrations and plotting kobs as a function of calcium concentration. While this can be done it does require very large amounts of the thin filament proteins Tm and Tn which are in limited supply when purified from Lethocerus. We therefore explored the use of a more efficient method using much lower concentrations of thin filament proteins. In principle the measurements can be repeated at lower concentrations of all proteins provided the actin concentration remains about tenfold in excess of the S1. However, in practice the binding reaction becomes slow and baseline artefacts begin to affect the quality of the data. Reducing S1 concentration much below 0.25 μM also begins to enter the region where the reaction may not proceed to complete binding of S1 and then analysis becomes more complex.
The assays can be performed by swapping the concentrations of S1 and actin used and observing the reaction when excess S1 binds to actinTmTn. Such assays have been performed17,19-21 but the analysis of the data is model dependent. A control data set using the rabbit proteins is shown in Figure 2 for both excess actin (2.5 μM) binding to 0.25 μM S1 and excess S1 (2.5 μM) binding to 0.25 μM rabbit thin filaments. As shown in Figure 2(a) for excess actin, the data show a series of exponentials (fitted line superimposed) at different calcium concentrations and a plot of kobs versus pCa is shown in Figure 2(c). The data can be described by a Hill equation with a mid point at pCa 6.02±0.02 and a Hill coefficient of 1.5±0.07 as we reported. In contrast, the observed transients for the excess S1 case, in all but the highest calcium concentrations, have a clear sigmoid shape as initially the S1 binds to the thin filaments in the switched-off conformation. As S1 begins to bind the filament switches to the on-state and the rate of binding accelerates. Full analysis of such curves requires a detailed model and requires parameters such as the fraction of the filament in the off-state as a function of calcium, the rate of binding of S1 to the off and on-states, the rate of switching between on and off states, and the extent of the cooperativity between off and on states. Such analyses have been attempted but there is no agreement on the models, nor the validity of the parameters derived from the models.19,22 We therefore took a simpler pragmatic approach, which should be independent of the details of the model of calcium regulation. We measured the mid-point (t1/2) of the transient curves and plotted these in Figure 2(c). As can be seen, the pCa plot is the mirror image of the data from Figure 2(a) and the mid-point of the fits to the Hill equation gave pCa of 6.02±0.02 and 6.09±0.05. The analysis appears therefore to return the same information as the more complete analysis in Figure 2(a) but requires one-tenth of the amount of thin filament proteins. Note, however, that the Hill coefficients (h) differ for the fits to the two curves. Over a series of measurements the Hill coefficient for the fit to the pCa curve was 1.5 (range 1.4-1.6) in the excess actin experiment but 2.5 to 3.5 for the excess S1 curves when using rabbit skeletal Tn. (When using cardiac Tn the differences were much smaller, increasing from 1.2 for excess actin to 1.8 for excess S1 (S.E.B. and M.A.G., unpublished observation)). The data analysis therefore appears to give the same value of the mid-point of the calcium sensitivity but a significantly larger value of the Hill coefficient. The larger Hill coefficient probably reflects that the cooperativity of activation in the excess S1 case includes the cooperativity in calcium and myosin binding.
Figure 2.
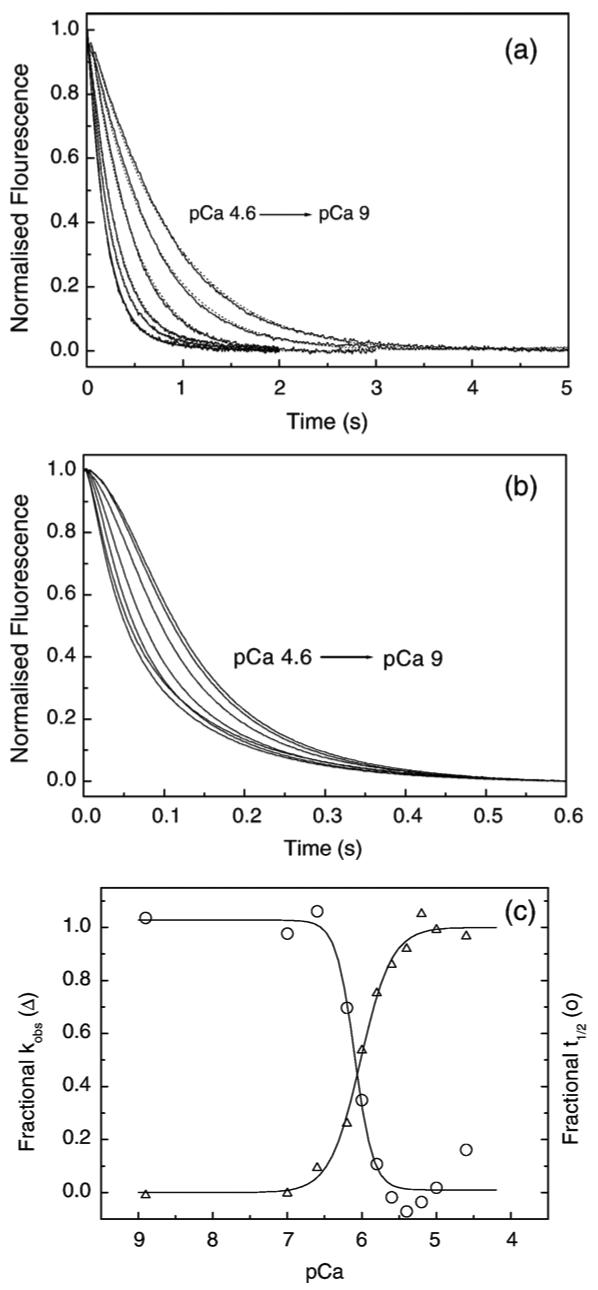
Dependence of the rate of S1 binding to pyr-actin. TmTn on calcium concentrations. (a) Observed transients for excess pyr-actin (2.5 μM and 1 μM skTmTn) binding to 0.25 μM S1. The best fit single exponentials (dotted line) are superimposed and gave kobs values of: 5.2 s-1 (pCa 4.6), 4.25 s-1 (pCa 5.6), 3.4 s-1 (pCa 6), 2.2 s-1 (pCa 6.2), 1.55 s-1 (pCa 6.6) and 1.2 s-1 (pCa 9). (b) Observed transients for excess S1 (2.5 μM) binding to 0.25 μM pyr-actin and 0.15 μM skeletal muscle TmTn. The same pCa values as for (a) were used. No fitted line was used. (c) Plot of kobs for S1 binding to excess pyr-actin (Δ, left axis, from (a)) and the t1/2 values for excess S1 binding to pyr-actin transients (circles, right axis, from (b)) plotted as a function of pCa. The data were fitted to a Hill equation and gave pCa50% values 6.02±0.02 and 6.09±0.05 and Hill coefficients (nCa) of 1.5±0.07, 2.8±0.15 for kobs and t1/2, respectively. Experimental conditions were as for Figure 1.
An experiment using the Lethocerus proteins is shown in Figure 3. Here an excess of S1 (2.5 μM) was rapidly mixed with a lower concentration of regulated actin filaments (0.25 μM actin + 0.071 μM TmTn). As shown in Figure 3(a) for both F1 and F2 TnC the transients are exponential when calcium is saturating but sigmoid when calcium is removed. The data here show that the two TnC isoforms behave in a similar way to each other (and to mammalian proteins). A series of transients using the F1 isoform at pCa values between 8.9 and 4.5 are shown in Figure 3(b) and a plot of the t1/2 values against pCa are shown in Figure 3(c) for both TnC isoforms. A best fit to the Hill equation gave a mid point for the curves (pK50%) of 6.92±0.06 and 5.96±0.03 for F1 and F2, respectively, with Hill coefficients of h of 0.51±0.3 and 2.17±0.5. The two TnCs therefore regulated the switch between the blocked and the other states of the filament at quite different pCa values and with different Hill coefficients.
Figure 3.
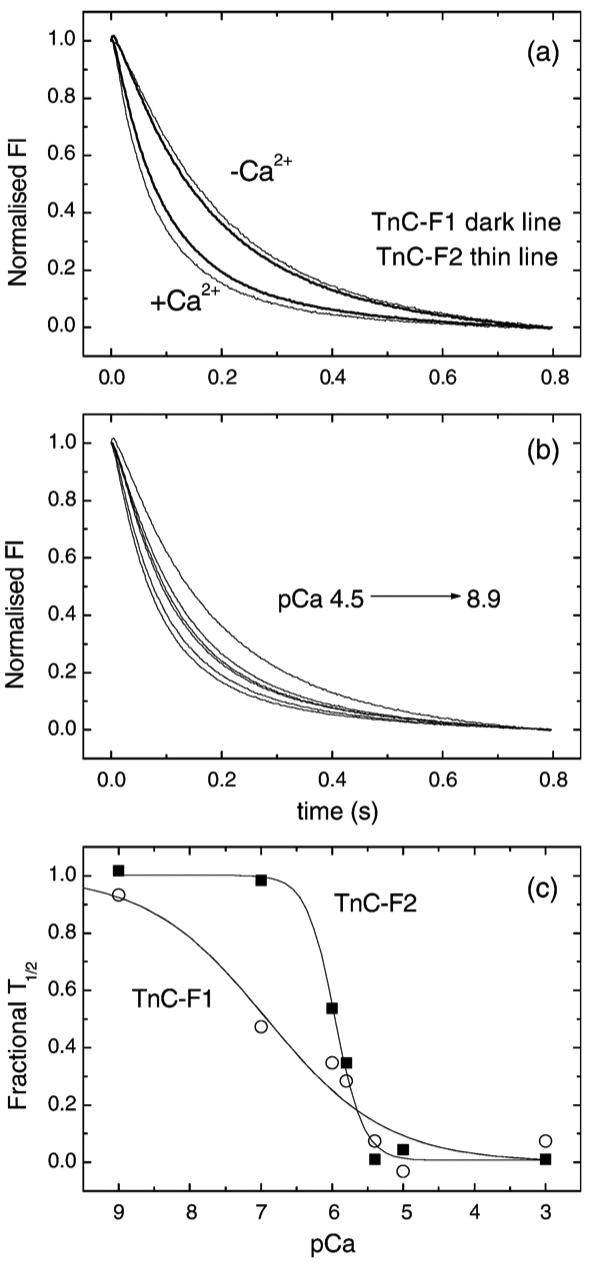
The calcium dependence of excess S1 binding to Lethocerus TmTn regulated thin filaments. The effect of calcium on the observed fluorescence transient when 2.5 μM S1 was rapidly mixed with 0.25 μM actin that had been pre-incubated with 0.2 μM Lethocerus TmTn. (a) The transients observed in the presence and absence of Ca2+ for Lethocerus TmTn with TnC-F1 or TnC-F2. (b) The calcium dependence of the observed transients for TnC-F1 over the pCa range 8.9 to 4.5. (c) A plot of the t1/2 time point against pCa for the two Lethocerus TnCs. The best fit to the Hill equation gives pK50% of 6.92±0.63 and 5.96±0.03 with nCa of 0.51±0.33 and 2.17±0.50 for TnC-F1 and F2, respectively.
Equilibrium binding assays and the C-to-M state
In equilibrium binding assays pyr-actin at 10 μM was pre-incubated with 10 μM phalloidin to stabilise the filaments for at least 3 h and then incubated with 2 μM Lethocerus TmTnTH with either the F1 or F2 isoform of Lethocerus TnC. This solution was diluted to 50 nM pyr-actin (with TmTn at 1 μM to maintain the fully assembled filament) in a standard fluorescence cuvette, which was stirred continuously. S1 (5 μM) was titrated into the cuvette from a syringe at a rate of 0.25 μl min-1 and the fluorescence recorded every 0.5 s. The resulting titration curves are shown in Figure 4(a) for the mammalian actin filament proteins and in Figure 4(b) for both Lethocerus TnC isoforms. The data for the mammalian proteins are in line with previously published titration curves. A titration curve for actin alone is also included in Figure 4 for reference. As can be seen the curve in the absence of TmTn is hyperbolic and the best fit to a simple binding curve is superimposed with a Kd value of 56 nM. This agrees with previously published values under these conditions. The presence of TmTn results in a sigmoid titration curve and a significant increase in overall affinity (as judged by the total S1 concentration at 50% saturation of the fluorescence change (approximately 40, 60, 100 nM S1 for TmTn+Ca2+, TmTn-Ca2+ and actin alone curves, respectively). An increase in the overall affinity of S1 for actin has been well documented for the mammalian and avian muscle proteins.23,24
Figure 4.
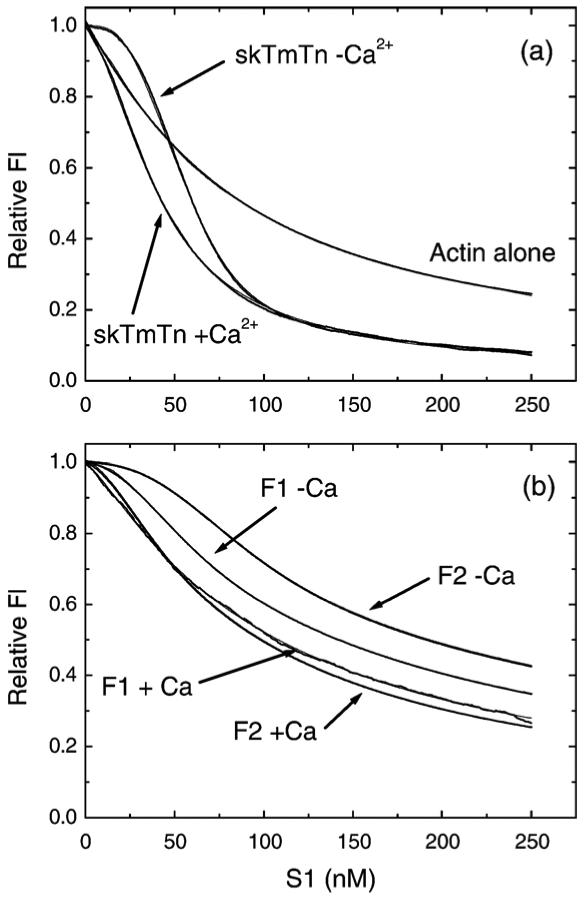
Fluorescence titration of pyr-actinTmTn with S1. The fluorescence changes observed when 50 nM pyr-actin pre-incubated with 1 μM TmTn was titrated with 0-250 nM rabbit S1 in the presence or absence of calcium. (a) Control titration using 50 nM pyr-actin with 0.3 μM rabbit TmTn (heavy line) with the best fit to the three-state model shown superimposed (thin line - barely visible on the fit). A titration using actin alone is also included with the best fit to a non-cooperative binding model Kd = 54 nM. (b) The titrations for Lethocerus TmTn (1 μM) with either the F1 or F2 form of TnC are shown (heavy line) with the best fit to the three-state model superimposed (thin line). The best-fit parameters to the three-state model are shown in Table 1.
The best least-squares fit to the McKillop and Geeves three-state model is superimposed on the TmTn fluorescence curves. The fit assumes that KB is ≥10 in the presence of calcium for each protein system and that KB is 0.38, the value obtained from Figure 1 in the absence of calcium. As can be seen from the Figures the fitted line gives a very good fit to the data but the values are not uniquely determined. The product of K1K2 is well defined by the overall affinity, the value of KT and K2 are linked and KT is also dependent upon the value of KB used. The value of n is not well defined by the data but a reasonable fit is generated if n is fixed at integer values between 5 and 12 and the value chosen which generates the minimum of the least-squares fit.25 In general the larger the value of n the larger the value of KT. The values returned from the fits are given in Table 1 and are in agreement with our published values.
Table 1.
Summary of data on calcium regulation of thin filament states
| F1+Ca | F1-Ca | F2+Ca | F2-Ca | Rab+Ca | Rab-Ca | |
|---|---|---|---|---|---|---|
| K1 (×103 M-1) | 60 | 42 | 67 | 30.5 | 282 | 292 |
| KT | 0.9 | 0.47(0.42) | 0.19 | 0.19 | 0.12 | 0.02 |
| n | 10-11 | 11-12 | 10 | 11 | 6 | 7 |
| KB | >10 | 0.21 | >10 | 0.28 | >10 | 0.36 |
| 1/K1K2 (nM) | 83.3 | 116 | 74.6 | 164 | 17 | 17 |
| Fmax/F∞ | 0.57 | 0.54 | 0.43 | 0.41 | 0.28 | 0.29 |
K1, K2, KB, KT and n are defined in Methods and are obtained from the fits to the titration data in Figure 4. The values are those from the best least-squares fit to the data. For TnC-F1 the fits were indistinguishable for values of n in the range shown. If n is assumed to be lower than the values quoted then the value of KT increases. The values shown therefore represent the minimum value of KT. Two values are given for KT for TnC-F1-Ca. The fit to the kinetic data assumes KT is small in order to calculate KB This value of KB is then required to fit the titration data to yield a value of KT. An iteration procedure is required to produce the best value of KT and KB This process has no significant effect on the values quoted except for KT-Ca for TnC-F1. The value in parentheses represents the value after iteration. Fmax is the fluorescence level for actin TmTn and F∞ represents the fluorescence on saturating the actin with S1. The ratio therefore defines the quench of fluorescence. The Fmax values for TnC-F1 and TnC-F2 were ∼10% lower than those for rabbit Tn.
The same procedure was followed using the Lethocerus TmTn actin filament. As can be seen from the data in Figure 4(b) the titrations (performed on the same day using the same actin and S1 preparations as Figure 4(a)) show clear calcium sensitive sigmoid titration curves but with a much weaker overall affinity of S1 for the thin filaments than seen for the rabbit proteins. The 50% saturation point occurs at 100-150 μM total added S1. The curves for F1 and F2 are quite similar in the presence of calcium with F1 being slightly less sigmoid in nature. In the absence of calcium both titration curves shift to the right indicating a more switched off filament in the absence of calcium (as expected from the change in KB) but the shift is much more marked for F2 than for F1. This difference on removal of calcium is primarily caused by the change in KB and the difference between the TnC-F1 and F2 curves is primarily caused by a weaker affinity of S1 in theTnC-F2 case.
When the data were fitted to the three-state model using the same procedure as for Figure 4(a) the data were well described by the model and the best fit parameters are shown in Table 1. The quality of the fits is shown by the fact that the fitted lines, which are superimposed on the original data in Figure 4, are barely visible so close is the fit. The quality of the information listed in Table 1 and the implications of the values for the mechanism of regulation will be considered in the Discussion.
Discussion
The data presented here demonstrate that the in vitro methods developed to analyse the properties of rabbit muscle thin filaments can be applied equally well to the filaments of rabbit actin with Lethocerus Tm and Tn. This then allows the three-state McKillop and Geeves model of regulation to be used to interpret the data. The results of the analysis are given in Table 1.
The startling result observed here is that the presence of Lethocerus TmTn reduces the second-order rate constant by a factor of 10. How the TmTn produces this result is not known but the constant is expected to be defined by two things, the frequency of successful collision between the actin and myosin and the rate constant of any subsequent protein isomerisation events. If actin sites were occluded by TmTn, thus limiting myosin binding, then this would be expected to be similar to the presence of a blocked-state and predicts a different binding profile when measured for excess S1 versus excess actin. No such difference is apparent. The simplest explanation is therefore that the Lethocerus TmTn is different, resulting in a lower frequency of successful collision. A charge change on the surface of actin could produce this effect.
The kinetic analysis shows that as for the rabbit TmTn, removal of calcium reduces the rate constant for rabbit S1 binding to actin by about a factor of 3. This predicts a value of the equilibrium between B and C states of the same order for the rabbit TmTn and both forms of TnC-Lethocerus filaments. However, the titration data predict a larger value of KT for the Lethocerus filaments and this results in the calculated values of KB being 0.21 and 0.28 for TnC-F1 and TnC-F2, respectively, compared to the value for rabbit of 0.36. The differences in KB appear relatively large but because of the differences in KT values for the TnC isoforms (discussed below) the predicted occupancy of the B-state, in the absence of calcium, is within 1% of 75% for the rabbit filament and for both Lethocerus proteins (Figure 5).
Figure 5.

The fractional occupancy of the three states of the TnC-F1 and TnC-F2 containing thin filaments as a function of calcium concentration. The occupancy at pCa 8.9 and pCa 4.5 are based on the values of KB and KT given in Table 1. (Calculated from fraction of actin as C states = 1/(1 + KT+1/KB), then B states = C states/KB and M states = C states KT). The values of KB at pCa 7 and 6 are based on the pCa plots of Figure 3(c) and were 0.37 and 1.4 for TnC-F1 and 1.03 and 9.5 for TnC-F2, respectively. KT was independent of calcium for TnC-F2 while for TnC-F1 it had a small dependence on calcium and was assumed to follow the same pCa dependence as KB with values of 0.55 at pCa 7 and 0.68 at pCa 6. Values for the rabbit TnC are not shown but were very similar to those for TnC-F2.
The novel method used to evaluate the calcium sensitivity of the thin filaments suggests that the midpoint of the calcium switch occurs at pCa 6.92 ([Ca2+]=0.12 μM) for TnC-F1. The midpoint of the curve is not well defined for such a tight binding site but it is clearly at a much lower pCa than for the TnC-F2 which occurred at pCa 5.96 ([Ca2+]=1.1 μM). The curve is apparently less cooperative for TnC-F1 than TnC-F2 and both are lower than for the rabbit proteins but the Hill coefficients are not well defined in these data sets and therefore caution should be used in interpreting the values. The differences in the values for the mid points of the curves are, however, consistent with the calcium affinities of the high affinity “structural” calcium binding site IV on TnC-F1 (Kd=0.16 μM) and TnC-F2 (Kd=2.6 μM). Note these are the calcium affinities of the isolated TnC constructs and the values may differ in the complex on the thin filament and in the presence of magnesium. Thus the data are consistent with the earlier work of Agianian et al.7 in suggesting that the C-terminal calcium binding site IV has regulatory role and that this is similar to the role of the N-terminal calcium sites in mammalian TnC.
The titration data of Figure 4 allow an estimation of the affinity of rabbit myosin S1 for the thin filaments, the value of KT, the equilibrium between the C and M states and the degree of cooperativity involved in the switch. The analysis shows that the overall affinity (1/K1K2) of S1 for actin is little affected by the presence of the Lethocerus proteins, 1/K1K2 (75-85 nM, +Ca) is similar to that of pure actin (54 nM). On removal of calcium the fitted value of 1/K1K2 is 1.5 to twofold larger. The precision of the measurements is such that no great significance can be given to a ≤2-fold change. The weaker affinity of S1 for the Lethocerus TmTn filaments compared to the rabbit TmTn is also associated with a smaller quench of the pyrene fluorescence, 0.41-0.57 compared to the well established value of 0.28-0.29 for rabbit actin (with or without rabbit TmTn).
The value of KT for TnC-F2 is 0.19 in both the presence and absence of calcium and similar to the value for the rabbit skeletal muscle proteins in the presence of calcium. The rabbit data, in the original experiment suggested little change in KT on removal of calcium but more recent studies with improved resolution do show a reduction in KT. However, data with either bovine or human cardiac troponin show little change in KT on removal of calcium.25 In contrast the value of KT for the F1 isoform is much larger than any reported previously, 0.9 in the presence of calcium and 0.47 in the absence of calcium. The twofold change is too small with the current data to have any mechanistic implication. The cooperativity of the switch is not well defined but is reasonably consistent across all of the titration data and has value of 10-12 similar to values for the mammalian proteins.
The two curves in Figure 4 in the absence of calcium appear quite distinct but the differences are largely caused by the differences in the affinity of the filament for S1 (116 nM for TnC-F1 and 164 nM for TnC-F2) the sigmoidicity is dominated by the values of KB. which do not differ by very much.
If we take the values of KB and KT given in Table 1 we can calculate the occupancy of the three states of the filament as a function of calcium concentration and these are shown in Figure 5. The calcium dependence of the occupancy of the three states is very similar for the rabbit thin filaments and those containing TnC-F2 and is not shown in the Figure. In the absence of calcium the occupancy of the B and C states is similar for both Lethocerus filaments, 74-76% B-state and 16-22% C-state. The M-state occupancy is small in both cases but apparently larger for F1 (7%) than F2 (4%) but since the occupancy is small it is not well defined. The differences are more apparent at pCa 4.5. In all cases the B-state has a low occupancy, we have set this arbitrarily at 10% of the C-state as this is at the limit of the measurement. The fully on M-state is, 15% for TnC-F2 and 45% for TnC-F1 so the TnC-F1 filament is significantly more switched on, as is clear in the raw titration data where the TnC-F1 data shows little sigmoidicity in the plot (see Figure 4). These differences are even more pronounced in the middle of the pCa range where the greater calcium sensitivity of the TnC-F1 further emphasises the differences. At pCa 7, TnC-F1 is at the mid point of its calcium-induced transition (40% C and 22% M-state) while the TnC-F2 filament remains almost fully in the low calcium state. At pCa 6 where the TnC-F2 is at the mid point of the calcium sensitivity the occupancy of B, C, and M states is 37, 52, and 10%, respectively, while the TnC-F1 filament occupancy is 6, 56 and 38%, respectively.
The implications of these results are of interest in terms of the different methods of activation of the muscle filaments. TnC-F2 appears very similar to TnC in mammalian muscle filaments in that the filament is largely off in the absence of calcium (75% B-state, ≤5% M-state). Addition of calcium removes almost all of the B-state but the filament remains in the inactive C-state (77%); strong binding of myosin cross-bridges is required to fully activate the filament.
TnC-F1 is similar to TnC-F2 in the absence of calcium and is turned off to a similar extent but is activated in a different way by calcium. Calcium switches the TnC-F1 almost completely on (45% M-state) and therefore requires very little myosin binding to switch on fully.
At the physiological level both TnC isoforms are present in the muscle fibre at a ratio of 7:1 for F1/F2 and must work together to regulate the different types of contraction observed. TnC-F1 has been implicated in calcium activation of the stretch activated oscillatory motion of the muscle fibres whereas TnC-F2 has been implicated in steady isometric contraction. Assuming a random distribution of isoforms then at moderate calcium concentrations (sufficient to allow stretch activated oscillatory contraction) the occasional TnC-F2 regulated actin7- TmTn units would be expected to be off (B and C states) between long segments of activated TnC-F1 regulated units (in C and M states). On average there will be six TnC-F1 units between TnC-F2 regulated units. These occasional off TnC-F2 units must therefore be sufficient to inhibit isometric contractions but not oscillatory contraction. The calcium is not expected to increase and decrease with each 30 Hz (200 Hz for Drosophila) contraction cycle. However, Gordon and Dickinson26 recently obtained evidence that intracellular Ca2+ does regulate the mechanical power output of Drosophila IFM during flight. The power output of the IFM varied with the (asynchronous) firing rate of spikes in the membrane potential of the muscle and the intracellular Ca2+ level increased with firing rate. These findings suggest that ambient Ca2+ levels during flight must be well below the level producing appreciable isometric tension, or raising Ca2+ could not increase power production. An interpretation of this behaviour is that low levels of calcium (<10-6 M) activate the majority TnC-F1 units which allow stretch activation and oscillatory contraction but that calcium binding to TnC-F2 may modulate the power output of the system. Full activation of the TnC-F2 units (>10-6 M) generates isometric contractions.
This description matches the properties of the two TnC isoforms revealed here. In low calcium both types of units will be off and no contraction occurs. Moderate calcium (∼10-7 M) activates most of the filament, with the TnC-F1 regulatory units being poised 50:50 between C and M states. Very little binding of myosin heads would be needed to induce full activation of these units and this myosin head binding could be induced by, for example, a small stretch altering the alignment between myosin heads and their target sites on the thin filament.27 The TnC-F2 units would, however, remain biased towards the blocked state and be more resistant to activation by myosin heads. Further increases in calcium would progressively activate the TnC-F2 units leading to greater power generation and eventually isometric contraction. This view is consistent with the observation that Lethocerus fibres in which endogenous TnC was substituted in vitro with recombinant TnC-F2 showed minimal stretch activation but normal isometric contraction.7 Testing such ideas will require more detailed physiological measurements with varying ratios of the two TnC isoforms and the level of calcium needed to induce the different types of contraction.
Asynchronous flight muscles in other insects are probably regulated in the same way as Lethocerus IFM. Drosophila and Anopheles (both Diptera) have isoforms of TnC homologous to those in Lethocerus IFM.10 But tropomyosin and TnI in Diptera are different from the proteins in other insects: the proline-alanine-rich sequence is at the C terminus of a tropomyosin isoform in the Diptera, and at the C terminus of TnI in Lethocerus, Apis and many other insects.10,28-30 It is of significance that electron micrographs of negatively stained Drosophila IFM thin filaments were indistinguishable from those of mammalian muscle fibres in the presence and absence of calcium.31 This is compatible with the Tm in these filaments being predominantly in the B or blocked state in the absence of calcium and moving to the calcium-induced or C-state on addition of calcium. If the Drosophila filaments are similar to the Lethocerus filaments, then the B-state is expected from the data presented here in the absence of calcium, with a mixture of M and C states in the presence of calcium. The calcium concentration used in activating conditions in the electron microscopy studies was relatively high. An intermediate calcium concentration (around pCa7) would result in a higher occupancy of the closed conformation than in vertebrate. In the presence of calcium the occupancy of the C and M states expected will depend upon how the properties of TnC-F1 and F2 combine in the isolated filament. But a higher proportion of M sates can be anticipated. Questions remain about the molecular mechanism of thin filament activation by these two novel TnC isoforms. The classical view emerging from studies of mammalian thin filaments is that calcium binding to the N-terminal lobe of TnC opens the lobe and allows the switch peptide of TnI to bind within the lobe. This then results in disruption of the TnI inhibitory peptide binding to actin and loss of this interaction leads to movement of Tm and thin filament activation. Since TnC-F1 lacks any calcium binding site in the N-terminal lobe the same mechanism cannot apply and the regulation observed here must involve the C-terminal lobe which has been viewed as “structural” with little regulatory role. TnC-F2 has the identical Ca2+-coordinating residues in the C-terminal site as the F1 isoform despite the weaker calcium affinity of the C-terminal site. This must mean that there is some influence of non-coordinating residues, or sequence variation in the rest of the TnC on the conformation of the C-lobe. Since TnC-F1 regulates via calcium binding to the C-terminal lobe then TnC-F2 may also regulate through the C-lobe, although a mechanism involving the N-terminal lobe similar to the mammalian TnC must remain a strong possibility. It is of interest that recent studies of calcium regulation in the scallop muscle thin filament have shown that the scallop TnC also has a single calcium binding site in the C-terminal lobe which can regulate the interaction with TnI.32-34 The affinity for calcium is lower in the scallop TnC than in Lethocerus TnC-F1. In scallop, calcium regulation occurs at two levels, via the TnC as here and also via the calcium binding sites in the neck of the myosin motor domain. Scallop TnC activates the thin filament at high calcium concentrations, while myosin-linked regulation inhibits the interation of actin and myosin at low calcium concentrations. Understanding the molecular mechanism of calcium activation in Lethocerus thin filaments will require detailed structural studies of the two TnC isoforms and these are currently underway (see note added in proof).
The differences reported here between TnC-F1 and F2 have been obtained using rabbit actin and rabbit S1. The actin is very highly conserved and is not expected to have a major influence on the results reported here. Lethocerus and Drosophila myosin have been shown to function with rabbit actin in motility assays and in a range of solution kinetic assay.35-39 Similarly Drosophila actin has been shown to function with rabbit myosin and S1 in similar assays.35,40 Thus, the actin differences may have subtle effects on the results reported here but are unlikely to differ in a major way. The rabbit S1 is used as a probe of the state of the thin filament but otherwise is not a direct influence on the values of KB and KT, which are quoted in the absence of any bound myosin.
Methods
Preparation of Lethocerus proteins
A complex of tropomyosin and the troponin components, TnT and TnH, was isolated from myofibrils prepared from 10 g of Lethocerus flight muscle, by a modification of the method described by Wendt et al.11 An extract containing Tm and Tn was loaded onto a butyl-Sepharose 4 Fast Flow column (Pharmacia) and eluted with a gradient of buffer containing from 25% to 0% (w/v) saturated ammonium sulphate. The complex of TmTn, without TnC, was eluted at about 15% saturated ammonium sulphate. The protein was concentrated by precipitating at 35% saturated ammonium sulphate; after centrifuging at 30,000g for 30 min, the pellet was rinsed in column buffer without ammonium sulphate, and resuspended in 0.5 M NaCl, 2 mM Tris-HCl (pH 7.9), 0.1% (v/v) β-mercaptoethanol, 0.1 mM PMSF. The yield was 4-5 mg Tm-TnT-TnH (TmTnTH) complex from 10 g IFM (15 waterbugs). Samples were stored at -20 °C.
Recombinant TnCs, F1 and F2, were prepared according to Qiu et al.10 An N-terminal 6His tag was cleaved from purified TnCs with TEV protease.
Figure 6 shows a typical SDS-PAGE gel of the Lethocerus proteins used. Note there are two isoforms of Tm present in roughly equal amounts.
Figure 6.
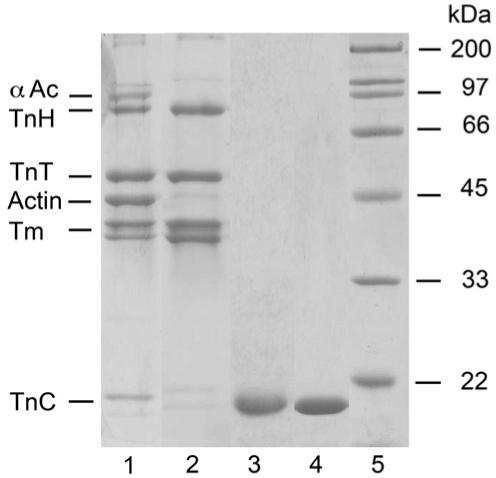
Lethocerus tropomyosin-TnT-TnH complex and TnC-F1 and F2. SDS-PAGE of samples: lane 1, IFM fraction applied to a butyl Sepharose column; lane 2, fraction from the column, containing pure TmTnTH; lane 3, F1 (TnC-F1); lane 4, F2 (TnC-F2); lane 5, markers. F1 and F2 were expressed in E. coli, and isolated from a cell lysate with Ni-agarose. An N-terminal 6His tag was removed with TEV protease. TnH is Lethocerus troponin I. αAc, α-actinin.
Preparation of rabbit proteins
Rabbit actin was prepared following the method described by Spudich and Watt.12 The concentration of actin was assayed at 280 nm UV absorbance using an extinction coefficient of 1.104 mg-1 ml cm-1 and a molecular mass of 42, 000 Da. Actin labelling with N-(1-pyrenyl)iodoacetamide was based on a procedure by Criddle et al.13 When used below 1 μM concentration the actin was stabilised by pre-incubation of 10 μM actin with 10 μM phalloidin for 3 h before use.
Rabbit myosin subfragment (S1) was prepared using the method described by Weeds and Taylor.14 Its concentration was measured at 280 nm with an extinction coefficient of 0.79 mg-1 ml cm-1 and a molecular mass of 115, 000 Da.
Rabbit skTmTn was obtained using the method by Ebashi et al.15 The concentration was measured by absorbance at 280 nm with an extinction coefficient of 0.385 mg-1 ml cm-1 and a molecular mass of 136, 000 Da.
Assembly of actin TmTn filaments
For stopped-flow experiments where pyr-actin was in large excess over S1, 2.5 μM pyr-actin was incubated with 1 μM skTmTn or 1 μM Lethocerus TmTnTH and 1 μM of either F1 or F2 for 3 h. The measurements were carried out in 140 mM KCl, 5 mM MgCl2, 1 mM DTT, 20 mM Mops (pH 7.0) at 20 °C,with either 2 mM CaCl2 or 2 mM EGTA. The pre-assembled thin filament complex was mixed with 0.25 μM S1 in the stopped-flow fluorimeter.
In the case where S1 was in large excess over actin the same procedure was followed except that 0.25 μM of phalloidin stabilized pyr-actin was incubated with 0.2 μM of TmTn and either TnC-F1 or F2 with 2 mM Ca2+ or EGTA. The S1 concentration was 2.5 μM.
For the S1 titration experiment, 50 nM of phalloidin--stabilised pyr-actin was incubated with an excess of 1 μM TmTn complex for at least 3 h in the same buffer as for the kinetic measurments in the presence of either 2 mM Ca2+ or EGTA. The mixture was then transferred to a cuvette to which S1 was titrated.
Transient kinetics
The rate of S1 binding to the assembled thin filaments at different pCa values was measured by monitoring the change in pyrene fluorescence in the stopped-flow fluorimeter (SF 61DX, Hi-Tech Scientific, Salisbury UK). The measurements were carried out at 20 °C. Pyrene was excited at 365 nm and its fluorescence emission was detected using a KV 389 nm cut off filter. Each transient analysed was the average of at least three shots. Data analysis was carried out using the software Kinetasyst, provided with the instrument. For the excess actin filament experiment the averaged trace was fitted to a single exponential and the obtained kobs was plotted as a function of pCa values. The Ca2+ sensitivity curve obtained was fitted to the following version of the Hill equation:
where k’obs is the relative value of kobs and is calculated as (kobs-kobs,8.9)/(kobs,4.5-kobs,8.9).
K is the calcium concentration at the midpoint of the pCa curve and h is the Hill coefficient.
The pCa values used were generated using mixtures of 2 mM EGTA and 2 mM Ca-EGTA to produce the free calcium concentration required based on the affinity constants.16
For the experiments using an excess of S1 the data are not described by a single exponential and the time at which the transient was 50% complete (t1/2) was estimated and the calcium sensitivity of the t1/2 values treated as for kobs.
S1 titration
The fluorescence titrations were carried out at 20 °C (in the Perkin Elmer LS50B) with excitation at 365 nm and emission at 405 nm. The cuvette which contains the pre-assembled thin filament components was constantly stirred. S1 was titrated into the cuvette from a 5 μM stock using a software operated Harvard Apparatus syringe infusion pump driving a 100 μl Hamilton glass syringe. Data points were collected over 250 s with a data-point being collected every 0.5 s.
Model of thin filament regulation
The data were analysed in terms of the three-state model by McKillop and Geeves.17 In this model the thin filament can exist in a combination of 3-states (blocked or B-state; closed, calcium-induced or C-state; open, myosin-induced or M-state,18) and the cooperativity of the thin filament can be defined by the number of actin monomers (n) within a filament which change between the states at the same time:
Note that n is not necessarily the same value for the two equilibrium constants. Regulation of muscle contraction arises because the three states differ in the way they interact with myosin. Actin monomers in the B-state do not have any significant interaction with myosin. This is normally the predominant state of a regulated actin filament in the absence of calcium. In the calcium induced C-state the myosin can only bind to form the weakly bound A-M form of the complex and in the M-state the myosin can bind weakly and isomerise to the strongly bound rigor-like A-M form:
i.e. step 1 of binding is possible in the closed or open state, only the open state allows step 2.
Equilibrium binding assays
In an equilibrium binding assay the fraction of actin sites (α) with a strongly bound A·M myosin is defined by the McKillop and Geeves model as:
where P=1+K1[M](1+K2), Q=1+K1[M] and [M] is the concentration of free, unbound myosin heads and n is the apparent cooperative unit size of the actin filament, i.e. the number of actin sites switching to the M-state for each myosin binding at low levels of saturation with myosin.
For the fluorescence titration using pyr-actin α =(F-F0)/(F0-F∞).
Where F is the fluorescence measured at any given myosin concentration, F0 is the initial fluorescence in the absence of any myosin and F∞ is the fluorescence at saturating myosin concentrations.
Kinetics binding assays
In a kinetic binding experiment the mixing of a large excess of actin monomers in a filament with myosin heads ([A]≫[M] pseudo-first-order in actin) results in an exponential binding reaction and the observed exponential rate constant (kobs) is given by:
where [A] is the concentration of available actin monomers for myosin to bind and k+1 is the second-order rate constant for binding. The fraction of actin sites available is given by:
If all of the actin is available in the presence of calcium (KB≫1) then the ratio (R) of the kobs values in the absence and presence of calcium can be defined by the values of KB and KT in the absence of calcium:
Rearranging KB=R/(1-R)(1+KT) and if KT is small this approximates to R/1-R.
The combination of both equilibrium titrations and kinetic binding experiments allows the values of K1K2, KB, KT and n to be evaluated.
The experiment can also be done under conditions where [M]≫[A] in this case the reaction is exponential in the presence of saturating calcium (no B-state) but sigmoid at low calcium. The initial myosin binding is slow as the actin sites are not available but myosin binding cooperatively activates the filament leading to an acceleration of the rate of binding. There is no simple analysis of such sigmoid binding curve, since the form of the sigmoidicity depends upon the details of the model used. For such experiments the t1/2 was recorded as an indication of the speed of the reaction.
Acknowledgements
This work was partly funded by an EU 6th Framework NOE grant (Myores), the NIH (no AR048776) and Wellcome Trust (program grant no 070021). B.A. was supported by a Marie Curie fellowship and S.E.B. by a British Heart Foundation Studentship (FS/2000014).
Abbreviations used
- Tn
troponin
- Tm
tropomyosin
- IFM
indirect flight muscle
- pyr-actin
pyrene-labelled rabbit actin
Note Added in Proof
See - G. De Nicola, G., Burkart, C., Qiu, F., Agianian, B., Labeit, S., Martin, S., Bullard, Pastore, A. (2007). Structure, 15, 813-824.
References
- 1.Pringle J. Stretch activation of muscle: function and mechanism. Proc. Roy. Soc. ser. B. 1978;201:107–130. doi: 10.1098/rspb.1978.0035. [DOI] [PubMed] [Google Scholar]
- 2.Josephson RK, Malamud JG, Stokes DR. Asynchronous muscle: a primer. J. Exp. Biol. 2000;203:2713–2722. doi: 10.1242/jeb.203.18.2713. [DOI] [PubMed] [Google Scholar]
- 3.Esch H, Goller F, Heinrich B. How do bees shiver? Naturwissenschaften. 1991;78:325–328. doi: 10.1007/BF01221422. [DOI] [PubMed] [Google Scholar]
- 4.Heinrich B. The Thermal Warriors: Strategies of Insect Survival. Harvard University Press; Cambridge, MA: 1996. [Google Scholar]
- 5.Kuhn HJ, Bletz C, Guth K, Ruegg JC. The effect of MgATP on forming and breaking actin-myosin linkages in contracted skinned insect flight muscle fibres. J. Mus. Res. Cell Motil. 1985;6:5–27. doi: 10.1007/BF00712308. [DOI] [PubMed] [Google Scholar]
- 6.Taylor KA, Schmitz H, Reedy MC, Goldman YE, Franzini-Armstrong C, Sasaki H, et al. Tomographic 3D reconstruction of quick-frozen, Ca2+-activated contracting insect flight muscle. Cell. 1999;99:421–431. doi: 10.1016/s0092-8674(00)81528-7. [DOI] [PubMed] [Google Scholar]
- 7.Agianian B, Krzic U, Qiu F, Linke WA, Leonard K, Bullard B. A troponin switch that regulates muscle contraction by stretch instead of calcium. EMBO J. 2004;23:772–779. doi: 10.1038/sj.emboj.7600097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linari M, Reedy MK, Reedy MC, Lombardi V, Piazzesi G. Ca-activation and stretch-activation in insect flight muscle. Biophys. J. 2004;87:1101–1111. doi: 10.1529/biophysj.103.037374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bullard B, Leonard K, Larkins A, Butcher G, Karlik C, Fyrberg E. Troponin of asynchronous flight muscle. J. Mol. Biol. 1988;204:621–637. doi: 10.1016/0022-2836(88)90360-9. [DOI] [PubMed] [Google Scholar]
- 10.Qiu F, Lakey A, Agianian B, Hutchings A, Butcher GW, Labeit S, et al. Troponin C in different insect muscle types: identification of two isoforms in Lethocerus, Drosophila and Anopheles that are specific to asynchronous flight muscle in the adult insect. Biochem. J. 2003;371:811–821. doi: 10.1042/BJ20021814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wendt T, Guenebaut V, Leonard KR. Structure of the Lethocerus troponin-tropomyosin complex as determined by electron microscopy. J. Struct. Biol. 1997;118:1–8. doi: 10.1006/jsbi.1996.3834. [DOI] [PubMed] [Google Scholar]
- 12.Spudich JA, Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J. Biol. Chem. 1971;246:4866–4871. [PubMed] [Google Scholar]
- 13.Criddle AH, Geeves MA, Jeffries T. The use of actin labelled with N-(1-pyrenyl)iodoacetamide to study the interaction of actin with myosin subfragments and troponin/tropomyosin. Biochem. J. 1985;232:343–349. doi: 10.1042/bj2320343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weeds AG, Taylor RS. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975;257:54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- 15.Ebashi S, Wakabayashi T, Ebashi F. Troponin and its components. J. Biochem. (Tokyo) 1971;69:441–445. doi: 10.1093/oxfordjournals.jbchem.a129486. [DOI] [PubMed] [Google Scholar]
- 16.Harrison SM, Bers DM. The effect of temperature and ionic strength on the apparent Ca-affinity of EGTA and the analogous Ca-chelators BAPTA and dibromo-BAPTA. Biochim. Biophys. Acta. 1987;925:133–143. doi: 10.1016/0304-4165(87)90102-4. [DOI] [PubMed] [Google Scholar]
- 17.McKillop DF, Geeves MA. Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys. J. 1993;65:693–701. doi: 10.1016/S0006-3495(93)81110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehman W, Hatch V, Korman V, Rosol M, Thomas L, Maytum R, et al. Tropomyosin and actin isoforms modulate the localization of tropomyosin strands on actin filaments. J. Mol. Biol. 2000;302:593–606. doi: 10.1006/jmbi.2000.4080. [DOI] [PubMed] [Google Scholar]
- 19.Head JG, Ritchie MD, Geeves MA. Characterization of the equilibrium between blocked and closed states of muscle thin filaments. Eur. J. Biochem. 1995;227:694–699. doi: 10.1111/j.1432-1033.1995.tb20190.x. [DOI] [PubMed] [Google Scholar]
- 20.Trybus KM, Taylor EW. Kinetic studies of the cooperative binding of subfragment 1 to regulated actin. Proc. Natl Acad. Sci. USA. 1980;77:7209–7213. doi: 10.1073/pnas.77.12.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gafurov B, Chen YD, Chalovich JM. Ca2+ and ionic strength dependencies of S1-ADP binding to actin-tropomyosin-troponin: regulatory implications. Biophys. J. 2004;87:1825–1835. doi: 10.1529/biophysj.104.043364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Yan B, Chalovich JM, Brenner B. Theoretical kinetic studies of models for binding myosin subfragment-1 to regulated actin: Hill model versus Geeves model. Biophys. J. 2001;80:2338–2349. doi: 10.1016/s0006-3495(01)76204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moraczewska J, Nicholson-Flynn K, Hitchcock-DeGregori SE. The ends of tropomyosin are major determinants of actin affinity and myosin subfragment 1-induced binding to F-actin in the open state. Biochemistry. 1999;38:15885–15892. doi: 10.1021/bi991816j. [DOI] [PubMed] [Google Scholar]
- 24.Landis C, Back N, Homsher E, Tobacman LS. Effects of tropomyosin internal deletions on thin filament function. J. Biol. Chem. 1999;274:31279–31285. doi: 10.1074/jbc.274.44.31279. [DOI] [PubMed] [Google Scholar]
- 25.Maytum R, Westerdorf B, Jaquet K, Geeves MA. Differential regulation of the actomyosin interaction by skeletal and cardiac troponin isoforms. J. Biol. Chem. 2003;278:6696–6701. doi: 10.1074/jbc.M210690200. [DOI] [PubMed] [Google Scholar]
- 26.Gordon S, Dickinson MH. Role of calcium in the regulation of mechanical power in insect flight. Proc. Natl Acad. Sci. USA. 2006;103:4311–4315. doi: 10.1073/pnas.0510109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wray JS. Structure of the backbone in myosin filaments of muscle. Nature. 1979;277:37–40. doi: 10.1038/277037a0. [DOI] [PubMed] [Google Scholar]
- 28.Peckham M, Cripps R, White D, Bullard B. Mechanics and protein content of insect flight muscle. J. Exp. Biol. 1992;168:57–76. [Google Scholar]
- 29.Herranz R, Mateos J, Mas JA, Garcia-Zaragoza E, Cervera M, Marco R. The co-evolution of insect muscle TpnT and TpnI gene isoforms. Mol. Biol. Evol. 2005;22:2231–2242. doi: 10.1093/molbev/msi223. [DOI] [PubMed] [Google Scholar]
- 30.Mateos J, Herranz R, Domingo A, Sparrow J, Marco R. The structural role of high molecular weight tropomyosins in dipteran indirect flight muscle and the effect of phosphorylation. J. Mus. Res. Cell Motil. 2006;27:189–201. doi: 10.1007/s10974-005-9044-3. [DOI] [PubMed] [Google Scholar]
- 31.Cammarato A, Hatch V, Saide J, Craig R, Sparrow JC, Tobacman LS, Lehman W. Drosophila muscle regulation characterized by electron microscopy and three-dimensional reconstruction of thin filament mutants. Biophys. J. 2004;86:1618–1624. doi: 10.1016/S0006-3495(04)74229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doi T, Satoh A, Tanaka H, Inoue A, Yumoto F, Tanokura M, et al. Functional importance of Ca2+- deficient N-terminal lobe of molluscan troponin C in troponin regulation. Arch. Biochem. Biophys. 2005;436:83–90. doi: 10.1016/j.abb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Ojima T, Koizumi N, Ueyama K, Inoue A, Nishita K. Functional role of Ca(2+)-binding site IV of scallop troponin C. J. Biochem. (Tokyo) 2000;128:803–809. doi: 10.1093/oxfordjournals.jbchem.a022818. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka H, Takeya Y, Doi T, Yumoto F, Tanokura M, Ohtsuki I, et al. Comparative studies on the functional roles of N- and C-terminal regions of molluskan and vertebrate troponin-I. FEBS J. 2005;272:4475–4486. doi: 10.1111/j.1742-4658.2005.04866.x. [DOI] [PubMed] [Google Scholar]
- 35.Miller BM, Nyitrai M, Bernstein SI, Geeves MA. Kinetic analysis of Drosophila muscle myosin isoforms suggests a novel mode of mechanochemical coupling. J. Biol. Chem. 2003;278:50293–50300. doi: 10.1074/jbc.M308318200. [DOI] [PubMed] [Google Scholar]
- 36.Silva R, Sparrow JC, Geeves MA. Isolation and kinetic characterisation of myosin and myosin S1 from the Drosophila indirect flight muscles. J. Mus. Res. Cell Motil. 2003;24:489–498. doi: 10.1023/b:jure.0000009809.69829.74. [DOI] [PubMed] [Google Scholar]
- 37.Bullard B, Dabrowska R, Winkelman L. The contractile and regulatory proteins of insect flight muscle. Biochem. J. 1973;135:277–286. doi: 10.1042/bj1350277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White DC, Ricigliano JW, Webb MR. Analysis of the ATPase mechanism of myosin subfragment 1 from insect fibrillar flight muscle in the presence and absence of actin, using phosphate-water oxygen exchange measurements. J. Mus. Res. Cell Motil. 1987;8:537–540. doi: 10.1007/BF01567912. [DOI] [PubMed] [Google Scholar]
- 39.White DC, Zimmerman RW, Trentham DR. The ATPase kinetics of insect fibrillar flight muscle myosin subfragment-1. J. Mus. Res. Cell Motil. 1986;7:179–192. doi: 10.1007/BF01753419. [DOI] [PubMed] [Google Scholar]
- 40.Razzaq A, Schmitz S, Veigel C, Molloy JE, Geeves MA, Sparrow JC. Actin residue glu(93) is identified as an amino acid affecting myosin binding. J. Biol. Chem. 1999;274:28321–28328. doi: 10.1074/jbc.274.40.28321. [DOI] [PubMed] [Google Scholar]


