Abstract
Background
Chronic alcoholism is associated with an elevated risk for pulmonary infection and a 3-fold chance for incidence and mortality of acute respiratory distress syndrome with critical injury. Limited sampling of the alveolar lining fluid has restricted clinical studies of the role of glutathione (GSH) redox balance in pulmonary function and diseased states. Non-invasive sampling in the exhaled breath condensate (EBC) to monitor alveolar GSH would facilitate research in pulmonary oxidative stress.
Methods
EBC was collected from otherwise healthy subjects with and without a history of alcohol abuse. Reduced and oxidized EBC glutathione (GSH and GSSG, respectively), pH, and hydrogen peroxide were measured.
Results
GSH was statistically decreased in alcohol abusers only when normalized to protein (4.7 nmol/mg protein [0.75, 11.4] vs 13.4 [7.8, 26.4], p=0.03). In contrast, GSSG was significantly elevated in the EBC from alcohol abusers when compared to controls, 5.62 [0.45. 8.94] vs 0.50 nM [0.38, 0.80], p= 0.03. Thus, a greater percentage was in the oxidized GSSG form when subjects abused alcohol, 35.3% [11.8, 58.1] vs 5.2 [3.6, 6.1], p<0.001). These concentrations represented a 40 mV shift in GSH redox state towards a more oxidized state.
Conclusions
Proper sample preparation was essential to prevent GSH loss and artificial oxidation. The shift in redox potential or %GSSG, which were not affected by dilution, may serve as better markers of pulmonary oxidative stress. Furthermore, these data suggested that the oxidant stress observed in the lavage fluid of otherwise healthy alcoholics could be measured non-invasively in the EBC.
Keywords: EBC, GSH, Alcoholism, Redox potential
Background
Chronic alcoholism is associated with an elevated risk for pulmonary infection and a 3-fold increase in the incidence of acute respiratory distress syndrome (ARDS) [1-3]. A multi-center study including more than 200 ARDS subjects reported a higher prevalence of a history of chronic alcohol abuse (50%) [1-3]. To date, the exact mechanisms for this susceptibility of chronic alcohol abusers to worsened mortality and morbidity with ARDS remain unclear. Studies reported an alcohol-induced depletion of glutathione (GSH) a major antioxidant in the epithelial lining fluid (ELF) of the lungs. GSH is present in the ELF at a high concentration (500 μM), the majority (96%) of which is in the reduced form [4]. As the alveolar spaces are exposed to environmental and endogenous oxidants, sufficient levels of GSH would be essential to protect the airway cells against oxidant damages [5] [6].
Alterations in the GSH redox balance and associated oxidative stress has been thought to induce inflammation and immune hypersensitivity [7, 8]. In clinical studies, subjects with positive chronic alcohol abuse without apparently altered pulmonary function had only 20% of GSH present in the ELF [9]. At the same time, this decrease in GSH was accompanied by a 4-fold increase in glutathione disulfide, GSSG. This suggested that chronic alcohol abuse was associated with chronic alveolar oxidant stress. Unfortunately, due to the limited opportunity for sampling of the airspace in humans the exact mechanism and progression linking the concentration or the redox balance of GSH/GSSG to the risk for pulmonary pathologies remains elusive. Developing a non-invasive marker for pulmonary GSH and GSSG could facilitate identification of at-risk alcohol abusers who may not yet present with any clinical symptoms of dysfunction but who may benefit from pulmonary antioxidant replacement for preventative or treatment strategies.
In the past decade, exhaled breath condensate (EBC) has been collected in subjects with various respiratory conditions including COPD, asthma, and cystic fibrosis with the goals to better understand the etiology and progression of diseases and to obtain accurate and non-invasive biomarkers for disease prevention or prognosis [10-14]. This EBC is thought to contain micro-droplets of the alveolar and/or airway lining fluid picked up along the respiratory tree with turbulent airflow of breathing. Cytokines, markers of oxidant stress including hydrogen peroxide, 8-isoprostanes, nitric oxide metabolites have been measured in EBC in normal and diseased individuals [11, 12, 14, 15].
Previous studies by this laboratory demonstrated that a history of alcohol abuse altered GSH homeostasis in the lavage fluid as defined by decreased GSH, increased GSSG, and an increased percentage of the total pool present as GSSG even when controlled for smoking [9, 16, 17]. These changes in GSH and GSSG resulted in a 50 mV change in the redox potential [16, 17], a measure of the electric potential required for transferring electrons from the oxidant to the reductant. The purpose of the current study is to determine if GSH and GSSG could be measured in EBC and to optimize and standardize measurements for maximal accuracy and sensitivity. A second goal was to test the applicability of EBC GSH measurements in otherwise healthy subjects with chronic alcohol abuse where BALF GSH has been previously shown to be significantly decreased and to be more oxidized than non-alcohol abusers.
Methods
Material
Iodoacetic acid, dansyl chloride, dithiothreitol, γ-glutamylglutamate (γ-Glu-Glu) and GSSG were purchased from Sigma Chemical (St. Louis, MO, USA). GSH was from ICN Biochemical (Cleveland, OH, USA). Boric acid, sodium tetraborate, potassium tetraborate, perchloric acid, acetic acid, sodium heparin were analytical grade and purchased from Fisher Scientific (Piscataway, NJ, USA). Methanol, acetone, and chloroform were HPLC grade and ordered from EMD Chemicals (Gibbstown, NJ, USA).
Subjects
To investigate the effects of chronic alcoholism on EBC GSH and GSSG, EBC was collected from 26 otherwise healthy alcohol abusers whose alcohol abuse was determined by a score greater than 3 on the Short Michigan Alcohol Screening Test (SMAST) questionnaire [18] or a score of greater than 8 on the AUDIT test [19]. Previously established exclusion criteria were used [20]. For comparison, 15 subjects without a history of alcohol abuse, verified by a SMAST score of zero, with similar age and smoking history as the alcohol abusers were recruited. All recruitment, processing, and analysis were in accordance with requirements set by the Investigation Review Board of Emory University.
For determination of proper sample processing, EBC samples were collected from 7 individuals without a history of smoking or alcohol abuse (28% males, median age of 34 years [26.0, 42.5]). Samples were collected from these same controls within the same hour on two consecutive days and were made more than one hour after the last food intake.
Sample collection
Using an R-tube (Respiratory Research, Charlottesville, VA) which included a sterile polypropylene collection tube, a mouthpiece, and a saliva trap, EBC was collected from subjects who breathed tidally for 10 minutes. The tube was kept cooled with an outer chilled aluminium sleeve (-70 ° C) during the collection period so that breath condensed at the inner wall of the tube. Subjects did not wear a nose-clip during collection and were instructed to swallow any fluid that built up in the mouth to avoid exhaling saliva into the collection tube. The one-way valve trap on the tube also served as a barrier against salivary contamination.
Salivary amylase activity
As a means to evaluate salivary contamination, the activity of amylase in EBC was measured using the EnzChek kit from Molecular Probes sufficiently sensitive to 1 × 10-4 U/mL (0.3 μg protein/mL).
pH measurements
Gas-standardization of the EBC was made by gently flushing 200 μl of the EBC sample in a microcentrifuge tube with a sufficient stream of argon to bubble the fluid but minimize sample loss using the same protocol established by other laboratories [21]. The pH of the EBC was measured before and after the 10-minute argon standardization. Using a microprobe from Beckman (Fullerton, CA) and a compatible Beckman pH meter, the pH was measured in a 100-μL aliquot.
Protein Measurements
The total protein content in the EBC was measured using a modified protocol of the Better Bradford Coomassie Reagent from Pierce (Piscataway, NJ). The standard concentrations used for the standard curve ranged from 0.025 to 25 μM. 50 μL of EBC was added to 50 μL of the Bradford Reagent. Absorbance measurements at 590 nm were initially made at 1, 5, 10, and 20 minutes after the addition of reagent. As no differences were found among these time points except a non-specific increase in background absorbance, measurements were made after 5 minutes of incubation and calculation of concentration based upon the standard curve.
Hydrogen peroxide (H2O2) Measurements
The concentration of H2O2 was determined in the EBC by the Amplex Red Assay available from Molecular Probes (Portland, OR). Employing a sensitive fluorescence dye activated upon exposure to H2O2, the kit permitted valid measurements for most of the EBC samples while other assay including ones using 2,7-dichlorofluorescin failed to be sufficiently sensitive.
Preparation of samples for GSH measurement
Immediately after collection, a 300 μL EBC aliquot was treated with a preservation solution that contained perchloric acid (5% final), iodoacetic acid (13.4 mM final), boric acid (0.1 M final) and an internal standard γ-Glu-Glu (5 nM final). Preserved samples were stored at -70° C for no longer than 2 months prior to analysis. Derivatization was performed by first thawing the acidified samples on ice then centrifuging at 4 °C for 5 minutes at 13,000Xg. A 300 μL was then transferred into a fresh tube and adjusted to pH 9.0 ± 0.2 with 3M KOH. After 20 minutes, the samples were dansylated and incubated in the dark for 24 hours and then terminated by the addition of chloroform.
HPLC Setup
The dansylated derivatives were separated on a 10 μm Ultrasil amino column (Waters) by normal phase gradient chromatography. The mobile solvent was 80% HPLC grade methanol in water and the salt solvent was 0.8 M sodium acetate in acetic acid and methanol as previously published [22]. An autosampler module (Waters 2690) was used to inject 100 μL of the aqueous layer of the derivatized sample. In addition to a Waters 474 fluorescence detector, a Gilson Fluorometer Model 121 was used because of the need for increased sensitivity. The concentration of the respective derivatives was calculated in reference to the area of the internal standard. GSH and GSSG standards were prepared from a range of 1-2000 nM. GSH standards were treated with 10 mM dithiotreitol to reduce reversible disulfide formation [22]. A standard curve was created from integrated area of peaks for this concentration range. Control EBC samples were spiked with varying amounts of GSH and GSSG in the nanomolar range to determine potential shifts in retention time or peak area in the EBC.
Evaluation of the effects of time and storage conditions on GSH measurements
Previous studies have indicated a need to preserve plasma samples immediately after collection for GSH measurements [22]. In order to determine whether similarly stringent conditions need to be applied to EBC samples, we collected samples from healthy subjects. EBC samples were aliquoted into separate tubes and kept on ice for 5, 10 or 20 minutes before addition of the preservation fluid was added. In order to determine the effect of storage and temperature, the EBC in the preservation fluid was kept at room temperature for 0, 5, 10, 20, or 60 minutes prior to freezing. All samples were then derivatized for GSH measurements. The day- to- day variation in GSH concentrations was investigated. EBC samples were collected from 5 healthy individuals on two different days to determine the within-person variation in GSH levels. Samples were immediately preserved and derivatized as described above.
Redox potential calculations
The redox potential (Eh) of the GSH/GSSG thiol pair in the EBC was calculated with the Nernst equation, Eh = Eo + RT/nF ln [disulfide]/([thiol1] [thiol2]). The Eo is the standard potential for the redox couple, R is the gas constant, T is the absolute temperature, n is 2 for the number of electrons transferred, and F is Faraday’s constant. The standard potential Eo for the 2 GSH/GSSG couple was -250 mV, pH = 7.0.
Statistical Analysis
Results were reported as median [25%, 75%]. Correlations were determined by Spearman correlation analysis. Group median comparisons were made with the non-parametric Mann-Whitney Test. Statistical significance was obtained when p<0.05 unless otherwise stated.
Results
Measurements of pH and H2O2 in the EBC
Patient demographics were reported in Table 1. Amylase is a major salivary enzyme. Although the collection protocol and tube design included efforts to minimize salivary contaminations, the activity of amylase was measured in all samples to verify its absence. None of the EBC samples had any detectable amylase activity. The pH value either before or after argon deaeration did not differ between EBC samples of controls and of alcohol abusers. Median pH for subjects with chronic alcoholism before and after deaeration was respectively 6.77 [6.25, 7.28] and 7.07 [6.77, 7.34] and not different from that of non-abusers, 6.94 [6.83, 7.13] and 7.07 [6.79, 7.22]. When stratified by smoking history, pH measurements were not different regardless of whether the sample was deaerated or not (p=0.3). There was no difference in EBC protein levels between subjects with and without alcohol history (p=0.3). Median protein concentration for all subjects was 1.15 μg/ml [0.320, 2.44]. Specifically, median protein concentration was 0.68 μg/ml [0.26, 1.14] and 1.10 [0.42, 2.43], respectively, for the non-alcoholic and alcoholic groups.
TABLE 1.
Subject Demographics
| NO ALCOHOL ABUSE | ALCOHOL ABUSE | p-value | |
|---|---|---|---|
| N | 15 | 26 | |
| AGE | 42.5 [36- 47] | 46 [44- 51] | NS |
| MALE (%) | 6 (40%) | 4 (86%) | NS |
| SMOKE (%) | 8 (53%) | 22 (79%) | NS |
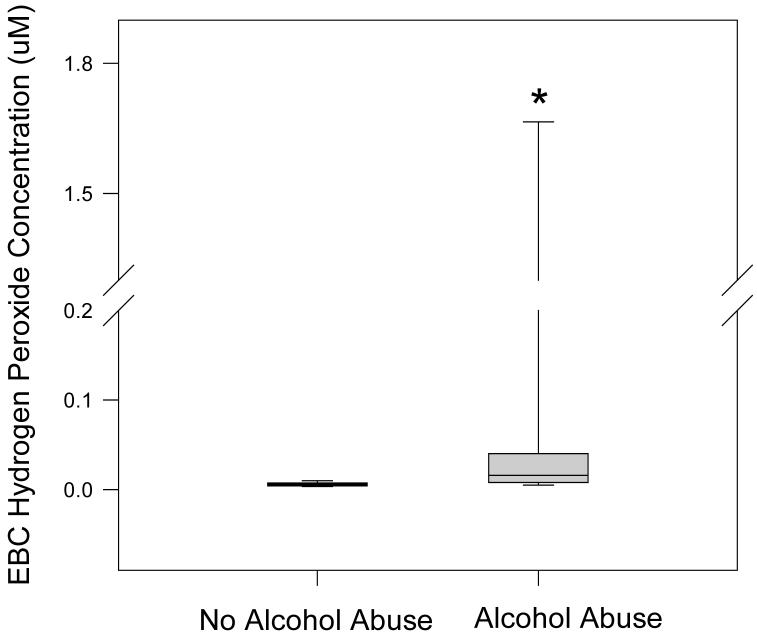
H2O2 was measured in fresh, non-deaerated EBC using the Amplex-Red fluorescent assay although some samples were below the detection limit. Median H2O2 value was elevated by nearly four-fold among subjects with alcohol abuse, 16 nM [8, 36] vs 6 [4, 7] (FIGURE 1; p<0.01). There were insufficient subjects by power analysis to stratify the H2O2 values with smoking status.
Figure 1. Concentration of hydrogen peroxide in EBC by alcohol status.
H2O2 concentration in EBC was measured using the Amplex Red Assay from Molecular Probes (Portland, OR). The data were presented as boxplots with median line, the first and third quartiles and whiskers to indicate the 90%ile and 10%ile values. * Denotes p < 0.03.
Parameters of GSH measurement
The detection of the internal standard γ-glutamyl-glutamate was possible and consistent at a final concentration of 5 nM for 100 μl of sample injected. GSH and GSSG standards in phosphate buffer were detected starting at 0.5 nM with the Gilson detector. However, this sensitivity level was possible only by using fresh columns and taking care to run under optimal HPLC solvent and machine conditions. Variation in instrumentation was partially corrected by normalizing to the internal standard.
a. Time Factor in Preparation of Samples
Immediate preservation of EBC in acid containing iodoacetic acid prevented the loss of GSH with time (FIGURE 2). Although GSH and GSSG were detected in samples acidified 20 minutes after collection, there was a 60% loss in GSH and an 80% loss in GSSG. The loss in GSH in these samples was about 2.6% for every minute the sample remained unpreserved. Preserved samples left at room temperature for one hour had a 50% decrease in GSH and GSSG when compared to samples cooled immediately after collection. This underlined the essentiality of proper sample storage for valid EBC measurements.
Figure 2. Loss of GSH and GSSG without proper preservation.
GSH and GSSG concentrations in EBC decreased with time when samples were not properly preserved.
b. Within-person variation in EBC GSH
The average within-person variation in EBC GSH was calculated based upon samples from 5 controls collected on two different days. The variation was 11.1 ± 3.3 %. Identifying and characterizing factors contributing to GSH variation may be essential for accurate interpretation of findings in clinical applications.
Chronic alcoholism associated with elevated oxidation of GSH in EBC
The median GSH concentration in the EBC of otherwise healthy subjects with a history of alcohol abuse was 5.5 nM [2.3-13.0] and not statistically different from the value of healthy controls, 9.2 nM [7.0-12.1], p=0.2. GSH concentrations were also normalized to EBC protein concentration. For alcohol abusers, the median was 25% that of non-abusers at 4.7 nmol/mg EBC protein [0.75, 11.4] vs 13.4 [7.8, 26.4] and these were statistically different (FIGURE 3; p=0.03). Chronic alcohol abusers had significant elevation of EBC GSSG when compared with alcohol non-abusers, (0.5 nM [0.4, 0.8] vs 5.6 [0.4. 8.9]) for the non-abusers and abusers, respectively (FIGURE 4; p=0.03). EBC GSSG adjusted by protein concentration was 0.9 nmol/mg EBC protein [0.4, 1.4] vs 3.3 [0.2, 13.7] for the non-abusers and abusers respectively but did not reach statistical significance (p=0.3).
Figure 3. GSH (A) and protein normalized GSH (B) concentration in the EBC.
Concentration of GSH in EBC of chronic alcohol abusers and non-abusers GSH were determined by HPLC. GSH concentrations were normalized to total EBC protein measured by a modified Bradford method. The data were presented as boxplots. * Denotes p = 0.03.
Figure 4. GSSG (A) and protein normalized GSSG (B) concentration in the EBC.
Concentration of GSSG in EBC of chronic alcohol abusers and non-abusers GSH were determined by HPLC. GSSG concentrations were normalized to total EBC protein measured by modified Bradford method. The data were presented as boxplots. * Denotes p = 0.03.
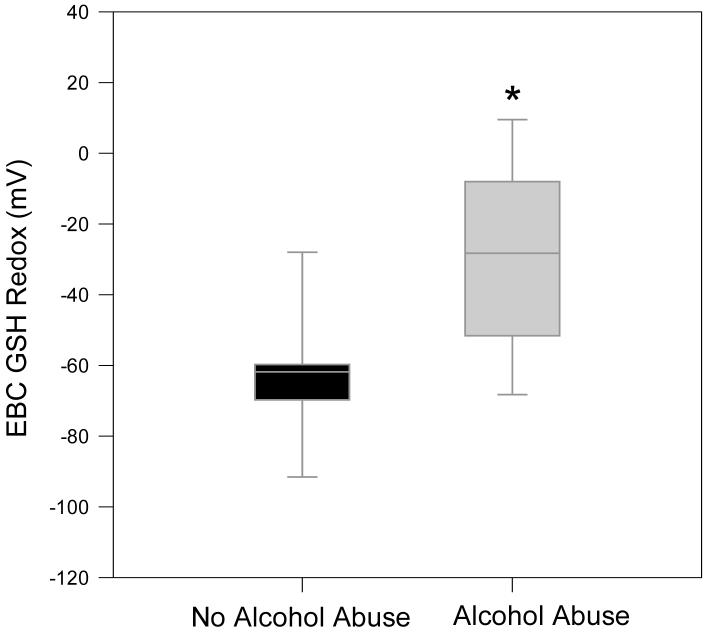
Given the concern of dilution by the condensate, the %GSSG was also determined. As described in the methods section, %GSSG represents the ratio of GSSG over total concentration of both the reduced and oxidized GSH (GSH+GSSG). The median %GSSG for alcohol abusers was 35.3%, nearly 10-fold that of controls (FIGURE 5; p<0.01). Using the GSH and GSSG concentrations, the redox state of GSH/GSSG Eh (GSH/GSSG) was calculated by the Nernst equation after adjustment for pH. When compared to control values of -61.8 mV, the redox potential of the chronic alcohol abusers was shifted by 40 mV to a more oxidized state (FIGURE 6; p<0.01).
Figure 5. Percentage of oxidized GSH in the EBC.
The percentage of the total GSH present as the oxidized moiety was determined in the EBC of chronic alcohol abusers and non-abusers (% GSSG = [GSSG] / ([GSH]+[GSSG]) × 100). Data presented as boxplots. * Denotes p< 0.01.
Figure 6. Redox potential values (Eh) for the GSH/GSSG thiol pair in the EBC.
The Eh values of EBC were calculated from the GSH and GSSG concentrations using the Nernst equation with E0 value of [GSSG]/[GSH]2 = -264 mV at a pH of 7.4. Data presented as boxplots. * Denotes p < 0.01.
Discussion
Previous works estimated EBC to be a 1000-fold dilution when compared to the bronchoalveolar lavage [23, 24]. Indeed, the range of GSH concentrations determined by this present method for the EBC were diluted approximately 1000-fold when compared to literature values for the lavage fluid for control subjects (1-5 nM in EBC vs 0.5-5 μM in the lavage fluid) [4, 9]. In 2003, Corradi et al reported a decrease in EBC GSH for asthmatic subjects during an exacerbation when compared to non-asthmatic subjects. The concentrations of GSH determined in our study (9.2 nM) correlated well with their reported value of 10 nM GSH [25]. They were able to detect an increase in GSH after proper treatment of asthma to the level of healthy subjects, but the GSSG content of the EBC was not determined.
The results of the current study underline the importance of properly preparing the samples for accurate measurement. Failure to preserve samples with perchloric acid and iodoacetic acid within 20 minutes of collection not only led to underestimation of GSH and GSSG but also distortion of the oxidized to reduced ratio for GSH. After preservation, the proper storage of the samples also proved to be essential. Therefore, measurements of EBC GSH and GSSG require particular care in sample collection, processing and storage for accurate measurements.
In order to optimize GSH measurements in the EBC and minimize artificial oxidation or loss, samples needed to be preserved within 20 minutes after collection. This was achieved by keeping the EBC on ice both before and after preservation as well as long-term storage at - 70 ° C. The use of a nose-clip was not necessary. In addition, GSH could be detected in the EBC only when the sample was collected with a polypropylene tube.
As Effros and colleagues suggested, proper normalization of EBC data may be necessary prior to useful interpretation of measured concentrations [26]. Attempts have been made to assess dilution of the ELF in EBC by using exhaled volume, urea, protein concentration, or exhaled ions [21, 27-29]. Reports from the EBC Task Force by the major American and European respiratory societies state that although dilution may be a factor influencing EBC data, it does not appear to improve reproducibility [24]. However, the current study demonstrated that %GSSG of EBC was not affected by dilution. The % GSSG was significantly elevated in chronic alcohol abusers and paralleled our previous reports of alcohol-associated increases of %GSSG in the lavage fluid [16, 17]. This increase suggested elevated oxidative stress throughout the respiratory tree that necessitated neutralization via the oxidation of GSH to its disulfide GSSG. As %GSSG avoids confounding variables such as those associated with dilution correction, it may be useful for comparison between individuals [24]. While we cannot rule out the possibility, it is unlikely there would be unequal dilution of the reduced and oxidized forms.
For controls, the redox potential for the GSH/GSSG couple was calculated to be -60 mV compared to -20 mV for those that abused alcohol. In recent work by this laboratory, GSH redox states in bronchoalveolar lavage samples of chronic alcohol abusers shifted towards the more oxidized by approximately 40 mV when compared to non-alcohol abusers [16, 17]. This was approximately the same shift in the redox state as observed in the EBC. The degree of this shift could correspond to a 10-fold increase in the ratio of oxidized to reduced forms of vicinal cysteines and potentially alter redox-sensitive pathways and processes [30-32]. This suggested that EBC GSH redox state may be a useful non-invasive tool to monitor oxidative states in the lungs previously evaluated only by BALF.
A recent paper reported a lack of correlation between nitric oxide metabolites, isoprostane or pH in BALF and EBC of healthy subjects or subjects requiring lung transplant [33]. However, their comparisons were limited by assay sensitivities and did not consider ratios or more specific metabolites. Thiols, particularly GSH, which is present in the lavage fluids at high concentrations (500 μM) may be more accurately detected in EBC than other markers present in the lavage fluids at lower concentrations (e.g. isoprostane ∼1 ug/ml). A well-characterized method to correct for dilution will be necessary prior to conclusive BALF and EBC comparisons.
Although some studies have reported acidification of EBC, particularly in asthmatics, this was not observed in the EBC of otherwise healthy chronic alcohol abusers [34, 35]. However, H2O2 was significantly elevated in chronic alcohol abusers. This increase in H2O2 paralleled the increase in %GSSG and the shift in the redox potential. Therefore, these three independent variables suggested that a history of alcohol abuse increased oxidant stress throughout the respiratory tree.
In asthmatic, but not COPD subjects, chronic and acute exposure to smoke were associated with elevated EBC H2O2 [15]. However, the current studies were not powered to address the effect of smoking on the EBC thiol measursements. Studies presently underway in which EBC and BALF, and even some direct microsamples of lavage fluid are collected will facilitate better characterization of the relationships among thiols and other biomarkers in the EBC, BALF and epithelial lining fluid.
Acknowledgements
We would like to thank Dean P. Jones for his expert advice on GSH/GSSG measurements and discussions of redox potential. This study was funded by The National Institute of Alcohol Abuse and Addiction Grant R21 AA015335 (LASB and MM), R01 AA-014435 (MM), K23AA013918 (EB) and 1 P50 AA135757 (LASB, MM and EB) as well as a Pre-doctoral Fellowship Training Grant from the American Heart Association 0515296B (MY).
Competing interests
None
Abbreviations
- EBC
Exhaled Breath Condensate
- H2O2
Hydrogen peroxide
- GSH
Glutathione
- GSSG
Oxidized Glutathione; Glutathione disulfide
- BALF
Bronchoalveolar lavage fluid
- Eh
Redox potential; redox state
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moss M, et al. The effect of chronic alcohol abuse on the incidence of ARDS and the severity of the multiple organ dysfunction syndrome in adults with septic shock: an interim and multivariate analysis. Chest. 1999;116(1 Suppl):97S–98S. [PubMed] [Google Scholar]
- 2.Moss M, et al. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit. Care Med. 2003;31:869–877. doi: 10.1097/01.CCM.0000055389.64497.11. [DOI] [PubMed] [Google Scholar]
- 3.Moss M, et al. Endothelial cell activity varies in patients at risk for the adult respiratory distress syndrome. Crit Care Med. 1996;24(11):1782–6. doi: 10.1097/00003246-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Cantin AM, et al. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol. 1987;63(1):152–7. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- 5.Davis WB, Pacht FR, Crystal RG, et al., editors. The Lung: Scientific Foundations. 2ed. Lippincott-Raven; Philadelphia, PA: 1997. Extracellular antioxidant defenses; pp. 2271–2278. [Google Scholar]
- 6.Cantin AM, et al. Antioxidant macromolecules in the epithelial lining fluid of the normal human lower respiratory tract. J Clin Invest. 1990;86(3):962–71. doi: 10.1172/JCI114798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowler RP, Crapo JD. Oxidative stress in airways: is there a role for extracellular superoxide dismutase? Am J Respir Crit Care Med. 2002;166(12 Pt 2):S38–43. doi: 10.1164/rccm.2206014. [DOI] [PubMed] [Google Scholar]
- 8.Crapo JD. Oxidative stress as an initiator of cytokine release and cell damage. Eur Respir J Suppl. 2003;44:4s–6s. doi: 10.1183/09031936.03.00000203a. [DOI] [PubMed] [Google Scholar]
- 9.Moss M, et al. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. Am J Respir Crit Care Med. 2000;161(2 Pt 1):414–9. doi: 10.1164/ajrccm.161.2.9905002. [DOI] [PubMed] [Google Scholar]
- 10.Chladkova J, et al. Validation of nitrite and nitrate measurements in exhaled breath condensate. Respiration. 2006;73(2):173–9. doi: 10.1159/000088050. [DOI] [PubMed] [Google Scholar]
- 11.Rahman I, Kelly F. Biomarkers in breath condensate: a promising new non-invasive technique in free radical research. Free Radic Res. 2003;37(12):1253–66. doi: 10.1080/10715760310001623331. [DOI] [PubMed] [Google Scholar]
- 12.Kostikas K, et al. Oxidative stress in expired breath condensate of patients with COPD. Chest. 2003;124(4):1373–80. doi: 10.1378/chest.124.4.1373. [DOI] [PubMed] [Google Scholar]
- 13.Kharitonov SA. Exhaled markers of inflammatory lung diseases: ready for routine monitoring? Swiss Med Wkly. 2004;134(1314):175–92. doi: 10.4414/smw.2004.10411. [DOI] [PubMed] [Google Scholar]
- 14.van Beurden WJ, et al. Markers of inflammation and oxidative stress during lower respiratory tract infections in COPD patients. Monaldi Arch Chest Dis. 2003;59(4):273–80. [PubMed] [Google Scholar]
- 15.Horvath I, et al. Exhaled nitric oxide and hydrogen peroxide concentrations in asthmatic smokers. Respiration. 2004;71(5):463–8. doi: 10.1159/000080630. [DOI] [PubMed] [Google Scholar]
- 16.Yeh M, et al. Monitoring of the Bornchoalveolar Oxidant Status in Chronic Alcoholics by Exhaled Breath Condensate and Segmental Lavage. Proceedings of the American Thoracic Society. 2006;3:A428. Abstracts. [Google Scholar]
- 17.Yeh MY, et al. Chronic Alcoholism Alters Systemic and Pulmonary Glutathione Redox Status. Am J Respir Crit Care Med. 2007 doi: 10.1164/rccm.200611-1722OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selzer ML, Vinokur A, van Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST) J Stud Alcohol. 1975;36(1):117–26. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- 19.O’Connor PG, Schottenfeld RS. Patients with alcohol problems. N Engl J Med. 1998;338(9):592–602. doi: 10.1056/NEJM199802263380907. [DOI] [PubMed] [Google Scholar]
- 20.Burnham EL, et al. Effects of chronic alcohol abuse on alveolar epithelial barrier function and glutathione homeostasis. Alcohol Clin Exp Res. 2003;27(7):1167–72. doi: 10.1097/01.ALC.0000075821.34270.98. [DOI] [PubMed] [Google Scholar]
- 21.Vaughan J, et al. Exhaled breath condensate pH is a robust and reproducible assay of airway acidity. Eur Respir J. 2003;22(6):889–94. doi: 10.1183/09031936.03.00038803. [DOI] [PubMed] [Google Scholar]
- 22.Jones DP, et al. Glutathione measurement in human plasma. Evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin Chim Acta. 1998;275(2):175–84. doi: 10.1016/s0009-8981(98)00089-8. [DOI] [PubMed] [Google Scholar]
- 23.Effros RM, et al. Epithelial lining fluid solute concentrations in chronic obstructive lung disease patients and normal subjects. J Appl Physiol. 2005;99(4):1286–92. doi: 10.1152/japplphysiol.00362.2005. [DOI] [PubMed] [Google Scholar]
- 24.Horvath I, et al. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J. 2005;26(3):523–48. doi: 10.1183/09031936.05.00029705. [DOI] [PubMed] [Google Scholar]
- 25.Corradi M, et al. Aldehydes and Glutathione in Exhaled Breath Condensate of Children with Asthma Exacerbation. Am. J. Respir. Crit. Care Med. 2003;167(3):395–399. doi: 10.1164/rccm.200206-507OC. [DOI] [PubMed] [Google Scholar]
- 26.Effros RM, et al. The effects of volatile salivary acids and bases on exhaled breath condensate pH. Am J Respir Crit Care Med. 2006;173(4):386–92. doi: 10.1164/rccm.200507-1059OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zacharasiewicz A, et al. Repeatability of sodium and chloride in exhaled breath condensates. Pediatr Pulmonol. 2004;37(3):273–5. doi: 10.1002/ppul.10431. [DOI] [PubMed] [Google Scholar]
- 28.Gessner C, et al. Factors influencing breath condensate volume. Pneumologie. 2001;55(9):414–9. doi: 10.1055/s-2001-16947. [DOI] [PubMed] [Google Scholar]
- 29.Effros RM, et al. Dilution of respiratory solutes in exhaled condensates. Am J Respir Crit Care Med. 2002;165(5):663–9. doi: 10.1164/ajrccm.165.5.2101018. [DOI] [PubMed] [Google Scholar]
- 30.Gruber CW, et al. Protein disulfide isomerase: the structure of oxidative folding. Trends Biochem Sci. 2006;31(8):455–64. doi: 10.1016/j.tibs.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- 32.Marin-Navarro J, Moreno J. Modification of the proteolytic fragmentation pattern upon oxidation of cysteines from ribulose 1,5-bisphosphate carboxylase/oxygenase. Biochemistry. 2003;42(50):14930–8. doi: 10.1021/bi035713j. [DOI] [PubMed] [Google Scholar]
- 33.Jackson AS, et al. Comparison of biomarkers in exhaled breath condensate and bronchoalveolar lavage. Am J Respir Crit Care Med. 2007;175(3):222–7. doi: 10.1164/rccm.200601-107OC. [DOI] [PubMed] [Google Scholar]
- 34.Hunt J, et al. Identification of acid reflux cough using serial assays of exhaled breath condensate pH. Cough. 2006;2:3. doi: 10.1186/1745-9974-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunt JF, et al. Endogenous airway acidification. Implications for asthma pathophysiology. Am J Respir Crit Care Med. 2000;161(3 Pt 1):694–9. doi: 10.1164/ajrccm.161.3.9911005. [DOI] [PubMed] [Google Scholar]