Abstract
F2-isoprostanes (IsoPs), lipid peroxidation products, are markers that quantitatively measure levels of oxidative stress. IsoP levels increase in tissues and serum of aging animals suggesting an increase in oxidative stress. This supports the Free Radical Theory of Aging which proposes that elevated levels of reactive oxygen species (ROS) cause macromolecular damage, and is a factor in the age-associated decline in tissue function. Numerous studies have shown that the longevity of long-lived mutant mice correlates with their resistance to oxidative stress. However, although the Ames dwarf (DW) mice show resistance to oxidative stress, it has not been shown that these mice have inherently lower levels of ROS. Our results show that the serum and liver IsoP levels in DW mice are lower at all ages suggesting that the lower levels of endogenous ROS production in DW mice may be a factor in their resistance to oxidative stress and longevity.
Keywords: Aging, Ames dwarf mice, F2-isoprostanes, longevity, oxidative stress
Introduction
Oxidative stress is a major characteristic of aging, the age-related decline in tissue function and in age-associated diseases. The Free Radical Theory of Aging proposes that an increase in ROS production is a major cause of macromolecular damage and age-associated decline in tissue function [1]. Mouse models that carry specific mutations affecting levels of oxidative stress (ROS production) and also exhibit increased life span have been identified. These models strongly support the hypothesis that levels of ROS production may play a key role in longevity determination. In particular, the Snell and Ames dwarf mutants, which lack growth hormone, thyroid stimulating hormone, and prolactin, and live ∼40-60% longer than their normal littermates [2] have been extensively used in studies of the role of oxidative stress in aging. These long-lived mutants have provided basic information on the role of oxidative metabolism and oxidative stress in longevity determination. Recent studies also suggest that these dwarf mice are more resistant to oxidative stress generated by environmental factors such as UV, hydrogen peroxide and paraquat [3-9]. These mice also have higher levels of antioxidant enzyme activities compared to the wild-type mice (WT) [6,10,11], thus, suggesting a possible mechanism of resistance to external oxidative stress in these long-lived mice. Although there is no direct evidence suggesting that resistance to oxidative stress by the DW mice is due to lower levels of endogenous ROS production our studies have shown that the ROS activated stress response pathway, p38 MAPK, is significantly down-regulated in young and aged Snell dwarf mutants [9]. We, therefore, propose that these data strongly suggest lower endogenous levels of ROS in the long-lived mutant.
Studies have indicated that F2-isoprostanes (IsoPs) are potentially important markers of the status of oxidative stress in eukaryotes [12-14]. IsoPs are products of arachidonic acid catalyzed by non-enzymatic free radicals and are present in both tissues and extracellular compartments [12]. Thus, IsoPs reflect the level of oxidative stress due to lipid peroxidation in vivo [15]. Measurment of the levels of these lipid molecules in biological fluids such as serum and urine, and in tissues is well established [16], and their levels are very reliable markers of the status of oxidative stress in normal aging, disease processes and pre- and post- acute injuries [12-14,17]. Based on these studies, we propose that serum and tissue IsoP levels may be markers of oxidative stress in Ames DW mice (compared to WT) at all ages. In these studies we have measured the differences in levels of IsoPs in the young, middle-aged and old Ames DW mutant and WT mice. Our results clearly show that the Ames DW mice have lower levels of IsoPs in both serum and liver at all ages, and provide further evidence in support of the Free Radical Theory on Aging. In addition, the differences in IsoP serum levels between aged DW and WT mice significantly decreased suggesting there is a delay in the development of aging characteristics by Ames DW mice. Thus we propose that Ames DW mice have endogenously lower levels of oxidative stress, that this plays a key role in resistance to oxidative stress generated by environmental factors and lead to longevity in these mice.
Materials and methods
Animals
Ames colony was maintained at the University of Texas Medical Branch (UTMB). Ames mice were generated by mating of Prop1+/- heterozygous males and females. The progeny was weaned at 1 month of age and tail DNA was collected to genotype Prop1+/+ (wild-type), Prop1+/- (heterozygous), and Prop1-/- (dwarf) mice. Real time RT-PCR was performed in Bio-Rad iCycler (Bio-Rad, CA) using TaqMan® probes for allelic discrimination of Prop1 gene.
| PCR probes: | Anti-sense, 5′-CGA GCC CAG ATG TCA GGA TAC-3′ |
| Sense, 5′-CAC CGC ACC ACC TTC AAC C-3′ | |
| TaqMan® probes: | Dwarf probe 5′-TCC CAA AGG CTG GCT CCA GC-3′ |
| WT probe 5′-TCC CAA AGG CTG ACT CCA GCT-3′ |
PCR protocol was as follows: 95°C for 1.5 min (1X), 95°C for 0.5 min, 63°C for 0.5 min, and 70°C for 0.5 min (50X). Reaction volume was 25 μL.
Mice were separated according to their genotype and wild-type and dwarf mice were aged for further use. Young (4-5 months), middle-aged (10-12 months) and old (20-26 months) male wild-type and dwarf mice were maintained in UTMB animal research facility with a 12h light/dark cycle and fed ad libitum on a standard chow diet before sacrifice.
Sample collection
Immediately after sacrifice of the animal by cervical dislocation and removal of head, blood was collected and allowed to coagulate on ice. Serum was collected by centrifugation of coagulated blood at 2500 X g for 10 min and collecting the supernatant. The serum samples were then snap-frozen in liquid nitrogen and stored at −80°C for further use. All steps were carried out quickly until the samples were frozen. Following the removal of blood, the liver of the animal was removed and ∼100 mg of liver piece was put in cryogenic tubes, snap-frozen in liquid nitrogen and stored at −80°C for further use.
Measurements of F2-Isoprostane levels
F2-Isoprostane levels were determined by extraction of isoprostanes from serum and liver samples as described previously [16]. IsoP values are reported as pg/mL of serum when obtained for serums from 7 young, 8 middle-aged, and 8 old wild-type and 12 young, 13 middle-aged, and 8 old dwarf mice. IsoP values are reported as ng/g of tissue when obtained for liver pieces from 5 young, 7 middle-aged, and 8 old wild-type and 12 young, 13 middle-aged, and 4 old dwarf mice. Statistical significance was calculated using the Student's T-test with p<0.05 and p<0.005 considered significant and highly significant, respectively.
Results
F2-isoprostane levels in serum of Ames WT and DW mice
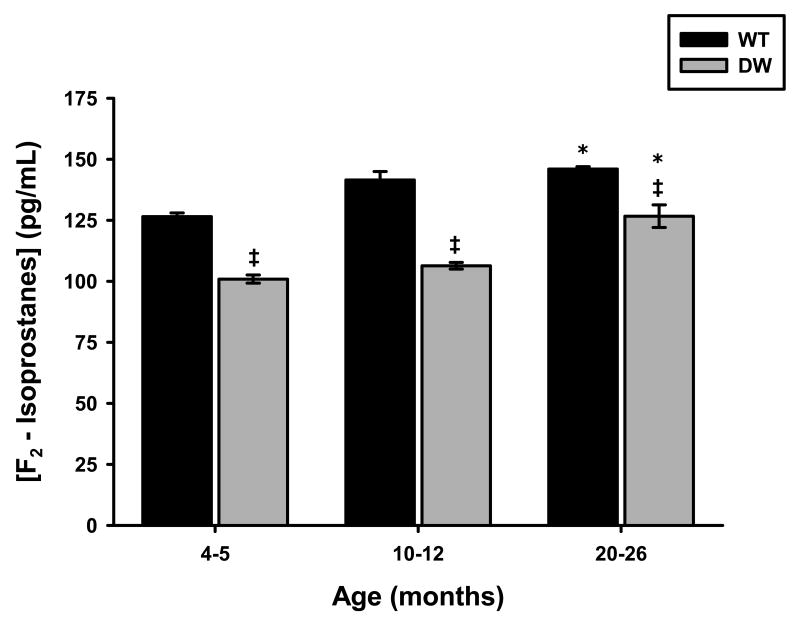
To determine whether there are changes in the levels of IsoPs that correlate with levels of oxidative stress in Ames WT and DW mice, we measured IsoP levels from sera of young, middle and old age mice. The results in Fig. 1 show that at all ages the levels of IsoPs are lower in DW mice compared to WT (∼20% at young age, ∼25% at middle age and ∼13% at old age). The small coefficients of variance of these data clearly show statistically significant differences between WT and DW IsoP levels at these ages. In addition, there is a significant increase in IsoP levels in both the mutant and normal mice at old age suggesting that the level of oxidative stress is elevated in the aged animals compared to young and middle age. However, the difference between WT and DW IsoP levels diminishes with age suggesting that the development of aging characteristics is attenuated in DW mice.
Fig. 1. Serum F2-Isoprostane levels from Ames wild-type and dwarf mice.
F2-Isoprostane (IsoP) levels were measured from serums collected from Ames wild-type and dwarf mice as described in Materials and Methods. IsoP levels were determined in young (4-5 months), middle-aged (10-12 months), and old (20-26 months) mice. The data represented are averages of IsoP values obtained for serums from 7 young, 8 middle-aged, and 8 old wild-type and 12 young, 13 middle-aged, and 8 old dwarf mice. Coefficients of variance for wild-type were 1.7% (young), 3.5% (middle-age), and 1% (old), respectively. Coefficients of variance for dwarf were 2.4% (young), 1.8% (middle-age), and 6.5% (old), respectively. * - p<0.05 compared to young and ‡ - p<0.05 compared to wild-type.
F2-Isoprostane levels in liver of Ames WT and DW mice
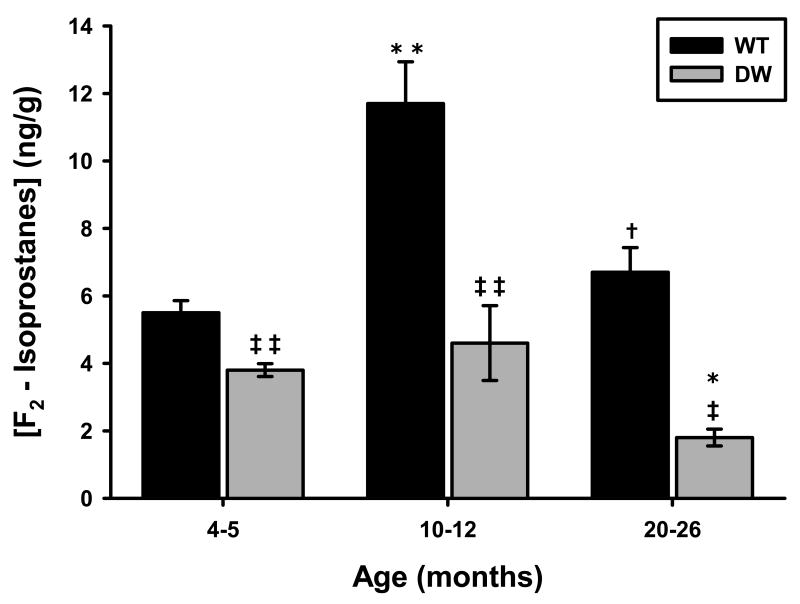
To determine if IsoP levels are changed in WT and DW tissues, we utilized liver samples to measure the amount of IsoPs at young, middle and old age. The results in Fig. 2 demonstrate dramatic differences between WT and DW IsoP levels in livers at all ages, i.e., WT mice have significantly higher levels of IsoPs compared to DW (∼45% at young age, ∼154% at middle age and ∼272% at old age). Interestingly, there is an age-related two-fold increase in IsoP levels from young to middle age in WT suggesting that these mice are undergoing significantly elevated levels of oxidative stress whereas the DW mice show a slight but non significant increase suggesting a corresponding lower level of oxidative stress. In contrast, both WT and DW mice show a decrease in IsoP levels from middle to old age where in WT the IsoP levels reverted to those of the young; in DW the levels are significantly lower than both young and middle age. These data suggest that in tissues such as liver both WT and DW revert to a lower level of endogenous oxidative stress at this age compared to middle age.
Fig. 2. Liver F2-Isoprostane levels from Ames wild-type and dwarf mice.
F2-Isoprostane (IsoP) levels were measured from liver pieces collected from Ames wild-type and dwarf mice as described in Materials and Methods. IsoP levels were determined in young (4-5 months), middle-aged (10-12 months), and old (20-24 months) mice. The data represented are averages of IsoP values obtained for liver pieces from 5 young, 7 middle-aged, and 8 old wild-type and 12 young, 13 middle-aged, and 4 old dwarf mice. Coefficients of variance for wild-type were 6.5% (young), 10.6% (middle-age), and 10.9% (old), respectively. Coefficients of variance for dwarf were 5% (young), 24.2% (middle-age), and 13.9% (old), respectively. * - p<0.05 compared to young, ** - p<0.005 compared to young, † - p<0.05 compared to middle-aged, ‡ - p<0.05 compared to wild-type and ‡‡ - p<0.005 compared to wild-type.
Discussion
The Ames dwarf mouse model, which exhibits an ∼40-60% increased life span, has been extensively used to study the molecular mechanisms of longevity [2]. The mutation of the Prop 1 locus in this mouse leads to incomplete development of the anterior pituitary gland and to a deficiency of growth hormone, thyroid stimulating hormone and prolactin [5]. Although numerous studies have indicated that the Ames dwarf mouse model as well as the parallel Snell dwarf model are more resistant to oxidative stress generated by extrinsic factors such as UV light, hydrogen peroxide and paraquat [3,4,6-8], no studies have conclusively shown that these mice generate less ROS and hence lower levels of oxidative stress. However, that the Ames DW mice exhibit a lower endogenous level of ROS is suggested by our observations that the oxidative stress induced p38 MAPK stress response pathway is down-regulated in the DW mice compared to WT [9,18-20]. Taken together, these studies suggest that the endogenous ROS levels are significantly lower in the Ames dwarf at all ages.
Our results from the sera of WT and DW mice show that the DW mice have lower levels of IsoPs in young, middle and old age. Thus, the production of IsoPs from non-enzymatic catalysis of arachidonic acid by free radicals is a more direct marker of decreased levels of endogenous oxidative stress. Furthermore, the lower levels of IsoPs in young, middle and old ages suggest that resistance to intracellular oxidative stress occurs in all tissues and at all ages of these mutant mice. We conclude that the increase in oxidative stress indicated by IsoP levels, with aging, support the Free Radical Theory of Aging.
Our studies show that the differences in levels of IsoP between WT and DW decrease by old age suggesting that the processes that generate higher levels of ROS in aging tissues are attenuated in DW mice. We propose that this delay is associated with lower levels of oxidative stress at young and middle age that correlates with resistance to oxidative stress. Thus the biological processes associated with lower endogenous ROS and resistance to oxidative stress are overcome or neutralized in the long-lived mutant but at a delayed rate. Finally we propose that resistance to oxidative stress promotes longevity in this animal.
The levels of liver IsoP show a similar profile to that of serum such that at any age DW mice have lower levels of IsoPs. However, the levels in liver, in contrast to the serum levels, are much higher, especially at the middle and old age. We propose that the dramatic increase in IsoP levels in livers of WT from young to middle age is indicative of a significant increase in oxidative stress in this tissue and that the mild age-related increase observed in DW mice suggests major differences in endogenous oxidative stress between the WT and long-lived DW mouse. Again, these results support the theory that DW mice show characteristics of delayed aging. In contrast, the decrease in IsoP levels from middle to old age suggests a major change in oxidative metabolism at this stage of the life cycle. This is the first time that such a dramatic change in oxidative metabolism has been reported to occur in normal aged tissue. We hypothesize that as the tissues age, they lose the ability to respire efficiently (normally) thereby decreasing the level of mitochondrial ROS production. Based on this hypothesis it is possible that tissue dysfunction may be due to lower levels of mitochondrial function and that the level of mitochondrial ROS leakage may also slow down. The fact that the decline of IsoP levels is much more dramatic in aged DW mice compared to WT also suggests a possible protective mechanism in response to oxidative stress in the long-lived DW mice which may involve decreased mitochondrial function or population.
In conclusion, we demonstrate that Ames dwarf mice have lower levels of oxidative stress compared to the wild-type mice; they show characteristics of delayed aging; and their resistance to intracellular oxidative stress may play a key role in delaying the aging phenomenon and conferring extended life-span in these dwarf mutants.
Acknowledgments
This publication was supported by U.S.P.H.S. grant 1P01 AG021830 awarded by the National Institute on Aging, and the National Institute on Aging 1 P30 AG024832-01; Claude D. Pepper Older Americans Independence Center grant and by the Sealy Center on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 2.Murakami S. Stress resistance in long-lived mouse models. Exp Gerontol. 2006;41:1014–1019. doi: 10.1016/j.exger.2006.06.061. [DOI] [PubMed] [Google Scholar]
- 3.Murakami S, Salmon A, Miller RA. Multiplex stress resistance in cells from long-lived dwarf mice. FASEB J. 2003;17:1565–1566. doi: 10.1096/fj.02-1092fje. [DOI] [PubMed] [Google Scholar]
- 4.Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller RA. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am J Physiol Endocrinol Metab. 2005;289:E23–E29. doi: 10.1152/ajpendo.00575.2004. [DOI] [PubMed] [Google Scholar]
- 5.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 6.Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol. 2004;63:189–225. doi: 10.1016/S0070-2153(04)63006-7. [DOI] [PubMed] [Google Scholar]
- 7.Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci U S A. 2001;98:6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flurkey K, Papaconstantinou J, Harrison DE. The Snell dwarf mutation Pit1(dw) can increase life span in mice. Mech Ageing Dev. 2002;123:121–130. doi: 10.1016/s0047-6374(01)00339-6. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh CC, Papaconstantinou J. Thioredoxin-ASK1 complex levels regulate ROS-mediated p38 MAPK pathway activity in livers of aged and long-lived Snell dwarf mice. FASEB J. 2006;20:259–268. doi: 10.1096/fj.05-4376com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown-Borg HM, Bode AM, Bartke A. Antioxidative mechanisms and plasma growth hormone levels: potential relationship in the aging process. Endocrine. 1999;11:41–48. doi: 10.1385/ENDO:11:1:41. [DOI] [PubMed] [Google Scholar]
- 11.Brown-Borg HM, Rakoczy SG. Catalase expression in delayed and premature aging mouse models. Exp Gerontol. 2000;35:199–212. doi: 10.1016/s0531-5565(00)00079-6. [DOI] [PubMed] [Google Scholar]
- 12.Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000;28:505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 13.Montuschi P, Barnes PJ, Roberts LJ. Isoprostanes: markers and mediators of oxidative stress. FASEB J. 2004;18:1791–1800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- 14.Montuschi P, Barnes P, Roberts LJ. Insights into oxidative stress: the isoprostanes. Curr Med Chem. 2007;14:703–717. doi: 10.2174/092986707780059607. [DOI] [PubMed] [Google Scholar]
- 15.Pratico D, Tangirala RK, Rader DJ, Rokach J, FitzGerald GA. Vitamin E suppresses isoprostane generation in vivo and reduces atherosclerosis in ApoE-deficient mice. Nat Med. 1998;4:1189–1192. doi: 10.1038/2685. [DOI] [PubMed] [Google Scholar]
- 16.Morrow JD, Roberts LJ. Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as measure of oxidant stress. Methods Enzymol. 1999;300:3–12. doi: 10.1016/s0076-6879(99)00106-8. [DOI] [PubMed] [Google Scholar]
- 17.Ward WF, Qi W, Van Remmen H, Zackert WE, Roberts LJ, Richardson A. Effects of age and caloric restriction on lipid peroxidation: measurement of oxidative stress by F2-isoprostane levels. J Gerontol A Biol Sci Med Sci. 2005;60:847–851. doi: 10.1093/gerona/60.7.847. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh CC, Papaconstantinou J. Akt/PKB and p38 MAPK signaling, translational initiation and longevity in Snell dwarf mouse livers. Mech Ageing Dev. 2004;125:785–798. doi: 10.1016/j.mad.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Madsen MA, Hsieh CC, Boylston WH, Flurkey K, Harrison D, Papaconstantinou J. Altered oxidative stress response of the long-lived Snell dwarf mouse. Biochem Biophys Res Commun. 2004;318:998–1005. doi: 10.1016/j.bbrc.2004.04.126. [DOI] [PubMed] [Google Scholar]
- 20.Papaconstantinou J, Deford JH, Gerstner A, Hsieh CC, Boylston WH, Guigneaux MM, Flurkey K, Harrison DE. Hepatic gene and protein expression of primary components of the IGF-I axis in long lived Snell dwarf mice. Mech Ageing Dev. 2005;126:692–704. doi: 10.1016/j.mad.2005.01.002. [DOI] [PubMed] [Google Scholar]