Abstract
MarA, a transcriptional regulator in Escherichia coli, affects functions such as multiple-antibiotic resistance (Mar) and virulence. Usually an activator, MarA is a repressor of hdeAB and other acid resistance genes. We found that, in wild-type cells grown in LB medium at pH 7.0 or pH 5.5, repression of hdeAB by MarA occurred only in stationary phase and was reduced in the absence of H-NS and GadE, the main regulators of hdeAB. Moreover, repression of hdeAB by MarA was greater in the absence of GadX or Lrp in exponential phase at pH 7.0 and in the absence of GadW or RpoS in stationary phase at pH 5.5. In turn, MarA enhanced repression of hdeAB by H-NS and hindered activation by GadE in stationary phase and also reduced the activity of GadX, GadW, RpoS, and Lrp on hdeAB under some conditions. As a result of its direct and indirect effects, overexpression of MarA prevented most of the induction of hdeAB expression as cells entered stationary phase and made the cells sevenfold more sensitive to acid challenge at pH 2.5. These findings show that repression of hdeAB by MarA depends on pH, growth phase, and other regulators of hdeAB and is associated with reduced resistance to acid conditions.
MarA, an AraC/XylS transcriptional regulator of Escherichia coli, directly activates or represses multiple chromosomal genes. As a result, it affects many functions including multiple-antibiotic resistance (Mar), virulence, and survival (1, 5). MarA is specified by the marRAB operon, whose expression is induced by sodium salicylate, menadione, and other chemicals which inactivate the autorepressor MarR (1, 10, 33, 38). Using gene arrays, Pomposiello et al. (33) found that overexpression of MarA from an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible plasmid repressed hdeAB and other genes involved in acid resistance (gadA, gadB, gadC, hdeD, and slp), whereas sodium salicylate activated these same genes. Direct repression of hdeAB by MarA has been demonstrated previously (37). However, whether MarA affects cell resistance to acid conditions remained unknown.
The hdeAB operon of E. coli specifies two periplasmic chaperones that are essential for cell survival in rich media at extremely low pH, such as that in the stomach (pH 1.5 to 3). Both chaperones are only active below pH 3 and prevent irreversible aggregation of acid-denatured periplasmic proteins (14, 21, 23, 28). HdeA seems to play a major role at pH 2, whereas HdeB is more active at pH 3 (23). The determined HdeA crystal structure and the model HdeB structure are similar (14, 45), in agreement with their genetic and functional similarities. HdeA is one of the most abundant periplasmic proteins in stationary phase (25).
Expression of hdeAB is affected by different environmental conditions. The hdeAB operon is induced in stationary phase (39, 44) and at acid pH (19, 43). Transcription of hdeAB depends on complex circuits of regulation involving RpoD (σ70), RpoS (σ38), H-NS, cyclic AMP receptor protein, GadE, GadX, GadW, GadY, EvgAS, YdeO, Lrp, MarA, SoxRS, TorRS, and TrmE, among other regulators (see Fig. 1 and references in the legend; see Fig. 2 for the DNA binding sites of some of these regulators in the hdeAB promoter).
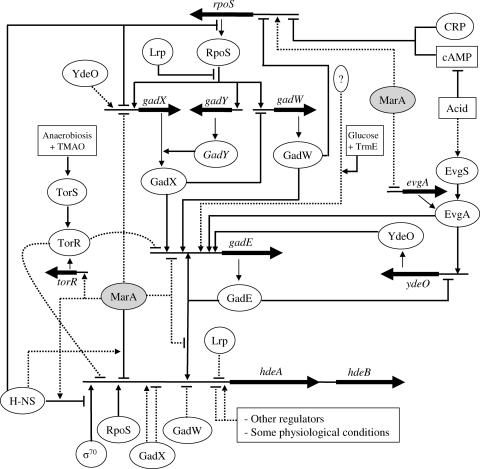
FIG. 1.
Regulation of the hdeAB operon. The figure has been produced using data from the literature (8, 13, 16, 20, 22, 24, 26, 27, 30, 33, 37, 39, 42, 43, 44, 46) as well as the results obtained in this study. Thick arrows represent genes. Direct regulation is shown as a continuous thin arrow. Indirect regulation, or regulation in which a direct effect has not been confirmed, is shown as a dotted thin arrow. See the supplemental material for a more detailed explanation of this figure. cAMP, cyclic AMP; CRP, cAMP receptor protein.
FIG. 2.
Binding sites in the hdeAB promoter for the selected regulators. The hdeAB promoter was analyzed from positions −168 to +54 as described in Materials and Methods. The binding sites of the selected transcriptional regulators are shown below the corresponding sequence as continuous arrows (known) or dotted arrows (putative). In parentheses are the number of nucleotides that match the consensus sequence/number of nucleotides of the consensus sequence. Even though hdeAB is transcribed from RNA polymerase containing σ70 or σ38 (39), its promoter does not have the −10 sequence (CTATACT) found in many promoters regulated by σ38 that replaces the −10 sequence (XTATAAT) found in σ70 promoters (12). TSS, transcription start site.
We have studied how MarA fits into the complex transcriptional control of hdeAB. We report the effect of pH, growth phase, and known regulators of hdeAB on hdeAB expression and repression by MarA, as well as the effect of MarA on the activities of these other regulators. We also describe the effect of MarA on acid resistance.
MATERIALS AND METHODS
Strains, plasmids, and growth media.
The bacterial strains and plasmids used in this study are listed in Table 1. Because HdeA and HdeB seem to be mainly important in rich medium (28), we used LB medium (per liter: 10 g of tryptone, 5 g of yeast extract, 10 g of NaCl) at pH 7.0 or buffered to pH 5.5 with 100 mM MES (morpholinoethanesulfonic acid). All cultures were grown at 37°C with agitation. Cells grown overnight (18 h) were subsequently diluted 1:100 in fresh media to an optical density at 600 nm (OD600) of 0.03. According to the growth curves obtained, exponential-phase cells were defined as those obtained from cultures in fresh media grown for 2 to 2.5 h (0.3 to 0.6 OD600) and early-stationary-phase cells as those obtained after 6 h (1.4 to 2.9 OD600). The final pH of the cultures was 7.0 to 8.0 in LB at pH 7.0 and 5.6 to 6.0 in LB at pH 5.5. The antibiotics were used at 100 μg ml−1 (ampicillin) and 25 μg ml−1 (chloramphenicol, kanamycin, and tetracycline). MarA expression from plasmid pMB102 was induced by inoculation of cells from the overnight cultures into fresh medium containing 0.5 mM IPTG.
TABLE 1.
Bacterial strains and plasmids used in this study
| Name | Genotype/relevant characteristics | Reference/source |
|---|---|---|
| E. coli strains | ||
| AG100 | argE3 thi-1 rpsL xyl supE44 λ lysogen | 15, also see reference 4; this studya |
| CV1008 | CV976 lrp-35::Tn10 (Tetr) | 32 |
| DT162 | MG1655 ΔgadX::kan | 43 |
| DT203 | MG1655 ΔgadW::kan | 43 |
| EF1155 | MG1655 ΔgadE(yhiE)::kan | 26 |
| GC4468 | Δ(lac)U169 rpsL | 9 |
| JHC1096 | GC4468 zdd-239::Tn9 del1738 (39 kb deleted, including marRAB locus) (Cmr) | 17 |
| LCB621 | MC4100 torR49::mini-Tn10 (Tetr) | 31 |
| PS2652 | ΔlacZ169 zch-506::Tn10 hns-1001::Tnseq1 (Kanr) | 6 |
| SPC105 | MC4100 marOII-lacZ (Ampr) in λatt site | 10 |
| ZK1000 | ZK126 ΔrpoS::kan | 7 |
| HdeA96 | JHC1096 hdeABp::lacZ (Ampr) in λatt site | This study |
| HdeA100 | HdeA96 transformed with pMB102 | This study |
| HdeA101 | HdeA100 hns-1001::Tnseq1 (Kanr) | P1 PS2652 × HdeA100 |
| HdeA103 | HdeA100 ΔrpoS::kan | P1 ZK1000 × HdeA100 |
| HdeA105 | HdeA100 lrp-35::Tn10 (Tetr) | P1 CV1008 × HdeA100 |
| HdeA107 | HdeA100 torR49::mini-Tn10 (Tetr) | P1 LCB621 × HdeA100 |
| HdeA109 | HdeA100 ΔgadX::kan | P1 DT162 × HdeA100 |
| HdeA111 | HdeA100 ΔgadW::kan | P1 DT203 × HdeA100 |
| HdeA113 | HdeA100 ΔgadE::kan | P1 EF1155 × HdeA100 |
| Plasmids | ||
| pHdeA415 | ori colE1 hdeABp-lacZ, AmpR | This study |
| pJPBH | ori colE1 lacI, AmpR | 37 |
| pMB102 | ori colE1 lacI lacZp::marA, AmpR | 33 |
| pRS415 | ori colE1 lacZ fusion vector, AmpR | 41 |
Determined in this study to be a single lysogen of λ by PCR as previously described (34).
Construction of the hdeABp-lacZ chromosomal transcriptional fusion and other DNA manipulations.
A 372-bp fragment from the hdeAB promoter (from −349 to +23 relative to the transcriptional start site) was amplified by PCR using chromosomal DNA from strain AG100 as template, primers HdeALF (5′AGGgaattcAAAATATCGCCAGAGACGAAC, EcoRI site in lowercase) and HdeALR (5′TATggatccAGCCGTCACGAATCAAT, BamHI site in lowercase), and Turbo Pfu polymerase (Stratagene, La Jolla, CA), using a Tm of 55°C. The amplified DNA was cloned into the vector pRS415 between the EcoRI and BamHI sites, yielding the plasmid pHdeA415, whose insert sequence was verified at the Tufts University Core Facility. Recombination between the hdeABp-lacZ region of pHdeA415 and λRZ5 (35) resulted in a lysate bearing λRZ5 (hdeABp-lacZ). This was used to infect strain JHC1096, and Ampr Lac+ lysogens were selected and purified on LB agar containing ampicillin (50 μg ml−1) and X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; 40 μg ml−1). Lysates from these lysogens were then used to infect strain JHC1096, and Ampr Lac+ lysogens were again isolated. The resulting strain, HdeA96, was confirmed by PCR, as previously described (34), to have a single copy of the transcriptional fusion located in the λatt site on the chromosome. Strain HdeA96 was subsequently transformed with plasmid pMB102 to obtain strain HdeA100. Plasmid CaCl2 transformation and phage P1 transduction to transfer mutations in different hdeAB regulatory genes to the strain HdeA100 were performed according to standard procedures (36).
Identification of the putative binding sites for the hdeAB regulators.
The hdeAB promoter was analyzed from positions −168 (transcriptional start site of the divergent gene hdeD) to +54 (translation start codon for HdeA) (Fig. 2). Putative binding sites were identified using the “search patterns” utility of Colibri (genolist.pasteur.fr/Colibri/). MarA (37) and H-NS (39) binding sites in the hdeAB promoter region have already been identified. The GadE binding site has been located between −158 and +4 but not identified (20). The consensus binding sequences we used were the following: 5′WYAGGMWWWWDYTWYWWW for binding of GadE, GadX, and GadW (gadbox [26, 43]); 5′YAGHAWATTWTDCTR for Lrp (11); and 5′CTGTTCATAT for TorR (2, 40); where D is A, T, or G; H is A, C, or T; M is A or C; R is A or G; W is A or T; and Y is C or T. Only sequences with 70% or higher identity to the consensus sequences were selected.
β-Galactosidase assays.
Expression of the marO-lacZ and hdeABp-lacZ chromosomal reporters was tested using exponential-phase and stationary-phase cells prepared as described above. The assays were performed using cells permeabilized with sodium dodecyl sulfate-chloroform as previously described (29). All assays were performed at least in triplicate; each replicate was the result of an independent assay performed in duplicate. Statistically significant differences were determined by t test (two independent samples with equal variance, two-tailed distribution) using Microsoft Excel 2003 software. Unless otherwise stated, differences were considered to be statistically significant only when P was <0.01.
Acid resistance assays at very low pH.
The assays were performed using early-stationary-phase cells (OD600 of 1.3 to 2.5) prepared as described above and obtained from cultures in fresh media grown for 6 h (cultures in LB broth) or 7 to 8 h (cultures in LB broth containing 5 mM sodium salicylate). Control cells were directly plated on LB pH 7.0 agar after serial dilution in fresh LB broth at pH 7.0. To mimic the conditions that E. coli cells would encounter when passing through the stomach, sample cells were diluted to about 106 CFU per ml in LB at pH 2.5 (acidified with HCl) and incubated for 2 h at 37°C without shaking. Cells were then serially diluted in fresh LB broth at pH 7.0 and plated on LB pH 7.0 agar plates to determine viability. The assays were performed at least in triplicate.
RESULTS
Effect of stationary phase and pH on hdeAB expression and repression by MarA.
We chose conditions that are known to have a major effect on the expression of hdeAB (exponential versus stationary phase and pH 7.0 versus pH 5.5 [19, 39, 43, 44]) and on the physiology of the HdeA and HdeB proteins (LB rich medium [28]). In a ΔmarRAB strain bearing an hdeABp-lacZ chromosomal reporter, the expression of hdeAB at pH 7.0 increased 1.7-fold from exponential phase to early stationary phase (Fig. 3). The β-galactosidase activity obtained and the increase in hdeAB expression observed are in agreement with a previous report (39). When the assays were performed using late-stationary-phase cells (overnight cultures), the activity found was about 10-fold higher than that in exponential-phase cells (data not shown). Such high expression is consistent with the high amount of HdeA protein reported under similar conditions (25). At pH 5.5, the expression of hdeAB in exponential phase showed no significant increase compared to that at pH 7.0, even though other authors have found acid induction in exponential-phase cells grown under different conditions (19, 43). In stationary phase, hdeAB expression at pH 5.5 was larger than that at pH 7.0 (a 2.5-fold increase compared to expression in exponential phase at pH 5.5; Fig. 3).
FIG. 3.
Effect of MarA on the expression of hdeAB. The β-galactosidase activity in Miller units of the hdeABp-lacZ chromosomal reporter was determined using HdeA100 cells grown in LB at pH 7.0 (left) or in LB at pH 5.5 (right) to exponential or stationary phase. The assays were performed in the absence of MarA (without IPTG) or in the presence of MarA (with IPTG). The results are presented as the averages ± the standard errors of the means (n ≥ 3). Statistically significant differences (P < 0.01) between related conditions (exponential versus stationary phase at the same pH; pH 7.0 versus pH 5.5 in the same growth phase; and presence versus absence of MarA under the same pH and growth phase) are indicated by asterisks.
When HdeA100 cells (bearing pMB102) were induced by IPTG to overexpress MarA, we found that MarA prevented nearly all of the 1.7- to 2.5-fold increase in hdeAB expression as cells entered stationary phase (Fig. 3). MarA did not repress hdeAB in exponential phase but did repress about twofold in stationary phase regardless of the pH (Fig. 3) to nearly the exponential-phase level. With the control plasmid pJPBH, IPTG led to no effect (data not shown).
Effect of known regulators on hdeAB expression: H-NS is the main repressor and GadE is the main activator of hdeAB.
We asked whether the changes in the repression produced by MarA on hdeAB occurred via other known regulators of hdeAB. Seven regulators were selected for the study: H-NS, RpoS (σ38), and GadE, which are known direct regulators of the hdeAB operon (see Fig. 1 and 2); and GadX, GadW, Lrp, and TorR, which are thought to be both direct (not demonstrated, but all of them have several putative binding sites in the hdeAB promoter; Fig. 2) and indirect regulators of hdeAB (they regulate H-NS, RpoS, or GadE; Fig. 1).
The effects of these regulators on hdeAB expression obtained earlier by others cannot be compared since these studies have been performed using dissimilar methods and conditions (in some cases only with microarrays of exponential-phase cells grown in minimal medium). Therefore, before studying their effects on MarA control of hdeAB expression, we evaluated the relative importance of each of these regulators individually on hdeAB expression under the same conditions. We chose the conditions described above that have a major effect on the expression of hdeAB and on the physiology of HdeA and HdeB.
The results obtained and their statistical significance are displayed in Table 2 (data in the absence of MarA). Under all the conditions studied, H-NS was the main repressor of hdeAB, although repression by H-NS was about half as great at pH 5.5 as at pH 7.0. GadE was the main activator. GadX was a direct or indirect activator of hdeAB only in stationary-phase cells at pH 5.5, but it was a repressor in exponential phase at pH 7.0; GadW was a repressor of hdeAB mainly in stationary phase at pH 5.5. RpoS had a small effect on hdeAB expression and only under certain conditions. Lrp only repressed hdeAB in exponential phase at pH 7.0. TorR did not significantly affect hdeAB expression.
TABLE 2.
Activation or repression of hdeAB expression by selected regulators in the absence and the presence of MarAa
| Regulator | Activation or repression in indicated growth phaseb,c
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| pH 7.0
|
pH 5.5
|
||||||||
| Exponential
|
Stationary
|
Exponential
|
Stationary
|
||||||
| - | + | - | + | - | + | - | + | ||
| H-NS | -10.5 | -9.9 | -9.9 | -13.7 | -6.0 | -6.1 | -5.7 | -8.7 | |
| GadE | 2.9 | 2.9 | 3.5 | 2.2 | 3.7 | 3.7 | 4.0 | 2.3 | |
| GadX | -1.9 | -1.5 | 1.1 | 1.2 | -1.1 | 1.0 | 1.7 | 1.6 | |
| GadW | -1.4 | -1.2 | -1.3 | -1.2 | -1.4 | -1.2 | -2.3 | -1.7 | |
| RpoS | -1.2 | -1.1 | 1.3 | 1.1 | 1.0 | 1.2 | -1.2 | 1.0 | |
| Lrp | -1.3 | 1.0 | 1.0 | 1.1 | -1.1 | -1.1 | -1.1 | 1.0 | |
| TorR | -1.1 | -1.1 | 1.0 | 1.1 | 1.0 | 1.1 | -1.1 | 1.0 | |
hdeAB expression in LB was determined by measuring the β-galactosidase activity of the hdeABp-lacZ chromosomal reporter in the ΔmarRAB strain HdeA100 (wild type) and in seven HdeA100-derivative strains inactivated for different hdeAB regulators (see Table 1). The assays were performed in the absence (−) of MarA (without IPTG) or in the presence (+) of MarA (with IPTG).
Results are the n-fold activation (positive values) or repression (negative values) produced by each regulator on hdeAB expression and were obtained from a ratio of the hdeAB expression in the parental strain and the hdeAB expression in the strain inactivated for a particular regulator under the same conditions; e.g., a regulator that increased hdeAB expression five times (ratio of the parental strain/inactivated strain of 5) would be represented in the table as “5” and would be said to produce a fivefold activation. On the contrary, a regulator that decreased hdeAB expression five times (ratio of the parental strain/inactivated strain of 0.2; thus, ratio of the inactivated strain/parental strain of 5) would be represented in the table as “-5” and would be said to produce a fivefold repression. Note that both regulators would have the same “strength” with respect to their effect on hdeAB expression, although in an opposite way. Results in bold indicate that the activation or repression produced by a regulator was statistically significant (P < 0.01).
Results in italics (P < 0.05) or underlined (P < 0.01) indicate that MarA produced a statistically significant change in the activation or repression produced by a regulator under the same conditions (e.g., the repression produced by H-NS on hdeAB in stationary phase at pH 5.5 was significantly stronger in the presence of MarA than in the absence of MarA).
MarA also controls hdeAB expression by modifying the effect of other regulators of hdeAB.
We studied whether MarA was able to alter the effect of the other regulators on hdeAB expression. Under each condition, we compared the regulator-inactivated strain/parental strain ratio obtained in the presence of MarA with that obtained without MarA (Table 2).
The activity of two major regulators, H-NS and GadE, under these conditions was strongly affected by MarA (Table 2). MarA enhanced hdeAB repression by H-NS in stationary phase at both pH 7.0 and pH 5.5, which indicates that MarA assists or collaborates with H-NS to repress hdeAB in stationary phase. On the contrary, MarA reduced the ability of GadE to activate hdeAB in stationary phase at pH 5.5, which suggests that MarA hinders hdeAB activation by GadE.
MarA also reduced the repression produced by GadX and Lrp in exponential phase at pH 7.0 and the repression produced by GadW and RpoS in stationary phase at pH 5.5 (Table 2).
Effect of the other regulators of hdeAB on repression by MarA.
After finding that MarA was able to alter the effect of the other regulators on hdeAB, we studied the converse possibility: whether the absence of the other regulators affected the ability of MarA to repress hdeAB (Table 3). A dramatic decrease in the repression produced by MarA in stationary phase was found in the hns and gadE mutants at both pH 7.0 and pH 5.5 (Table 3). Therefore, both H-NS and GadE were involved in the increased hdeAB repression by MarA that occurred as cells enter stationary phase.
TABLE 3.
hdeAB repression by MarA in strains inactivated for selected regulators of hdeAB
| Strain | Repression in indicated growth phasea
|
|||
|---|---|---|---|---|
| pH 7.0
|
pH 5.5
|
|||
| Exponential | Stationary | Exponential | Stationary | |
| WTb | -1.1 | -1.7 | -1.1 | -2.0 |
| hns | -1.1 | -1.2 | -1.1 | -1.3 |
| gadE | -1.1 | -1.0 | -1.1 | -1.1 |
| gadX | -1.3 | -1.7 | -1.2 | -1.9 |
| gadW | -1.2 | -1.7 | -1.2 | -2.6 |
| rpoS | -1.2 | -1.5 | -1.3 | -2.5 |
| lrp | -1.4 | -1.8 | -1.1 | -2.3 |
| torR | -1.1 | -1.7 | -1.1 | -2.0 |
Results are the n-fold repression (since the values are negative) of hdeAB expression produced by MarA (the ratio calculated as hdeAB expression without MarA divided by hdeAB expression with MarA in the same strain and conditions). Results in bold (P < 0.05) or in bold and underlined (P < 0.01) indicate when the inactivation of a regulator produced a statistically significant change in the repression produced by MarA compared to the repression produced by MarA in the parental strain under the same conditions (same column). hdeAB expression was measured as described in the footnotes of Table 2.
Repression of hdeAB expression produced by MarA in the parental strain (see Fig. 3) was statistically significant (P < 0.01) only in stationary phase (values in italics). WT, wild type.
On the contrary, repression by MarA increased in the gadX and lrp mutants in exponential phase at pH 7.0 and in the gadW, rpoS, and lrp mutants in stationary phase at pH 5.5 (Table 3). These results might be due to competition of GadX, GadW, and Lrp with MarA, while the decreased repression by MarA produced by RpoS may be a direct or indirect effect (see Fig. 1).
Effect of growth phase and pH on the expression of marA without and with the inducer sodium salicylate.
To understand how the native promoter of marA responded to growth phase and pH, we used marO-lacZ, a chromosomal reporter of the marRAB operon (in SPC105). We found that expression of marA at pH 7.0 was slightly, but significantly, induced in stationary phase compared to the level in exponential phase; greater induction occurred at pH 5.5 (Table 4). Induction of marA expression by sodium salicylate (5 mM) depended also on the growth phase and the pH. At pH 7.0, salicylate produced a strong induction after 1 h when added to cells growing in exponential phase (Table 4), as previously reported (10, 38), whereas it had much less effect when added to stationary-phase cells (as measured relative to expression in cells in stationary phase without salicylate) (Table 4). At pH 5.5, salicylate produced little or no effect on marA expression after 1 h when added to cells in exponential or in stationary phase. When cells grown overnight without salicylate were inoculated into medium at pH 7.0 with 5 mM salicylate, cell growth was slower than that without inducer. Under these conditions, the induction of marA expression by salicylate was very strong when tested in exponential phase and even stronger in stationary phase (Table 4). Note that this set of cells was exposed longer to salicylate (Table 4). Growth was arrested when overnight cultures grown without salicylate were diluted into medium at pH 5.5 with 5 mM salicylate.
TABLE 4.
Effect of different growth conditions on the expression of marRABa with and without the inducer sodium salicylate
| Condition | Expression ratiob
|
|
|---|---|---|
| Exponential phasec | Stationary phasec | |
| No salicylate | ||
| pH 7.0 | 1.0 | 1.3 |
| pH 5.5 | 1.6 | 1.8 |
| 5 mM salicylate for 1 hd | ||
| pH 7.0 | 7.7 | 1.7 |
| pH 5.5 | 1.4 | 1.8 |
| 5 mM salicylate, added to diluted stationary-phase cellse | ||
| pH 7.0 | 13.9 | 27.4 |
| pH 5.5 | ND | ND |
The expression of the marRAB operon in LB medium was studied using the chromosomal marO-lacZ reporter of the strain SPC105.
The β-galactosidase activity under each condition was normalized to the activity without salicylate at pH 7.0 in exponential phase (154 ± 7.5 Miller units), defined as expression ratio 1.0. The expression of marRAB under all the conditions studied was statistically different (P < 0.01) from that found without salicylate at pH 7.0 in exponential phase. The pH of the cultures was unaffected by sodium salicylate.
Exponential- and stationary-phase assays were performed at OD600 of 0.3 to 0.6 (achieved 2 h after inoculation from an overnight culture) and at OD600 of 1.9 to 2.7 (achieved after 6 h), respectively.
Sodium salicylate was added to the cultures after 2 h of growth (exponential phase) or after 6 h of growth (stationary phase); the reporter expression was measured 1 h later.
Overnight cultures grown without sodium salicylate were used to inoculate fresh media containing sodium salicylate. The cultures needed 4 to 5 h to reach OD600 of 0.3 to 0.6 and 17 h to reach OD600 of 1.9 to 2.7. The induction in LB at pH 5.5 was not measured because of cessation of cell growth under these conditions (ND).
Overexpression of marA decreases acid resistance in E. coli.
To know whether repression of hdeAB by MarA affects acid resistance in E. coli under conditions similar to those in the stomach, we compared the survivals at pH 2.5 for 2 h at 37°C of a wild-type strain and a ΔmarRAB strain (Fig. 4). We used cells grown in LB with or without 5 mM sodium salicylate to early stationary phase (where MarA produces the highest repression of hdeAB). In the absence of salicylate, the two strains showed similar survivals (20 to 34%) when grown at both pH 7.0 (Fig. 4) and pH 5.5 (not shown) prior to the acid challenge. However, growing the cells in LB at pH 7.0 in the presence of the marRAB inducer salicylate produced a fivefold decrease in the acid resistance of the ΔmarRAB strain but a 35-fold decrease in the acid resistance of the wild-type strain (ratio between gray and black bars in Fig. 4). These results show that (i) although salicylate induces the expression of hdeAB and other acid resistance genes (33), salicylate itself was toxic, decreasing the acid resistance of the cells, and (ii) the strong overexpression of marA produced by salicylate in wild-type cells made them sevenfold more sensitive than ΔmarRAB cells to the acid challenge.
FIG. 4.
Effect of MarA on the acid resistance of E. coli. The acid challenge at pH 2.5 was performed as described in Materials and Methods, using early-stationary-phase cells grown in LB broth at pH 7.0 without (gray) or with (black) 5 mM sodium salicylate to induce marRAB. Two different strains were compared: the ΔmarRAB strain JHC1096 (ΔmarRAB) and the wild-type strain GC4468 (WT). The percent survival at pH 2.5 after incubation for 2 h was determined in comparison with untreated samples. The results are presented as the averages ± the standard errors of the means (n = 3). The n-fold decrease in survival produced by sodium salicylate in each strain (ratio between gray and black bars) is indicated above the bars.
DISCUSSION
We studied the role of MarA in the complex regulation of hdeAB and in acid resistance of E. coli growing in rich medium at both pH 7.0 and pH 5.5. Overexpression of MarA largely prevented the induction of hdeAB expression as cells enter stationary phase, in part because MarA-mediated repression of hdeAB increased in stationary phase. Growth phase or pH-dependent changes on transcriptional regulation by MarA have not been reported before for any other member of the mar regulon. Thus, we asked if such changes occurred via other regulators of hdeAB.
Seven other regulators were selected to study how they affected hdeAB repression by MarA. These regulators were chosen because they regulate hdeAB directly and/or indirectly (Fig. 1), and because all of them have known or putative DNA binding sites in the hdeAB promoter (Fig. 2). MarA is known to bind a “marbox” partially overlapping the −35 hexamer of the promoter (37). Therefore, competition with RNA polymerase for DNA binding and/or direct interactions between MarA and σ70, σ38, or other subunits of RNA polymerase are possible. Moreover, several putative binding sites for the other selected regulators, except H-NS, are adjacent to or partially overlap the marbox (Fig. 2). Thus, interaction and/or competition between MarA and these regulators is also possible.
Before studying how these regulators affected MarA repression on hdeAB, we examined their own effect on hdeAB under the chosen growth conditions. H-NS was the main repressor of hdeAB under all the conditions studied, in agreement with previous findings at pH 7.0 (3, 39). However, hdeAB repression by H-NS was significantly lower at pH 5.5, which has not been described before.
GadE was the main activator of hdeAB in LB medium under all the conditions studied, whereas GadX repressed or activated hdeAB depending on the growth conditions, and GadW repressed hdeAB. The different effects that GadX and GadW had under diverse conditions, sometimes opposite to that of GadE, support the idea that they can regulate hdeAB independently of GadE. The effect of the Gad regulators on hdeAB has not been studied before in stationary phase; thus we can only compare our results with previous data obtained using exponential-phase cells grown in minimal medium (20, 43). In general, our results are in agreement with the previous findings (20, 43), although some differences were found, probably because of the different culture media used. (i) In exponential phase, we found that GadX repressed hdeAB in LB medium at pH 7.0 (Table 2), while others observed no significant effect of GadX in minimal medium at pH 7.4 (43). (ii) In exponential phase, we found that GadX produced no effect on hdeAB expression in LB medium at pH 5.5 (Table 2), while others reported activation of hdeAB in minimal medium at pH 5.5 (20, 43) to be even greater than that produced by GadE (20).
RpoS had little effect on hdeAB expression under the conditions studied, probably because of a balance between its direct and indirect effects (Fig. 1). Lrp had little effect on hdeAB expression in LB medium, in contrast with the strong repression of hdeAB by Lrp that occurs in minimal media (42). TorR had no effect on hdeAB expression, which suggests that TorR only regulates hdeAB after induction of the TorRS system by trimethylamine N-oxide and anaerobiosis (8).
We then found that some of the hdeAB regulators examined can affect MarA-mediated repression. Moreover, in turn, MarA can modify the effect of some of these other regulators. This is the first report of functional interactions between MarA and other gene regulators. H-NS and GadE were associated with the increase in hdeAB repression by MarA that occurred in stationary phase. Conversely, MarA helped H-NS repression and interfered with GadE activation of hdeAB in stationary phase. Given that the H-NS and MarA binding sites are not close (Fig. 2), and that H-NS can produce complex DNA superstructures in the DNA (39), it is not yet clear how H-NS and MarA interact to repress hdeAB.
It seems unlikely that GadE, the main activator of hdeAB, assists MarA in repression. More likely, MarA interferes with GadE-mediated activation of hdeAB directly (the marbox partially overlaps one “gadbox” [Fig. 2]; thus, MarA and GadE might compete for DNA binding or for interacting with RNA polymerase) and/or indirectly (MarA represses the expression of gadE and the gadE activators gadX and evgA [33]).
MarA seems also to compete functionally with GadX, GadW, RpoS, and Lrp under some conditions, which might ensure that hdeAB expression is not repressed more than needed. Such interference might be direct, given the partial overlap between the MarA binding site and some binding sites of these regulators (Fig. 2), or indirect by means of an intermediary (Fig. 1).
To know whether repression of hdeAB by MarA was correlated with decreased acid resistance, we studied the effect of pH and growth phase on the expression of marA and the effect of MarA on acid resistance. We found that, like hdeAB expression (Fig. 3), marA expression was induced in stationary phase and at acid pH (Table 4). However, this moderate induction was presumably not enough to significantly change hdeAB expression, since the acid resistance of the cells remained almost unaffected (Fig. 4); but it might be important in affecting the expression of other genes of the mar regulon with a less complex regulation or with marboxes with higher affinity for MarA.
Sodium salicylate induced the expression of marA at pH 7.0 but not at pH 5.5 (Table 4), which might relate to a change in its interaction with MarR (5) or to the unknown mechanisms involved in the induction of marRAB by acid pH. However, this lack of induction at pH 5.5 could be important to maintain hdeAB expression and the resistance of the cells under acid conditions.
At pH 7.0, induction of marA expression by salicylate was very strong, mainly when the cells were exposed to salicylate for longer periods of time. This strong induction made stationary-phase cells sevenfold more sensitive to acid challenge at pH 2.5 in the presence of marA than in its absence (Fig. 4), consistent with the repression produced by MarA on hdeAB (and probably other acid resistance genes) in stationary phase.
Further research is needed to understand the biological meaning of MarA repression of hdeAB at pH 7.0 in response to salicylate. Such repression might allow cell resources to be directed to the (unknown, but perhaps more important) MarA-mediated response to salicylate. Once salicylate was no longer present, the fast degradation of MarA (18) would allow a rapid recovery of the expression of hdeAB and other acid resistance genes, minimizing the impact of MarA on acid resistance.
In conclusion, MarA plays an active and complex role in the regulation of hdeAB and acid resistance in E. coli that depends on the growth conditions, on other regulators of hdeAB, and on the degree of induction of marA expression.
Supplementary Material
Acknowledgments
We thank J. Calvo, V. Mejean, J. Foster, A. Sayad, R. Kolter, and P. Bertin for kindly providing the strains mutated in the different hdeAB regulators.
This work was supported by United States Public Health Service grant AI56021 from the National Institutes of Health.
Footnotes
Published ahead of print on 14 December 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Alekshun, M. N., and S. B. Levy. 1999. The mar regulon: multiple resistance to antibiotics and other toxic chemicals. Trends Microbiol. 7410-413. [DOI] [PubMed] [Google Scholar]
- 2.Ansaldi, M., G. Simon, M. Lepelletier, and V. Mejean. 2000. The TorR high-affinity binding site plays a key role in both torR autoregulation and torCAD operon expression in Escherichia coli. J. Bacteriol. 182961-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnqvist, A., A. Olsen, and S. Normark. 1994. Sigma S-dependent growth-phase induction of the csgBA promoter in Escherichia coli can be achieved in vivo by sigma 70 in the absence of the nucleoid-associated protein H-NS. Mol. Microbiol. 131021-1032. [DOI] [PubMed] [Google Scholar]
- 4.Barbosa, T. M., and S. B. Levy. 2000. Differential expression of over 60 chromosomal genes in Escherichia coli by constitutive expression of MarA. J. Bacteriol. 1823467-3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbosa, T. M., and P. J. Pomposiello. 2005. The mar regulon, p. 209-223. In D. G. White, M. N. Alekshun, and P. F. McDermott (ed.), Frontiers in antimicrobial resistance: a tribute to Stuart B. Levy. ASM Press, Washington, DC.
- 6.Bertin, P., E. Terao, E. H. Lee, P. Lejeune, C. Colson, A. Danchin, and E. Collatz. 1994. The H-NS protein is involved in the biogenesis of flagella in Escherichia coli. J. Bacteriol. 1765537-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohannon, D. E., N. Connell, J. Keener, A. Tormo, M. Espinosa-Urgel, M. M. Zambrano, and R. Kolter. 1991. Stationary-phase-inducible “gearbox” promoters: differential effects of katF mutations and role of sigma 70. J. Bacteriol. 1734482-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bordi, C., L. Theraulaz, V. Mejean, and C. Jourlin-Castelli. 2003. Anticipating an alkaline stress through the Tor phosphorelay system in Escherichia coli. Mol. Microbiol. 48211-223. [DOI] [PubMed] [Google Scholar]
- 9.Carlioz, A., and D. Touati. 1986. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 5623-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen, S. P., S. B. Levy, J. Foulds, and J. L. Rosner. 1993. Salicylate induction of antibiotic resistance in Escherichia coli: activation of the mar operon and a mar-independent pathway. J. Bacteriol. 1757856-7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui, Y., Q. Wang, G. D. Stormo, and J. M. Calvo. 1995. A consensus sequence for binding of Lrp to DNA. J. Bacteriol. 1774872-4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espinosa-Urgel, M., C. Chamizo, and A. Tormo. 1996. A consensus structure for sigma S-dependent promoters. Mol. Microbiol. 21657-659. [DOI] [PubMed] [Google Scholar]
- 13.Foster, J. W. 2004. Escherichia coli acid resistance: tales of an amateur acidophile. Nat. Rev. Microbiol. 2898-907. [DOI] [PubMed] [Google Scholar]
- 14.Gajiwala, K. S., and S. K. Burley. 2000. HDEA, a periplasmic protein that supports acid resistance in pathogenic enteric bacteria. J. Mol. Biol. 295605-612. [DOI] [PubMed] [Google Scholar]
- 15.George, A. M., and S. B. Levy. 1983. Amplifiable resistance to tetracycline, chloramphenicol, and other antibiotics in Escherichia coli: involvement of a non-plasmid-determined efflux of tetracycline. J. Bacteriol. 155531-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giangrossi, M., S. Zattoni, A. Tramonti, D. De Biase, and M. Falconi. 2005. Antagonistic role of H-NS and GadX in the regulation of the glutamate decarboxylase-dependent acid resistance system in Escherichia coli. J. Biol. Chem. 28021498-21505. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg, J. T., J. H. Chou, P. A. Monach, and B. Demple. 1991. Activation of oxidative stress genes by mutations at the soxQ/cfxB/marA locus of Escherichia coli. J. Bacteriol. 1734433-4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffith, K. L., I. M. Shah, and R. E. Wolf, Jr. 2004. Proteolytic degradation of Escherichia coli transcription activators SoxS and MarA as the mechanism for reversing the induction of the superoxide (SoxRS) and multiple antibiotic resistance (Mar) regulons. Mol. Microbiol. 511801-1816. [DOI] [PubMed] [Google Scholar]
- 19.Hayes, E. T., J. C. Wilks, P. Sanfilippo, E. Yohannes, D. P. Tate, B. D. Jones, M. D. Radmacher, S. S. BonDurant, and J. L. Slonczewski. 2006. Oxygen limitation modulates pH regulation of catabolism and hydrogenases, multidrug transporters, and envelope composition in Escherichia coli K-12. BMC Microbiol. 689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hommais, F., E. Krin, J. Y. Coppee, C. Lacroix, E. Yeramian, A. Danchin, and P. Bertin. 2004. GadE (YhiE): a novel activator involved in the response to acid environment in Escherichia coli. Microbiology 15061-72. [DOI] [PubMed] [Google Scholar]
- 21.Hong, W., W. Jiao, J. Hu, J. Zhang, C. Liu, X. Fu, D. Shen, B. Xia, and Z. Chang. 2005. Periplasmic protein HdeA exhibits chaperone-like activity exclusively within stomach pH range by transforming into disordered conformation. J. Biol. Chem. 28027029-27034. [DOI] [PubMed] [Google Scholar]
- 22.Ishihama, A. 2000. Functional modulation of Escherichia coli RNA polymerase. Annu. Rev. Microbiol. 54499-518. [DOI] [PubMed] [Google Scholar]
- 23.Kern, R., A. Malki, J. Abdallah, J. Tagourti, and G. Richarme. 2007. Escherichia coli HdeB is an acid stress chaperone. J. Bacteriol. 189603-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim, E. Y., M. S. Shin, J. H. Rhee, and H. E. Choy. 2004. Factors influencing preferential utilization of RNA polymerase containing sigma-38 in stationary-phase gene expression in Escherichia coli. J. Microbiol. 42103-110. [PubMed] [Google Scholar]
- 25.Link, A. J., K. Robison, and G. M. Church. 1997. Comparing the predicted and observed properties of proteins encoded in the genome of Escherichia coli K-12. Electrophoresis 181259-1313. [DOI] [PubMed] [Google Scholar]
- 26.Ma, Z., N. Masuda, and J. W. Foster. 2004. Characterization of EvgAS-YdeO-GadE branched regulatory circuit governing glutamate-dependent acid resistance in Escherichia coli. J. Bacteriol. 1867378-7389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masuda, N., and G. M. Church. 2003. Regulatory network of acid resistance genes in Escherichia coli. Mol. Microbiol. 48699-712. [DOI] [PubMed] [Google Scholar]
- 28.Mates, A. K., A. K. Sayed, and J. W. Foster. 2007. Products of the Escherichia coli acid fitness island attenuate metabolite stress at extremely low pH and mediate a cell density-dependent acid resistance. J. Bacteriol. 1892759-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 30.Opdyke, J. A., J. G. Kang, and G. Storz. 2004. GadY, a small-RNA regulator of acid response genes in Escherichia coli. J. Bacteriol. 1866698-6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pascal, M. C., M. Lepelletier, G. Giordano, and M. Chippaux. 1991. A regulatory mutant of the trimethylamine N-oxide reductase of Escherichia coli K12. FEMS Microbiol. Lett. 62297-300. [DOI] [PubMed] [Google Scholar]
- 32.Platko, J. V., D. A. Willins, and J. M. Calvo. 1990. The ilvIH operon of Escherichia coli is positively regulated. J. Bacteriol. 1724563-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pomposiello, P. J., M. H. Bennik, and B. Demple. 2001. Genome-wide transcriptional profiling of the Escherichia coli responses to superoxide stress and sodium salicylate. J. Bacteriol. 1833890-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powell, B. S., M. P. Rivas, D. L. Court, Y. Nakamura, and C. L. Turnbough, Jr. 1994. Rapid confirmation of single copy lambda prophage integration by PCR. Nucleic Acids Res. 225765-5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roland, K. L., C. G. Liu, and C. L. Turnbough, Jr. 1988. Role of the ribosome in suppressing transcriptional termination at the pyrBI attenuator of Escherichia coli K-12. Proc. Natl. Acad. Sci. USA 857149-7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 37.Schneiders, T., T. M. Barbosa, L. M. McMurry, and S. B. Levy. 2004. The Escherichia coli transcriptional regulator MarA directly represses transcription of purA and hdeA. J. Biol. Chem. 2799037-9042. [DOI] [PubMed] [Google Scholar]
- 38.Seoane, A. S., and S. B. Levy. 1995. Characterization of MarR, the repressor of the multiple antibiotic resistance (mar) operon in Escherichia coli. J. Bacteriol. 1773414-3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin, M., M. Song, J. H. Rhee, Y. Hong, Y. J. Kim, Y. J. Seok, K. S. Ha, S. H. Jung, and H. E. Choy. 2005. DNA looping-mediated repression by histone-like protein H-NS: specific requirement of Esigma70 as a cofactor for looping. Genes Dev. 192388-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simon, G., C. Jourlin, M. Ansaldi, M. C. Pascal, M. Chippaux, and V. Mejean. 1995. Binding of the TorR regulator to cis-acting direct repeats activates tor operon expression. Mol. Microbiol. 17971-980. [DOI] [PubMed] [Google Scholar]
- 41.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 5385-96. [DOI] [PubMed] [Google Scholar]
- 42.Tani, T. H., A. Khodursky, R. M. Blumenthal, P. O. Brown, and R. G. Matthews. 2002. Adaptation to famine: a family of stationary-phase genes revealed by microarray analysis. Proc. Natl. Acad. Sci. USA 9913471-13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tucker, D. L., N. Tucker, Z. Ma, J. W. Foster, R. L. Miranda, P. S. Cohen, and T. Conway. 2003. Genes of the GadX-GadW regulon in Escherichia coli. J. Bacteriol. 1853190-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weber, H., T. Polen, J. Heuveling, V. F. Wendisch, and R. Hengge. 2005. Genome-wide analysis of the general stress response network in Escherichia coli: σS-dependent genes, promoters, and sigma factor selectivity. J. Bacteriol. 1871591-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang, F., K. R. Gustafson, M. R. Boyd, and A. Wlodawer. 1998. Crystal structure of Escherichia coli HdeA. Nat. Struct. Biol. 5763-764. [DOI] [PubMed] [Google Scholar]
- 46.Zhou, Y., and S. Gottesman. 2006. Modes of regulation of RpoS by H-NS. J. Bacteriol. 1887022-7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.