Abstract
We report evidence that CotC and CotU, two previously identified components of the Bacillus subtilis spore coat, are produced concurrently in the mother cell chamber of the sporulating cell under the control of σK and GerE and immediately assembled around the forming spore. In the coat, the two proteins interact to form a coat component of 23 kDa. The CotU-CotC interaction was not detected in two heterologous hosts, suggesting that it occurs only in B. subtilis. Monomeric forms of both CotU and CotC failed to be assembled at the surface of the developing spore and accumulated in the mother cell compartment of cells mutant for cotE. In contrast, neither CotU nor CotC accumulated in the mother cell compartment of cells mutant for cotH. These results suggest that CotH is required to protect both CotU and CotC in the mother cell compartment of the sporangium and that CotE is needed to allow their assembly and subsequent interaction at the spore surface.
In Bacillus subtilis, the spore coat is a complex multiprotein structure that plays an important role in spore germination and resistance to toxic chemicals (8, 11, 13). Recently, new functions have been assigned to the coat, from sensing the external environment through active enzymes present on its surface (4, 12, 24, 40) to protecting the spore from predation by phagocytic protozoans (21). In addition, the coat is a novel system for the display at the spore surface of heterologous antigens (16, 18, 25), enzymes (22), and bioactive molecules (20).
The coat is a dynamic structure, able to adapt to changes in the spore volume by expanding and contracting in response to dehydration and rehydration occurring during the B. subtilis life cycle (3). A heterogeneous group of over 50 polypeptides form the three main structural layers of the coat: a diffuse undercoat, a laminated lightly staining inner layer, and a thick electron-dense outer coat (11, 13). Several of these polypeptides have been studied, and their structural genes (cot genes) have been identified. Expression of all cot genes is governed by a cascade of four transcription factors acting specifically in the mother cell compartment of the sporangium in the sequence SigmaE-SpoIIID-SigmaK-GerE; SigmaE and SigmaK are RNA polymerase sigma factors, and SpoIIID and GerE are DNA-binding proteins acting in conjunction with SigmaE- and SigmaK-driven RNA polymerase (8, 11, 13). An additional transcription factor, GerR, has recently been implicated in the control of some coat genes (10).
In addition to the transcriptional regulation, control exerted at the protein level seems to be particularly important for the assembly of the multiprotein structure of the coat. A variety of posttranslational modifications have been shown to occur during coat formation: some coat-associated polypeptides appear to be glycosylated (11, 13), others are derived from proteolytic processing of larger precursors (1, 6, 34, 37), and some others are highly cross-linked as a result of reactions that take place at the spore surface (12, 40). In addition, a small subset of coat proteins, known as morphogenetic proteins, play an important role in controlling the assembly of most of the coat. These proteins have no effects on coat protein synthesis but act posttranslationally to guide the assembly of the various coat components around the forming spore (19). SpoVM, a 26-amino-acid peptide, is believed to adhere to the outer forespore membrane and to allow the localization of SpoIVA around the forming spore (30). The SpoIVA layer then directs the assembly of the morphogenetic protein CotE in a ringlike structure around the forespore (9). Inner coat components are thought to infiltrate through the CotE ring, while outer coat proteins assemble on the outside of the E ring (8, 11, 13). Additional proteins with morphogenetic functions are needed at later stages of coat formation. For instance, SpoVID has the dual role of directing SafA to the forming spore and maintaining the CotE ring around the forespore (4, 28). Another example is CotH, which plays a role in the assembly of various outer coat components, partially controls assembly of CotE, and is required for the development of the normal morphological features of spores (19, 26, 41). A recent study has shown that CotH controls the assembly of the coat proteins CotB, CotC, CotG, CotS, CotSA, CotQ, CotU, CotZ, and YusA (19). In different studies, it has been proposed that the role of CotH in the assembly of CotC, CotG, and CotB is to stabilize CotC (17) and CotG, which in turn is needed for the assembly and dimerization of CotB (41). In particular, CotC does not accumulate in the mother cell compartment, where it is synthesized, but is immediately assembled around the forming spore (17). Assembly of CotC requires expression of both cotH and cotE, but CotC does not accumulate in the mother cell compartment when its assembly is prevented by mutation of cotH (17). In contrast, overexpression of cotH allows the accumulation of CotC in the mother cell compartment, suggesting that CotH, or a CotH-dependent factor, acts to prevent degradation of CotC in the mother cell and then allows its assembly within the coat (2). The mechanism of assembly of CotC is of interest, as the abundant CotC protein has been used as a vehicle for the display of heterologous proteins at the spore surface (18).
Here, we report that CotU, a recently identified structural homologue of CotC (23), interacts with CotC, forming an alkali-soluble coat protein of 23 kDa in a CotE- and CotH-dependent manner. CotC and CotU share almost identical N-terminal regions, with 23 out of 24 identical amino acid residues, and less conserved C-terminal parts (Fig. 1A) (7). In addition, both CotU and CotC contain high numbers of tyrosine, lysine, and aspartic acid residues that account for over 70% of their total numbers of amino acids. This peculiar primary structure likely causes the unusual migration of the two proteins on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), with CotC and CotU having deduced masses of 8.8 and 11.4 kDa and apparent masses of 12 and 17 kDa, respectively.
FIG. 1.
(A) CotC and CotU amino acid alignment. (B) Schematic representation of the cotC-cotU chromosomal region. The arrows and numbers indicate the directions of transcription and the positions on the B. subtilis chromosome, respectively. (C) cotU promoter region. The translational start site (TTG) is in boldface, the transcriptional start site is indicated as +1, and the putative promoter sequences are underlined. The arrow indicates oligonucleotide U-pr-Anti, used for the primer extension experiment shown in Fig. 2B.
We show that like CotC, the CotU protein does not accumulate in the mother cell compartment of a cotH mutant. In contrast both CotC and CotU (but not the 23-kDa species) accumulated in the mother cell of a cotE mutant but failed to be assembled. These results reinforce the view that CotH has a role in the stabilization of certain coat proteins in the mother cell cytoplasm. The results also indicate that formation of the 23-kDa CotC-CotU species takes place at the spore surface, following the assembly of both proteins.
MATERIALS AND METHODS
Bacterial strains and transformation.
The B. subtilis strains utilized are listed in Table 1. Plasmid amplification for nucleotide sequencing, subcloning experiments, and transformation of Escherichia coli competent cells were performed with E. coli strain DH5α (33). Bacterial strains were transformed by previously described procedures: CaCl2-mediated transformation of E. coli competent cells (33) and two-step transformation of B. subtilis (5).
TABLE 1.
B. subtilis strains
| Strain | Relevant genotype | Source |
|---|---|---|
| PY79 | Wild type | 36 |
| BD063 | cotA::cat | 7 |
| RH248 | ΔamyE::cotU-lacZ Cmr | This work |
| RH249 | gerE ΔamyE::cotU-lacZ Cmr | This work |
| AZ153 | gerE36 | Lab collection |
| AZ198 | SPβC2cotC::lacZ Cmr | Lab collection |
| RH228 | cotC::His6cotC::spc | This work |
| RH208 | cotU::neo cotU::His6 | This work |
| RH209 | cotU::neo cotC::cat | 18 |
| RH210 | cotU::neo cotC::cat cotU::His6 | This work |
| RH225 | ΔamyE::cotC-His6 Cmr | This work |
| RH232 | ΔamyE::cotC-His6 Ermr | This work |
| RH254 | cotU::neo cotC::cm ΔamyE::cotU-His6 | This work |
| RH256 | oxdD::cat | This work |
| RH255 | sodA::cat | This work |
| RH233 | tgl::spc | 40 |
| ER203 | cotG::Δerm | 32 |
| RH101 | cotC::spc | 17 |
| RH202 | cotU::neo | 17 |
Plasmid and strain construction.
Isolation of plasmids, restriction digestion, and ligation of DNA were carried out by standard methods (33). Chromosomal DNA from B. subtilis was isolated as described elsewhere (5). Fragments of cotC and cotU DNAs were PCR amplified from the B. subtilis chromosome; the amplification was primed with the synthetic oligonucleotides listed in Table 2.
TABLE 2.
Synthetic oligonucleotides
| Oligonucleotide | Sequence (5′-3′)a | Restriction site | Position of annealingb |
|---|---|---|---|
| Y3s | gtcgacGATTTAATGCATTGTATTTTACC | SalI | −494/−471 |
| Ua | aagcttTTCCAAGTATAATACTCTTC | HindIII | +43/+24 |
| SodA-3F | ggtaccGCTTACGAACTTCC | KpnI | +4/+18 |
| SodA-445R | ggatccGGTTTGGCGTGC | BamHI | +446/+434 |
| OxdD-3F | ggtaccCTGTTGGAACAA | KpnI | +4/+20 |
| OxdD-647R | ggatccCGGCACATTCCC | BamHI | +648/+635 |
| Ycoding | ctcgagTTGGGTTATTATAAA | XhoI | +1/+15 |
| Ya2 | gcatgcTTATAAATAGGGGAAGGC | SphI | +449/+430 |
| CotCp | ACATgcatgcTGTAGGATAAATCGTTTG | SphI | −181/−63 |
| CotCSTOP | gtcgacTTATTAGTAGTGTTTTTTATGC | SalI | +357/+338 |
| C-His1 | GTCATCGTCATGGTGGTGATGATGATGCATATATACTCCTCC | −13/+1 | |
| C-His2 | CATCACCACCATGACGATGACGATAAGATGGGTTATTACAAA | +15/+1 | |
| Y-His1 | CCATGATGATGATGATGATGCAATTAAATTCCTCC | +3/−12 | |
| Y-His2 | TTGCATCATCATCATCATCATGGTTATTATAAAAAA | +4/+18 | |
| AmyA | CGAGAAGCTATCACCGCCCAGC | +2128/+2150 | |
| AmyS | CCAATGAGGTTAAGAGTATTCC | +569/+590 | |
| U-pr-Anti | AAGTTTAAATGATATGGTATGG | +39/+60 |
Uppercase and lowercase letters indicate nucleotides complementary to corresponding cotU or cotC DNA and unpaired flanking sequences carrying a restriction site, respectively.
Positions refer to cotU, cotC, or amyE sequences, considering the first nucleotide of the translational start site as +1.
The cotU::lacZ translational fusion was obtained as follows. A DNA fragment containing 476 bp upstream of the cotU translational start site was PCR amplified using chromosomal DNA as a template and oligonucleotides Y3s and Ua (Table 2) and inserted into plasmid pJM783 (40) upstream of the promoterless lacZ gene. The resulting plasmid, pRH92, carrying the cotU::lacZ fusion, was introduced by single reciprocal (Campbell-like) recombination between B. subtilis DNA sequences in pRH92 and the corresponding region of homology in the chromosome in the PY79 wild-type strain of B. subtilis. Several chloramphenicol-resistant (Cmr) clones were analyzed by PCR, and one of them, RH248, was used for further studies. Chromosomal DNA containing the integrated fusion-bearing plasmid was then used to transform a congenic collection of sporulation mutants.
cotU::His6 and cotC::His6 gene fusions were constructed by using the gene splicing by overlap extension technique as described by Horton et al. (14). The B. subtilis chromosome was used as a template, and the synthetic oligonucleotides Y3s/Y2anti and Y-His1/Y-His2 for cotU::His6 and CotCp/C-His1 and C-His2/Cstop for cotC::His6 were used as primers (Table 2). The 680- and 402-bp PCR products obtained for cotU::His6 and cotC::His6 were cloned into the pGEM-T easy vector (Promega). The inserts in the resulting plasmids were analyzed by DNA sequencing to verify the absence of unwanted mutations, released with SphI and SalI, and introduced into pDG364 (5) previously digested with the same two enzymes. E. coli competent cells were transformed with the ligation mixture, and the selected ampicillin-resistant clones were screened by restriction analysis of their plasmids.
The obtained plasmids, pRH42 (cotU::His6) and pRH48 (cotC::His6), were linearized and used to transform competent cells of the B. subtilis strains RH202 and RH101 (Table 1), yielding strains RH208 (cotU::His6) and RH228 (cotC::His6), respectively. Cmr clones were the result of double-crossover recombination, due to the interruption of the nonessential amyE gene on the B. subtilis chromosome. Several Cmr clones were tested by PCR using chromosomal DNA as a template and oligonucleotides AmyA and AmyS (Table 2). Two clones, one from each transformation, were named RH208 (cotU::His6) and RH228 (cotC::His6) and kept for further studies.
To obtain a B. subtilis strain carrying both cotC::His6 and cotU::His6 fusions, the Cmr determinant (cat) of strain RH225 was replaced with an erythromycin resistance gene cassette (erm) by using plasmid pECE72 (Bacillus Genetic Stock Center, Columbus, OH). Chromosomal DNA of the resulting strain, RH232, was used to transform competent cells of strain RH210 (cotU::His6). Several clones resistant to erythromycin were tested by PCR, and one, RH254, was selected for further studies.
sodA null mutation was obtained by PCR amplifying an internal part of the gene using B. subtilis chromosomal DNA as a template and oligonucleotides SodA-3F and SodA-445R (Table 2). The 445-bp PCR product was cloned into plasmid pER19 (31), and the resulting plasmid, pRH97, was used to transform competent cells of the B. subtilis strain PY79, yielding strain RH255.
An oxdD null mutation was obtained by PCR amplifying an internal part of the gene using B. subtilis chromosomal DNA as a template and oligonucleotides OxdD-3F and OxdD-647R (Table 2). The 647-bp PCR product was cloned into plasmid pER19 (31), and the resulting plasmid, pRH121, was used to transform competent cells of the B. subtilis strain PY79, yielding strain RH256.
Primer extension analysis.
Total RNA was extracted from a wild-type strain 8 h after the onset of sporulation using the Qiagen Mini Kit (Qiagen, Milan, Italy) according to the manufacturer's instructions. Total RNAs were dissolved in 50 μl of RNase-free water and stored at −80°C. The final concentration and quality of the RNA samples were estimated either spectrophotometrically or by agarose gel electrophoresis with ethidium bromide staining. Total RNAs were treated with RNase-free DNase (1 U/μg of total RNA; Turbo DNA-free; Ambion) for 30 min at 37°C, and the reaction was stopped with DNase inactivation reagent. For primer extension experiments, 10 μg of total RNA was used with [γ32-P]dATP (GE Healthcare)-labeled oligonucleotide U-pr-Anti (Table 2), deoxynucleoside triphosphate, and avian myeloblastosis virus reverse transcriptase (BRL) to prime cDNA synthesis, as previously described (26). The reaction products were fractionated on 6 M urea-6% polyacrylamide gels, along with DNA-sequencing reactions using pRH85 (pGEM-T easy/cotU) as the template primed with the same oligonucleotide.
Expression in E. coli.
The cotU coding region was PCR amplified from B. subtilis chromosomal DNA with primers Ycoding and Y2a (Table 2). The 302-bp PCR product was cleaved with XhoI and EcoRI and ligated into the expression vector pRSETA, previously digested with the same restriction enzymes (Invitrogen). The recombinant plasmid carrying an in-frame fusion of the 3′ end of the cotU coding region to six histidine codons under the transcriptional control of a T7 promoter was used to transform competent cells of E. coli BL21(DE3) (Invitrogen), yielding strain RH59. This strain was grown in ampicillin-supplemented (50 μg/ml) tryptone-yeast extract medium to an optical density of 0.7 at 600 nm. The T7 promoter was then induced by the addition of IPTG (isopropyl-β-d-thiogalactopyranoside; 0.5 mM final concentration) to the culture and incubating it for 2 h at 37°C. The His6-tagged CotU protein was purified under denaturing conditions via Ni2+-nitrilotriacetic acid affinity chromatography as recommended by the manufacturer (Qiagen, Inc.) and used to raise specific antibodies (IGtech, Salerno, Italy).
Western blotting.
B. subtilis sporulation of wild-type and recombinant strains was induced by the exhaustion method (5, 27). After a 30-h incubation at 37°C, spores were collected, washed four times, and purified by lysozyme treatment as previously described (5, 27). The number of purified spores obtained was measured by direct counting with a Bürker chamber under an optical microscope (Olympus BH-2 with 40× lenses). Aliquots of 1010 spores suspended in 0.3 ml of distilled water were used to extract coat proteins by 0.1 N NaOH treatment at 4°C as previously reported (2). The concentration of the extracted coat proteins was determined by the Bio-Rad DC (Detergent Compatible) Protein Assay to avoid potential interference by the NaOH present (0.2 to 0.6 mN, final concentration) in the extraction buffer and 15 μg of total proteins fractionated on 18% denaturing polyacrylamide gels. The proteins were then electrotransferred to nitrocellulose filters (Bio-Rad) and used for Western blot analysis by standard procedures. For the analysis of sporulating cells, samples were harvested at various times during sporulation and disrupted by sonication in 25 mM Tris (pH 7.5), 0.1 M NaCl, 1 mM EDTA, 15% (vol/vol) glycerol, and 0.1 mg/ml of phenylmethylsulfonyl fluoride. The sonicated material was then fractionated by centrifugation at 10,000 rpm for 20 min. The pellet, containing the forming spores resistant to the sonication treatment, was solubilized by 0.1 N NaOH treatment at 4°C, and the total protein concentration was determined as described above. Fifty micrograms (mother cell extract) or 15 μg (forespore extract) of total proteins was fractionated on 18% denaturing polyacrylamide gels. Western blot filters were visualized by the SuperSignal West Pico Chemiluminescence (Pierce) method as specified by the manufacturer.
RESULTS
The cotU gene of B. subtilis is under σK-GerE control.
The cotU (formerly ynzH) and cotC genes, coding for CotU and CotC, respectively, are located about 4 kb apart in the the B. subtilis chromosome (Fig. 1B). Previous reports (15, 39) showed that cotC transcription is driven by σK-containing RNA polymerase and that the DNA-binding protein GerE acts as a transcriptional activator of cotC expression. Recently, studies using DNA arrays have suggested that cotU is also transcribed under the control of σK-driven RNA polymerase (10, 35). To analyze cotU expression in more detail, we constructed a transcriptional gene fusion between the cotU promoter region and the lacZ gene of E. coli and measured the activities of β-galactosidase at various times after the onset of sporulation in an otherwise wild-type strain and a collection of congenic sporulation mutants. In all cases, the fusion was integrated at the cotU locus as a result of a single reciprocal crossover event (see Materials and Methods). In agreement with the expectation that cotU is under the control of σK, we observed that β-galactosidase production commenced between 6 and 7 h after the onset of sporulation (Fig. 2A), at the same time as expression of a cotC::lacZ fusion in a congenic strain (39) (Fig. 2A). Moreover, expression of cotU::lacZ was severely reduced in a σK mutant (spoIIIC) (data not shown), as well as in mutants (spoIIG, spoIIID, spoIIIG, and spoIVF) (data not shown) known to be impaired in σK production. cotU::lacZ-driven synthesis of β-galactosidase was also impaired in a gerE mutant (Fig. 2A), indicating that efficient transcription of cotU requires the presence of the gerE-encoded DNA-binding protein.
FIG. 2.
(A) Expression of a cotU::lacZ transcriptional fusion during sporulation in an otherwise wild-type (open diamonds) or a gerE null mutant (closed squares) strain and of a cotC::lacZ translational fusion in an otherwise wild-type strain (closed diamonds). Background levels of β-galactosidase activity were determined in a wild-type strain bearing no lacZ gene (open squares). Samples were collected at various times after the onset of sporulation. Enzyme activity is expressed in Miller units. The data are the means of three independent experiments. (B) Primer extension analysis of the cotU promoter region performed with total RNA extracted from sporulating cells 8 hours (T8) after the onset of sporulation. Primer extension and sequencing reactions were primed with the synthetic oligonucleotide U-pr-Anti (Table 2).
By analogy with the case of cotC (15, 39) and in extension of previous observations (10, 35), we inferred that cotU is transcribed by σK-containing RNA polymerase acting in conjunction with GerE.
A primer extension experiment was performed to map the cotU promoter and transcriptional start site. The extension product obtained (Fig. 2B) allowed us to localize the 5′ terminus of cotU mRNA 91 bp upstream of the beginning of the open reading frame (Fig. 1C). Sequences upstream of the 5′ terminus (+1) resembled the conserved features of a σK promoter, matching in four of six positions the consensus −10 (consensus, CATANNNTA; cotU, CAgANNNTg; differences are in lowercase) and in both positions the consensus −35 (AC) (Fig. 1C).
CotU and CotC directly interact in the spore coat.
In order to study CotU assembly within the spore coat, we overexpressed a His6-tagged version of cotU in E. coli and used the protein partially purified by Ni2+ affinity chromatography to raise a polyclonal antibody (see Materials and Methods). Due to the high similarity between CotU and CotC, our antibody reacted to the gene products of both cotU and cotC, and this recognition appeared to be specific, since no proteins were recognized in a cotC cotU double-null mutant (Fig. 3). Our anti-CotU antibodies recognized six polypeptides in the coat protein fractions of wild-type spores; four of them (of 12, 12.5, 21, and 30 kDa) were also detected in the coat extracts from cotU mutant spores (Fig. 3) and thus corresponded to the previously identified products of cotC expression (17). The 17-kDa protein was absent from the coats of cotU null mutant spores but present in the coats of cotC null mutant spores (Fig. 3), suggesting that it was the product of cotU expression. The conclusion that the 17-kDa protein is the product of cotU is also supported by the results of a previous study (7), in which N-terminal amino acid sequence analysis revealed a perfect match with the deduced sequence of CotU for the first 20 N-terminal positions of the 17-kDa protein.
FIG. 3.
Western blot analysis with anti-CotU antibody of coat proteins extracted from a wild type (wt) and congenic strains with null mutations in cotU, cotC, and cotU cotC. The proteins were fractionated on an 18% polyacrylamide gel and, upon electrotransfer onto nitrocellulose membranes, reacted with CotU-specific rabbit antibodies and then with peroxidase-conjugated secondary antibodies and visualized by the Pierce method. The arrows indicate the apparent molecular weights of the observed proteins. The molecular masses of a marker are also indicated. Identical results were obtained with anti-CotC antibody.
The remaining polypeptide of 23 kDa was detected only among the proteins extracted from the coats of wild-type spores (Fig. 3), suggesting that it is dependent on the expression of both cotC and cotU (17). The genetic dependence of the 23-kDa protein on cotC and cotU expression obviously suggests the possibility that it is the result of an interaction between CotC and CotU. To test this possibility, we constructed two recombinant strains of B. subtilis carrying a His-tagged version of either cotC (RH228; see below) or cotU (RH208) as the only copy of cotC or cotU present in those strains. As shown in Fig. 4A, the 23-kDa proteins of strains RH208 and RH228 showed similarly slower migrations on SDS-PAGE than the 23-kDa protein of the congenic wild-type strain. The slower migration was most likely due to the presence of the His tag, as suggested by the observation that other tagged proteins also displayed altered electrophoretic properties. For instance, in strain RH228 (CotC-His6) the presence of the His6 tag slowed the migration of the four CotC forms and of the 23-kDa protein, although in the experiment shown in Fig. 4A, this effect is observable only for the higher-molecular-mass proteins (21, 23, and 30 kDa); in strain RH208 (CotU-His6), the presence of the His6 tag reduced the migration of both cotU-dependent proteins of 17 and 23 kDa (Fig. 4A). Moreover, when spore coat proteins were purified on Ni2+ columns, fractionated on SDS-PAGE, and analyzed by Western blotting with anti-CotC (17) or anti-CotU antibodies, the 23-kDa species was found in extracts from strain RH228 (CotC-His6) (Fig. 4B) or RH208 (CotU-His6) (Fig. 4C) but not those from a congenic wild-type strain (not shown). The variation in the relative abundances of the 23-kDa protein in the Western blots in Fig. 4 (compare panel A with panels B and C) most likely depended on poor recovery of this less well represented protein species from Ni2+ columns. Thus, the 23-kDa polypeptide was purified by Ni2+ affinity chromatography when the His6 tag was joined to either CotC or CotU.
FIG. 4.
Western blot analysis with anti-CotU (A and C) or anti-CotC (B) antibody. Coat proteins were extracted from a wild-type (wt) strain and a congenic strain carrying a cotC::His6 (C::His6) or a cotU::His6 (U::His6) fusion as indicated. Proteins were fractionated and then blotted (A) or purified through an Ni column and then blotted (B and C). In panels B and C, the wt lane contains unpurified proteins from wild-type spores. The arrows indicate the apparent molecular weights of the observed proteins. Molecular mass markers are also indicated.
The genetic dependence and the biochemical evidence shown in Fig. 4 together indicate that CotC and CotU directly interact to form the 23-kDa polypeptide. To gain insight into the interaction between CotC and CotU, we constructed gene fusions in which the His6 tag was placed in frame to the 3′ or 5′ end of both the cotC and cotU genes. All fusions were integrated on the chromosome of strain RH209, carrying null mutations in both cotC and cotU (18), and the extracted coat proteins were analyzed by Western blotting. A wild-type pattern of CotC- and CotU-dependent proteins was observed when CotU was tagged at either end (not shown) and when CotC was tagged at its N-terminal end (Fig. 5A). The presence of the His6 tag at the C terminus of CotC did not impair assembly of the CotC species of 12 kDa (presumably the CotC monomer) (Fig. 5A): the level of CotC (12 kDa) was not reduced relative to the amount found in the coats of wild-type spores (Fig. 5A), yet formation of all other forms of CotC and of the 23-kDa CotC-CotU species was severely reduced. These results, therefore, suggest that the C-terminal end of CotC is involved in the formation of both the CotC-CotC homodimer and the CotC-CotU heterodimer. Moreover, these results strongly support the idea that multimerization of CotC and CotU occurs at the spore surface, following assembly of both proteins.
FIG. 5.
Western blot analysis with anti-CotC antibody. (A) Proteins extracted from recombinant B. subtilis strains containing the His6 tag fused at the 5′ (C::His6 N-term) or 3′ (C::His6 C-term) end of the cotC gene and from a congenic wild-type strain (wt). The indicated molecular masses refer to the various CotC- and CotU-dependent proteins from a wild-type strain (without the His6 tag). The arrow indicates the 12-kDa species of CotC. (B) Proteins were extracted from spores of a wild-type B. subtilis strain and of a collection of congenic strains with null mutations in cotA, oxdD, sodA, or tgl. Identical results were obtained with anti-CotU antibody.
CotU and CotC do not interact in heterologous hosts.
Yeast two-hybrid experiments were performed using cotU and cotC coding sequences fused to the activation or the DNA-binding domain of the Saccharomyces cerevisiae transcriptional activator GAL4, as previously described (17, 28). The gene fusions were then introduced into the yeast reporter strains Y187/Y190 (28), and the expression of a lacZ reporter gene followed. As previously reported, an interaction was detected between CotC and itself, indicating that CotC molecules self-interact (17). In contrast, and under similar conditions, no interaction was detected between CotC and CotU (data not shown), indicating that the two proteins do not interact when produced in yeast cells.
To verify whether CotU and CotC were able to interact in a different heterologous host, we coexpressed cotU and cotC in E. coli. Plasmid pRH51 (17), carrying the cotC coding region His6 tagged at its 5′ end under the transcriptional control of the T7lac promoter, was engineered to replace the ampicillin resistance gene with a kanamycin resistance gene cassette, and the resulting plasmid was used to transform strain BL21(DE3) (Novagen), already containing plasmid pRH59 with a version of cotU His6 tagged at its 3′ end. Upon IPTG induction, three bands corresponding in size to those produced by expressing cotU or cotC separately were produced and recognized by anti-CotU, anti-CotC, and His6 antibodies. No additional proteins due to the interaction of CotC and CotU were observed (data not shown). In contrast, the 23-kDa CotC-CotU species was detected by both anti-CotC and anti-CotU antibodies in coat extracts of a B. subtilis strain expressing both His6-cotC (His6 at the 5′ end) and cotU-His6 (His6 at the 3′ end) (not shown). Therefore, the same proteins that failed to interact in E. coli were able to interact in B. subtilis, thus excluding the possibility that the interaction was inhibited in E. coli by the simultaneous presence of the His6 tag in both partners involved in the interaction.
Since the CotC-CotU interaction occurs in B. subtilis but not in two heterologous hosts (S. cerevisiae and E. coli), we hypothesized that a specific factor might be needed to mediate that interaction.
CotC-CotU interaction does not require CotA, OxdD, Tgl, or SodA.
CotA, OxdD, SodA, and Tgl are coat components with laccase (24), oxalate decarboxylase (4), superoxide dismutase (12), and transglutaminase (40) activities, respectively. It has been proposed that spore coat-associated enzymatic activities may be involved in mediating specific protein-protein interactions, including protein cross-linking reactions within the coat (11).
Based on this, we used a collection of congenic strains of B. subtilis mutant for cotA (BD063), oxdD (RH256), sodA (RH255), or tgl (RH233) to analyze whether the laccase, oxalate decarboxylase, superoxide dismutase, or transglutaminase activity was involved in mediating the CotC-CotU interaction. The 23-kDa protein (Fig. 5B), as well as all other CotC- and CotU-dependent proteins (not shown), was present in the coat fractions of spores of all mutant strains analyzed. Therefore, none of the enzymatic activities tested is required for formation of the 23-kDa CotC-CotU species.
Assembly of CotU and of the CotU-CotC heterodimer.
Western blotting of proteins extracted from the mother cell or the forespore compartment of sporulating cells had indicated that none of the CotC forms accumulated in the mother cell, suggesting their rapid assembly onto the forming spore (17). Here, we have extended this analysis to the assembly of CotU and of the CotU-CotC polypeptide of 23 kDa. Sporulating cells of a wild-type strain were harvested at various times during sporulation and lysed by sonication as described in Materials and Methods, and the forming spores (forespore fraction) were separated from the mother cell cytoplasm (mother cell fraction). The forming spores were then extracted by alkali treatment, and the released proteins were compared with those present in the mother cell cytoplasm. For each time point, both protein fractions were analyzed by Western blotting with anti-CotU antibodies. At all time points analyzed, we detected no cotU-dependent polypeptides in the mother cell fraction (not shown). In the forespore fraction, we observed the four CotC forms (12, 12.5, 21, and 30 kDa) from hour 6 onward; as previously reported (17), CotU (17 kDa) and the CotU-CotC species (23 kDa) were detected from hour 8 onward (Fig. 6A).
FIG. 6.
Western blot of proteins extracted at various times (t7 to t16) after the onset of sporulation from the forespores of sporulating cells of a wild-type (wt) (A) and a cotC null mutant (B) strain of B. subtilis. The proteins were fractionated on a 15% polyacrylamide gel and, upon electrotransfer onto nitrocellulose membranes, reacted with CotU-specific rabbit antibodies and then with peroxidase-conjugated secondary antibodies and visualized by the Pierce method. The estimated sizes of CotC- and CotU-dependent polypeptides are indicated in kilodaltons.
In the time course experiment shown in Fig. 6A, CotU, as well as the 23-kDa protein, appeared 1 hour later than CotC. Since the analysis of the cotU::lacZ transcriptional fusion has shown that the timing of expression of cotU is identical to that of cotC (Fig. 2), the delayed appearance of CotU could be due to a posttranscriptional control on cotU expression. Alternatively, it could be that the high concentration of CotC in wild-type spores would not allow a sufficiently long exposure of the membrane, needed to visualize the less abundant CotU. To discriminate between the two possibilities, we repeated a similar time course experiment with a cotC null strain. As shown in Fig. 6B, when CotC was not present and the membrane could be exposed for enough time, CotU was detected 8 h after the onset of sporulation, at the same time as the appearance of CotC (Fig. 6A). Thus, although CotC and CotU accumulate at different rates, the onsets of their syntheses coincide.
The absence of CotC and CotU forms from the mother cell fraction suggests their rapid assembly at the surface of the developing spore. This again suggests that formation of the 23-kDa species takes place only at the spore surface.
CotU assembly and CotU-CotC interaction depend on CotE and CotH.
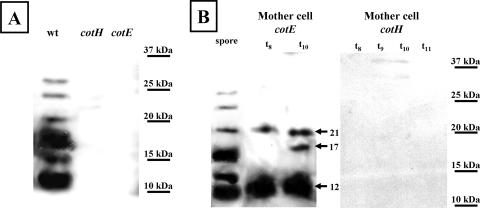
To analyze the requirements for CotU assembly and for the CotC-CotU interaction, we performed a Western blot analysis with anti-CotU antibody and proteins extracted from mature spores of the wild type and spores of various congenic strains deficient in the production of key coat morphogenetic factors. CotU and the CotU-CotC proteins of 23 kDa were found in the coats of wild-type spores but were not found in cotE or cotH spores (Fig. 7A). Therefore, CotU assembly and CotC-CotU interaction appear to be CotE and CotH dependent. Since CotE and CotH do not affect cotC or cotU expression (26, 38), we predicted that in cotE and cotH null mutants, CotU and CotC would accumulate in the mother cell. This was true for the cotE null mutant, in which CotU and the two main forms of CotC (12 and 21 kDa) (17) were found in the mother cell compartment (Fig. 7B). However, the CotC-CotU protein of 23 kDa was not found in the mother cell compartment of a cotE mutant (Fig. 7B) (17). This is in agreement with the idea that the 23-kDa CotC-CotU protein is formed at the spore surface. The absence of the two additional forms of CotC (12.5 and 30 kDa) in the mother cell of a cotE null mutant has been previously reported and discussed (17). In the cotH null mutant, CotU and the CotC-CotU form, as well as all CotC forms, were not found to accumulate in the mother cell (Fig. 7B). It has been previously proposed that the assembly of all CotC forms depends on the presence of CotH, which protects and/or stabilizes them in the mother cell compartment, allowing their assembly around the forming spore (2, 17). By analogy, we propose here that CotH is also involved in the stabilization/protection of CotU in the mother cell and possibly in its assembly on the coat.
FIG. 7.
Western blot analysis with anti-CotU antibody. (A) Coat proteins were extracted from a wild-type (wt) and congenic strains with null mutations in cotH or cotE. (B) Proteins were extracted at the indicated times (t8 to t11) during sporulation from the mother cell compartments of sporulating cultures of a cotE or a cotH mutant strain. The lane labeled “spore” contains coat proteins extracted from spores of a wild-type strain.
Therefore, formation of the 23-kDa CotC-CotU species is under dual control: CotH stabilizes and/or protects both interaction partners in the mother cell compartment, whereas CotE governs their assembly and subsequent interaction at the spore surface.
DISCUSSION
In the present work, we have shown that the cotU gene is transcribed during sporulation under the joint control of the σK factor of RNA polymerase and the transcriptional factor GerE. Therefore, cotU and cotC, both of which code for highly similar tyrosine-rich components of the coat, are expressed coincidentally in the mother cell compartments of sporulating cells. The interaction of GerE with the cotU promoter region was not directly observed but was inferred on the basis of the effect of a gerE mutation on cotU-driven β-galactosidase activity.
We have also studied the assembly of CotU and expanded previous work on the assembly of the related protein CotC. We present genetic and biochemical evidence that CotU and CotC specifically interact, forming a protein of 23 kDa extractable from the coats of mature spores. The CotC-CotU species appears shorter than expected on SDS-PAGE if the apparent sizes of CotC and CotU are added to each other. A possible explanation is that a fragment of one of the two components is cleaved when the interaction occurs. Our data do not allow us to establish whether such a proteolytic event actually occurs. However, the data in Fig. 4 showing that the CotC-CotU species can be purified on an Ni2+ column when a His tag is placed at the C-terminal end of CotU or at the N-terminal end of CotC allow us to exclude the possibility that those two regions are involved in a potential proteolytic cleavage. Several lines of evidence suggest that the interaction of CotC and CotU to form the CotU-CotC species of 23 kDa takes place at the spore surface. First, neither CotU nor CotC is detected in the mother cell compartment of wild-type cells, suggesting that their assembly occurs rapidly following their synthesis (reference 17 and this work). Second, a cotE null mutation prevented the assembly of both CotU and CotC, causing the accumulation of the monomeric forms of both proteins in the mother cell but not the accumulation of the 23-kDa CotU-CotC form. Finally, expression of an allele of cotC fused at its 3′ end to the sequence for the His6 tag resulted in the assembly of wild-type amounts of monomeric CotC but drastically impaired the formation of all the multimeric forms of CotC and of the CotC-CotU heterodimer. Although specific interactions between coat components have often been suggested as an important aspect of coat formation (19), only a few cases of direct interaction have been reported for coat proteins. In this respect, the observation that the CotU-CotC interaction occurs in B. subtilis but not in two heterologous hosts is particularly intriguing. It seems to suggest that the interaction involves an additional factor active at the spore surface. It is tempting to speculate that this factor could be a cross-linking enzyme, possibly assembled under CotE control (formation of the CotC-CotU species of 23 kDa was not detected in the mother cell of cotE mutant cells). The CotU-CotC interaction is not affected by extraction under alkaline conditions or by boiling in the presence of SDS and reducing agents. Both CotU and CotC are tyrosine rich, and formation of irreversible peroxidase-catalyzed o,o-dityrosine cross-links, which may occur within the coats, could account for the stability of the 23-kDa species. We note that cross-linking reactions have been shown to take place at the spore surface even after spore release from the mother cell, including the multimerization of another tyrosine-rich abundant coat protein, CotG (29, 40). However, we also note that a coat-associated peroxidase has not yet been found. Further work is needed to characterize the nature of the CotU-CotC interaction and the putative factor involved. In any case, our results also indicate that the C-terminal end of CotC is likely to be involved in mediating the interaction of CotC with itself and with CotU. It may be that the C-terminal region of CotC represents a nucleation point for multimer formation, because the presence of the His6 tag in this region drastically reduces formation of all multimeric forms of CotC (including the CotC-CotU species).
Our results also expand on previous observations related to the role of the morphogenetic protein CotH in assembly of the coat structure. CotU does not accumulate in the mother cell cytoplasm of a cotH mutant. This cannot be due to reduced stability of the protein in the mother cell cytoplasm, since it was detected by Western blotting in the cytoplasm of a cotE mutant, as well as in E. coli. We believe it is more likely that a specific factor (a protease?) degrades CotU in the absence of CotH (or a cotH-dependent protein). Similarly to what has been proposed for CotC (17) and CotG (42), we hypothesize that in a wild-type strain, CotH (or a cotH-dependent protein) would prevent CotU degradation either by interacting in a chaperone-like manner with CotU or its specific protease in the mother cell or by immediately recruiting CotU into the coat of the forming spore.
In a recent paper, CotU assembly within the coat was analyzed by fluorescence microscopy and found to be only partially dependent on CotE and CotH (19). In the present study, our Western blotting experiments indicated that CotU assembly is totally dependent on both morphogenetic proteins. As previously proposed for other coat components (19), this discrepancy could be explained by the presence of CotU in both the soluble (extractable) and insoluble (resistant to extraction) fractions of coat proteins. Fluorescence microscopy would then allow the detection of all CotU molecules, while Western blotting would detect only soluble CotU molecules. An alternative possibility is that CotU and the chimeric protein CotU-green fluorescent protein used in the previous study (19) have different stability and/or assembly properties. Since none of the mutants described here has any obvious phenotypic consequences for the properties of the coat, the functionality of a CotU-green fluorescent protein fusion is difficult to assess.
Future work will aim at characterizing in detail the interaction between CotC and CotU, including the identification and role of the putative factor involved, and the role of CotH in the process.
Acknowledgments
We thank L. Di Iorio for technical assistance.
This work was partially supported by a C.N.R. (PRIN 2006) grant to E.R., by a Centro Regionale di Competenza BioTekNet (Naples, Italy) grant to M.D.F., and by grant POCI/BIA-BCM/60855/2004 from the Fundação para a Ciência e a Tecnologia (FCT) to A.O.H.
Footnotes
Published ahead of print on 7 December 2007.
REFERENCES
- 1.Aronson, A. I., H.-Y. Song, and N. Bourne. 1988. Gene structure and precursor processing of a novel Bacillus subtilis spore coat protein. Mol. Microbiol. 3437-444. [DOI] [PubMed] [Google Scholar]
- 2.Baccigalupi, L., G. Castaldo, G. Cangiano, R. Isticato, R. Marasco, M. De Felice, and E. Ricca. 2004. GerE-independent expression of cotH leads to CotC accumulation in the mother cell compartment during Bacillus subtilis sporulation. Microbiology 1503441-3449. [DOI] [PubMed] [Google Scholar]
- 3.Chada, V. G., E. A. Sanstad, R. Wang, and A. Driks. 2003. Morphogenesis of Bacillus spore surfaces. J. Bacteriol. 1856255-6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa, T. V., L. O. Martins, U. Voelker, and A. O. Henriques. 2004. Assembly of an oxalate decarboxylase produced under σK control into the Bacillus subtilis spore coat. J. Bacteriol. 1861462-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cutting, S., and P. B. Vander Horn. 1990. Genetic analysis, p. 27-74. In C. Harwood and S. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, United Kingdom.
- 6.Cutting, S., L. Zheng, and R. Losick. 1991. Gene encoding two alkali-soluble components of the spore coat from Bacillus subtilis. J. Bacteriol. 1732915-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donovan, W., L. Zheng, K. Sandman, and R. Losick. 1987. Genes encoding spore coat polypeptides from Bacillus subtilis. J. Mol. Biol. 1961-10. [DOI] [PubMed] [Google Scholar]
- 8.Driks, A. 1999. Bacillus subtilis spore coat. Microbiol. Mol. Biol. Rev. 631-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Driks, A., S. Roels, B. Beall, C. P. Moran, Jr., and R. Losick. 1994. Subcellular localization of proteins involved in the assembly of the spore coat of Bacillus subtilis. Genes Dev. 8234-244. [DOI] [PubMed] [Google Scholar]
- 10.Eichenberger, P., M. Fujita, S. T. Jensen, E. M. Conlon, D. Z. Rudner, S. T. Wang, C. Ferguson, K. Haga, T. Sato, J. S. Liu, and R. Losick. 2004. The program of gene transcription for a single differentiating cell type during sporulation in Bacillus subtilis. PLoS Biol. 2e328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henriques, A. O., T. Costa, L. O. Martins, and R. Zilhão. 2004. The functional architecture and assembly of the spore coat, p. 65-85. In E. Ricca, A. O. Henriques, and S. M. Cutting (ed.), Bacterial spore formers: probiotics and emerging application. Horizon Bioscences Press, Norfolk, United Kingdom.
- 12.Henriques, A. O., L. R. Melsen, and C. P. Moran, Jr. 1998. Involvement of superoxide dismutase in spore coat assembly in Bacillus subtilis. J. Bacteriol. 1802285-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henriques, A. O., and C. P. Moran, Jr. 2007. Structure, assembly and function of the spore surface layers. Annu. Rev. Microbiol. 61555-588. [DOI] [PubMed] [Google Scholar]
- 14.Horton, R. M., H. D. Hunt, S. N. Ho, J. K. Pullen, and L. R. Pease. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 7761-68. [DOI] [PubMed] [Google Scholar]
- 15.Ichikawa, H., and L. Kroos. 2000. Combined action of two transcription factors regulates genes encoding spore coat proteins of Bacillus subtilis. J. Biol. Chem. 27513849-13855. [DOI] [PubMed] [Google Scholar]
- 16.Isticato, R., G. Cangiano, T.-H. Tran, A. Ciabattini, D. Medaglini, M. R. Oggioni, M. De Felice, G. Pozzi, and E. Ricca. 2001. Surface display of recombinant proteins on Bacillus subtilis spores. J. Bacteriol. 1836294-6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isticato, R., G. Esposito, R. Zilhão, S. Nolasco, G. Cangiano, M. De Felice, A. O. Henriques, and E. Ricca. 2004. Assembly of multiple CotC forms into the Bacillus subtilis spore coat. J. Bacteriol. 1861129-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isticato, R., D. Scotto Di Mase, E. M. F. Mauriello, M. De Felice, and E. Ricca. 2007. Amino terminal fusion of heterologous proteins to CotC increases display efficiencies in the Bacillus subtilis spore system. BioTechniques 42151-156. [DOI] [PubMed] [Google Scholar]
- 19.Kim, H., M. Hahn, P. Grabowski, D. McPherson, M. M. Otte, R. Wang, C. C. Ferguson, P. Eichenberger, and A. Driks. 2006. The Bacillus subtilis spore coat protein interaction network. Mol. Microbiol. 59487-502. [DOI] [PubMed] [Google Scholar]
- 20.Kim, J.-H., C.-S. Lee, and B.-G. Kim. 2005. Spore-displayed streptavidin: a live diagnostic tool in biotechnology. Biochem. Biophys. Res. Commun. 331210-214. [DOI] [PubMed] [Google Scholar]
- 21.Kloblutcher, L. A., K. Ragkousi, and P. Setlow. 2006. The Bacillus subtilis spore coat provides “eat resistance” during phagocytic predation by the protozoan Tetrahymena thermophila. Proc. Natl. Acad. Sci. USA 103165-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwon, S. J., H. C. Jung, and J. P. Pan. 2007. Transgalactosylation in a water-solvent biphasic reaction system with beta-galactosidase displayed on the surface of Bacillus subtilis spores. Appl. Environ. Microbiol. 732251-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai, E.-M., N. D. Phadke, M. T. Kachman, R. Giorno, S. Vazquez, A. J. Vazquez, J. R. Maddock, and A. Driks. 2003. Proteomic analysis of the spore coats of Bacillus subtilis and Bacillus anthracis. J. Bacteriol. 1951443-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martins, L. O., C. M. Soares, M. M. Pereira, M. Teixeira, T. Costa, G. H. Jones, and A. O. Henriques. 2002. Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the Bacillus subtilis endospore coat. J. Biol. Chem. 27718849-18859. [DOI] [PubMed] [Google Scholar]
- 25.Mauriello, E. M. F., L. H. Duc, R. Isticato, G. Cangiano, H. A. Hong, M. De Felice, E. Ricca, and S. M. Cutting. 2004. Display of heterologous antigens on the Bacillus subtilis spore coat using CotC as a fusion partner. Vaccine 221177-1187. [DOI] [PubMed] [Google Scholar]
- 26.Naclerio, G., L. Baccigalupi, R. Zilhao, M. De Felice, and E. Ricca. 1996. Bacillus subtilis spore coat assembly requires cotH gene expression. J. Bacteriol. 1784375-4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination and outgrowth, p. 391-450. In C. Harwood and S. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, United Kingdom.
- 28.Ozin, A. J., C. S. Samford, A. O. Henriques, and C. P. Moran, Jr. 2001. SpoVID guides SafA to the spore coat in Bacillus subtilis. J. Bacteriol. 1833041-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ragkousi, K., and P. Setlow. 2004. Transglutaminase-mediated cross-linking of GerQ in the coats of Bacillus subtilis spores. J. Bacteriol. 1865567-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramamurthi, K. S., K. S. Clapham, and R. Losick. 2006. Peptide anchoring spore coat assembly to the outer forespore membrane in Bacillus subtilis. Mol. Microbiol. 621547-1557. [DOI] [PubMed] [Google Scholar]
- 31.Ricca, E., S. Cutting, and R. Losick. 1992. Characterization of bofA, a gene involved in inter-compartmental regulation of pro-σK processing during sporulation in Bacillus subtilis. J. Bacteriol. 1743177-3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sacco, M., E. Ricca, R. Losick, and S. Cutting. 1995. An additional GerE-controlled gene encoding an abundant spore coat protein from Bacillus subtilis. J. Bacteriol. 177372-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 34.Serrano, M., R. Zilhão, E. Ricca, A. Ozin, C. Moran, and A. Henriques. 1999. A Bacillus subtilis secreted protein with a role in endospore coat assembly and function. J. Bacteriol. 1813632-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steil, L., M. Serrano, A. O. Henriques, and U. Völker. 2005. Genome-wide analysis of temporally-regulated and compartment-specific gene expression during spore development in Bacillus subtilis. Microbiology 151399-420. [DOI] [PubMed] [Google Scholar]
- 36.Youngman, P., J. B. Perkins, and R. Losick. 1984. A novel method for the rapid cloning in Escherichia coli of Bacillus subtilis chromosomal DNA adjacent to Tn917 insertion. Mol. Gen. Genet. 195424-433. [DOI] [PubMed] [Google Scholar]
- 37.Zhang, J., H. Ichikawa, R. Halberg, L. Kroos, and A. I. Aronson. 1994. Regulation of the transcription of a cluster of Bacillus subtilis spore coat genes. J. Mol. Biol. 240405-415. [DOI] [PubMed] [Google Scholar]
- 38.Zheng, L., W. P. Donovan, P. C. Fitz-James, and R. Losick. 1988. Gene encoding a morphogenic protein required in the assembly of the outer coat of the Bacillus subtilis endospore. Genes Dev. 21047-1054. [DOI] [PubMed] [Google Scholar]
- 39.Zheng, L. B., and R. Losick. 1990. Cascade regulation of spore coat gene expression in Bacillus subtilis. J. Mol. Biol. 212645-660. [DOI] [PubMed] [Google Scholar]
- 40.Zilhão, R., R. Isticato, L. O. Martins, L. Steil, U. Völker, E. Ricca, C. P. Moran, Jr., and A. O. Henriques. 2005. Assembly and function of a transglutaminase at the surface of the developing spore in Bacillus subtilis. J. Bacteriol. 1877753-7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zilhão, R., G. Naclerio, L. Baccigalupi, A. Henriques, C. Moran, and E. Ricca. 1999. Assembly requirements and role of CotH during spore coat formation in Bacillus subtilis. J. Bacteriol. 1812631-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zilhão, R., M. Serrano, R. Isticato, E. Ricca, C. P. Moran, Jr., and A. O. Henriques. 2004. Interactions among CotB, CotG, and CotH during assembly of the Bacillus subtilis spore coat. J. Bacteriol. 1861110-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]