Abstract
Pseudomonas aeruginosa is an opportunistic human pathogen which relies on several intercellular signaling systems for optimum population density-dependent regulation of virulence genes. The Pseudomonas quinolone signal (PQS) is a 3-hydroxy-4-quinolone with a 2-alkyl substitution which is synthesized by the condensation of anthranilic acid with a 3-keto-fatty acid. The pqsABCDE operon has been identified as being necessary for PQS production, and the pqsA gene encodes a predicted protein with homology to acyl coenzyme A (acyl-CoA) ligases. In order to elucidate the first step of the 4-quinolone synthesis pathway in P. aeruginosa, we have characterized the function of the pqsA gene product. Extracts prepared from Escherichia coli expressing PqsA were shown to catalyze the formation of anthraniloyl-CoA from anthranilate, ATP, and CoA. The PqsA protein was purified as a recombinant His-tagged polypeptide, and this protein was shown to have anthranilate-CoA ligase activity. The enzyme was active on a variety of aromatic substrates, including benzoate and chloro and fluoro derivatives of anthranilate. Inhibition of PQS formation in vivo was observed for the chloro- and fluoroanthranilate derivatives, as well as for several analogs which were not PqsA enzymatic substrates. These results indicate that the PqsA protein is responsible for priming anthranilate for entry into the PQS biosynthetic pathway and that this enzyme may serve as a useful in vitro indicator for potential agents to disrupt quinolone signaling in P. aeruginosa.
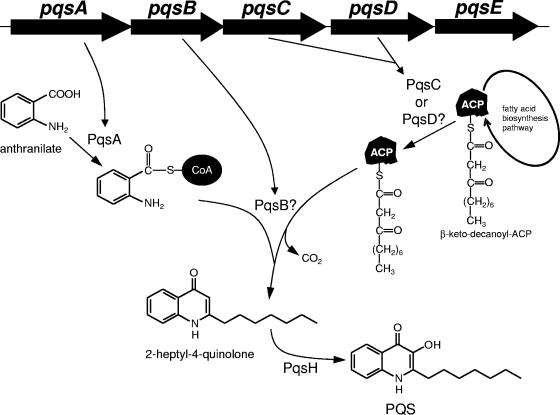
Pseudomonas aeruginosa is a common environmental organism and also an opportunistic human pathogen. A large number of the virulence factors which it produces during infection are regulated by low-molecular-weight molecules which are part of the cell-to-cell signaling systems of this organism. One class of signals, which are common in gram-negative bacteria, is the acyl homoserine lactone molecules. In the case of P. aeruginosa, the molecules in this class are N-(3-oxo-dodecanoyl)-l-homoserine lactone, which is the signal of the las quorum-sensing system, and N-butanoyl-homoserine lactone, which is the signal of the rhl quorum-sensing system (28, 40, 43-45). The second signal class, the members of which thus far appear to be synthesized only by P. aeruginosa and certain Burkholderia species (22), is based on the 2-alkyl-4-quinolone structure. The compound 2-heptyl-3-hydroxy-4-quinolone, referred to as the Pseudomonas quinolone signal (PQS), has been shown to control the expression of multiple virulence factors in P. aeruginosa (20, 37, 47). PQS has been shown to act as a ligand for PqsR, the transcriptional activator which controls the pqsABCDE operon (57). The biosynthesis of PQS appears to involve the condensation of anthranilate with a 10-carbon fatty acid and requires the expression of multiple genetic operons (Fig. 1) (10, 12, 19, 27). Based on protein similarities, the pqsABCDE operon is thought to code for the activating and condensation enzymes (except for pqsE, which is not required for biosynthesis but is required for PQS signaling) (27). The pqsH gene, at another chromosomal location, is most likely responsible for the introduction of the 3-hydroxy group (21, 27). The anthranilate precursor can be derived either from a unique anthranilate synthase operon (phnAB), from tryptophan via the kynurenine pathway, or from exogenous anthranilate (26).
FIG. 1.
Proposed pathway for PQS biosynthesis. Anthranilate is activated by PqsA to form anthraniloyl-CoA, and this is condensed with 2-oxo-decanoyl-ACP which is recruited from the fatty acid biosynthetic pathway. The recruitment and condensation are postulated to be catalyzed by PqsB, PqsC, and PqsD. The resulting 2-heptyl-4-quinolone is then hydroxylated at the third carbon by the PqsH monooxygenase to produce PQS.
To elucidate the actual biosynthetic route to PQS, this study focused on establishing the role of the pqsA gene product. The predicted PqsA protein is homologous to a variety of acyl coenzyme A (acyl-CoA) ligases, and the obvious role of PqsA is to convert either anthranilate or the 10-carbon fatty acid into a CoA thioester. This product would then be the precursor for the condensation steps that give the final 4-quinolone framework. In this report, we show that PqsA is responsible for anthranilate activation and provide the initial biochemical characterization of this enzyme. We also provide data to show that this enzyme may be useful as a means to screen inhibitors of PQS biosynthesis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
P. aeruginosa strain PAO1 was maintained at −70°C in 10% skim milk (Becton Dickinson). P. aeruginosa was grown at 37°C with shaking in PTSB medium (41), and freshly plated cells from skim milk stocks were used to begin all experiments. Cultures of Escherichia coli strain Rosetta 2(DE3) (EMD-Novagen) were grown at 37°C with shaking in Luria-Bertani broth (42) supplemented with 30 μg of chloramphenicol/ml and 100 μg of ampicillin/ml to maintain plasmids when appropriate.
Plasmid construction.
To obtain a native PqsA expression plasmid, PCR was used to generate a 1,681-bp DNA fragment that began at the pqsA start codon (ATG) and ended 127 bp downstream from the stop codon. Chromosomal DNA from P. aeruginosa strain PAO1 was used as a template for PCR. The oligonucleotide primers for PCR were engineered to contain a blunt end at the start codon and a single HindIII restriction site downstream from the stop codon. Plasmid pEX1.8, a tac promoter expression vector with origins of replication for both E. coli and P. aeruginosa (46), was digested with EcoRI, treated with Klenow fragment to fill in the 5′ overhang, and then digested with HindIII. After digestion with HindIII, the pqsA-containing fragment was ligated into pEX1.8 to yield pSLM20. The expression of His-tagged PqsA was accomplished using plasmid pMWC-trc996. This plasmid was constructed using the same strategy described above by ligating a PCR-derived DNA fragment that contained the coding region of pqsA into pTrcHis A (Invitrogen). Primers containing an upstream BamHI site and a downstream HindIII site were used in this case to enable cloning of the PCR fragment into the respective sites of the vector. The pqsA gene with a sequence encoding a His tag was also inserted into the E. coli-P. aeruginosa shuttle vector plasmid pUCP18 to generate plasmid pCAL996H for use in complementation experiments. This insertion was accomplished by digesting pMWC-trc996 with SphI and HindIII and ligating the resulting 3,333-bp fragment to pUCP18 that had been digested with the same enzymes. All gene fusions were sequenced to ensure cloning integrity.
In vivo PQS synthesis inhibition assays.
One-milliliter aliquots of a P. aeruginosa strain PAO1 subculture, adjusted to an A660 of 0.05 with fresh medium, were added to tubes with and without anthranilate analogs at a final concentration of 1 mM, and the tubes were incubated for 24 h at 37°C with shaking (all chemicals and enzymes were purchased from Sigma-Aldrich, Inc.). At 24 h, culture optical densities were measured to ensure that no growth inhibition had occurred, and 900 μl of acidified ethyl acetate was added to 300 μl of cell culture. After vortexing for 1 min and centrifugation at 16,000 × g for 5 min, an 800-μl aliquot of the organic layer was transferred to a new tube and the solvent was evaporated to dryness. For thin-layer chromatography (TLC) analysis, the dried extract was dissolved in 50 μl of 1:1 acidified ethyl acetate-acetonitrile and 5 μl of each extract was resolved by TLC as described previously (12). Fifty nanograms of synthetic PQS was also loaded as a standard. The resolved plates were photographed under long-wave UV light. For the comparison of PQS production in untreated strain PAO1 versus that in analog-treated strain PAO1, extracts in decreasing amounts were resolved by TLC as described above and digitized images of the resulting PQS spots were quantitated using ImageQuant software (version 3.3; Molecular Dynamics).
CoA ligase activity assays.
Continuous assays with anthranilate as the substrate were performed as described elsewhere (58) with 0.5-ml reaction mixtures containing 100 mM HEPES, pH 8.0, 0.2 mM dithiothreitol (DTT), 2 mM MgCl2, 0.4 mM CoA, 1 mM ATP, and protein. After equilibration of the mixture in the sample cuvette at 37°C for 1 min, reactions were initiated by the addition of anthranilate (potassium salt) to a final concentration of 0.5 mM. The formation of anthraniloyl-CoA was monitored at 365 nm. The extinction coefficient at 365 nm (ɛ365) was 5.5 mM−1·cm−1. The effect of inhibitors on the formation of anthraniloyl-CoA was monitored using the same conditions. The optimum reaction mixture pH was determined using the same reaction mixture described above except that 100 mM HEPES was replaced by 100 mM Tris-HCl buffer set to pHs of 7.0, 7.5, 8.0, 8.5, and 9.0.
Alternative substrates were assayed using either the same conditions as those for anthranilate or a continuous coupled assay mixture containing the following, in a final volume of 1 ml: 100 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 1.5 mM ATP, 0.4 mM CoA, 0.24 mM NADH, 1.5 mM phosphoenolpyruvate, myokinase (7.2 U), pyruvate kinase (2 U), lactate dehydrogenase (2.8 U), and 1 mM substrate. After equilibration of the reaction mixtures at 37°C for 1 min, reactions were initiated by the addition of purified PqsA protein. Depending on product CoA thioester absorption, NADH oxidation was monitored at either 334, 340, or 365 nm (ɛ334 = 6.15 mM−1·cm−1; ɛ340 = 6.3 mM−1·cm−1; ɛ365 = 3.4 mM−1·cm−1) (6). A two-step process was also used in assays with anthranilate analogs to qualitatively verify product formation or reaction inhibition. These reaction mixtures (0.5 ml) had the same composition as that for the direct assay with 1 mM substrate. Before the addition of the enzyme, a 0.25-ml aliquot was removed to be used as a time zero blank. The enzyme was then added to the remaining 0.25-ml sample, and the reaction mixture was incubated at 37°C for 10 min before the addition of 35 μl of formic acid to both the blank and the reaction sample. Samples were placed on ice for 5 min and then centrifuged (16,000 × g) for 5 min in a tabletop microcentrifuge. Each reaction sample was read in an ND-1000 spectrophotometer (NanoDrop Technologies, Inc.) in scan mode between 250 and 400 nm, after blanking with the corresponding time zero sample. The absorbance at the λmax for each individual product was recorded and expressed relative to the absorbance obtained with anthranilate as a substrate at 365 nm. The amount of enzyme used was pretested to give a linear reaction over the 10-min time course with anthranilate as a substrate.
Thioester formation with acyl carrier protein (ACP) was tested using the filter paper precipitation assay (50). The complete reaction mixture contained 100 mM HEPES, pH 8.0, 0.2 mM DTT, 2 mM MgCl2, 5 mM ATP, 15 μM holo-ACP (61.1 mCi/mmol; Sigma, St. Louis, MO), 60 μM [U-14C]anthranilate (61.1 mCi/mmol; Sigma, St. Louis, MO), and enzyme. Control reaction mixtures had individual substrates omitted. Time zero samples were removed before the addition of the enzyme. Reaction mixtures were incubated for 10 min at 37°C, and then aliquots were spotted onto Whatman 3MM filter paper disks (2.4 cm in diameter). The disks were washed twice in chloroform-methanol-acetic acid (10:20:3, vol/vol/vol), air dried, and then subjected to liquid scintillation counting.
Heterologous expression and purification of anthranilate-CoA ligase.
The native enzyme was purified using the following procedure. E. coli strain Rosetta 2(DE3) containing pSLM20 was grown to an optical density at 600 nm of 0.6, IPTG (isopropyl-β-d-thiogalactopyranoside) was added to 1 mM, and growth was continued for 2 h. After harvesting of the cells, cell pellets were suspended in 2 volumes of a mixture of 100 mM Tris-HCl, 2 mM MgCl2, and 2 mM DTT, pH 7.8 (buffer A), and disrupted by two passages through a French pressure cell. The lysate was centrifuged at 12,000 × g for 30 min to remove cell debris and then subjected to centrifugation at 100,000 × g for 30 min. The supernatant was brought to 35% saturation by the addition of solid ammonium sulfate and centrifuged at 12,000 × g for 30 min. The supernatant was removed and brought to 50% saturation with solid ammonium sulfate and centrifuged as described above. The 35 to 50% ammonium sulfate fractionation pellet was resuspended in buffer A containing 0.5 M ammonium sulfate (buffer B) and applied to a butyl-Sepharose 4 fast-flow column (2.5 by 6.0 cm; Amersham) at a flow rate of 1.2 ml/min. The column was washed with buffer B until the A280 baseline had returned to 0, and then bound protein was eluted with a 50-ml-decreasing salt gradient (0.5 to 0 M ammonium sulfate in buffer A). Active fractions were diluted 1:1 with a mixture of 20 mM triethanolamine, 0.2 M NaCl, and 1 mM DTT, pH 7.8 (buffer Q), and applied to a HiTrap Q HP column (5 ml; Amersham) equilibrated with buffer Q. The column was washed with buffer Q until the A280 baseline had returned to 0, and then bound protein was eluted with a 40-ml linear gradient of 0.2 to 1.0 M NaCl.
The His-tagged protein was purified using the following procedure. E. coli strain Rosetta 2(DE3) containing pMWC-trc996 was grown, induced with 1 mM IPTG, harvested, and disrupted as described above. Lysates were centrifuged at 100,000 × g. The supernatant was brought to 50% saturation by the addition of solid ammonium sulfate and then centrifuged at 12,000 × g for 30 min. The pellet was resuspended in a 1:1 mixture of buffer A and His-Select wash buffer (10 mM imidazole, 0.3 M NaCl, 50 mM sodium phosphate, pH 8.0), and the suspension was applied in 200-mg batches to a 6.4-ml His-Select cartridge (Sigma-Aldrich). The column was washed with wash buffer until the A280 baseline had returned to 0, and then bound proteins were eluted with His-Select elution buffer (250 mM imidazole, 0.3 M NaCl, 50 mM sodium phosphate, pH 8.0). The active fractions eluted from the His-Select cartridge were subjected to hydrophobic interaction chromatography on a butyl-Sepharose column and anion exchange chromatography on a HiTrap Q HP column as described above for the native enzyme.
Column fractions during purification were monitored by measurements at 280 and 260 nm with a NanoDrop ND-1000 spectrophotometer. Protein concentrations for enzyme assays and polyacrylamide gel electrophoresis (PAGE) analysis were determined by the Bradford assay using Bio-Rad reagents (9).
Gel permeation chromatography.
A column packed with Sephacryl S-200 (GE Healthcare, Piscataway, NJ) with bed dimensions of 1.5 by 47 cm was equilibrated with a mixture of 100 mM Tris-HCl, 200 mM NaCl, 2 mM DTT, and 2 mM MgCl2, pH 7.8, calibrated with marker proteins, and used to determine the mass of the PqsA protein. The flow rate was approximately 1.0 ml/min, and the elution of proteins was monitored at 280 nm using a Bio-Rad model EM-1 Econo UV monitor coupled to a Bio-Rad model 1327 Econo-Recorder. Gel filtration molecular mass markers (Sigma-Aldrich, St. Louis, MO) included blue dextran (2,000 kDa), sweet potato β-amylase (200 kDa), yeast alcohol dehydrogenase (150 kDa), bovine serum albumin (66 kDa), bovine erythrocyte carbonic anhydrase (29 kDa), and horse heart cytochrome c (12.4 kDa).
PAGE.
Sodium dodecyl sulfate (SDS)-10% PAGE was performed by the method of Laemmli (36).
Reaction product identification.
To identify the anthranilate adduct formed, samples (0.4 ml) from a 2-ml enzymatic reaction mixture were removed at timed intervals, treated with 50 μl of ice-cold formic acid, and centrifuged to remove precipitated protein. Samples (50 μl) were evaporated to dryness and resuspended in 5 μl of water, the suspensions were applied to silica gel G TLC plates (with fluorescent indicators) along with standards (synthetic anthraniloyl-CoA, anthranilic acid, and CoA), and the plates were developed with a butanol-acetic acid-water (60:35:25) solvent system. After drying under air, plates were photographed under 365-nm UV light. Synthetic anthraniloyl-CoA was prepared by the reaction of CoA with isatoic anhydride under the conditions described for the reaction of succinic anhydride with CoA (56). For reactions to identify the fate of ATP, 200-μl reaction mixtures were run under the continuous-assay conditions except that [8-14C]ATP (51 mCi/mmol; MP Biomedicals Inc., Irvine, CA) was used in place of unlabeled ATP. Samples were removed at timed periods, and reactions were stopped with a 1/10 volume of cold formic acid. Following centrifugation, supernatant samples were applied to polyethyleneimine-cellulose plates along with ATP, ADP, and AMP standards. The plates were developed in 0.75 M Tris-0.45 M HCl (8) and then exposed to film to detect radiolabeled spots. A handheld UV lamp was used to determine the positions of standards.
Kinetic parameters and inhibitors.
Kinetic constants for anthranilate-based compounds, ATP, and CoA were tested using the direct assay at 365 nm. Kinetic constants for benzoate-based compounds and 3-hydroxydecanoic acid were determined using the coupled assay at 340 nm. Kinetic constants were calculated by using direct linear plots (17) and Hanes plots (33). Inhibitors were tested using the direct assay by monitoring their effects on the reaction course with anthranilate as the substrate. Inhibition constants were calculated as described by Hamilton-Miller (32).
RESULTS
PqsA has anthranilate-CoA ligase activity.
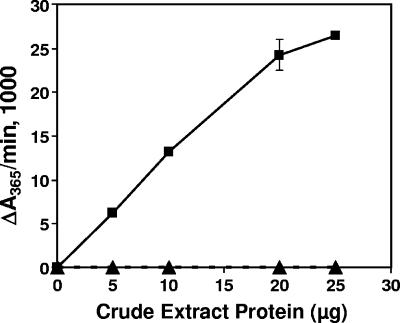
Previous data from our laboratory and other groups showed that anthranilate was a direct precursor for PQS (10, 12, 16, 52). Based on the structure of PQS and the proposed involvement of a condensation reaction between anthranilate and 3-oxo-decanoic acid in 4-quinolone synthesis, we expected that the carbon-carbon bond formation which occurs would involve a CoA-activated precursor. This prediction led us to consider that the possibilities for an activated precursor moiety were anthranilate and 3-oxo-decanoic acid. An examination of the pqsABCDE gene cluster showed that the pqsA reading frame encoded a protein with significant homology to numerous acyl- and aryl-CoA ligases. Therefore, we focused on the PqsA protein to determine if it was, in fact, a CoA ligase and whether it was responsible for the thioesterification of anthranilate or of a 3-oxo-decanoic acid (or both). Using either the direct assay or the coupled assay, we were unable to demonstrate that anthranilate-CoA ligase activity was present in crude extracts prepared from P. aeruginosa strain PAO1. This result was due presumably to high levels of endogenous ATP hydrolysis or CoA esterase activity present in crude extracts of P. aeruginosa strain PAO1. For this reason, heterologously expressed native and N-terminal His-tagged PqsA proteins were purified after expression in E. coli. We found that crude E. coli cell lysates containing native PqsA demonstrated CoA ligase activity with anthranilate as a substrate (Fig. 2). The control strains containing a vector plasmid had no such activity (Fig. 2). In order to facilitate purification, we then repeated this experiment with His-tagged PqsA and determined that this recombinant enzyme had activity identical to that of the native protein (data not shown). (Note that as a control to ensure that the PqsA-His fusion had native activity, we showed that PQS production was fully complemented in a P. aeruginosa pqsA mutant that was transformed with a plasmid harboring this fusion [pCAL996H] [data not shown]). Subsequently, the His-tagged enzyme was used for all future assays because of the higher enzyme yield obtained with this form than with the native protein and because of the ease of purification. The purification of His-tagged PqsA is summarized in Table 1, and the results of an SDS-PAGE analysis of the purification steps are shown in Fig. 3. The final preparation was estimated to be >95% homogenous by SDS-PAGE analysis and represented a purification factor of approximately 58-fold (Table 1). The initial specific activity was estimated from the total yield in ammonium sulfate-precipitated fractions because of interfering activities in the crude extract. Most likely, this interference was due to ATPase activity or CoA esterase activity at this stage. Contaminating proteins present after the Ni-chelate column chromatography step were removed by sequential chromatography on butyl-Sepharose and HiTrap Q columns, though this resulted in a substantial decrease in the overall yield (16%) (Table 1; Fig. 3).
FIG. 2.
Dependence of CoA thioester formation on the presence of PqsA. The indicated amounts of crude extract protein prepared from E. coli Rosetta 2(DE3) cells containing either pSLM20 (▪) or the control vector pEX1.8 (▴) were assayed as described in Materials and Methods by using the direct spectrophotometric assay with potassium anthranilate as the substrate. CoA thioester formation was monitored at 365 nm, the maximum λ of anthraniloyl-CoA.
TABLE 1.
Purification of anthranilate-CoA ligase
| Purification step | Total activity (U) | Amt of total protein (mg) | Sp act (U/mg protein) | Yield (%) | Purification (fold) |
|---|---|---|---|---|---|
| Crude extract | 93.4a | 4,192 | 0.022a | 100 | 1 |
| 50% ammonium sulfate pellet | 88.7 | 820 | 0.108 | 95 | 4.9 |
| His-Select fraction (Ni2+ affinity) | 61.8 | 80 | 0.773 | 66 | 35 |
| Butyl-Sepharose fraction | 21.4 | 21 | 1.02 | 23 | 46 |
| HiTrap Q fraction | 15.2 | 12 | 1.27 | 16 | 58 |
Enzymatic activity values for the crude extract are extrapolated from the sum of the total activities in the pellet and the supernatant from the 50% ammonium sulfate precipitation. Interfering activities gave artificially low values when the activity in the crude extract was measured directly.
FIG. 3.
SDS-PAGE analysis of steps of purification of His-tagged anthranilate-CoA ligase. Samples from stages of purification (described in Materials and Methods) of His-tagged anthranilate-CoA ligase were subjected to SDS-PAGE on an 8% polyacrylamide gel. Lanes: 1, crude extract, 20 μg; 2, ammonium sulfate pellet, 20 μg; 3, His-Select column peak fractions, 2 μg; 4, butyl-Sepharose peak fractions, 2 μg; 5, HiTrap Q column peak fractions, 2 μg; and 6, molecular mass markers, with corresponding kilodalton values indicated on the right.
Molecular properties of anthranilate-CoA ligase.
The native PqsA gene product is 516 amino acids in length and has a predicted molecular weight of 56,608, while the predicted molecular weight of the His-tagged PqsA gene product is 60,639. SDS-PAGE analyses of the His-tagged protein showed a molecular weight of approximately 57,000 (Fig. 3). This anomalously fast migration may be due to the high hydrophobicity of this protein, which may cause a higher degree of SDS binding and consequently a higher charge-to-mass ratio (49). In this regard, we also observed that PqsA bound very tightly to a phenyl-Sepharose matrix and was not effectively precipitated by the deoxycholate-trichloroacetic acid procedure which we have commonly used for preparing samples for SDS-PAGE analysis. Similarly strong binding to phenyl-Sepharose has been reported previously for the benzoate-CoA ligase from Rhodopseudomonas palustris (29). Gel filtration of the enzyme on a calibrated column of Sephacryl S-200 indicated that the enzyme most likely exists as a native monomer, with an elution volume corresponding to a molecular weight of approximately 62,900 (data not shown). Most of the previously characterized aryl-CoA ligases have been shown to have similar subunit molecular weights and to exist either as monomers (7, 29, 38, 54) or dimers (34), though tetrameric (35) and octameric (2) structures have been reported.
Products and stoichiometry.
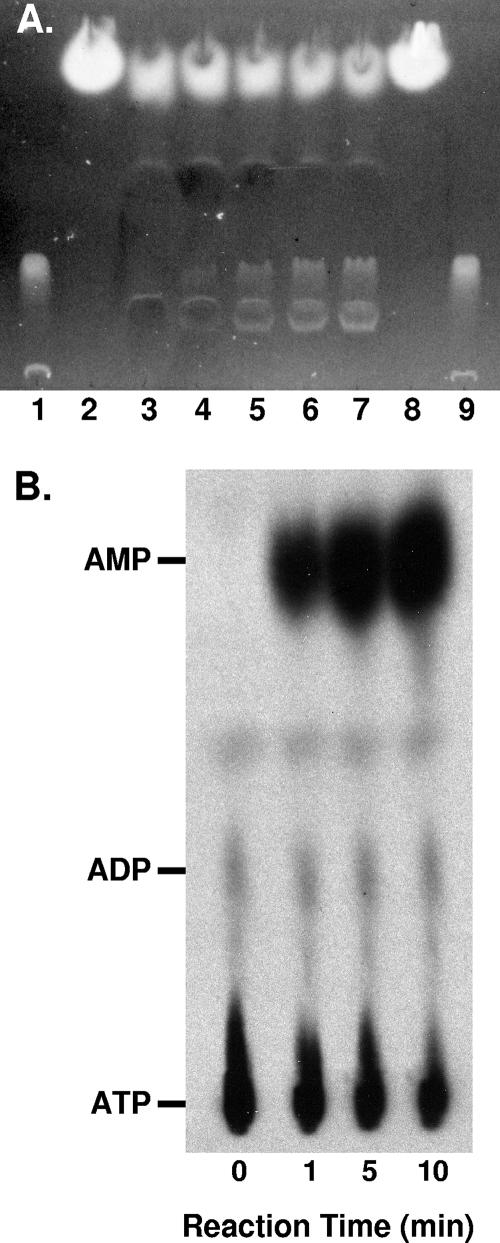
In order to learn more about the enzymatic activity of PqsA, we measured several basic biochemical parameters. UV/visible spectra of reaction mixes containing anthranilate, CoA, ATP, and PqsA protein showed a time-dependent increase in absorbance with an observed maximum at ∼365 nm, which is the maximal absorption wavelength of anthraniloyl-CoA (11). TLC analyses of extracts of the reaction mixes sampled at timed intervals showed increased fluorescence in a band comigrating with chemically synthesized anthraniloyl-CoA (Fig. 4A). No absorbance increase was observed with the methyl esterified derivative, methyl anthranilate (data not shown). This result is further evidence that the enzymatic reaction involves the carboxylic acid group of anthranilate. A comparison of reaction rates between the direct assay (responsive to the formation of anthraniloyl-CoA) and the coupled assay (responsive to the oxidation of NADH to regenerate ATP from either AMP or ADP) was consistent with a stoichiometry of 2 mol of NADH oxidized per mol of anthraniloyl-CoA formed. This pattern is consistent with the formation of AMP rather than ADP (58) and was confirmed by performing the assay with anthranilate, CoA, and [8-14C]ATP and subjecting the products to TLC analysis. Autoradiography of the TLC-separated reaction mixes demonstrated the conversion of the ATP substrate into AMP, with insignificant amounts of ADP formed (Fig. 4B). Thus, the net reaction is as follows: anthranilate + ATP + coenzyme A → anthraniloyl-CoA + AMP + PPi. This reaction is in agreement with the two-step mechanism proposed for members of the CoA ligase family, which involves the formation of an initial adenylated intermediate which serves as the acyl donor to coenzyme A for thioester bond formation (13).
FIG. 4.
TLC analysis of anthranilate-CoA ligase reaction products. (A) A 2-ml CoA ligase reaction mixture was sampled at time zero (before enzyme addition) and at 5, 15, 30, and 60 min. After the addition of the enzyme, the progress of the reaction was monitored in a spectrophotometer at 365 nm. Samples (0.4 ml) were removed at various times, added to 50 μl of ice-cold formic acid, and then centrifuged to remove precipitated proteins. A 50-μl aliquot of each sample was evaporated to dryness, redissolved in 5 μl of water, and spotted onto a silica gel TLC plate. The plate was developed in butanol-acetic acid-water (60:35:25) and photographed under long-wave UV light. Lanes: 1 and 9, synthetic anthraniloyl-CoA; 2 and 8, anthranilate; 3, 0 min (A365 = 0); 4, 5 min (A365 = 0.07); 5, 15 min (A365 = 0.17); 6, 30 min (A365 = 0.28); and 7, 60 min (A365 = 0.39). (B) Autoradiograph of polyethyleneimine-cellulose-separated reaction samples containing [8-14C]ATP in place of unlabeled ATP. Samples were treated and subjected to chromatography as described in Materials and Methods, and TLC plates were then exposed to X-ray film. Unlabeled ATP, ADP, and AMP were spotted alongside samples, and their positions were determined using a handheld UV lamp.
Kinetic and physicochemical properties.
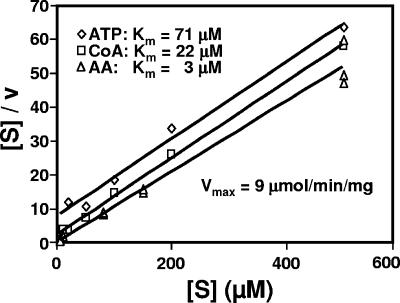
The pqsA-encoded enzyme displayed activity at physiological pH values, with demonstrable activity between pH 7.0 and pH 9.0 and an optimum at pH 8.5. Michaelis constants for anthranilate and select aromatic substrates are shown in Table 2. The Km values for anthranilate, CoA, and ATP were 3, 22, and 71 μM, respectively, as determined from Hanes plots (Fig. 5). These values are all within physiological ranges and are similar to values obtained for other benzoate- and anthranilate-CoA ligases (1, 7, 54).
TABLE 2.
Effect of substrate analogs on in vitro anthranilate-CoA ligase activity and in vivo PQS production in P. aeruginosa
| Compound | Efficacy as CoA ligase substratea | CoA ligase Km (μM)b | CoA ligase Ki (μM)c | PQS level (%)d |
|---|---|---|---|---|
| Benzoate | ++ | 150 | ||
| 2-Aminobenzoate (anthranilate) | ++ | 8 | ||
| 2-Amino-5-bromobenzoate | + | 78 | 100 | |
| 2-Amino-3-chlorobenzoate | − | 12.9 | 18 ± 7 | |
| 2-Amino-4-chlorobenzoate | ++ | 480 | 10 ± 1 | |
| 2-Amino-5-chlorobenzoate | ++ | 703 | 35 ± 13 | |
| 2-Amino-6-chlorobenzoate | ++ | 122 | 52 ± 11 | |
| 2-Amino-4-fluorobenzoate | +++ | 70 | 42 ± 12 | |
| 2-Amino-5-fluorobenzoate | +++ | 120 | 36 ± 13 | |
| 2-Amino-6-fluorobenzoate | ++ | 11 | 0 | |
| 2-Amino-3-methylbenzoate | + | 2,830 | 100 | |
| 2-Amino-5-methylbenzoate | ++ | 980 | 100 | |
| 2-Amino-3-methoxybenzoate | + | 159 | 100 | |
| 2-Hydroxybenzoate (salicylate) | − | 18.3 | 76 ± 2 | |
| Anthranilonitrile | − | 1,300 | 13 ± 3 | |
| 5-Nitroanthranilonitrile | − | 37.0 | 6 ± 1 | |
| 3-Fluoro-O-anisidine | − | 89.7 | 41 ± 12 | |
| Methylanthranilate (2-aminobenzoate methyl ester) | − | 5,800 | 50 ± 5 | |
| N-Methylanthranilate | ++ |
Based on the observation of product formation in direct assays and in NanoDrop UV spectra. Symbols refer to the reaction rate relative to that with anthranilate as the substrate under identical reaction conditions. +++, >100%; ++, 50-100%; +, <50%; −, no reaction.
Based on direct assays at wavelengths determined by NanoDrop UV spectra and Km values calculated by the direct linear plot method (25).
Based on direct assays with anthranilate as the substrate with Ki values calculated by the 50% inhibition method (32).
Determined from triplicate TLC assays as described in Materials and Methods. Results are means ± standard deviations of PQS levels relative to that obtained with anthranilate as the substrate.
FIG. 5.
Hanes plots for anthranilate, CoA, and ATP. Each substrate was varied independently in reaction mixes containing saturating amounts of the other two substrates. The substrate concentration ([S]) is plotted on the abscissa, and the reaction velocity (v; micromoles per minute per milligram of protein) is plotted on the ordinate. Values represent the average of results from three assays. Km value estimates were determined from the x intercept (x intercept = −Km), and Vmax values were determined from the slope (slope = 1/Vmax) as described by Hanes (33).
Substrate specificity.
In order to gain knowledge of the substrate specificity of the PqsA protein, we tested a variety of aromatic and aliphatic substrates using either the direct or the indirect assay. Activity on a variety of aromatic carboxylic acids with structural similarity to anthranilic acid was observed (Table 2). No activity was observed with acetate, decanoate, or 3-hydroxydecanoate (data not shown). An unblocked carboxylic acid group was required (no activity with anthranilate methyl ester) for enzymatic activity. However, analogs with substitutions on the amino group were active as substrates (N-methyl anthranilate). The presence of the amino group was not required, as activity was seen with the compound without substitutions (benzoate) and with 2- and 3-fluorobenzoates. Aminobenzoates with halogen substitutions were active in general, with the highest reaction rates observed with the compounds with fluoro substitutions and the lowest observed with the compound with the 5-bromo substitution. Ring methyl substitutions on anthranilate resulted in decreased activity (5-methyl activity was greater than 3-methyl activity). Furthermore, reactions performed with E. coli ACP in place of CoA showed no thioester product (data not shown). Based on these results, it appears that the PqsA protein is a CoA ligase specific for aromatic carboxylic acid substrates.
In vivo and in vitro inhibition studies.
In order to determine if there is a correlation between the ability of compounds to inhibit in vivo PQS formation and their in vitro inhibition of one specific target, anthranilate-CoA ligase, we tested various anthranilate analogs for their effects on PQS production in vivo and also tested the same compounds for their effects on the anthranilate-CoA ligase reaction. The amount of PQS produced after the growth of P. aeruginosa in the presence of analogs was determined by the fluorescence of TLC-resolved extracts (Table 2). These same analogs were used in the anthranilate-CoA ligase direct assay to determine Km values (for compounds which were substrates) or Ki values (for nonsubstrates). The anthranilates with chloro and fluoro substitutions were generally effective PQS synthesis inhibitors, and all except for the 3-chloro compound were effective substrates (tested at 1 mM). The anthranilates with 3-methyl, 5-methyl, and 3-methoxy substitutions were effective substrates but did not appear to inhibit PQS formation. As expected, four compounds which lacked a carboxyl group, anthranilonitrile, 5-nitroanthranilonitrile, methylanthranilate, and 3-fluoro-O-anisidine, did not function as substrates. However, all four significantly inhibited PQS formation. Although the most effective PQS synthesis inhibitor of the four, 5-nitroanthranilonitrile, had the lowest calculated Ki (37 μM), the inhibition constants for the other compounds did not correlate well with their degrees of inhibition of PQS synthesis. Nevertheless, the observations are consistent with a competitive inhibition mechanism, although action at other steps in the PQS pathway is possible. The same is true of the 2-hydroxy homolog of anthranilate, salicylate, which was not an effective substrate when tested at 1 mM but had a very low Ki and inhibited PQS formation in vivo. No inhibition of activity with anthranilate as a substrate was observed with decanoate or 3-hydroxydecanoate in the reaction mix, evidence that aliphatic carboxylic acids are not the natural substrates for the enzyme. Overall, these results demonstrate that in vitro screening using the PqsA enzyme is of utility in identifying compounds of interest for disrupting PQS-mediated virulence factor expression.
DISCUSSION
The data presented in this work clearly demonstrate that the PqsA gene product has 2-aminobenzoate-CoA ligase activity. In the presence of anthranilate, ATP, and CoA, the purified enzyme catalyzed the formation of a product which comigrated on TLC plates with synthetic anthraniloyl-CoA. The product also had an absorption peak at 365 nm, which is in agreement with properties reported for anthraniloyl-CoA (11). Although activity on other aromatic acids, including benzoate, was also observed, the presence of the PqsA gene in an operon required for PQS biosynthesis supports the prediction that the natural substrate for PqsA is anthranilate and that this enzyme serves to activate anthranilate as a priming step for entry into the PQS biosynthetic pathway, which involves the condensation (with decarboxylation) of a 10-carbon fatty acid with anthranilic acid. Biological condensation reactions such as this typically require that one or both of the substrates are activated as phosphopantetheine thioesters (with CoA or ACP) or, more rarely, as adenylates. Others have demonstrated this general scheme using isotope experiments along with mass spectrometry and nuclear magnetic resonance analyses (10, 16, 52). As to the question of whether a CoA ligase is required for the activation of the anthranilate or the fatty acid, our data support a synthetic scheme whereby fatty acids are drawn from their biosynthetic pathway at the 10-carbon stage as ACP thioesters and funneled into the PQS biosynthetic pathway to condense with anthraniloyl-CoA (Fig. 1). This scheme is also an energetically more feasible mechanism than the alternative, since the de-esterification and reesterification of fatty acids from the biosynthetic pathway would seem wasteful. The question still remains as to the nature of the fatty acid cosubstrate. Specifically, is the fatty acid cosubstrate an ACP or a CoA thioester? If it is in the form of an ACP-linked substrate, the fatty acid could be used directly from the fatty acid biosynthesis pathway, as occurs in acyl homoserine lactone biosynthesis (39, 42). Alternatively, it is possible that the fatty acid is transacylated from AcpP, which is involved in fatty acid biosynthesis, to one of the other two ACPs synthesized by P. aeruginosa, Acp1 (thought to be involved in acyl homoserine lactone biosynthesis) or Acp3 (51). A transacylation such as this could be catalyzed by the pqsC or pqsD gene product and could proceed directly or through a CoA thioester intermediate. Alternatively, either of these same proteins may transacylate the fatty acyl-ACP into fatty acyl-CoA, which would then serve as the condensing substrate with anthraniloyl-CoA. In none of these cases would the fatty acid pass through a free carboxyl stage, so there would not seem to be a requirement for an acyl-CoA ligase to form CoA thioesters from free fatty acids. Anthranilate, on the other hand, would exist as the free acid form, whether drawn from its biosynthetic pathway or from exogenous sources, and would require an activating enzyme to form the CoA thioester.
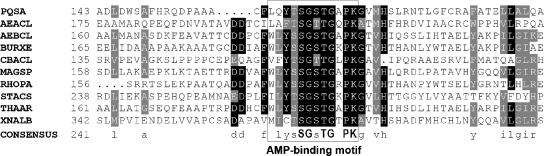
The subunit size and monomeric native conformation of the PqsA protein are similar to those of other previously characterized carboxylic acid-CoA ligases. Its predicted amino acid sequence also is similar to those of confirmed CoA ligases that are active on acyl or aryl substrates. Most of the previously characterized aromatic acid-CoA ligases, including those with anthranilate-CoA ligase activity, are involved in catabolic reactions during growth on aromatic substrates. It is interesting that a large number of predicted CoA synthase sequences are present in genomic databases and that the PqsA protein displays as high as 34% identity to most of these (data not shown). However, database searches revealed that PqsA has no very close relatives other than those corresponding to predicted open reading frames in other P. aeruginosa strains. Even in Burkholderia species which have a gene cluster homologous to pqsABCDE that appears to be part of an entire 4-quinolone biosynthetic operon, the identity between the PqsA homologs is only 31% (22). The reason for this low degree of identity is unclear, but the low level may indicate that the PQS synthetic pathway evolved very early and diverged in the Pseudomonas and Burkholderia lineages. Key residues shown to be involved in catalysis in other CoA ligases are present (Fig. 6), and a motif (residues 161 to 172; LQYTSGSTGAPK) of the adenylate-forming enzyme superfamily is also present (http://ca.expasy.org/prosite/-sequence PS00455) (15, 18). This motif constitutes an AMP-binding domain which is involved in the initial adenylation reaction that precedes thioester bond formation in the acyl and aryl-CoA synthetase subfamily of this superfamily (3, 23, 53).
FIG. 6.
Sequence comparisons of PqsA with other functionally verified CoA ligases. The region containing the AMP-binding domain is shown (15, 18). Gray and black shading designates similar and identical residues, respectively. Alignments were performed using AlignX (Vector NTI Advance; Invitrogen Corp.), and the figure was produced using Boxshade 3.21 (http://www.ch.embnet.org/software/BOX_form.html). The sequences used were as follows: PQSA, anthranilate-CoA ligase (this work); AEACL, 2-aminobenzoate-CoA ligase from Azoarcus evansii (55); AEBCL, benzoate-CoA ligase from Azoarcus evansii (30); BURXE, benzoate-CoA ligase from Burkholderia xenovorans LB400 (4); CBACL, 4-chlorobenzoate-CoA ligase from Pseudomonas sp. strain CBS-3 (14); MAGSP, benzoate-CoA ligase from Magnetospirillum sp. strain TS-6 (34); RHOPA, benzoate-CoA ligase from Rhodopseudomonas palustris (24); STACS, acetate-CoA ligase from Salmonella enterica serovar Typhimurium LT2 (31); THAAR, benzoate-CoA ligase from Thauera aromatica (54); XNALB, benzoate-CoA ligase from Xanthomonas albilineans (GenBank accession no. EF117322) (S. M. Hashimi and R. G. Birch, unpublished results). The numbers at the left indicate the starting residue for each sequence. Consensus residues are shown on the bottom line, with lowercase letters indicating conserved residues (present in >6 of 10 sequences) and bold uppercase letters indicating residues identical in all sequences. The location of the AMP-binding motif described previously (15, 18) is shown.
Our earlier observation that PQS biosynthesis was inhibited by the methyl ester of anthranilate (12) led to the testing of other anthranilate analogs for their in vitro activity as substrates or inhibitors of the purified anthranilate-CoA ligase, followed by in vivo testing for effects on PQS synthesis. This in vitro assay allows for the rapid identification of potential inhibitors of PQS biosynthesis. We anticipated that useful analog inhibitors could be broadly categorized into those which were substrates and those which were not. Compounds which are not substrates may act as inhibitors by competitive binding to the anthranilate-binding site. Salicylate is in this category and has been shown previously to down-regulate virulence factor expression in P. aeruginosa (5, 48). Compounds which are substrates may inhibit PQS formation either by competition at the anthranilate-binding site or by the inhibition of downstream enzymes in the PQS biosynthetic pathway by their CoA thioesters. The chlorinated and fluorinated anthranilate derivatives that were tested all inhibited PQS biosynthesis, and all except for 3-chloroanthanilate (2-amino-3-chlorobenzoate) served as substrates for the ligase when tested at 1 mM. There was no general correlation between the Km or Ki and the effect on PQS synthesis, although the most effective synthesis inhibitor, 6-fluoroanthranilate, had the lowest Km (11 μM). It remains to be seen whether the inhibition of PQS synthesis by these compounds is due to competition with anthranilate or is caused by the inhibition of downstream enzymes by CoA thioesters that form through the action of PqsA on the anthranilate analogs. It is also possible that the inhibition of PQS biosynthesis may be the result of effects on the kynurenine pathway, which has been demonstrated to be an alternate route for the supply of anthranilate for PQS biosynthesis (26). In any case, it is apparent that targeting specific parts of the PQS synthetic pathway will allow for the discovery of inhibitory compounds that will serve as potential therapeutics that decrease PQS production and thereby lessen P. aeruginosa virulence.
Acknowledgments
This work was supported by a research grant from the National Institute of Allergy and Infectious Diseases (grant R01-AI076272) and a Research Development Award grant from East Carolina University.
Footnotes
Published ahead of print on 14 December 2007.
REFERENCES
- 1.Altenschmidt, U., and G. Fuchs. 1992. Novel aerobic 2-aminobenzoate metabolism. Purification and characterization of 2-aminobenzoate-CoA ligase, localisation of the gene on a 8-kbp plasmid, and cloning and sequencing of the gene from a denitrifying Pseudomonas sp. Eur. J. Biochem. 205721-727. [DOI] [PubMed] [Google Scholar]
- 2.Auburger, G., and J. Winter. 1992. Purification and characterization of benzoyl-CoA ligase from a syntrophic, benzoate-degrading, anaerobic mixed culture. Appl. Microbiol. Biotechnol. 37789-795. [DOI] [PubMed] [Google Scholar]
- 3.Babbitt, P. C., G. L. Kenyon, B. M. Martin, H. Charest, M. Slyvestre, J. D. Scholten, K. H. Chang, P. H. Liang, and D. Dunaway-Mariano. 1992. Ancestry of the 4-chlorobenzoate dehalogenase: analysis of amino acid sequence identities among families of acyl:adenyl ligases, enoyl-CoA hydratases/isomerases, and acyl-CoA thioesterases. Biochemistry 315594-5604. [DOI] [PubMed] [Google Scholar]
- 4.Bains, J., and M. J. Boulanger. 2007. Biochemical and structural characterization of the paralogous benzoate CoA ligases from Burkholderia xenovorans LB400: defining the entry point into the novel benzoate oxidation (box) pathway. J. Mol. Biol. 373965-977. [DOI] [PubMed] [Google Scholar]
- 5.Bandara, M. B., H. Zhu, P. R. Sankaridurg, and M. D. Willcox. 2006. Salicylic acid reduces the production of several potential virulence factors of Pseudomonas aeruginosa associated with microbial keratitis. Investig. Ophthalmol. Vis. Sci. 474453-4460. [DOI] [PubMed] [Google Scholar]
- 6.Bergmeyer, H. L. 1975. New values for the molar extinction coefficients of NADH and NADPH for the use in routine laboratories. Z. Klin. Chem. Klin. Biochem. 13507-508. (In German.) [PubMed] [Google Scholar]
- 7.Beuerle, T., and E. Pichersky. 2002. Purification and characterization of benzoate:coenzyme A ligase from Clarkia breweri. Arch. Biochem. Biophys. 400258-264. [DOI] [PubMed] [Google Scholar]
- 8.Bochner, B. R., and B. N. Ames. 1982. Complete analysis of cellular nucleotides by two-dimensional thin layer chromatography. J. Biol. Chem. 2579759-9769. [PubMed] [Google Scholar]
- 9.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72248-254. [DOI] [PubMed] [Google Scholar]
- 10.Bredenbruch, F., M. Nimtz, V. Wray, M. Morr, R. Muller, and S. Haussler. 2005. Biosynthetic pathway of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines. J. Bacteriol. 1873630-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buder, R., K. Ziegler, G. Fuchs, B. Langkau, and S. Ghisla. 1989. 2-Aminobenzoyl-CoA monooxygenase/reductase, a novel type of flavoenzyme. Studies on the stoichiometry and the course of the reaction. Eur. J. Biochem. 185637-643. [DOI] [PubMed] [Google Scholar]
- 12.Calfee, M. W., J. P. Coleman, and E. C. Pesci. 2001. Interference with Pseudomonas quinolone signal synthesis inhibits virulence factor expression by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 9811633-11637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang, K. H., and D. Dunaway-Mariano. 1996. Determination of the chemical pathway for 4-chlorobenzoate:coenzyme A ligase catalysis. Biochemistry 3513478-13484. [DOI] [PubMed] [Google Scholar]
- 14.Chang, K. H., P. H. Liang, W. Beck, J. D. Scholten, and D. Dunaway-Mariano. 1992. Isolation and characterization of the three polypeptide components of 4-chlorobenzoate dehalogenase from Pseudomonas sp. strain CBS-3. Biochemistry 315605-5610. [DOI] [PubMed] [Google Scholar]
- 15.Chang, K. H., H. Xiang, and D. Dunaway-Mariano. 1997. Acyl-adenylate motif of the acyl-adenylate/thioester-forming enzyme superfamily: a site-directed mutagenesis study with the Pseudomonas sp. strain CBS3 4-chlorobenzoate:coenzyme A ligase. Biochemistry 3615650-15659. [DOI] [PubMed] [Google Scholar]
- 16.Cornforth, J. W., and A. T. James. 1956. Structure of a naturally occurring antagonist of dihydrostreptomycin. Biochem. J. 63124-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornish-Bowden, A., and R. Eisenthal. 1978. Estimation of Michaelis constant and maximum velocity from the direct linear plot. Biochim. Biophys. Acta 523268-272. [DOI] [PubMed] [Google Scholar]
- 18.Cukovic, D., J. Ehlting, J. A. VanZiffle, and C. J. Douglas. 2001. Structure and evolution of 4-coumarate:coenzyme A ligase (4CL) gene families. Biol. Chem. 382645-654. [DOI] [PubMed] [Google Scholar]
- 19.D'Argenio, D. A., M. W. Calfee, P. B. Rainey, and E. C. Pesci. 2002. Autolysis and autoaggregation in Pseudomonas aeruginosa colony morphology mutants. J. Bacteriol. 1846481-6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deziel, E., S. Gopalan, A. P. Tampakaki, F. Lepine, K. E. Padfield, M. Saucier, G. Xiao, and L. G. Rahme. 2005. The contribution of MvfR to Pseudomonas aeruginosa pathogenesis and quorum sensing circuitry regulation: multiple quorum sensing-regulated genes are modulated without affecting lasRI, rhlRI or the production of N-acyl-l-homoserine lactones. Mol. Microbiol. 55998-1014. [DOI] [PubMed] [Google Scholar]
- 21.Deziel, E., F. Lepine, S. Milot, J. He, M. N. Mindrinos, R. G. Tompkins, and L. G. Rahme. 2004. Analysis of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs) reveals a role for 4-hydroxy-2-heptylquinoline in cell-to-cell communication. Proc. Natl. Acad. Sci. USA 1011339-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diggle, S. P., P. Lumjiaktase, F. Dipilato, K. Winzer, M. Kunakorn, D. A. Barrett, S. R. Chhabra, M. Camara, and P. Williams. 2006. Functional genetic analysis reveals a 2-alkyl-4-quinolone signaling system in the human pathogen Burkholderia pseudomallei and related bacteria. Chem. Biol. 13701-710. [DOI] [PubMed] [Google Scholar]
- 23.Dunaway-Mariano, D., and P. C. Babbitt. 1994. On the origins and functions of the enzymes of the 4-chlorobenzoate to 4-hydroxybenzoate converting pathway. Biodegradation 5259-276. [DOI] [PubMed] [Google Scholar]
- 24.Egland, P. G., J. Gibson, and C. S. Harwood. 1995. Benzoate-coenzyme A ligase, encoded by badA, is one of three ligases able to catalyze benzoyl-coenzyme A formation during anaerobic growth of Rhodopseudomonas palustris on benzoate. J. Bacteriol. 1776545-6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenthal, R., and A. Cornish-Bowden. 1974. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem. J. 139715-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farrow, J. M., and E. C. Pesci. 2007. Two distinct pathways supply anthranilate as a precursor of the Pseudomonas quinolone signal. J. Bacteriol. 1893425-3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallagher, L. A., S. L. McKnight, M. S. Kuznetsova, E. C. Pesci, and C. Manoil. 2002. Functions required for extracellular quinolone signaling by Pseudomonas aeruginosa. J. Bacteriol. 1846472-6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gambello, M. J., and B. H. Iglewski. 1991. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J. Bacteriol. 1733000-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geissler, J. F., C. S. Harwood, and J. Gibson. 1988. Purification and properties of benzoate-coenzyme A ligase, a Rhodopseudomonas palustris enzyme involved in the anaerobic degradation of benzoate. J. Bacteriol. 1701709-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gescher, J., A. Zaar, M. Mohamed, H. Schagger, and G. Fuchs. 2002. Genes coding for a new pathway of aerobic benzoate metabolism in Azoarcus evansii. J. Bacteriol. 1846301-6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gulick, A. M., V. J. Starai, A. R. Horswill, K. M. Homick, and J. C. Escalante-Semerena. 2003. The 1.75 A crystal structure of acetyl-CoA synthetase bound to adenosine-5′-propylphosphate and coenzyme A. Biochemistry 422866-2873. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton-Miller, J. M. 1966. A novel method for evaluating Ki/Km and its application to the competitive inhibition of staphylococcal penicillinase by cephalosporins. Biochem. J. 10140C-42C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanes, C. S. 1932. Studies on plant amylases: the effect of starch concentration upon the velocity of hydrolysis by the amylase of germinated barley. Biochem. J. 261406-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawaguchi, K., Y. Shinoda, H. Yurimoto, Y. Sakai, and N. Kato. 2006. Purification and characterization of benzoate-CoA ligase from Magnetospirillum sp. strain TS-6 capable of aerobic and anaerobic degradation of aromatic compounds. FEMS Microbiol. Lett. 257208-213. [DOI] [PubMed] [Google Scholar]
- 35.Kwon, O., D. K. Bhattacharyya, and R. Meganathan. 1996. Menaquinone (vitamin K2) biosynthesis: overexpression, purification, and properties of o-succinylbenzoyl-coenzyme A synthetase from Escherichia coli. J. Bacteriol. 1786778-6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 37.McKnight, S. L., B. H. Iglewski, and E. C. Pesci. 2000. The Pseudomonas quinolone signal regulates rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 1822702-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohamed, M. E.-S., and G. Fuchs. 1993. Purification and characterization of phenylacetate-coenzyme A ligase from a denitrifying Pseudomonas sp., an enzyme involved in the anaerobic degradation of phenylacetate. Arch. Microbiol. 159554-562. [DOI] [PubMed] [Google Scholar]
- 39.More, M. I., L. D. Finger, J. L. Stryker, C. Fuqua, A. Eberhard, and S. C. Winans. 1996. Enzymatic synthesis of a quorum-sensing autoinducer through use of defined substrates. Science 2721655-1658. [DOI] [PubMed] [Google Scholar]
- 40.Ochsner, U. A., A. K. Koch, A. Fiechter, and J. Reiser. 1994. Isolation and characterization of a regulatory gene affecting rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. J. Bacteriol. 1762044-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohman, D. E., S. J. Cryz, and B. H. Iglewski. 1980. Isolation and characterization of Pseudomonas aeruginosa PAO mutant that produces altered elastase. J. Bacteriol. 142836-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parsek, M. R., D. L. Val, B. L. Hanzelka, J. E. Cronan, Jr., and E. P. Greenberg. 1999. Acyl homoserine-lactone quorum-sensing signal generation. Proc. Natl. Acad. Sci. USA 964360-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Passador, L., J. M. Cook, M. J. Gambello, L. Rust, and B. H. Iglewski. 1993. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 2601127-1130. [DOI] [PubMed] [Google Scholar]
- 44.Pearson, J. P., K. M. Gray, L. Passador, K. D. Tucker, A. Eberhard, B. H. Iglewski, and E. P. Greenberg. 1994. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc. Natl. Acad. Sci. USA 91197-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pearson, J. P., L. Passador, B. H. Iglewski, and E. P. Greenberg. 1995. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 921490-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pearson, J. P., E. C. Pesci, and B. H. Iglewski. 1997. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 1795756-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pesci, E. C., J. B. Milbank, J. P. Pearson, S. McKnight, A. S. Kende, E. P. Greenberg, and B. H. Iglewski. 1999. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 9611229-11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prithiviraj, B., H. P. Bais, T. Weir, B. Suresh, E. H. Najarro, B. V. Dayakar, H. P. Schweizer, and J. M. Vivanco. 2005. Down regulation of virulence factors of Pseudomonas aeruginosa by salicylic acid attenuates its virulence on Arabidopsis thaliana and Caenorhabditis elegans. Infect. Immun. 735319-5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rais, I., M. Karas, and H. Schagger. 2004. Two-dimensional electrophoresis for the isolation of integral membrane proteins and mass spectrometric identification. Proteomics 42567-2571. [DOI] [PubMed] [Google Scholar]
- 50.Ray, T. K., and J. E. Cronan, Jr. 1976. Activation of long chain fatty acids with acyl carrier protein: demonstration of a new enzyme, acyl-acyl carrier protein synthetase, in Escherichia coli. Proc. Natl. Acad. Sci. USA 734374-4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raychaudhuri, A., A. Jerga, and P. A. Tipton. 2005. Chemical mechanism and substrate specificity of RhlI, an acylhomoserine lactone synthase from Pseudomonas aeruginosa. Biochemistry 442974-2981. [DOI] [PubMed] [Google Scholar]
- 52.Ritter, C., and M. Luckner. 1971. Biosynthesis of 2-n-alkyl-4-hydroxyquinoline derivates (pseudane) in Pseudomonas aeruginosa. Eur. J. Biochem. 18391-400. (In German.) [DOI] [PubMed] [Google Scholar]
- 53.Scholten, J. D., K. H. Chang, P. C. Babbitt, H. Charest, M. Sylvestre, and D. Dunaway-Mariano. 1991. Novel enzymic hydrolytic dehalogenation of a chlorinated aromatic. Science 253182-185. [DOI] [PubMed] [Google Scholar]
- 54.Schuhle, K., J. Gescher, U. Feil, M. Paul, M. Jahn, H. Schagger, and G. Fuchs. 2003. Benzoate-coenzyme A ligase from Thauera aromatica: an enzyme acting in anaerobic and aerobic pathways. J. Bacteriol. 1854920-4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schuhle, K., M. Jahn, S. Ghisla, and G. Fuchs. 2001. Two similar gene clusters coding for enzymes of a new type of aerobic 2-aminobenzoate (anthranilate) metabolism in the bacterium Azoarcus evansii. J. Bacteriol. 1835268-5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simon, E. J., and D. Shemin. 1953. The preparation of S-succinyl coenzyme A. J. Am. Chem. Soc. 752520. [Google Scholar]
- 57.Wade, D. S., M. W. Calfee, E. R. Rocha, E. A. Ling, E. Engstrom, J. P. Coleman, and E. C. Pesci. 2005. Regulation of Pseudomonas quinolone signal synthesis in Pseudomonas aeruginosa. J. Bacteriol. 1874372-4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ziegler, K., R. Buder, J. Winter, and G. Fuchs. 1989. Activation of aromatic acids and aerobic 2-aminobenzoate metabolism in a denitrifying Pseudomonas strain. Arch. Microbiol. 151171-176. [Google Scholar]