Abstract
The activity of copolymer 1 (Cop 1, Copaxone, glatiramer acetate) in suppressing experimental autoimmune encephalomyelitis (EAE) and in the treatment of multiple sclerosis patients when injected parenterally has been extensively demonstrated. In the present study we addressed the question of whether Cop 1 can induce oral tolerance to EAE similar to myelin basic protein (MBP). We now have demonstrated that oral Cop 1 inhibited EAE induction in both rats and mice. Furthermore, oral Cop 1 was more effective than oral MBP in suppressing EAE in rats. The beneficial effect of oral Cop 1 was found to be associated with specific inhibition of the proliferative and Th1 cytokine secretion responses to MBP of spleen cells from Cop 1-fed mice and rats. In all of these assays, oral Cop 1 was more effective than oral MBP. The tolerance induced by Cop 1 could be adoptively transferred with spleen cells from Cop 1-fed animals. Furthermore, Cop 1-specific T cell lines, which inhibit EAE induction in vivo, could be isolated from the above spleen cells. These T cell lines secrete the anti-inflammatory cytokines IL-10 and transforming growth factor type β, but not IL-4, in response to both Cop 1 and MBP. In conclusion, oral Cop 1 has a beneficial effect on the development of EAE that is associated with down-regulation of T cell immune responses to MBP and is mediated by Th2/3 type regulatory cells. These results suggest that oral administration of Cop 1 may modulate multiple sclerosis as well.
Keywords: oral tolerance, multiple sclerosis, autoimmunity
The mucous membranes covering the aerodigestive and urogenital tracts are endowed with a large and highly specialized immune system, mucosa-associated lymphoid tissues. Mucosal administration of antigens (for instance, by ingestion or inhalation) may result in the development of a state of peripheral immunological tolerance (1). Recently, oral and nasal administration of autoantigens were shown to suppress a variety of experimental autoimmune diseases, including experimental allergic encephalomyelitis (EAE) (2–4). Three distinct mechanisms have been elucidated for the systemic-antigen-specific immune suppression associated with oral tolerance: clonal deletion, clonal anergy, and active cellular suppression. The mechanism by which tolerance is generated depends on the amount of antigen administered. Feeding high doses of antigen induces either deletion or anergy of antigen-specific cells (5–7), whereas feeding multiple low doses of antigen induces regulatory T cells that mediate suppression by secreting the cytokines IL-4, IL-10, and transforming growth factor (TGF) β1. In the EAE system, feeding a low dose of myelin basic protein (MBP) induces cells that are structurally identical to Th1 encephalitogenic CD4+ clones in their T cell receptor usage, MHC class II restriction, and epitope recognition, but secrete the above suppressive cytokines. Such T cell clones isolated from mesenteric lymph nodes of mice orally tolerized with low doses of MBP were demonstrated to suppress ongoing EAE induced by either MBP or proteolipid protein (PLP) (8). This finding indicates that antigen-specific regulatory T cells generated in the gut can migrate to the target organ and suppress ongoing inflammatory reactions to a nonrelated antigen as well, a phenomenon termed bystander suppression (9).
The investigations in the experimental models of autoimmune diseases have led to a series of phase II/III double-blind clinical trials of oral tolerance in subjects with multiple sclerosis (MS), rheumatoid arthritis, and uveitis (10–13). The results of these trials were equivocal. Thus, although phase I/II trials in MS have shown some positive results, in a large phase III trial, patients fed with myelin showed no difference from placebo in the number of relapses. Double-blind studies of patients with rheumatoid arthritis treated with oral collagen showed positive effects that were dose dependent (11). In uveitis, oral administration of purified S antigen, but not the retinal mixture, showed positive effects (12, 13). These results highlight the need for further experimental studies to successfully apply oral tolerance as therapeutic modality for human autoimmune diseases.
Copolymer 1 (Cop 1, Copaxone, glatiramer acetate) is a synthetic amino acid copolymer composed of l-alanine, l-glutamic acid, l-lysine, and l-tyrosine. It was demonstrated to suppress EAE induced by various encephalitogens (MBP, PLP, and myelin oligodendrocyte glycoprotein) in a variety of species when administered parenterally (14–16). In phase III clinical trials, daily s.c. injections of Copaxone were found to slow progression of disability and reduce the relapse rate in exacerbating-remitting MS (17, 18).
The mechanisms underlying the therapeutic activity of Cop 1 in EAE and MS have been extensively studied (16). Binding of Cop 1 to the MHC class II molecules is a prerequisite for its activity by any mechanism. Cop 1 exhibits a very rapid, high, and efficient binding to different MHC class II haplotypes (19). As a result of the efficient MHC binding, Cop 1 can compete for MHC class II with several myelin-associated antigens, e.g., MBP, PLP, and myelin oligodendrocyte glycoprotein (MOG), resulting in inhibition of antigen-specific effector functions. In addition, complexes of Cop 1 with MHC class II molecules can compete with MBP complexes at the level of the T cell receptor to MBP p82–100 (20). Cop 1 binding to the relevant MHC leads also to activation of T suppressor (Ts) cells of the Th2 type, which are activated by shared suppressive determinants between MBP and Cop 1 to secrete anti-inflammatory Th2 cytokines. These cytokines may mediate the therapeutic effect of Cop 1 in disease induced not only with MBP but also with PLP and MOG by the mechanism of bystander suppression (21, 22).
It was therefore of interest to test the effect of oral administration of Cop 1 on the development of EAE and the immune response to MBP. We report that oral Cop 1 exerts a beneficial effect on the development of EAE. This effect is associated with down-regulation of T cell immune responses to MBP and is mediated by Cop 1-specific suppressor cells secreting TGFβ.
MATERIALS AND METHODS
Antigens.
Cop 1 b.05392 (used in the rat studies) and b.54496 (used in the mice studies) were obtained from TEVA Pharmaceutical Industries (Petach-Tikva, Israel). Guinea pig myelin basic protein (GP-MBP) and mouse MBP were prepared from spinal cord by acid extraction and ammonium sulfate precipitation (23). The synthetic peptide p82–100 of MBP (DENPVVHFFKNIVTPRTPP) was synthesized by the Merrifield solid-phase method (24), using the peptide synthesizer 430A of Applied Biosystems and purified by HPLC. Purified protein derivative of tuberculin was obtained from Statens Serum Institute (Copenhagen).
Animals.
(SJL/JxBALB/c) F1 female mice, 8–10 weeks old, and female Lewis rats, 8–12 weeks old, were obtained from Harlan Laboratories (Jerusalem).
Induction and Assessment of EAE.
(SJL/x BALB/c)F1 mice were injected in all four footpads with 2 mg per mouse of spinal cord homogenate emulsified at a 1:1 ratio with complete Freund’s adjuvant (CFA) containing 1 mg/ml Mycobacteria H37Ra (Difco). Pertussis toxin (250 ng/mouse, Sigma) was injected i.v. immediately afterward and 48 h later. Rats were immunized with 25 μg of GP-MBP emulsified 1:1 in CFA containing 4 mg/ml of H37Ra. The emulsion, a total volume of 0.1 ml, was injected into the two hind foot pads. Animals were examined daily from day 10 postinjection for signs of disease. EAE was scored as follows: 0-no disease, 1-limp tail, 2-hind limb paralysis, 3-paralysis of all four limbs, 4-moribund condition, and 5-death. Statistical analysis of differences between groups used Student’s t test for comparison of the mean maximal EAE score, and the Fisher exact test was used to compare the incidence of clinical symptoms.
Induction of Oral Tolerance.
Mice were fed with 250 μg of mouse MBP or Cop 1 (50–500 μg) dissolved in PBS, on days −7, −5, −3, 0, 2, 4, and 6, relative to the day of EAE induction or, in other experiments, 10 feedings every alternate day after disease induction, by gastric intubation with an 18-gauge stainless steel feeding needle (Thomas Scientific, Swedeboro, NJ). Rats were fed with 1 mg of GP-MBP or Cop 1 (0.5–2 mg) dissolved in PBS by gastric intubation using a sterile feeding tube (Uno Plast, Hundested, Denmark). Rats were fed five times (a total dose of 5 mg) before disease induction, at intervals of 2–3 days. EAE was induced 2 days after the last feeding. Control mice and rats were mock-fed with PBS.
Isolation of Cop 1-Specific T Cell Lines.
Lewis rats were fed five times with 1 mg of Cop 1 and (SJL/Jx BALB/c) F1 mice seven times with 250 μg of Cop 1, at intervals of 2–3 days. Four to 12 days after the last feeding, animals were sacrificed, and their spleens were removed. Spleen cells of three animals were pooled and incubated (50 × 106 cells per plate) with Cop 1 (50 μg) in medium containing 1% autologous serum, for 4 days. Every 14–21 days cells (4–6 × 106/plate) were restimulated by 3 days of exposure to Cop 1 (500 μg) presented on syngeneic irradiated (3,000 rad) rat thymocytes (100 × 106/plate) or mouse splenocytes (50 × 106/plate), respectively. Stimulation was followed by propagation in medium containing 10% supernatant of Con A-activated normal mouse spleen cells as T cell growth factor.
Proliferation Assay.
The proliferation response of spleen cells was tested 10–11 days after EAE induction. Cells from three animals in each group were pooled and cultured in triplicate (5 × 105 mouse cells and 2 × 105 rat cells) in microtiter plates with various antigen concentrations in a final volume of 0.2 ml in RPMI culture medium supplemented with 1% autologous serum. After 72 hr of incubation, the cells were pulsed with 1 μCi [3H]thymidine for 18 hr and then harvested onto filter papers, and radioactivity was counted. T cell lines (1 × 104 cells) were cultured with irradiated (3,000 rad) rat thymocytes (1 × 106) or mouse splenocytes (5 × 105) and with the indicated antigens in a final volume of 0.2 ml in microtiter plates. At the end of 48 hr of incubation, the cultures were pulsed with [3H]thymidine and harvested 6–12 hr later. Results are expressed as the mean cpm for triplicate cultures. SDs were under 20% of the mean cpm.
Cytokine Secretion Assay.
Spleens were removed 10–11 days after EAE induction, and cells of three animals from each group were pooled. Cells (5 × 106/ml) were cultured in duplicate in 24-well plates in RPMI supplemented with 10% FCS in the presence or absence of antigen. T cell lines (1 × 106/ml) were incubated with the indicated antigens presented on irradiated rat thymocytes (10 × 106/ml) or mouse spleen cells (5 × 106/ml). Supernatants were harvested after 24–40 hr of culture. Quantitative ELISA assays for IL-2, IFN-γ, IL-4, IL-6, and IL-10 were performed by using paired mAbs specific for the corresponding cytokines (PharMingen) according to the manufacturer’s instructions. TGFβ was measured by using an ELISA kit (R&D Systems) in 72 hr supernatants of cultured splenocytes, incubated in serum free media-DCCM-1 (Biological Industries, Beit Haemek, Israel). Results are expressed as the mean concentration of duplicate culture supernatants, measured in duplicate wells (SD under 10%).
Adoptive Transfer of Disease Suppression.
Adoptive transfer with spleen cells. Donor rats were fed with either Cop 1 or BSA (1 mg), five times at 2–3 day intervals. Two to 16 days after the last feeding, the rats were sacrificed, and spleen suspensions were prepared. Cells were activated with Con A (1.5 μg/ml) in proliferation media for 48 hr. Cells (100–200 × 106) were injected i.p. into recipient rats.
Adoptive transfer with T cell lines.
T cell lines from rat or mouse origin were incubated with Cop 1 (50–100 mg/ml) and irradiated antigen-presenting cells for 72 hr as described above. The cells were washed and injected into recipient rats (20 × 106 cells per rat injected i.p.). or mice (15 × 106 cells per mouse injected i.v.). Recipient animals were challenged for EAE induction, as described above, immediately after cell transfer.
RESULTS
Suppression of EAE by Oral Administration of Cop 1.
The ability of orally administered Cop 1 to prevent clinical manifestations of EAE in Lewis rats was assayed under conditions previously reported to induce oral suppression by low doses of MBP (25). The results summarized in Table 1 demonstrate that Cop 1 could reduce both the incidence and the clinical signs of EAE in comparison to rats fed with PBS. A maximum effect was achieved with a dose of 1 mg of Cop 1, whereas a dose of 2 mg of Cop 1 was somewhat less efficient, although not significantly, in suppressing EAE. We then compared the efficacy of suppression induced by the most effective dose of Cop 1 (1 mg) to that of MBP. Feeding with either MBP or Cop 1 resulted in significant suppression of EAE with Cop 1 being somewhat more effective than MBP. It induced a 52% reduction in disease incidence and a 57% inhibition of disease severity as compared with PBS-fed control rats (Table 1).
Table 1.
Oral suppression of EAE in rats
| Fed Ag | Incidence | Mean max. score | Mean onset, days | Suppression of EAE, %
|
|
|---|---|---|---|---|---|
| Incidence | Score | ||||
| PBS, control | 7/9 | 1.05 ± 0.7 | 11.2 | ||
| Cop 1, 0.5 mg | 5/9 | 0.61 ± 0.6 | 11.6 | 29 | 42 |
| Cop 1, 1 mg | 3/9 | 0.33 ± 0.55 (P = 0.01) | 13.0 | 57 | 69 |
| Cop 1, 2 mg | 4/9 | 0.44 ± 0.5 | 11.0 | 43 | 58 |
| PBS, control | 27/28 | 1.8 ± 0.5 | 11.9 | ||
| MBP, 1 mg | 10/17 (P = 0.0026) | 0.9 ± 0.5 (P < 0.00001) | 11.4 | 39 | 50 |
| Cop 1, 1 mg | 13/28 (P = 0.00005) | 0.78 ± 0.4 (P < 0.00001) | 12.6 | 52 | 57 |
Each incidence figure represents the cumulative results of 2–5 individual experiments.
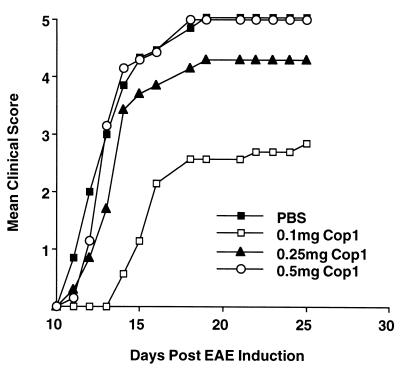
The effect of oral Cop 1 on the development of EAE in mice was tested by using a protocol reported by Al-Sabbagh et al. (26) for MBP. As shown in Fig. 1, Cop 1 given orally was found to effectively suppress EAE, the most effective dose being 100–250 μg. The results in Fig. 1 demonstrate a representative experiment in which the most effective dose was 100 μg of Cop 1, (43% suppression, P = 0.03) whereas in other experiments the most effective dose was 250 μg (data not shown). The dose of 500 μg was always completely inefficient. The ability of oral Cop 1 to suppress EAE also was tested when treatment began only after EAE induction. Cop 1 was found effective under these conditions as well, and a dose of 250 μg of Cop 1, which was the most efficient one, decreased the incidence of disease (40% reduction) and disease severity (44%) and delayed the mean onset of disease from 12.3 days in the control group to 20.8 days in the Cop 1-fed group.
Figure 1.
Oral suppression of EAE in mice by Cop 1. (SJL/JxBALB/c) F1 mice (10 per group) were fed with PBS or various doses of Cop 1 and challenged for EAE induction by injection of mouse spinal cord homogenate.
Down-Regulation of MBP-Specific Immune Responses by Oral Administration of Cop 1.
The effect of oral administration of Cop 1 on the immune response to the disease-inducing antigen MBP was evaluated in both rats and mice. We compared the proliferative response and cytokine production of spleen cells from animals receiving either PBS or Cop 1 orally and then challenged with MBP.
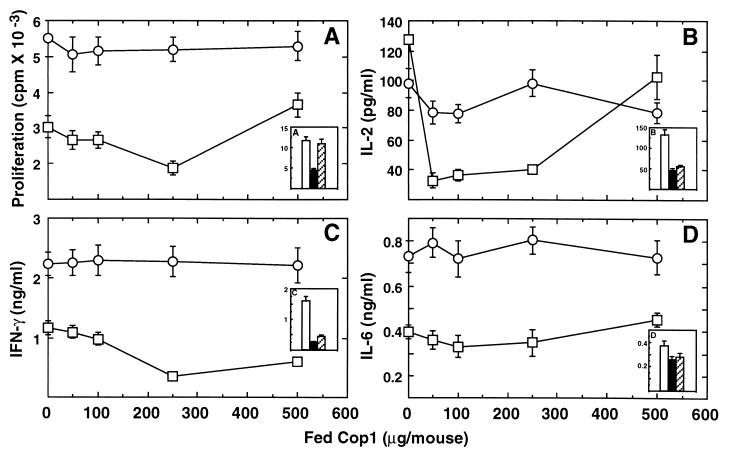
Splenocytes from the control PBS-treated rats proliferated in response to MBP and secreted high levels of IFN-γ, but not IL-2 or the Th2 cytokines IL-4 and IL-10. Both proliferation and IFN-γ secretion in response to MBP were markedly inhibited in spleen cells from Cop 1-fed rats, i.e., 54% inhibition of proliferation and 88% inhibition of IFN-γ secretion were observed (Fig. 2). Maximum effect was obtained with 1 mg of Cop 1, a dose that also was found as most effective in disease suppression (Table 1). As also shown in Fig. 2, the inhibitory effect induced by oral Cop 1 was similar to that of oral MBP, as regards both proliferation and IFN-γ secretion.
Figure 2.
Effect of antigen feeding on the immune response to MBP in rats. Rats were fed with PBS, various doses of Cop 1, or GP-MBP (1 mg/feeding), and primed with GP-MBP 2 days after the last feeding. Spleens were removed 10 days later, and cells from three animals in each group were pooled and tested for (A) proliferation and (B) IFN-γ secretion, in response to GP-MBP.
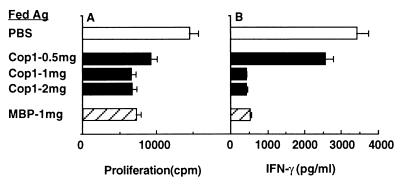
Similar studies also were conducted in mice that were fed with either PBS or various doses of Cop 1. Control mice fed with PBS proliferated in response to the encephalitogenic epitope MBP p82–100 and secreted IL-2, IFN-γ, and IL-6, whereas antigen-specific production of IL-4 or IL-10 could not be detected. In mice fed with Cop 1, both the proliferation and the secretion of the Th1 cytokines IL-2 and IFN-γ were considerably reduced, whereas IL-6 secretion was only slightly affected (Fig. 3). Thus 38% inhibition of proliferation, 74% inhibition of IL-2 secretion, 70% inhibition of IFN-γ secretion, and only 16% inhibition of IL-6 secretion were observed. The inhibition of MBP-specific responses by oral Cop 1 was a dose-dependent bell-shaped curve and consistent with the dose response inhibition of clinical manifestations of EAE. The down-regulation of the immune response to MBP was antigen specific, because in the Cop 1-fed mice no inhibition was observed in the response to purified protein derivative, an antigen that is included in the encephalitogenic inoculum, compared with control mice (Fig. 3). Mice fed with MBP demonstrated reduced proliferation and cytokine secretion in response to MBP p82–100 (Fig. 3, Insets). However, as shown, Cop 1 was more effective in suppressing the proliferative response and at least as effective in inhibiting the antigen-specific cytokine secretion.
Figure 3.
Suppression of antigen-specific responses in mice by Cop 1 feeding. (SJL/JxBALB/c)F1 mice were fed with PBS various doses of Cop 1 or MBP and primed with MBP p82–100. Ten days later, spleens were removed, and cells of three animals in each group were pooled and tested for (A) proliferation, (B) IL-2 secretion, (C) IFN-γ secretion, and (D) IL-6 secretion, in response to (□) MBP p82–100 and (○) purified protein derivative. (Insets) Feeding with PBS (empty columns), Cop 1 (250 μg) (filled columns), and MBP (250 μg) (striped columns).
Adoptive Transfer of Protection Against EAE.
To understand the mechanism involved in the suppression of EAE after oral administration of Cop 1, we tested whether this unresponsiveness could be adoptively transferred by spleen cells of Cop 1-fed donors or by Cop 1-specific T cell lines established from such splenocytes. The results summarized in Table 2 demonstrate that Con A-activated splencoytes from rats fed with BSA failed to transfer protection against EAE. On the other hand, Con A-stimulated spleen cells from Cop 1-fed donors transferred into naive recipient rats inhibited EAE manifestations as evidenced in two independent experiments by reduction of both disease incidence and clinical score (Table 2). The less effective suppression obtained in experiment 2 may be caused by either fewer cells that were transferred or to the longer interval between feeding and cell transfer. These results, however, clearly indicate that oral administration of Cop 1 induced regulatory cells that could adoptively transfer resistance to EAE induction.
Table 2.
Adoptive transfer of protection against EAE in rats by splenocytes from Cop 1-fed rats
| Pretreatment | EAE in recipients
|
||
|---|---|---|---|
| Incidence | Mean max. score | Mean onset | |
| Experiment 1 | |||
| None | 4/5 | 1.0 ± 0.7 | 12.7 |
| 200 × 106 SPC from BSA-fed donors | 3/5 | 0.8 ± 0.8 | 13.5 |
| 200 × 106 SPC from Cop 1-fed donors | 0/5 | 0.0 ± 0. (P = 0.006) | |
| Experiment 2 | |||
| None | 6/6 | 2.3 ± 0.8 | 11.7 |
| 100 × 106 SPC from BSA-fed donors | 5/6 | 2.2 ± 1.2 | 12.3 |
| 100 × 106 SPC from Cop 1-fed donors | 2/6 | 0.5 ± 0.8 (P = 0.001) | 13.0 |
In experiment 1 spleen cells were taken 2 days after last feeding and in experiment 2 after 16 days.
SPC, spleen cells.
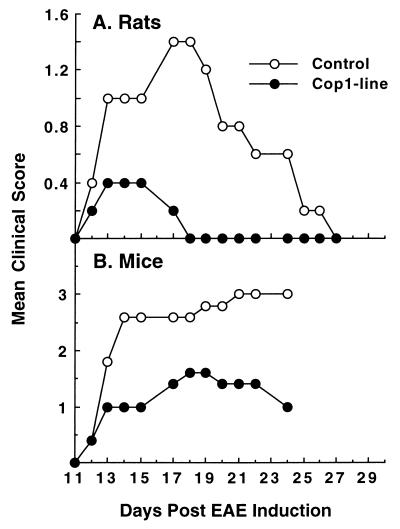
We further addressed the issue of the type of cells involved in Cop 1-induced oral tolerance, by using Cop 1-specific T cell lines obtained from spleens of Cop 1-fed rats and mice, by repeated in vitro stimulations with Cop 1. The ability of these T cell lines to prevent EAE in vivo was followed. The results illustrated in Fig. 4 demonstrate that the disease was considerably inhibited in the recipient animals, as reflected in the clinical score, i.e., 44% inhibition in mice and 88% inhibition in rats. Thus, both the rat and murine Cop 1-specific T cell lines derived from splenocytes of Cop 1-fed donors are Ts cells capable of transferring the orally induced unresponsiveness to EAE.
Figure 4.
Inhibition of EAE by T cell lines derived from Cop 1-fed donors. Activated Ts cell lines derived from spleens of Cop 1-fed donors were injected 3 days after stimulation with Cop 1 to naive (A) rats (20 × 106/rat i.p.) and (B) to mice (15 × 106/mouse) i.v. Recipient animals then were challenged for EAE induction.
Characterization of Ts Cell Lines from Cop 1 Oral Tolerized Animals.
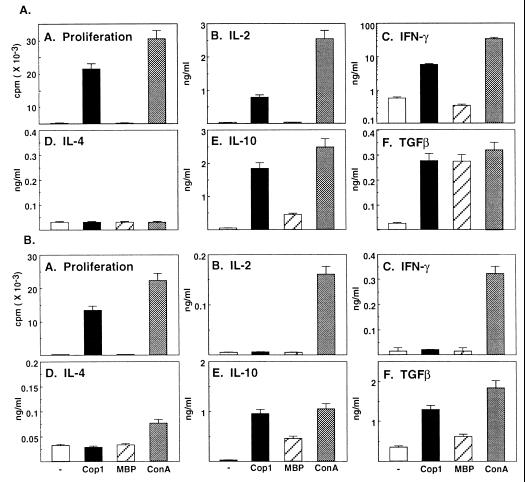
To characterize the Ts-Cop 1 lines, we studied their proliferation response and the pattern of cytokine secretion in response to Cop 1 and the autoantigen MBP. The detailed proliferation and cytokine secretion profile of the Ts line derived from rats is depicted in Fig. 5A. The line proliferated in response to Cop 1 and secreted some IL-2, IFN-γ, and also IL-10 and TGFβ but not IL-4. A response to MBP could not be observed in the proliferation assay, neither by secretion of the Th1 cytokines IL-2 or IFN-γ. On the other hand, some IL-10 and large amounts of TGFβ were secreted in response to MBP, similar to the secretion induced by Cop 1 and Con A. A similar pattern of response also was demonstrated with the Ts derived from Cop 1-fed mice (Fig. 5B). The line proliferated in response to Cop 1, but Cop 1 could not induce either IL-2 or IFN-γ secretion. It induced, however, IL-10 and TGFβ, but not IL-4. MBP did not induce proliferation or secretion of Th1 cytokines (i.e., IL-2 and IFN-γ) and, as in the case of the rat T cell line, MBP triggered the secretion of IL-10 and TGFβ. Thus, considerable crossreaction between Cop 1 and MBP at the level of these anti-inflammatory cytokines IL-10 and TGFβ was demonstrated with both the rat and murine Ts lines.
Figure 5.
Proliferation and cytokine secretion profile of rat (A) and murine (B) Cop 1-specific Ts cell lines. Cells were cultured with no antigen, Cop 1 (50 μg/ml), GP-MBP (100 μg/ml), and Con A (5 μg/ml). (A) Proliferation and cytokine secretion, (B) IL-2, (C) IFN-γ, (D) IL-4, (E) IL-10, and (F) TGFβ were measured.
DISCUSSION
Feeding autoantigens was shown to down-regulate autoimmune responses in a variety of autoimmune diseases. In the EAE system, several studies have demonstrated the effectiveness of orally administered myelin antigens (MBP and PLP) in suppressing disease in both rat and mouse models (12). Recently there has been a renewed interest in the potential of this method for modulating human autoimmune diseases, including MS (12). In the present study, we addressed the question of whether Cop 1, which had been found effective in suppressing EAE and MS when injected parenterally, is also effective when given orally. The results presented in this paper demonstrate that feeding both rats and mice with Cop 1 effectively inhibited subsequent EAE induction (Table 1, Fig. 1). Furthermore, oral Cop 1 was more effective than oral MBP in suppressing EAE in rats (Table 1). The activity of Cop 1 in both mice and rats is dose dependent, and exceeding the effective dose in mice resulted in inefficient suppression of EAE. It is interesting that the optimal dose for inducing oral tolerance by Cop 1 in both species was very similar to that reported previously for MBP (25, 26). The effective dose found is consistent with low dose tolerance. Whether high dose tolerance also could be induced by Cop 1 is an open question.
The beneficial effect of oral Cop 1 was found to be paralleled with specific down-regulation of T cell immune responses to MBP as demonstrated by the inhibition of the ex vivo proliferative and Th1 cytokine secretion responses of spleen cells from Cop 1-fed mice and rats (Figs. 2 and 3). The response of these lymphocytes to purified protein derivative, which is one of the components in the EAE inducing inoculum, was not affected, indicating that feeding with Cop 1 resulted in an antigen-specific tolerance to MBP. Similar inhibition of Th1 responses also was obtained with MBP. Cop 1, however, was somewhat superior to MBP in down-regulating the immune response to MBP in both rats and mice (Figs. 2 and 3). Similar results recently were reported by Maron et al. (27) in MBP T cell receptor-transgenic mice, where Cop 1 feeding resulted in inhibition of EAE and decreased proliferation and IL-2, IL-6, and IFN-γ production. Interestingly, in this system, too, Cop 1 was more efficient than MBP in inducing oral tolerance. It is noteworthy that there is a good correlation between the dose-response range for disease suppression and inhibition of the immune response to MBP by Cop 1, indicating that the two activities of Cop 1 are intimately associated.
The inhibition of EAE and the specific immune response to MBP by oral Cop 1 can result from different mechanisms, such as induction of anergy, deletion state, or regulatory cells. To study which of the above mechanisms is involved, we tested whether resistance to EAE induced by oral Cop 1 could be adoptively transferred by spleen cells of Cop 1-fed donors and by Cop 1-specific T cell lines originating from these spleen cells. Our results (Table 2, Fig. 4) indicate that regulatory T cells that can suppress EAE are induced by Cop 1 feeding, in accordance with the finding that low doses of antigen favor the generation of regulatory cells (12). The regulatory cells induced by Cop 1 were demonstrated to be of the Th2/3 type as revealed by the cytokine secretion profile of Cop 1-specific Ts cell lines isolated from spleens of Cop 1-fed donors (Fig. 5). Both the rat and the murine T cell lines secreted large amounts of IL-10 and TGFβ, but not IL-4, in response to Cop 1. Although the rat line also secreted some Th1 cytokines (IL-2 and IFN-γ) in response to Cop 1, the murine line was confined to the secretion of IL-10 and TGFβ. Both lines also could be stimulated by MBP to secrete those anti-inflammatory cytokines, but did not proliferate or secrete Th1 cytokines. These results indicate that feeding with Cop 1 induces regulatory cells that can suppress not only MBP-induced responses but also other encephalitogenic responses (e.g., to PLP and myelin oligodendrocyte glycoprotein) involved in EAE and MS, by bystander suppression, a phenomenon demonstrated by regulatory cells induced by MBP feeding (9). Indeed, we have demonstrated in mice that both Cop 1 feeding and injection of Cop 1-specific Ts cell lines originating from Cop 1-fed mice were capable of suppressing EAE induced by whole spinal cord homogenate that contains several autoantigens, suggesting that bystander suppression actually occurs in vivo.
We previously have demonstrated that similar Cop 1-specific Th2 type Ts cells are induced by parenteral administration of Cop 1. These Ts cells crossreacted with MBP at the level of Th2 cytokine secretion and mediated in vitro and in vivo bystander suppression of encephalitogenic responses (21, 22). A major difference, however, between these Ts cells and those induced by Cop 1 feeding is in the IL-4 secretion activity. Whereas the latter cells secreted almost no IL-4 while secreting IL-10 and TGFβ, the Cop 1 Ts cells induced by s.c. injection secreted large amounts of IL-4 and the crossreactivity with MBP was most prominent at the level of IL-4 secretion. It thus can be concluded that Cop 1 induces Th2/3 type responses irrespective of the route of administration; however, the spectrum of the cytokines they secrete depends on the site of their induction. Indeed, it was demonstrated that gut-associated lymphoid tissue favors the induction of unique T cells (termed Th3) secreting primarily TGFβ (8). On the other hand, Th2 cells induced in the periphery secrete high levels of IL-4 (28).
Mucosal (oral or nasal) tolerance induction offers several important advantages over the parenteral route, such as minimization of adverse effects and easy delivery. However, it may represent a two-edged sword (29) when an autoantigen is used, because the development of active immune response may follow mucosal processing of antigen. Thus, oral administration of MBP to neonatal animals resulted in enhanced disease expression during adulthood (30) and nasal administration of MBP enhanced clinical signs of ongoing EAE induced in DA rats (29). In this respect, the use of Cop 1, which is devoid of any encephalitogenic activity (14), yet is at least as effective as MBP in inducing oral tolerance to EAE, may be advantageous as a potential treatment of MS.
Acknowledgments
We thank Ada Wexler and Carmit Bar-Natan for their skillful technical assistance. This work was supported by a grant from TEVA Pharmaceutical Industries, Israel.
ABBREVIATIONS
- EAE
experimental autoimmune encephalomyelitis
- Cop 1
copolymer 1
- MBP
myelin basic protein
- GP-MBP
guinea pig MBP
- MS
multiple sclerosis
- PLP
proteolipid protein
- Ts
T suppressor
- TGF
transforming growth factor
Footnotes
A Commentary on this article begins on page 3333.
References
- 1.Czerkinsky C, Holmgren J. Immunologist. 1995;3:97–103. [Google Scholar]
- 2.Hafler D A, Weiner H L. Chem Immunol. 1995;60:126–149. [PubMed] [Google Scholar]
- 3.Metzler B, Wraith D C. Ann NY Acad Sci. 1995;778:228–242. doi: 10.1111/j.1749-6632.1996.tb21131.x. [DOI] [PubMed] [Google Scholar]
- 4.Al-Sabbagh A, Nelson P A, Akselband Y, Sobelm R A, Weiner H L. Cell Immunol. 1996;171:111–119. doi: 10.1006/cimm.1996.0180. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Inobe J I, Marks R, Gonnella P, Kuchroom V K, Weiner H L. Nature (London) 1995;367:177–180. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- 6.Friedman A, Weiner H L. Proc Natl Acad Sci USA. 1991;91:6688–6692. doi: 10.1073/pnas.91.14.6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitacre C C, Gienapp I E, Orosz C G, Bitar D M. J Immunol. 1991;147:2155–2163. [PubMed] [Google Scholar]
- 8.Chen Y, Kuchroo K, Inobe J-I, Hafler D A, Weiner H L. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 9.Miller A, Lider O, Weiner H L. J Exp Med. 1991;174:791–798. doi: 10.1084/jem.174.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiner H L, Macklin G A, Matsui M, Orav E J, Khoury S K, Dawson D M, Hafler D A. Science. 1993;259:1321–1324. doi: 10.1126/science.7680493. [DOI] [PubMed] [Google Scholar]
- 11.Trentham D E, Dynesius-Trentham R A, Orav E J, Combitchi D C, Lorenzo C, Sewall K L, Hafler D A, Weiner H L. Science. 1993;261:1727–1730. doi: 10.1126/science.8378772. [DOI] [PubMed] [Google Scholar]
- 12.Weiner H L. Immunol Today. 1997;18:335–343. doi: 10.1016/s0167-5699(97)01053-0. [DOI] [PubMed] [Google Scholar]
- 13.Nussenblatt R B, Gery I, Weiner H L, Ferris F L, Shibach J, Reema N, Perry C, Caspi R R, Foster C S, Whitecup S M. Am J Ophthalmol. 1997;123:583–592. doi: 10.1016/s0002-9394(14)71070-0. [DOI] [PubMed] [Google Scholar]
- 14.Teitelbaum D, Meshorer A, Hirshfeld T, Arnon R, Sela M. Eur J Immunol. 1971;1:242–248. doi: 10.1002/eji.1830010406. [DOI] [PubMed] [Google Scholar]
- 15.Teitelbaum D, Webb C, Meshorer A, Arnon R, Sela M. Eur J Immunol. 1973;3:273–279. doi: 10.1002/eji.1830030505. [DOI] [PubMed] [Google Scholar]
- 16.Teitelbaum D, Arnon R, Sela M. Cell Mol Life Sci. 1997;53:24–28. doi: 10.1007/PL00000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson K P, Brooks B R, Cohen J A, Ford C C, Goldstein J, Lisak R P, Myers L W, Panitch H S, Rose J, Schiffer W, et al. Neurology. 1995;45:1268–1276. doi: 10.1212/wnl.45.7.1268. [DOI] [PubMed] [Google Scholar]
- 18.Johnson K P, Brooks B R, Cohen J A, Ford C C, Goldstein J, Lisak R P, Myers L W, Panitch H S, Rose J W, Schiffer R B, et al. Neurology. 1998;50:701–708. doi: 10.1212/wnl.50.3.701. [DOI] [PubMed] [Google Scholar]
- 19.Fridkis-Hareli M, Teitelbaum D, Gurevich E, Pecht I, Brautbar C, Kwon O J, Brenner T, Arnon R, Sela M. Proc Natl Acad Sci USA. 1994;91:4872–4876. doi: 10.1073/pnas.91.11.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aharoni R, Teitelbaum D, Arnon R, Sela M. Proc Natl Acad Sci USA. 1999;96:634–639. doi: 10.1073/pnas.96.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aharoni R, Teitelbaum D, Sela M, Arnon R. Proc Natl Acad Sci USA. 1997;94:10821–10826. doi: 10.1073/pnas.94.20.10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aharoni R, Teitelbaum D, Sela M, Arnon R. J Neuroimmunol. 1998;91:135–146. doi: 10.1016/s0165-5728(98)00166-0. [DOI] [PubMed] [Google Scholar]
- 23.Hirshfeld H, Teitelbaum D, Arnon R, Sela M. FEBS Lett. 1970;7:317–320. doi: 10.1016/0014-5793(70)80193-4. [DOI] [PubMed] [Google Scholar]
- 24.Merrifield R B. Science. 1965;150:178–185. doi: 10.1126/science.150.3693.178. [DOI] [PubMed] [Google Scholar]
- 25.Higgins P J, Weiner H L. J Immunol. 1998;140:440–445. [PubMed] [Google Scholar]
- 26.Al-Sabbagh A, Miller A, Santos L M B, Weiner H L. Eur J Immunol. 1994;24:2104–2109. doi: 10.1002/eji.1830240926. [DOI] [PubMed] [Google Scholar]
- 27.Maron R, Slavin A, Weiner H L. J Neuroimmunol. 1998;90:82. (Abstr.). [Google Scholar]
- 28.Abbas A K, Murphy K M, Sher A. Nature (London) 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 29.Xiao B-G, Link H. Clin Immunol Immunopathol. 1997;85:119–128. doi: 10.1006/clin.1997.4432. [DOI] [PubMed] [Google Scholar]
- 30.Miller A, Lider O, Abramsky O, Weiner H L. Eur J Immunol. 1994;24:1024–1032. doi: 10.1002/eji.1830240503. [DOI] [PubMed] [Google Scholar]