Abstract
Chemotaxis, a means for motile bacteria to sense the environment and achieve directed swimming, is controlled by flagellar rotation. The primary output of the chemotaxis machinery is the phosphorylated form of the response regulator CheY (P∼CheY). The steady-state level of P∼CheY dictates the direction of rotation of the flagellar motor. The chemotaxis signal in the form of P∼CheY is terminated by the phosphatase CheZ. Efficient dephosphorylation of CheY by CheZ requires two distinct protein-protein interfaces: one involving the strongly conserved C-terminal helix of CheZ (CheZC) tethering the two proteins together and the other constituting an active site for catalytic dephosphorylation. In a previous work (J. Guhaniyogi, V. L. Robinson, and A. M. Stock, J. Mol. Biol. 359:624-645, 2006), we presented high-resolution crystal structures of CheY in complex with the CheZC peptide that revealed alternate binding modes subject to the conformational state of CheY. In this study, we report biochemical and structural data that support the alternate-binding-mode hypothesis and identify key recognition elements in the CheY-CheZC interaction. In addition, we present kinetic studies of the CheZC-associated effect on CheY phosphorylation with its physiologically relevant phosphodonor, the histidine kinase CheA. Our results indicate mechanistic differences in phosphotransfer from the kinase CheA versus that from small-molecule phosphodonors, explaining a modest twofold increase of CheY phosphorylation with the former, observed in this study, relative to a 10-fold increase previously documented with the latter.
The specific swimming behavior of a bacterial cell subject to its chemical environment is governed by the direction of flagellar rotation (2, 3, 48). The response regulator CheY is a central component of bacterial chemotaxis. Along with its cognate histidine kinase, CheA, CheY constitutes the well-conserved two-component phosphotransfer pathway transducing signals from the environment via the transmembrane chemoreceptors to the flagellar motor. This signal transduction cascade regulates a steady-state level of phosphorylated CheY (P∼CheY), which dictates the direction of flagellar rotation. Phosphorylation of CheY enhances its affinity for FliM, a component of the flagellar motor, inducing clockwise flagellar rotation and tumbly behavior.
Cellular concentrations of P∼CheY are regulated by a delicate balance of incoming phosphoryl groups from the kinase CheA and outgoing phosphoryl groups owing to both CheY autodephosphorylation and dephosphorylation mediated by its phosphatase, CheZ. Efficient dephosphorylation of CheY by CheZ requires the formation of an active site for dephosphorylation and tethering of the two proteins to each other (35, 52). The latter involves interaction of P∼CheY with the strongly conserved C-terminal region of CheZ (CheZC) (4). The binding of CheZC to P∼CheY is essential for CheZ-mediated dephosphorylation (4, 35). The minimum region sufficient for binding to CheY spans the C-terminal 19 residues of CheZ (CheZC19), residues 196 to 214, bearing the sequence AGVVASQDQVDDLLDSLGF (4).
In a previous article (13) describing high-resolution crystal structures of both inactive CheY and CheY activated with the phosphoryl analog BeF3− in complex with a synthetic CheZC peptide (CheZC15), we showed that the CheZC15 peptide binds to both inactive and active CheY in alternate orientations (state-specific dual binding model) (see Fig. 1), and in the case of inactive CheY, it binds to a meta-active conformation, providing an explanation for the previously observed increase in binding affinity for the CheZC19 peptide with CheY phosphorylation (26) and the enhancement in the CheY phosphorylation rate with small-molecule phosphodonors (35). However, the extent of lattice bias on both the observed alternate binding mode and the meta-active conformation of CheY in the inactive CheY-CheZC15 structures was unclear. Here we present equilibrium binding studies with mutant CheZC peptides, a 2.6-Å crystal structure of CheY-BeF3− bound to the CheZC19 peptide with an extended N terminus relative to the one previously used (13) and a 2.7-Å crystal structure of the inactive CheY-CheZC15 complex in a crystal lattice different from the one formerly described (13). All support the conformational state-specific dual-binding-mode hypothesis unbiased by lattice conformations. In addition, our binding studies reveal the significance of the strongly conserved C-terminal Phe214 residue of CheZ as an anchor in the molecular recognition mechanism between CheY and CheZC.
FIG. 1.
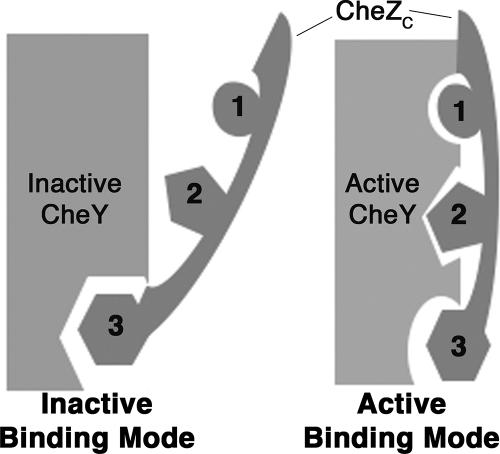
Schematic illustration of the state-specific dual-binding model. Discrete modes of CheZC peptide binding to CheY are correlated with different conformational states of CheY, i.e., inactive binding mode for binding to inactive CheY and active binding mode for binding to activated CheY. The CheZC peptide binds to the α4-β5-α5 surface of CheY in both modes. In the inactive mode, the majority of the interactions are through C-terminal Phe214 (residue no. 3; depicted as hexagons) and the N terminus is exposed to the solvent; in the active mode, the surface buried by C-terminal Phe214 is reduced while electrostatic contacts involving N-terminal residues Gln202 (residue no. 1; depicted as circles) and Asp206 (residue no. 2; depicted as pentagons) are responsible for burying the N terminus of the CheZC peptide to the α4-β5-α5 face of CheY. In the absence of CheY activation, when the CheZC peptide is bound in the inactive mode, CheY is in a meta-active state (13).
Furthermore, studies on CheZC-associated enhancement of CheY phosphorylation have thus far been limited to phosphotransfer from small-molecule phosphodonors (13, 35). Here we present kinetic studies of CheZC-associated acceleration of CheY phosphorylation from its physiologically relevant phosphodonor, phosphorylated CheA (P∼CheA). Our results suggest mechanistic differences between two sources of phosphoryl groups for CheY—small molecules and P∼CheA. Their significance in the context of bacterial chemosensory signaling is discussed.
MATERIALS AND METHODS
Peptides and oligonucleotides.
CheZC peptides and oligonucleotides are listed in Tables 1 and 2, respectively. Unless otherwise indicated to be nonacetylated with the subscript “nonac”, all peptides used in this study were acetylated at the N termini. The CheZC peptides used for crystallization were obtained from the peptide synthesis core facility of Massachusetts General Hospital (Boston, MA) and those for binding and quench flow experiments from the Louisiana State University Protein Facility (Baton Rouge, LA). The oligonucleotides used for CheA plasmid constructions were obtained from Integrated DNA Technologies (Coralville, IA).
TABLE 1.
Peptides and corresponding sequences
| Peptide | Sequencea |
|---|---|
| WT CheZC15 | Ac-ASQDQVDDLLDSLGF-OH |
| WT CheZC19 | Ac-AGVVASQDQVDDLLDSLGF-OH |
| WTnonacCheZC19 | AGVVASQDQVDDLLDSLGF-OH |
| CheZC19amide | Ac-AGVVASQDQVDDLLDSLGF-NH2 |
| CheZC19214Phe→Ala | Ac-AGVVASQDQVDDLLDSLGA-OH |
| CheZC19202Gln→Ala206 Asp→Ala | Ac-AGVVASADQVADLLDSLGF-OH |
N-terminal acetyl groups are denoted by “Ac” and C-terminal carboxylate and amide groups by “OH” and “NH2,” respectively.
TABLE 2.
Primers and sequences
| Construct | Primer | Sequence |
|---|---|---|
| CheAFL, CheAΔP2 | NdeI_forward | GGTGATCATATGAGCATGGATATTAGC |
| CheAFL, CheAΔP2 | SalI_reverse | CTTCTCGTCGACTCAGGCGGCTGTGATCGC |
| CheAΔP2 | BamHI_reverse | TGGGGATCCTGCTGCTGGTGCTGGCTGGATCGCCGCGCTTAG |
| CheAΔP2 | BamHI_forward | GCAGGATCCCCACCACGAGCGTCAGCGGAACATCATGCGGGG |
| CheAΔP2 | Linker_forward | TCGACGGATCCTCTAGA |
| CheAΔP2 | Linker_reverse | AGCTTCTAGAGGATCCG |
Lyophilized peptides were initially dissolved in a minimal amount of 1 M triethyl ammonium acetate (TEAA) buffer (90358; Fluka), followed by dilution in water to a final concentration of 20 mM. The peptide solutions, subsequently stored as lyophilized aliquots at −80°C, were dissolved in respective buffers to yield 20 mM working stock solutions and subjected to centrifugation before each use. The peptide content for peptides used in the binding experiments was determined by amino acid analyses by the LSU protein facility (Baton Rouge, LA), and peptide stability was assessed by UV absorbance at 220 nm on a Nanodrop ND-1000 spectrophotometer before each use.
Chemicals.
The ammonium salt of the small-molecule phosphodonor phosphoramidate was synthesized as previously described (36). [γ-32P]ATP with a specific activity of 5,000 Ci per mmol was purchased from GE Healthcare. All other reagent-grade chemicals used in the experiments were obtained from standard commercial sources.
Proteins.
A pUC12-based Salmonella enterica CheY expression vector, pME124 (27, 43), was used for CheY expression. The S. enterica cheA gene (accession code J03611) (41) from the T7 expression vector pJES307 (46) (pAS2) was subcloned using a double-stranded-DNA linker (Table 2) into the NdeI-HindIII site, replacing the cheR gene within the CheR expression plasmid, pME43 (38), to create the CheAFL expression plasmid pJG4. The S. enterica CheAΔP2 construct was created by removing the P2 coding region (residues 150 to 263) and joining the flanking linker regions with a proline-rich linker having the sequence PAPAAGSPPRASA, similar but not identical to the previously described Escherichia coli CheAΔP2 construct (40). The cheAΔP2 coding region was first cloned into the T7 expression vector, pJES307 (46), to create the CheAΔP2 expression vector pJG2 using the BamHI_forward and BamHI_reverse primers (Table 2) encoding the 13-residue flexible linker and then cloned into the NdeI-HindIII site of pME43 to create the CheAΔP2 expression plasmid pJG3.
Protein expression and purification.
Expression plasmids for CheY, CheAFL, and CheAΔP2 were transformed into HB101 cells. All proteins were purified with fast-performance liquid chromatography using an AKTA system (GE Healthcare), using columns from GE Healthcare. The CheY protein was purified by a modification of previously described procedures (42). The previously used ion exchange and gel filtration columns were replaced with a HiTrap Q Fast Flow 26/20 column and a Superdex 75 26/60 column, respectively. Both CheA proteins were purified by a series of DEAE-Sepharose 26/20, CHT-II 26/20, butyl Sepharose, and Superdex 200 26/60 columns. The purified proteins were quantitated by measuring UV absorbance at 280 nm using extinction coefficients (ɛ280) of 0.493, 0.220, and 0.258 ml mg−1 cm−1 for CheY, CheAFL, and CheAΔP2, respectively, calculated from the amino acid composition (12).
Crystallization.
The crystallization protocol using the hanging drop method was followed as previously described (13). Crystallization of the CheY-BeF3−-CheZC19 complex was achieved with 1.0 mM CheY and 1.5 mM CheZC19 in 50 mM HEPES (pH 7.0) (buffer A) with 10 mM MgCl2, 5 mM BeCl2, and 30 mM NaF and with reservoir solutions containing 0.1 M ammonium thiocyanate and 37.5% PEG-8000 in 0.1 M 2-(N-morpholino)-ethanesulfonic acid (MES) (pH 6.0). The CheY-CheZC15 crystals in this study were generated using CheY and the CheZC15 peptide in buffer A at 1:1.5 molar ratios with CheY concentrations ranging between 0.1 and 1.0 M and the peptide concentrations between 0.15 and 1.5 M, respectively, under identical precipitant conditions. Cryoprotection was achieved as previously described (13). NaF and BeCl2 were included in the cryosolution in the case of the BeF3−-bound crystal.
Data collection and processing.
In the case of CheY-BeF3−-CheZC19, 200° of data with 1° width were collected to 2.6 Å at beamline X4C, and in the case of CheY-CheZC15, 360° of data with 1° width were collected to 2.7 Å, at beamline X4A at the National Synchrotron Light Source at Brookhaven National Laboratory, Upton, NY. Reflections were indexed and integrated using the DENZO software program (29), and the processed images were scaled using the SCALEPACK software program (29). Data collection and processing statistics are listed in Table 3. Space groups were determined using a combination of SCALEPACK statistics, output from the Matthews probability calculator server (http://www.ruppweb.org/mattprob/) (14, 24), and inspection of systematic absences along the screw axes using the HKLVIEW program (8). For the CheY-BeF3−-CheZC19 and CheY-CheZC15 structures, the data processed in the P21212 and P1 space groups with three and four molecules per asymmetric unit with a Matthews coefficient of 2.4 Å3/Da and 2.1 Å3/Da, yielding a solvent content of 49% and 41.5%, respectively.
TABLE 3.
Data collection and refinement statistics
| Parameter | Value for data or structurea
|
|
|---|---|---|
| CheY-CheZC15 | CheY-CheZC19 | |
| Data collection | ||
| Wavelength | 0.97931 | 0.9795 |
| Lattice | P1 | P21212 |
| Cell | ||
| a, b, c (Å) | 34.8, 53.7, 65.6 | 65.1, 163.0, 37.3 |
| α, β, γ (°) | 90.2, 102.9, 90.2 | 90.0, 90.0, 90.0 |
| Resolution (Å) | 30.0-2.7 (2.8-2.7) | 30.0-2.6 (2.7-2.6) |
| Rsymb | 0.061 (0.508) | 0.137 (0.350) |
| Completeness (%) | 98.8 (98.4) | 99.5 (99.2) |
| Redundancy | 3.9 (3.9) | 8.4 (8.6) |
| I/σI | 22.4 (2.8) | 15.4 (6.4) |
| Refinement | ||
| Resolution (Å) | 30.0-2.7 | 30.0-2.6 |
| Rworkc | 0.205 | 0.213 |
| Rfreed | 0.285 | 0.274 |
| No. of reflections | 11,332 | 12,004 |
| No. of protein atoms | 2039 | 3308 |
| No. of small molecules | ||
| Magnesium ion | 2 | 3 |
| BeF3− | 0 | 3 |
| MES | 0 | 1 |
| Water | 16 | 54 |
| rmsd values from ideality | ||
| Bond lengths (Å) | 0.012 | 0.014 |
| Bond angles (°) | 1.033 | 1.124 |
| Avg B value for all atoms (Å2) | 33.6 | 22.2 |
Values corresponding to highest-resolution shells are shown in parentheses.
Rsym, (Σ|Iobs − Iavg|)/(ΣIavg), where Iobs is the observed integrated intensity and Iavg is the average integrated intensity from multiple measurements.
Rwork, (Σ‖Fobs|(hkl) − |Fcalc|(hkl)|)/(Σ|Fobs|(hkl)), where Fobs and Fcalc are the observed and calculated structure factor amplitudes for hkl indices, respectively.
Rfree is identical to Rwork but is calculated from 10% of the reflections set aside as a disjoint set prior to refinement.
Structure determination and refinement.
Both the CheY-BeF3−-CheZC19 and CheY-CheZC15 structures were solved using the molecular replacement program Phaser (33, 44), using as a search molecule a polyalanine model of the CheY-BeF3− crystal structure (PDB identifier [ID] 1FQW) lacking the β4-α4 loop (residues 88 to 92) (19). Subsequent steps in structure refinement were performed using the CCP4 4.2.2 program suite (8). The intensities obtained from SCALEPACK were converted to structure factor amplitudes using the software program TRUNCATE (11), followed by a rigid-body refinement, density modification with solvent flipping, histogram matching, and noncrystallographic symmetry averaging using the software program Refmac 5.1.24 (28). Subsequently, iterative cycles of maximum likelihood and isotropic temperature factor refinement and model building using the software program O were performed until convergence. Water molecules were initially modeled using the ARP-wARP routine (18), and subsequently only waters and other small molecules with Fourier difference peaks greater than 3σ were included in the final models. The translation/libration/screw motion determination protocol using the TLSMD server (http://skuld.bmsc.washington.edu/∼tlsmd/) (30, 31) was used to determine the optimal number of translation/libration/screw groups and generate parameters that were used in the final stages of refinement in the software program Refmac 5.1.2.4 (28). Initial models for MES were obtained from the Hic-up server (http://xray.bmc.uu.se/hicup/) (17). Refinement statistics are listed in Table 3. In the case of CheY-CheZC19, the three CheY molecules in each asymmetric unit have low main-chain root mean square deviation (rmsd) values ranging between 0.32 Å and 0.39 Å, whereas the three CheZC19 molecules differ in the degree of disorder in the solvent-exposed terminal regions, limiting the inclusion of terminal residues in the final model. In the case of CheY-CheZC15, two CheY chains have bound Mg2+ and two have bound water molecules at the active sites in spite of the absence of MgCl2 from the crystallization solutions. The two CheY chains with bound Mg2+ differ from the latter by having main-chain rmsd values ranging between 0.55 Å and 0.57 Å.
Structural analyses.
Least-square superpositions of atomic models and estimates of differences in tertiary structure by δ3-rmsd analyses were performed as previously described (13, 15). Peptide-CheY contact analyses were performed using the software programs CNS 1.1 (6) and iMOLTALK version 2.0 (10) and the protein-protein interaction server (http://www.biochem.ucl.ac.uk/bsm/PP/server/). The lattice contact analyses were performed using the WHATIF web interface (http://swift.cmbi.kun.nl/WIWWWI/) (47). All structural figures were generated using the Pymol software program (9).
Equilibrium binding analyses.
Dissociation constants (Kd values) for CheY-CheZC binding were measured by equilibrium binding analyses, following the change in CheY intrinsic fluorescence in the conserved Trp58 adjacent to the active site Asp57 (23). Fluorescence measurements were performed with a CheY sample in a 2-ml reaction buffer containing 0.1 M HEPES and 10 mM MgCl2 (pH 7.0) (buffer B) in a 10- by 10- by 40-mm quartz cuvette with continuous stirring on a Fluoromax-2 spectrofluorometer (Jobin Yvon; Spex Instruments S.A., Inc.) with an excitation wavelength at 280 nm. For each set, 5-μl aliquots of a peptide stock in buffer B were added to the CheY sample using the PB-600 repeating dispenser attached to a 250-μl Hamilton syringe (Hamilton Company) and stirred for 1 min before fluorescence measurement. Optimal fits subject to individual binding affinities were obtained using different concentrations of CheY and the peptide stock, different emission wavelengths (340 to 410 nm), and different excitation and emission slit widths (0.6 to 2.0 nm), ensuring a linear range for all measured intensities. Each set was repeated three times, and the average values for the dissociation constants and the respective standard errors are reported (Table 4). Fluorescence measurements with P∼CheY were performed as previously described using phosphoramidate as the phosphodonor (23, 26). The effect of increased ionic strength due to phosphoramidate on peptide binding was examined with the CheZC19amide peptide in the presence and absence of 0.1 M phosphoramidate in a buffer containing 0.1 M HEPES (pH 7.0)-0.1 mM EDTA, without Mg2+, a condition under which CheY phosphorylation does not occur. The Kd values obtained after fitting the data, as described below, were within 1 standard deviation (∼7%), although the absolute fluorescence change is significantly greater in the presence of 0.1 M phosphoramidate (data not shown).
TABLE 4.
Binding analyses
| Peptide |
Kd (μM)a
|
|
|---|---|---|
| Inactive CheY | P∼CheY | |
| WTnonacCheZC19 | 206.9 ± 15.4 | 15.1 ± 0.1 |
| WT CheZC19 | 67.4 ± 4.5 | 2.2 ± 0.2 |
| CheZC19202Q→A,206D→A | 110.0 ± 9.2 | 19.5 ± 0.6 |
| CheZC19amide | 156.3 ± 12.5 | 7.9 ± 0.1 |
| CheZC19214F→A | 471.9 ± 26.0 | 53.2 ± 0.9 |
| WT CheZC15 | 64.7 ± 3.1 | 13.2 ± 2.1 |
Standard errors from triplicate experiments are listed.
The data were treated as in a previous bimolecular binding study (20). The fluorescence values were corrected for sample dilution according to equation 1,
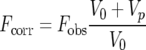 |
(1) |
where Fcorr is the corrected fluorescence, Fobs is the observed fluorescence, V0 is the initial sample volume, and Vp is the cumulative volume of the peptide added. The fluorescence intensities from the peptide samples by themselves were not significant. The corrected fluorescence intensities were plotted against the total peptide concentration and were fitted to equations 2 and 3,
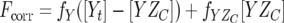 |
(2) |
where fY is the fluorescence coefficient of free CheY, 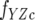 is the fluorescence coefficient of CheY bound to the CheZC peptide, [Yt] is the total CheY protein concentration, and [YZC] is the concentration of CheY bound to the CheZC peptide, which can be further defined as
is the fluorescence coefficient of CheY bound to the CheZC peptide, [Yt] is the total CheY protein concentration, and [YZC] is the concentration of CheY bound to the CheZC peptide, which can be further defined as
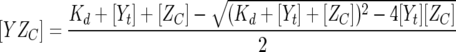 |
(3) |
where Kd is the dissociation constant and [ZC] is the total CheZC peptide concentration. The data were analyzed using the Sigmaplot 8.0 software program (Systat Software, Inc.) with fY, 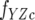 , and Kd treated as fittable parameters.
, and Kd treated as fittable parameters.
Phosphorylation of CheA proteins.
The purified CheAFL and CheAΔP2 proteins, at final concentrations of 5 μM, were phosphorylated with 1 mM [γ-32P]ATP with a specific activity of ∼2,000 cpm/pmol of phosphoryl groups at 20°C for 20 min in a buffer containing 50 mM Tris (pH 7.5), 50 mM KCl, 5 mM MgCl2, and 0.5 mM dithiothreitol (buffer C) with 0.5 mM EDTA. Phosphorylated proteins were then purified as the void volume eluent on EconoPak 10DG prepacked disposable columns (Bio-Rad), preequilibrated with buffer C. The concentration of phosphorylated protein was determined by filter binding assays using nitrocellulose paper squares following four consecutive washes under highly alkaline conditions with 50 mM Na2CO3 to maintain the acid-labile phosphohistidine bonds. From the specific activity and protein concentration, it is estimated that under these conditions, ∼90% of CheA is phosphorylated. Purified proteins were stored at −20°C for up to a week prior to use.
CheY phosphorylation kinetics.
All CheY phosphotransfer experiments with radiolabeled P∼CheA proteins were performed using a KinTek Corporation RQF-3 rapid quench instrument with a buffer containing 50 mM Tris, 50 mM KCl, and 10 mM MgCl2 (pH 7.5) (buffer D), with temperatures maintained at 8°C for experiments with P∼CheAFL and at 25°C for experiments with P∼CheAΔP2 using a circulating water bath, by rapidly mixing 30.5 μl of 0.1 μM P∼CheAFL or P∼CheAΔP2 with 27.5 μl of CheY at concentrations ranging between 2 μM and 40 μM in the presence or absence of 2 mM CheZC15 peptide. At predetermined time points ranging from 5 to 200 ms, achieved by using a variable reaction loop volume, each reaction was terminated with ∼96.9 μl of 0.1 M EDTA in 50 mM Tris (pH 7.5) (quench buffer) and expelled into a collection tube containing 51.5 μl of 10% sodium dodecyl sulfate in bromophenol blue solution (loading buffer) with no further second delay time.
A 12-μl aliquot of each sample was loaded onto a 26-well 18% Tris-HCl Criterion gel (Bio-Rad) and run in duplicate for 45 min in a Criterion cell (Bio-Rad) at 170 V at 4°C. Complete time courses were analyzed on the same gel. Gels were immediately blotted with Kimwipes, wrapped in plastic wrap, exposed to a PhosphorImager screen (Molecular Dynamics) for 12 h, and scanned using a PhosphorImager scanner (Molecular Dynamics). The band intensity (arbitrary units) was determined using ImageQuant software. Each reaction was performed in two independent experiments, and within each experiment, duplicate aliquots were analyzed on separate gels. The levels of P∼CheA in the samples relative to those at the zero time points were averaged and used for the analysis.
Observed rate constants (kobs) were generated by fitting the time courses to single exponentials by using the Sigmaplot 8.0 program (Systat Software, Inc.). The kobs values were then plotted against the final CheY concentrations and were treated according to the kinetic model as previously described (25). Under the experimental conditions, phosphotransfer from P∼CheA to CheY follows pseudo-first-order kinetics (25), displaying saturation kinetics with P∼CheAFL, described by a rectangular hyperbola, treating the first-order phosphorylation rate and the dissociation constant for the P∼CheAFL·CheY complex as fittable parameters (39), and no saturation with P∼CheAΔP2, described by a straight line, treating the second-order phosphorylation rate as a fittable parameter (40) in kobs-versus-[CheY] plots.
Protein data bank accession numbers.
The coordinates and structure factors for BeF3−-bound CheY-CheZC19 and for BeF3−-free CheY-CheZC15 have been deposited in the RCSB Protein Data Bank with the PDB IDs 2PL9 and 2PMC, respectively.
RESULTS
Equilibrium binding analyses of CheY-CheZC.
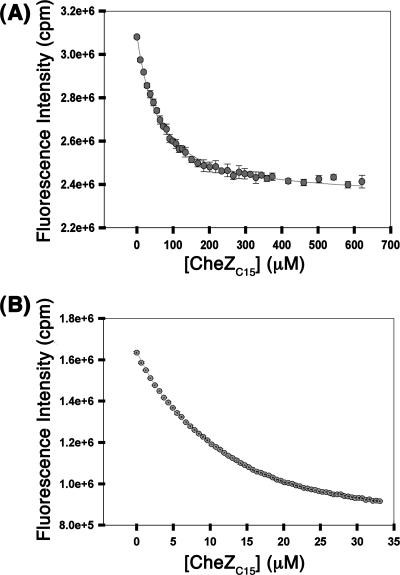
Previously reported structures of inactive and activated CheY bound to the CheZC15 peptide (13) clearly demonstrate the correlation between alternate binding modes and CheY conformation that forms the basis of the state-specific binding model, schematically depicted in Fig. 1. To test this binding model, equilibrium binding analyses were performed with inactive CheY and CheY phosphorylated with phosphoramidate (see Materials and Methods) using peptides with mutations disrupting each binding mode-specific contact (Fig. 1) revealed by the previous structural analyses (13). Representative binding curves for the wild-type (WT) CheZC15 peptide for binding inactive CheY and P∼CheY are shown in Fig. 2, and average Kd values for all peptides analyzed are reported in Table 4.
FIG. 2.
Binding of the WT CheZC19 peptide to inactive CheY (A) or CheY activated with 0.1 M phosphoramidate (B). Peptide binding was assayed by measurement of intrinsic fluorescence of CheY Trp58 using excitation and emission wavelengths of 280 nm and 346 nm, respectively. The average scaled fluorescence intensities with standard errors of replicates are shown. The data for inactive CheY were generated with 10 μM CheY using slit widths of 0.6 nm and for P∼CheY, with 2 μM CheY using slit widths of 2 nm.
Peptides used in the biochemical and structural analyses were typically acetylated at their N termini to more closely replicate the noncharged backbone of the extended polypeptide chain. However, we examined one nonacetylated peptide (WTnonacCheZC19) to allow comparison with data from a previous study (26). Dissociation constants (Kd) for the S. enterica WT CheZC19 peptide without N-terminal acetylation (WTnonacCheZC19) are similar (within twofold) to those previously reported for the E. coli system (26). Acetylation of the N terminus of the WT peptide (WT CheZC19) increases binding affinities (decreases Kd) for inactive CheY by 2.3-fold and those to P∼CheY by 6.8-fold.
The mutant peptide CheZC19 Gln202Ala Asp206Ala, in which the active binding mode-specific contacts involving residues Gln202 and Asp206 are disrupted, displayed a marginally weaker binding affinity (1.6-fold higher Kd) for inactive CheY but a significantly weaker affinity (8.9-fold higher Kd) for P∼CheY relative to results for the WT CheZC19 peptide. The CheZC19amide mutant peptide, in which the inactive binding mode-specific contact involving the C-terminal carboxyl group is disrupted, showed almost equally weak affinities for both inactive CheY and P∼CheY. The effect of the mutation of the C-terminal Phe214 residue to Ala (i.e., CheZC19 Phe214Ala) was the most drastic, with 7-fold-weaker binding to inactive CheY and 24-fold-weaker binding to P∼CheY. Additionally, binding analyses were performed with the WT CheZC15 peptide, identical to the one used in the previously reported CheY-CheZC15 structural studies, in which four residues at the N terminus are truncated relative to the WT CheZC19 peptide. N-terminal truncation had no effect on binding to inactive CheY but weakened binding to activated CheY (sixfold higher Kd) relative to results for the WT CheZC19 peptide.
Structural analysis of BeF3−-bound CheY-CheZC19.
Equilibrium binding analyses indicated that P∼CheY binds to CheZC19 with higher binding affinity than that for CheZC15 while inactive CheY binds to both peptides with similar affinities (Table 4). To explain the difference in binding affinities between the CheZC19 and CheZC15 peptides and activated CheY, crystal structures were pursued. While biochemical studies typically employ phosphoramidate as the small-molecule phosphodonor to phosphorylate CheY, the phospho-Asp thus generated is too labile for crystallographic analysis. Hence, BeF3− was used as a noncovalent phosphoryl analog to mimic the activated CheY protein in the structural studies (51). A crystal of S. enterica WT BeF3−- activated CheY in complex with a synthetic CheZC19 peptide was generated as detailed in Materials and Methods, diffracted to 2.6 Å, and found to belong to the P21212 space group. The structure was solved and refined as described in Materials and Methods. Data collection and refinement statistics are presented in Table 3.
In the CheY-BeF3−-CheZC19 structure, CheY is fully activated, similar to CheY-BeF3− (PDB ID 1FQW) (19), since a comparison of the CheY molecules in the two structures yields the following: (i) low average values of rmsd upon superposition of main-chain atoms of CheY (0.39 Å) and (ii) identical active-site geometry, similar positions of switch regions (Thr87, β4-α4 loop, and Tyr106), and a smaller magnitude of tertiary structure difference in the switch regions, as quantified by δ3-rmsd (data not shown).
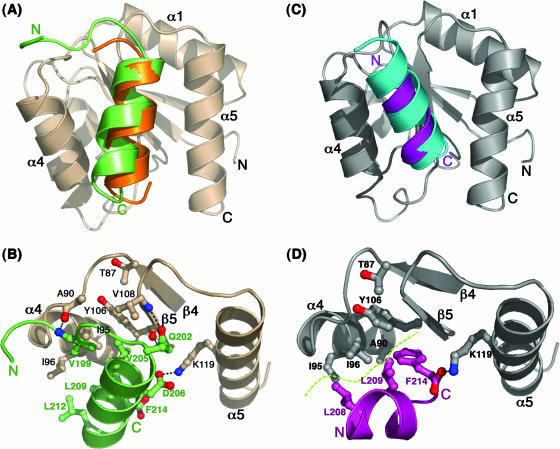
Superposition of the main-chain atoms of the CheY molecules in the previously reported CheY-BeF3−-CheZC15 (PDB ID 2FMK) (13) and CheY-BeF3−-CheZC19 structures shows high similarity except for a small deviation in the angle of the peptide helices (Fig. 3A). Contact analysis shows that the CheZC19 peptides in the two complexes exhibit the same set of H-bond and Van der Waals contacts (Fig. 3B). The position of the CheZC19 peptide does not appear to be influenced by any noncrystallographic or specific symmetry contacts. Lattice interactions involving the peptide include only three nonspecific van der Waals contacts at distances more than 3.7 Å with symmetry-related CheY molecules (data not shown). In addition to the common set of contacts characterizing the active binding mode, the CheZC19 peptide participates in three additional specific contacts (an H bond between the backbone carbonyl of CheY Ala90 and the backbone amide of CheZC Val199 and between CheY Lys92 and backbone groups of CheZC Ala196 and Gly197 and hydrophobic contacts involving Val199), as well as several nonspecific van der Waals contacts between the N-terminal extended region of the CheZC19 peptide and the CheY β4-α4 loop. These additional contacts offered by the extended N terminus in CheZC19 relative to CheZC15 account for the increased binding affinities for activated CheY and the CheZC19 and CheZC15 peptides (Table 4). These contacts are likely to exist in the full-length protein. Thus, the CheZC19 peptide is a more physiologically relevant model than the CheZC15 peptide.
FIG. 3.
Mode of CheZC binding in CheY-CheZC complexes. (A) Orientations of the CheZC helices in BeF3−-bound CheY-CheZC19 (PDB ID 2PL9) and BeF3−-bound CheY-CheZC15 (PDB ID 2FMK) structures upon superposition of CheY main-chain atoms. The CheY molecule is shown in wheat and the CheZC peptides in green and orange. (B) Specific interactions between the α4-β5-α5 region of CheY (wheat) and the CheZC19 peptide (green) in BeF3−-bound CheY-CheZC19. (C) Orientations of the CheZC helices in the BeF3−-free CheY-CheZC15 structure solved from a P1 crystal (PDB ID 2PMC) and the BeF3−-free CheY-CheZC15 structure solved from an F432 crystal (PDB ID 2FMH) upon superposition of CheY main-chain atoms. The CheY molecule is shown in gray and the CheZC peptides in purple and cyan. (D) Specific interactions between the α4-β5-α5 region of CheY (gray) and the CheZC15 peptide (purple) in BeF3−-free CheY-CheZC15 solved from a P1 crystal (2PMC). The side chains of only the contacting residues in both the CheY and CheZC peptides are shown as ball-and-stick models. The electrostatic contacts are shown as dotted lines, and the hydrophobic interface is illustrated by a dashed green curve.
Structural analysis of inactive CheY-CheZC15 in the P1 lattice.
In the previously characterized complexes of inactive CheY-CheZC15, crystallized in the F432 space group, the peptide molecules are involved in symmetry contacts through nonspecific van der Waals interactions, raising a question of the influence of lattice contacts on the observed peptide orientation (13). Crystallization and characterization of the inactive CheY-CheZC15 peptide complex in a space group different from F432 was undertaken to rule out this lattice bias. A crystal of S. enterica CheY bound to the CheZC15 peptide, diffracting to 2.7 Å with data processing in the triclinic P1 space group, was used for structure determination (Materials and Methods). Data collection and refinement statistics are presented in Table 3.
The CheZC15 peptide in the structure displays a complicated binding mode shared between two symmetry-related CheY molecules, imposed by lattice restrictions (see Fig. S1 in the supplemental material). Apparently as a consequence of this shared binding, electron density in this region was diffuse and peptide atoms refined with high B-factors, although all side chains for CheZC15 residues 206 to 214 were included in the final model. Of these, side chains for Phe214 and Asp210 were well ordered; density for the side chains of Leu208 and Leu212 was weak, and that for the remaining residues was nonexistent. However, based on the angle of the helical axis, it is clear that the CheZC15 peptide in the structure is bound in the inactive binding mode (Fig. 3C). Furthermore, conformational analyses indicated that the conformation of CheY in the structure approximates a “meta-active” state, similar to the one observed in the previously reported CheY-CheZC15 structures solved in the F432 lattice (13) (Fig. 3D; also data not shown). The highly similar peptide orientation and CheY conformation in structures of the inactive complex solved from crystals belonging to two different space groups with different lattice interactions provide structural evidence against lattice bias in the mode of interaction of the peptide with inactive CheY.
Kinetic analysis of CheY phosphorylation in the presence and absence of the CheZC15 peptide with P∼CheA.
Peptide-associated acceleration of the rate of phosphoryl transfer to CheY from small-molecule phosphodonors has been established (35). To address the effect of the CheZC peptide on CheY phosphotransfer from its physiologically relevant phosphodonor, kinase CheA, the rates of phosphotransfer from CheA to CheY in the presence and absence of the CheZC15 peptide were determined. CheY binds to the P2 domain of CheA, and binding of P2 and the CheZC peptide to CheY is mutually exclusive since the two binding interfaces on CheY overlap (53). The effect of the CheZC peptide on CheA-mediated CheY phosphorylation can be most clearly delineated by using a CheA protein lacking the P2 domain (CheAΔP2; see Materials and Methods). The in vivo relevance of this deletion construct is discussed below. A similar E. coli CheAΔP2 protein was shown to exhibit 25-fold-slower CheY phosphorylation kinetics than the full-length E. coli CheA protein (CheAFL), still orders of magnitude faster than CheY phosphotransfer kinetics reported with small-molecule phosphodonors (40).
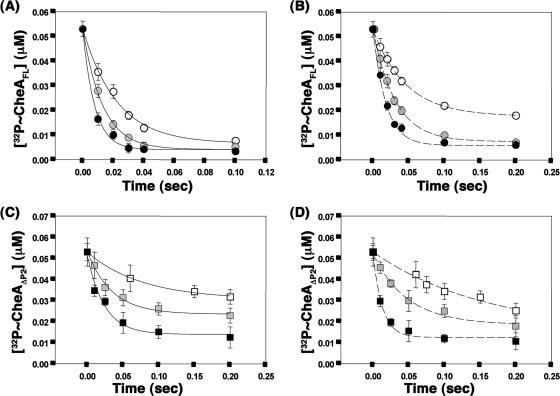
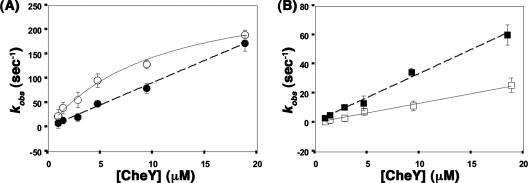
Since the E. coli CheAFL and CheAΔP2 proteins follow subsecond kinetics of phosphoryl group transfer to CheY, the effect of the presence of the CheZC peptide on CheY phosphorylation with the S. enterica CheAFL and CheAΔP2 proteins was investigated using a rapid quench instrument similar to that previously used with the E. coli proteins (39, 40). When CheA proteins phosphorylated with [γ-32P]ATP were rapidly mixed with various concentrations of CheY in the presence or absence of the CheZC15 peptide, the intensity of the radiolabeled band corresponding to P∼CheAFL (73 kDa) or P∼CheAΔP2 (64 kDa) decreased with time due to phosphotransfer while the intensity of the radiolabeled band corresponding to P∼CheY (14 kDa) increased. Due to the greater lability of phospho∼Asp in P∼CheY, the decrease in P∼CheA was used in the kinetic analyses. Observed rate constants (kobs) were obtained by fitting the relative concentration of P∼CheA over time to a single exponential (Fig. 4). First- and second-order phosphorylation rates were estimated from a titration analysis with increasing concentrations of CheY.
FIG. 4.
Single exponential fits to representative data for phosphotransfer from P∼CheA to CheY. Phosphoryl transfer from P∼CheAFL (A and B) or P∼CheAΔP2 (C and D) to CheY was assayed with 1.4 μM CheY (white), 4.7 μM CheY (gray), or 18.8 μM CheY (black) in the absence (A and C) or presence (B and D) of 2 mM CheZC15 peptide. Single exponential fits used for estimation of kobs are shown. Within each experiment, two aliquots from each time point were analyzed on separate gels, and results were averaged. The data points shown are average values from two independent experiments with the standard errors indicated.
Phosphoryl transfer from S. enterica P∼CheAFL to CheY followed saturation kinetics (Fig. 5A), as previously observed with E. coli proteins (39). Rectangular hyperbola fits to the data (Fig. 5A) defined a first-order rate constant (kphos) of 290 ± 100 s−1 and a KS value, describing the dissociation of the P∼CheA·CheY complex, of 10.3 ± 2.8 μM, compared to 250 ± 40 s−1 and 7 ± 2 μM, respectively, previously reported with E. coli proteins performed at an identical temperature (8°C) (39). The presence of the CheZC15 peptide in the phosphorylation experiments with P∼CheAFL eliminated saturation kinetics, and the calculated second-order rate constant (kphos/KS) for the CheAFL phosphotransfer reaction (28.2 μM−1 s−1) was threefold higher than the observed second-order rate constant in the presence of the CheZC15 peptide (9.3 ± 2.2 μM−1 s−1) (Fig. 5A).
FIG. 5.
Kinetic fits to kobs versus [CheY]. Estimates of the kobs value were obtained from phosphotransfer data from P∼CheAFL (A) or P∼CheAΔP2 (B) to CheY in the presence (closed symbols) or absence (open symbols) of the CheZC15 peptide. The data corresponding to phosphotransfer from P∼CheAFL to CheY in the absence of the CheZC15 peptide are fit to a rectangular hyperbola to define a second-order rate constant, kphos; the remaining data are fit linearly to define first-order rates (kphos/KS). Error bars denote standard errors of replicates.
Phosphoryl transfer from S. enterica P∼CheAΔP2 to CheY also did not follow saturation kinetics (Fig. 5B), similar to that observed for E. coli CheAΔP2 (40). Linear fits of the data defined an effective second-order rate constant (kphos/KS) of 1.3 ± 0.5 μM−1 sec−1, compared to 1.5 μM−1 sec−1 for E. coli CheAΔP2 at 25°C (40). In the presence of the CheZC15 peptide, phosphoryl transfer from P∼CheAΔP2 still did not follow saturation kinetics but was accelerated by 2.6-fold (Fig. 5B) compared to phosphorylation of peptide-free CheY. Linear fits of the data defined a second-order rate constant, kphos/KS of 3.4 ± 1.2 μM−1 s−1. The 2.6-fold acceleration of phosphoryl transfer from CheA to CheY in the presence of the CheZC15 peptide contrasts with the 10-fold acceleration of phosphoryl transfer to CheZC15-bound CheY from the small-molecule phosphodonor phosphoramidate (35).
DISCUSSION
Evidence for CheY conformation-specific alternate binding modes of CheZC.
Previous structural studies with CheZC15 peptide-bound complexes of inactive and activated CheY revealed alternate modes of binding of the CheZC peptide to CheY that are subject to the conformational state of the CheY protein (Fig. 1). The equilibrium binding studies provide strong biochemical evidence in support of the state-specific dual-binding-mode hypothesis (Table 4). While inactive CheY binds to both the CheZC19 and CheZC15 peptides with similar affinities, the binding affinity of P∼CheY is sixfold greater for CheZC19 than for CheZC15. Furthermore, the CheZC19 peptide containing the Gln202Ala and Asp206Ala mutations, which eliminate the active mode-specific contacts, shows close to 10-fold lower affinity for P∼CheY and less than 2-fold weaker binding to inactive CheY.
Structural studies of CheY-BeF3−-CheZC19 revealed additional specific contacts between the switch region in CheY and the extended N terminus in CheZC19, accounting for the lower Kd value for activated CheY binding to CheZC19 than for binding to CheZC15 (Table 4). Further, in the structures of the inactive CheY-CheZC15 complexes, the C terminus of the CheZC15 peptide accounts for a substantial portion of the CheY α4-β5-α5 binding interface while the N terminus is exposed to the solvent (13) (Fig. 3D). With the assumption that CheZC19 binds to inactive CheY using the inactive binding mode, the extended N terminus would be predicted to have little or no effect, accounting for the observed identical binding affinities for inactive CheY with both the CheZC19 and CheZC15 peptides (Table 4).
Molecular recognition by the CheZC peptide for inactive and activated CheY.
The importance of the CheZ C-terminal Phe214 residue has long been recognized, and several studies have addressed the consequences of mutations at this site. The cheZ Phe214Cys mutants in both E. coli (5) and S. enterica (37) are chemotactic, and a cheZ Phe214Leu mutant in E. coli displays a gain-of-function phenotype (34). The current study addresses the importance of this residue by explaining the mechanism of molecular recognition in the CheY-CheZC interaction. A single-site mutation to Ala (CheZC19 Phe214Ala) drastically impacts binding to both inactive and P∼CheY (Table 4). Phe214 is completely buried in the inactive CheY-CheZC15 structures, contributing to >40% of the buried surface at the interface, while it is only partially buried in the activated CheY-CheZC structures, contributing to <15% of the buried surface at the interface (13).
The structural and mutational analyses presented in this study establish the role of Phe214 as an anchor residue in the molecular recognition mechanism between CheZC and CheY. Phe214 is highly conserved as the C-terminal residue in CheZ proteins of most eubacterial species. A rare exception occurs in Xanthomonas spp., where the C-terminal residue is a Leu and residues in CheY that are predicted to form the CheY-CheZC interface also differ, consistent with the identification of this residue as a key determinant of molecular recognition. The use of Phe as an anchor is not unique to CheY-CheZC recognition in bacterial chemotaxis. The pentapeptide motif (NWETF) present at the C termini of high-copy-number E. coli methyl-accepting proteins (Tar and Tsr) also uses the C-terminal Phe to tether methyltransferase CheR for methylation (50).
Evolutionarily conserved anchor residues provide specific side chains that penetrate into a structurally constrained binding groove of the binding partner during molecular recognition between two interacting proteins (32). Such recognition motifs bury the maximum surface area after complexation. The existence of such anchors might allow binding pathways to bypass kinetically expensive structural rearrangements at the binding interface. It is postulated that once the anchors are docked, solvent-exposed flexible side chains latch onto the binding interface at the periphery of the pocket through an induced-fit mechanism.
Consistent with its role as a molecular anchor, Phe214 hooks into the hydrophobic pocket created by the solvent-buried conformation of CheY Tyr106 on its α4-β5-α5 face, irrespective of the phosphorylation or activation state of the active site Asp57, since the rotameric conformation of Tyr106 is almost identical in meta-active CheY of the inactive CheY-CheZC15 structures and in fully activated CheY-BeF3− of the CheZC15- and CheZC19-bound structures. However, in the absence of phosphorylation, the lack of complete reorientation of Thr87 and the β4-α4 loop leaves only a hydrophobic patch and the solvent-exposed Lys119 side chain for interaction at the inactive CheY binding interface, rendering the inactive binding mode with the N terminus exposed to the solvent. In contrast, in the presence of phosphorylation, complete reorientation of Thr87 and the β4-α4 loop offers additional contacts for the N terminus of the CheZC peptide, which can now latch onto the activated CheY interface using solvent-exposed side chains of Gln202 and Asp206 of CheZC. In essence, conformation-dependent differences in surface profiles of the α4-β5-α5 signaling face favor binding of the CheZC helix in different orientations.
Effect of CheZC peptide on CheY phosphorylation kinetics with P∼CheA.
The CheZC peptide-associated acceleration of CheY phosphorylation was previously reported with small-molecule phosphodonors (35). This is the first report investigating the effect of the CheZC peptide on CheY phosphorylation with the more physiologically relevant phosphodonor kinase CheA. Phosphoryl transfer from CheAFL to CheY follows saturation kinetics, as previously reported (39), indicating the formation of a P∼CheAFL·CheY complex prior to phosphotransfer. The existence of a CheY binding domain on CheAFL (P2) is the basis for the observed saturation. In the presence of the CheZC15 peptide, the rate of phosphorylation is threefold lower and saturation is eliminated, indicating loss of the binding site on CheA for CheY. Binding of CheZC and that of CheA-P2 to CheY are mutually exclusive due to steric overlap of the binding sites. With the Kd values reported to be ∼2 μM for P2-CheY binding (21) and determined to be ∼70 μM for CheZC15-CheY binding, in these experiments, ∼97% of the CheY molecules are estimated to exist as a complex with the peptide and CheA-P2 is predicted to compete with the CheZC15 peptide for binding to CheY at the final protein and peptide concentrations used, accounting for the lower rate of phosphoryl transfer. In the absence of the CheA-P2 domain (CheAΔP2), saturation kinetics is eliminated (40). The presence of the CheZC15 peptide in this case has only a modest effect (2.6-fold) on the rate of phosphoryl transfer to CheY.
It should be noted that one of the CheAFL-CheY phosphotransfer time courses in the presence of the CheZC15 peptide (Fig. 4B) and two of the CheAΔP2-CheY phosphotransfer time courses, in both the absence and presence of the CheZC15 peptide (Fig. 4C and D), reach an equilibrium rather than completion. In all of the above cases, the effective concentration of CheY available to bind to CheA is below the Kd value for CheA-CheY interaction. It is also possible that the rate of reverse phosphotransfer from P∼CheY to CheA might be affected by the CheZC peptide, although such a conclusion is beyond the scope of this study. In either case, the initial rates of phosphotransfer, and hence conclusions drawn from these observations, are not affected.
Effects of the CheZC peptide on phosphotransfer kinetics from P∼CheA to CheY are relevant within the context of a bacterial cell. Localization of key components plays an important role in chemotaxis signal transduction. Both the kinase CheA and the phosphatase CheZ localize to the cytoplasmic faces of membrane-bound chemoreceptors that cluster at cell poles (1, 7, 16, 22, 45, 49). This potentially poises CheZ to present CheY to CheA for phosphoryl transfer through the CheZC-mediated tether. Such a scenario is supported by stoichiometric quantification of chemotaxis components (22), together with estimated binding affinities, imparting an ancillary level of regulation in the form of futile cycles of phosphorylation by CheA and dephosphorylation by CheZ that might play a role in modulating sensitivity in the system (13). Whether the modest 2.6-fold enhancement of CheA-mediated phosphorylation of CheY by CheZC binding observed in vitro is itself important or whether it is further amplified in the intracellular environment is still an open question.
Supplementary Material
Acknowledgments
We thank the beamline staff at X4A and X4C at the National Synchrotron Light Source at Brookhaven National Laboratory for technical assistance during data collection, Rajiv Bandwar and Guo-Qing Tang for scientific interpretation of the kinetic data, Vasanti S. Anand and Vaishnavi Rajagopal for technical help with the rapid quench instrument, and J. Kavanaugh for the delta rmsd fortran codes.
This work was supported by U.S. National Institutes of Health grant 2R37GM47958. A.M.S. is an investigator of the Howard Hughes Medical Institute.
We have no competing financial interests.
Footnotes
Published ahead of print on 14 December 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Ames, P., C. A. Studdert, R. H. Reiser, and J. S. Parkinson. 2002. Collaborative signaling by mixed chemoreceptor teams in Escherichia coli. Proc. Natl. Acad. Sci. USA 997060-7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker, M. D., P. M. Wolanin, and J. B. Stock. 2006. Signal transduction in bacterial chemotaxis. Bioessays 289-22. [DOI] [PubMed] [Google Scholar]
- 3.Baker, M. D., P. M. Wolanin, and J. B. Stock. 2006. Systems biology of bacterial chemotaxis. Curr. Opin. Microbiol. 9187-192. [DOI] [PubMed] [Google Scholar]
- 4.Blat, Y., and M. Eisenbach. 1996. Conserved C-terminus of the phosphatase CheZ is a binding domain for the chemotactic response regulator CheY. Biochemistry 355679-5683. [DOI] [PubMed] [Google Scholar]
- 5.Blat, Y., and M. Eisenbach. 1996. Oligomerization of the phosphatase CheZ upon interaction with the phosphorylated form of CheY. J. Biol. Chem. 2711226-1231. [DOI] [PubMed] [Google Scholar]
- 6.Brünger, A. T., P. D. Adams, G. M. Clore, W. L. DeLano, P. Gros, R. W. Grosse-Kunstleve, J. S. Jiang, J. Kuszewski, M. Nilges, N. S. Pannu, R. J. Read, L. M. Rice, T. Simonson, and G. L. Warren. 1998. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54905-921. [DOI] [PubMed] [Google Scholar]
- 7.Cantwell, B. J., R. R. Draheim, R. B. Weart, C. Nguyen, R. C. Stewart, and M. D. Manson. 2003. CheZ phosphatase localizes to chemoreceptor patches via CheA-short. J. Bacteriol. 1852354-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collaborative Computational Project, Number 4. 1994. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50760-763. [DOI] [PubMed] [Google Scholar]
- 9.Delano, W. L. 2002. The Pymol molecular graphics system. DeLano Scientific, San Carlos, CA.
- 10.Diemand, A. V., and H. Scheib. 2004. iMolTalk: an interactive, internet-based protein structure analysis server. Nucleic Acids Res. 32W512-W516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.French, S., and K. Wilson. 1978. On the treatment of negative intensity observations. Acta Crystallogr. A 34517-525. [Google Scholar]
- 12.Gill, S. C., and P. H. von Hippel. 1989. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 182319-326. [DOI] [PubMed] [Google Scholar]
- 13.Guhaniyogi, J., V. L. Robinson, and A. M. Stock. 2006. Crystal structures of beryllium fluoride-free and beryllium fluoride-bound CheY in complex with the conserved C-terminal peptide of CheZ reveal dual binding modes specific to CheY conformation. J. Mol. Biol. 359624-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kantardjieff, K. A., and B. Rupp. 2003. Matthews coefficient probabilities: improved estimates for unit cell contents of proteins, DNA, and protein-nucleic acid complex crystals. Protein Sci. 121865-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kavanaugh, J. S., J. A. Weydert, P. H. Rogers, and A. Arnone. 1998. High-resolution crystal structures of human hemoglobin with mutations at tryptophan 37β: structural basis for a high-affinity T-state. Biochemistry 374358-4373. [DOI] [PubMed] [Google Scholar]
- 16.Kim, K. K., H. Yokota, and S.-H. Kim. 1999. Four-helical-bundle structure of the cytoplasmic domain of a serine chemotaxis receptor. Nature 400787-792. [DOI] [PubMed] [Google Scholar]
- 17.Kleywegt, G. J., and T. A. Jones. 1998. Databases in protein crystallography. Acta Crystallogr. D 541119-1131. [DOI] [PubMed] [Google Scholar]
- 18.Lamzin, V. S., and K. S. Wilson. 1993. Automated refinement of protein models. Acta Crystallogr. D. 49129-147. [DOI] [PubMed] [Google Scholar]
- 19.Lee, S. Y., H. S. Cho, J. G. Pelton, D. Yan, E. A. Berry, and D. E. Wemmer. 2001. Crystal structure of activated CheY. J. Biol. Chem. 27616425-16431. [DOI] [PubMed] [Google Scholar]
- 20.Levin, M. K., and S. S. Patel. 2002. Helicase from hepatitis C virus, energetics of DNA binding. J. Biol. Chem. 27729377-29385. [DOI] [PubMed] [Google Scholar]
- 21.Li, J., R. V. Swanson, M. I. Simon, and R. M. Weis. 1995. The response regulators CheB and CheY exhibit competitive binding to the kinase CheA. Biochemistry 3414626-14636. [DOI] [PubMed] [Google Scholar]
- 22.Li, M., and G. L. Hazelbauer. 2004. Cellular stoichiometry of the components of the chemotaxis signaling complex. J. Bacteriol. 1863687-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lukat, G. S., W. R. McCleary, A. M. Stock, and J. B. Stock. 1992. Phosphorylation of bacterial response regulator proteins by low molecular weight phospho-donors. Proc. Natl. Acad. Sci. USA 89718-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews, B. W. 1968. Solvent content of protein crystals. J. Mol. Biol. 33491-497. [DOI] [PubMed] [Google Scholar]
- 25.Mayover, T. L., C. J. Halkides, and R. C. Stewart. 1999. Kinetic characterization of CheY phosphorylation reactions: comparison of P-CheA and small-molecule phosphodonors. Biochemistry 382259-2271. [DOI] [PubMed] [Google Scholar]
- 26.McEvoy, M. M., A. Bren, M. Eisenbach, and F. W. Dahlquist. 1999. Identification of the binding interfaces on CheY for two of its targets, the phosphatase CheZ and the flagellar switch protein FliM. J. Mol. Biol. 2891423-1433. [DOI] [PubMed] [Google Scholar]
- 27.Messing, J. 1983. New M13 vectors for cloning. Methods Enzymol. 10120-78. [DOI] [PubMed] [Google Scholar]
- 28.Murshudov, G. N., A. A. Vagin, and E. J. Dodson. 1997. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D 53240-255. [DOI] [PubMed] [Google Scholar]
- 29.Otwinowski, Z., and W. Minor. 1997. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276307-326. [DOI] [PubMed] [Google Scholar]
- 30.Painter, J., and E. A. Merritt. 2005. A molecular viewer for the analysis of TLS rigid-body motion in macromolecules. Acta Crystallogr. D 61465-471. [DOI] [PubMed] [Google Scholar]
- 31.Painter, J., and E. A. Merritt. 2006. Optimal description of a protein structure in terms of multiple groups undergoing TLS motion. Acta Crystallogr. D 62439-450. [DOI] [PubMed] [Google Scholar]
- 32.Rajamani, D., S. Thiel, S. Vajda, and C. J. Camacho. 2004. Anchor residues in protein-protein interactions. Proc. Natl. Acad. Sci. USA 10111287-11292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossmann, M. G. 1990. The molecular replacement method. Acta Crystallogr. A 4673-82. [DOI] [PubMed] [Google Scholar]
- 34.Sanna, M. G., and M. I. Simon. 1996. In vivo and in vitro characterization of Escherichia coli protein CheZ gain- and loss-of-function mutants. J. Bacteriol. 1786275-6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuster, M., R. E. Silversmith, and R. B. Bourret. 2001. Conformational coupling in the chemotaxis response regulator CheY. Proc. Natl. Acad. Sci. USA 986003-6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheridan, R. C., J. F. McCullough, and Z. T. Wakefield. 1971. Phosphoramidic acid and its salts. Inorg. Synth. 1323-26. [Google Scholar]
- 37.Silversmith, R. E. 2005. High mobility of carboxyl-terminal region of bacterial chemotaxis phosphatase CheZ is diminished upon binding divalent cation or CheY-P substrate. Biochemistry 447768-7776. [DOI] [PubMed] [Google Scholar]
- 38.Simms, S. A., A. M. Stock, and J. B. Stock. 1987. Purification and characterization of the S-adenosylmethionine: glutamyl methyltransferase that modifies membrane chemoreceptor proteins in bacteria. J. Biol. Chem. 2628537-8543. [PubMed] [Google Scholar]
- 39.Stewart, R. C. 1997. Kinetic characterization of phosphotransfer between CheA and CheY in the bacterial chemotaxis signal transduction pathway. Biochemistry 362030-2040. [DOI] [PubMed] [Google Scholar]
- 40.Stewart, R. C., K. Jahreis, and J. S. Parkinson. 2000. Rapid phosphotransfer to CheY from a CheA protein lacking the CheY-binding domain. Biochemistry 3913157-13165. [DOI] [PubMed] [Google Scholar]
- 41.Stock, A., T. Chen, D. Welsh, and J. Stock. 1988. CheA protein, a central regulator of bacterial chemotaxis, belongs to a family of proteins that control gene expression in response to changing environmental conditions. Proc. Natl. Acad. Sci. USA 851403-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stock, A., D. E. Koshland, Jr., and J. Stock. 1985. Homologies between the Salmonella typhimurium CheY protein and proteins involved in the regulation of chemotaxis, membrane protein synthesis, and sporulation. Proc. Natl. Acad. Sci. USA 827989-7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stock, A. M., J. M. Mottonen, J. B. Stock, and C. E. Schutt. 1989. Three-dimensional structure of CheY, the response regulator of bacterial chemotaxis. Nature 337745-749. [DOI] [PubMed] [Google Scholar]
- 44.Storoni, L. C., A. J. McCoy, and R. J. Read. 2004. Likelihood-enhanced fast rotation functions. Acta Crystallogr. D 60432-438. [DOI] [PubMed] [Google Scholar]
- 45.Studdert, C. A., and J. S. Parkinson. 2004. Crosslinking snapshots of bacterial chemoreceptor squads. Proc. Natl. Acad. Sci. USA 1012117-2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tabor, S., and C. C. Richardson. 1985. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc. Natl. Acad. Sci. USA 841074-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vriend, G. 1990. WHAT IF: a molecular modeling and drug design program. J. Mol. Graph. 852-56, 29. [DOI] [PubMed] [Google Scholar]
- 48.Wadhams, G. H., and J. P. Armitage. 2004. Making sense of it all: bacterial chemotaxis. Nat. Rev. Mol. Cell Biol. 51024-1037. [DOI] [PubMed] [Google Scholar]
- 49.Wang, H., and P. Matsumura. 1996. Characterization of the CheAs/CheZ complex: a specific interaction resulting in enhanced dephosphorylating activity on CheY-phosphate. Mol. Microbiol. 19695-703. [DOI] [PubMed] [Google Scholar]
- 50.Wu, J., J. Li, G. Li, D. G. Long, and R. M. Weis. 1996. The receptor binding site for the methyltransferase of bacterial chemotaxis is distinct from the sites of methylation. Biochemistry 354984-4993. [DOI] [PubMed] [Google Scholar]
- 51.Yan, D., H. S. Cho, C. A. Hastings, M. M. Igo, S. Y. Lee, J. G. Pelton, V. Stewart, D. E. Wemmer, and S. Kustu. 1999. Beryllofluoride mimics phosphorylation of NtrC and other bacterial response regulators. Proc. Natl. Acad. Sci. USA 9614789-14794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao, R., E. J. Collins, R. B. Bourret, and R. E. Silversmith. 2002. Structure and catalytic mechanism of the E. coli chemotaxis phosphatase CheZ. Nat. Struct. Biol. 9570-575. [DOI] [PubMed] [Google Scholar]
- 53.Zhu, X., K. Volz, and P. Matsumura. 1997. The CheZ-binding surface of CheY overlaps the CheA- and FliM-binding surfaces. J. Biol. Chem. 27223758-23764. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.