Abstract
SecA is an essential component in the Sec-dependent protein translocation pathway and, together with ATP, provides the driving force for the transport of secretory proteins across the cytoplasmic membrane of Escherichia coli. Previous studies established that SecA undergoes monomer-dimer equilibrium in solution. However, the oligomeric state of functional SecA during the protein translocation process is controversial. In this study, we provide additional evidence that SecA functions as a dimer in the membrane by (i) demonstration of the capability of the presumably monomeric SecA derivative to be cross-linked as dimers in vitro and in vivo, (ii) complementation of the growth of a secA(Ts) mutant with another nonfunctional SecA or (iii) in vivo complementation and in vitro function of a genetically tandem SecA dimer that does not dissociate into monomers, and (iv) formation of similar ring-like structures by the tandem SecA dimer and SecA in the presence of lipid bilayers. We conclude that SecA functions as a dimer in the membrane and dissociation into monomers is not necessary during protein translocation.
The Sec-dependent protein translocation pathway is responsible for the majority of protein exportation in bacteria. SecA is an essential and ubiquitous component (4, 21) and, together with ATP, provides the driving force for the transport of secretory proteins across the cytoplasmic membrane of Escherichia coli (20).
The structure of SecA in solution has been studied extensively. SecA has been previously described as a homodimer of 102-kDa subunits that undergoes monomer-dimer equilibrium in solution (1, 8, 32). However, the structure of SecA in the membrane is not well understood and the oligomeric state of functional SecA in the membrane is controversial. Or et al. (23) advocated that SecA functions as a monomer in protein translocation on the basis of evidence that acidic phospholipids and a synthetic signal peptide induce dissociation of SecA dimers. Later, Benach et al. (3) reported that monomeric SecA is the transient stage of SecA as signal peptides redimerize it during preprotein translocation, concluding that functional SecA is a dimer. Similar results were also reported by Shin et al. (29), who found that signal peptides induce SecA oligomerization and consequently enhance its ability for membrane insertion. A more recent study by Alami et al. using Nanodiscs, an emerging technology, showed that the SecYEG complex triggers dissociation of the SecA dimer; however, no evidence was shown that the Nanodisc-reconstituted SecYEG monomer was active in protein translocation (2).
Or et al. (22) generated a reported monomeric SecA derivative mutant, SecAΔ11/N95, which lacks extreme terminal residues and showed in vitro and in vivo activities, suggesting that functional SecA is a monomer. Contradictory results have been reported for a similar SecA monomer, SecAΔ11, in which no in vivo or in vitro activities were detected (14), indicating that the SecA dimer is the functional unit of protein translocation (13, 14, 25, 27). Interestingly, however, a recombinant SecA protein (amino acids 9 to 861, lacking both the N and C termini) from E. coli was recently crystallized as an antiparallel dimer at 2 Å resolution, revealing that neither the N nor the C terminus, as previously reported (15), is related to dimerization (25). Or et al. (24) showed that a disulfide-cross-linked SecA dimer is inactive, suggesting that dissociation of SecA dimers into monomers is required during protein translocation. However, a similar cross-linked SecA-Cys637/801 dimer retained its in vitro activity (13). In addition, a nondissociable SecA dimer, crossed-linked at its C terminus, is functional in protein translocation in vitro, demonstrating that dissociation of the SecA dimer is not an essential step in preprotein translocation (6).
In the present work, we first determined the oligomeric state of the proposed monomeric SecAΔ11/N95 protein by various means and showed its ability to form dimers. In addition, we demonstrated that combination of two nonfunctional SecA molecules leads to activity. Furthermore, we generated genetically a nondissociable SecA tandem dimer and showed its full functionality in vivo and in vitro. Moreover, the structure of the tandem SecA dimer in the membrane is strikingly similar to that of wild-type SecA, as observed by atomic force microscopy (AFM). Together, our results further support the ideas that functional SecA is a dimer in the membrane and that dissociation into monomer form is not an essential step in vivo or in vitro for SecA to function in protein translocation.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
E. coli DH5α was used for plasmid isolation and for subcloning of DNA fragments. E. coli BL21.19 (18) harboring pET5a/secAΔ11/N95 or pET5a/secAA was used for complementation assays, and BL21(DE3) was used for overproduction of SecA derivatives. Cells for SecA derivative overproduction were cultured in TAG medium (10 g/liter tryptone, 5 g/liter NaCl, A salts [30], 0.5% glucose) supplemented with 100 μg/ml ampicillin. E. coli MM52 (recA) was obtained from D. Oliver (28). To construct pET5a/secAΔ11/N95, the N-terminal NdeI/EcoRI fragment of pET5a/secA/N95 (laboratory stock; unpublished data) was replaced with a PCR product, the corresponding fragment of 411 bp amplified from pET5a/secAΔ2-12 (laboratory stock; unpublished data) with the SecA 11th amino acid inserted after the first codon, treated with the same enzymes. To construct pET5a/secA826, the KpnI/SnaBI fragment of pET5a/secA was replaced with a PCR fragment that was amplified with 5′ primer 5′ GGCCTGCATATCATCGGTACC3′ and 3′ primer 5′TTATTACAGCGTACTGATAACTTCATA3′ and introduced with two consecutive stop codons, followed by blunt-end ligation. In this construction, pET5a/secA was used as the template and Pfu DNA polymerase (Stratagene) was used to generate the blunt-end PCR fragment. The resulting PCR fragment was then digested with KpnI alone and swapped with the KpnI/SnaBI fragment of secA located between bp 1676 and 2478. pET5a/secAL826Q was generated in a similar manner, except that site-directed mutagenesis was introduced by a three-round asymmetric PCR method (33) to obtain the desired point mutation. pET5a/secAA was constructed from pET5a/secA. The entire PCR-amplified secA gene was inserted into the NdeI site of pET5a/secA treated with the same restriction enzyme to yield pET5a/secAA without a linker. All of the plasmid constructions were verified at the DNA Sequencing Core Facility (Department of Biology, Georgia State University).
Expression and purification of SecAA or SecAΔ11/N95.
All protein purification steps and centrifugations were performed at 4°C. SecAΔ11/N95- and SecAA-overexpressing cells were cultured at 30°C to an optical density at 600 nm (OD600) of 0.8 and induced with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG). Proteins were overexpressed at 20°C for 16 h or at 30°C for 4 h. Cells were harvested by centrifugation in a Beckman JA-10 rotor at 5,000 rpm for 15 min and resuspended in 2 ml/g wet cells of TKMD buffer (10 mM Tris-acetate, pH 7.6, 50 mM KCl, 10 mM Mg acetate, 1 mM dithiothreitol [DTT]). Protease inhibitor cocktail (Roche) was added before cell suspensions were passed through a French pressure cell (SLM-Aminco) at 15,000 lb/in2. Cell lysates were centrifuged at 30,000 rpm for 60 min with a Beckman TLA-100.3 rotor to remove membranes and debris. After centrifugation to remove any aggregates, the supernatant was diluted five times with PPB buffer (25 mM sodium phosphate, pH 7.6, 1 mM DTT) and then loaded onto a Q-Sepharose 26/10 (Amersham Bioscience Corp.) anion-exchange column. SecAA was eluted at about 0.4 M NaCl. Fractions containing SecAA detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were pooled and concentrated by a centrifugal filter device with a cutoff of 30 kDa (Amicon). The recovered supernatant fraction was diluted five times with PPB buffer (25 mM sodium phosphate, pH 6.4, 1 mM DTT) and then applied to an SP-Sepharose 16/5 (Amersham Bioscience Corp.) cation-exchange column. SecAA was eluted at about 0.39 M NaCl. Fractions containing SecAA detected by SDS-PAGE were pooled and concentrated by the same centrifugal filter device to a volume of 0.5 ml and loaded onto a Superose 12 HR gel filtration column (Amersham Bioscience Corp.) and eluted in 100 mM (NH4)HCO3 buffer containing 1 mM DTT at a flow rate of 0.5 ml/min. Fractions that contained SecAA were combined, aliquoted, and stored at −80°C. SecAΔ11/N95 was purified similarly with only two chromatography steps, without the Q-Sepharose column step. SecAΔ11/N95 was eluted from an SP-Sepharose column at 0.34 M NaCl. Protein concentration was determined by the Bradford assay (Bio-Rad) with bovine serum albumin as the standard.
Cross-linking assay.
In vitro cross-linking was conducted with 1 mM EDC [1-ethyl-3-(3-dimethyl-aminopropyl)carbodiimide; Sigma] in 0.1 M MES buffer, pH 6.4, at room temperature for the indicated times. In vivo cross-linking was performed with 0.1 or 1% formaldehyde (HCHO; Sigma Chemical Co.) as previously described (17). Cross-linked products were analyzed by SDS-PAGE, followed by immunoblotting with rabbit SecA antibody.
Complementation assay.
Overnight-growing BL21.19 or MM52(recA) cells harboring vector pET5a alone or the indicated SecA derivatives were cultured in fresh LB/Amp at 30°C. Cells were adjusted to the same OD600 of 0.5. For the LB/Amp plate assay, 1 μl of each indicated dilution was spotted onto the plate and incubated at 42°C. A duplicate control plate was incubated at 30°C. For the CFU assay (26), 50- to 100-μl volumes of serial 10-fold dilutions were applied to plates prepared from the same medium and incubated at 42°C. Duplicate control plates were incubated at 30°C. Plates containing 30 to 300 colonies were used for plating efficiency counts.
ATPase activity assays.
ATPase activity assays were carried out as previously reported (16). Liposomes were prepared from E. coli total-lipid mixtures (Avanti Polar Lipids, Inc.) by sonication in a water bath.
In vitro protein translocation assay.
Protein translocations were carried out as previously described (5), with SecA-depleted BA13 membrane vesicles (4). Protein synthesis was performed with an S30 extract of SecA-depleted BL15.5 as previously described (4). The messenger was total RNA from strain HJM114 containing plasmids pOmp9 and pCI857 (9). The membrane vesicles containing the 35S-labeled proteins after proteinase K treatment were isolated, analyzed by 12% SDS-PAGE, autoradiographed, and quantified with a densitometer. The amount of 35S-labeled pro-OmpA used in the translocation assay was defined as 100%. The results shown are averages from four separated experiments.
AFM.
AFM images were obtained with a CP-Autoprobe (Park Scientific, Sunnyvale, CA) by using the noncontact mode as previously described (31).
RESULTS AND DISCUSSION
Functional SecAΔ11/N95 is a dimer.
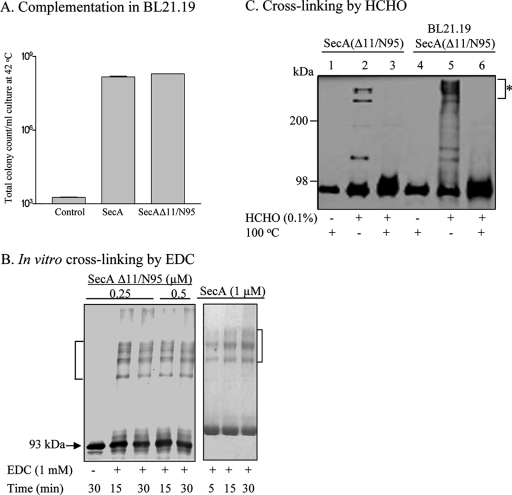
As part of our ongoing studies of the roles of the N and C termini of SecA, we constructed a SecAΔ11/N95 derivative identical to that of Or et al. (22) and confirmed its activities in vivo by growth complementation by a CFU assay as previously described (26). Consistent with the previous reports (13, 14, 22), SecAΔ11/N95 was able to complement secA(Ts) amber mutant strain BL21.19 to the same extent as wild-type SecA (18) at the nonpermissive temperature (Fig. 1A). In addition, we purified the truncated SecA protein and examined its oligomeric state by cross-linking with the zero-length cross-linker EDC and size exclusion chromatography. In contrast to the previous finding of monomers without detectable dimers (22), we found that SecAΔ11/N95 showed strong dimer-oligomer (indicated by brackets) cross-linking (Fig. 1B).
FIG. 1.
Complementation and cross-linking analyses of SecAΔ11/N95. (A) SecAΔ11/N95 restored the growth of secA(Ts) amber strain BL21.19 at 42°C. SecAΔ11/N95 could be cross-linked as a dimer in vitro (B) and in vivo (C). Purified SecAΔ11/N95 and SecA were subjected to EDC cross-linking as described in Materials and Methods (B). (C) Cross-linking by HCHO. BL21.19 harboring pETsecAΔ11/N95 was first grown at 30°C in LB containing 100 μg/ml ampicillin until the OD reached 1.0; cells were then diluted 10-fold in fresh medium and further cultured at 42°C to an OD600 of 1.2. Cells were harvested by centrifugation, and in vivo cross-linking was performed as previously described (17). Purified SecAΔ11/N95 protein was used as the control. Samples were analyzed by 5% (EDC cross-linking) or 10% (HCHO cross-linking) SDS-PAGE and detected by Western blotting with anti-SecA serum.
This discrepancy could possibly be due to different salt concentrations in the reaction buffers, to which SecA monomer-dimer equilibrium is sensitive (32). Benach et al. (3) also pointed out that the behavior of SecA could be altered in different cross-linking buffers. The exact physiological ionic strength and SecA concentration where the translocations take place remain to be determined; however, in vitro protein translocation activity was inhibited at an approximately physiological ionic strength of 150 mM NaCl (5). We therefore conducted direct cross-linking in vivo (17) by adding 0.1% formaldehyde to pETsecAΔ11/N95/BL21.19 intact cells grown at 42°C. Again, SecAΔ11/N95 was able to cross-link under physiological conditions, as dimers and/or higher-order oligomers were observed in a pattern similar to that produced by in vitro cross-linking with purified SecAΔ11/N95. Formaldehyde-dependent cross-linking of products (indicated by the asterisk) was observed as generated in vitro (Fig. 1C, lanes 2 and 5), and the cross-linked products were readily reversed upon heating (lanes 3 and 6), indicating that those complexes represented dimeric (and/or higher-order) forms of this particular construct. This result is in contrast to the report by Or et al. (22) showing that double-cysteine-mutated SecAΔ11/N95 remained in monomeric form in vivo.
Next, we examined the oligomeric state of SecAΔ11/N95 in parallel with SecA by size exclusion chromatography with an approximately physiological ionic strength buffer (150 mM NaCl, 20 mM sodium phosphate buffer, pH 7.0, 1 mM DTT). In agreement with previous observations (32), our data show that both SecAΔ11/N95 and SecA were equally sensitive to salt and protein concentrations (data not shown). At low protein and high salt concentrations, as described above, SecAΔ11/N95 and SecA (4 to 5 μM) eluted at the same volume as a monomer, whereas in a lower-ionic-strength buffer [100 mM (NH4)HCO3], SecAΔ11/N95 eluted earlier with an estimated molecular mass of 158 kDa, indicating an equilibrium shift toward dimers (data not shown). The cellular concentration of SecA was reported to be 8 μM (1), which is higher than the concentration in our assay. Additionally, increased temperatures favor dimer formation (32); however, we observed dimer formation even at 4°C. Together, our data show that dimer formation by SecAΔ11/N95 and SecA in vitro was similar. The results were further supported by those of Gold et al., which predicted that monomeric mutant SecAΔ11/N95 is a dimer as illustrated by equilibrium and velocity analysis by analytical ultracentrifugation (10). Taken together, our in vivo and in vitro cross-linking results strongly support the conclusion that the presumably monomeric SecA derivative in fact functions as a dimer.
Though our results are contradictory to the findings reported by Or et al. (22, 23), it is not surprising that SecAΔ11/N95 could be dimerized since N95 was reported to be a dimer and the C-terminal domain of SecA (residues 662 to 831), but not the N-terminal region, was reported to be essential for SecA dimerization (11). Furthermore, a recent report based on crystal structures of the recombinant E. coli SecA (amino acids 9 to 861) homodimer indicates that dimerization is mediated solely through extensive contacts of the DEAD motor domain but not the C or N terminus (25).
A defective SecA protein complemented the growth of MM52 [secA51(Ts)/recA].
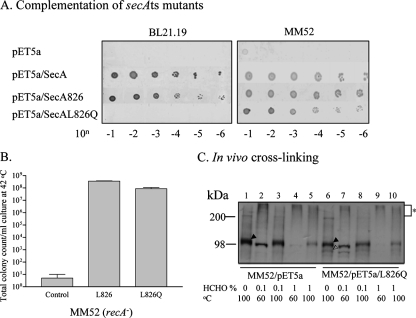
Another line of evidence that SecA may function as a dimer came from complementation studies with two defective SecA mutants, i.e., well-known temperature-sensitive point mutant MM52 (28) and secA(Ts) amber mutant BL21.19 (18). In our study of the function of C-terminal SecA, we constructed SecA826 containing a deletion of the C-terminal 76 amino acids and a derivative, SecAL826Q, with an additional point mutation at amino acid 826. SecA826 was able to complement secA(Ts) amber mutant BL21.19 at 42°C, whereas its derivative SecAL826Q, with the last hydrophobic amino acid residue replaced with a polar residue, was not (Fig. 2A), indicating that SecAL826Q is not functional. However, SecAL826Q restored the growth of MM52 [secA51(Ts)], which contains a nonfunctional point mutation, SecAL43P (28), at 42°C (Fig. 2A).
FIG. 2.
Heterodimeric SecA is functional and forms complexes in vivo. (A) Complementation of secA(Ts) strains BL21.19 and MM52 by secA and its derivatives at the nonpermissive temperature. SecA, wild-type SecA; SecA826, truncated SecA lacking C-terminal residues 827 to 901; SecAL826Q, SecA826 derivative containing the point mutation L826Q. (B) Complementation of the MM52 recA strain by a CFU assay. (C) In vivo cross-linking. MM52 (recA) harboring pETsecAL826Q or the pET5a vector alone (control) was cultured, cross-linked, and detected as described in the legend to Fig. 1C. Filled arrowheads, SecAL43P; open arrowhead, SecAL826Q.
To exclude the possibility of homologous recombination, a recA derivative of MM52 (a gift kindly provided by D. Oliver) transformed with pET5a/secAL826Q was tested by the CFU assay. The data showed complementation of growth at 42°C (Fig. 2B) with a plating efficiency that was 28% of that at 30°C. These results indicate that combination of two inactive SecA molecules, presumably forming heterodimers, leads to complementation activity. To test whether the two inactive SecA molecules form oligomers, in vivo cross-linking was carried out with the contact site cross-linker formaldehyde. As expected, cross-linking of SecAL43P and SecAL826Q with 1% formaldehyde was observed (Fig. 2C, lane 9, indicated by the asterisk). Since the amount of SecAL826Q in the cells is significantly less than that of SecAL43P, it is conceivable that little SecAL826Q was cross-linked at a lower concentration of formaldehyde (lane 7). This could explain the low plating efficiency (28%) of MM52/pET5a/secAL826Q at 42°C. The specificity of cross-linked products was also verified by reduction by heating as described above (lane 10).
The complementation result indicates that monomeric SecA is not active, which is in agreement with previous reports (7, 8, 12, 14, 32). There are at least two possible mechanisms by which a defective SecA protein (SecAL826Q) complements the growth of MM52 (SecAL43P), (i) heterodimer formation from two nonfunctional SecA molecules and (ii) reassembly of functional monomeric SecA from functional domains provided by the two nonfunctional SecA variants. Although the complementation experiment cannot discriminate between the two possibilities, our other data suggest that functional SecA is mostly dimeric. Therefore, the latter is less likely to happen. In addition, E. coli SecA has been crystallized as a dimer and the dimeric form was proposed to facilitate its catalytic activity by a hand-over-hand mechanism for preprotein chain relay (25). Our results strongly suggest that heterodimeric SecA consisting of two inactive variants, not a monomer, is functional in the membrane.
Genetically dimerized SecA is functional in vivo and in vitro.
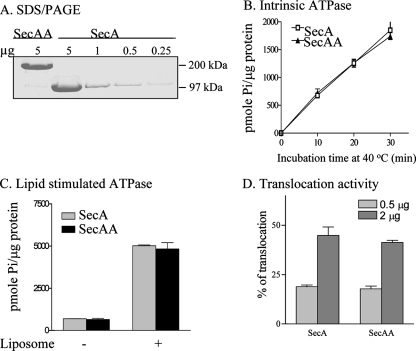
To provide additional direct evidence that SecA dimers are indeed functional in vivo, we constructed plasmid pET5a/secAA containing wild-type secA genes in tandem, without linkers in between, to yield head-to-tail tandem SecA dimers. The genetically linked SecA dimers fully complemented secA(Ts) amber mutant BL21.19 at the nonpermissive temperature in the presence of the protease inhibitor phenylmethylsulfonyl fluoride to prevent the cleavage of dimeric SecAA (Fig. 3A), with a plating efficiency of 92.5% of that at 30°C. The dimeric SecAA protein was stable, as the cells at 42°C lacked detectable monomeric SecA (Fig. 3B). Furthermore, the purified tandem SecA dimers (with a molecular mass of about 202 kDa and >97% purity, as analyzed by SDS-PAGE; Fig. 4A) were fully functional (Fig. 4), as shown below.
FIG. 3.
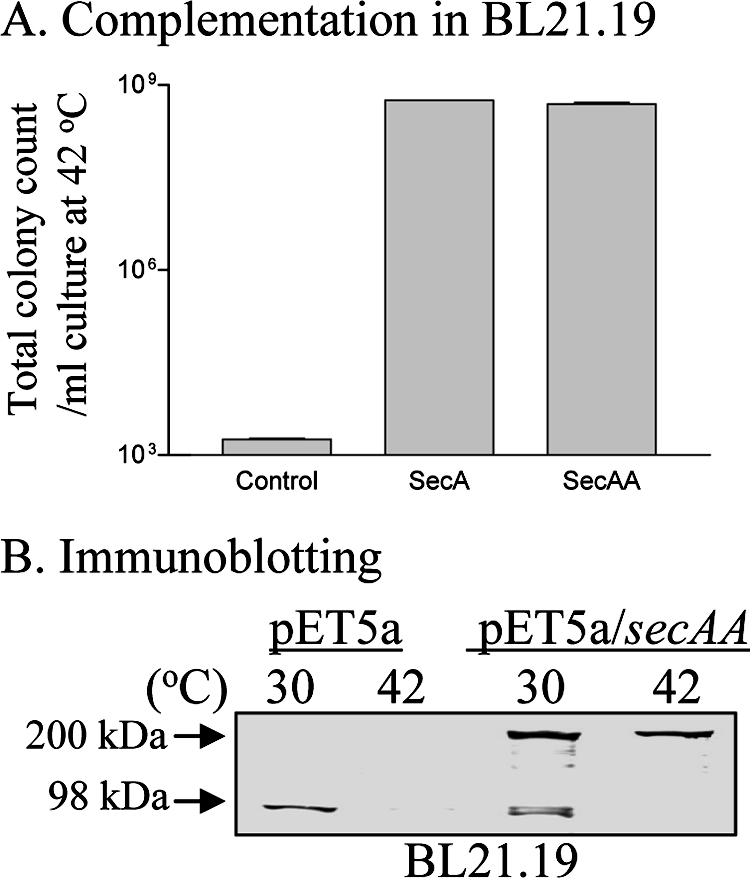
Tandem SecA is functional and stable in vivo. (A) A complementation assay was carried out with BL21.19 cells harboring the vector pET5a alone, pET5a/secA, or pET5a/secAA (B) SDS-PAGE immunoblotting of SecA and SecAA from cells collected prior to and after SecA depletion (2 h after a shift from 30 to 42°C) with anti-SecA serum. One millimolar phenylmethylsulfonyl fluoride was added to cultures to prevent protein degradation.
FIG. 4.
The isolated SecAA protein is functional in vitro. (A) SDS-PAGE and Coomassie blue staining of purified SecAA and SecA showing >97% purity. (B) Intrinsic ATPase activity of SecA and SecAA as a function of incubation time. Five micrograms each of purified SecA and SecAA was incubated in reaction mixtures at 40°C for the times indicated. (C) Lipid-stimulated ATPase activity of genetically linked SecA dimers. Two micrograms each of purified SecA and SecAA was incubated with 10 μg of liposomes at 30°C for 30 min. (D) In vitro protein translocation activity of 35S-Met-labeled pro-OmpA in the presence of the indicated amount of wild-type SecA or SecAA.
The intrinsic ATPase activity of tandem SecAA dimers, like SecA, was low, as previously reported (19), and the activity was also stimulated by liposomes to a level similar to that of wild-type SecA (Fig. 4B and C). Moreover, the in vitro translocation activity of purified SecAA (Fig. 4D) was significantly higher than that of the proposed monomeric SecA mutant (22) and similar to that of the reported conjugated SecA dimer which no longer supports SecB-dependent protein translocation (6). The inactive cross-linked dimers reported by Or et al. (24) were most likely due to loss of other dynamic features of SecA in the protein translocation process. Our observations of fully active tandem SecAA suggest that the head-to-tail SecA dimers are the functional units in protein translocation, consistent with the antiparallel dimeric SecA observed in X-ray structure (25).
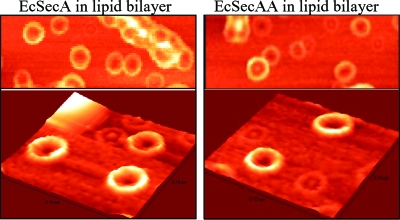
We have shown previously that SecA formed a ring-like structure upon interacting with anionic phospholipids by AFM (31). We therefore examined the structures of SecAA in lipids. Interestingly, the ring-like pore structures of SecAA and SecA are remarkably similar in size and shape (Fig. 5), implying that dimeric (or higher-order) lipid-bound SecA is the channel-forming unit. These observations indicate that SecA exists in lipid bilayers likely as dimers, not monomers or tethered monomers; otherwise, one would expect to observe side-by-side twin ring-like pore monomer structures in tandem dimerized SecA preparations.
FIG. 5.
SecAA forms ring-like pore structures, like SecA, as observed by AFM. Purified SecA and SecAA were incubated with lipid bilayers prepared from extracted E. coli membrane lipids and mounted on freshly cleaved mica as previously described (31). The lower panels represented three-dimensional structures.
These data indicate that it is not essential for SecA to dissociate into monomers to be functional. The possibility that only one of the protomers of the tandem SecA dimer is actively involved in translocation cannot be excluded. However, the complementation and in vivo cross-linking of inactive SecAL43P and SecAL826Q, the structure and function of SecAA tandem dimers, and the dimeric crystal structure of E. coli SecA provide strong evidence that SecA functions in the membrane as dimers. Though different possibilities exist for each of the observations above, the simplest interpretation is that SecA functions as a dimer. Taken together, the evidence presented here provides additional support for the idea that SecA functions as a dimer and dissociation of SecA dimers is not an essential step for its function in protein translocation in the membrane.
Acknowledgments
This work was supported in part by NIH research grant GM 34766. Bing Na is a fellow of the Molecular Basis of Disease Program at Georgia State University.
We thank Quynh Ho for assistance and discussions, P. M. Johnson for review of the manuscript, and Donald Oliver for kindly providing the strains. We also thank Ping Jiang for DNA sequencing and Hui Zhao for AFM in the GSU Biology Core Facility, which is supported by the Georgia Research Alliance.
Footnotes
Published ahead of print on 7 December 2007.
REFERENCES
- 1.Akita, M., A. Shinkai, S. Matsuyama, and S. Mizushima. 1991. SecA, an essential component of the secretory machinery of Escherichia coli, exists as homodimer. Biochem. Biophys. Res. Commun. 174211-216. [DOI] [PubMed] [Google Scholar]
- 2.Alami, M., K. Dalal, B. Lelj-Garolla, S. G. Sligar, and F. Duong. 2007. Nanodiscs unravel the interaction between the SecYEG channel and its cytosolic partner SecA. EMBO J. 261995-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benach, J., Y. T. Chou, J. J. Fak, A. Itkin, D. D. Nicolae, P. C. Smith, G. Wittrock, D. L. Floyd, C. M. Golsaz, L. M. Gierasch, and J. F. Hunt. 2003. Phospholipid-induced monomerization and signal-peptide-induced oligomerization of SecA. J. Biol. Chem. 2783628-3638. [DOI] [PubMed] [Google Scholar]
- 4.Cabelli, R. J., L. L. Chen, P. C. Tai, and D. B. Oliver. 1988. SecA protein is required for secretory protein translocation into Escherichia coli membrane-vesicles. Cell 55683-692. [DOI] [PubMed] [Google Scholar]
- 5.Chen, L., D. Rhoads, and P. C. Tai. 1985. Alkaline phosphatase and OmpA protein can be translocated posttranslationally into membrane vesicles of Escherichia coli. J. Bacteriol. 161973-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Keyzer, J., E. O. van der Sluis, R. E. Spelbrink, N. Nijstad, B. de Kruijff, N. Nouwen, C. van der Does, and A. J. Driessen. 2005. Covalently dimerized SecA is functional in protein translocation. J. Biol. Chem. 28035255-35260. [DOI] [PubMed] [Google Scholar]
- 7.Ding, H., J. F. Hunt, I. Mukerji, and D. Oliver. 2003. Bacillus subtilis SecA ATPase exists as an antiparallel dimer in solution. Biochemistry 428729-8738. [DOI] [PubMed] [Google Scholar]
- 8.Driessen, A. J. 1993. SecA, the peripheral subunit of the Escherichia coli precursor protein translocase, is functional as a dimer. Biochemistry 3213190-13197. [DOI] [PubMed] [Google Scholar]
- 9.Geller, B. L., N. R. Movva, and W. Wickner. 1986. Both ATP and the electrochemical potential are required for optimal assembly of pro-OmpA into Escherichia coli inner membrane vesicles. Proc. Natl. Acad. Sci. USA 834219-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gold, V. A., A. Robson, A. R. Clarke, and I. Collinson. 2007. Allosteric regulation of SecA: magnesium-mediated control of conformation and activity. J. Biol. Chem. 28217424-17432. [DOI] [PubMed] [Google Scholar]
- 11.Hirano, M., S. Matsuyama, and H. Tokuda. 1996. The carboxyl-terminal region is essential for Sec-A dimerization. Biochem. Biophys. Res. Commun. 22990-95. [DOI] [PubMed] [Google Scholar]
- 12.Hunt, J. F., S. Weinkauf, L. Henry, J. J. Fak, P. McNicholas, D. B. Oliver, and J. Deisenhofer. 2002. Nucleotide control of interdomain interactions in the conformational reaction cycle of SecA. Science 2972018-2026. [DOI] [PubMed] [Google Scholar]
- 13.Jilaveanu, L. B., and D. Oliver. 2006. SecA dimer cross-linked at its subunit interface is functional for protein translocation. J. Bacteriol. 188335-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jilaveanu, L. B., C. R. Zito, and D. Oliver. 2005. Dimeric SecA is essential for protein translocation. Proc. Natl. Acad. Sci. USA 1027511-7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karamanou, S., G. Sianidis, G. Gouridis, C. Pozidis, Y. Papanikolau, E. Papanikou, and A. Economou. 2005. Escherichia coli SecA truncated at its termini is functional and dimeric. FEBS Lett. 5791267-1271. [DOI] [PubMed] [Google Scholar]
- 16.Lill, R., W. Dowhan, and W. Wickner. 1990. The ATPase activity of SecA is regulated by acidic phospholipids, SecY, and the leader and mature domains of precursor proteins. Cell 60271-280. [DOI] [PubMed] [Google Scholar]
- 17.Manting, E. H., C. van der Does, and A. J. Driessen. 1997. In vivo cross-linking of the SecA and SecY subunits of the Escherichia coli preprotein translocase. J. Bacteriol. 1795699-5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell, C., and D. Oliver. 1993. Two distinct ATP-binding domains are needed to promote protein export by Escherichia coli SecA ATPase. Mol. Microbiol. 10483-497. [DOI] [PubMed] [Google Scholar]
- 19.Nakatogawa, H., H. Mori, and K. Ito. 2000. Two independent mechanisms down-regulate the intrinsic SecA ATPase activity. J. Biol. Chem. 27533209-33212. [DOI] [PubMed] [Google Scholar]
- 20.Oliver, D. B. 1993. SecA protein: autoregulated ATPase catalysing preprotein insertion and translocation across the Escherichia coli inner membrane. Mol. Microbiol. 7159-165. [DOI] [PubMed] [Google Scholar]
- 21.Oliver, D. B., and J. Beckwith. 1981. E. coli mutant pleiotropically defective in the export of secreted proteins. Cell 25765-772. [DOI] [PubMed] [Google Scholar]
- 22.Or, E., D. Boyd, S. Gon, J. Beckwith, and T. Rapoport. 2005. The bacterial ATPase SecA functions as a monomer in protein translocation. J. Biol. Chem. 2809097-9105. [DOI] [PubMed] [Google Scholar]
- 23.Or, E., A. Navon, and T. Rapoport. 2002. Dissociation of the dimeric SecA ATPase during protein translocation across the bacterial membrane. EMBO J. 214470-4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Or, E., and T. Rapoport. 2007. Cross-linked SecA dimers are not functional in protein translocation. FEBS Lett. 5812616-2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papanikolau, Y. P., M. Ravelli, R. B. G. McCarthy, A. A. Cusack, S. Economou, and K. Petratos. 2007. Structure of dimeric SecA, the Escherichia coli preprotein translocase motor. J. Mol. Biol. 3661545-1557. [DOI] [PubMed] [Google Scholar]
- 26.Rahman, M. S., J. A. Simser, K. R. Macaluso, and A. F. Azad. 2003. Molecular and functional analysis of the lepB gene, encoding a type I signal peptidase from Rickettsia rickettsii and Rickettsia typhi. J. Bacteriol. 1854578-4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Randall, L. L., J. M. Crane, A. A. Lilly, G. Liu, C. Mao, C. N. Patel, and S. J. Hardy. 2005. Asymmetric binding between SecA and SecB two symmetric proteins: implications for function in export. J. Mol. Biol. 348479-489. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt, M. G., E. E. Rollo, J. Grodberg, and D. B. Oliver. 1988. Nucleotide-sequence of the secA gene and secA(Ts) mutations preventing protein export in Escherichia coli. J. Bacteriol. 1703404-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin, J. Y., M. Kim, and T. Ahn. 2006. Effects of signal peptide and adenylate on the oligomerization and membrane binding of soluble SecA. J. Biochem. Mol. Biol. 39319-328. [DOI] [PubMed] [Google Scholar]
- 30.Tai, P. C., B. J. Wallace, and B. D. Davis. 1978. Streptomycin causes misreading of natural messenger by interacting with ribosomes after initiation. Proc. Natl. Acad. Sci. USA 75275-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, H. W., Y. Chen, H. Yang, X. Chen, M. X. Duan, P. C. Tai, and S. F. Sui. 2003. Ring-like pore structures of SecA: implication for bacterial protein-conducting channels. Proc. Natl. Acad. Sci. USA 1004221-4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woodbury, R. L., S. J. Hardy, and L. L. Randall. 2002. Complex behavior in solution of homodimeric SecA. Protein Sci. 11875-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu, K. H., and P. C. Tai. 2004. Cys32 and His105 are the critical residues for the calcium-dependent cysteine proteolytic activity of CvaB, an ATP-binding cassette transporter. J. Biol. Chem. 279901-909. [DOI] [PubMed] [Google Scholar]