Abstract
Similarities between Mycobacterium tuberculosis phoP-phoR mutants and the attenuated laboratory strain M. tuberculosis H37Ra in terms of morphological and cytochemical properties, lipid content, gene expression and virulence attenuation prompted us to analyze the functionality of this two-component regulator in the latter strain. Sequence analysis revealed a base substitution resulting in a one-amino-acid change in the likely DNA-binding region of PhoP in H37Ra relative to H37Rv. Using gel-shift assays, we show that this mutation abrogates the ability of the H37Ra PhoP protein to bind to a 40-bp segment of its own promoter. Consistent with this result, the phoP gene from H37Rv but not that from H37Ra was able to restore the synthesis of sulfolipids, diacyltrehaloses and polyacyltrehaloses in an isogenic phoP-phoR knock-out mutant of M. tuberculosis Moreover, complementation of H37Ra with phoP from H37Rv fully restored sulfolipid, diacyltrehalose and polyacyltrehalose synthesis, clearly indicating that the lack of production of these lipids in H37Ra is solely due to the point mutation in phoP. Using a pks2-3/4 knock-out mutant of M. tuberculosis H37Rv, evidence is further provided that the above-mentioned polyketide-derived acyltrehaloses do not significantly contribute to the virulence of the tubercle bacillus in a mouse model of infection. Reasons for the attenuation of H37Ra thus most likely stand in other virulence factors, many of which are expected to belong to the PhoP regulon and another of which, unrelated to PhoP, appears to be the lack of production of phthiocerol dimycocerosates in this strain.
H37Rv and H37Ra are two variants of an Mycobacterium tuberculosis strain named H37 that was originally isolated from the sputum of a tuberculosis patient in 1905. Serial passaging of H37 through different media led to the dissociation of this isolate into two variants, a virulent one known as H37Rv and an avirulent one known as H37Ra, which also differ in their colonial morphology and cording properties (27, 39). With the goal of identifying the molecular determinants underlying the virulence attenuation of H37Ra, numerous approaches including genetic complementation of H37Ra with H37Rv genomic DNA (29), gene expression profiling (15, 28, 32), subtractive RNA hybridization (22) and comparative genomics, proteomics and lipidomics (4, 7, 10, 18, 26) have been undertaken. Although these studies have led to the identification of a number of genes or gene products the expression of which differs between H37Rv and H37Ra, it is still at present unclear to what extent these products account for the virulence attenuation of H37Ra. Furthermore, as attempts to restore the virulence of the H37Ra strain through genetic complementation with H37Rv DNA have so far yielded negative results (4, 29), it has been concluded that the virulence attenuation of H37Ra was certainly the result of multiple mutations and/or rearrangements affecting multiple chromosomal loci.
With the recent study by different groups of the two-component regulator PhoP-PhoR from M. tuberculosis (16, 24, 31, 37, 40), striking similarities between H37Ra and different M. tuberculosis phoP-phoR knock-out mutants began to emerge. PhoP-PhoR is a two-component regulator whose disruption was shown to affect dramatically the ability of M. tuberculosis to replicate in animal and cellular models (31, 40). Like the H37Ra strain (14, 26, 27, 39), phoP-phoR mutants of M. tuberculosis form smaller colonies on agar plates (31), tend to lose acid fastness (40), do not stain with neutral red (16) and fail to grow as serpentine cords (16, 31). Most of these attenuation, morphological and cytochemical properties have been proposed to be a direct consequence of the inability of both H37Ra and the phoP-phoR mutants to produce three classes of polyketide-derived acyltrehaloses known as sulfolipids (SL), diacyltrehaloses (DAT) and polyacyltrehaloses (PAT) (7, 10, 16, 26). Definitive proof for this assumption was, however, lacking. With the more widespread use of microarrays, other striking parallels between the two types of strains in terms of gene expression became obvious as nine of the twenty-two genes whose expression was found to be consistently lower in H37Ra than in H37Rv were also among the most down-regulated in a phoP-phoR mutant of M. tuberculosis H37Rv (15, 40). These observations led us to investigate whether the H37Ra strain expressed a functional PhoP-PhoR two-component system.
MATERIALS AND METHODS
Bacterial strains.
The M. tuberculosis strains used in this study were M. tuberculosis H37Rv (ATCC 25618), M. tuberculosis H37Ra (ATCC 25177), the phoPR mutant of the M. tuberculosis clinical isolate 1237, 1237ΔphoPR::hyg (16) and the msl3 strain (12).
Production and purification of PhoP-Rv and PhoP-Ra.
The PhoP proteins from H37Rv and H37Ra (thereafter named PhoP-Rv and PhoP-Ra, respectively) were produced in Escherichia coli BL21(DE3)pLysS using the pET15b expression plasmid (Novagen) and purified using a HiTrap Chelating HP affinity column on an ÁKTA-prime FPLC (Amersham Biosciences). His tags were cleaved using the Thrombin CleanCleavage kit from Sigma. PhoP-Ra was obtained by producing a serine-to-leucine mutation at position 219 of PhoP-Rv using the QuikChange XL site-directed mutagenesis kit (Stratagene).
Gel shift assays.
The 40-bp fragment of the phoP promoter (phoP40) used in the gel shift assay experiments was designed according to results of DNase I protection assays (C. Martín, unpublished results) and encompassed region −31 to −70, relative to the translational start site of phoP. This DNA fragment was labeled at the 5′ end with IRD700 (InfraRed Dye 700, Li-Cor, Inc.). 25 nM double-stranded labeled phoP promoter probe and an increasing concentration of PhoP-Rv and PhoP-Ra proteins were mixed together in binding buffer [50 mM Tris HCl, pH 7.0, 100 mM KCl, 5% glycerol, 5 mM dithiothreitol, 1 mM EDTA, 0.5% Tween, 50 μg ml−1 bovine serum albumin, 10 μg ml−1 poly(dI-dC)] and applied to a 20-cm × 20-cm 5% native polyacrylamide gel. PhoP-Rv and PhoP-Ra were phosphorylated by incubation with 50 mM acetyl phosphate in a buffer consisting of 100 mM Tris HCl (pH 7.0), 10 mM MgCl2 and 150 mM KCl.
Circular dichroism.
Far-UV circular dichroism spectra were acquired at 25°C with an Aviv 215 spectropolarimeter (Aviv Biomedical) using a cylindrical cell with a 0.02-cm path-length. PhoP-Rv (0.7 mg ml−1) and PhoP-Ra (0.22 mg ml−1), both in 20 mM phosphate, 500 mM NaF, pH 8.0, were scanned three times from 260 to 190 nm (0.5-nm step) with an averaging time of 5 s per step. The spectra, corrected using buffer baselines measured under the same conditions, were normalized to the molar peptide bond concentration and path length (1 cm) as mean molar differential extinction coefficient per residue (Δɛ). Secondary structure estimations were derived from the normalized spectra using the CONTIN/LL method included in the CDPro software and the SP43 protein reference set (38).
Complementation studies.
Plasmid pSO5K was described earlier (16). The same primers as were used to amplify phoP from M. tuberculosis H37Rv in pSO5K were used to amplify phoP from M. tuberculosis H37Ra in pOMKPhoP-Ra (19). In pSO5K and pOMKphoP-Ra, the phoP genes are expressed from their own promoter. pVVphoP-Rv and pVVphoP-Ra were constructed by PCR-amplifying the entire coding sequences of the phoP genes from M. tuberculosis H37Rv and H37Ra using the primers PhoP.9 (5′-gggcgcccatatgcggaaaggggttgatctcgtg-3′) and PhoP.10 (5′-gggaagctttcgaggctcccgcagtacgtagc-3′) and cloning these fragments into the NdeI and HindIII restriction sites of the expression vector pVV16 (23).
Lipid analyses, neutral red staining and cording properties.
Radiolabeling of whole M. tuberculosis cells with [1-14C]propionate (specific activity, 56.7 Ci mol−1, MP Biomedicals Inc.), extraction and analyses of lipids were performed as described (16). Neutral red staining of tubercle bacilli was performed as described by Soto and collaborators (36). For the analysis of cording properties, a drop of M. tuberculosis culture grown in Sauton's medium was smeared onto a glass slide, stained by the standard Kinyoun procedure and observed under a light microscope at ×1,000 magnification.
Generation of a pks2-pks3-4 mutant of M. tuberculosis H37Rv.
The ts-sacB method (30) was used to achieve allelic replacement at the pks2 locus of the pks3/4 M. tuberculosis mutant, msl3 (12). The M. tuberculosis H37Rv pks2 gene and flanking regions were extracted from cosmid MTCY409 on a 6,576-bp EcoRI restriction fragment and a disrupted allele, pks2::kan, was obtained by replacing 3,049 bp of the coding sequence of this gene bracketed between two NruI sites with the kanamycin resistance cassette from pUC4K (Amersham Pharmacia Biotech). pks2::kan was then cloned into the BamHI-cut and blunt-ended pPR27 (30) to obtain pPR27pks2K, the construct used for allelic replacement in the msl3 strain. Allelic replacement at the pks2 locus of the msl3 strain was confirmed by PCR using primers pks2.1 (5′-gacggtgaccggatcctggcg-3′) and pks2.2 (5′-gacgtacatgcgcggcaccgc-3′).
Virulence studies.
6- to 8-week-old female BALB/c mice were infected intravenously with 105 CFU of M. tuberculosis H37Rv, M. tuberculosis H37Ra and the M. tuberculosis H37Rv pks2-3/4 mutant as described (20). Five mice were used per experimental point and per strain.
RESULTS AND DISCUSSION
Identification of a point mutation in PhoP-Ra.
Bacterial response regulator transcription factors can be classed into subfamilies based on structural similarity within their DNA-binding effector domains. Sequence similarities of the C-terminal effector domain identify PhoP from M. tuberculosis as a member of the OmpR/PhoB subfamily, characterized by a winged-helix DNA-binding domain (25). Sequencing of 2.9 kb of the chromosomal DNA of M. tuberculosis H37Ra encompassing the entire coding sequences of phoP and phoR as well as 600 bp of the promoter region of phoP and 80 bp of downstream DNA revealed only one single nucleotide polymorphism affecting codon 219 (TCG → TTG) in phoP and changing a serine to a leucine. S219 in PhoP of M. tuberculosis is equivalent to R200 in PhoB of E. coli which is comprised in the helix that penetrates the major groove of DNA and has been shown to be implicated in DNA binding (3). Data thus suggested that PhoP-Ra might be impaired in DNA binding.
PhoP-Ra is unable to bind its own promoter.
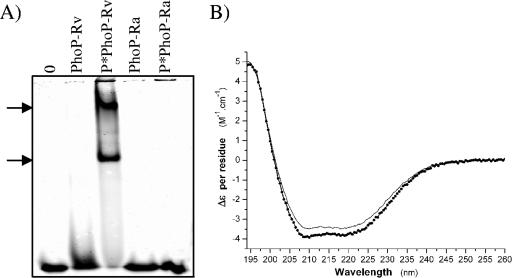
PhoP from M. tuberculosis has been proposed to repress its own expression (17). The ability of PhoP-Ra to bind its own promoter was thus investigated in vitro using gel shift assays. To this end, the PhoP-Rv and PhoP-Ra proteins were overproduced in E. coli as C-terminal His-tagged proteins and purified to homogeneity by Ni2+-NTA affinity chromatography. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis showed that they migrated with apparent molecular masses of approximately 27.5 kDa, consistent with the calculated molecular masses of PhoP-Rv (27,514 Da) and its S219L mutant (27,539 Da) (data not shown). PhoP-Ra and PhoP-Rv DNA binding experiments were carried out in the presence of 10 μg ml−1 poly(dI-dC) to prevent non-specific binding, an excess of recombinant proteins and a 40-bp DNA fragment (phoP40) comprising the DR1 and DR2 direct repeats identified by Gupta et al. (17). The ability of the recombinant proteins to bind DNA was checked before and after phosphorylation with acetyl phosphate. As shown in Fig. 1A, in this experimental setting, the binding of PhoP-Rv to phoP40 was phosphorylation-dependent and resulted in the formation of two major higher-order complexes, most likely reflecting the existence of two PhoP-binding sites in phoP40. Under the same experimental conditions, neither PhoP-Ra nor its phosphorylated counterpart P*PhoP-Ra bound to phoP40. The phosphorylation-dependent binding of PhoP-Rv to phoP40 is in apparent conflict with the recent report of Gupta and collaborators (17) of a phosphorylation-independent assembly of PhoP-DNA complexes at elevated concentrations of PhoP. Although both studies were performed in the presence of an excess of PhoP proteins, the fact that a non-His-tagged version of PhoP was used in our assays and important differences in the sizes of the DNA probes used in the gel shift experiments—a 40-bp fragment encompassing two direct repeats of the promoter region of phoP in our study, 160- to 410-bp DNA probes encompassing all three direct repeats and more than 100 bp of upstream region in the Gupta et al. study (17)—most likely account for this discrepancy.
FIG. 1.
Production of recombinant forms of PhoP-Rv and PhoP-Ra and comparative analysis of their binding to a 40-bp region of the promoter of phoP by mobility shift assay. (A) 11 μM of native or phosphorylated PhoP-Rv and PhoP-Ra (P*PhoP-Rv and P*PhoP-Ra) were analyzed for their ability to bind phoP40 using 25 nM of labeled DNA probe as described under Materials and Methods. 0, no protein added to the assay. The arrows indicate the positions of the two shifted P*PhoP-Rv-DNA complexes. (B) Circular dichroism spectra of PhoP-Rv (bold line) and PhoP-Ra (thin line).
To ensure that the overall folding of the PhoP-Ra recombinant protein had not been compromised and that the point mutation alone accounted for the lack of binding of this protein to phoP40, circular dichroism tests were carried out on PhoP-Rv and PhoP-Ra. Far-UV circular dichroism analysis of PhoP-Rv and PhoP-Ra revealed virtually identical spectral properties (Fig. 1B), indicating that the global secondary structure of the two variants was very close. The double trough shape around 208 and 222 nm is characteristic of proteins with high α-helix content. Estimations of the secondary structure content of PhoP-Rv and PhoP-Ra by single-value decomposition showed in both cases that about 40% of the residues were involved in α-helices, 15% in β-strands, and 20% in turns, while 25% remained unordered.
PhoP-Ra is unable to restore polyketide-derived acyltrehalose synthesis in a phoP-phoR knock-out mutant of M. tuberculosis.
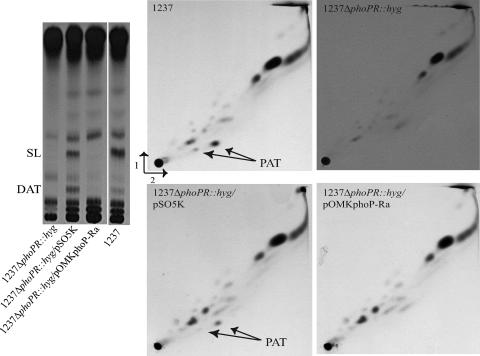
Because PhoP was shown to be a positive regulator of the synthesis of SL, DAT and PAT in M. tuberculosis (16, 40), we then set out to determine whether PhoP-Ra was able to restore the synthesis of these complex lipids in 1237ΔphoPR::hyg, a phoP-null mutant generated earlier in the M. tuberculosis clinical isolate 1237 (16). Two types of constructs were used in the complementation studies. In pOMKphoP-Ra and pSO5K, the phoP genes from H37Ra and H37Rv, respectively, are expressed from their own promoters. As the inability of PhoP-Ra to bind its promoter might have resulted in poor expression from this plasmid, other constructs in which the phoP genes from H37Ra and H37Rv were placed under control of the phsp60 promoter were also generated, resulting in pVVphoP-Ra and pVVphoP-Rv. All of these plasmids were electroporated into 1237ΔphoPR::hyg and the production of PhoP-Ra and PhoP-Rv from pVVphoP-Ra and pVVphoP-Rv was confirmed by immunoblotting with monoclonal anti-His antibodies (see Fig. S1 in the supplemental material). As reported earlier, complementation of the mutant with phoP-Rv expressed from either plasmid (pSO5K and pVVphoP-Rv) restored the production of SL, DAT and PAT in 1237ΔphoPR::hyg (Fig. 2) (16). In contrast, the expression of phoP-Ra in 1237ΔphoPR::hyg had no effect on the production of these lipids (Fig. 2), suggesting that PhoP-Ra is unable to activate the promoters of the genes governing their synthesis.
FIG. 2.
Acyltrehalose composition of phoP-phoR knock-out mutants of M. tuberculosis complemented with the phoP genes from M. tuberculosis H37Ra and M. tuberculosis H37Rv. Autoradiograms of thin-layer chromatograms of lipids derived from [1-14C]propionate are shown. A) For the analysis of DAT and SL, 20,000 cpm of [1-14C]propionate-derived total lipids were subjected to thin-layer chromatography with chloroform:methanol:water (90:10:1, by vol.) as the solvent. B) PAT were analyzed by loading 20,000 cpm of [1-14C]propionate-derived total lipids on thin-layer chromatography plates and developing the plates thrice in petroleum ether (60/80°C):acetone (92:8, by vol.) in the first direction and once in toluene:acetone (95:5, by vol.) in the second direction. Only the lipid profiles of the 1237 mutants complemented with the pSO5K and pOMKphoP-Ra plasmids are shown. Similar profiles were obtained for the mutants complemented with pVVphoP-Rv and pVVphoP-Ra.
PhoP-Ra is responsible for the absence of SL, DAT and PAT but not PDIM in the H37Ra strain.
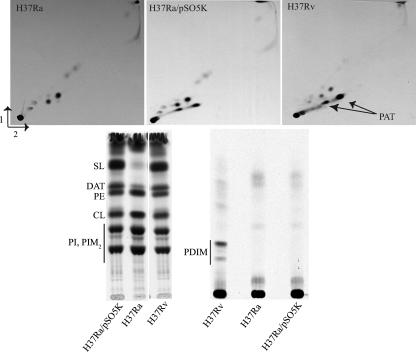
The introduction of pSO5K and pVVphoP-Rv in H37Ra induced the synthesis of SL, DAT and PAT (Fig. 3), indicating that the S219L mutation in PhoP-Ra is the sole reason for the absence of these lipids in the attenuated laboratory strain. Interestingly, lipid analyses also revealed that H37Ra is deficient in the production of phthiocerol dimycocerosates (PDIM), another family of polyketide-derived lipids implicated in the virulence of M. tuberculosis (5, 9, 21, 35) (Fig. 3). In contrast to the production of SL, DAT and PAT, the synthesis of PDIM was not restored upon complementation with phoP-Rv, consistent with our earlier observation that this family of lipids is apparently not under the regulatory control of PhoP-PhoR (16).
FIG. 3.
Complementation of the H37Ra strain with the phoP-Rv gene. Autoradiograms of thin-layer chromatograms of lipids derived from [1-14C]propionate are shown (20,000 cpm were loaded per lane). For the analysis of DAT and SL, lipids were subjected to thin-layer chromatography with chloroform:methanol:water (60:30:6, by vol.) as the solvent. The minor compound(s) displaying similar migration properties as DAT in the H37Ra strain might correspond to minor forms of diacyltrehaloses esterified with hydroxylated long-chain methyl-branched fatty acids or short-chain unsaturated mono-methyl-branched fatty acids (2, 13). PDIM were analyzed with petroleum ether (60/80°C):ethyl acetate (98:2, by vol., three developments) as the solvent. The solvent systems used for the analysis of PAT were identical to those described in Fig. 2. Only the lipid profiles of H37Ra complemented with the pSO5K are shown. Similar profiles were obtained for H37Ra complemented with pVVphoP-Rv. CL, cardiolipin; PE, phosphatidylethanolamine; PI, phosphatidyl-myo-inositol; PIM2, phosphatidyl-myo-inositol dimannosides.
Complementation of H37Ra with phoP-Rv also resulted in the partial restoration of the cording properties of H37Ra and in a colonial morphology resembling that of M. tuberculosis H37Rv (data not shown). Complementation, however, did not significantly enhance the growth rate of H37Ra on agar plates and did not confer to it the ability to stain with neutral red (data not shown).
Effect of the combined loss of SL, DAT and PAT on the virulence of M. tuberculosis H37Rv.
Because of their likely involvement in the neutral red staining and cording properties of the tubercle bacillus, SL, DAT and PAT have often been proposed to be one of the major causes of the virulence attenuation of the H37Ra strain. This was further supported by the important decrease in virulence of phoP mutants of M. tuberculosis lacking the same families of lipids and the decreased pathogenicity of mmpL8 mutants of M. tuberculosis deficient in the production of mature forms of SL (8, 11, 16, 31, 40). Arguing against this assumption, however, isogenic pks3/4 and pks2 mutants of M. tuberculosis H37Rv, specifically deficient in the production of DAT and PAT or all forms of SL, respectively, were reported to be as virulent as their wild-type parent in the mouse or guinea pig models of infection (33, 34). Given that the synthesis of SL, DAT and PAT is coordinately regulated by PhoP (16, 40), it was thus proposed that these families of lipids might be able to compensate for one another and that their roles in virulence might only become clearly visible when their synthesis is abolished simultaneously (21).
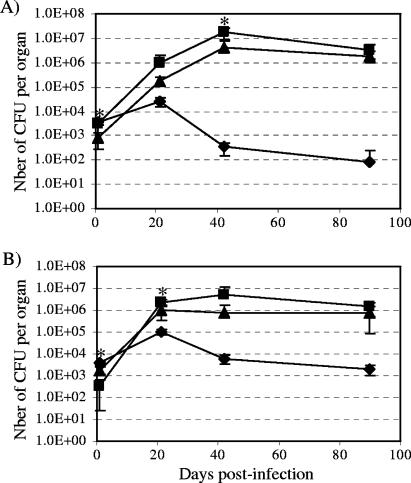
In order to investigate this hypothesis and address the question of the contribution of these lipids to the virulence attenuation of H37Ra, we undertook to generate an SL/DAT/PAT mutant of M. tuberculosis H37Rv and to compare its virulence to that of the wild-type H37Rv and H37Ra strains in a murine model. A pks2-3/4 knock-out mutant was constructed by allelic replacement at the pks2 locus of the msl3 (Δpks3/4) mutant of H37Rv (12) using the ts-sacB method (see Fig. S2 in the supplemental material) (30). As expected, the msl3Δpks2 mutant was totally devoid of SL, DAT and PAT (see Fig. S3 in the supplemental material). It was also partially impaired in its ability to grow as serpentine cords in liquid medium and displayed an intermediate phenotype between H37Rv and H37Ra in this respect (data not shown). However, it grew similarly to wild-type H37Rv and displayed a normal morphology on 7H11 agar plates. The mutant was used, along with the H37Rv and H37Ra strains, to infect BALB/c mice. As shown in Fig. 4, the ability of the mutant to multiply and persist in mice over a 90-day period was comparable to that of wild-type H37Rv, indicating that the combined loss of the three families of acyltrehaloses had no impact on the virulence of M. tuberculosis H37Rv in this model. Interestingly, while the H37Ra strain failed to fix neutral red, the mutants were as proficient as M. tuberculosis H37Rv at staining with the dye (data not shown). It can thus be concluded from these studies that the loss of SL, DAT and PAT per se is unlikely to be a major cause of the inability of M. tuberculosis H37Ra to produce progressive disease in animal models. Moreover, the simultaneous loss of these three families of lipids does not appear to have any significant impact on the growth rate and neutral red staining properties of the tubercle bacillus.
FIG. 4.
Multiplication and persistence of M. tuberculosis H37Ra, M. tuberculosis H37Rv and msl3Δpks2 in the lungs (A) and spleen (B) of intravenously infected BALB/c mice. Results are expressed as means ± standard deviations (error bars) of CFU counts for five infected mice. H37Ra (diamonds); wild-type M. tuberculosis H37Rv (triangles); msl3Δpks2 (squares). Asterisks indicate P values < 0.05 (Student's t test) for a comparison of H37Rv- versus msl3Δpks2-infected mice.
Conclusions.
Evidence is provided in this study that a point mutation in the DNA-binding region of the PhoP response regulator from M. tuberculosis H37Ra is responsible for the inability of this strain to produce SL, DAT and PAT. This mutation also partially accounts for the particular morphology of H37Ra.
Observations made on an msl3Δpks2 knock-out mutant of M. tuberculosis H37Rv further allowed us to conclude that while SL, DAT and PAT are likely to account, at least in part, for the inability of H37Ra to grow as serpentine cords, they have by themselves no significant impact on the growth rate, virulence and ability of the tubercle bacillus to stain with neutral red. A recent study on the neutral red staining properties of lipid-deficient mutants of M. tuberculosis (6) suggests, however, that the loss of PDIM in addition to SL, DAT and PAT is the likely cause of the inability of H37Ra to fix the dye.
Given the central role of PhoP-PhoR in M. tuberculosis virulence (31, 40), it is most likely that the identified mutation, along with the absence of PDIM, whose roles in pathogenicity have been well documented (5, 9, 21, 35), is responsible for much of the virulence attenuation of H37Ra. A study aimed at evaluating the virulence and immunological properties of H37Ra complemented with phoP-Rv has been undertaken by some of us to verify this hypothesis and will be reported elsewhere (R. Brosch and S. T. Cole, unpublished results). It is expected that the characterization of the PhoP regulon will allow other molecular determinants contributing to the attenuation phenotype of M. tuberculosis H37Ra to be identified. Possible such members of this regulon include the lipF, fbpA and mmpL8 genes (40), the disruption of all of which has been reported to affect M. tuberculosis virulence and/or pathogenicity (1, 5, 8, 11).
Supplementary Material
Acknowledgments
This work was supported by the Institut Pasteur (GPH-05, Tuberculose) and the European Commission contract LSHP-CT-2003-503367.
We are grateful to Dr. Patrick England (Biophysics core facility, Pasteur Institute) for assistance with acquisition and discussion of the CD spectra.
Footnotes
Published ahead of print on 7 December 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Armitige, L. Y., C. Jagannath, A. R. Wanger, and S. J. Norris. 2000. Disruption of the genes encoding antigen 85A and antigen 85B of Mycobacterium tuberculosis H37Rv: effect on growth in culture and in macrophages. Infect. Immun. 68767-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Besra, G. S., R. Bolton, M. R. McNeil, M. Ridell, K. E. Simpson, J. Glushka, H. van Halbeek, P. J. Brennan, and D. E. Minnikin. 1992. Structure elucidation and antigenicity of a novel family of glycolipid antigens from Mycobacterium tuberculosis H37Rv. Biochemistry 319832-9837. [DOI] [PubMed] [Google Scholar]
- 3.Blanco, A. G., M. Sola, F.-X. Gomis-Ruth, and M. Coll. 2002. Tandem DNA recognition by PhoB, a two-component signal transduction activator. Structure 10701-713. [DOI] [PubMed] [Google Scholar]
- 4.Brosch, R., W. J. Philipp, E. Stavropoulos, J. M. Colston, S. T. Cole, and S. V. Gordon. 1999. Genomic analysis reveals variation between Mycobacterium tuberculosis H37Rv and the attenuated M. tuberculosis H37Ra strain. Infect. Immun. 675768-5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camacho, L. R., D. Ensergueix, E. Pérez, B. Gicquel, and C. Guilhot. 1999. Identification of a virulence gene cluster of Mycobacterium tuberculosis by signature-tagged transposon mutagenesis. Mol. Microbiol. 34257-267. [DOI] [PubMed] [Google Scholar]
- 6.Cardona, P.-J., C. Y. Soto, C. Martin, B. Gicquel, G. Agusti, E. Guirado, T. D. Sirakova, P. E. Kolattukudy, E. Julian, and M. Luquin. 2005. Neutral red reaction is related to virulence and cell wall methyl-branched lipids in Mycobacterium tuberculosis. Microbes Infect. 8183-190. [DOI] [PubMed] [Google Scholar]
- 7.Cason, J., C. Freeman Allen, W. DeAcetis, and G. J. Fonken. 1956. Fatty acids from the lipides of non-virulent strains of the tubercle bacillus. J. Biol. Chem. 220893-904. [PubMed] [Google Scholar]
- 8.Converse, S. E., J. D. Mougous, M. D. Leavell, J. A. Leary, C. R. Bertozzi, and J. S. Cox. 2003. MmpL8 is required for sulfolipid-1 biosynthesis and Mycobacterium tuberculosis virulence. Proc. Natl. Acad. Sci. USA 1006121-6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox, J. S., B. Chen, M. McNeil, and W. R. Jacobs, Jr. 1999. Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature 40279-83. [DOI] [PubMed] [Google Scholar]
- 10.Daffé, M., C. Lacave, M.-A. Lanéelle, M. Gillois, and G. Lanéelle. 1988. Polyphthienoyl trehalose, glycolipids specific for virulent strains of the tubercle bacillus. Eur. J. Biochem. 172579-584. [DOI] [PubMed] [Google Scholar]
- 11.Domenech, P., M. B. Reed, C. S. Dowd, C. Manca, G. Kaplan, and C. E. Barry III. 2004. The role of MmpL8 in sulfatide biogenesis and virulence of Mycobacterium tuberculosis. J. Biol. Chem. 27921257-21265. [DOI] [PubMed] [Google Scholar]
- 12.Dubey, V. S., T. D. Sirakova, and P. E. Kolattukudy. 2002. Disruption of msl3 abolishes the synthesis of mycolipanoic and mycolipenic acids required for polyacyltrehalose synthesis in Mycobacterium tuberculosis H37Rv and causes cell aggregation. Mol. Microbiol. 451451-1459. [DOI] [PubMed] [Google Scholar]
- 13.Dubey, V. S., T. D. Sirakova, M. H. Cynamon, and P. E. Kolattukudy. 2003. Biochemical function of msl5 (pks8 plus pks17) in Mycobacterium tuberculosis H37Rv: biosynthesis of monomethyl branched unsaturated fatty acids. J. Bacteriol. 1854620-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubos, R. J., and G. Middlebrook. 1948. Cytochemical reaction of virulent tubercle bacilli. Amer. Rev. Tuberc. 58698-699. [DOI] [PubMed] [Google Scholar]
- 15.Gao, Q., K. Kripke, Z. Arinc, M. Voskuil, and P. Small. 2004. Comparative expression studies of a complex phenotype: cord formation in Mycobacterium tuberculosis. Tuberculosis 84188-196. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalo Asensio, J., C. Maia, N. L. Ferrer, N. Barilone, F. Laval, C. Soto, N. Winter, M. Daffé, B. Gicquel, C. Martin, and M. Jackson. 2006. The virulence-associated two-component PhoP-PhoR system controls the biosynthesis of polyketide-derived lipids in Mycobacterium tuberculosis. J. Biol. Chem. 2811313-1316. [DOI] [PubMed] [Google Scholar]
- 17.Gupta, S., A. Sinha, and D. Sarkar. 2006. Transcriptional autoregulation by Mycobacterium tuberculosis PhoP involves recognition of novel direct repeat sequences in the regulatory region of the promoter. FEBS Lett. 5805328-5338. [DOI] [PubMed] [Google Scholar]
- 18.He, X.-Y., Y.-H. Zhuang, X.-G. Zhang, and G.-L. Li. 2003. Comparative proteome analysis of culture supernatant proteins of Mycobacterium tuberculosis H37Rv and H37Ra. Microbes Infect. 5851-856. [DOI] [PubMed] [Google Scholar]
- 19.Jackson, M., F. X. Berthet, I. Otal, J. Rauzier, C. Martin, B. Gicquel, and C. Guilhot. 1996. The Mycobacterium tuberculosis purine biosynthetic pathway: isolation and characterization of the purC and purL genes. Microbiology 1422439-2447. [DOI] [PubMed] [Google Scholar]
- 20.Jackson, M., S. W. Phalen, M. Lagranderie, D. Ensergueix, P. Chavarot, G. Marchal, D. N. McMurray, B. Gicquel, and C. Guilhot. 1999. Persistence and protective efficacy of a Mycobacterium tuberculosis auxotroph vaccine. Infect. Immun. 672867-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson, M., G. Stadthagen, and B. Gicquel. 2007. Long-chain multiple methyl-branched fatty acid-containing lipids of Mycobacterium tuberculosis: biosynthesis, transport, regulation and biological activities. Tuberculosis 8778-86. [DOI] [PubMed] [Google Scholar]
- 22.Kinger, A. K., and J. S. Tyagi. 1993. Identification and cloning of genes differentially expressed in the virulent strain of Mycobacterium tuberculosis. Gene 131113-117. [DOI] [PubMed] [Google Scholar]
- 23.Korduláková, J., M. Gilleron, K. Mikusová, G. Puzo, P. J. Brennan, B. Gicquel, and M. Jackson. 2002. Definition of the first mannosylation step in phosphatidylinositol synthesis: PimA is essential for growth of mycobacteria. J. Biol. Chem. 27731335-31344. [DOI] [PubMed] [Google Scholar]
- 24.Martín, C., A. Williams, R. Hernandez-Pando, P. J. Cardona, E. Gormley, Y. Bordat, C. Y. Soto, S. O. Clark, G. J. Hatch, D. Aguilar, V. Ausina, and B. Gicquel. 2006. The live Mycobacterium tuberculosis phoP mutant strain is more attenuated than BCG and confers protective immunity against tuberculosis in mice and guinea pigs. Vaccine 243408-3419. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Hackert, E., and A. M. Stock. 1997. Structural relationships in the OmpR family of winged-helix transcription factors. J. Mol. Biol. 269301-312. [DOI] [PubMed] [Google Scholar]
- 26.Middlebrook, G., C. M. Coleman, and W. B. Schaefer. 1959. Sulfolipid from virulent tubercle bacilli. Proc. Natl. Acad. Sci. USA 451801-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Middlebrook, G., R. J. Dubos, and C. Pierce. 1947. Virulence and morphological characteristics of mammalian tubercle bacilli. J. Exp. Med. 86175-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mostowy, S., C. Cleto, D. R. Sherman, and M. A. Behr. 2004. The Mycobacterium tuberculosis complex transcriptome of attenuation. Tuberculosis 84197-204. [DOI] [PubMed] [Google Scholar]
- 29.Pascopella, L., F. M. Collins, J. M. Martin, M. H. Lee, G. F. Hatfull, C. K. Stover, B. R. Bloom, and W. R. Jacobs. 1994. Use of in vivo complementation in Mycobacterium tuberculosis to identify a genomic fragment associated with virulence. Infect. Immun. 621313-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pelicic, V., M. Jackson, J. M. Reyrat, W. R. Jacobs, Jr., B. Gicquel, and C. Guilhot. 1997. Efficient allelic exchange and transposon mutagenesis in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 9410955-10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pérez, E., S. Samper, Y. Bordat, C. Guilhot, B. Gicquel, and C. Martin. 2001. An essential role for phoP in Mycobacterium tuberculosis virulence. Mol. Microbiol. 41179-187. [DOI] [PubMed] [Google Scholar]
- 32.Rindi, L., N. Lari, and C. Garzelli. 1999. Search for genes potentially involved in Mycobacterium tuberculosis virulence by mRNA differential display. Biochem. Biophys. Res. Commun. 25894-101. [DOI] [PubMed] [Google Scholar]
- 33.Rousseau, C., O. Neyrolles, Y. Bordat, S. Giroux, T. D. Sirakova, M.-C. Prevost, P. E. Kolattukudy, B. Gicquel, and M. Jackson. 2003. Deficiency in mycolipenate- and mycosanoate-derived acyltrehaloses enhances early interactions of Mycobacterium tuberculosis with host cells. Cell. Microbiol. 5405-415. [DOI] [PubMed] [Google Scholar]
- 34.Rousseau, C., O. C. Turner, E. Rush, Y. Bordat, T. D. Sirakova, P. E. Kolattukudy, S. Ritter, I. M. Orme, B. Gicquel, and M. Jackson. 2003. Sulfolipid deficiency does not affect the virulence of Mycobacterium tuberculosis H37Rv in mice and guinea pigs. Infect. Immun. 714684-4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rousseau, C., N. Winter, E. Pivert, Y. Bordat, O. Neyrolles, P. Avé, M. Huerre, B. Gicquel, and M. Jackson. 2004. Production of phthiocerol dimycocerosates protects Mycobacterium tuberculosis from the cidal activity of reactive nitrogen intermediates produced by macrophages and modulates the early immune response to infection. Cell. Microbiol. 6277-287. [DOI] [PubMed] [Google Scholar]
- 36.Soto, C. Y., N. Andreu, I. Gibert, and M. Luquin. 2002. Simple and rapid differentiation of Mycobacterium tuberculosis H37Ra from M. tuberculosis clinical isolates through two cytochemical tests using neutral red and Nile blue stains. J. Clin. Microbiol. 403021-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soto, C. Y., M. C. Menéndez, E. Pérez, S. Samper, A. B. Gomez, M. J. Garcia, and C. Martin. 2004. IS6110 mediates increased transcription of the phoP virulence gene in a multidrug-resistant clinical isolate responsible for tuberculosis outbreaks. J. Clin. Microbiol. 42212-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sreerama, N., and R. W. Woody. 2000. Estimation of protein secondary structure from circular dichroism spectra: comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal. Biochem. 87252-260. [DOI] [PubMed] [Google Scholar]
- 39.Steenken, W., W. H. Oatway, and S. A. Petroff. 1934. Biological studies of the tubercle bacillus. III. Dissociation and pathogenicity of the R and S variants of the human tubercle bacillus (H37). J. Exp. Med. 60515-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walters, S. B., E. Dubnau, I. Kolesnikova, F. Laval, M. Daffé, and I. Smith. 2006. The Mycobacterium tuberculosis PhoPR two-component system regulates genes essential for virulence and complex lipid biosynthesis. Mol. Microbiol. 60312-330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.