Abstract
The cellular localization and processing of the endo-xylanases (1,4-β-d-xylan-xylanohydrolase; EC 3.2.1.8) of the hyperthermophile Thermotoga maritima were investigated, in particular with respect to the unusual outer membrane (“toga”) of this gram-negative bacterium. XynB (40 kDa) was detected in the periplasmic fraction of T. maritima cells and in the culture supernatant. XynA (120 kDa) was partially released to the surrounding medium, but most XynA remained cell associated. Immunogold labeling of thin sections revealed that cell-bound XynA was localized mainly in the outer membranes of T. maritima cells. Amino-terminal sequencing of purified membrane-bound XynA revealed processing of the signal peptide after the eighth residue, thereby leaving the hydrophobic core of the signal peptide attached to the enzyme. This mode of processing is reminiscent of type IV prepilin signal peptide cleavage. Removal of the entire XynA signal peptide was necessary for release from the cell because enzyme purified from the culture supernatant lacked 44 residues at the N terminus, including the hydrophobic part of the signal peptide. We conclude that toga association of XynA is mediated by residues 9 to 44 of the signal peptide. The biochemical and electron microscopic localization studies together with the amino-terminal processing data indicate that XynA is held at the cell surface of T. maritima via a hydrophobic peptide anchor, which is highly unusual for an outer membrane protein.
Thermotoga maritima was the first hyperthermophilic species of the domain Bacteria to be discovered (18), and to date it is the best-studied bacterial hyperthermophile. For the organisms related to Thermotoga, the phylum Thermotogae was proposed (35). The hyperthermophilic bacteria represent a deep branch within the phylogenetic tree of the Bacteria, suggesting that these organisms may have retained archetypal features resembling those of primordial bacterial cells. This expectation is apparently supported by the fact that some of the biomolecules and properties of T. maritima have indeed revealed quite unusual features (19). Its peptidoglycan is thin and with a low degree of cross-linking, devoid of meso-diaminopimelic acid but containing d-lysine and l-lysine, which are absent from other gram-negative bacteria (17). A common morphological characteristic of the members of the order Thermotogales is the presence of an outer sheath-like envelope (“toga”) (19). For cells of the genus Thermotoga, the toga forms large balloons at the cell ends and in this way encloses a periplasmic compartment with a volume comparable to or sometimes even larger than that of the cytoplasm. The only components of the toga studied in any detail are the coiled-coil protein Ompα and the porin Ompβ of T. maritima (13, 14, 34). Previous studies have indicated that besides Ompβ, certain enzymes are also probably localized in the toga (reference 36 and our unpublished results).
The genome of T. maritima strain MSB8 encodes all enzymes needed for the degradation of complex xylans consisting of a poly-β-1,4-linked xylose backbone substituted with acetyl, arabinofuranosyl and 4-O-methylglucuronyl groups, i.e., xylanases, β-xylosidase, arabinofuranosidase, α-glucuronidase, and acetyxylan esterase. This paper deals with the localization of the endo-xylanases (1,4-β-d-xylan-xylanohydrolase; EC 3.2.1.8) XynB and, in particular, XynA of T. maritima strain MSB8. XynA is an extremely thermostable 120-kDa enzyme whose precursor sequence, based on the primary structure derived from the nucleotide sequence of the gene, is composed of an apparently typical N-terminal signal peptide, followed by five domains in the order A1-A2-B-C1-C2 (42). The central part (domain B, ∼340 amino acids), which represents the catalytic domain, belongs to family 10 of glycoside hydrolases. The N-terminal ∼150-amino-acid repeated domains (A1 and A2) have no significant similarity to the C-terminal ∼170-amino-acid repeated domains (C1 and C2). Domain A2 belongs to family 22 of carbohydrate-binding modules (CBMs) and has been shown to display xylan-binding ability (24, 30). Domain C2 has been shown to represent a cellulose-binding module (42) which belongs to family 9 of CBMs and is unique in that it specifically binds to the reducing end of cellulose and soluble polysaccharides as well as to a variety of mono-, di-, and oligosaccharides. After binding to microcrystalline cellulose, elution of C2 can be achieved with a 0.2 M cellobiose solution (10, 22, 32, 42). The data reported here clearly demonstrate the toga association of the multidomain xylanase XynA and provide information about the processing and the mechanism of attachment of this enzyme to the cell envelope of T. maritima.
MATERIALS AND METHODS
Strain and growth conditions.
Thermotoga maritima strain MSB8 (DSM 3109) was propagated in Difco marine broth medium 2216 (Difco, Detroit, MI) supplemented with 0.25% soluble starch or xylose as described before (42). For large batch cultures, cells from a fresh overnight culture were inoculated into a reduced medium consisting of 0.5% peptone, 0.1% yeast extract, 0.25% soluble starch or xylose, 1% (vol/vol) Difco marine broth, 3% NaCl, 0.5% Na2S, and 0.0001% resazurin dissolved in tap water and preheated to 80°C. Disruption of T. maritima cells for the preparation of crude extracts was achieved by passing suspensions of cells in 20 mM bis-Tris buffer (pH 6.2) through a French pressure cell (American Instrument Company, Silver Spring, MD) at 6.9 MPa.
Protein purification, analytical methods, and xylanase assay.
Previously published methods (25, 26) were used for the determination of protein concentrations and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Detection of protein bands with thermostable xylanase activity after separation by SDS-PAGE was done with a zymogram staining technique described before (42). Amino-terminal sequencing of protein samples via sequential Edman degradation was done on an Applied Biosystems 477A sequencer.
For purification of XynA from T. maritima culture supernatant, the supernatant obtained from 50 liters of culture was concentrated to a final volume of 300 ml by ultrafiltration with a Sartocon ultrafiltration system followed by a second step with a Sartocon mini-ultrafiltration system (Sartorius, Göttingen, Germany), both equipped with cellulose triacetate membranes (10,000-Da cutoff). The concentrated supernatant was applied to a column with a 400-ml bed volume packed with microcrystalline cellulose. Washing of the column and elution of the 120-kDa enzyme with 0.1 M cellobiose were done as described previously (42).
For expression of xynA in Escherichia coli, a synthetic oligonucleotide primer with the sequence 5′-GGACTGAATTCATGCAAGTCAGGAAGAGACGGGG-3′ was used in combination with primer 6 (42) to generate via PCR a DNA fragment carrying the complete xynA coding region. The PCR product was cut at the ends with EcoRI and SmaI before insertion into the accordingly double-digested Ptac expression vector pJF118ut (8), a derivative of pJF118EH (16). This plasmid construction, designated p86, was introduced into E. coli WCM105. The secreted recombinant xylanase was purified to apparent electrophoretic homogeneity via ultrafiltration and cellulose affinity chromatography.
Standard assay mixtures for the determination of xylanase activity contained 0.8% oat spelt xylan (Roth, Karlsruhe, Germany), 250 mM NaCl, 50 mM bis-Tris buffer (pH 6.2), and appropriately diluted enzyme (in some cases in the form of a suspension of washed cells; see below), and assays were performed in a total volume of 0.5 ml. Unless mentioned otherwise, incubation was carried out for 10 min at 75°C. The amount of reducing groups liberated during the enzymatic hydrolysis of xylan was quantitated by the dinitrosalicylic acid method (7) as described previously (43). One unit of xylanase activity is the amount of enzyme necessary to liberate 1 μmol of reducing groups (as xylose equivalents) per minute.
Osmotic shock treatment of T. maritima cells.
Cells of T. maritima (1 g) grown in medium with soluble starch (0.2%), oat spelt xylan (0.1%), or xylose (0.5%) were suspended in 8 ml of 500 mM glucose-1 mM EDTA-200 mM Tris-HCl, pH 7.4. After addition of 0.8 ml of a lysozyme solution (20 mg ml−1) and 5 ml polymyxin B sulfate (1 mg ml−1) and incubation at ambient temperature for 30 min, 8 ml distilled water was added; 30 min later, 32 ml distilled water was added. The supernatant obtained after centrifugation for 10 min at 11,000 × g was used as the periplasmic protein preparation. Control experiments demonstrated the efficient separation of the T. maritima periplasmic maltose-binding protein with this spheroplasting and osmotic shock treatment (not shown).
Immunogold labeling and electron microscopy.
A suspension of T. maritima cells from a fresh overnight culture was used for fixation (30 min, 20°C) with 2% paraformaldehyde, 1% glutaraldehyde, and 0.5% uranyl acetate. The cells were embedded in 2% low-gelling agarose type VII (Sigma) before the preparation was subjected to multistep infiltration with Lowicryl K4M (Lowi, Waldkraiburg, Germany) using the PLT method (23) as described before (14). After polymerization of the sample with UV light, thin sections (60 nm) were collected on nickel grids.
For immunolabeling, the nickel grids with the T. maritima thin sections were incubated for 1 h in labeling solution (TBS [10 mM Tris {pH 7.5}, 0.15 M NaCl] supplemented with 0.05% Tween 20, 5% milk powder, and 0.5 M NaCl) and then for 3 h in labeling solution mixed with 0.01 volume of a polyclonal anti-XynA antiserum, which was a rabbit-derived preparation raised against a recombinant truncated xylanase derivative consisting of the central catalytic domain of XynA (42). After five brief washing steps in labeling solution, the grids were incubated for 1 h with anti-rabbit immunoglobulin G (IgG) gold (10 nm) conjugate (Sigma, Deisenhofen, Germany) diluted 1:10 to 1:50 in labeling solution. After washing repeatedly in labeling solution and once in water (5 min), the grids were air dried for 10 min. Poststaining was done by incubation for 10 min in 4% uranyl acetate and, after three washes with water, for 5 min in lead citrate, followed by a final wash with water. After shadowing with carbon, electron micrographs were recorded with a Philips CM12 apparatus at a nominal magnification of ×16,000.
Immunofluorescence labeling and epifluorescence microscopy.
Cells from a fresh overnight culture of T. maritima grown in medium with 0.25% xylose were collected by centrifugation and suspended in TBS. The cells were used for immunolabeling either after fixation, which was accomplished by mixing the suspension with 3 volumes TBS containing 4% paraformaldehyde and incubating at 4°C for 16 h, or without prior fixation. For immunolabeling, 0.01 volume of rabbit anti-XynA antiserum was added. After 90 min, excess primary antibody was removed by washing the cells with TBS. The cells were then suspended in TBS and mixed with 0.025 volume fluorescein isothiocyanate-anti-rabbit antibody conjugate (Sigma) for 90 min before immobilization on a gelatin-coated glass slide. Fluorescence microscopy was carried out at 495 nm with a Zeiss Axioplan epifluorescence microscope. In some experiments, immunolabeling of the fixed cells was preceded by successive 2-min incubation steps in 30%, 50%, and 70% ethanol. In another set of experiments, intact cells were directly immobilized on gelatin-coated microscopic slides, fixation with formaldehyde was omitted, and the cells were air dried before immunolabeling on the slide surface. The apparent intactness of the cells was generally checked by phase-contrast microscopic examination. Dried cells and ethanol-treated cells displayed relatively weak phase contrast.
RESULTS
Cell-associated and extracellular xylanase production by Thermotoga maritima.
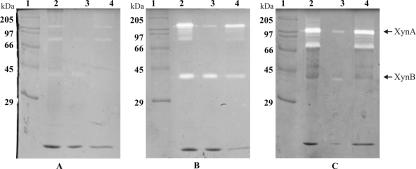
Upon growth of T. maritima strain MSB8 in medium supplemented with either soluble starch (0.2%, wt/vol), oat spelt xylan (0.1% wt/vol), or xylose (0.5%, wt/vol), 0.5 g cells from each culture were harvested, and the xylanase activities present in whole cells, in the soluble fraction from spheroplasting and osmotic shock treatment (periplasmic fraction), and in the non-shock-extractable fractions of the cells were compared by SDS-PAGE and zymogram staining (Fig. 1). While activity bands from starch-grown cells were barely visible, both the 120-kDa XynA and the 40-kDa XynB were found in xylan- or xylose-grown cells, in accordance with previous results (42), confirming that xylanase production in T. maritima underlies an induction mechanism. Obviously, more XynB was present after growth on xylan than after growth on xylose, which indicates that xylan-derived oligosaccharides are more efficient inducers of XynB synthesis than the monosaccharide xylose. While most XynB was released from the cells with spheroplast formation and osmotic shock (Fig. 1B and C, lanes 3), XynA (and variable amounts of smaller activity bands presumably representing C-terminally shortened derivatives) remained cell bound during this procedure.
FIG. 1.
Xylanase zymogram staining of SDS-polyacrylamide gel of fractions of T. maritima cells grown on soluble starch (A), oat spelt xylan (B), or xylose (C). Lanes 1, molecular mass marker proteins; lanes 2, total crude extract; lanes 3, soluble fraction after spheroplast preparation (cleared supernatant; periplasmic fraction); lanes 4, residual fraction after spheroplast preparation (nonperiplasmic cell-bound proteins). In each gel, the samples applied to lanes 2, 3, and 4 were derived from identical amounts of cells. Dark bands are Coomassie blue-stained protein bands, and white bands against the gray background correspond to proteins with xylanase activity. The mobilities of full-length XynA (120 kDa) and XynB (40 kDa) are marked at the right.
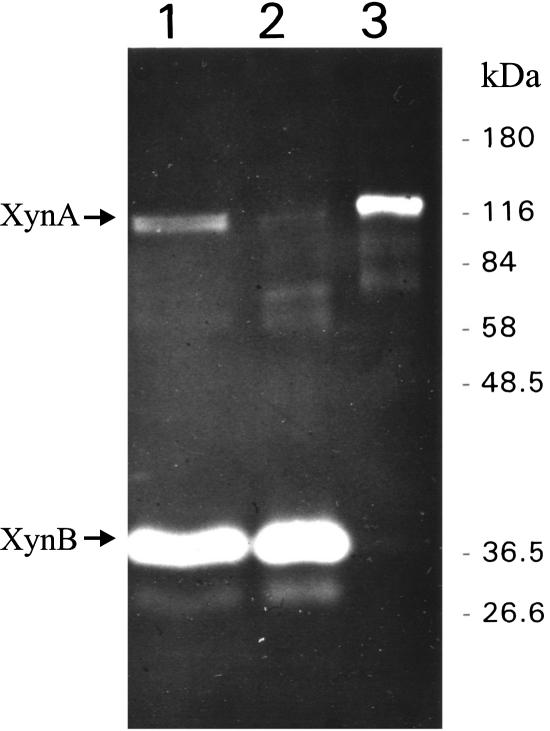
In several independent experiments where T. maritima strain MSB8 was grown in xylose-supplemented medium, we have observed that xylanase activity was present in the culture supernatant. The activity in the supernatant represented about 16% to 30% of the total xylanase activity (cell-associated plus extracellular) of the culture (data not shown). In order to characterize the extracellular xylanase(s), the supernatant obtained from 50 liters of culture was concentrated by ultrafiltration (10,000-Da cutoff) as described in Materials and Methods. SDS-PAGE coupled with zymogram staining for thermoactive xylanase activity revealed two bands with mobilities of 120 kDa and 40 kDa (Fig. 2, lane 1). Thus, it appeared that both T. maritima xylanases previously purified from cell extracts (42), i.e., XynA (120 kDa) and XynB (40 kDa), were also present in the culture supernatant. In case the 120-kDa secreted enzyme was indeed XynA, which has been shown to contain a cellulose-binding domain (42), cellulose affinity chromatography was thought to be an adequate method for purification. Indeed, it was possible to separate the secreted xylanases with this method (Fig. 2). The N-terminal sequence determined for the secreted 40-kDa xylanase was NH2-Phe-Gln-Asn-Val-Ser-Leu-Arg-Glu-Leu-Ala-Xxx-Lys-Leu-Asn- Ile-Tyr-Ile-Gly-Phe-Ala-Ala-Ile, which corresponds to positions 20 to 38 of the XynB precursor sequence, with the exception of the first residue, which according to the translation of the xynB open reading frame is Ser and not Phe. An aliquot of the purified secreted 120-kDa enzyme was also subjected to N-terminal sequencing. The 14 N-terminal residues were NH2-Gly-Asp-Ser-Ser-Leu-Glu-Thr-Val-Leu-Ala-Leu-Ser-Phe-Glu, which corresponds to positions 45 to 58 of the pre-XynA primary structure.
FIG. 2.
Zymogram of SDS-polyacrylamide gel with xylanases from the supernatant of a T. maritima MSB8 culture and their separation via cellulose affinity chromatography. Lane 1, concentrated culture supernatant (10 μg protein) before cellulose affinity chromatography; lane 2, material with no affinity to microcrystalline cellulose (100 μg protein); lane 3, XynA eluted from the cellulose affinity column with 0.1 M cellobiose. Note that it is not possible to accurately estimate the relative amounts of XynA and XynB on the basis of the intensity of the active bands due to the fact that the two enzymes differ significantly in specific activity, pH and temperature optima, and molecular mass (43). The numbers at the right indicate the positions of molecular mass markers.
Upon expression of the complete xynA coding region in E. coli WCM105, which is a strain with a leaky outer membrane that releases proteins exported across the cytoplasmic membrane to the culture medium, the secreted recombinant xylanase was purified to apparent electrophoretic homogeneity and subjected to N-terminal sequencing. The 10 N-terminal residues were NH2-Gly-Asp-Ser-Ser-Leu-Glu-Thr-Val-Leu-Ala, which corresponds to positions 45 to 54 of pre-XynA. Thus, the recombinant xylanase exported by E. coli had the same N terminus as authentic XynA isolated from the T. maritima MSB8 culture supernatant (data not shown). However, we observed that the signals obtained on the phenylthiohydantoin amino acid analyzer during sequencing of the secreted recombinant XynA were weaker than expected for the amount of protein used for the analysis, which could be an indication of partial N-terminal modification.
Amino-terminal amino acid sequence of membrane-bound XynA.
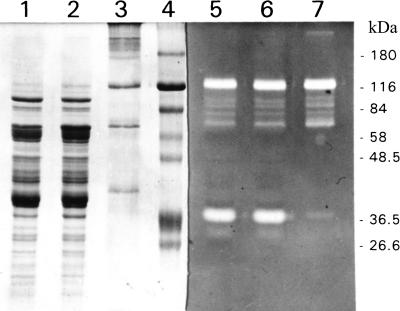
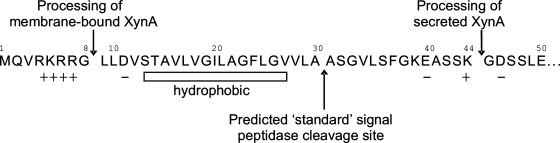
Since previous experiments had indicated the presence of XynA in the membrane fraction of T. maritima cells, a membrane fraction was prepared from 12 g of T. maritima cells grown in the presence of 0.25% xylose by French press lysis of the cells suspended in 30 mM bis-Tris (pH 7), removal of remaining intact cells by centrifugation at 5,000 × g for 15 min, and high-speed centrifugation (140,000 × g) for 1.5 h in a TST41.14 swing-out rotor (Kontron Instruments). The membrane fraction was suspended in 50 mM bis-Tris (pH 6.2), recentrifuged, and solubilized in 50 mM bis-Tris (pH 6.2) containing 2% Triton X-100 with the aid of sonication. Of the total xylanase activity of the crude lysate (note that not only XynA but also XynB contributes to the total activity of the crude lysate), 43.5% was recovered from the membrane fraction (Table 1). SDS-PAGE analysis coupled with zymogram staining for xylanase activity showed that there was almost no XynB left in this membrane fraction (Fig. 3). The 120-kDa xylanase XynA was purified from the membrane preparation with the chromatographic steps described earlier (43). The enzyme preparation obtained was homogenous as judged by SDS-PAGE analysis. Seven cycles of automated N-terminal Edman degradation yielded the sequence NH2-Leu-Leu-Asp-Val-Ser-Thr-Ala for the membrane-derived xylanase. This sequence is present once, i.e., at positions 9 to 15, in the pre-XynA primary structure derived from the nucleotide sequence of the gene (42) and thus indicates proteolytic processing of the precursor after residue Gly8, which is on the N-terminal side of the hydrophobic core region of the XynA signal peptide.
TABLE 1.
Xylanase activity in fractions of a T. maritima crude cell extracta
| Fraction | Protein (mg) | Xylanase activity (U)b | % of total activity |
|---|---|---|---|
| Soluble | 648 | 3,950 | 51.2 |
| Wash supernatant | 52 | 406 | 5.3 |
| Membrane | 68 | 3,360 | 43.5 |
| Total | 768 | 7,716 | 100.0 |
The extract was obtained by disruption of cells by French press lysis followed by high-speed centrifugation (140,000 × g, 1.5 h). The membrane pellet was washed once with 50 mM bis-Tris buffer, pH 6.2.
Xylanase activities are expressed as units of oat spelt xylan-hydrolyzing activity and were determined in 100 mM sodium phosphate-citrate buffer (pH 6.2) at 90°C.
FIG. 3.
SDS-PAGE analysis of crude extract and membrane proteins of T. maritima. See footnote a of Table 1 for details of sample preparation. Lanes 1 and 5, crude extract; lanes 2 and 6, wash supernatant; lanes 3 and 7, washed membrane fraction; lane 4, molecular mass standard proteins. The sizes of the markers are indicated at the right. Ten micrograms of protein was applied to each lane. The left half of the gel was stained with Coomassie brilliant blue, while the right half of the gel was stained for xylanase activity.
Immunogold labeling experiments for investigation of the subcellular localization of XynA.
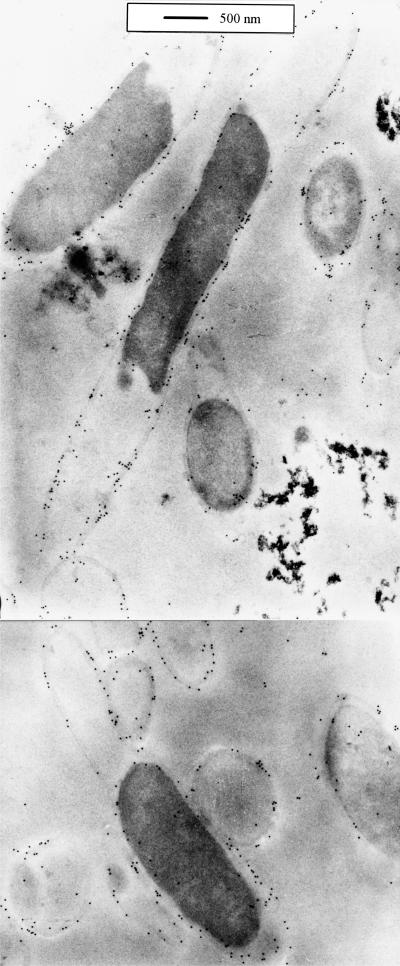
The biochemical evidence pointing to the membrane localization of cell-associated XynA raised the question of whether the enzyme is associated with the cytoplasmic membrane or the outer membrane of T. maritima cells. In order to determine the precise subcellular localization, the organism was grown in Difco marine broth supplemented with 0.25% xylose. The cells were harvested, fixed, and embedded as described in Materials and Methods before the preparation of thin sections (60 nm). A polyclonal antiserum raised against a recombinant truncated xylanase derivative consisting of the central catalytic domain of XynA (42) was used to probe the xylanase in the thin sections. Specifically bound anti-XynA antibodies were labeled with anti-rabbit IgG conjugated with 10-nm gold particles, which were then detected via transmission electron microscopy (Fig. 4). The location of the gold spheres in relation to the cellular substructures demonstrated that XynA is evenly distributed along the entire cell envelope. The unique cellular morphology of T. maritima, particularly the large distance between the cytoplasmic membrane and the outer membrane at the cell poles, facilitates the interpretation of the immunogold labeling results. Since the outer membrane parts ballooning over the cell ends were labeled as well as the cylindrical part of the cells, but on the other hand, the cytoplasmic membrane at the cell ends carried no significant amount of label, it seems clear that XynA is localized mainly in the toga. The frequency of gold particles within the cytoplasmic and periplasmic compartments was similar to the background level outside of the cells.
FIG. 4.
Electron micrograph of immunogold-labeled thin sections of fixed, Lowicryl K4M-embedded Thermotoga maritima cells. The primary antibody used for labeling of XynA was raised against the recombinantly synthesized, purified catalytic domain of XynA. The secondary antibody was an anti-rabbit IgG gold (10 nm) conjugate.
Immunofluorescence labeling and xylanase activity of intact T. maritima cells.
The immunogold labeling method successfully used as described above is not suited to discriminate whether the antigen is located on the outer or the inner side of the toga of T. maritima cells. Therefore, we attempted to address this question by the determination of xylanase activity with intact cells. For this purpose, cells from a fresh overnight culture in Difco marine broth supplemented with 0.25% xylose were harvested and washed once with 3% NaCl, and half of the washed cells were subjected to French press disintegration. Comparison of the total xylanase activity of the intact cells with the activity of the crude lysate, as determined with the standard assay for XynA (43), revealed that the total activity of the crude lysate was only 1.3-fold higher than the activity of the intact cells (data not shown), indicating the accessibility of XynA to high-molecular-mass substrate. The 30% increase of xylanase activity upon cell lysis can be attributed to the release of the 40-kDa enzyme XynB, which is partially cell associated as it can be purified from a crude cell extract (43), apparently localized mainly in the periplasm but not membrane bound (see above).
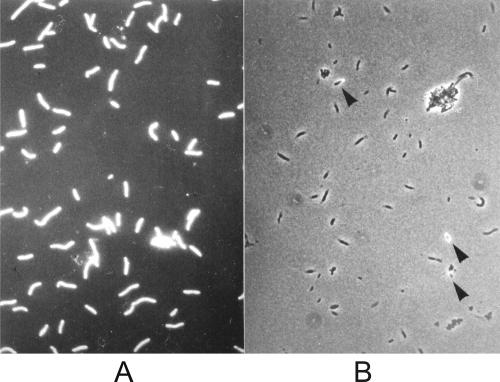
XynA in whole cells was inaccessible to immunofluorescence labeling when untreated or formaldehyde-fixed T. maritima cells grown in the presence of xylose were used. In this case, fewer than 5% of the cells revealed fluorescence when examined with an epifluorescence microscope after the labeling procedure (Fig. 5). This could mean that the antigenic epitopes of the catalytic domain of XynA were not accessible for antibody binding, perhaps due to steric hindrance by the noncatalytic domains of the enzyme itself or by other cell surface structures such as proteins or carbohydrates, or that XynA has an inward orientation facing the periplasmic compartment. The latter possibility seems unlikely because most of the xylanase activity of whole cells of T. maritima was accessible from the outside (see previous paragraph). Alternatively, the treatment with anti-XynA serum could have released XynA from the cell surfaces of the nonfixed intact cells, which would also result in nonfluorescent cells. On the other hand, when the cells were air dried on the microscopic slide or when formaldehyde-fixed cells were treated with 70% ethanol, subsequent immunofluorescence labeling was positive for 100% of the cells. Both methods of pretreatment did not affect the overall cell morphology, as judged by microscopic examination (not shown), but presumably caused (partial) dehydration/denaturation of toga components.
FIG. 5.
Epifluorescence micrographs of cells of T. maritima strain MSB8 grown in medium containing 0.25% xylose after immunolabeling with anti-XynA antiserum and anti-rabbit IgG fluorescein isothiocyanate conjugate. (A) Formaldehyde-fixed cells labeled without ethanol pretreatment. This micrograph represents a double exposure under phase-contrast and epifluorescence microscope conditions in order to visualize both fluorescent (arrowheads) and nonfluorescent cells. (B) Epifluorescence micrograph of cells labeled after pretreatment with ethanol as described in Materials and Methods. In this case, all cells displayed green fluorescence.
Finally we attempted to proteolytically degrade surface-exposed XynA from whole T. maritima cells. However, this method was not useful to characterize the orientation of XynA, since the proteinase K treatment (2 to 10 mg ml−1, 30 min, 55°C) led to the loss of the rod-shaped morphology of more than 95% of the T. maritima cells (not shown).
DISCUSSION
Localization of xylanases in T. maritima cells.
The amount of xylanase released to the medium by T. maritima MSB8 cells was in the range of 16 to 30% of the total activity (data not shown). The electrophoretic mobilities (120 kDa and 40 kDa, respectively) and cellulose-binding properties provided indirect evidence that the two xylanases found in the supernatant (Fig. 2) were extracellular derivatives of the same xylanases, XynA (120 kDa) and XynB (40 kDa), that initially were found in crude cellular extracts prepared from washed T. maritima MSB8 cells (43). N-terminal sequencing of the secreted 40-kDa xylanase revealed that this enzyme represented XynB devoid of its 19-residue signal peptide. Thus, cleavage of the XynB precursor had occurred at the position predicted by sequence analysis with the program SignalP 3.0 (6). The software LipoP 1.0 (21) did not predict a lipoprotein cleavage site. It is currently unknown whether a specific mechanism for transport across the outer membrane exists or whether nonspecific phenomena such as leakage or autolysis lead to the appearance of XynB in the surrounding medium after its export to the periplasmic space (Fig. 1). N-terminal sequencing of the purified secreted 120-kDa xylanase demonstrated that this enzyme was indeed a product of the same gene as cell-bound XynA. The secreted XynA species was processed after residue 44, i.e., on the C-terminal side of the hydrophobic region of the signal peptide (Fig. 6) of pre-XynA.
FIG. 6.
Summary of processing events observed with toga-associated and secreted XynA derivatives produced by T. maritima strain MSB8. The hydrophobic core of the XynA signal peptide as well as charged residues and the predicted standard signal peptidase cleavage site (which apparently is not utilized in T. maritima) are also indicated.
More than 40% of the total cell-bound xylanase activity was found in the membrane fraction after high-speed centrifugation of T. maritima crude extract (Fig. 3; Table 1). XynA most likely accounts for most of the membrane-bound activity (Fig. 2). Also, the immunogold labeling experiments with polyclonal anti-XynA antibodies (Fig. 4) demonstrate the membrane association of XynA. We found uniformly distributed labeling along the entire surface of the cells. From the lack of specific labeling of the cytoplasmic membrane at the cell ends, where the outer membrane (toga) and the cytoplasmic membrane are far apart, we conclude that the main subcellular location of XynA is the toga. It cannot be excluded that part of XynA may be (perhaps intermediately) bound to the cytoplasmic membrane along the cylindrical part of the cell. However, about 77% of the total cell-associated xylanase activity is accessible to the polymeric substrate from outside without disintegration of the cells (see below). This fact together with the immunodetection of much of the XynA in the toga regions supports a functional localization for access to extracellular xylan.
The high labeling density found in the immunogold-labeled ultrathin sections (Fig. 4) indicates a large abundance of XynA molecules in the toga, which is in agreement with earlier biochemical experiments. Based on the fact that about sixfold purification was sufficient to obtain >90% pure XynA from a membrane fraction of T. maritima cells (43) and assuming that roughly 50% of the membrane fraction is outer membrane (which due to its ballooning at the cell ends is an underestimate), XynA under appropriate conditions of xylanase induction may represent as much as one-third of the outer membrane proteins of T. maritima cells. Using different growth conditions, lower apparent levels of membrane-bound xylanase have been observed (unpublished data).
In intact T. maritima cells, the xylanase was largely accessible to polymeric oat spelt xylan. The total activity of a crude lysate prepared from washed T. maritima cells was only 1.3-fold higher than the activity of a corresponding amount of intact cells, meaning that about 77% of the total xylanase activity of T. maritima cells, as determined under optimal XynA assay conditions, is accessible from outside without cell disruption. Taking into account that part of the crude extract activity (but not of the undisrupted whole-cell activity) must be attributed to the soluble 40-kDa xylanase XynB, it seems clear that toga-associated XynA must be responsible for most of the activity measured with washed whole cells. Previous studies on the toga proteins of T. maritima, especially the porin Ompβ, which appears to have properties similar to those of other bacterial porins (13), make it seem unlikely that xylan can enter the periplasm without prior cleavage. We conclude that XynA is faced toward the exterior medium. Curiously, however, immunofluorescence labeling of XynA on untreated or formaldehyde-fixed whole cells was not possible, indicating that under these conditions the central catalytic domain of the enzyme (note that the antibodies were raised against this part of XynA) is inaccessible to antibody binding. Possibly, the noncatalytic domains of XynA (24) or other cell surface structures such as proteins or carbohydrates prevented antibody binding. Alternatively, treatment of the nonfixed cells with anti-XynA serum may have caused the release of XynA from the cell surface, a phenomenon that was reported to occur with pullulanase bound to Klebsiella oxytoca (K. pneumoniae) cells (12).
Mode of outer membrane (toga) anchorage of XynA.
Some members of the Firmicutes (low-GC gram-positive bacteria) have cell wall-anchored xylanases with a modular structure similar to that of XynA, with a central glycoside hydrolase family 10 catalytic domain flanked on the N-terminal side by family 22 CBMs and on the C-terminal side by family 9 CBMs (e.g., Paenibacillus sp. Xyn5, Clostridium josui Xyn10A, Clostridium stercorarium Xyn10B, Thermoanaerobacterium thermosulfurigenes XynA, and Thermoanaerobacterium saccharolyticum XynA). Cell surface display of these enzymes is mediated by two or three C-terminal S-layer-homologous domains of 50 to 60 residues (15, 28) which are thought to bind to secondary cell wall polymers (4, 20, 44). In Rhodothermus marinus and related bacteria, a different, approximately 80-residue C-terminal domain is involved in cell attachment (31). Finally, some clostridial xylanases are cell wall bound within large enzyme complexes called cellulosomes. We now show that in the gram-negative T. maritima, a member of a deeply branching phylogenetic lineage, a completely different mechanism of xylanase cell surface display is used.
At first glance, the N terminus of pre-XynA looks like a typical standard signal peptide, with positively charged residues near the N terminus, a ∼15-amino-acid hydrophobic core region, and a possible cleavage site for signal peptidase (predicted as described by von Heijne [41] to be between Ala30 and Ala31). However, in membrane-bound XynA, processing had occurred between Gly8 and Leu9, leaving the hydrophobic core attached to the enzyme. On the other hand, the N terminus of the XynA species isolated from the culture supernatant began at residue 45 and therefore lacked the entire signal peptide. We conclude that the N terminus of XynA (residues 9 to 44), most likely the hydrophobic region of the signal peptide, represents the anchor responsible for the attachment of the enzyme to the outer membrane of T. maritima. Signal peptide cleavage on the N- instead of the C-terminal side of the hydrophobic core, as found with toga-associated XynA, is reminiscent of type IV prepilin processing but to our knowledge is unprecedented for an outer membrane-bound enzyme. A comparison of the signal peptides of pre-XynA and various type IV prepilins is shown in Fig. 7.
FIG. 7.
Alignment of typical type IV prepilin signal peptides with the N-terminal sequence of T. maritima XynA. The highly conserved glycine and glutamic acid residues are boxed. An aspartic acid residue near the processing site is boxed in the case of the T. maritima enzyme, which lacks the glutamic acid at position +5. The arrow indicates the processing site.
Type IV pilin proteins undergo unique processing and modification steps in the course of their maturation and finally assemble to cell surface appendages in diverse gram-negative bacteria (39). The signal peptides of type IV prepilins are cleaved after an invariant glycine residue on the N-terminal side of the hydrophobic core region (38). The amino acid at position +1, usually a phenylalanine or a methionine, often is N methylated. An invariant (with exceptions in the GspK family of pseudopilins [9]) glutamic acid residue at position +5, although not necessary for prepilin cleavage, appears to be essential for N methylation and pilus assembly (39). Cleavage and modification are performed by a bifunctional enzyme called type IV prepilin peptidase (33). After endoproteolytic cleavage, the type IV pilins are thought to accumulate in the cytoplasmic membrane before assembly, but they may be less firmly anchored due to the lack of a positively charged N terminus (33). It is noteworthy that the genome of T. maritima contains only one open reading frame with a classical type IV pilin-like signal peptide (TM1271).
Like type IV prepilins, the XynA precursor of T. maritima is processed on the N-terminal side of the hydrophobic core region of its signal peptide after the first glycine (Gly8) of the sequence. A type IV prepilin peptidase-like enzyme putatively encoded by a PilD gene ortholog in the T. maritima genome (TM1696) may be involved in cleavage of pre-XynA. A glutamic acid residue at position +5 relative to the cleavage site, which in type IV pilins apparently is necessary for methylation (39), is not present in XynA, but N methylation of the N-terminal residue (Leu9) of membrane-bound XynA also could not be detected (data not shown).
In this context, it is interesting to note that the flagellins and exported membrane-anchored proteins such as sugar-binding proteins of ABC transporters of archaea have type IV pilin-like signal peptides with a positively charged amino terminus that connects to a highly hydrophobic stretch of amino acids through a conserved glycine. These preproteins, like XynA, do not contain a conserved glutamate at position +5, and they do not undergo methylation upon signal peptide cleavage (2, 3).
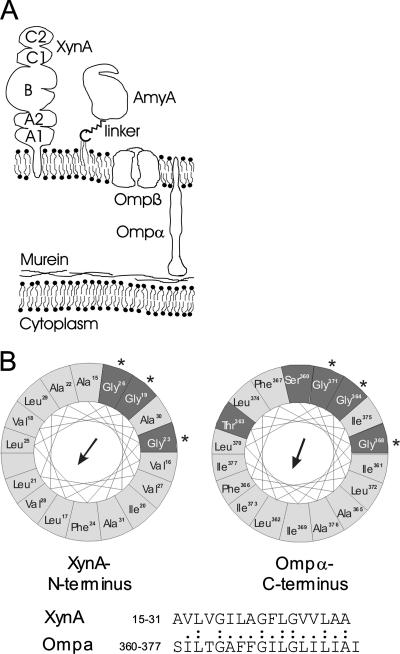
Typical outer membrane proteins of gram-negative bacteria differ from integral cytoplasmic membrane proteins in that their transmembrane segments are formed by amphipathic β-sheets rather than hydrophobic α-helices (40). On the contrary, long stretches of hydrophobic amino acids can act as stop-transfer sequences, preventing release from the cytoplasmic membrane and sorting to the outer membrane (1, 29, 37). A different common mode of attachment to the outer membrane is via N-terminal lipoprotein modification which results in a fatty-acylated cysteine residue at the extreme N terminus of the mature protein. Our data suggest that binding of the xylanase XynA to the outer membrane of T. maritima cells is accomplished with neither of the two modes just discussed but rather via an N-terminal hydrophobic membrane anchor (Fig. 8), which is highly unusual for an outer membrane protein. Interestingly, another protein of T. maritima, called Ompα, a coiled-coil protein which apparently spans the periplasmic space, was proposed to be anchored to the outer membrane via a carboxy-terminal hydrophobic peptide tail (14). Intriguingly, the hydrophobic core sequences of the postulated outer membrane anchors of XynA (N terminal) and Ompα (C terminal) bear striking similarity to one another, including three conserved glycine residues (Fig. 8). We are aware of only one other case, i.e., the phospholipase PldA of E. coli, where export to the outer membrane without proteolytic removal of the putative (but in our opinion far from optimal because quite hydrophilic) signal peptide has been suggested (11).
FIG. 8.
(A) Schematic model of the T. maritima cell envelope, showing the postulated mode of anchoring of XynA in the toga via a hydrophobic N-terminal insertion signal. The five-domain modular structure of XynA consists of the central catalytic domain (B) flanked by repeated N-terminal domains (A1 and A2) and repeated C-terminal domains (C1 and C2), which represent CBMs. The most abundant proteins of the T. maritima cell envelope, i.e., Ompα (a dimeric coiled-coil protein apparently spanning the periplasm [14]) and Ompβ (a porin [13, 34]), are also indicated. The lipid content and composition of the T. maritima outer membrane and the mode of association of Ompα with murein or the cytoplasmic membrane are not clear. (B) Helical wheel representation of the hydrophobic cores of the postulated outer membrane anchors of XynA and Ompα of T. maritima. Hydrophobic residues are shaded in light gray. In both cases the glycine residues (marked with asterisks) occupy similar positions in the structure.
The unusual mode of outer membrane anchoring of T. maritima XynA raises a number of interesting questions. (i) What are the sorting signals that lead to outer membrane localization? (ii) What does the terminal part of the secretion pathway, leading to the final location of the enzyme in the outer membrane, look like? (iii) Where and how does the processing of pre-XynA after residue 44 (Fig. 6), which leads to the secreted XynA species found in the culture supernatant, take place? Clearly more work is necessary to come to a better understanding of protein transport and localization in the ancestral eubacterium T. maritima.
Interestingly, most of the amylase activity of T. maritima MSB8 cells also is associated with the toga (reference 36 and our unpublished data). According to sequence data, the mode of attachment to the outer membrane of at least one α-amylase, AmyA, appears to be based on a bacterial lipoprotein modification (27) and therefore is different from the case for XynA. Thus, the hyperthermophile T. maritima has various polysaccharide hydrolases anchored via different mechanisms in the outermost cell layer. The retention of these enzymes at the cell surface is a plausible strategy to avoid the rapid loss of secreted enzymes in an extremely hot marine environment. Perhaps the unique ballooning of the outer membrane over the ends of Thermotoga cells serves to enlarge the surface area spiked with depolymerases.
Acknowledgments
We thank B. Kühlmorgen and B. Schumacher for skillful technical assistance, F. Lottspeich for N-terminal sequencing of protein samples, and A. Engel for valuable advice during the course of immunogold labeling experiments and electron microscopy. We are also grateful to R. Amann for assistance with epifluorescence microscopy.
Financial support from the Deutsche Forschungsgemeinschaft (Li 398/7), Consortium für elektrochemische Chemie GmbH, Munich, and the Federal Ministry for Education and Science (BMBF) to W.L. is gratefully acknowledged.
Footnotes
Published ahead of print on 14 December 2007.
REFERENCES
- 1.Agterberg, M., H. Adriaanse, A. van Bruggen, M. Karperien, and J. Tommassen. 1990. Outer membrane PhoE protein of Escherichia coli K-12 as an exposure vector: possibilities and limitations. Gene 8837-45. [DOI] [PubMed] [Google Scholar]
- 2.Albers, S.-V., W. N. Konings, and A. J. M. Driessen. 1999. A unique short signal sequence in membrane-anchored proteins of archaea. Mol. Microbiol. 311595-1596. [DOI] [PubMed] [Google Scholar]
- 3.Albers, S.-V., and A. J. M. Driessen. 2002. Signal peptides of secreted proteins of the archaeon Sulfolobus solfataricus: a genomic survey. Arch. Microbiol. 177209-216. [DOI] [PubMed] [Google Scholar]
- 4.Ali, M. K., T. Kimura, K. Sakka, and K. Ohmiya. 2001. The multidomain xylanase Xyn10B as a cellulose-binding protein in Clostridium stercorarium. FEMS Microbiol. Lett. 19879-83. [DOI] [PubMed] [Google Scholar]
- 5.Reference deleted.
- 6.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340783-795. [DOI] [PubMed] [Google Scholar]
- 7.Bernfeld, P. 1955. Amylases α and β. Methods Enzymol. 1149-158. [Google Scholar]
- 8.Binder, F. 1987. Genetische und biochemische Analyse der Cyclodextrin-Glycosyl-Transferase aus Klebsiella pneumoniae M5α1. Ph.D. thesis. Ludwig-Maximilians-Universität München, Munich, Germany.
- 9.Bleves, S., R. Voulhoux, G. Michel, A. Lazdunski, J. Tommassen, and A. Filloux. 1998. The secretion apparatus of Pseudomonas aeruginosa: identification of a fifth pseudopilin, XcpX (GspK family). Mol. Microbiol. 2731-40. [DOI] [PubMed] [Google Scholar]
- 10.Boraston, A. B., A. L. Creagh, M. Alam Md, J. M. Kormos, P. Tomme, C. A. Haynes, A. J. Warren, and D. G. Kilburn. 2001. Binding specificity and thermodynamics of a family 9 carbohydrate-binding module from Thermotoga maritima xylanase 10A. Biochemistry 406240-6247. [DOI] [PubMed] [Google Scholar]
- 11.de Geus, P., M. Verheij, N. H. Riegman, W. P. M. Hoekstra, and G. H. de Haas. 1984. The pro- and mature forms of the E. coli K-12 outer membrane phospholipase A are identical. EMBO J. 31799-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.d'Enfert, C., C. Chapon, and A. P. Pugsley. 1987. Export and secretion of the lipoprotein pullulanase by Klebsiella pneumoniae. Mol. Microbiol. 1107-116. [DOI] [PubMed] [Google Scholar]
- 13.Engel, A. M., M. Brunen, and W. Baumeister. 1993. The functional properties of Ompβ, a regularly arrayed porin of the hyperthermophilic bacterium Thermotoga maritima. FEMS Microbiol. Lett. 109231-236. [Google Scholar]
- 14.Engel, A. M., Z. Cejka, A. Lupas, F. Lottspeich, and W. Baumeister. 1992. Isolation and cloning of Ompα, a coiled-coil protein spanning the periplasmic space of the ancestral eubacterium Thermotoga maritima. EMBO J. 114369-4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujino, T., P. Beguin, and J. P. Aubert. 1993. Organization of a Clostridium thermocellum gene cluster encoding the cellulosomal scaffolding protein CipA and a protein possibly involved in attachment of the cellulosome to the cell surface. J. Bacteriol. 1751891-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fürste, J. P., W. Pansegrau, R. Frank, H. Blöcker, P. Scholz, M. Bagdasarian, and E. Lanka. 1986. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene 48119-131. [DOI] [PubMed] [Google Scholar]
- 17.Huber, R., and M. Hannig. 2006. Thermotogales, p. 899-922. In M. Dworkin et al. (ed.), The prokaryotes. An evolving electronic resource for the microbiological community, 3rd ed., vol. 7. Springer-Verlag, New York, NY. [Google Scholar]
- 18.Huber, R., T. A. Langworthy, H. König, M. Thomm, C. R. Woese, U. B. Sleytr, and K. O. Stetter. 1986. Thermotoga maritima sp. nov. represents a new genus of unique extremely thermophilic eubacteria growing up to 90°C. Arch. Microbiol. 144324-333. [Google Scholar]
- 19.Huber, R., and K. O. Stetter. 1992. The order Thermotogales, p. 3809-3815. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K. H. Schleifer (ed.), The prokaryotes. A handbook on the biology of bacteria, 2nd ed., vol. II. Ecophysiology, isolation, identification, applications. Springer-Verlag, New York, NY. [Google Scholar]
- 20.Ito, Y., T. Tomita, N. Roy, A. Nikano, N. Sugawara-Tomita, S. Watanabe, N. Okai, N. Abe, and Y. Kamio. 2003. Cloning, expression and cell surface localization of Paenibacillus sp. strain W-61 xylanase 5, a multidomain xylanase. Appl. Environ Microbiol. 696969-6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juncker, A. S., H. Willenbrock, G. von Heijne, H. Nielsen, S. Brunak, and A. Krogh. 2003. Prediction of lipoprotein signal peptides in Gram-negative bacteria. Protein Sci. 121652-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kavoosi, M., J. Meijer, E. Kwan, A. L. Creagh, D. G. Kilburn, and C. A. Haynes. 2004. Inexpensive one-step purification of polypeptides expressed in Escherichia coli as fusions with the family 9 carbohydrate-binding module of xylanase 10A from T. maritima. J. Chromatogr. B 80787-94. [DOI] [PubMed] [Google Scholar]
- 23.Kellenberger, E., E. Carlemalm, W. Villiger, J. Roth, and R. M. Garavito. 1980. Low denaturation embedding for electron microscopy of thin sections. Chem. Werke Lowi, Waldkraiburg, Germany.
- 24.Kleine, J., and W. Liebl. 2006. Comparative characterization of deletion derivatives of the modular xylanase XynA of Thermotoga maritima. Extremophiles 10373-381. [DOI] [PubMed] [Google Scholar]
- 25.Liebl, W., R. Feil, J. Gabelsberger, J. Kellermann, and K. H. Schleifer. 1992. Purification and characterization of a novel thermostable 4-α-glucanotransferase of Thermotoga maritima cloned in Escherichia coli. Eur. J. Biochem. 20781-88. [DOI] [PubMed] [Google Scholar]
- 26.Liebl, W., J. Gabelsberger, and K. H. Schleifer. 1994. Structural analysis of the bglA gene encoding a β-glucosidase of the hyperthermophilic bacterium Thermotoga maritima and comparison of the deduced amino acid sequence with other β-1,4-glycosyl hydrolases. Mol. Gen. Genet. 242111-115. [DOI] [PubMed] [Google Scholar]
- 27.Liebl, W., I. Stemplinger, and P. Ruile. 1997. Properties and gene structure of the Thermotoga maritima α-amylase AmyA, a putative lipoprotein of a hyperthermophilic bacterium. J. Bacteriol. 179941-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lupas, A., H. Engelhardt, J. Peters, U. Santarius, S. Volker, and W. Baumeister. 1994. Domain structure of the Acetogenium kivui surface layer revealed by electron crystallography and sequence analysis. J. Bacteriol. 1761224-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacIntyre, S., R. Freudl, M.-L. Eschbach, and U. Henning. 1988. An artificial hydrophobic sequence functions as either an anchor or a signal sequence at only one of two positions within the Escherichia coli outer membrane protein OmpA. J. Biol. Chem. 26319053-19059. [PubMed] [Google Scholar]
- 30.Meissner, K., D. Wassenberg, and W. Liebl. 2000. The ‘thermostabilising domain’ of the modular xylanase XynA of the hyperthermophilic bacterium Thermotoga maritima represents a novel xylan-binding domain. Mol. Microbiol. 36898-912. [DOI] [PubMed] [Google Scholar]
- 31.Nordberg Karlsson, E., M. Abou Hachem, S. Ramchuran, H. Costa, O. Holst, A. F. Svenningsen, and G. O. Hreggvidsson. 2004. The modular xylanase Xyn10A from Rhodothermus marinus is cell-attached, and its C-terminal domain has several putative homologues among cell-attached proteins within the phylum Bacteroidetes. FEMS Microbiol. Lett. 241233-242. [DOI] [PubMed] [Google Scholar]
- 32.Notenboom, V., A. B. Boraston, D. G. Kilburn, and D. R. Rose. 2001. Crystal structure of the family 9 carbohydrate-binding module from Thermotoga maritima xylanase 10A in native and ligand-bound forms. Biochemistry 406248-6256. [DOI] [PubMed] [Google Scholar]
- 33.Pugsley, A. P. 1993. The complete general secretory pathway in gram-negative bacteria. Microbiol. Rev. 5750-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rachel, R., A. M. Engel, R. Huber, K. O. Stetter, and W. Baumeister. 1990. A porin is the main constituent of the cell envelope of the ancestral eubacterium Thermotoga maritima. FEBS Lett. 26264-68. [Google Scholar]
- 35.Reysenbach, A.-L. 2001. Phylum BII: Thermotogae phy. nov., p. 369. In D. R. Boone and R. W. Castenholz (ed.), Bergey's manual of systematic bacteriology, 2nd ed. Springer-Verlag, New York, NY.
- 36.Schumann, J., A. Wrba, R. Jaenicke, and K. O. Stetter. 1991. Topographical and enzymatic characterization of amylases from the extremely thermophilic eubacterium Thermotoga maritima. FEBS Lett. 282122-126. [DOI] [PubMed] [Google Scholar]
- 37.Shinkai, A., H. Yamada, T. Mizuno, and S. Mizushima. 1989. Insertion of a signal peptide-derived hydrophobic segment into the mature domain of OmpC, an outer membrane protein, does not interfere with the export of the following polypeptide chain across the cytoplasmic membrane of E. coli. J. Biochem. 106323-330. [DOI] [PubMed] [Google Scholar]
- 38.Strom, M. S., and S. Lory. 1991. Amino acid substitutions in pilin of Pseudomonas aeruginosa. Effect on leader peptide cleavage, amino-terminal methylation, and pilus assembly. J. Biol. Chem. 2661656-1664. [PubMed] [Google Scholar]
- 39.Strom, M. S., and S. Lory. 1993. Structure-function and biogenesis of the type IV pili. Annu. Rev. Microbiol. 47565-596. [DOI] [PubMed] [Google Scholar]
- 40.Tommassen, J., M. Struyve, and H. deCock. 1992. Export and assembly of bacterial outer membrane proteins. Antonie van Leeuwenhoek. 6181-85. [DOI] [PubMed] [Google Scholar]
- 41.von Heijne, G. 1986. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 144683-4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winterhalter, C., P. Heinrich, A. Candussio, G. Wich, and W. Liebl. 1995. Identification of a novel cellulose-binding domain within the multi-domain 120 kDa xylanase XynA of the hyperthermophilic bacterium Thermotoga maritima. Mol. Microbiol. 15431-444. [DOI] [PubMed] [Google Scholar]
- 43.Winterhalter, C., and W. Liebl. 1995. Two extremely thermostable xylanases of the hyperthermophilic bacterium Thermotoga maritima strain MSB8. Appl. Environ Microbiol. 611810-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao, G., E. Ali, M. Sakka, T. Kimura, and K. Sakka. 2006. Binding of S-layer homology modules from Clostridium thermocellum SdbA to peptidoglycans. Appl. Microbiol. Biotechnol. 70464-469. [DOI] [PubMed] [Google Scholar]