Abstract
Circadian rhythms are endogenous cycles with periods close to, but not exactly equal to, 24 h. In mammals, circadian rhythms are generated in the suprachiasmatic nucleus (SCN) of the hypothalamus as well as several peripheral cell types, such as fibroblasts. Protein kinases are key regulators of the circadian molecular machinery. We investigated the role of the c-Jun N-terminal kinases (JNK), which belong to the mitogen-activated protein kinases family, in the regulation of circadian rhythms. In rat-1 fibroblasts, the p46 kDa, but not the p54 kDa, isoforms of JNK expressed circadian rhythms in phosphorylation. The JNK-inhibitor SP600125 dose-dependently extended the period of Period1-luciferase rhythms in rat-1 fibroblasts from 24.23±0.17–31.48±0.07 h. This treatment also dose-dependently delayed the onset of the bioluminescence rhythms. The effects of SP600125 on explant cultures from Period1-luciferase transgenic mice and Period2Luciferase knockin mice appeared tissue-specific. SP600125 lengthened the period in SCN, pineal gland, and lung explants in Period1-luciferase and Period2Luciferase mice. However, in the kidneys circadian rhythms were abolished in Period1-luciferase, while circadian rhythms were not affected by SP600125 treatment in Period2Luciferase mice. Valproic acid, already known to affect period length, enhanced JNK phosphorylation and, as predicted, shortened the period of the Period1-bioluminescence rhythms in rat-1 fibroblasts. In conclusion, our results showed that SP600125 treatment, as well as valproic acid, alters JNK phosphorylation levels, and modulates the period length in various tissues. We conclude that JNK phosphorylation levels may help to set the period length of mammalian circadian rhythms.
Keywords: SCN, peripheral oscillators, Period genes, valproic acid, real time bioluminescence monitoring
Developed through evolution to adjust to environmental conditions, self-sustained circadian oscillators generate daily fluctuations in behavior and physiology with a period of approximately 24 h. The study of the mammalian circadian clock at molecular levels has progressed dramatically over the past decade (Fukuhara and Tosini, 2003; Schibler et al., 2003; Gachon et al., 2004). In mammals, the central circadian clock is localized to the suprachiasmatic nucleus (SCN) of the hypothalamus, which synchronizes circadian events within the body. Peripheral circadian clocks have been found in many types of cells and tissues, e.g. fibroblasts, pineal gland, heart, liver, kidney, lung, and so on. Sustained circadian rhythm can be generated not only in a SCN neuron, but also in a fibroblast and other tissues and organs. Furthermore, expression patterns of circadian clock genes and proteins are similarly observed between the SCN neurons and peripheral cells, tissues and organs (Ko and Takahashi, 2006). These observations suggest that circadian machinery is likely the same or similar between the central and peripheral cells. Therefore, the findings enable us to use peripheral cells and organs to study machinery of circadian rhythm generation.
Numerous studies of the molecular clock mechanism have suggested that these circadian pacemakers consist of transcriptional/translational autoregulatory feedback loops of several clock gene products (Hastings and Herzog, 2004; Ko and Takahashi, 2006). The stability of clock proteins, regulated through phosphorylation and dephosphorylation via protein kinases and phosphatases, is now recognized as a critical component to properly regulate the period of circadian oscillations. In the Syrian hamster, the tau mutation located in the locus of the casein kinase Iε (CKIε) gene (Lowrey et al., 2000), modifies PERIOD protein phosphorylation (Gallego et al., 2006) and shortens the period of the behavioral locomotor activity rhythms (Ralph and Menaker, 1988).
The mitogen-activated protein kinases (MAPK), members of the superfamily of serine/threonine kinases, are intracellular signal transduction enzymes that respond to a wide range of external stimuli such as mechanical stimulation, growth factors, hormones, or cytokines. They play an essential role in regulating several intracellular processes, such as gene expression, growth, cell survival, differentiation or death (Barr and Bogoyevitch, 2001; Pearson et al., 2001). To date, four main subfamilies have been identified: extracellular-regulated kinases (ERK), p38MAPK, c-Jun N-terminal kinases (JNK) and big MAPK (BMK/ERK5) (Pearson et al., 2001). The role of JNK in the regulation of the circadian molecular machinery is of particular interest since JNK are involved in numerous illnesses such as neurodegenerative diseases, cancers, and stroke (Bozyczko-Coyne et al., 2002; Manning and Davis, 2003; Peng and Andersen, 2003; Resnick and Fennell, 2004), in which disturbed circadian organization has been reported (Mormont and Levi, 1997; Garcia-Borreguero et al., 2003; Onen and Onen, 2003; Walters et al., 2003; Morton et al., 2005).
In the present study, we investigated what role JNK might play in the regulation of the molecular clockwork in rat-1 fibroblasts using a JNK inhibitor SP600125. Since SP600125 affected period of circadian rhythm expression, we further studied the effects of the anticonvulsant valproic acid, which has been used to treat bipolar disorder patients and is known to affect period (Rietveld and van Schravendijk, 1987; Klemfuss and Kripke, 1995; Dokucu et al., 2005), on JNK phosphorylation levels. By means of a real-time bioluminescence monitoring apparatus, we monitored the effects of the chemicals on the expression patterns of Period1-bioluminescence rhythms in rat-1 fibroblasts, and the effects of SP600125 on Period1- and Period2-bioluminescence rhythms in central and peripheral tissues using Period1-luciferase transgenic mice and Period2Luciferase knockin mice.
EXPERIMENTAL PROCEDURES
Animals
Transgenic mice carrying a Period1-luciferase transgene as a reporter of activity were generously provided by Dr. Hajime Tei (Mitsubishi Kagaku Institute of Life Sciences, Tokyo, Japan), and Period2Luciferase knockin mice harboring a luciferase reporter were obtained from Dr. Joseph S. Takahashi (Northwestern University, Evanston, IL, USA) (Yamazaki et al., 2000; Herzog et al., 2004; Yoo et al., 2004). Period1-luciferase and Period2Luciferase mice were maintained at Morehouse School of Medicine and at Smith College, respectively. All animals were housed under a 12-h light/dark cycle (lights on, 07:00–19:00 h) in a temperature-controlled environment and had access to food and water ad libitum. Zeitgeber time (ZT) 0 was defined as light onset and ZT 12 as lights off. Both heterozygous and homozygous mice were used in our experiments. All experiments were run under the oversight of IACUC of the Atlanta University Center and the Smith College IACUC and NIH guidelines. The minimum number of animals necessary to obtain a significant signal/noise ratio was used. The animals were decapitated under anesthesia to minimize their suffering.
Cell and tissue cultures
Three days before starting experiments, 106 rat-1 fibroblasts, stably transfected with the Period1-luciferase reporter gene (Izumo et al., 2003) (generous gift from Carl H. Johnson, Vanderbilt University, Nashville, TN, USA), were seeded in 35 mm Petri dishes. Cells were cultured at 36 °C under 5% CO2–95% air atmosphere in 2 mL of Dulbecco’s Eagle Modified Medium (Gibco, Carlsbad, CA, USA) supplemented with 5% fetal bovine serum (Gibco), 100 units/mL penicillin and 100 μg/mL streptomycin (Gibco). For all experiments, circadian rhythms were synchronized among the cells by replacing the culture medium with 2 mL of assay medium (Hirota et al., 2002), consisting of serum-free DMEM (Cellgro, Herndon, VA, USA), 10 mM Hepes (Gibco), 350 mg/mL sodium bicarbonate (Gibco), 2% B27 Supplement (Gibco), 25 units/mL penicillin, and 25 μg/mL streptomycin. For bioluminescence analysis, 0.1 mM luciferin (Promega, Madison, WI, USA) was added to the assay medium.
For explant cultures, brain, pineal gland, lungs, and kidneys were quickly removed from transgenic/knockin mice decapitated following overdose halothane anesthesia at ZT 11 (Yoshikawa et al., 2005), and were placed in cold Hanks’ Balanced Salt Solution (HBSS, Invitrogen, Carlsbad, CA, USA). Brain coronal sections at 300-μm thickness were obtained using a vibratome (World Precision Instruments, Sarasota, FL, USA). The brain section containing the SCN was further dissected as 1×1 mm square. Lungs and kidneys were dissected into pieces as ≈1 mm3 in volume. Each piece of lung and kidney was cultured individually in a 35 mm Petri dish and SCN slices and the pineal gland were cultured on Millicell culture membranes (Millipore, Billerica, MA, USA) placed in 35 mm dishes, sealed with silicon grease and a circular glass coverslip.
For both fibroblasts and tissue explant cultures, SP600125 (1,9-pyrazoloanthrone, Calbiochem, San Diego, CA, USA), JNK inhibitor negative control (N1-methyl-1,9-pyrazoloanthrone, Calbiochem), vehicle or valproic acid (Sigma, St. Louis, MO, USA) was added at the beginning of the record in the assay medium.
Cell survival assay
Fibroblast preparations were obtained as described previously. The cells were cultured for 3 days in the presence or absence of 30 μM SP600125 or vehicle. Cells were then trypsinized and incubated with 4% Trypan Blue dye for 5 min at room temperature. Fibroblasts were examined under an inverted microscope using a hematocytometer and cell survival was expressed in percentage, as the ratio of living cells on total number of cells.
Western blot analysis
Detailed procedures have been described in a previous study (Chansard et al., 2005). In brief, protein extracts (30 μg) were separated on a SDS-PAGE gel and further transferred to a nitrocellulose membrane. Each blot was successively incubated with primary antibodies recognizing phosphorylated forms of JNK at Thr183 and Tyr185 (Cell Signaling Technology, Danvers, MA, USA), total JNK (Cell Signaling Technology), and ACTIN (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The intensity of each band was measured using the NIH ImageJ analysis software and was normalized toward the corresponding ACTIN band intensity.
Bioluminescence monitoring and data analysis
Detailed procedures for bioluminescence monitoring and data analysis have been described elsewhere (Yamazaki et al., 2000; Izumo et al., 2003). Bioluminescence rhythms were recorded in a real-time bioluminescence monitoring system (Lumicycle, Actimetrics, Wilmette, IL, USA). Petri dishes to be assayed were placed in a light-tight chamber placed in a 36 °C air incubator. Photons emitted from cells in each Petri dish were monitored by photomultipliers for 60 s at 10-min intervals. The data were acquired using a PCI-6601 card and a NI-DAQ 7.1 acquisition driver (National Instruments, Austin, TX, USA) associated with an acquisition software (Lumicycle Software, Actimetrics).
Data analyses were performed using the Lumicycle Data Analysis Software (Actimetrics). The raw data were subjected to baseline correction, performed by fitting a polynomial curve to the data. The baseline fit curve obtained was subtracted from the raw data and the resulting baseline-subtracted data were used to determine the period. A chi-square periodogram analysis was performed on all baseline-subtracted data to establish whether or not there was a significant circadian rhythm (Sokolove and Bushell, 1978). In this study, all the circadian rhythms recorded from fibroblasts and tissues, except for some of the SP600125-treated Period1-luciferase and Period2Luciferase mice kidneys, showed sig-nificant circadian rhythms (P<0.05). The estimated period was obtained from a Fourier transform of the records: the software fits the data to a sine wave multiplied by an exponential decay:
where L is the bioluminescence in counts/s, i.e. the photon counts emitted per second as represented in the corresponding figures, P is the period in hours, t0 is the phase in cycles relative to the start of the fitted portion of the data, A is the amplitude in counts/s and d is the exponential damping time constant in days. The time points of the peaks and troughs of the baseline-subtracted data are fitted to the dominant sine wave and then fitted to an exponential decay. The damping rate was defined as the number of days necessary for the amplitude of the rhythm to decrease to 1/e (≈36.79%) of its initial value.
The baseline level of bioluminescence, which corresponds to the background level, was evaluated at 28.79±7.66 counts/s.
Statistics
Data are expressed as means±S.E.M. and statistical analysis was performed using parametric and non-parametric statistics (ANOVA, Student’s t-test).
RESULTS
Phosphorylation patterns of p46 kDa and p54 kDa JNK isoforms in rat-1 fibroblasts
First, the profiles of JNK phosphorylation throughout 24 h in rat-1 Period1-luciferase fibroblasts were analyzed. The experiment was designed according to preliminary data, which assessed the Period1-driven bioluminescence rhythms in Period1-luciferase rat-1 fibroblasts after medium change, so that the samples were taken on a full circadian cycle (Fig. 1). Over 24 h, p46 kDa isoforms showed a rhythmic phosphorylation (Fig. 1A, ANOVA, P<0.05). The peak of p46 kDa isoforms phosphorylation occurred 44 h after medium change (P<0.05 as compared with the 36 h time point). As compared with the Period1-luciferase bioluminescence rhythm, which reached its peak 28 h following synchronization, p46 kDa phosphorylation peaked 16 h later, as assessed by the record of the Period1-driven bioluminescence oscillations on the same time duration. p54 kDa did not display any significant fluctuations (Fig. 1B, ANOVA, P>0.1). Total amount of p46 kDa and p54 kDa isoforms remained unchanged over the 24-h period (ANOVA, both cases, P>0.1).
Fig. 1. Phosphorylation of JNK isoforms in synchronized rat-1 fibroblasts.
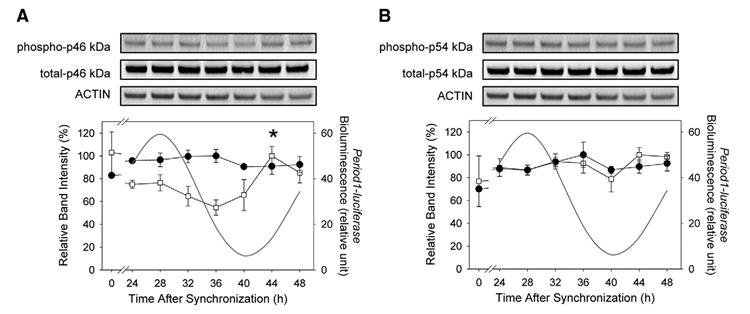
Twenty-four hours after synchronization, cells were harvested at 4-h intervals on a circadian cycle. (A) Phosphorylation of p46 kDa isoforms showed a rhythmic pattern (open square, P<0.05) over the circadian cycle with a peak of phosphorylation reached 44 h after synchronization. Total amount of p46 kDa was unchanged (filled circle, P>0.1). (B) p54 kDa isoform phosphorylation (open square) as well as total protein amounts (filled circle) did not show significant variations (both cases, P>0.1). Period1-luciferase rhythmic bioluminescence expression on the same time window is represented (A and B). Values are means±S.E.M. n=4–5 For each time point. *P<0.05. For symbols without standard error bars, the bars are smaller than the size of symbols.
The effects of the JNK inhibitor SP600125 on the period of Period1-luciferase rhythms in rat-1 fibroblasts
To investigate whether JNK phosphorylation is required to maintain normal circadian oscillations, the JNK specific inhibitor SP600125 was used. As compared with the control condition, the period length was significantly extended in the presence of 30 μM SP600125 (P<0.0005, Table 1, Fig. 2). The amplitude of the oscillations was not affected by the SP600125 treatment (P>0.1, Table 1). Similarly, the damping rate was not significantly affected by the SP600125 treatment (P>0.1, Table 1). Culturing cells in the presence of the SP600125 for 3 days did not affect the cell survival rate (control, 78.90±2.83%; SP600125, 75.93±0.43%).
Table 1.
Period, amplitude and damping rate analyses of bioluminescence patterns following various treatments
| Cell type/tissue | Treatment | Period (h) | Amplitude (counts/s) | Damping rate (d) | |
|---|---|---|---|---|---|
| Period1-luciferase rat-1 fibroblasts | Control | 24.23 ± 0.17 | 188.11 ± 21.20 | 1.72 ± 0.23 | Fig. 2 |
| DMSO | 24.28 ± 0.20 ns | 285.06 ± 12.52* | 1.57 ± 0.09 ns | ||
| SP600125 30μM | 31.48 ± 0.07*** | 260.27 ± 50.62 ns | 1.30 ± 0.05 ns | ||
| SP, negative control 30μM | 24.83 ± 0.38 ns | 181.85 ± 21.47 ns | 2.06 ± 0.35 ns | ||
| Mouse Period1-luciferase SCN | Control | 24.63 ± 0.59 | 234.81 ± 84.02 | 1.22 ± 0.25 | Fig. 5A |
| SP600125 30μM | 32.45 ± 0.99** | 197.75 ± 119.46 ns | 1.05 ± 0.23 ns | ||
| Mouse Period2Luciferase SCN | Control | 24.10 ± 0.25 | 150.74 ± 55.86 | 4.12 ± 1.16 | Fig. 5A |
| SP600125 30μM | 32.96 ± 1.19* | 75.35 ± 10.11 ns | 2.93 ± 0.48 ns | ||
| Mouse Period1-luciferase pineal gland | Control | 23.55 ± 0.25 | 44.51 ± 12.59 | 2.60 ± 0.57 | Fig. 5B |
| SP600125 30μM | 32.83 ± 1.04** | 330.23 ± 138.32 ns | 1.01 ± 0.03 ns | ||
| Mouse Period2Luciferase pineal gland | Control | 23.87 ± 0.13 | 79.56 ± 20.47 | 1.70 ± 0.33 | Fig. 5B |
| SP600125 30μM | 35.35 ± 0.93*** | 87.22 ± 19.68 ns | 1.73 ± 0.09 ns | ||
| Mouse Period1-luciferase lungs | Control | 24.63 ± 0.27 | 175.35 ± 48.60 | 1.88 ± 0.17 | Fig. 6A |
| SP600125 30μM | 30.56 ± 1.45* | 151.03 ± 68.12 ns | 2.80 ± 0.86 ns | ||
| Mouse Period2Luciferase lungs | Control | 23.65 ± 0.12 | 33.29 ± 19.64 | 3.29 ± 0.55 | Fig. 6A |
| SP600125 30μM | 32.78 ± 1.34*** | 63.44 ± 23.26 ns | 1.92 ± 0.16 ns | ||
| Mouse Period1-luciferase kidneys | Control | 24.54 ± 0.14 | 15.52 ± 4.42 | 3.97 ± 0.53 | Fig. 6B |
| SP600125 30μM | N/A | N/A | N/A | ||
| Mouse Period2Luciferase kidneys | Control | 24.16 ± 0.20 | 258.96 ± 201.11 | 1.34 ± 0.25 | Fig. 6B |
| SP600125 30μM | 25.46 ± 0.78 ns | 21.78 ± 10.92 ns | 1.40 ± 0.15 ns | ||
| Period1-luciferase rat-1 fibroblasts | Control | 23.98 ± 0.12 | 141.60 ± 19.99 | 1.70 ± 0.14 | Fig. 7A |
| Valproic acid 1mM | 23.35 ± 0.17* | 503.25 ± 119.58* | 1.59 ± 0.44 ns |
N/A, analysis not possible due to a lack of rhythmicity; ns, not significant; SP, SP600126.
p<0.05.
p<0.005.
p<0.0005.
Fig. 2. The effects of SP600125 on the period of Period1-luciferase bioluminescence rhythms in rat-1 fibroblasts.
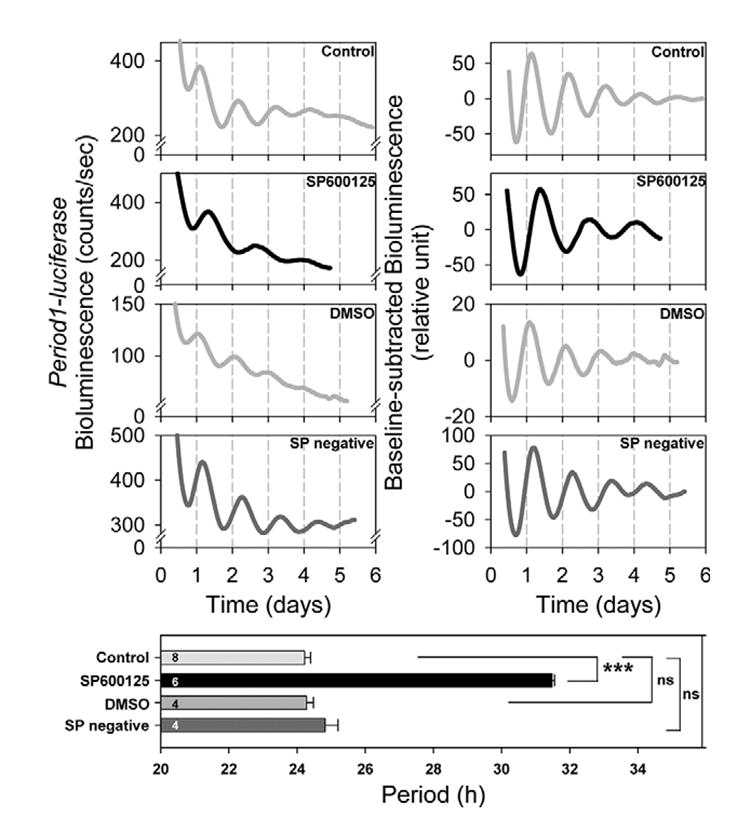
Fibroblasts were synchronized by medium change and Period1-driven bioluminescence was recorded for several days. SP600125 (30 μM) lengthened the period of the bioluminescence rhythms. SP600125 negative control (30 μM) showed no significant effect. The left panels show the representative raw bioluminescence recordings and the right panels the baseline-corrected bioluminescence data. The columns in the lower panel show the estimated period length in each experimental condition. Values are means±S.E.M. *** P<0.005; ns, not significant. In all figures a number shown in a column indicates sample size.
To determine whether the vehicle by itself affected the period length of the Period1-driven bioluminescence rhythm, we treated fibroblasts with dimethyl sulfoxide (DMSO). Oscillations showed a period, which was not significantly different from the control condition (P>0.1, Table 1, Fig. 2). Although amplitude was significantly increased (P<0.05, Table 1), the damping rate was unchanged (P>0.1, Table 1). DMSO did not affect the cell survival rate (81.60±2.19%, P>0.1).
Then, the effect of a JNK inhibitor negative control (30 μM) was tested. The period was not affected by the negative control (P<0.1, Table 1, Fig. 2). Similarly, both amplitude and damping rate stayed unchanged (both cases, P>0.1, Table 1).
The effects of SP600125 on phosphorylated JNK levels
The acute and chronic effects of SP600125 treatment on the phosphorylation levels of p46 kDa and p54 kDa isoforms were further analyzed. Both p46 kDa and p54 kDa isoforms were highly and significantly phosphorylated 20 min after medium change (both cases, P<0.0005, Fig. 3A). SP600125 prevented this acute phosphorylation (p46 kDa, P>0.1, p54 kDa, P>0.1). Total JNK levels of p46 kDa and p54 kDa were not significantly affected 20 min after medium change with or without SP600125 (P>0.05 in all cases). The effect of a chronic application of SP600125 by harvesting the cells on a full circadian cycle was determined. Based on circadian expression patterns of Period1-bioluminescence rhythms (Fig. 2), we decided to harvest cells every 5 h for 30 h starting 30 h after medium change. As compared with the control condition (Fig. 1), rhythmic p46 kDa phosphorylation was abolished by the SP600125 and phosphorylation levels were low (Fig. 3B, ANOVA, P>0.1). As expected from the control condition (Fig. 1), p54 kDa phosphorylation remained arrhythmic after SP600125 treatment (ANOVA, P>0.1). The total levels of the two isoforms were not affected by the SP600125 treatments (both cases, ANOVA, P>0.1).
Fig. 3. The effects of SP600125 on the acute and chronic activation of JNK in rat-1 fibroblasts.
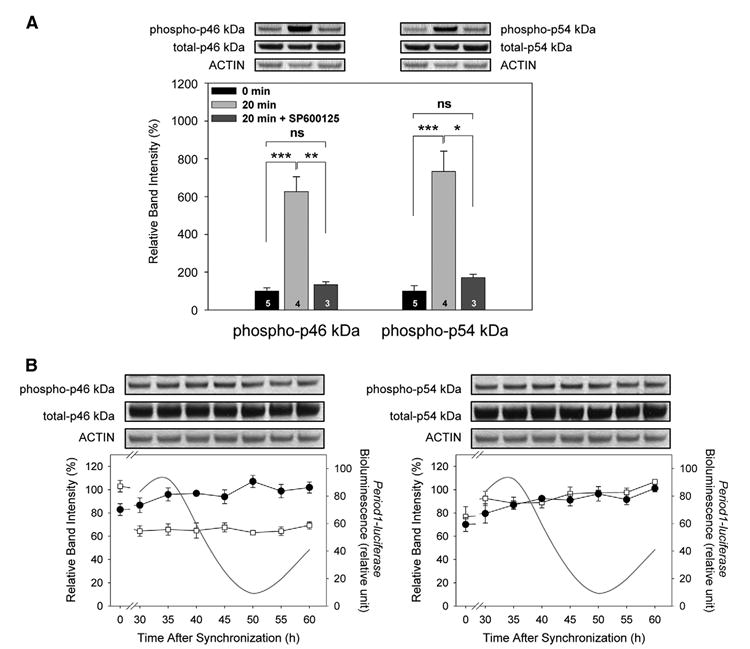
(A) Fibroblasts were synchronized by medium change with or without SP600125. The acute phosphorylation of p46 kDa and p54 kDa isoforms, measured 20 min after medium change was inhibited by SP600125. The lower panel shows quantitative data. Control (0 min) levels were normalized as 100. See detailed description in Fig. 1. (B) Cells were harvested on a full circadian cycle at 5-h intervals starting 30 h after medium change. p46 kDa and p54 kDa isoforms (open square), normalized toward the respective peak time level of expression in the control condition (shown in Fig. 1), did not show significant rhythmicity (both cases, P>0.01). Total amounts of both isoforms remained unvaried (both cases, P>0.1). Values are means±S.E.M. n=3 For each time point. Period1-luciferase rhythmic expression on the same time window is represented. * P<0.05; ** P<0.005; *** P<0.0005.
Characteristics of SP600125 in the regulation of the Period1-bioluminescence rhythms
Described in the literature as a reversible inhibitor (Bennett et al., 2001), we tested whether the effect of SP600125 on the period length was reversible or not. We monitored oscillations in bioluminescence levels for 3 days in the presence of 30 μM SP600125 before refreshing the preparation with fresh medium without the inhibitor. After the medium change, the period was then significantly shortened (before washout, 30.73±0.16 h; after washout, 24.44±0.10 h, P<0.0005, Fig. 4A), which was not significantly different from the control condition, or that from the vehicle condition (both cases, P>0.1).
Fig. 4. Characteristics of SP600125 in the regulation of the Period1-driven bioluminescence rhythm in rat-1 fibroblasts.
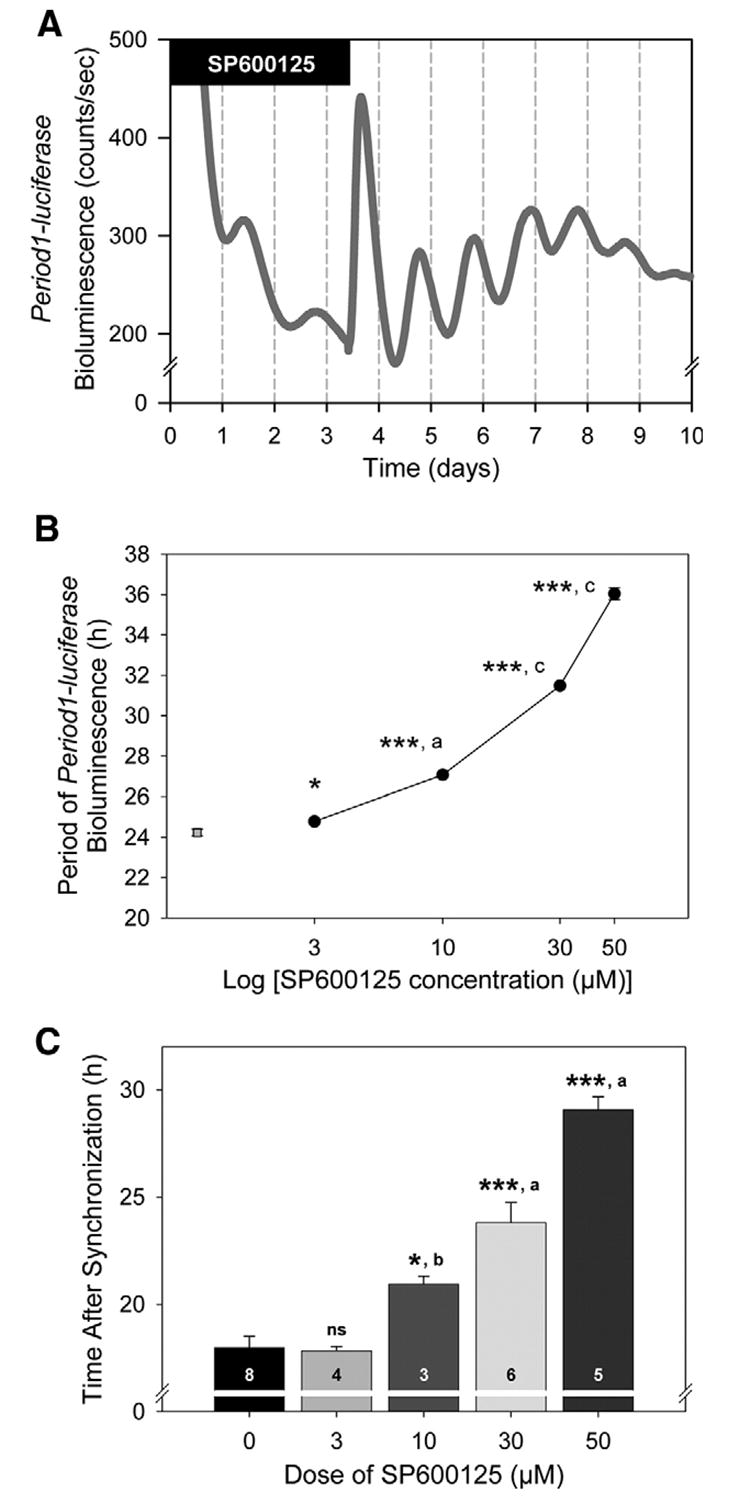
(A) Circadian rhythmic expression of Period1-bioluminescence in fibroblasts were synchronized and recorded for 3 days in the presence of 30 μM SP600125 (filled bar, 30.73±0.16 h). Medium was then replaced by assay medium without SP600125. After washout, the period was significantly shortened (24.44±0.10 h, P<0.0005). The graph is representative of three independent experiments. n=7. (B) SP600125 showed dose-dependent effects on the period lengthening. Data represent means±S.E.M. n=3–8 For each dose. Comparisons with the control condition (“0”): * P<0.05; *** P<0.005. Comparisons among SP600125 conditions: a P<0.05; c P<0.005. (C) SP600125 dose-dependently delayed the time of the onset. Data shown represent means±S.E.M. Comparisons with the control condition: * P<0.05; *** P<0.0005. Comparisons among SP600125 conditions: a P<0.05; b P<0.0005.
We further found that SP600125 dose-dependently extended the period of the bioluminescence rhythms (Fig. 4B); period was lengthened from 24.23±0.17 h (control, 0 μM) to 24.78±0.06 h (3 μM, P<0.05), 27.07±0.09 h (10 μM, P<0.0005), 31.48±0.06 h (30 μM, P<0.0005) and 36.04±0.30 h (50 μM, P<0.0005). Moreover, the effect of 3 μM SP600125 was significantly different from the effect of 10 μM inhibitor (P<0.05). Similarly, the effect of 10 μM SP600125 was significantly different from 30 μM (P<0.0005), and 30 μM from 50 μM (P<0.0005).
We also noticed that the onset of the oscillations, after synchronization, was dose-dependently delayed by SP600125 treatment (Fig. 4C). We defined the onset of the oscillations as the time at which the bioluminescence level reached its first trough after medium change. As compared with the control condition, where the onset was at 17.98±0.53 h, 3 μM SP600125 did not delay the onset (17.83±0.20 h, P>0.1). On the contrary, 10 μM (20.94±0.36 h, P<0.05), 30 μM (23.80±0.94 h, P<0.0005), and 50 μM inhibitor (29.06±0.61 h, P<0.0005) significantly delayed the onset. The delay observed was also significantly higher after 10 μM treatment than 3 μM treatment (P<0.0005). Similarly, 30 μM SP600125 treatment delayed the onset of the oscillations more than 10 μM SP600125 treatment (P<0.05), and 50 μM more than 30 μM (P<0.005).
The effects of SP600125 on the period length in various tissues
The effects of 30 μM SP600125 were further evaluated in diverse tissues. In the SCN, the period of the bioluminescence rhythm was significantly extended in Period1-luciferase transgenic mice (P<0.005; Table 1, Fig. 5A) and Period2Luciferase SCN slices (P<0.05, Table 1, Fig. 5A).
Fig. 5. The effects of SP600125 treatment on the period of Period1- and Period2-driven bioluminescence rhythms in SCN and pineal gland of transgenic/knockin mice.
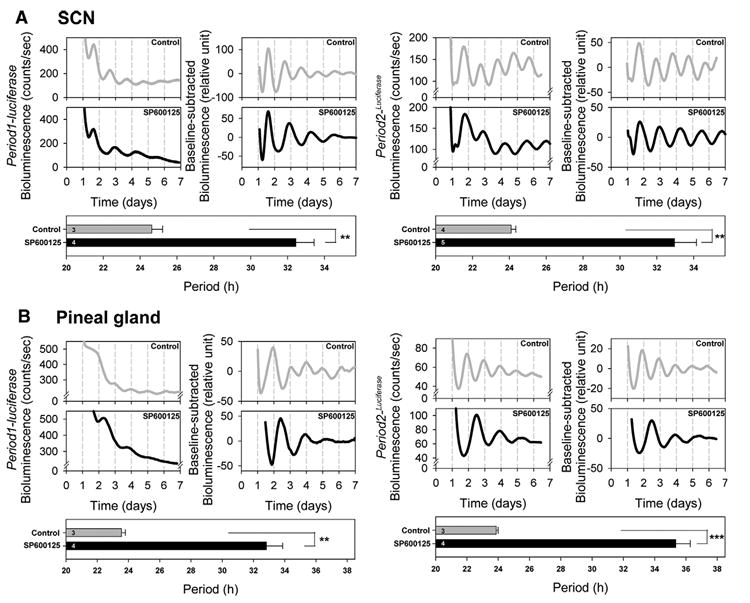
SCN slices from Period1-luciferase transgenic mice (left panels) and Period2Luciferase knockin mice (right panels) showed a significant period lengthening in the presence of SP600125 (A). A similar effect of SP600125 was found in the pineal gland (B). Bioluminescence values for each corresponding daily time are plotted on the chart with time 0 as midnight (00:00 h) on the starting day of the cultures. For each tissue, the left panel represents the raw bioluminescence record and the right panel the baseline-corrected bioluminescence data. The lower panel shows the estimated period length. Each graph is representative of three to four independent experiments. Values are means±S.E.M. ** P<0.005; *** P<0.0005.
We then studied the effects of SP600125 on the pineal gland, lungs and kidneys. First, in the pineal gland of Period1-luciferase mice, the period was significantly extended (P<0.005, Table 1, Fig. 5B). Similar results were obtained with the Period2Luciferase pineal gland (P<0.0005, Table 1, Fig. 5B). In lungs obtained from Period1-luciferase mice, the period length was significantly increased with the SP600125 treatment (P<0.05, Table 1, Fig. 6A). In Period2Luciferase mice, the period was significantly lengthened as well (P<0.0005, Table 1, Fig. 6A).
Fig. 6. The effects of SP600125 treatment on the period of Period1- and Period2-driven bioluminescence rhythms in peripheral tissues of transgenic/knockin mice.
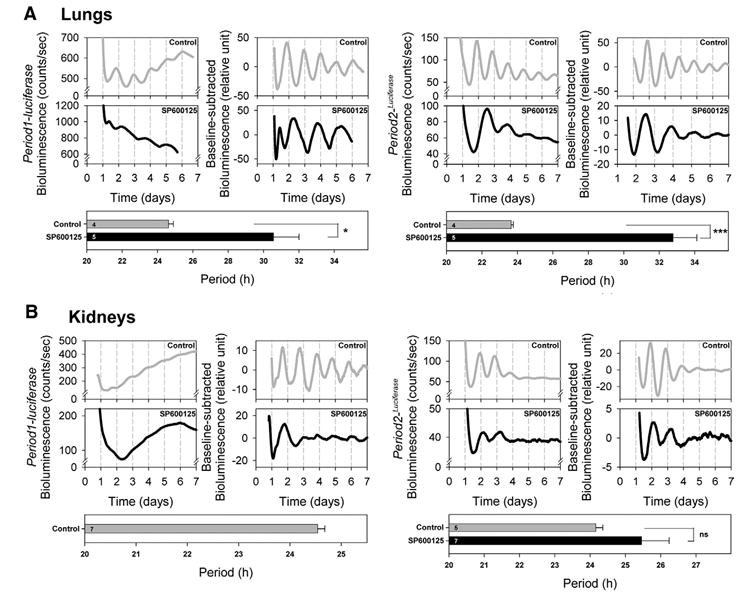
Lung explants from Period1-luciferase transgenic mice (left panels) and Period2Luciferase knockin mice (right panels) showed a significant period lengthening in the presence of SP600125 (A). However, in the kidneys, circadian rhythms were abolished in Period1-luciferase mice, while circadian rhythms were not affected by SP600125 treatment in Period2Luciferase mice (B). Bioluminescence values for each corresponding daily time are plotted on the chart with time 0 as midnight (00:00 h) on the starting day of the cultures. For each tissue, the left panel represents the raw bioluminescence record and the right panel the baseline-corrected bioluminescence data. The lower panel shows the estimated period length. For Period1-luciferase kidneys, only control samples are shown because no significant circadian rhythms or period was obtained in the presence of SP600125. Each graph is representative of four to seven independent experiments. Values are means ±S.E.M. ns, Not significant; * P<0.05; *** P<0.0005.
In the SCNs, pineal glands and lungs obtained from Period1-luciferase and Period2Luciferase mice, amplitudes and damping rates were not affected by SP600125 treatment (P>0.1, Table 1, Figs. 5A, B and 6A).
However, oscillations in kidneys observed in the presence of SP600125 showed more compound results (Table 1, Fig. 6B). When analyzing the entire 7 day sample, none of the SP600125-treated Period1-luciferase samples showed significant circadian oscillation (P>0.05), whereas seven of nine Period2Luciferase samples did show significant circadian oscillations in the presence of SP600125 (P<0.05). Interestingly, the Period2Luciferase samples showed oscillations that were much stronger for the first 2 days in cultures, strongly damping subsequently, and 7 of 16 Period1-luciferase samples showed some evidence for circadian oscillations on the first 2 days of culture. The rhythmic Period2Luciferase cultures did not show a circadian period significantly different from that of controls (P>0.05, Table 1, Fig. 6B). So were the amplitude and damping rate (P>0.05, Table 1).
The effects of valproic acid on JNK phosphorylation levels and the period of circadian oscillations
We treated rat-1 Period1-luciferase fibroblasts with 1 mM valproic acid since it has been shown to affect the period length of locomotor behavior in Drosophila and mammals. We found that the period of the bioluminescence oscillations was significantly shortened as compared with the control condition (P<0.05; Table 1, Fig. 7A). Although the amplitude of the oscillations was significantly increased by the treatment (P<0.05, Table 1), the damping rate remained unchanged (P>0.1, Table 1). Both p46 kDa and p54 kDa JNK isoforms showed hyper-phosphorylation after 12 h of valproic acid treatment (both cases, P<0.005, Fig. 7B). For comparison, when cells were treated with 30 μM SP600125 for 12 h, p46 kDa and p54 kDa phosphorylation levels were not significantly different from control groups (P>0.05 and P>0.1 respectively), but they were significantly lower than those in the valproic acid-treated groups (both cases, P<0.0005; Fig. 7B). Total levels of p46 kDa and p54 kDa were not affected by any treatments (in all the cases, P>0.1).
Fig. 7. The effects of valproic acid treatment on the period of Period1-bioluminescence rhythms and the phosphorylation level of JNK in rat-1 fibroblasts.
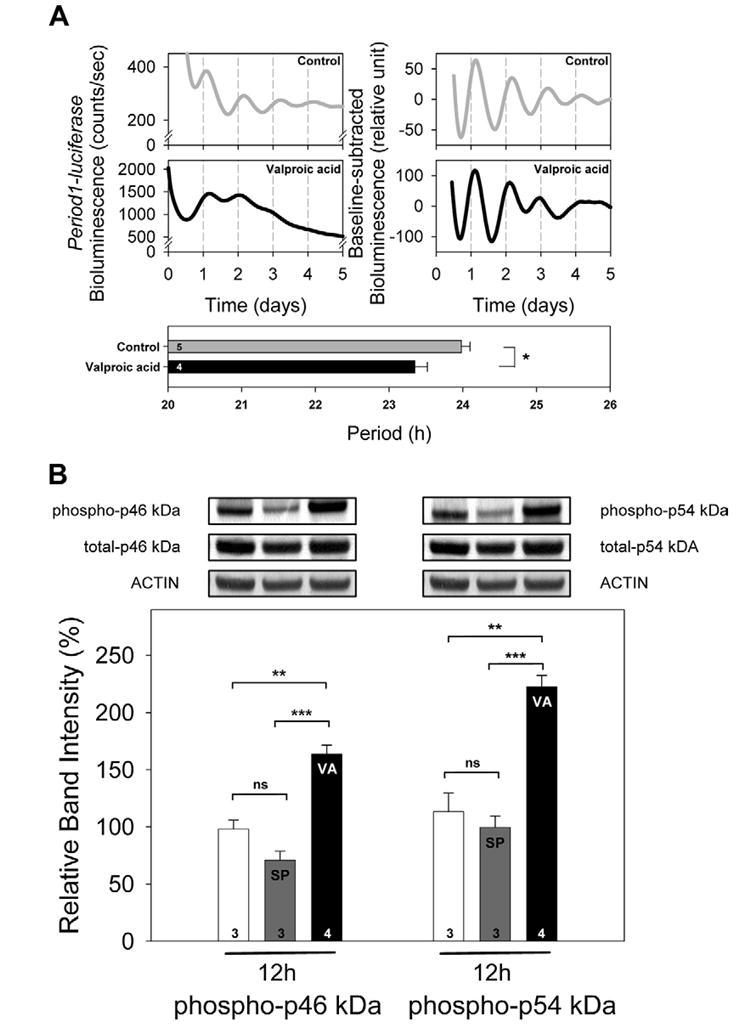
(A) Rat-1 fibroblasts were synchronized and treated with 1 mM valproic acid. Period was significantly shortened in the presence of valproic acid. Values are means±S.E.M. In each graph, a number shown in a column indicates sample size. * P<0.05. Student’s t-test. (B) Rat-1 fibroblasts were cultured and treated for 12 h with either 30 μM SP600125 or 1 mM valproic acid. SP600125 (SP) did not significantly affect the phosphorylation levels of p46 kDa and p54 kDa, while valproic acid (VA) hyper-phosphorylated both isoforms. Total JNK levels were not affected by SP or VA treatment. Values are means±S.E.M. n=3–4 For each treatment. ** P<0.005; *** P<0.0005; ns, not significant.
DISCUSSION
Our studies demonstrate an important role of JNK activation in the regulation of circadian rhythms. We found that p46 kDa and p54 kDa JNK isoforms were both acutely phosphorylated after synchronization of rat-1 fibroblasts and that p46 kDa phosphorylation level was rhythmic over the circadian cycle (Fig. 1, 3). The JNK inhibitor SP600125 dramatically lengthened the period of reporter gene expression in a dose-dependent fashion (Fig. 2, 4B). This effect was found in the SCN and some peripheral oscillators of both Period1-luciferase transgenic mice and Period2Luciferase knockin mice (Fig. 5, 6). In contrast, we demonstrated that increasing phosphorylated forms of JNK with valproic acid shortened the circadian period (Fig. 7).
Ten JNK isoforms of either 46 kDa or 54 kDa have been identified (Derijard et al., 1994; Gupta et al., 1996). These are coded from three genes (Jnk1, Jnk2, Jnk3) through alternative splicing. Jnk3 gene expression is restricted to the CNS, and to a lesser extent to the heart and testis. Therefore, p46 kDa and p54 kDa isoforms, found in the present study, represent JNK1 and JNK2 in fibroblasts (Figs. 1, 3, 7). Although p46 kDa and p54 kDa isoforms are both acutely phosphorylated upon medium change, only p46 kDa isoforms display a rhythmic phosphorylation profile over the next 24 h. (Fig. 1). This finding suggests that JNK isoforms may mediate distinct signals and information in these cells. Support for the latter idea comes from the study of JNK isoforms roles in other systems and the finding that JNK1 and JNK2 isoforms activation can drive opposite responses, as it has been demonstrated for c-JUN-dependent cell proliferation (Sabapathy et al., 2004). The JNK phosphorylation that we report here is consistent with a previous study in which JNK show circadian activation in the SCN of Syrian hamster, in vivo (Pizzio et al., 2003). The related p38MAPK and ERK subfamilies activation were found rhythmic in tissues containing circadian oscillators. First, p38MAPK phosphorylation levels oscillate in the rat and chick pineal gland where these kinases have been shown to control the period and phase of circadian rhythms (Hayashi et al., 2003; Hasegawa and Cahill, 2004). Second, daily and circadian rhythms of ERK activations occur respectively in the rat pineal gland (Ho et al., 2003), and in both Syrian hamster and mouse SCN (Obrietan et al., 1998; Pizzio et al., 2003). In the mouse SCN, ERK is known to participate to the entrainment of rhythms by light cycles (Butcher et al., 2002; Dziema et al., 2003). These data allowed us to raise the hypothesis that JNK might play a role in the circadian machinery.
To determine the role of JNK phosphorylation in circadian rhythm regulation, we utilized an inhibitor of JNK activation, SP600125. This potent and selective ATP-competitive inhibitor is effective on all isoforms of JNK and exhibits over 300-fold greater selectivity for JNK as compared with related kinases of the ERK and p38MAPK families (Bennett et al., 2001). SP600125 is described as a reversible and dose-dependent inhibitor (Bennett et al., 2001). Using the real-time bioluminescence monitoring system to record Period1-driven bioluminescence rhythms, we notably found that SP600125 dose-dependently lengthens the period of the oscillations in a reversible manner, and delays the onset of the oscillations (Fig. 4). This effect was intriguing by its magnitude, although a similar phenomenon but with a lesser degree, has been described by Hayashi et al. (2003). Following inhibition of p38MAPK activation, using its inhibitor SB203580, Hayashi et al. (2003) found the free-running period of the rhythm of melatonin release in cultured chick pineal cells extended from 24.0±0.3 h to 28.7±1.1 h. They also reported that a 4 h-pulse of SB203580 given during the subjective day could delay the phase of the melatonin release rhythm. SP600125 could also dose-dependently delay the phase of the rhythm of melatonin release from Xenopus photoreceptor layers (Hasegawa and Cahill, 2004). The similar effects of SB203580 and SP600125 might suggest that p38MAPK and JNK are both involved in the regulation of the circadian molecular clockwork. Moreover, Hasegawa and Cahill (2004) suggested that SB203580 not only inhibits activation of p38MAPK but also other kinases, including JNK1, JNK2 and JNK3. This finding implies that the effects of SB203580 on lengthening the period, as well as delaying the phase, reported by Hayashi et al. (2003), might actually be an effect of both p38MAPK and JNK inhibitions.
In the SCN from Period1-luciferase transgenic mice and Period2Luciferase knockin mice, we observed a comparable SP600125-induced lengthening of the period (Table 1, Fig. 5A). Likewise, an extra-SCN brain region (pineal gland) and a peripheral tissue (lung) known to contain circadian clocks (Sakamoto et al., 1998; Yamazaki et al., 2002; Karolczak et al., 2004; Simonneaux et al., 2004; Fukuhara et al., 2005) demonstrated an analogous effect of SP600125 (Table 1, Figs. 5B and 6A). It is noteworthy that in the SCN and the pineal gland, in which the three Jnk genes are expressed, we cannot exclude a putative involvement of JNK3 isoforms in the modulation of the period by SP600125 treatment.
Interestingly, kidneys that rhythmically express circadian clock genes (Sakamoto et al., 1998; Yamazaki et al., 2002; Yoo et al., 2004) are affected by SP600125 treatment in a different fashion than other tissues (Fig. 6B). The discrepancy between results of Period1-luciferase and Period2Luciferase could be explained by the different DNA constructs used to produce transgenic and knockin animals. Indeed, the Period2Luciferase mice have been produced by insertion of the luciferase reporter gene after the Period2 endogenous coding sequence (Yoo et al., 2004) and thus are likely to drive more stable oscillations than the Period1-luciferase transgenic mice (Yamazaki et al., 2000; Herzog et al., 2004). Alternative explanations will be either a higher sensitivity of kidney tissue to the inhibitor, or an organ-specific effect of SP600125 on regulation of circadian oscillations. To test the first hypothesis, we assayed the Period1-bioluminescence rhythms in the presence of 15 μM SP600125, but found similar rhythm disruption as seen with the 30 μM-treatment (data not shown). It is therefore likely that the effects of SP600125 on circadian oscillations are tissue-specific.
What might be the mechanisms of the action of SP600125 in the modulation of the period length? First, it is noticeable that the effects of SP600125 are not due to toxicity, because we demonstrated that after 3 days of SP600125 treatment, when the clock is already slowed down due to the inhibitor, the cell survival rate is identical in the SP600125-treated and non-treated samples.
It is most likely that SP600125 lengthens period through changes in JNK phosphorylation levels. First, we show that the lengthening effect of SP600125 in fibroblasts is associated with both a complete inhibition of the acute phosphorylation of p46 kDa and p54 kDa isoforms, confirming that SP600125 efficiently worked in our model, and an abolishment of the rhythmic phosphorylation of p46 kDa isoforms (Fig. 3). Contrary to p46 kDa whose baseline phosphorylation levels were reduced during the 24 h of SP600125 treatment, p54 kDa phosphorylation levels remained unchanged as compared with the control condition (Fig. 1, 3B). This finding suggests that the p54 kDa phosphorylation levels detected in the control condition correspond to the baseline levels and that p54 kDa is likely to be acutely but only transiently phosphorylated, as opposed to p46 kDa. We also show that valproic acid both increases activation of both isoforms of JNK (Fig. 7B), and shortens the period of bioluminescence oscillations in fibroblasts (Fig. 7A). Current literature on the effect of valproic acid on circadian rhythms is not consistent, though. Valproic acid has been shown to lengthen the period of free-running locomotor activity rhythms and increase arrhythmicity in Drosophila housed in constant darkness (Dokucu et al., 2005), but it does not seem to alter any rhythmic parameters in Syrian hamster maintained in constant darkness (Klemfuss and Kripke, 1995). Another group showed that valproic acid increases activity duration of rats while shortening the period length of their free-running circadian rhythmicity (Rietveld and van Schravendijk, 1987). To provide firm evidence that valproic acid is affecting the mammalian circadian clock, we treated Period1-luciferase SCN slices using 1 mM valproic acid, and found that the period was shortened (22.60 h, data not shown). Although we suggest here that valproic acid may regulate the period length through an effect on JNK, it should be noted that the related kinase ERK is also activated by valproic acid (Yuan et al., 2001). We thus cannot exclude the possibility that valproic acid utilizes multiple pathways to mediate period shortening. Taken together, these results suggest that JNK phosphorylation levels may participate in the regulation of circadian rhythms’ period length. As bipolar disorder patients experience unstable circadian rhythms, and treating patients with valproic acid remarkably stabilizes their mood (Goodwin and Jamison, 1990; Tsujimoto et al., 1990; Jones et al., 2005), it would be interesting to further investigate whether the effect of SP600125 on period and perhaps other circadian parameters is related to mood stabilizing effect.
A possible mechanism for the action of JNK might be alteration of the activity levels of some of the downstream targets of the JNK. As it has been demonstrated that ERK1/2 interacts with and phosphorylates BMAL1 (Sanada et al., 2002) and CRY1/2 (Sanada et al., 2004) proteins to control their stability, a similar function of JNK is conceivable. Alternatively, JNKs are potent activators of c-JUN, which participates in the formation of the activator protein-1 (AP-1) transcription activator complex. AP-1 is known to regulate gene expression through binding to the tetradecanoyl phorbol acetate response element (TRE, consensus sequence 5’-TGAG/CTCA-3’) binding site located in the promoter of numerous genes (Hess et al., 2004). Therefore, changing JNK activation levels by treating with SP600125 or valproic acid might affect the transcription of circadian clock genes through an AP-1 regulation. Period1 promoters of mouse and rat genes, including the 6.7 kb used to create the Period1-luciferase DNA construct for Period1-luciferase mice (Herzog et al., 2004), seem to contain four AP-1-mediated regulatory elements as described in a previous study (Chansard et al., 2005). Likewise, we found two putative TRE binding sites in the mouse Period2 promoters that localize at −1459 bp and −4470 bp (GenBank accession number NT_078297), from a transcription start site located at 6063573 bp. Taken together, it strengthens the possibility that the periods of the bioluminescence rhythms might be modulated through an AP-1 activity.
Although SP600125 has been described as a highly specific inhibitor for JNK activation (Bennett et al., 2001), other kinases may also be affected by this drug (Bain et al., 2003). For example, the activity of cyclin-dependent kinase 2 (CDK-2), which controls the cell cycle, is inhibited by SP600125 (Malumbres and Barbacid, 2005). Previous reports showed that olomoucine, an inhibitor of this kinase (CDK-2), dose-dependently lengthens the period in the eye of the marine snail Bulla gouldiana (Krucher et al., 1997), and phase-delays the rhythm of Aplysia ocular circadian rhythm (Sankrithi and Eskin, 1999). Despite a lower magnitude of the period lengthening effect, those effects on the circadian oscillations are similar to what we report here using SP600125; thus, we cannot exclude the possibility that both JNK and CDK-2 inhibitions are responsible for the period lengthening. Additional investigations will be necessary to understand the participation of other kinases in the modulation of period length by SP600125.
Over the past few years, protein kinases have been proven to play a critical role in the regulation of circadian rhythm generation. However, the real importance of this post-translational modification in the mechanisms of circadian clocks remains unclear. Data collected in several organisms have shown that the phosphorylation status of some of the circadian clock proteins affects their stability and their participation in the regulation of the core transcriptional loops of circadian clocks. For example, circadian period in Drosophila, is controlled by casein kinase II (Akten et al., 2003) and DOUBLETIME (Price et al., 1998; Bao et al., 2001) which destabilize, and PROTEIN PHOSPHATASE 2A, which stabilizes, the core loop component, PERIOD protein (Sathyanarayanan et al., 2004). This tight regulation of PERIOD phosphorylation eventually determines the timing of the PERIOD- and TIMELESS-driven inhibition of Period and Timeless transcription through an action on the CLOCK-CYCLE heterodimer. Some data collected in several organisms suggest that phosphorylation of clock proteins might even play a greater role than initially suspected. The most striking of these data have come from a study performed by Nakajima and colleagues (2005) on the cyanobacteria circadian clock. In this study, the three main components of the cyanobacteria circadian machinery, the KaiA, KaiB and KaiC proteins, were incubated in vitro in the presence of ATP. In these conditions and thus in the absence of any transcription, the KaiC phosphorylation robustly oscillated with a period of about 24 h and this phenomenon was temperature-compensated, a characteristic of circadian rhythms (Nakajima et al., 2005). This astonishing result raises questions about the nature of the role of both transcription and translation into the molecular mechanisms of circadian clocks. The magnitude of the effect we observed when changing JNK phosphorylation levels with SP600125 supports the idea of a major role for clock protein phosphorylation by JNK in the mechanisms of circadian clocks. Additional studies will allow us to understand the mechanism of JNK-specific period regulation.
Acknowledgments
The authors would like to thank Drs. Hajime Tei, Akiko Hida-Fukuda and Shin Yamazaki for providing us Pe-riod1-luciferase transgenic mice, Dr. Joseph S. Takahashi and Dr. Eric D. Herzog for providing us Period2Luciferase mice, and Dr. Carl H. Johnson for providing rat-1 fibroblasts stably transfected with a Period1-luciferase reporter gene. We are very grateful to Drs. Shin Yamazaki and Valérie Simonneaux for fruitful discussions. This investigation was supported by NS034194, CBN, NSF IIS 0423200, NSF0423200, the Keck Foundation and the Fondation Recherche Médicale.
Abbreviations
- AP-1
activator protein-1
- CDK-2
cyclin-dependent kinase 2
- DMSO
dimethyl sulfoxide
- ERK
extracellular-regulated kinases
- JNK
c-Jun N-terminal kinases
- MAPK
mitogen-activated protein kinases
- SCN
suprachiasmatic nucleus
- TRE
tetradecanoyl phorbol acetate response element
- ZT
zeitgeber time
References
- Akten B, Jauch E, Genova GK, Kim EY, Edery I, Raabe T, Jackson FR. A role for CK2 in the Drosophila circadian oscillator. Nat Neurosci. 2003;6:251–257. doi: 10.1038/nn1007. [DOI] [PubMed] [Google Scholar]
- Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Rihel J, Bjes E, Fan JY, Price JL. The Drosophila double-timeS mutation delays the nuclear accumulation of period protein and affects the feedback regulation of period mRNA. J Neurosci. 2001;21:7117–7126. doi: 10.1523/JNEUROSCI.21-18-07117.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr RK, Bogoyevitch MA. The c-Jun N-terminal protein kinase family of mitogen-activated protein kinases (JNK MAPKs) Int J Biochem Cell Biol. 2001;33:1047–1063. doi: 10.1016/s1357-2725(01)00093-0. [DOI] [PubMed] [Google Scholar]
- Bennett BL, Sasaki DT, Murray BW, O’Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozyczko-Coyne D, Saporito MS, Hudkins RL. Targeting the JNK pathway for therapeutic benefit in CNS disease. Curr Drug Targets CNS Neurol Disord. 2002;1:31–49. doi: 10.2174/1568007023339472. [DOI] [PubMed] [Google Scholar]
- Butcher GQ, Dziema H, Collamore M, Burgoon PW, Obrietan K. The p42/44 mitogen-activated protein kinase pathway couples photic input to circadian clock entrainment. J Biol Chem. 2002;277:29519–29525. doi: 10.1074/jbc.M203301200. [DOI] [PubMed] [Google Scholar]
- Chansard M, Iwahana E, Liang J, Fukuhara C. Regulation of cAMP-induced arylalkylamine N-acetyltransferase, Period1, and MKP-1 gene expression by mitogen-activated protein kinases in the rat pineal gland. Brain Res Mol Brain Res. 2005;139:333–340. doi: 10.1016/j.molbrainres.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Derijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Dokucu ME, Yu L, Taghert PH. Lithium- and valproate-induced alterations in circadian locomotor behavior in Drosophila. Neuropsychopharmacology. 2005;30:2216–2224. doi: 10.1038/sj.npp.1300764. [DOI] [PubMed] [Google Scholar]
- Dziema H, Oatis B, Butcher GQ, Yates R, Hoyt KR, Obrietan K. The ERK/MAP kinase pathway couples light to immediate-early gene expression in the suprachiasmatic nucleus. Eur J Neurosci. 2003;17:1617–1627. doi: 10.1046/j.1460-9568.2003.02592.x. [DOI] [PubMed] [Google Scholar]
- Fukuhara C, Tosini G. Peripheral circadian oscillators and their rhythmic regulation. Front Biosci. 2003;8:d642–d651. doi: 10.2741/1042. [DOI] [PubMed] [Google Scholar]
- Fukuhara C, Yamazaki S, Liang J. Pineal circadian clocks gate arylalkylamine N-acetyltransferase gene expression in the mouse pineal gland. J Neurochem. 2005;93:156–162. doi: 10.1111/j.1471-4159.2004.03008.x. [DOI] [PubMed] [Google Scholar]
- Gachon F, Nagoshi E, Brown SA, Ripperger J, Schibler U. The mammalian circadian timing system: from gene expression to physiology. Chromosoma. 2004;113:103–112. doi: 10.1007/s00412-004-0296-2. [DOI] [PubMed] [Google Scholar]
- Gallego M, Eide EJ, Woolf MF, Virshup DM, Forger DB. An opposite role for tau in circadian rhythms revealed by mathematical modeling. Proc Natl Acad Sci U S A. 2006;103:10618–10623. doi: 10.1073/pnas.0604511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Borreguero D, Larrosa O, Bravo M. Parkinson’s disease and sleep. Sleep Med Rev. 2003;7:115–129. doi: 10.1053/smrv.2002.0229. [DOI] [PubMed] [Google Scholar]
- Goodwin FK, Jamison KR. Manic depressive illness. New York: Oxford University Press; 1990. [Google Scholar]
- Gupta S, Barrett T, Whitmarsh AJ, Cavanagh J, Sluss HK, Derijard B, Davis RJ. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996;15:2760–2770. [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M, Cahill GM. Regulation of the circadian oscillator in Xenopus retinal photoreceptors by protein kinases sensitive to the stress-activated protein kinase inhibitor, SB 203580. J Biol Chem. 2004;279:22738–22746. doi: 10.1074/jbc.M401389200. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Herzog ED. Clock genes, oscillators, and cellular networks in the suprachiasmatic nuclei. J Biol Rhythms. 2004;19:400–413. doi: 10.1177/0748730404268786. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Sanada K, Hirota T, Shimizu F, Fukada Y. p38 Mitogen-activated protein kinase regulates oscillation of chick pineal circadian clock. J Biol Chem. 2003;278:25166–25171. doi: 10.1074/jbc.M212726200. [DOI] [PubMed] [Google Scholar]
- Herzog ED, Aton SJ, Numano R, Sakaki Y, Tei H. Temporal precision in the mammalian circadian system: a reliable clock from less reliable neurons. J Biol Rhythms. 2004;19:35–46. doi: 10.1177/0748730403260776. [DOI] [PubMed] [Google Scholar]
- Hess J, Angel P, Schorpp-Kistner M. AP-1 subunits: quarrel and harmony among siblings. J Cell Sci. 2004;117:5965–5973. doi: 10.1242/jcs.01589. [DOI] [PubMed] [Google Scholar]
- Hirota T, Okano T, Kokame K, Shirotani-Ikejima H, Miyata T, Fukada Y. Glucose down-regulates Per1 and Per2 mRNA levels and induces circadian gene expression in cultured Rat-1 fibroblasts. J Biol Chem. 2002;277:44244–44251. doi: 10.1074/jbc.M206233200. [DOI] [PubMed] [Google Scholar]
- Ho AK, Mackova M, Price L, Chik CL. Diurnal variation in p42/44 mitogen-activated protein kinase in the rat pineal gland. Mol Cell Endocrinol. 2003;208:23–30. doi: 10.1016/s0303-7207(03)00260-0. [DOI] [PubMed] [Google Scholar]
- Izumo M, Johnson CH, Yamazaki S. Circadian gene expression in mammalian fibroblasts revealed by real-time luminescence reporting: temperature compensation and damping. Proc Natl Acad Sci U S A. 2003;100:16089–16094. doi: 10.1073/pnas.2536313100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SH, Hare DJ, Evershed K. Actigraphic assessment of circadian activity and sleep patterns in bipolar disorder. Bipolar Disord. 2005;7:176–186. doi: 10.1111/j.1399-5618.2005.00187.x. [DOI] [PubMed] [Google Scholar]
- Karolczak M, Burbach GJ, Sties G, Korf HW, Stehle JH. Clock gene mRNA and protein rhythms in the pineal gland of mice. Eur J Neurosci. 2004;19:3382–3388. doi: 10.1111/j.0953-816X.2004.03444.x. [DOI] [PubMed] [Google Scholar]
- Klemfuss H, Kripke DF. Antimanic drugs stabilize hamster circadian rhythms. Psychiatry Res. 1995;57:215–222. doi: 10.1016/0165-1781(95)02687-r. [DOI] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(Spec No 2):R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Krucher NA, Meijer L, Roberts MH. The cyclin-dependent kinase (cdk) inhibitors, olomoucine and roscovitine, alter the expression of a molluscan circadian pacemaker. Cell Mol Neurobiol. 1997;17:495–507. doi: 10.1023/A:1026358821640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrey PL, Shimomura K, Antoch MP, Yamazaki S, Zemenides PD, Ralph MR, Menaker M, Takahashi JS. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science. 2000;288:483–492. doi: 10.1126/science.288.5465.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem Sci. 2005;30:630–641. doi: 10.1016/j.tibs.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Manning AM, Davis RJ. Targeting JNK for therapeutic benefit: from junk to gold? Nat Rev Drug Discov. 2003;2:554–565. doi: 10.1038/nrd1132. [DOI] [PubMed] [Google Scholar]
- Mormont MC, Levi F. Circadian-system alterations during cancer processes: a review. Int J Cancer. 1997;70:241–247. doi: 10.1002/(sici)1097-0215(19970117)70:2<241::aid-ijc16>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Morton AJ, Wood NI, Hastings MH, Hurelbrink C, Barker RA, Maywood ES. Disintegration of the sleep-wake cycle and circadian timing in Huntington’s disease. J Neurosci. 2005;25:157–163. doi: 10.1523/JNEUROSCI.3842-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Imai K, Ito H, Nishiwaki T, Murayama Y, Iwasaki H, Oyama T, Kondo T. Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro. Science. 2005;308:414–415. doi: 10.1126/science.1108451. [DOI] [PubMed] [Google Scholar]
- Obrietan K, Impey S, Storm DR. Light and circadian rhythmicity regulate MAP kinase activation in the suprachiasmatic nuclei. Nat Neurosci. 1998;1:693–700. doi: 10.1038/3695. [DOI] [PubMed] [Google Scholar]
- Onen F, Onen SH. Sleep rhythm disturbances in Alzheimer’s disease. Rev Med Interne. 2003;24:165–171. doi: 10.1016/s0248-8663(02)00018-8. [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Peng J, Andersen JK. The role of c-Jun N-terminal kinase (JNK) in Parkinson’s disease. IUBMB Life. 2003;55:267–271. doi: 10.1080/1521654031000121666. [DOI] [PubMed] [Google Scholar]
- Pizzio GA, Hainich EC, Ferreyra GA, Coso OA, Golombek DA. Circadian and photic regulation of ERK, JNK and p38 in the hamster SCN. Neuroreport. 2003;14:1417–1419. doi: 10.1097/00001756-200308060-00002. [DOI] [PubMed] [Google Scholar]
- Price JL, Blau J, Rothenfluh A, Abodeely M, Kloss B, Young MW. Double-time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell. 1998;94:83–95. doi: 10.1016/s0092-8674(00)81224-6. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Menaker M. A mutation of the circadian system in golden hamsters. Science. 1988;241:1225–1227. doi: 10.1126/science.3413487. [DOI] [PubMed] [Google Scholar]
- Resnick L, Fennell M. Targeting JNK3 for the treatment of neurodegenerative disorders. Drug Discov Today. 2004;9:932–939. doi: 10.1016/S1359-6446(04)03251-9. [DOI] [PubMed] [Google Scholar]
- Rietveld WJ, van Schravendijk K. Food intake and antiepileptic drugs: evidence for a role of GABA in circadian time keeping. Prog Clin Biol Res. 1987;227B:507–511. [PubMed] [Google Scholar]
- Sabapathy K, Hochedlinger K, Nam SY, Bauer A, Karin M, Wagner EF. Distinct roles for JNK1 and JNK2 in regulating JNK activity and c-Jun-dependent cell proliferation. Mol Cell. 2004;15:713–725. doi: 10.1016/j.molcel.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Nagase T, Fukui H, Horikawa K, Okada T, Tanaka H, Sato K, Miyake Y, Ohara O, Kako K, Ishida N. Multitissue circadian expression of rat period homolog (rPer2) mRNA is governed by the mammalian circadian clock, the suprachiasmatic nucleus in the brain. J Biol Chem. 1998;273:27039–27042. doi: 10.1074/jbc.273.42.27039. [DOI] [PubMed] [Google Scholar]
- Sanada K, Harada Y, Sakai M, Todo T, Fukada Y. Serine phosphorylation of mCRY1 and mCRY2 by mitogen-activated protein kinase. Genes Cells. 2004;9:697–708. doi: 10.1111/j.1356-9597.2004.00758.x. [DOI] [PubMed] [Google Scholar]
- Sanada K, Okano T, Fukada Y. Mitogen-activated protein kinase phosphorylates and negatively regulates basic helix-loop-helix-PAS transcription factor BMAL1. J Biol Chem. 2002;277:267–271. doi: 10.1074/jbc.M107850200. [DOI] [PubMed] [Google Scholar]
- Sankrithi N, Eskin A. Effects of cyclin-dependent kinase inhibitors on transcription and ocular circadian rhythm of Aplysia. J Neurochem. 1999;72:605–613. doi: 10.1046/j.1471-4159.1999.0720605.x. [DOI] [PubMed] [Google Scholar]
- Sathyanarayanan S, Zheng X, Xiao R, Sehgal A. Posttranslational regulation of Drosophila PERIOD protein by protein phosphatase 2A. Cell. 2004;116:603–615. doi: 10.1016/s0092-8674(04)00128-x. [DOI] [PubMed] [Google Scholar]
- Schibler U, Ripperger J, Brown SA. Peripheral circadian oscillators in mammals: time and food. J Biol Rhythms. 2003;18:250–260. doi: 10.1177/0748730403018003007. [DOI] [PubMed] [Google Scholar]
- Simonneaux V, Poirel VJ, Garidou ML, Nguyen D, Diaz-Rodriguez E, Pevet P. Daily rhythm and regulation of clock gene expression in the rat pineal gland. Brain Res Mol Brain Res. 2004;120:164–172. doi: 10.1016/j.molbrainres.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Sokolove PG, Bushell WN. The chi square periodogram: its utility for analysis of circadian rhythms. J Theor Biol. 1978;72:131–160. doi: 10.1016/0022-5193(78)90022-x. [DOI] [PubMed] [Google Scholar]
- Tsujimoto T, Yamada N, Shimoda K, Hanada K, Takahashi S. Circadian rhythms in depression. Part II: Circadian rhythms in inpatients with various mental disorders. J Affect Disord. 1990;18:199–210. doi: 10.1016/0165-0327(90)90037-9. [DOI] [PubMed] [Google Scholar]
- Walters J, Skene D, Hampton SM, Ferns GA. Biological rhythms, endothelial health and cardiovascular disease. Med Sci Monit. 2003;9:RA1–RA8. [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Effects of aging on central and peripheral mammalian clocks. Proc Natl Acad Sci U S A. 2002;99:10801–10806. doi: 10.1073/pnas.152318499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2∷LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T, Yamazaki S, Menaker M. Effects of preparation time on phase of cultured tissues reveal complexity of circadian organization. J Biol Rhythms. 2005;20:500–512. doi: 10.1177/0748730405280775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan PX, Huang LD, Jiang YM, Gutkind JS, Manji HK, Chen G. The mood stabilizer valproic acid activates mitogen-activated protein kinases and promotes neurite growth. J Biol Chem. 2001;276:31674–31683. doi: 10.1074/jbc.M104309200. [DOI] [PubMed] [Google Scholar]


