Abstract
Activation of inositol-1,4,5-trisphosphate receptors (InsP3Rs) and ryanodine receptors (RyRs) can lead to the release of Ca2+ from intracellular stores and propagating Ca2+ waves. Previous studies of these proteins in neurons have focused on their distribution in adult tissue, whereas, recent functional studies have examined neural tissue extracted from prenatal and young postnatal animals. In this study we examined the distribution of InsP3R isotypes 1–3 and RyR isotypes 1–3 in rat hippocampus during postnatal maturation, with a focus on InsP3R1 because it is most prominent in the hippocampus. InsP3R1 was observed in pyramidal cells and granule cells, InsP3R2 immunoreactivity was observed in perivascular astrocytes and endothelial cells, and InsP3R3 immunoreactivity was detected in axon terminals located in stratum pyramidale of CA1 and microvessels in stratum radiatum. RyR1 immunolabeling was enriched in CA1, RyR2 was most intense in CA3 and the dentate gyrus, and RyR3 immunolabeling was detected in all subfields of the hippocampus, but was most intense in stratum lacunosum-moleculare. During maturation from 2 to 10 weeks of age there was a shift in InsP3R1 immunoreactivity from a high density in the proximal apical dendrites to a uniform distribution along the dendrites. Independent of age, InsP3R1 immunoreactivity was observed to form clusters within the primary apical dendrite and at dendritic bifurcations of pyramidal neurons. As CA1 pyramidal neurons matured, InsP3R1 was often co-localized with the Ca2+ binding protein calbindin D-28k. In contrast, InsP3R1 immunolabel was never co-localized with calbindin D-28k immunopositive interneurons located outside of stratum pyramidale or with parvalbumin, typically found in hippocampal basket cells, suggesting that InsP3R1s do not play a role in internal Ca2+ release in these interneurons. These findings should help to interpret past functional studies and inform future studies examining the characteristics and consequences of InsP3R-mediated internal Ca2+ release and Ca2+ waves in hippocampal neurons.
Keywords: calcium release, calcium wave, CA1, CA3, IP3, calbindin D, 28k
Inositol-1,4,5-trisphosphate receptors (InsP3Rs) and ryanodine receptors (RyRs) are two families of proteins located on membranes of cellular organelles that store calcium (Ca2+) (for review, see Berridge, 1998). They are channels permeable to Ca2+, predominantly located on the smooth endoplasmic reticulum (SER), and are ubiquitous across cell types. Activation of InsP3Rs and RyRs by InsP3 and Ca2+, respectively, lead to transient opening of the channels and the flow of Ca2+ down a steep concentration gradient into the cytosol. The process can occur progressively along the SER resulting in the propagation of Ca2+ waves. Surprisingly little is known, however, about the basic characteristics and consequences of internal Ca2+ release and Ca2+ waves in neurons, despite the ubiquitous role that Ca2+ plays in virtually all aspects of neuronal function.
There are three known isotypes of InsP3Rs and three known isotypes of RyRs (designated types 1, 2, or 3 for both families). Activation of a given isotype leads to distinct Ca2+ release characteristics as determined by their binding properties and their intrinsic regulatory features. In some cell types, InsP3Rs and RyRs interact functionally to coordinate Ca2+ release and/or the propagation of Ca2+ waves through a regenerative process in which the InsP3-initiated release of Ca2+ leads to subsequent activation of RyRs and additional release along the SER (Berridge, 1998; Leite et al., 2002). Because each receptor isotype has unique biochemical and biophysical properties (Berridge, 1998), and thus are likely to have different consequences on internal Ca2+ release, the distribution of individual isotypes both within neurons and across neuron types is of particular interest to researchers who study internal Ca2+ release and its role in neuronal function. It is also important to understand whether these distributions change during development and aging.
There have been a number of seminal studies investigating the distribution of intracellular Ca2+ release channels in neural tissue (Sharp et al., 1993; Sharp et al., 1999). These studies have focused on characterizing the distribution of the type 1 InsP3Rs and type 2 RyRs in adult tissue (Nakanishi et al., 1991; Fotuhi et al., 1993; Sharp et al., 1993; Dent et al., 1996; Sharp et al., 1999), mostly likely due to their predominance in the brain (McPherson and Campbell, 1990). The distribution of type 2 InsP3Rs have been described to a lesser extent (Bourguignon et al., 1994; Mountian et al., 1999; Sharp et al., 1999), and the distribution of RyR isotypes 1–3 have been studied using in situ hybridization techniques (Furuichi and Mikoshiba, 1995; Giannini et al., 1995; Mori et al., 2000; Zhao et al., 2000).
Despite the prevalence of these proteins in neurons, functional studies of InsP3R- and RyR-mediated internal Ca2+ release have lagged behind their anatomical descriptions. Further complicating a comprehensive understanding of their role in neuronal function is the mismatch between the ages of animals used in anatomical studies and the ages of animals used to characterize internal Ca2+ release. Whereas anatomical studies have been performed on tissue from adult animals, functional studies have been performed almost exclusively on reduced preparations from young rats > 3 weeks old. The primary goal of this study was to characterize the distribution of InsP3Rs and RyRs in neurons of hippocampus during their maturation from 2 to 10 weeks old. In particular, we focused on the distribution of InsP3R1s, and their overlap with the Ca2+ binding proteins calbindin D-28k and parvalbumin, because it is known that internal Ca2+ release in these neurons depend on InsP3R activation and not RyR activation (Nakamura et al., 1999; Kapur et al., 2001), and because Ca2+ binding proteins are thought to be potent regulators of internal Ca2+ release and Ca2+ waves, in general (Dargan and Parker, 2003; Dargan et al., 2004).
Experimental Procedures
Animals and Tissue Preparation
Tissue was obtained from rats (Sprague-Dawley, Charles River) using experimental procedures consistent with those outlined in National Institutes of Health publication 91-3207, Preparation and Maintenance of Higher Animals During Neuroscience Experiments, and approved by the Institutional Animal Care and Use Committee at the Yale University School of Medicine. Animals were deeply anesthetized with a mixture of ketamine, xylazine and acepromazine and perfused transcardially with 4% paraformaldehyde/0.05% glutaraldehyde in 0.1 M phosphate buffer (PB; pH = 7.4) and finally by aldehyde-free PB. After perfusion, the brains were removed from the skull, sliced coronally or sagitally into 60 μm sections using a vibratome (VT 1000S, Leica) and washed in PB overnight. We used rats of the following ages: two weeks old (n = 9), five weeks old (n = 6), 8–10 weeks old (n = 10), and one year old (n = 3). In the text, we often refer to 2 week-old animals as “young” and 8–10 week-old rats as “mature.”
Antibodies
For primary antibodies and references see Table 1.
Table 1.
Primary antibodies utilized in immunoprocedures.
| Antibody | Clonality | Source | Reference |
|---|---|---|---|
| anti-InsP3R1 | polyclonal | Research Genetics (Huntsville, AL) | Johenning et al., 2002
Koulen et al., 2000 |
| CT2 (anti-InsP3R2) | polyclonal | R. J. Wojcikiewicz (SUNY, Syracuse) | Wojcikiewicz, 1995 |
| anti-InsP3R3 | monoclonal | BD Transduction Laboratories (Lexington, KY) | Blondel et al., 1993 |
|
| |||
| MA3-925 | monoclonal | Affinity Bioreagents (Golden, CO) | Airey et al., 1993 |
| MA3-916 | Affinity Bioreagents (Golden, CO) | Morgan and Jacob, 1994 | |
|
| |||
| anti-RYR1 | polyclonal | Vincenzo Sorrentino (University of Siena) | Balschun et al., 1999 |
| anti-RYR2 | |||
| anti-RYR3 | |||
|
| |||
| anti-calbindin D-28k | monoclonal | Sigma (St. Louis, MO) | |
| anti-parvalbumin | monoclonal | Swant (Bellinzona, Switzerland) | |
Immunohistochemistry
Free-floating sections were washed in 0.05 M Tris-buffered saline (TBS, pH = 7.4) and treated in sodium borohydride/TBS for 10 minutes to reduce free aldehyde groups. Sections were washed in TBS and preincubated for 1 hour in 10% normal goat serum (NGS) and 0.05% Tween 20 in TBS. Incubation with primary antibodies was performed for 36 h at 4°C in 1% NGS and 0.01% Tween 20 in TBS. Control experiments contained no primary antibody. Sections were washed in TBS and incubated for 2 hours in anti-rabbit or anti-mouse biotinylated F(ab′)2 (Jackson Immunoresearch, West Grove, PA), diluted 1:500 in TBS. Sections were washed repeatedly in TBS and incubated in avidin-biotin-peroxidase complex (ABC-Elite, Vector Laboratories) for 45 minutes. After washing in TBS, sections were preincubated for 10 minutes in 0.025% 3,3-diaminobenzidine (DAB) solution in TBS. Peroxidase reaction was started by the addition of 0.001% hydrogen peroxide; the reaction was stopped by transferring the tissue into TBS. Within an individual experiment, all reactions were terminated at the same time point. For fluorescence probes, sections were washed in TBS and incubated for 2 hours in dye-conjugated anti-rabbit and anti-mouse F(ab′)2, and diluted 1:500 in TBS. Sections were washed repeatedly in TBS, mounted on glass slides and stored at +4°.
Microscopy
InsP3R1 fluorescence was viewed using either an inverted confocal microscope (Nikon TE3000, Melville, NY) or an upright two-photon microscope (Zeiss LSM 510, Carl Zeiss, Göttingen, Germany) excited with a Ti:Sapphire laser (Chameleon, Coherent Inc., Santa Clara, CA). Stacks of digital micrographs (0.4 μm between images, 0.29 μm pixels) were saved, and clusters of InsP3R1 staining were identified by visual inspection. A cluster was defined as an area of intense fluorescence surrounded by little fluorescence and visible in at least 3 consecutive micrographs. Zeiss LSM Imaging Software was used to measure the distance between cluster edges and to construct 2D projections from image stacks.
Semi-Quantitive Analysis
Immunohistochemical data were analyzed using custom routines written in IGOR Pro (Wavemetrics, Lake Oswego, OR). Analysis focused on determining the relative density of immunolabeling across the layers of the CA1 subfield in individual tissue sections, and on determining whether this distribution of label density differed across age groups. Digital micrographs were taken at 20x magnification under similar lighting conditions using a Zeiss Axiophot. First, regions including stratum pyramidale, stratum radiatum and stratum lacunosum-moleculare with no visible irregularities were selected from digital micrographs. Next, label density in the selected regions was determined and normalized to the overall density in each selected CA1 area. At least 4 mm of the CA1 subfield across the longitudinal extent of the hippocampus of individual animals was analyzed. To optimize the process, all digital micrographs where taken at the same light intensity and multiple non-overlapping areas were measured on each digital micrograph. Data were processed by normalization to overall intensity in each selected region. In addition, the arithmetic average of label density was calculated for each micrograph, each animal, and each age group. Traces in figures include data from several animals, as indicated.
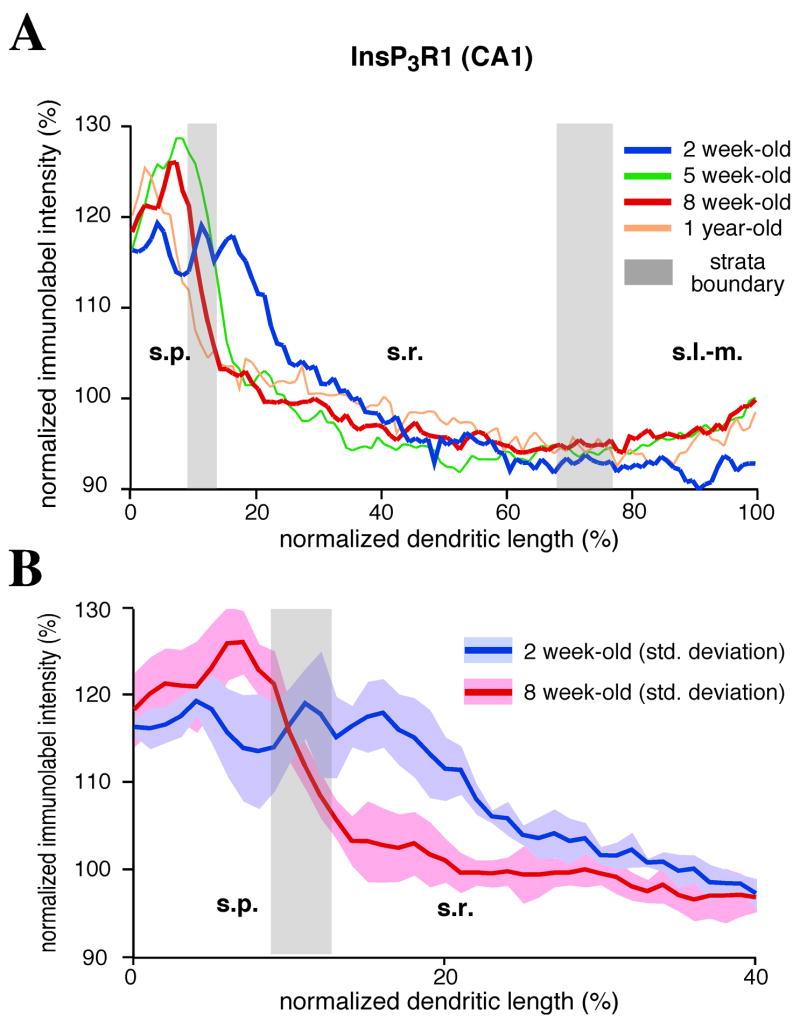
To normalize the distribution of InsP3R1 for dendritic length, we measured the distance from the starting point of the rectangle to the edge of stratum pyramidale, as well as the distance from the beginning and end of stratum lacunosum-moleculare. From these measurements, we calculated the width of all CA1 strata in our data (n = 5 for each age group; mean ± Std Dev.). The first area, containing two cell rows of stratum pyramidale, had a similar length in both age groups. Stratum radiatum was measured to be significantly shorter in 2 week-old rat hippocampus compared to 8–10 week-old rat hippocampus (young, 254 ± 25 μm; mature, 357 ± 27 μm; p < 0.01). The change in the length of stratum radiatum resulted in a small increase in the overall length (young, 410 ± 39 μm; mature, 448 ± 42 μm; p < 0.01), but was partially compensated for by a decrease of the width of stratum lacunosum-moleculare (young, 131 ± 21 μm; mature, 111 ± 2 μm; p < 0.01). After identifying the extent of developmentally associated changes in the widths of CA1 strata, we normalized the lengths of our densitometric traces to 100% and then plotted them in the same graph (see Figure 3).
Figure 3. InsP3R1 redistribution during maturation of CA1 pyramidal dendrites.
(A and B) Densitometric analysis of InsP3R1 immunolabel (DAB) shows that as CA1 pyramidal neurons mature there is a shift in the distribution of InsP3R1s from a relatively higher density in proximal apical dendrites to a more even distribution in the apical dendrites. The relative density of InsP3R1 label was determined by normalizing the intensity of the immunolabel signal continuously along the neuron from stratum pyramidale to stratum lacunosum-moleculare to the average intensity of label across the CA1 subfield (see Methods). The blue lines represent the average InsP3R1 distribution from 2 week-old tissue (n = 5; light blue shadow in B shows standard deviation). The red lines represent eight week-old data (n = 5; light red shadow in B shows standard deviation). (B) The graph has been expanded to highlight the region along the proximal apical dendrites that showed a significant difference in the distribution of InsP3R1 during maturation. This proximal region is the area where synaptically elicited internal Ca2+ release and Ca2+ waves are typically observed in CA1 and CA3 pyramidal neurons (Kapur et al, 2001; Nakamura et al., 1999).
RESULTS
InsP3R Distribution During Postnatal Development
General InsP3R Isotype 1–3 Immunoreactivity In Mature Rat Hippocampus
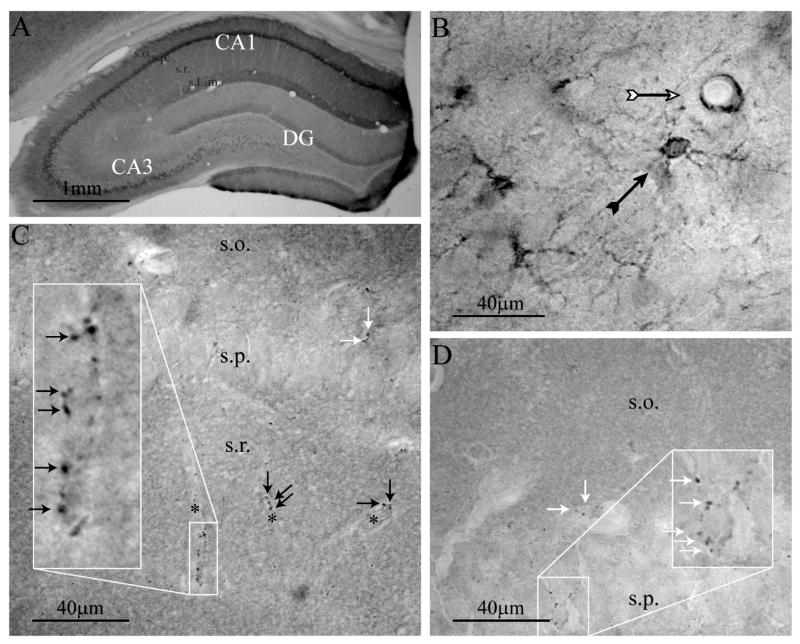
Consistent with previous reports (Nakanishi et al., 1991; Sharp et al., 1993; Dent et al., 1996, Sharp et al., 1999), InsP3R1 immunoreactivity was found throughout the mature rat hippocampus (> 8 weeks old) with the strongest labeling in area CA1 (Figures 1A and 2A). InsP3R1 immunostaining appeared to be exclusively located in neurons, while InsP3R2 immunoreactivity was only found in a subpopulation of astrocytes (Sharp et al., 1999) and in endothelial cells of capillaries (Figure 1B). Fine astrocytic protrusions immunopositive for InsP3R2 extended to endothelial cells and formed loose perivascular networks (Figure 1B). InsP3R isotype 3 antibody labeled sparsely distributed axonal boutons of unknown origin throughout the hippocampus (see Sharp et al., 1993). The immunoreactive boutons were found on somata of small neurons in stratum pyramidale of CA1 (Figures 1C and D), and in association with microvessels throughout the hippocampus (Figure 1C). In some cases, cell bodies in the stratum oriens of CA1 and CA3, adjacent to the alveus, displayed weak InsP3R3 immunoreactivity. A summary, describing the distribution of hippocampal InsP3R isotypes can be found in Table 2.
Figure 1. Expression pattern of InsP3R isotypes 1–3 immunoreactivity in mature rat hippocampus.
(A) Coronal section showing InsP3R1 immunostaining. The CA1 subfield exhibited a greater density of immunolabel than that observed in the CA3 subfield and the dentate gyrus. (B) InsP3R2 immunoreactivity was detected in astrocytes (black arrow) and in a neighboring endothelial cell (white arrow). (C and D) InsP3R3 immunolabeling was detected in structures resembling en passant axon terminals that were associated with microvessels in stratum radiatum (black arrows) and somata in stratum pyramidale (white arrows); asterisk marks the capillary lumen. Abbreviations for hippocampal strata: s.o., stratum oriens; s.p., stratum pyramidale; s.r., stratum radiatum.
Figure 2. InsP3R1 immunolabeling of 2 week-old and 8 week-old rat hippocampus.
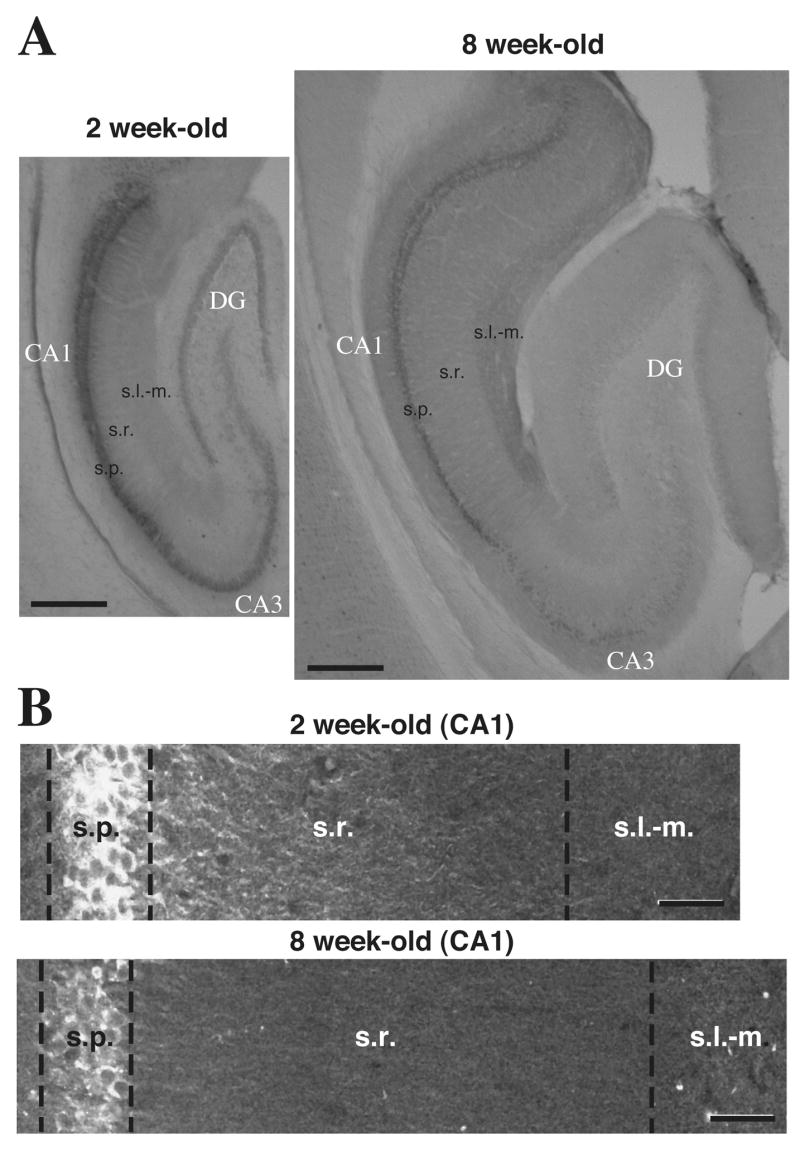
(A) Hippocampal sagittal sections show InsP3R1 immunolabeling and the relative sizes of hippocampi at 2 weeks and 8 weeks of age. (B) Confocal composite images of InsP3R1 fluorescence immunoreactivity in CA1 pyramidal neurons from 2 week-old and from an 8 week-old rat coronal hippocampal sections. (scale bars: A, 500 μm; B, 40 μm).
Table 2.
Summary of detected Immunolabeling for InsP3Rs and RYRs.
| CA1 | CA3 | Dentate Gyrus | |
|---|---|---|---|
| InsP3R1 | +++ | ++ | + |
| InsP3R2 | + | + | + |
| InsP3R3 | + | ||
|
| |||
| RyR1 | +++ | ++ | + |
| RyR2 | + | ++ | +++ |
| RyR3 | + | + | + |
detected
strong labeling
very strong labeling
Changes In InsP3R Distribution During Late Postnatal Development
Because most functional studies examining InsP3R-mediated internal Ca2+ release are performed on hippocampal pyramidal neurons in reduced preparations from young animals < 3 weeks old (Pozzo-Miller et al., 1996; Nakamura et al., 1999; Yeckel et al., 1999; Nakamura et al., 2002), we were particularly interested in comparing the distribution of InsP3R1 in the CA1 subfield of two week-old (young) and 8–10 week-old (mature) rats (Figures 2A and 2B). InsP3R1 immunolabeling in CA1 pyramidal neurons of young rats revealed intense immunoreactivity in somata and proximal primary dendrites, with a decrease in intensity more distally along the dendrite (Figure 1B). In contrast, tissue from mature animals exhibited InsP3R1 immunostaining that was distributed with relative uniformity along the entire length of the apical dendrites (Figure 2B). It should be noted that there were no obvious differences in the distribution of the other InsP3R isotypes or in the distribution of RyRs in the dendrites of young vs. mature hippocampal pyramidal neurons.
To quantify the differences in InsP3R1 immunoreactivity, we performed densitometric measurements on DAB immunolabeling within the CA1 subfield of young and mature rat hippocampus. Rectangular regions of interest were drawn over digital micrographs of area CA1, which included stratum pyramidale, stratum radiatum, and stratum lacunosum-moleculare. Label intensity for each marked area was normalized to the average intensity across the entire region in order to reveal differences in the distribution of InsP3R1 along the length of the dendrites, starting with the somal region. In young animals, quantitative analysis showed a significantly greater density of InsP3R1 immunolabel in stratum pyramidale and the most proximal portion of the apical dendrites compared to a relatively weak density of label in the distal portions of the apical dendrites in stratum radiatum and stratum lacunosum-moleculare (Figure 3). In tissue from 8–10 week-old rats, analysis of InsP3R1 showed that label intensity was strongest in stratum pyramidale, followed by a sharp decline along the proximal dendrites and throughout stratum radiatum (Figure 3), and then a slight rise in immunolabel density in the distal dendrites in stratum lacunosum-moleculare.
Dendritic development is quite dynamic up to 4–6 weeks of age. In addition to the redistribution of a host of ion and ligand channels, the dendritic tree grows longer and more elaborate (Pokorny and Yamamoto, 1981). This development is reflected by increases in the overall size of the different hippocampal subfields (see Figure 2A). To address whether differences in dendritic length might confound our interpretation of the densitometry analysis, we normalized the distribution of InsP3R1 along CA1 pyramidal neurons during development. We performed this analysis on DAB immunohistochemical data and our previously identified regions of analysis by measuring the length of strata relative to the total length of CA1 neurons from edge of the first two cell rows of stratum pyramidale and the distal edge of stratum lacunosum-moleculare (see Methods). The results of this analysis supports our fundamental observation that the distribution of InsP3R1 shifts from being concentrated in the proximal primary dendrites to being distributed more uniformly along the length of the dendrites during the maturation of CA1 pyramidal neurons (Figure 3). In CA3 pyramidal neurons we observed a qualitatively similar redistribution during maturation. In young granule cells of the dentate gyrus the intensity of IP3R1 immunolabel was more intense in the soma region compared to mature tissue. In general, there was very little IP3R1 immunolabel in granule cell dendrites of either young or mature animals compared to pyramidal neurons of CA1.
High-resolution Microscopy Reveals InsP3R1 Clustering in CA1 Pyramidal Neurons
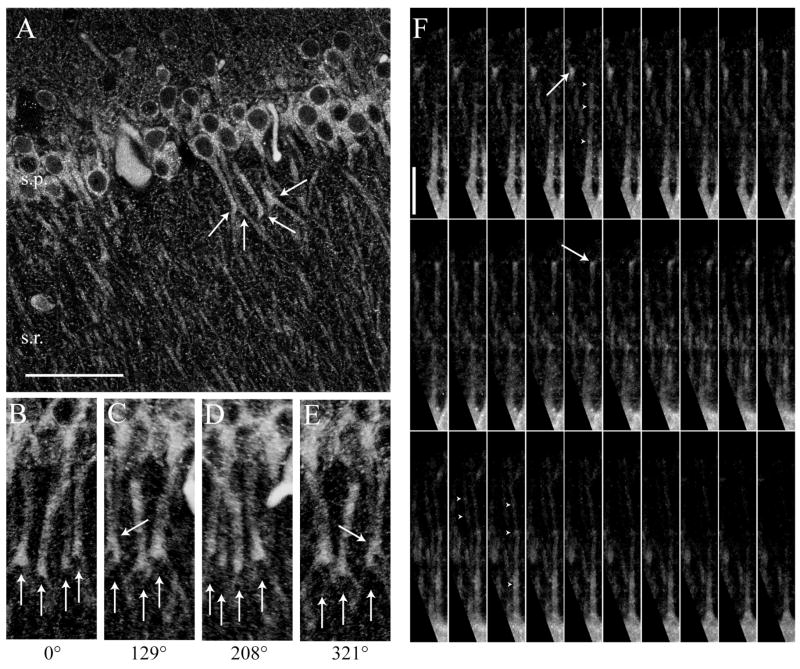
Additional analyses performed on tissue using fluorescent Alexa 488-labeled secondary antibodies and confocal microscopy, confirmed our finding that the subcellular expression of InsP3R1 differs in CA1 neurons during postnatal development of the hippocampus (Figures 2B). Confocal microscopy further revealed that InsP3R1 immunolabel tended to form aggregates of label within the primary apical dendrite of individual pyramidal neurons (Figure 4). This clustering of InsP3R1s occurred along the length of the primary apical dendrite of CA1 pyramidal neurons, and was particularly prominent at dendritic bifurcations (Figure 4A–F). Analysis of 3-D reconstructions of confocal images showed that clusters of InsP3R1 label had an average inter-cluster distance of 18.2 ± 7 μm. As will be discussed, the clustered expression of InsP3R1 has important implications for the functional properties of internal Ca2+ release and the ability of Ca2+ waves to propagate CA1 pyramidal neurons.
Figure 4. “Clustering” of InsP3R1 immunofluorescence in CA1 pyramidal cells.
(A) Two-photon image of InsP3R1 immunofluorescence in hippocampus showing clusters of immunolabeling at branch points in the proximal apical dendrites of CA1 pyramidal neurons (large white arrows). (B–E) 3-D reconstruction of InsP3R1 immunofluorescent tissue presented at the listed rotation angles showing that clustering is not part of another dendrite crossing above or underneath the indicated cell. (F) In many cells from both mature and immature tissue, distinct clustering of InsP3R1 was detected in the proximal apical dendrites of CA1 pyramidal neurons (white arrowheads) and branch points (white arrows). (scale bars: A–E, 50 μm; F, 20 μm).
RyR Distribution in the Hippocampus
RyR Isotype 1–3 Immunoreactivity In Mature Rat Hippocampus
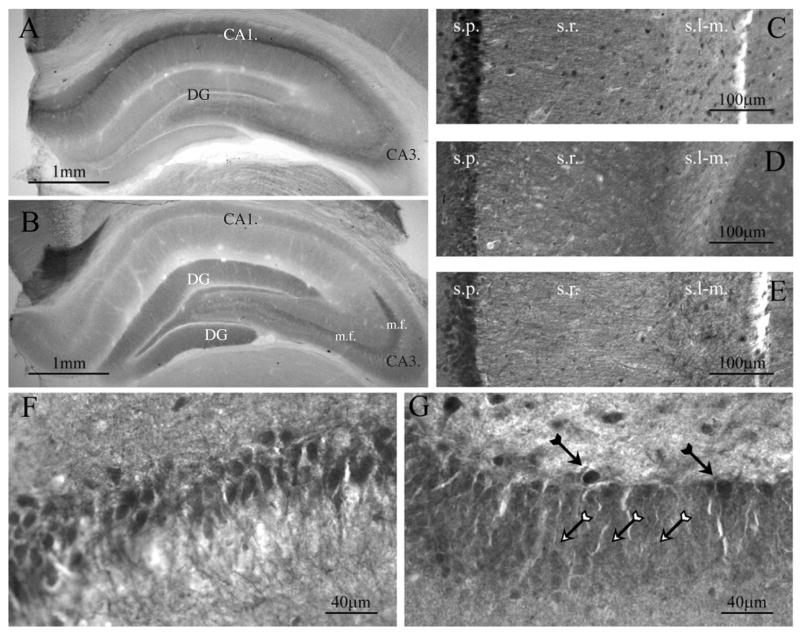
In complementary experiments, we examined the expression of RyR isotypes 1–3 in hippocampal tissue from young and mature rats. We used five different antibodies to investigate the distribution of RyR immunoreactivity in 8–10 week-old rat hippocampus (see Methods). Consistent with in situ hybridization studies of RyR mRNA expression in the hippocampus (Lai et al., 1992; Furuichi and Mikoshiba, 1995; Giannini et al., 1995; Mori et al., 2000; Zhao et al., 2000), we found immunolabeling of RyR isotypes in neurons and glia located in the hippocampus. RyR1 immunoreactivity was strongest in the CA1 region, with immunolabel found in stratum oriens, stratum pyramidale and stratum radiatum (Figure 5A). More specifically, we observed the densest RyR1 labeling in the somata of pyramidal cells within stratum pyramidale, as well as in portions of their apical dendrites in stratum radiatum. RyR1 label was not observed in the most distal dendrites in stratum-lacunosum moleculare (Figures 5A and C). Similar to the distribution of RyR1 in the CA1 subfield, immunolabeling for RyR1 in the CA3 subfield was observed in somata of pyramidal neurons in stratum pyramidale and in the apical dendrites within stratum radiatum. In the dentate gyrus, immunolabeling for RyR1 was present, but relatively weak compared to CA1 and CA3 regions (Figure 5A).
Figure 5. Expression pattern of RyR isotypes 1–3 immunoreactivity in hippocampus of mature rat.
(A) Immunolabeling with monoclonal RyR1. RyR1 immunoreactivity shows intense labeling of the CA1 region and weaker labeling of the CA3 region and the dentate gyrus. (B) Immunolabeling with monoclonal RyR2 antibody. RyR2 immunolabeling reveals intense labeling of the dentate gyrus and the mossy fiber tract. (C–G) Nickel-enhanced immunostaining using polyclonal antibodies. (C) RyR1 labeling in CA1 stratum pyramidale and stratum radiatum, but not in stratum lacunosum-moleculare (see also A). (D) RyR2 immunolabel was similarly observed in CA1 stratum pyramidale and stratum radiatum, but not in stratum lacunosum-moleculare (see also B). (E) RyR3 immunoreactivity in CA3 stratum pyramidale. (F) Higher magnification image showing RyR3 immunoreactivity in CA3 stratum pyramidale. (G) RyR3 immunolabeling of the dentate gyrus. The majority of granule cells show weak RyR3 immunolabeling (white arrows). A small subpopulation of cells, however, exhibited intense immunoreactivity (black arrows). Abbreviations for hippocampal strata: s.p., stratum pyramidale; s.r., stratum radiatum; s.l.m., stratum lacunosum-moleculare; m.f., mossy fiber tract. (scale bars: A–B, 1 mm; C–E, 100 μm; F–G, 40 μm).
The distribution of RyR2 immunoreactivity was consistent with the general distribution of RyR2 mRNA as described by others (Lai et al., 1992). In particular, RyR2 immunolabeling was primarily detected in the cells of the dentate gyrus and in the stratum lucidum of CA3 presumably in mossy fiber axons of the dentate gyrus granule neurons (Figure 5B). Within the hippocampus proper, we found weakly labeled pyramidal cells in stratum pyramidale (Figures 5B and D). In the CA1 region, RyR2 immunoreactivity was detected in stratum radiatum, but not in stratum lacunosum-moleculare (Figures 5B and D).
The polyclonal antibody directed against RyR3 labeled the entire CA1 subfield, including stratum lacunosum-moleculare (Figure 5E). Pyramidal cells within stratum pyramidale of the CA3 subfield also exhibited strong immunoreactivity (Figure 5F). Although stratum granulosum of the dentate gyrus was only weakly labeled for RyR3, intense immunoreactivity was observed in neurons located along the dentate gyrus/hilus border and within the hilus. In addition, labeling for RyR3 revealed diffuse immunoreactivity in the neuropil, which is consistent with previous reports of labeling of astrocytic processes (Matyash et al., 2002; Beck et al., 2004). A summary of the distribution of hippocampal RyR isotypes can be found in Table 2.
Comparative Analysis of RyR1 and InsP3R1 Distribution During Postnatal Maturation
We also examined whether the distribution of RyR1s in CA1 pyramidal neurons changed during postnatal neuronal maturation. Densitometric analysis of RyR1 immunoreactivity in area CA1 from 2 and 10 week-old animals revealed that during maturation the relative density of immunolabel increased in the cell bodies of CA1 pyramidal neurons and slightly decreased in the dendrites in stratum radiatum (Figure 6A and B). This finding contrasts with the change observed in InsP3R1 immunoreactivity during maturation in which the relative density of immunolabel significantly decreased in stratum radiatum of CA1 pyramidal neurons and only slightly increased in the cell body region (Figure 6C).
Figure 6. Changes in RyR1 distribution in CA1 strata during maturation.
(A) RyR1 immunoreactivity of area CA1 at 2 and 8 weeks of age (scale bars, 40 μm). (B) Densitometric measurements of RyR1 immunolabeling in CA1. Gray and black traces represent normalized label intensity in young and mature animals, respectively (see Methods and Figure 3 for description). The gray fill represents the difference between the relative distribution in young animals (black line) and mature animals (gray line). (C) Comparison of the changes in RyR1 distribution and InsP3R1 distribution during maturation.
Co-expression of InsP3R1 with Calbindin D-28k, But Not Parvalbumin
Ca2+ acts as a co-agonist on InsP3R1s (Bezprozvanny et al., 1991; Finch et al., 1991). It has been shown that regulation of cytosolic Ca2+ by Ca2+ binding proteins can shape the spatial and temporal properties of internal Ca2+ release in Xenopus oocytes (Dargan and Parker, 2003; Dargan et al., 2004). Based on the capacity for Ca2+ binding proteins to regulate internal Ca2+ release through the regulation of transient rises in cytosolic Ca2+ concentration, we examined whether two prominent Ca2+ binding proteins in the hippocampus, calbindin D-28k and parvalbumin, were co-localized with InsP3R1 in the hippocampus.
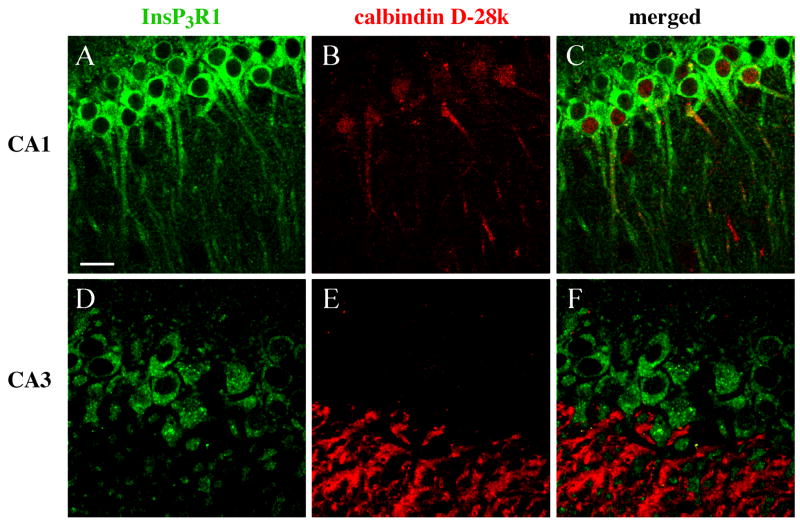
In CA1 pyramidal neurons, calbindin D-28k expression was not observed until after 4 weeks of age, and when present in maturing pyramidal neurons, it was always co-localized with InsP3R1 immunolabel (Figures 7A–C). Parvalbumin expression was never observed in CA1 pyramidal neurons. Calbindin D-28k immunolabeled CA1 pyramidal neurons appeared to be restricted to a subpopulation of CA1 pyramidal neurons with somata in the superior layer of stratum pyramidale adjacent to stratum radiatum. In contrast, somata of pyramidal neurons in the inferior layer adjacent to stratum oriens were not labeled with calbindin D-28k antibody. InsP3R1 immunoreactivity was found in pyramidal neuron somata throughout stratum pyramidale, suggesting that calbindin D-28k is positioned to influence InsP3R-mediated internal Ca2+ release in a specific subpopulation of CA1 pyramidal cells. In contrast to the CA1 region, InsP3R1-positive pyramidal cells in the CA3 region did not show immunoreactivity for calbindin D-28k (Figures 7D–F).
Figure 7. Dual immunofluorescence labeling of InsP3R1 and calbindin D-28k in mature rat hippocampus.
(A–C) CA1 tissue from an 8 week-old rat. (A) InsP3R1 immunoreactivity. (B) Calbindin D-28k immunoreactivity. (C) Overlay of InsP3R1 (green) and calbindin D-28k (red) immunolabeling in the CA1 region. InsP3R1 immunolabeling was found in the somata and dendrites of pyramidal cells of stratum pyramidale and co-localized with calbindin D-28k. (D–F) CA3 tissue from an 8 week-old animal. (D) InsP3R1 immunoreativity. (E) Calbindin D-28k immunoreactivity. (F) Overlay of InsP3R1 (green) and calbindin D-28k (red) immunolabeling in the CA3 region. InsP3R1 immunopositive CA3 pyramidal cells did not show immunoreactivity for calbindin D-28k. Immunofluorescence for calbindin D-28k in area CA3 was primarily limited to stratum lucidum, presumably in mossy fiber axons and axon terminals, as reported by others (Celio, 1990, Freund and Buzsaki, 1996). (scale bar, 20 μm).
Consistent with numerous reports, interneurons throughout the hippocampus of young and mature animals were immunolabeled for calbindin D-28k and parvalbumin (Freund and Buzsaki, 1996). For example, calbindin D-28k labeled interneurons lying outside of stratum pyramidale did not exhibit InsP3R1 labeling. Similarly, parvalbumin-labeled interneurons, presumed to be basket cells within stratum pyramidale (Sloviter, 1989, Ribak et al., 1990, Baimbridge et al., 1992), showed no evidence for co-localization of parvalbumin and InsP3R1 (Figure 8A–C). The lack of InsP3Rs in CA1 interneurons is consistent with at least one observation that CA1 interneurons do not exhibit internal Ca2+ release (Nakamura et al., 2002).
Figure 8. Dual immunofluorescence labeling of InsP3R1 and parvalbumin in mature rat hippocampus.
(A–C) CA1 tissue from an 8 week-old rat. (A) InsP3R1 immunoreactivity. (B) parvalbumin immunoreactivity. (C) Overlay of InsP3R1 (green) and parvalbumin (red) immunolabeling. Parvalbumin-positive basket cells did not show immunoreactivity for InsP3R1 in the CA1 region. (D–F) CA3 tissue for an 8 week-old rata. (D) InsP3R1 immunoreactivity. (E) Parvalbumin immunoreactivity. (F) Overlay of InsP3R1 and parvalbumin immunolabeling in the CA3 region. As in area CA1, InsP3R1 and parvalbumin immunoreactivity in area CA3 were found in distinct types of neurons. Specifically, InsP3R1 was localized to the somata and dendrites of pyramidal neurons from CA3 stratum pyramidale, while parvalbumin immunofluorescence was found in basket cells. (scale bar, 20 μm).
DISCUSSION
In this study we examined the distribution of the intracellular Ca2+ channel proteins InsP3R1–3 and RyR1–3 in the hippocampus of maturing rat. The greatest changes occurred with type 1 InsP3Rs. Because synaptically elicited internal Ca2+ release in hippocampal pyramidal neurons has been reported to depend exclusively on InsP3Rs (Pozzo-Miller et al., 1996; Nakamura et al., 1999, Kapur et al., 2001), we focused our analysis on this protein. Our principle novel findings are the following: (1) during maturation there was a shift in the distribution of InsP3R1 within CA1 pyramidal neurons from being relatively dense in proximal apical dendrites to being more evenly distributed along the length of their dendrites in mature cells, (2) InsP3R1s formed clusters within the proximal apical dendrites and at dendritic branch points of CA1 pyramidal neurons, (3) InsP3R1 was co-localized with calbindin D-28k in CA1 pyramidal neurons following the onset of the calbindin D-28k during postnatal week 4, (4) mature CA1 pyramidal neurons expressing both InsP3R1 and calbindin D-28k were limited to the superficial layer of stratum pyramidale, and (5) InsP3R1 was not co-localized with calbindin D-28k labeled interneurons outside of stratum pyramidale or with parvalbumin immunolabeled interneurons. In addition, we found that the relative density of RyR1 increased in CA1 stratum pyramidale during maturation. These findings expand on previous studies (Sharp et al., 1993; Dent et al., 1996; Sharp et al., 1999), and provide important details that are likely to help explain and guide functional studies investigating internal Ca2+ release in hippocampal neurons and their consequences on cellular function.
Distribution of InsP3R Isotypes 1–3 In Mature Rat Hippocampus
Consistent with previous descriptions of InsP3R1 immunoreactivity in adult rat hippocampus (Nakanishi et al., 1991; Sharp et al., 1993; Dent et al., 1996; Sharp et al., 1999), we found high levels of InsP3R1 in the CA1 subfield compared to the CA3 subfield and the dentate gyrus. InsP3R1 immunolabel was particularly prominent in stratum pyramidale of CA1. As InsP3R1 label was only found in neurons with pyramid shaped somata in stratum pyramidale, it appears that the distribution of InsP3R1s in the hippocampus is largely, if not exclusively, localized to pyramidal cells of CA1 and CA3, and granule cells of the dentate gyrus. Additional support for the conclusion that InsP3R1s are in principal cells and not present in at least some interneurons comes from our data showing that there was no overlap between cells immunolabeled with InsP3R1 and parvalbumin, a Ca2+ binding protein found in basket cells of the hippocampus (Sloviter, 1989; Ribak et al., 1990; Baimbridge et al., 1992), or with calbindin D-28k immunolabeled neurons lying outside of stratum pyramidale.
InsP3R2 immunoreactivity was observed in a subpopulation of astrocytes and in endothelial cells (Bourguignon et al., 1994, Mountian et al., 1999, Grayson et al., 2004). Interestingly, there is evidence for propagation of Ca2+ waves from endothelial cells to astrocytes, but there is no evidence that internal Ca2+ release in astrocytes propagate to endothelial cells (Leybaert et al., 1998; Braet et al., 2001; Simard et al., 2003). It is not known why there is unidirectional propagation of Ca2+ waves in this network, but based on our data showing similar InsP3R2 expression profiles between astrocytes and endothelial cells it is not likely to be due to InsP3R2, but rather some other factor in the intracellular Ca2+ signalling toolkit (Leybaert et al., 1998).
The distribution of InsP3R3 immunoreactivity is not as easy to interpret in the hippocampus compared to the distribution of the other InsP3R isotypes. Punctate InsP3R3 immunolabeling in axon terminals and more diffuse labeling of glia has been previously described (Sharp et al., 1999). We detected similar levels of diffuse labeling in the neuropil using a different antibody. Virtually nothing is known about the synaptic targets of InsP3R3-immunolabeled boutons in the hippocampus. We identified two potential targets for InsP3R3-immunostained boutons en passant: small neuronal cells within stratum pyramidale and microvessels within the hippocampus. The associations between neurons and vessels are thought to be non-synaptic (Cervos-Navarro and Matakas, 1974; Rennels and Nelson, 1975; McDonald and Rasmussen, 1977). InsP3R isotype 3 immunolabeled terminals have been previously described as forming axosomatic and axodendritic synapses with flared, symmetric densities (Sharp et al., 1999), albeit outside the hippocampus. The origin and the synaptology of InsP3R3-immunopositive axon terminals in the hippocampus will need to be addressed in future studies.
Distribution of RyR Isotypes 1–3 In Mature Rat Hippocampus
Although the distribution of mRNA for RyR isotypes 1–3 in the hippocampus has been well described (Lai et al., 1992; Furuichi and Mikoshiba, 1995; Giannini et al., 1995; Mori et al., 2000; Zhao et al., 2000), little is known about the distribution of RyR proteins. The studies examining RyR protein have primarily focused on RyR2, possibly because it is considered to be the most abundant RyR isotype in the brain (Lai et al., 1992,; Sharp et al., 1993). Our analysis showed that RyR2 immunolabeling in axons is far greater than that observed in dendrites. More specifically, RyR2 was most densely distributed in the hippocampal mossy fiber pathway and in axon bundles traversing the cortical laminae. In hippocampus, we also observed a paucity of both RyR2 and RyR1 immunolabeling in stratum lacunosum-moleculare. This finding is consistent with the absence of RyR2 immunolabeling of fine dendritic processes on mouse hippocampal cells (Seymour-Laurent and Barish, 1995). Notably, RyR3 immunolabeling appears to fill the “gap” left in stratum lacunosum-moleculare by RyR1 and RyR2, as can be seen in their respective distribution patterns (RyRs1-2, Figure 6C and D; RyR3, Figure 6E). From a functional perspective, RyR3 has been reported to be important for memory consolidation (Balschun et al., 1999; Futatsugi et al., 1999), and its presence in stratum lacunosum-moleculare is consistent with reports that these afferents from the entorhinal cortex to the CA3 and CA1 subfields are particularly important for some forms of learning and memory (Yeckel and Berger, 1990; Remondes and Schuman, 2004).
InsP3Rs and RyRs During Maturation
The most dramatic change that we observed in hippocampal neurons as they matured from 2 to 10 weeks old was in a redistribution of InsP3R1s in pyramidal neurons. More specifically, our study revealed a distinct and gradual redistribution of InsP3R1s from a relatively high density in the primary proximal apical dendrites of CA1 pyramidal neurons in young cells to an even distribution along the dendrite in stratum radiatum and then an increase in InsP3R1 label in the distal dendritic region in stratum lacunosum-molecular as cells matured. Although we did not perform semi-quantitative analysis on the distribution of InsP3R1s in the CA3 subfield, we observed a qualitatively similar change in the pattern of IP3R1s during maturation as was observed in the CA1 subfield. In the dentate gyrus, there appeared to be a decrease in the intensity of label in the granule cell layer as the cells matured, and little InsP3R1 immunolabel in the granule cell dendrites at any age that we examined. To date, InsP3R-mediated internal Ca2+ release has yet to be described in granule cells of the dentate gyrus.
In contrast to changes to InsP3R1 that occurred during maturation, there was little change to RyR1 immunoreactivity in the proximal apical dendrites of CA1 pyramidal neurons, and an increase in the intensity of RyR1 immunolabel in stratum pyramidale during maturation. These findings supplement an earlier investigation describing RyR1 mRNA development in the hippocampus, where almost no change was observed between two weeks of age and maturity (Lai et al., 1992; Mori et al., 2000).
InsP3R1 Clusters
Similar to the clustering of InsP3R1s that we observed, clusters have been observed in cultured hippocampal neurons (Seymour-Laurent and Barish, 1995), cerebellar granule cells (Oberdorf et al., 1997), and astrocytes (Simpson et al., 1997). Consistent with clustering of InsP3R1s, functional studies report “hotspots” of internal Ca2+ release and “Ca2+ sparks” in non-neuronal cell types (Cheng et al., 1993; Callamaras et al., 1998; Mak et al., 2001; Dargan and Parker, 2003; Dargan et al., 2004). Computational studies provide additional support for clusters of InsP3Rs playing an important role in salutatory propagation of Ca2+ waves within cells (Dawson et al., 1999; Shuai and Jung, 2003; Strier et al., 2003). Finally, clusters of InsP3R1s at dendritic bifurcations are consistent with the observation that internal Ca2+ release in hippocampal pyramidal cells tends to initiate at branch points (Nakamura et al., 2002). One novel perspective is that the areas adjacent to InsP3R clusters that lack InsP3Rs (i.e., cold spots) might provide functional control over the ability of Ca2+ waves to propagate along CA1 pyramidal cell dendrites by requiring waves to be of sufficient magnitude to diffuse across the empty region or by having Ca2+ binding proteins shuttle the Ca2+ to the next cluster (Dargan and Parker, 2003; Dargan et al., 2004).
Co-Localization of InsP3R1 With Ca2+ Binding Proteins
The capability of Ca2+ binding proteins to regulate internal Ca2+ release and Ca2+ waves has been well-described in an elegant series of experiments showing that the spatial and temporal properties of in InsP3-mediated internal Ca2+ release in Xenopus oocytes was differentially effected by loading the cells with Ca2+ binding proteins with different binding affinities and kinetics (Dargan and Parker, 2003; Dargan et al., 2004). To date, regulation of internal Ca2+ release and Ca2+ waves by Ca2+ binding proteins in neurons has not been reported, although indirect evidence for this possibility has been observed in CA1 pyramidal neurons loaded with different concentrations of a Ca2+ indicator dye that acts to chelate Ca2+ (Nakamura et al., 1999). In this study we show that InsP3R1 and calbindin D-28k are co-expressed in CA1 pyramidal neurons of mature neurons. In addition, CA1 pyramidal neurons with both proteins were located in the superficial portion of stratum pyramidale. Interneurons lying outside stratum pyramidale that expressed calbindin D-28K did not exhibit co-expression with InsP3R1. Immunolabeling for parvalbumin, the other primary Ca2+ buffer in the hippocampus, is known to be restricted to basket cells (Sloviter, 1989, Ribak et al., 1990, Baimbridge et al., 1992). We found no co-expression of InsP3R1 and parvalbumin within stratum pyramidale of either the CA1 or the CA3 region (Figure 8). These data suggest that internal Ca2+ release and Ca2+ waves in immature CA1 pyramidal neurons without calbindin D-28k is likely to be different from internal Ca2+ release in at least those mature CA1 pyramidal neurons that express calbindin D-28k. It also suggests that InsP3R-mediated internal Ca2+ is not a prominent feature of hippocampal parvalbumin-containing interneurons.
Functional Significance
Despite our considerable knowledge of internal Ca2+ release and its basic components in non-excitable, non-neural cells, relatively little is known about internal Ca2+ release and Ca2+ waves in the brain. This is particularly surprising given the importance of Ca2+ as a signaling molecule in general, but also because of its critical role in both developmental and synaptic plasticity. In neurons, with their elaborate dendritic architecture, the capacity for InsP3R-mediated internal Ca2+ release to travel as a wave raises the intriguing possibility that Ca2+ waves carry information from one region of the neuron to another. As a prerequisite to understanding this process, it is important to first identify the first order signaling components of internal Ca2+ release and their spatial organization. For example, the data presented here showing a higher density of InsP3R1s in proximal apical dendrites of young CA1 pyramidal neurons, and their lack of Ca2+ binding proteins, suggest the possibility that internal Ca2+ release might be permissive in young animals compared to mature animals, and that internal waves might propagate more readily into the perisomatic region and nucleus. This scenario has profound implications for synapse-to-nuclear signaling in young CA1 pyramidal neurons, and perhaps their ongoing dendritic development and arborization. Finally, the data presented here highlight the importance of matching characterization of the anatomical and structural constituents that comprise the Ca2+ signaling toolbox with functional studies and the interpretation of those studies.
Acknowledgments
We gratefully acknowledge Constantinos Paspalas for his generous help with this project and for his insightful comments on this manuscript. We thank Rick Matthews for use of his confocal microscope. We also thank John Fitzpatrick for writing our computer analysis software, Anna Hagenston for a critical reading of the manuscript, Amanda Sleeper for her initial help with the experiments, and Keith Gipson for many thoughtful discussions. This research was supported by Whitehall Foundation, Kavli Foundation, Hellman Family Fund, NIH/NIMH (RO1-MH067830 and P50-MH068789).
Abbreviations
- Ca2+
calcium
- DAB
diaminobenzidine
- InsP3
inositol-1,4,5-trisphosphate
- InsP3R
inositol-1,4,5-trisphosphate receptor
- NGS
normal goat serum
- RyR
ryanodine receptor
- TBS
Tris-buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Baimbridge KG, Celio MR, Rogers JH. Calcium-binding proteins in the nervous-system. Trends In Neurosciences. 1992;15:303–308. doi: 10.1016/0166-2236(92)90081-i. [DOI] [PubMed] [Google Scholar]
- Balschun D, Wolfer DP, Bertocchini F, Barone V, Conti A, Zuschratter W, Missiaen L, Lipp HP, Frey JU, Sorrentino V. Deletion of the ryanodine receptor type 3 (RyR3) impairs forms of synaptic plasticity and spatial learning. Embo J. 1999;18:5264–5273. doi: 10.1093/emboj/18.19.5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Nieden RZ, Schneider HP, Deitmer JW. Calcium release from intracellular stores in rodent astrocytes and neurons in situ. Cell Calcium. 2004;35:47–58. doi: 10.1016/s0143-4160(03)00171-4. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Bourguignon LY, Iida N, Sobrin L, Bourguignon GJ. Identification of an IP3 receptor in endothelial cells. J Cell Physiol. 1994;159:29–34. doi: 10.1002/jcp.1041590105. [DOI] [PubMed] [Google Scholar]
- Braet K, Paemeleire K, D’Herde K, Sanderson MJ, Leybaert L. Astrocyte-endothelial cell calcium signals conveyed by two signalling pathways. Eur J Neurosci. 2001;13:79–91. [PubMed] [Google Scholar]
- Callamaras N, Marchant JS, Sun XP, Parker I. Activation and co-ordination of InsP3-mediated elementary Ca2+ events during global Ca2+ signals in Xenopus oocytes. J Physiol. 1998;509(Pt 1):81–91. doi: 10.1111/j.1469-7793.1998.081bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celio MR. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience. 1990;35:375–475. doi: 10.1016/0306-4522(90)90091-h. [DOI] [PubMed] [Google Scholar]
- Cervos-Navarro J, Matakas F. Electron microscopic evidence for innervation of intracerebral arterioles in the cat. Neurology. 1974;24:282–286. doi: 10.1212/wnl.24.3.282. [DOI] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Dargan SL, Parker I. Buffer kinetics shape the spatiotemporal patterns of IP3-evoked Ca2+ signals. J Physiol. 2003;553:775–788. doi: 10.1113/jphysiol.2003.054247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargan SL, Schwaller B, Parker I. Spatiotemporal patterning of IP3-mediated Ca2+ signals in Xenopus oocytes by Ca2+-binding proteins. J Physiol. 2004;556:447–461. doi: 10.1113/jphysiol.2003.059204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson SP, Keizer J, Pearson JE. Fire-diffuse-fire model of dynamics of intracellular calcium waves. PNAS. 1999;96:6060–6063. doi: 10.1073/pnas.96.11.6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent MA, Raisman G, Lai FA. Expression of type 1 inositol 1,4,5-trisphosphate receptor during axogenesis and synaptic contact in the central and peripheral nervous system of developing rat. Development. 1996;122:1029–1039. doi: 10.1242/dev.122.3.1029. [DOI] [PubMed] [Google Scholar]
- Finch EA, Turner TJ, Goldin SM. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991;252:443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Fotuhi M, Sharp AH, Glatt CE, Hwang PM, von KM, Snyder SH, Dawson TM. Differential localization of phosphoinositide-linked metabotropic glutamate receptor (mGluR1) and the inositol 1,4,5-trisphosphate receptor in rat brain. Journal Of Neuroscience. 1993;13:2001–2012. doi: 10.1523/JNEUROSCI.13-05-02001.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Furuichi T, Mikoshiba K. Inositol 1, 4, 5-trisphosphate receptor-mediated Ca2+ signaling in the brain. J Neurochem. 1995;64:953–960. doi: 10.1046/j.1471-4159.1995.64030953.x. [DOI] [PubMed] [Google Scholar]
- Futatsugi A, Kato K, Ogura H, Li ST, Nagata E, Kuwajima G, Tanaka K, Itohara S, Mikoshiba K. Facilitation of NMDAR-independent LTP and spatial learning in mutant mice lacking ryanodine receptor type 3. Neuron. 1999;24:701–713. doi: 10.1016/s0896-6273(00)81123-x. [DOI] [PubMed] [Google Scholar]
- Giannini G, Conti A, Mammarella S, Scrobogna M, Sorrentino V. The ryanodine receptor/calcium channel genes are widely and differentially expressed in murine brain and peripheral tissues. J Cell Biol. 1995;128:893–904. doi: 10.1083/jcb.128.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson TH, Haddock RE, Murray TP, Wojcikiewicz RJ, Hill CE. Inositol 1,4,5-trisphosphate receptor subtypes are differentially distributed between smooth muscle and endothelial layers of rat arteries. Cell Calcium. 2004;36:447–458. doi: 10.1016/j.ceca.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Kapur A, Yeckel M, Johnston D. Hippocampal mossy fiber activity evokes Ca2+ release in CA3 pyramidal neurons via a metabotropic glutamate receptor pathway. Neuroscience. 2001;107:59–69. doi: 10.1016/s0306-4522(01)00293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai FA, Dent M, Wickenden C, Xu L, Kumari G, Misra M, Lee HB, Sar M, Meissner G. Expression of a cardiac Ca2+-release channel isoform in mammalian brain. Biochem J. 1992;288(Pt 2):553–564. doi: 10.1042/bj2880553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite MF, Burgstahler AD, Nathanson MH. Ca2+ waves require sequential activation of inositol trisphosphate receptors and ryanodine receptors in pancreatic acini. Gastroenterology. 2002;122:415–427. doi: 10.1053/gast.2002.30982. [DOI] [PubMed] [Google Scholar]
- Leybaert L, Paemeleire K, Strahonja A, Sanderson MJ. Inositol-trisphosphate-dependent intercellular calcium signaling in and between astrocytes and endothelial cells. Glia. 1998;24:398–407. [PubMed] [Google Scholar]
- Mak DO, McBride S, Foskett JK. ATP-dependent adenophostin activation of inositol 1,4,5-trisphosphate receptor channel gating: kinetic implications for the durations of calcium puffs in cells. J Gen Physiol. 2001;117:299–314. doi: 10.1085/jgp.117.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyash M, Matyash V, Nolte C, Sorrentino V, Kettenmann H. Requirement of functional ryanodine receptor type 3 for astrocyte migration. Faseb J. 2002;16:84–86. doi: 10.1096/fj.01-0380fje. [DOI] [PubMed] [Google Scholar]
- McDonald DM, Rasmussen GL. An ultrastructural analysis of neurites in the basal lamina of capillaries in the chinchilla cochlear nucleus. J Comp Neurol. 1977;173:475–495. doi: 10.1002/cne.901730306. [DOI] [PubMed] [Google Scholar]
- McPherson PS, Campbell KP. Solubilization and biochemical characterization of the high affinity [3H]ryanodine receptor from rabbit brain membranes. J Biol Chem. 1990;265:18454–18460. [PubMed] [Google Scholar]
- Mori F, Fukaya M, Abe H, Wakabayashi K, Watanabe M. Developmental changes in expression of the three ryanodine receptor mRNAs in the mouse brain. Neurosci Lett. 2000;285:57–60. doi: 10.1016/s0304-3940(00)01046-6. [DOI] [PubMed] [Google Scholar]
- Mountian I, Manolopoulos VG, De Smedt H, Parys JB, Missiaen L, Wuytack F. Expression patterns of sarco/endoplasmic reticulum Ca2+-ATPase and inositol 1,4,5-trisphosphate receptor isoforms in vascular endothelial cells. Cell Calcium. 1999;25:371–380. doi: 10.1054/ceca.1999.0034. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Barbara JG, Nakamura K, Ross WN. Synergistic release of Ca2+ from IP3-sensitive stores evoked by synaptic activation of mGluRs paired with backpropagating action potentials. Neuron. 1999;24:727–737. doi: 10.1016/s0896-6273(00)81125-3. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Lasser-Ross N, Nakamura K, Ross WN. Spatial segregation and interaction of calcium signalling mechanisms in rat hippocampal CA1 pyramidal neurons. J Physiol. 2002;543:465–480. doi: 10.1113/jphysiol.2002.020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S, Maeda N, Mikoshiba K. Immunohistochemical localization of an inositol 1,4,5-trisphosphate receptor, P400, in neural tissue: studies in developing and adult mouse brain. J Neurosci. 1991;11:2075–2086. doi: 10.1523/JNEUROSCI.11-07-02075.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdorf J, Vallano ML, Wojcikiewicz RJ. Expression and regulation of types I and II inositol 1,4,5-trisphosphate receptors in rat cerebellar granule cell preparations. J Neurochem. 1997;69:1897–1903. doi: 10.1046/j.1471-4159.1997.69051897.x. [DOI] [PubMed] [Google Scholar]
- Pokorny J, Yamamoto T. Postnatal ontogenesis of hippocampal CA1 area in rats. I. Development of dendritic arborisation in pyramidal neurons. Brain Research Bulletin. 1981:113–120. doi: 10.1016/0361-9230(81)90075-7. [DOI] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Petrozzino JJ, Golarai G, Connor JA. Ca2+ release from intracellular stores induced by afferent stimulation of CA3 pyramidal neurons in hippocampal slices. J Neurophysiol. 1996;76:554–562. doi: 10.1152/jn.1996.76.1.554. [DOI] [PubMed] [Google Scholar]
- Remondes M, Schuman EM. Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory. Nature. 2004;431:699–703. doi: 10.1038/nature02965. [DOI] [PubMed] [Google Scholar]
- Rennels ML, Nelson E. Innervation of capillaries in the cat brain: an electron microscopic study. Trans Am Neurol Assoc. 1975;100:232–233. [PubMed] [Google Scholar]
- Ribak CE, Nitsch R, Seress L. Proportion of parvalbumiin-positive basket cells in the GABAergic innervation of pyramidal and granule cells of the rat hippocampal formation. J Comp Neurol. 1990;300:449–461. doi: 10.1002/cne.903000402. [DOI] [PubMed] [Google Scholar]
- Seymour-Laurent KJ, Barish ME. Inositol 1,4,5-trisphosphate and ryanodine receptor distributions and patterns of acetylcholine- and caffeine-induced calcium release in cultured mouse hippocampal neurons. J Neurosci. 1995;15:2592–2608. doi: 10.1523/JNEUROSCI.15-04-02592.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp AH, McPherson PS, Dawson TM, Aoki C, Campbell KP, Snyder SH. Differential immunohistochemical localization of inositol 1,4,5- trisphosphate- and ryanodine-sensitive Ca2+ release channels in rat brain. J Neurosci. 1993;13:3051–3063. doi: 10.1523/JNEUROSCI.13-07-03051.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp AH, Nucifora FC, Jr, Blondel O, Sheppard CA, Zhang C, Snyder SH, Russell JT, Ryugo DK, Ross CA. Differential cellular expression of isoforms of inositol 1,4,5-triphosphate receptors in neurons and glia in brain. J Comp Neurol. 1999;406:207–220. [PubMed] [Google Scholar]
- Shuai JW, Jung P. Optimal ion channel clustering for intracellular calcium signaling. Proc Natl Acad Sci U S A. 2003;100:506–510. doi: 10.1073/pnas.0236032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23:9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson PB, Mehotra S, Lange GD, Russell JT. High density distribution of endoplasmic reticulum proteins and mitochondria at specialized Ca2+ release sites in oligodendrocyte processes. J Biol Chem. 1997;272:22654–22661. doi: 10.1074/jbc.272.36.22654. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Calcium-binding protein (calbindin-D28k) and parvalbumin immunocytochemistry: localization in the rat hippocampus with specific reference to the selective vulnerability of hippocampal neurons to seizure activity. J Comp Neurol. 1989;280:183–196. doi: 10.1002/cne.902800203. [DOI] [PubMed] [Google Scholar]
- Strier DE, Ventura AC, Dawson SP. Saltatory and Continuous Calcium Waves and the Rapid Buffering Approximation. Biophys J. 2003;85:3575–3586. doi: 10.1016/S0006-3495(03)74776-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeckel MF, Berger TW. Feedforward excitation of the hippocampus by afferents from the entorhinal cortex: Redefinition of the role of the trisynaptic pathway. Proc Natl Acad Sci. 1990;87:5832–5836. doi: 10.1073/pnas.87.15.5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeckel MF, Kapur A, Johnston D. Multiple forms of LTP in hippocampal CA3 neurons use a common postsynaptic mechanism. Nat Neurosci. 1999;2:625–633. doi: 10.1038/10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Meiri N, Xu H, Cavallaro S, Quattrone A, Zhang L, Alkon DL. Spatial learning induced changes in expression of the ryanodine type II receptor in the rat hippocampus. Faseb J. 2000;14:290–300. doi: 10.1096/fasebj.14.2.290. [DOI] [PubMed] [Google Scholar]