Abstract
The importance of the face in social interaction and social intelligence is widely recognized in anthropology. Yet the adaptive functions of human facial expression remain largely unknown. An evolutionary model of human facial expression as behavioral adaptation can be constructed, given the current knowledge of the phenotypic variation, ecological contexts, and fitness consequences of facial behavior. Studies of facial expression are available, but results are not typically framed in an evolutionary perspective. This review identifies the relevant physical phenomena of facial expression and integrates the study of this behavior with the anthropological study of communication and sociality in general. Anthropological issues with relevance to the evolutionary study of facial expression include: facial expressions as coordinated, stereotyped behavioral phenotypes, the unique contexts and functions of different facial expressions, the relationship of facial expression to speech, the value of facial expressions as signals, and the relationship of facial expression to social intelligence in humans and in nonhuman primates. Human smiling is used as an example of adaptation, and testable hypotheses concerning the human smile, as well as other expressions, are proposed.
Keywords: nonverbal communication, social intelligence, signaling systems
INTRODUCTION
Facial expressions as adaptations
One of the central questions in human evolution is the origin of human sociality and ultimately, human culture. In the search for the origin of social intelligence in humans, much attention is focused on the evolution of the brain and consciousness. Many aspects of human cognition and behavior are best explained with reference to millions of years of evolution in a social context (Byrne, 1995; Cosmides et al., 1992; Humphrey, 1976). Human brainpower can thus be explained, in part, by increasing social demands over the course of human prehistory (Dunbar, 1998). Social intelligence, however, is not reflected only in the brain, but in every adaptation that allows successful interaction in social groups. New advances in studying the biology of social behavior have not fully explored that most visibly social part of the human body, the face. The face is a visible signal of others’ social intentions and motivations, and facial expression continues to be a critical variable in social interaction.
Although social intelligence is an increasingly rich source of hypotheses of cognitive and behavioral adaptations, the anthropological study of facial expression remains focused on essentially nonadaptive questions. Current anthropological views of facial expression tend to focus on the contrasts between universal and culture-specific explanations of facial expressions. Facial expression is either interpreted as a human universal, with basic expressions represented in all known human populations (Brown, 1990), or it is conceptualized as the natural outgrowth of cultural differences, with little overlap in expression from population to population (Birdwhistell, 1975). Physical anthropologists, with important exceptions (Blurton Jones, 1972; Fessler, 1999; and see Chevalier-Skolnikoff, 1973; Goodall, 1986; Hauser, 1996; Preuschoft and van Hooff, 1995 for comparison with nonhuman primates), have generally avoided the study of human facial expressions and nonverbal communication, leaving the interpretation of facial expression largely to psychology and to other branches of anthropology (Birdwhistell, 1970; LaBarre, 1947). The current state of research in facial expression, combined with the current interest in social intelligence as a driving force in human evolution, calls for the re-emergence of the study of facial adaptation in physical anthropology.
Establishing human facial expressions as biological adaptations requires a rigorous review of our current knowledge and ultimately the formation and testing of evolutionarily based hypotheses. The definition by Reeve and Sherman (1993) of adaptation is a guideline for developing evolutionary hypotheses, and allows the exploration of behavioral adaptations that have remained relatively unknown in physical anthropology. They define an adaptation as “a phenotypic variant that results in the highest fitness among a specified set of variants in a given environment.” This definition is particularly suited to adaptive hypotheses of human behavior, because its requirements can be met with observation of current phenomena, and reference to phylogenetic factors is not required (Reeve and Sherman, 1993). What is required, however, is evidence of phenotypic variation, well-defined ecological contexts, and fitness consequences for a particular adaptation.
The purpose of this review is to provide a framework for asking evolutionary, adaptive questions about human facial expression. First, we establish human facial expression as a potential behavioral adaptation, by detailing the phenotypic variation, ecological contexts, and fitness consequences of facial behavior. A particular expression, the human smile, is used as an example of the potential of the adaptationist approach for understanding human facial expression in an evolutionary perspective. Finally, facial behavior is compared to that of nonhuman primates to provide some further phylogenetic perspective on the evolution of facial expression and its role in the evolution of human social intelligence.
BEHAVIORAL PHENOTYPE SETS
Human universal facial expressions1 of emotion are perhaps the most familiar examples of facial expression, at least among anthropologists. Six basic expression categories have been shown to be recognizable across cultures (see Fig. 1), and this finding is generally accepted by psychologists working on facial expression. The six basic emotional expressions,2 or facial configurations associated with particular emotional situations, have been shown to be universal in their performance and in their perception (Ekman and Keltner, 1997), although there is some objection to the idea that these expressions signal similar emotions in people of different cultures (Fridlund, 1994; Russell and Fernandez-Dols, 1997). The controversy surrounding the attribution of universal emotions to universal facial expressions of emotion is important for understanding emotions cross-culturally. Nevertheless, even those who disagree on the emotion concede the cross-cultural consistency of the combinations of facial movements (behavioral phenotypes) that make up expressions of “disgust,” “fear,” “joy,” “surprise,” “sadness,” and “anger” (Russell and Fernandez-Dols, 1997). These expressions were first discussed by Darwin (1872/1998) as universals, and have been recognized in people from widely divergent cultural and social backgrounds, as well as in the faces of individuals born deaf and blind (Darwin, 1872/1998; Eibl-Eibesfeldt, 1989; Izard, 1977; Ekman and Keltner, 1997).
Fig. 1.

Basic facial expression phenotypes. 1, disgust; 2, fear; 3, joy; 4, surprise; 5, sadness; 6, anger. Posed images from Kanade et al. (2000).
In addition to the six basic facial expressions, there are also coordinated, stereotyped nonverbal displays that include stereotyped facial expression components. These include the eyebrow flash, yawning, startle, the coy display, and embarrassment and shame displays (Eibl-Eibesfeldt, 1989; Grammer et al., 1988; Keltner and Buswell, 1997; Keltner and Harker, 1998; Provine, 1997). These displays typically combine both facial and postural or gestural elements and are found in widely distributed populations, suggesting species rather than cultural specificity.
The eyebrow flash is a good example of this kind of display. The frontalis muscle is consistently used to raise both medial and lateral parts of the eyebrow (Ekman and Friesen, 1978; Grammer et al., 1988). A common repertoire of synchronous facial movements occurs in combination with the eyebrow flash, including most frequently the raising of the lip corners (smile), and lifting of the upper lid (Grammer et al., 1988). In addition to consistency in muscle action, the timing of the eyebrow flash is also consistent cross-culturally in the three non-Western populations analyzed. The onset of the eyebrow flash typically follows a pause in all other facial movements, and takes about 100 msec, with very little variation across cultural groups. In addition, coordinated head movements are found in association with eyebrow flashes, extending the display beyond the face itself (Grammer et al., 1988; and see Fig. 2).
Fig. 2.

Eyebrow flash of greeting (Eibl-Eibesfeldt, 1989).
In order to produce the eyebrow flash and other recognizable, universal expressions, humans presumably use the same facial musculature, and move it into a similar configuration under similar circumstances. Thus each of these coordinated facial displays can be considered a behavioral phenotype. Within these facial expression phenotypes, however, and across individual humans, there is a great deal of physical variation in structure, movement, and perception. Universal displays, together with variation around the basic components of these displays, comprise what can be considered phenotype sets of facial expression. Sources of this variation include anatomical and neurobiological differences, as well as demographic differences such as sex, age, and cultural background. In addition, the perception of facial expression, important for understanding communicative adaptations, is also a source of individual variation.
Anatomical variation and facial expression
The structure of human facial muscles has been known for some time (Duchenne, 1859/1990; Huber, 1931; and see Fig. 3). The anatomical basis of facial expression has been described in detail, and an anatomically based coding system is available for the objective study of facial action (Facial Action Coding System; Ekman and Friesen, 1978). This system outlines specific actions produced by particular facial muscles. The quality of these actions, however, likely varies with differences in the facial muscles. Different facial muscles produce different types of movements, and they are most likely heterogeneous in their structure and innervation. Goodmurphy and Ovalle (1999), for example, have shown that muscle fiber types, shapes, and sizes in orbicularis oculi, pars palpebralis, and corrugator supercilii are significantly different, although these two muscles share the same innervation and embryonic origin and are found in the same region of the face (lower eyelid and lower mid forehead, respectively). The orbicularis oculi consists of 89% fast-twitch fibers, significantly more than the corrugator, implying some difference in the movements produced by the two muscles (Goodmurphy and Ovalle, 1999). Zygomaticus major and zygomaticus minor muscles are similar to the orbicularis oculi in their high proportions of fast-twitch fibers, relative to other muscles, indicating a possible specialization for fast movements (Stal et al., 1987).
Fig. 3.
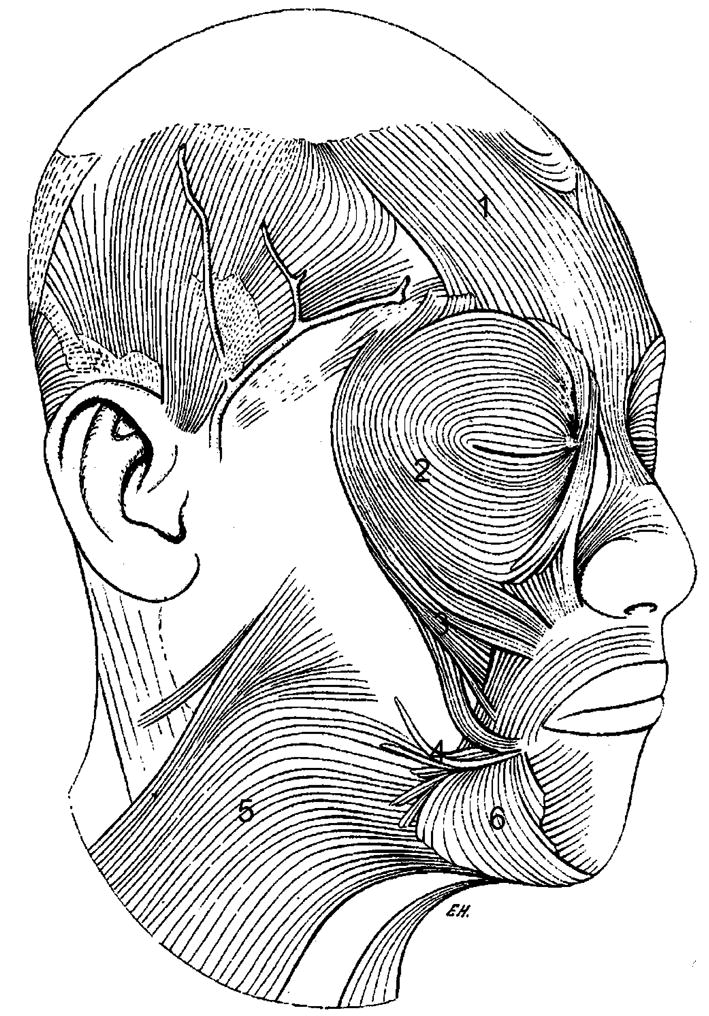
Muscles of facial expression. 1, frontalis; 2, orbicularis oculi; 3, zygomaticus major; 4, risorius; 5, platysma; 6, depressor anguli oris. Original drawing from Huber (1931). (Reprinted by permission of The Johns Hopkins University Press.)
There are also individual differences in the structure and differentiation of facial muscles. For example, a differentiated muscle bundle, the risorius, thought to be unique to humans, is highly variable. As many as 22 of 50 specimens in a recent study lacked this muscle (Pessa et al., 1998a), and Huber believed that it was absent completely in people of Melanesian ancestry, although this finding has not been replicated (Huber, 1931). Various furrows and other deformations of the facial skin are produced by variations in facial muscles, and these may contribute to individual differences in expression. In most individuals, the platysma muscle inserts on the skin over the inferior margin of the mandible, but it is occasionally observed inserting in the lateral cheek, causing a vertical depression or furrow to appear there. The zygomaticus major muscle also varies, appearing in a bifid version with two separate insertion points in 17 of 50 of specimens in an anatomical study (Pessa et al., 1998a). The tension caused by the two heads of the muscle at the corner of the mouth is believed to cause a dimple or small depression during the contraction of the muscle in smiling (Pessa et al., 1998b). Changes in facial texture, such as dimples that appear with a smile in some individuals, could be of added value in making an expression noticeable, or in providing information about the intensity of the expression.
A study of facial musculature in living humans noted a significant sex difference in the thickness of the zygomaticus major muscle (McAlister et al., 1998). This study also investigated differences in musculature, and found no significant differences in either levator labii superioris or zygomaticus major muscle thickness between Asians and Caucasians (McAlister et al., 1998). In general, there is not a great deal of published information on populational or sex-based variation in facial muscles, and findings of populational differences described above have not been replicated.
The effects of interindividual anatomical variation, including genetically based variation on facial expression, are even less well-known. The muscles themselves are highly variable, with some muscles appearing in some individuals and not in others (Pessa et al., 1998b). The presence of anatomical variation raises important questions about the link between facial actions and specific muscles. The relationship between muscle activity and displacement of facial features in expression is individualized to some degree; during posed eyebrow raises, muscle activity is roughly equal to brow displacement squared. Yet there is wide variation for individual brows and left brows rise higher, given the same amount of muscle activity (Pennock et al., 1999).
On the other hand, if the action of the face is the same, although there is variation in the underlying muscular structure, the resulting facial expressions may not be meaningfully discriminated. The universal recognition of some basic expressions indicates that facial expressions may not depend on a one-to-one anatomical correspondence in any two facial signalers. Basic facial expressions are also recognizable in abbreviated form, without the complete set of facial actions described for the prototype expression. Regardless of the degree of variation that can be detected empirically, perceivers may take no notice of these slight variations (Fridlund, 1997; Shor, 1978), or may categorize them similarly, with high agreement (Campbell et al., 1999; Cashdan, 1998). More importantly, it is unknown whether such phenotypic variation in facial expression meets these criteria of “just meaningful difference” (Hauser, 1996) by causing differences in receiver behavior or judgment to slightly variant displays of the same type.
Variation in neural control of facial muscles
Neurobiologically, facial expressions are dually controlled by extrapyramidal and pyramidal tracts, providing for automatic and voluntary control of facial expression. Based on observations of individuals suffering from various neurological conditions, Rinn (1984) described both systems of facial movement, along with the differential voluntary control over upper and lower face, related to greater asymmetry and voluntary control over the mouth region than the eyes. This difference is especially apparent in the facial expressions of people with cortical vs. extrapyramidal deficits. Those lacking cortical control produce largely asymmetrical voluntary (posed) expressions, but symmetrical spontaneous expressions. Extrapyramidal deficits produce the opposite effect (Rinn, 1984; Ross and Mathiesen, 1998).
Potential asymmetry due to differences in innervation of sides of the face may be related to sex differences in the brain, and therefore produce variation in facial expression among individuals. This is especially true if increased lateralization of cortical function in males includes more lateralized facial movement during expressions (Richardson et al., 2000). Spontaneity of expression also may play a role, with more spontaneous facial expressions under the control of a different neural pathway, and therefore more symmetric (Gazzaniga and Smylie, 1990; Rinn, 1984).
Research has not clearly confirmed the predictions of Rinn (1984) for asymmetry in lower face motion, especially in spontaneous expressions. Borod et al. (1998), in a meta-analysis of facial expression and asymmetry, concluded that facial expressions could generally be considered left-sided. However, they did not find that men were more likely to have asymmetry in their facial expressions, although individual studies had previously suggested this. Other researchers, using more objective quantitative methods (direct assessment of digitized images, rather than observer judgments or observer coding) found that the upper face was much more asymmetric than expected, particularly during spontaneous expression (Richardson et al., 2000).
In addition, the complex connections that have been proposed between the experience of positive and negative emotions and facial expression and cerebral laterality are still in question (Borod et al., 1998; Hager and Ekman, 1997). Borod et al. (1998) found significant left-sidedness of facial expression in studies involving muscle quantification and trained observer ratings (38 of 66 total studies showed left-sidedness as compared to 3 of 66 showing right-sidedness). The evidence of right-sidedness for positive expressions (smile), and left-sidedness for other emotion expressions, was much weaker (Borod et al., 1998). Although the role of asymmetry in the quality of facial signals is not clear, asymmetric facial expression is likely to be an important variable in considering facial displays as adaptations, particularly as it relates to spontaneity or deliberateness of expression.
Asymmetry in facial display is also probably related to individual differences in structural asymmetry that play a powerful role in our perceptions of human attractiveness and mate quality (Thornhill and Gangestad, 1994). Interestingly, most studies of facial expression asymmetry do not take into account whether or not structural asymmetry or movement asymmetry is responsible for asymmetry of facial expression. It is possible that asymmetry in expression is largely determined by asymmetries in the structure of the face at rest (Smith, 1998). By necessity, observer judgments of expression intensity can only be collected from faces at the height of expression, where both structure and movement have played a role in generating asymmetry. Electromyographic (EMG) studies of facial expression seem to support the idea that asymmetry in expression is simply a result of asymmetry in the structure of the face, because similar amounts of muscle activity are found on both sides of the face (Borod et al., 1998). In at least one case, the differential effects of similar muscle activity on facial features has been demonstrated (Pennock et al., 1999).
In more indirect fashion, the evolved perceptual preference for symmetry in structure may also extend to a preference for symmetry in movement. Spontaneous smiles, for example, are more symmetric than are posed smiles (Frank et al., 1993). They are also considered more sincere and possibly more attractive.
Variation within facial expression phenotype sets
Universal facial expressions, though distinct, are not uniformly produced or perceived. Asymmetry, due to neurobiological constraints and the relative spontaneity of facial movement, is one source of variation, but there are many others. Part of the difficulty is that expression has too often been studied opportunistically, without prior expectation or theoretical outlook on why particular facial movements should be grouped, or how the display as a whole came to be in the first place. Smiling is a good example of this problem. For example, smiling or the joy display typically involves upturned lip corners, and may also involve the squeezing and wrinkling of skin around the lateral corner of the eye (orbicularis oculi). Smiles that include both orbicularis oculi and zygomaticus major activity have been called Duchenne smiles in honor of the French anatomist, while smiles lacking orbicularis oculi activity are non-Duchenne smiles (Frank et al., 1993). Smiles also vary in their intensity, in the associated activity of other facial muscles such as frontalis, and in the open or closed position of the mouth (Blurton Jones, 1972; Cheyne 1976; Jones et al., 1990; Messinger et al., 1999) (see Figs. 4, 5). The significance of open or closed mouth smiling is also unknown, although discussions of nonhuman primate and human homology in expression suggest an appeasement function for closed bared teeth displays (smiles) and a play readiness function for open mouth displays (laughter and open mouth smiling) (Preuschoft, 1992; van Hooff 1972).
Fig. 4.

Non-Duchenne and Duchenne smiles. a: Non-Duchenne smile. b: Duchenne smile. Images from Kanade et al. (2000).
Fig. 5.
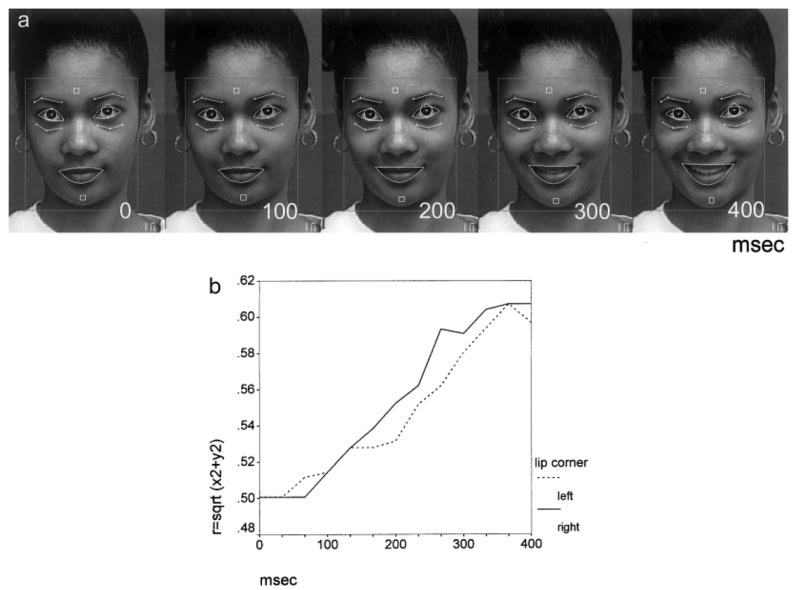
Automated method for quantifying facial movement. a: Posed smile with outlined features tracked automatically (Tian et al., 2001). b: Movement (radius length relative to mouth center) of right and left lip corners calculated from positional change in tracked features over first 400 msec of expression (Schmidt and Cohn, 2001).
Although the anatomical basis of facial expression in general is fairly well-established, important questions about the timing and patterning of facial movement also remain unanswered. Spontaneous enjoyment smiles appear to function within time constraints, typically lasting between 0.5–4 sec and having smoother transitions between onset, apex, and offset of movement (Frank et al., 1993). In a study of adult women’s smiles toward children, varied onset and offset timing of smiles were differentially interpreted by child observers. Smiles with relatively quicker offset periods were interpreted as less genuine by these children. Individual adult differences were found in patterns of onset and offset timing of smiles, and approximately 16% (122 of 763) of these smiles had multiple peaks, reinforcing the difficulty in assessing the temporal course of a smile (Bugental, 1986). Using a quantitative measure of change in smile appearance, Leonard et al. (1991) found that observers perceived the maximum difference in levels of happiness within the same few frames in which the smile changed the most. They interpreted the temporal change in smile, typically occurring within in a 100-msec window, as a template for smile perception. This timing may correspond to the limits of the perceptual system (Fridlund, 1994), and is remarkably similar to the timing of onset in the spontaneous eyebrow flash (Grammer et al., 1988). Speed of smiling (and possibly other expressions) is likely a feature of both expression and perception.
Also of interest are the patterns of coordinated movements occurring during expression. In the case of the eyebrow flash, the apex of the expression is also relatively stable, unaccompanied by the appearance of new facial movements (Grammer et al., 1988). This suggests that even for the very mobile and flexible human face, there may be limits on the types and time course of spontaneous expressions. Head and eye movements may also occur during facial expression, and are probably coordinated with facial movements, as components in a multicomponent display (Ekman, 1979; Niemitz et al., 2000). Lyons et al. (2000) demonstrated that head position has significant effects on the perception of facial expression; head position changes were perceived as facial expression changes, even in the absence of facial muscle activity.
Given the great diversity of human facial expression, anthropologists tend to note the flexibility, complexity, and voluntary nature of facial expression (Birdwhistell, 1970). Compared even to relatively expressive nonhuman primates, like chimpanzees, human facial expression seems to be too variable to be divided into well-defined phenotype sets. Voluntary control over facial muscles, especially over the muscles of the mouth, is a hallmark of human nonverbal expression, and is likely due to the articulatory demands of human language (Rinn, 1984). The diversity of voluntary and spontaneous human facial expression, however, should not be mistaken for infinite variability in facial expressions as actually performed. Current research on the configuration and temporal course of facial expression seems to support this idea, as it has so far shown a great degree of regularity in expression across cultures and individuals.
Individual differences in observable facial expression behavior
In addition to underlying physical variation in the face and in movement, empirically measured facial behavior varies according to factors such as sex (Briton and Hall, 1995; Chapell, 1997), age (Chapell, 1997), and cultural background (Ekman, 1973; Kupperbusch et al., 1999). Also important in facial expression are individualized factors, such as sociality of situation (Fridlund, 1994; Friedman and Miller-Herringer, 1991; Jakobs et al., 1999) and the emotion-eliciting nature of visual or other stimuli (Cohn and Tronick, 1983).
Humans vary in their ability and tendency to produce facial expressions, and this variation is presumably related to underlying muscular, neurobiological, or social differences. Nevertheless, variation in the signal itself, the visible changes in the face, is important to addressing hypotheses of the signaling value of facial expressions. Dimensions of nonpathological variability include interpersonal success in nonverbal communication (Strahan and Conger, 1998) and overall expressiveness (DePaulo, 1992). There are also sex differences in facial expression, especially for smiling, with women smiling more (Briton and Hall, 1995; Chapell, 1997; LaFrance and Hecht, 1999). LaFrance and Hecht (1999) maintain that women are not comfortable unless they are smiling. Women have also been shown to have thicker zygomaticus major muscles, although it is unknown whether this is a cause or consequence of increased smiling (McAlister et al., 1998). Sex differences in facial expression are not only frequency-based, however; there is also some evidence that women specialize in expressions of happiness, while men are better performers of angry expressions (Coats and Feldman, 1996). Individual differences in facial expression may be apparent even in neonates (Manstead, 1991). Most of these results are from industrial societies, and therefore, the range of human variation is probably not fully represented. Yet behavioral phenotypes, including universal expressions and facial displays, are clearly variable.
Methods in facial expression research
Hauser (1996) stresses the importance of methods that allow rigorous comparison among studies of communication. Currently, there are relatively few detailed objective methods, although recent work in facial expression research holds promise for improved expression analysis. The development and use of the Facial Action Coding System (FACS), an anatomically based coding system for recording appearance changes caused by the action of individual muscles, was the first to make possible the collection of a large body of reliable empirical data on these expressions (Ekman and Friesen, 1978; Ekman and Rosenberg, 1997). New methods of facial measurement allow even more objective, quantitative measurement of facial movement (see Fig. 5). Automated versions of FACS, which will automate the process of studying facial action, are currently being developed (Cohn et al., 1999). Other automated methods have been used for studying nonverbal signals in general (Grammer et al., 1999), and facial expressions in particular (Richardson et al., 2000; Scriba et al., 1999). These methods rely mainly on overall change in images of the face (or entire body) over the course of nonverbal expression. Amounts of change in the image, assuming all images are collected in the same manner, are interpreted as movement patterns (see Fig. 5).
Phenotypic variation in facial perception
Along with the study of the neurobiological and anatomical and behavioral aspects of facial action, the perceptual aspects of face recognition and facial expression recognition have also received a great deal of attention in the past several decades. In proposing that patterns of facial movement are communicative adaptations, there is also the task of understanding the coevolution of perceptual mechanisms (Chovil, 1997; Endler 1993; Fridlund, 1997; Hauser, 1996). There is good evidence that neurons in particular areas of the brain have specific sensitivity to social stimuli, including but not limited to static faces. The primary evidence for the existence of separate neurobiological mechanisms of facial expression recognition comes from studies of dissociable deficits: the ability to recognize faces is retained, while the ability to recognize expressions is lost in brain damage (Ellis and Young, 1998; Young et al., 1998) or in cases of autism (Celani et al., 1999). This evidence supports a theory of neurobiological specialization for social stimuli (Adolphs, 1999; Brothers et al., 1990).
Facial expressions are processed by several distinct brain regions. The amygdala is largely responsible for perception of fear and sadness expressions. Some researchers suggest that the amygdala may in fact be specialized for perception of fearful expressions only (Morris et al., 1996). Other regions, including the somatosensory and orbito-frontal cortex, are involved in recognizing emotion blends and anger (Adolphs, 1999; Blair et al., 1999; Morris et al., 1996). The difference between explicit processing (subjects instructed to judge facial expressions) and implicit processing (subjects instructed to judge sex of actor) may affect the region of brain used for processing, with implicit processing handled largely by the amygdala (Critchley et al., 2000).
Differences in facial expression processing likely relate to differences in adaptive behavioral responses to expressions. For example, Dimberg (1997) found that conditioning to faces worked much better for fear than for any other expression. Neurobiological specialization for facial expression processing also corresponds to the suggestion that perception of certain signals requires greater sensitivity (perception of anger at very low levels is likely to give a fitness advantage; Gosselin et al., 1997).
While the variation inherent in neurobiological perceptual systems is unknown, there is evidence for individual differences in facial expression perception. The most striking finding is that women appear to be more accurate and more sensitive decoders of facial expression than men, and that this ability develops in children as young as 3 years (Boyatzis et al., 1993; Hall, 1984; McClure, 2000). Women in one study gave higher ratings than men did to happy expressions and lower ratings to neutral expressions, suggesting they differentiate more among facial expressions of varying intensities (Katsikitis et al., 1997). Otta et al (1996) also found slightly greater discrimination among women, as perceivers of smiles.
A more specific ability, detection of deceit, also varies among individuals, and appears to be based on a more widely applicable ability—being able to detect small, extremely rapid changes in facial expression (Frank and Ekman, 1997). This ability to detect subtle changes in facial movements may also be an important individual difference. For example, some people were better able to judge smiles of enjoyment accurately, especially those who paid more attention to the eye region (Frank et al., 1993).
Finally, cultural background plays an important role in the attribution of personality traits to particular facial expressions. People with smiling faces are interpreted as more sociable than people with neutral faces by both Japanese and American subjects. However, the difference between the sociability of neutral and smiling faces is much higher for Americans than it is for Japanese subjects (Matsumoto and Kudoh, 1993).
Diversity among facial expression phenotypes
Although the concept of basic universal expressions has allowed us to consider human facial expressions as evolved behavioral phenotypes, an emphasis on universality obscures the abundance of variation within and between facial expressions. All facial expression phenotypes are not created equal. For example, some expressions are produced much more often than others (smile; Bavelas and Chovil, 1997), as frequently as two or three separate displays per minute of face-to-face interaction (Schmidt, 2000). Others, such as the Duchenne smile and the fear expression, cannot be as easily produced voluntarily. Some are quite infrequent and/or may not be as universally recognizable as others (disgust, fear, contempt; Fridlund, 1994). Different expressions are also associated with specific variation in autonomic functioning, suggesting different roles in emotional, self-regulatory processes. For example, fear expressions increased heart rate and sadness expressions decreased it (Levenson et al., 1990).
Even expressions that seem superficially similar, such as smiling and laughing (to be discussed later), are now best studied as separate phenotypes, given our current knowledge of their forms and functions (Keltner and Bonanno, 1997; Preuschoft and van Hooff, 1995; Provine, 1996, 1997). Smiling typically bares the teeth, but does not necessarily involve vocalization, unlike laughter, which has a characteristic pattern of open mouth and exhalation/vocalization associated with it, and may also include repetitive eye and mouth closure patterns (Niemitz et al., 2000). Extensive variation both among and within facial expression phenotypes clearly requires multiple adaptive explanations, rather than a single hypothesis of facial expression. The fit between behavioral phenotype, ecological context, and fitness consequences for facial expressions is specific to each particular expression phenotype. In general, however, facial expressions can be considered social signals, which is a good starting point from which to diverge into multiple adaptive contexts.
ECOLOGICAL CONTEXTS AND FITNESS CONSEQUENCES
Facial expressions as social signals
Facial expression is unambiguously social, in that the expressions are produced with greater frequency and intensity in social situations and can be directly linked to interactive consequences (Fridlund, 1994; Jancke and Kaufmann, 1994). If facial expressions (including displays of emotion) are social adaptations, then they are signals, produced primarily with a social purpose. Whether or not emotion is associated with most facial expressions, or even any facial expression, is an issue of great importance in the psychology of emotion. If facial expressions are simply outward expressions of emotion, and there is no specific fitness consequence proposed for the expressions themselves, then they are subject to other levels of analysis. When facial expressions are considered as potential adaptations, however, the relationship between experience of emotion and expression is not necessarily relevant (Hauser, 1996; although see Keltner and Gross, 1999, for a functional hypothesis of emotions in general). Forming hypotheses about the adaptive nature of facial expressions as social signals requires only that specific ecological contexts and fitness consequences of these behaviors be described. Adaptive explanations of the signals themselves may or may not include adaptive explanations of human emotions that underlie much of facial expression.3
Because they occur largely in the interactive context, human facial expressions are generally considered to be cooperative signaling systems, benefiting both signaler and receiver (Fridlund, 1997). Although it is not generally discussed, facial expressions can probably be considered low cost, as the energy required to produce an individual spontaneous expression is small. Such low-cost, frequent signals are expected in mutually beneficial interactions (Krebs and Dawkins, 1984), or within groups of related individuals (Bergstrom and Lachmann, 1998).
Facial expressions, however, occur in many different social contexts, not all of them cooperative. The complex relationships between social contexts and shared and conflicting interests suggests that human facial expressions have multiple signaling functions. As extensive modeling, and some empirical work in nonhuman primates has shown, the ecological contexts and fitness consequences of behavior are critical in determining the nature of the signals that evolve (Godfray and Johnstone, 2000; Grafen and Johnstone, 1993; Krebs and Dawkins, 1984).
Socioecological contexts of human facial expression
Human ecological contexts are undeniably socio-ecological contexts. Even nonsocial contexts may be framed in social ways, because of past selective pressures on the design of the mind (Brothers, 1997; Humphrey, 1976; Kummer et al., 1997). Absent or semipresent individuals provide enough social context to produce facial expression (speaking with someone on the phone, or knowing a friend is doing the same activity in the next room; Fridlund, 1994). These types of contexts are, of course, unlikely to have been important until recently in our evolutionary history, but they illustrate our readiness and willingness to produce facial expressions at even the suggestion of social interaction.
Humans are not completely unresponsive in the absence of social partners, but facial expressions vary in their susceptibility to audience effects, with changes in frequency and/or type of movement with different receivers (Jancke and Kauffmann, 1994; Kraut and Johnson, 1979). Some expressions vary little along social dimensions (startle), while others vary significantly (smile). For example, Fridlund (1994) and others have found that smiling decreased monotonically with decreasing social contexts (viewing a tape with a friend, viewing a tape that a friend is watching in the next room, viewing a tape while friend is off completing tests, viewing a tape alone). The knowledge that a friend was performing the same task led subjects to smile significantly more. Kraut and Johnson (1979) observed a similar phenomenon among bowlers, who smiled at friends more often than they smiled at a spare or strike. Hess et al. (1995), Jancke and Kaufmann (1994), and Manstead et al. (1999) found similar effects of sociality, concurrent with the effects of emotion-eliciting stimuli. Obviously, the natural context of facial expression is social interaction, and anthropological, adaptive explanations of facial behavior require attention to specific, naturalistic ecological contexts.
This type of approach has been suggested by Preuschoft (2000) with respect to the positive emotions, and could be adapted for the positive facial expressions of emotion. She includes several contexts for recategorizing the positive emotions, including interacting with babies, interacting with others playfully, seeking mates, and other social contexts. Bradbury and Vehrencamp (1998) suggested a similar list: conflict resolution, territory defense, sexual signals, parent/offspring interaction, social integration, environmental contexts, and auto-communication.
In this paper, we propose the following set of socioecological contexts for a beginning exploration of facial expression as adaptation: infant/caregiver interaction, cooperative interactions, speech, and intraspecific competitive interactions. We consider each separately defined ecological context as a source of variation in costs and benefits of facial signaling, with separate fitness consequences for each.
Infant/caregiver interaction
Both cooperative and competing interests of caregivers (parents) and offspring are probably reflected in the signaling systems that develop in the context of this interaction (Trivers, 1974). Infants may produce honest signals of need (Grafen, 1990; Godfray and Johnstone, 2000). Another possibility is that infant signals of need are exploitative, or represent the outcome of sibling conflict (Godfray and Johnstone, 2000). Infant facial expressions can be interpreted in these signaling contexts.
Infants display basic facial expressions that are similar to or the same as those of adults (Fig. 1). Some of the component movements of facial expression are probably innate (Izard and Malatesta, 1987; Meltzoff, 1996). In addition, studies of infant facial expression are most accessible to adaptive perspectives, in that researchers are unable to rely on self-report and must instead look at expression ethologically and with an eye toward consequences of behavior.
Human infant displays have been discussed in the literature from a signaling perspective. Infant crying is generally interpreted as an honest signal of need, as expected theoretically (Fridlund, 1997; Hauser, 1996). Facial expressions associated with crying are part of this multicomponent signal, and crying is effective in getting attention.
Infant smiling as a display is also coordinated with caregiver behavior. Infants look at their mothers whether the mother is attentive or not, but they only give the signal (smile) if she is attentive (Jones et al., 1991). The probability that an infant will smile at its mother is greatest when the mother is most attentive and also smiling (Cohn and Tronick, 1987). Infant bouts of smiling end quite abruptly when the mother ceases smiling (Cohn and Elmore, 1988). These results suggest that infants’ production of smiles is sensitive to the potential costs and benefits of making this expression. Since infants are subject to both maintenance and growth needs, they could be expected to modify even these seemingly inexpensive signals, depending on the potential for positive response. Positive responses, in this case, would be directly related to positive fitness consequences, including increased opportunities to interact, leading to earlier and better performance in social interaction. The function of infant smiling and mother infant interaction in the development of secure attachment is also significant in that secure attachment in infancy has been demonstrated to predict social competence later in childhood, while insecure attachment has negative consequences (Belsky and Nezworski, 1988).
One of the positive consequences of facial expression seems simply to get attention focused on oneself. Faces and facial expressions draw attention (Vuilleumier, 2000). Much of the work on this most simple effect of facial expression comes from the study of infant interaction. Human infants use facial expression to induce their mothers to pay attention, and mothers typically vocalize or smile in response (Jones et al., 1990). The bared teeth smile seems particularly useful in this context; Jones et al. (1990) found bared teeth smiles are more likely to be produced in social situations, when the infant is paying attention to its mother and not to toys. These bared teeth smiles subsequently elicit responsive and attentive behavior from the mother, a positive fitness consequence. The fitness effects of parental attention for the human infant are potentially great, considering the intense social and nutritional needs of the infant, as well as possible risks associated with lack of maternal attention, including failure to thrive, physical danger, and at the extreme, death from neglect or abandonment. Whether or not these social signals are honest signals of need or not, however, is difficult to assess given our current knowledge of infant smiling and attentional needs. The relationship between infant facial expressions and honest signals of need could be investigated, if need were operationalized as continuing fitness costs (length of time since last feeding, distance from mother, presence of sibling competitors), and signal intensity and frequency were operationalized for specific expressions.
As infants develop, they more often initiate bouts of smiling (Cohn and Tronick, 1987). They coordinate facial displays with their caregivers (parents), signaling only in what are considered appropriate contexts. This coordination of signals may represent a coordination of interests among healthy offspring and healthy parents (those with incentive to continue investment). Among older children, it becomes apparent that ability in sending and receiving facial signals varies, and is associated with positive fitness consequences of personal and social adjustment (Nowicki and Duke, 1994). As children get older, they use their perceptions of smiles to fine-tune their expressions until their deliberate posed smiles are relatively indistinguishable from their spontaneous smiles. There is strong evidence that this process relies on visual observation. Deliberate smiles of blind children were distinguishable from their spontaneous smiles, when viewed by untrained observers, while those of sighted children were not (Castanho and Otta, 1999). As adults, persons blind from birth produce voluntary expressions of emotion that are significantly less recognizable than those of sighted individuals (Galati et al., 1997).
The specialization for infant signals of need is also specific to target receivers of the signal. Infants vary their signals, depending on the parent (Forbes et al., 2000). Infant signals are differentially perceived by men and women, and infant smiles increase accuracy in infant recognition in women only (Jones, 1984). Jones (1984) suggested that the smile was a signal specifically directed toward women. Infants may vary signaling, based on experience of increased benefits for the behavior. For example, in infant smiling during parent/infant interaction, infants often begin with a non-Duchenne smile and then convert this smile to a Duchenne smile, by adding the contraction of the orbicularis oculi around the lateral edge of the eye (60% of all infant Duchenne smiles observed; Messinger et al., 1999). It is not inconceivable that this change in expression could be related to dynamic changes in the infant’s perception of benefits of the energetically more expensive Duchenne smile.
Long-term cooperative social interaction
Infants’ and children’s competence in producing and perceiving facial expressions eventually develops into adult skill at using facial expression with peers and other members of the social group. Relationships among relatives and other group members are characterized by speech and also by extensive facial expression behavior. The complexity of relationships in these groups means that contexts for facial expression will vary from interaction to interaction, on a frequent basis.
Because of their unusual stability and tendency to involve long-term relationships, human groups are a likely context in which reciprocal altruism can develop (Trivers, 1971). Reciprocal altruism would clearly be greatly facilitated by the use of signals for willingness to reciprocate or negative sanction of failure to reciprocate. It is in this context of repeated long term interactions that patterns of repeated facial expression can provide interactants with exactly the information they need with regard to others’ intentions, altruistic or otherwise (Brown and Moore, 2000; Silk et al., 2000). Brown and Moore (2000) hypothesized that in some cases, an “altruist detector” might be more valuable than a “cheater detector” mechanism. Presumably this detector would depend on input from stimuli such as facial expression, tone of voice, and other nonverbal signals. In this context a conventional signal of relatively small magnitude, but repeated performance would be predicted (Dawkins, 1993). If a pattern of signaling that corresponds with reliable altruism can be maintained, then the pattern could become ritualized as a representation of the sender’s altruistic intent. Continuous performance of this expression pattern might indicate that others can rely on the actor to act in an altruistic fashion. Zahavi (1993) notes that these types of reliable conventional signals can work in the following way: “a signal which seems to be performed alike by all individuals in a set is in fact a ‘standard’ against which the quality of different individuals of the same set is judged” (Zahavi, 1993, p. 228).
This explanation of facial expression in cooperative interaction is congruent with the suggestion by DePaulo (1992) that the primary goal of facial expression, conscious or unconscious, is self-presentation. According to DePaulo (1992), although deception could be adaptive, people are not trying to make themselves appear as something they are not. Instead, people typically try to enhance the accuracy of qualities they already display (DePaulo, 1992; Schmidt et al., 2000). For positive facial expressions, in particular, it could be proposed that they are trying to appear more altruistic (Brown and Moore, 2000).
Several human facial expression patterns support this view of altruistic signaling. Social status in housemates was associated with smiling, with high status housemates smiling more (Cashdan, 1998). A closed mouth bared-teeth display in nonhuman primates is typically interpreted as a submissive or appeasement display (van Hooff, 1972; Preuschoft, 1992; and see discussion below). This kind of display is not expected to be most frequent in the highest status individuals. However, another perspective, based on cooperative signaling, could explain these data. The individuals with high social status could be signaling their high potential for, or willingness to reciprocate (Brown and Moore, 2000). In fact, smiling in this study was quite frequent, for both males and females (smiling about 10% of the time they were observed; Cashdan, 1998).
Grammer et al. (1988) found a similar frequent signaling pattern in eyebrow flashes, with the added point that initial eyebrow flash was typically the most intense (getting attention of partner), followed by conversational eyebrow flashes (regularly signaling to attentive partner; Grammer et al., 1988).
One problem with this explanation is the possibility of nonreciprocators using repeated low-cost conventional signals, such as smiles, in a deceptive manner. Then somehow these repeated signals must be costly, or at least so costly as to negate the benefit for a cheater that uses them correctly, but deceptively (Zahavi and Zahavi, 1997). Or the signals, if cheaply produced, could represent coordinating activity among individuals whose interests are sometimes in conflict, but who benefit by coordinating activities nonetheless (Silk et al., 2000).
A signal has to truthfully represent the interactant’s intentions to reciprocate: honest signals are hard to fake (Keltner and Bonanno, 1997). An automatic, nonconscious response (electromyographically measured response of the receiver’s own facial muscles) to the smiling of others, occurs only to Duchenne smiles that are more energetically expensive and harder to fake (Surakka and Hietanen, 1998). A similar response occurs to the nonverbal vocal components of speech. The corrugator muscle activated in the case of anger, and the orbicularis oculi muscle activated when hearing contented voices (Hietanen et al., 1998). Contrary to Fridlund (1997), these reflexive displays of emotion may be quite adaptive, especially in contexts where automatic, quick response is critical. Emotional readout can be a powerful social tool, especially if it brings about positive fitness consequences for senders and receivers. For example, facial expressions play a role in creating and supporting empathy, an emotional, yet also adaptive phenomenon (Brothers, 1989; Buck and Ginsburg, 1997; Keltner and Bonanno, 1997). Other positive fitness consequences could include the likelihood of receivers sharing food and other resources with the signaler, benefits that arise from the reciprocally altruistic relationship in general.
The predicted characteristics of spontaneous smiles in the context of long-term cooperators, therefore, include honest signals such as the Duchenne sign (orbicularis oculi activity) and characteristic timing (see Fig. 5). We know that spontaneous smiles differ in quality, with more simultaneous muscle actions that are more coordinated, as compared to deliberate, posed smiles (Gosselin et al., 1997; Hager and Ekman, 1997; Hess et al., 1995). The contrast between Duchenne and non-Duchenne smiles is often interpreted as the difference between truly felt and less than sincere expressions of emotion. Ekman and Friesen (1982) maintain that the Duchenne smile is not easily faked, although a database of 105 posed smiles contained as many as 67% Duchenne smiles (Kanade et al., 2000; and see Fig. 4). Whether or not the signal can be faked, it is still consistent with an adaptive signal hypothesis of smiling. More honest signals tend to be more costly signals, and the Duchenne smile, to the extent that it is more sincere, is certainly more costly (involves at least two muscles instead of just one; see Fig. 3). In addition, Bugental (1986) found that longer, and therefore more costly smiles, were interpreted as more sincere. For laughter, Niemitz et al. (2000) found that longer laugh expressions, with more dynamic eye and mouth movements, were judged to be the most positive and sincere expressions by adult observers.
The range of contexts under which spontaneous smiles occur, and the resulting degrees of honesty expected, have not been considered. More research into the quality of nonverbal signals may be the key to distinguishing expressions along a continuum from more honest facial signals to those that are less than sincere (Grammer et al., 1997). For example, observer response toward spontaneous Duchenne and non-Duchenne smiles from the same individual would be predicted to differ, with the longest spontaneous Duchenne smiles generating the most positive responses.
Yet this perspective also suggests that even over-learned or automatic signals, like the non-Duchenne or “social smile,” could be important signals of cooperative intention, rather than emotion. If social smiles are performed according to a regular pattern, then signaling of positive intention could consist of a regular pattern or script for signaling, and deviations from the expected expression pattern would signal negative intentions. Social smiles, and other expressions, are coordinated and timed with attention to the listener. They typically occur as an alternate response to verbal “back channels,” such as “uh-huh” and “yeah,” that signal continued participation in a conversation (Bavelas and Chovil, 1997; Brunner, 1979).
We propose a new perspective on the costs associated with these social signals. In addition to the energetic costs of repeated signaling that add up during a social encounter, there are significant costs to the signaler if he is to maintain the signaling pattern properly. These costs are attentional. Timing requires attention, and attention requires the redirection of the sender’s neural processing and perception toward one interactant and away from others. Only by paying attention to the receiver and the course of the social interaction, can the sender continue to signal correctly. The smile, for example, is not a randomly performed signal. The small amount of evidence available shows that smiling is highly coordinated with the meaning as well as the nonverbal aspects of speech. Smiling “too early” or “too late” can cause an individual to appear insincere (Bugental, 1986; Ekman and Friesen, 1982). Smiling when the topic of speech prohibits smiling is a significant social faux pas.
For non-Duchenne smiling then, the cost of the signal is the cost of the attention paid to the interactant, rather than the added physiological costs associated with a spontaneous Duchenne smile. This prediction could be tested by observing the rate and timing of social smiles and other facial expressions during conditions in which attention is disrupted. Alternatively, one could measure the loss of attention to outside events during a typical interaction, as an approximation of the risk in focusing on one individual while other potentially important events or interactions are taking place. The level of involvement in an interaction as measured by number or intensity of social signals or gaze, among other measures, is predicted to correlate with decreased attention to other stimuli in the vicinity.
If regular signaling that one focuses on the speaker is accomplished by smiling, then a violation could be detrimental to individual fitness. Self-presentation is arguably more important among potential reciprocators, i.e., people whom one already knows. Regular smiling, then, could be a sign of altruistic intentions and would be expected to be more frequent around friends (Jakobs et al., 1999). The social smile, with its high frequency, could be the redundant signal that Johnstone (1997) proposes is the best for getting across a message to conspecifics. Krebs and Dawkins (1984) predicted that cooperative signals should be small and relatively inexpensive. An analysis of EMG or other measure of facial exertion would be predicted to show that this type of smile is much cheaper than other expressions, like anger, fear, or disgust, which corresponds with the fact that it is used frequently in cooperative interactions.
Positive fitness consequences
In proposing that smiling, or any other facial expression, is an adaptive social signal, it is important to establish the positive fitness consequences of such a behavioral phenotype. There is evidence to suggest that facial expressions function to increase cooperation and affiliation during interaction. The positive fitness consequences of facial expression include the promotion of social acceptance and affiliation, and the moderation of the effects of socially negative actions. While the effects of facial expressions, and smiling in particular, have not typically been operationalized as fitness consequences in the past, a review of recent studies indicates that these consequences are significant, at least with regard to social variables such as status. Smiling affects perception of the smiler’s other important characteristics, by increasing attributions of intelligence (Otta et al., 1993, 1996), happiness (Otta et al., 1996), and social status (LaFrance and Hecht, 1999). Although not typically associated with grief, smile displays during conversation, especially those that include orbicularis oculi activity around the lateral edge of the eye, increase perceiver sympathy for bereaved persons (Keltner and Bonanno, 1997). People associate smiling with happiness and with positive intentions directed toward them (liking) (Floyd and Burgoon, 1999). Smiling, in both the United States and Japan, is associated with increased sociability (Matsumoto and Kudoh, 1993).
These responses are mostly determined by self-report in psychological studies, but there is also direct physiological evidence that facial displays can bring about positive responses, even in the absence of overt emotional response. For example, a study of the effects of viewing Ronald Reagan’s reassuring smile display (raised brows, tilted head, relaxed open mouth smile) brought about physiological signs of positive response, even in viewers who said they did not support Reagan politically (Sullivan and Masters, 1991).
Clearly, smiling displays can bring about positive fitness consequences, and perhaps even convince others of ones’ willingness to reciprocate. There may be some individuals who fail to signal regularly, however. In this case, the failure to signal socially is in itself a signal (Bergstrom and Lachmann, 1998). The failure to smile correctly, or to produce an appropriate level of facial expression during cooperative social interaction, may be at the root of some of the social difficulties of people with flat affect as a result of schizophrenia, Parkinson’s disease, and depression, or facial paralysis (Mueser et al., 1997; Sakamoto et al., 1997; VanSwearingen et al., 1999).
Signaling altruism also implies the presence of remedial social signals. For individuals who fail to act in an altruistic manner, and get caught, there are also embarrassment and shame displays. Interestingly, in embarrassment displays, there is also a smile, although here it is interpreted as an appeasement signal (Keltner and Buswell, 1997). Shame displays are slightly different, and do not typically include a smile (Keltner, 1997). Although there is no consensus on whether or not the smile is an honest signal or not, the blushing response seems much closer to the definition of an honest costly signal. Also a component of the shame display, blushing actually works, with significant differences in the perceptions of interactants depending on whether or not an individual blushed. Blushing, which can be part of a voluntary shame display including glancing around and lowering the head, actually reduces negative evaluations of actor behavior (deJong, 1999). As a remedial gesture, blushing may be particularly potent, because it is a gesture that is generally believed to be impossible to fake. It is not surprising then that it produces a better response in observers than does the glancing around display, another remedial gesture (deJong, 1999). Keltner et al. (1997) interpreted both shame and embarrassment displays as appeasement displays, and supported this hypothesis with evidence that they are negatively correlated with aggression in adolescent boys.
Facial expressions during speech
Facial expression during social interaction is possibly an honest signal of affiliation, or willingness to reciprocate. Among humans, however, social interaction almost invariably involves speech, and there are unique considerations in the adaptiveness of the relationship between facial expression and speech. Facial expression is coordinated with speech at several levels: the use of muscles of facial expression to articulate speech sounds (Massaro, 1998), the contribution of facial expressions to the syntactic structure and meaning of particular utterances (Bavelas and Chovil, 1997; Ekman 1979), and graded or qualitative conversational signals that apply to the overall meaning of speech (Ekman 1979).
In addition to its functions for the speaker, facial expression is also an important part of listener activity (as many as 20% of conversational expressions were back channel cues, performed while the individual was not talking; Bavelas and Chovil 1997). Smiles are produced with the same timing as other back channel conversational cues, such as “uh-huh,” that signal continued participation in a conversation (Brunner, 1979), and are also produced at the end of utterances (Schmidt, 2000). Laughter occurs along with speech in a coordinated pattern (Provine, 1997). Ekman (1979) detailed the multiple patterns of association of brow movements with speech: as “batons” stressing a particular word, as question marks, or as “underliners” emphasizing a sequence of words, among others. Although the role of facial expressions and other gestures in language evolution may be limited, understanding the coevolution of language and the “gesture-call” system is critical to understanding human speaking behavior (Burling, 1993, with comment by Blount, p. 38–39).
Facial expressions might also be related to the social functions of speech. Although humans practice some social grooming (Schievenhovel, 1997), Dunbar (1996) suggests that the role of grooming in human society has largely been taken over by conversation. If nonverbal signals, including facial expressions, are coordinated with speech, they might also assist in the grooming function of speech. Andrew (1962) notes that lip smacking and other lip movements are intention signals for grooming in nonhuman primates. It is also possible that humans, while potentially “grooming” partners with speech, have retained these intentional signals in the form of lip sucking, lip wipes and lip biting (action units 28, 37, and 32, Ekman and Friesen, 1978). Fridlund (1997) refers to lip biting as a sign of agitation, but it may also be a sign of desire to groom which is often is associated with agitation. It is an open question whether or not these movements are more frequent during “vocal grooming” than in other human social settings.
Courtship and facial expression
Facial expressions are only a small proportion of the large number of human courtship signals. They are typically embedded in coordinated displays, including both whole body and whole head movements, as well as other signals. As such, they have a role in signaling both interest and mate quality. Signaling mate quality is accomplished by a number of physical trait-based characteristics such as symmetry, glossiness of hair and skin, and healthiness of eyes (Gangestad and Thornhill, 1997).
Because of the numerous available indicators of potential mate quality, one might argue that facial expression was unnecessary. However, it could also be expected that facial expression would act in such a way to enhance positive traits, while disguising or concealing less positive traits (enhancing self-presentation as a potential mate). Eibl-Eibesfeldt (1989) describes how clothing and makeup in many cases, accomplish these goals, but facial expression could provide an intermediate behavioral level between cultural modification and physical, biological constraints.
The description of the courtship displays of young German and Japanese women, for example, shows the importance of both facial expression and other nonverbal movements in signaling attraction (willingness to mate or to consider mating). Grammer et al. (1999) found that during mixed sex interactions between young men and women, a woman’s interest in her partner was related to the regularity of her nonverbal signals. Smaller, more regular movements in women’s nonverbal displays were associated with increased levels of interest (Grammer et al., 1999). This could be the case for facial expression as well. In the case of opposite sex interactants, repeated signaling with honest signals could also be interpreted as a sign of willingness to invest in a mate, and therefore increase the likelihood of reproductive opportunities for the signaler. While there is little positive evidence to support this idea, there is some negative evidence in that the lack of coordination of facial movements in people with schizophrenia can cause significant social problems for people with schizophrenia (Dworkin, 1992).
With regard to symmetry and mate selection, it is possible that symmetric facial expressions (typically spontaneous and difficult to produce voluntarily) evoke attractiveness in the same way that structural facial symmetry (also very difficult to fake) does (Gangestad and Thornhill, 1997; Grammer and Thornhill, 1994). Finally, facial and other nonverbal signals during courtship can be expected to be relevant to other signals of mate quality and to coordinate with them. The flirtatious hair flip (Grammer et al., 1999), for example, shows dynamically the quality of the hair, while open-mouthed expressions may reveal the quality of the teeth, as well as the tissues inside the mouth.
Strangers, competitors, and conflicts of interest
A variety of individual ecological contexts are included here, mainly because of the general lack of empirical information on facial expressions in these different contexts. In contrast to previously described situations, however, interactions with strangers and other interactions where potential conflicts of interest are likely to share some basic signaling properties.
Krebs and Dawkins (1984) suggest signals to those whose interests potentially conflict with the signaler should be stereotyped and clear, rather than hidden. Facial expressions are expected to revert to conventional forms that reveal as little extra information as possible (Wagner and Lee, 1999). In a classic study of Japanese and American students responding to films, facial expressions differ least in the presence of an interviewer, demonstrating the increased regularity of facial signals in a socially risky situation (with interviewer, rather than alone; Ekman and Friesen, 1978; Wagner and Lee, 1999). Signalers have some conscious perception of their signaling patterns: students can predict, based on context, their own likelihood of smiling at a joke told by a professor as compared to a joke told by another student (Nagashima and Schellenberg, 1997). Stereotyped nonverbal bodily displays are also characteristic of interactions in which strangers meet (Grammer et al., 1997).
The sender may modify normally beneficial honest expressions in these contexts, depending on potential costs or benefits of revealing information. When meeting strangers, humans sometimes attempt to hide or suppress expression. For example, children and young adults from a variety of cultures responded to a friendly stranger with a similar pattern of direct gaze with expression, followed by hiding the face, and then another glance (Eibl-Eibesfeldt, 1989). Concealment also occurs in competitive interactions with known individuals. In a study of individual differences of the tendency to self-monitor facial expressions, some individuals were found to conceal expressions of joy after winning. These smiles were concealed either by covering with a hand, or by twisting other muscles in the face into positions that minimized the appearance of the smile (Friedman and Miller-Herringer, 1991). Yawns and laughs are also sometime concealed by covering (Provine, 1997).
According to Fridlund (1997), concealment of emotion is highly desirable, and coordinates with the “dictate of privacy” in social interaction. This is misleading, though, in that the desirability of concealment necessarily varies with the particular socioecological context. Only those facial signals that depend on privacy between signaler and receiver for positive consequences are expected to be concealed from the rest of the group. Although there are likely to be contexts in which people seek to hide their expressions from others, facial expressions toward strange conspecifics in many cases are expected to be clear and easily readable, whether they signal internal state or not (Endler, 1993).
Meetings between strangers represent an obvious opportunity for deception, in that the altruistic tendencies of the signaler are unknown to the recipient. However, actively deceptive signals are expected to disappear eventually, because they do not supply the receiver with useful information (Zahavi and Zahavi, 1997). Actively deceptive signals of this type can be contrasted with concealment of basically honest signals, as in the effective suppression of facial expression in contexts where food resource, personal reputation, or other positive outcome is at stake (Ekman et al., 1997; Mitchell, 1999). A third strategy, the modification of internal states associated with expressions (emotional states), may be the most adaptive form of deception. Expressions generated under these conditions of self-deception would then appear spontaneous and automatic (Alexander, 1987). Emotional readout is not necessarily an honest signal: it may be the result of self-deception, and so appear honest when it is not.
Various facial behaviors associated with deceit have been described and tested empirically (Ekman, 1985; Ekman et al., 1997). These studies provide interesting information on social intelligence and Machiavellian intelligence in particular. However, the same researchers also found that there is substantial variation in the ability of human observers to detect these nonverbal signs of deceit. Although some individuals have this capability, many, even those with extensive experience or training (e.g., police officers), are unable to reliably detect cheating individuals using these cues (Frank and Ekman, 1997). In fact, at least for facial expression, human cheater detection mechanisms seem to be remarkably bad at detecting cheating, at least among strangers (DePaulo, 1992; Ekman, 1985; Gosselin et al., 1997). Either these mechanisms do not provide a clear fitness benefit, and have therefore not been subject to selection pressure (Bradbury and Vehrencamp, 2000; Zahavi, 1993), or else the relevant context of cheater detection is found not among strangers, but among social group members. Cheater detection ability may depend on an almost statistical knowledge of the normal facial expression pattern for a given interaction partner, rather than a stranger.
Cheater detection mechanisms are undoubtedly an important feature in the evolution of human social intelligence, but in the case of facial expression may not be very fine-tuned (DePaulo, 1992). The answer to these puzzling findings probably lies in the structure of human social interaction, especially in the distant past. Cheater detection would be expected for individuals with whom we have the most contact (strangers would likely not have been trusted in any case). We hypothesize that detection of deception by observation of nonverbal behavior may be limited to those individuals that have regular contact with the deceiver, and might not be expected anyway in those who have no prior knowledge of this person (such as observers described in Ekman, 1985 and Ekman et al., 1997). We are not aware of any studies of the relative difference in cheater detection performance based on the facial expression of known and unknown subjects, but predict that cheater detection would be significantly better for known individuals.
If facial expressions are generally used as honest signals, as part of an ordered sequence of actions appropriate to a particular social context, relatively infrequent use of these signals for deceitful purposes is a possibility. Mitchell (1999) describes these regular sequences of events as social scripts, where deception is dependent upon successful maintenance of behavior patterns (including facial expressions), that signal cooperation while individuals act deceitfully in their own self-interest. For example, the maintenance of friendly facial expression, followed by negative actions toward others, constitutes the deceptive use of a social script, facial expressions associated with affiliative interaction.
Signal properties of facial displays
Facial expressions are usually thought of as intimate, based in dyads such as parent/infant interactions, or conversations. More attention, however, needs to be drawn to the differential properties of facial signals, such as their clarity across relatively large distances (as far as 45 m; Hager and Ekman, 1979) and from angles other than directly face-to-face. Although facial expressions are typically observed at close range, their signal properties may allow for accurate reading by individuals in the periphery (eavesdroppers). The detectability of signals is an important factor in their ability to provide positive fitness consequences for signalers (Endler, 1993). A comparison between the distance of facial expression recognition and average social distance in other species would be interesting in this regard.
The functions of facial expression
By using these adaptive frameworks, and clarifying phenotype, ecological context, and fitness consequences of facial signaling, it becomes possible to investigate facial expression from an evolutionary perspective (Fridlund, 1994). Both social and emotional expressive functions of facial expression can be investigated from an evolutionary perspective and represent different levels of analysis (Hauser, 1996, p.495; see Jakobs et al., 1999, for a discussion of this issue from the psychological perspective). Fitness consequences are particularly hard to detect, especially given the cross-sectional nature of many psychological studies of facial expression. A single facial expression, with few exceptions, cannot be expected to provide a dramatic fitness benefit or cost. Rather, repeated signaling of intention or emotion is probably the biggest contributor to fitness accruing from facial expression.
In addition, the critical factor in the fitness of a particular expression such as a smile may be the difference between it and the average smile of the actor. Psychological studies generally take a cross-sectional approach, measuring only a few minutes of expression in a large number of individuals. Longitudinal studies of facial expression are few and far between (but see Messinger et al., 1999). Still, we can begin to get a picture of the importance of facial expression to fitness by considering some of the results of facial expression research.
PHYLOGENETIC PERSPECTIVES ON FACIAL EXPRESSION
Homology in facial expressions
Game theoretic models of facial signaling assume the actors have the alternative of not signaling at all, and may refer to a past generation where signaling did not yet exist (Bradbury and Vehrencamp, 2000). Although these issues are theoretically important, the phylogeny of humans and the long prehistory of sociality in the Primate order make it somewhat unlikely that signaling with human facial expression would disappear completely in humans. This is immediately apparent when considering the drastic and deeply damaging social consequences of facial paralysis (VanSwearingen et al., 1999). These and other forms of complete facial paralysis have such negative social consequences that it is difficult to imagine the lack of facial signaling as an alternative. In some cases, the amount of depression associated with facial paralysis is directly related to the degree of disability in producing a prototypical smile (VanSwearingen et al., 1999). It remains to be demonstrated, however, whether human facial expressions function adaptively, or whether they are simply remnants of a prelinguistic past (Darwin, 1872/1998).
Clearly, though, there are differences in the frequency and intensity of facial expression across normal individuals. Facial expressions are not always produced when they would be advantageous, and this may lead to negative fitness consequences that are less dramatic than those discussed above, but still potentially costly in social interaction, depending on cultural and social context. Negative fitness consequences here are conceptualized as reduced access to cooperative relationships that tend to enhance survival and reproductive potential. Given the long history of sociality in our lineage and the ubiquity of facial expression in observations of naturalistic social interaction, we hypothesize that a certain level of facial expression must be obtained, or the individual risks losing the fitness benefits acquired during earlier interactions.
The homology of human and nonhuman primate facial expression may illustrate continuity among facial expression phenotypes, especially between apes and humans. Preuschoft and van Hooff (1995) note the remarkable stereotypy of facial expression across an order that is usually known for its behavioral flexibility. Production of basic facial expressions, such as the fear grimace in rhesus macaques, also appears to be highly canalized (Geen, 1992). It would be surprising if the level of stereotypy and homology in nonhuman primate expression did not extend to at least some patterns of human expression.
Particular expressions, notably the silent bared teeth and relaxed open mouth displays, have been compared to the human smile and laughter (Chevalier-Skolnikoff, 1973; Darwin, 1872/1998; Preuschoft, 1992; Preuschoft and van Hooff, 1995; van Hooff, 1972). Given the similar structure of the facial muscles and adaptations for focusing on the face, homology between nonhuman primate and human facial expressions has been suggested (van Hooff, 1972). The smile has been proposed as a homologue to the silent bared teeth display (SBT), and the laugh as homologue to relaxed open mouth displays of monkeys and apes (van Hooff, 1972; Preuschoft, 1992; Preschoft and van Hooff, 1995). Preuschoft (2000) suggests that the apparent contrast between the smile of humans (upward lip corners) and the SBT of nonhuman primates may simply be the result of similar muscles stretched over a very different-shaped muzzle (Preuschoft, 2000; see Fig. 6).
Fig. 6.

Homologous displays in human and nonhuman primates. a: Rhesus macaque submissive display. Photograph by Frans DeWaal, 1989.(Silent bared teeth display.) b: Human smile. From Kanade et al.,2000. (Silent bared teeth display.) c: Bonobo play face. Photograph by Frans DeWaal, 1988. (Relaxed open mouth display.) d: Human play face, from Forbes et al., 2000. (Relaxed open mouth display.)
In addition, there are common neurobiological bases among humans and other primates in the control of facial expression. A recent study of projections from the cortex to the facial nerve nucleus in rhesus macaques found a pattern similar to that described by Rinn (1984). Bilateral cortical projections to facial nuclei control frontalis and orbicularis oculi muscles, and contralateral projections to the opposite facial nucleus, control the muscles around the mouth, allowing hemispheric differences in expressiveness to influence the lower face in particular (Morecraft et al., 2001). Researchers have begun to demonstrate asymmetry in facial expressions of monkeys, indicating that these underlying mechanisms of control also produce similar effects in non-human primates. Hook-Costigan and Rogers (1998) found that positive social calls and expressions of marmosets were lateralized to the right, while negative social expressions (fear) were lateralized to the left. Hauser and Akre (2001) also found that expressions were seen earlier on the left side of the face in rhesus macaques, although they found no different between expressions interpreted as socially positive or negative, expanding on the original finding by Hauser (1996) that rhesus expressions were left-sided.
Although at least some degree of neurobiological and physical homology of expression seems likely, there is also the problem of differential function. Can human smiles really be homologous to the SBT display when human smiles signal joy and nonhuman primate smiles signal appeasement or fear? From the perspective of human facial expression, the roles of the smile continue to expand with new research, and the equation of human smiles with happiness has been called into question (Ekman, 1985; Fridlund, 1994). Nonhuman primate bared teeth displays (as in Fig. 6) may be similar in meaning and function to human smiles, depending on the socioecological context.
Human anger expressions and ape anger/fear expressions may also be homologous (Chevalier-Skolnikoff, 1973). Embarrassment has been proposed as a homologue of primate appeasement displays, because it shares characteristics such as withdrawal, minimizing appearance, and smile with downward glance (Keltner and Buswell, 1997). Homology has also been proposed for the yawn, which shows evidence of being a social signal of transition between activity states both in humans and in non-human primates (Deputte, 1994; Provine, 1997).
Homology may also apply to aspects of hiding or minimizing expression. There are several examples of apes preventing conspecifics from seeing their expression by covering the face with a hand (Tomasello and Call, 1997), and one report of a chimp that was able to control a grimacing mouth by pressing down on his lips with his hand (Mitchell, 1999). Humans also move their hands to the face to hide expressions, possibly because such expressions would be detrimental to their interests if openly recognized (Keltner and Buswell, 1997) for embarrassment; see Provine (1997) for yawning. Humans have the additional ability of hiding their expressions with the actions of other facial muscles, especially around the mouth where the downward pull of the depressor anguli oris muscle can somewhat conceal the rise of the lip corners due to the action of zygomaticus major (Ekman, 1985). Spontaneous expressions may have elements of concealment associated with them, such as “twisting” the smile to avoid appearing too pleased in front of others (Friedman and Miller-Herringer, 1991). In the same study, the authors noted that hand contact increased with increased smiling. It could be that the action of other facial muscles in some cases was inadequate to the task of concealing the increasingly obvious smile signal.
Consideration of nonhuman primate facial expression, of course, is necessarily be specific to each individual species. Divergent facial signaling systems, as well as some homology, are to be expected. For the relationship between overall amount of expression and the structure of the facial nerve nucleus, Peburn et al. (2000) found variation between species, with the relatively more expressive macaques (Macaca fascicularis) showing significantly larger and better defined facial subnuclei than the relatively less expressive patas monkeys (Erythrocebus patas). Aotus, the only nocturnal anthropoid, has virtually no facial expression, and its facial musculature is also relatively less differentiated (Chevalier-Skolnikoff, 1973; Huber, 1931).
Likewise, many features of human facial expression, although universal, are specific to our species. The relative hairlessness of the human face, compared to the majority of nonhuman primates, suggests that the combination of exposed skin and retained hair may have some signaling value. Brows are an important component of human greeting and surprise displays, as well as some smiles. Upturned inner brows also signal sadness or grief (Eibl-Eibesfeldt, 1989; Ekman, 1979). Results of an intriguing study in which cross-species perception of facial expression was tested, the brows, a highly visible signal on the hairless human face, are not used by macaques seeking to identify sad human facial expressions. They rely instead on other, more phylogenetically conserved traits, such as cheek movement (Kanazawa, 1998). Retention of facial hair in adult male humans may also play a role in accentuating or concealing expression. Unfortunately, there has been no research on male facial hair as it relates to facial expression.
Functions of facial expressions in nonhuman primates
Like humans, many nonhuman primate species pay close attention to the faces of other group members. The complexity of face perception and facial expression among the great apes, for example, is just beginning to be demonstrated. Chimpanzees can use facial features to recognize both kin and unfamiliar conspecifics, and to categorize facial expressions of other chimpanzees (Parr et al., 1998, 2000; Parr and deWaal, 1999). Chimpanzees can follow the gaze of another individual by paying attention to its face (Povinelli and Eddy, 1996). Gorillas, chimpanzees, and bonobos have all been reported to monitor the gaze of others, in ways that suggest they may be able to anticipate the impact of their facial expressions on others’ behavior (Mitchell, 1999). Clearly, facial expressions are important signals in the social lives of most nonhuman primates, just as they are for humans.
By monitoring facial expressions, the intentions of others can be inferred. Because of their relatively stable existence, social groups provide an environment in which low-cost, redundant signals can help primates to keep track of the other individual, possibly similar to that which Chadwick-Jones (1998) described for baboons. Cheater males may fail to give continuous signals, and so must be monitored. The failure to adhere to social scripts, including facial expression patterns, draws attention to uncooperative individuals (Mitchell, 1999). Groups of nonhuman primates may also utilize redundant, “cheap” vocal signals in order to coordinate their activities (Silk et al., 2000). Haslam (1997) provided a list of social grammars for primate interaction, including communal sharing, authority ranking, equality matching, and market pricing. These are behavioral contexts of the type that could correspond to functional variation in primate facial expression. As in humans, considering facial expressions as adaptations would require specification of relevant phenotypes, ecological contexts, and positive and negative fitness consequences.
CONCLUSIONS
Current functional approaches to facial expression are largely framed in terms of the proximate functions of emotions associated with that expression (Keltner and Gross, 1999; Keltner and Haidt, 1999; Yik and Russell, 1999). Fridlund (1994) has argued from a critical psychological perspective for the consideration of all facial behavior in a behavioral ecological or evolutionary framework. Evolutionary approaches, however, are necessarily based on the study of facial expressions as biological adaptations, and much of the recent work on facial expression has been conducted outside this perspective. Nevertheless, results from this type of functional study of facial expression provide a basis on which to base evolutionary hypotheses of facial behavior. By detailing the phenotypic variation in facial expression, clearly defining the ecological contexts of facial expression, and describing some of the positive fitness consequences of these behaviors, we have outlined an evolutionary approach to the study of human facial expressions as adaptations.
By proposing testable hypotheses about the nature of facial expression, we can also provide a firm biological basis to other anthropological studies of communication. The unfortunate, and probably unintended, view of nonverbal expression as representing an ancestral-primate primitive ability, while language is uniquely specialized in humans, can also be challenged from this perspective (Eibl-Eibesfeldt, 1989). Instead of just assuming that non-verbal expression has much in common with primate relatives, but is also culturally patterned, we may be in a position to specify more clearly the adaptive role of facial expression in human social interaction.
Human social intelligence is obviously a major contributor to human brain evolution and intellectual abilities. Our most specialized and adaptive system of social signaling is human language (Pinker, 1994). Remarkable in its complexity and also because of its seeming discontinuity with non-human primate vocalization and communication, human language has clear adaptive consequences for human evolution (Burling, 1993; Pinker, 1994). There is no doubt that language has been a driving force in the evolution of human behavior, and the social context is dominated by language (Dunbar, 1996). Yet there is more to social intelligence than language. Deficits in other social skills, including nonverbal skills, make it very clear that language alone is not sufficient for successful social interaction, and the positive consequences of these skills are only now being described. This argues for increased attention to the evolution of nonverbal signaling systems, including facial expression, from the perspective of physical anthropology.
Acknowledgments
We thank John S. Allen and Melissa Panger for careful reading and helpful criticism of this article. This research was supported by NIH grant MH12579 to K. Schmidt and by NIH grant MH56193 to J. Cohn.
Footnotes
Notwithstanding Fridlund’s compelling arguments concerning the use of the alternate term “facial display,” to describe facial actions, this review will rely on the older term, “facial expression” for the sake of simplicity. No emotional input is necessarily implied by the use of the word expression.
Contempt and embarrassment have also been proposed as universal facial expressions of emotion, with varying degrees of universality in expression recognition reported (Haidt and Keltner, 1999).
Adaptive explanations of facial expressions as emotions are based primarily on the adaptiveness of experiencing emotions. Expressing emotion is an important part of signaling hypotheses of expression. The exploration of relationships between internally experienced emotion and facial expression is an important part of adaptive hypotheses of emotions, but the link between expressions of emotion and fitness consequences does not necessarily require that other adaptive consequences of that emotion be explained.
LITERATURE CITED
- Adolphs R. Social cognition and the human brain. Trends Cogn Sci. 1999;3:469–479. doi: 10.1016/s1364-6613(99)01399-6. [DOI] [PubMed] [Google Scholar]
- Alexander RD. The biology of moral systems. Hawthorne, NY: Aldine deGruyter; 1987. [Google Scholar]
- Andrew RJ. The origin and evolution of the calls and facial expressions of the primates. Behaviour. 1962;20:1–107. [Google Scholar]
- Bavelas JB, Chovil N. Faces in dialogue. In: Russell JA, Fernandez-Dols JM, editors. The psychology of facial expression. New York: Cambridge University Press; 1997. pp. 334–346. [Google Scholar]
- Belsky J, Nezworski T. Clinical implications of attachment. In: Belsky J, Nezworski T, editors. Clinical implications of attachment. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. pp. 3–17. [Google Scholar]
- Bergstrom CT, Lachmann M. Signaling among relatives. III. Talk is cheap. Proc Natl Acad Sci USA. 1998;95:5100–5105. doi: 10.1073/pnas.95.9.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdwhistell RL. Kinesics and context. Philadelphia: University of Pennsylvania Press; 1970. [Google Scholar]
- Birdwhistell RL. Background considerations to the study of the body as a medium of “expression”. In: Benthall J, Polhemus T, editors. The body as a medium of expression. New York: E.P. Dutton and Co., Inc; 1975. pp. 36–58. [Google Scholar]
- Blair R, Morris J, Frith C, Perrett D, Dolan R. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122:883–893. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Blurton Jones NG. Non-verbal communication in children. In: Hinde RA, editor. Non-verbal communication. New York: Cambridge University Press; 1972. pp. 271–295. [Google Scholar]
- Borod JC, Koff E, Yecker S, Santschi C, Schmidt JM. Facial asymmetry during emotional expression: gender, valence and measurement technique. Psychophysiology. 1998;36:1209–1215. doi: 10.1016/s0028-3932(97)00166-8. [DOI] [PubMed] [Google Scholar]
- Boyatzis CJ, Chazan E, Ting CZ. Preschool children’s decoding of facial emotions. J Genet Psychol. 1993;154:375–382. doi: 10.1080/00221325.1993.10532190. [DOI] [PubMed] [Google Scholar]
- Bradbury JW, Vehrencamp SL. Principles of animal communication. Sunderland, MA: Sinauer Associates, Inc; 1998. [Google Scholar]
- Bradbury JW, Vehrencamp SL. Economic models of animal communication. Anim Behav. 2000;59:259–268. doi: 10.1006/anbe.1999.1330. [DOI] [PubMed] [Google Scholar]
- Briton NJ, Hall JA. Gender-based expectancies and observer judgments of smiling. J Nonverb Behav. 1995;19:49–65. [Google Scholar]
- Brothers L. A biological perspective on empathy. Am J Psychiatry. 1989;146:10–19. doi: 10.1176/ajp.146.1.10. [DOI] [PubMed] [Google Scholar]
- Brothers L. Friday’s footprint: how society shapes the human mind. New York: Oxford University Press; 1997. [Google Scholar]
- Brothers L, Ring B, Kling A. Response of neurons in the macaque amygdala to complex social stimuli. Behav Brain Res. 1990;41:199–213. doi: 10.1016/0166-4328(90)90108-q. [DOI] [PubMed] [Google Scholar]
- Brown D. Human universals. New York: McGraw-Hill, Inc; 1990. [Google Scholar]
- Brown WM, Moore C. Is prospective altruist-detection an evolved solution to the adaptive problem of subtle cheating in cooperative ventures? Supportive evidence using the Wason selection task. Evol Hum Behav. 2000;21:25–37. [Google Scholar]
- Brunner LJ. Smiles can be back channels. J Pers Soc Psychol. 1979;37:728–734. [Google Scholar]
- Buck R, Ginsburg B. Communicative genes and the evolution of empathy. In: Ickes W, editor. Empathic accuracy. New York: Guilford Press; 1997. pp. 17–43. [Google Scholar]
- Bugental DB. Unmasking the “polite smile”: situational and personal determinants of managed affect in adult-child interaction. Pers Soc Psychol Bull. 1986;12:7–16. [Google Scholar]
- Burling R. Primate calls, human language, and nonverbal communication. Curr Anthropol. 1993;34:25–53. [Google Scholar]
- Byrne RW. The ape legacy: the evolution of Machiavellian intelligence and anticipatory interactive planning. In: Goody EN, editor. Social intelligence and interaction. New York: Cambridge University Press; 1995. pp. 37–52. [Google Scholar]
- Campbell R, Woll B, Benson PJ, Wallace SB. Categorical perception of face actions: their role in sign language and in communicative facial displays. Q J Exp Psychol [A] 1999;52:67–95. doi: 10.1080/713755802. [DOI] [PubMed] [Google Scholar]
- Cashdan E. Smiles, speech, and body posture: how women and men display sociometric status and power. J Nonverb Behav. 1998;22:209–228. [Google Scholar]
- Castanho AP, Otta E. Decoding spontaneous and posed smiles of children who are visually impaired and sighted. J Vis Impair Blind. 1999;93:659–662. [Google Scholar]
- Celani G, Battacchi M, Arcidiacono L. The understanding of the emotional meaning of facial expressions in people with autism. J Autism Dev Disord. 1999;29:57–66. doi: 10.1023/a:1025970600181. [DOI] [PubMed] [Google Scholar]
- Chadwick-Jones J. Developing a social psychology of monkeys and apes. East Sussex, UK: Psychology Press, Ltd; 1998. [Google Scholar]
- Chapell MS. Frequency of smiling across the life span. Percept Mot Skills. 1997;85:1326. doi: 10.2466/pms.1997.85.3f.1326. [DOI] [PubMed] [Google Scholar]
- Chevalier-Skolnikoff S. Facial expression of emotion in nonhuman primates. In: Ekman P, editor. Darwin and facial expression. New York: Academic Press; 1973. pp. 11–90. [Google Scholar]
- Cheyne JA. Development of forms and functions of smiling in preschoolers. Child Dev. 1976;47:820–823. [Google Scholar]
- Chovil N. Facing others: a social communicative perspective on facial displays. In: Russell JA, Fernandez-Dols JM, editors. The psychology of facial expression. New York: Cambridge University Press; 1997. pp. 321–333. [Google Scholar]
- Coats EJ, Feldman RS. Gender differences in nonverbal correlates of social status. Pers Soc Psychol Bull. 1996;22:1014–1022. [Google Scholar]
- Cohn JF, Elmore M. Effect of contingent changes in mothers’ affective expression on the organization of behavior in 3-month-old infants. Infant Behav Dev. 1988;11:493–505. [Google Scholar]
- Cohn JF, Tronick EZ. Three-month-old infants’ reaction to simulated maternal depression. Child Devel. 1983;54:185–193. [PubMed] [Google Scholar]
- Cohn JF, Tronick EZ. Mother-infant interaction: the sequence of dyadic states at three, six, and nine months. Dev Psychol. 1987;23:68–77. [Google Scholar]
- Cohn JF, Zlochower A, Lien J, Kanade T. Automated face analysis by feature point tracking has high concurrent validity with manual FACS coding. Psychophysiology. 1999;36:35–43. doi: 10.1017/s0048577299971184. [DOI] [PubMed] [Google Scholar]
- Cosmides L, Tooby J, Barkow J. Evolutionary psychology and conceptual integration. In: Cosmides L, Tooby J, Barkow J, editors. The adapted mind. New York: Oxford University Press; 1992. pp. 3–18. [Google Scholar]
- Critchley H, Daly E, Phillips M, Brammer M, Bullmore E, Williams S, Van Amelsvoort T, Robertson D, David A, Murphy D. Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Hum Brain Mapp. 2000;9:93–105. doi: 10.1002/(SICI)1097-0193(200002)9:2<93::AID-HBM4>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. In: The expression of the emotions in man and animals. 3. Ekman P, editor. New York: Oxford University Press; 18721998. [Google Scholar]
- Dawkins MS. Are there general principles of signal design? Philos Trans R Soc Lond [Biol] 1993;340:251–255. doi: 10.1098/rstb.1993.0065. [DOI] [PubMed] [Google Scholar]
- deJong PJ. Communicative and remedial effects of social blushing. J Nonverb Behav. 1999;23:197–217. [Google Scholar]
- DePaulo BM. Nonverbal behavior and self-presentation. Psychol Bull. 1992;111:203–243. doi: 10.1037/0033-2909.111.2.203. [DOI] [PubMed] [Google Scholar]
- Deputte BL. Ethological study of yawning in primates: quantitative analysis and study of causatiion in 2 species of Old-World monkeys (Cercopithecus albigena and Macaca fascicularis) Ethology. 1994;98:221–245. [Google Scholar]
- DeWaal FBM. The communicative repertoire of captive bonobos(Pan paniscus), compared to that of chimpanzees. Behaviour. 1988;106:183–251. [Google Scholar]
- DeWaal FBM. Peacemaking among primates. Cambridge, MA: Harvard University3 Press; 1989. [Google Scholar]
- Dimberg U. Psychophysiological reactions to facial expressions. In: Segerstrale U, Molnar P, editors. Nonverbal communication: where nature meets culture. Mahwah, NJ: Lawrence Erlbaum Associates; 1997. pp. 27–60. [Google Scholar]
- Duchenne GBA. The mechanism of human facial expression. New York: Cambridge University Press; 18591990. [Google Scholar]
- Dunbar R. Gossip, grooming and the evolution of human language. Cambridge, MA: Harvard University Press; 1996. [Google Scholar]
- Dunbar R. The social brain hypothesis. Evol Anthropol. 1998;6:178–190. [Google Scholar]
- Dworkin RH. Affective deficits and social deficits in schizophrenia: what’s what? Schizophr Bull. 1992;18:59–64. doi: 10.1093/schbul/18.1.59. [DOI] [PubMed] [Google Scholar]
- Eibl-Eibesfeldt I. Human ethology. New York: Aldine de Gruyter; 1989. [Google Scholar]
- Ekman P. Cross-cultural studies of facial expression. In: Ekman P, editor. Darwin and facial expression. New York: Academic Press; 1973. pp. 169–222. [Google Scholar]
- Ekman P. About brows: emotional and conversational signals. In: von Cranach M, Foppa K, Lepenies W, Ploog D, editors. Human ethology: claims and limits of a new discipline. New York: Cambridge University Press; 1979. pp. 169–222. [Google Scholar]
- Ekman P. Telling lies: clues to deceit in the marketplace, politics, and marriage. New York: W.W. Norton; 1985. [Google Scholar]
- Ekman P, Friesen WV. Facial action coding system. Palo Alto: Consulting Psychologists Press; 1978. [Google Scholar]
- Ekman P, Friesen W. False, felt, and miserable smiles. J Nonverb Behav. 1982;6:238–252. [Google Scholar]
- Ekman P, Keltner D. Universal facial expressions of emotion: an old controversy and new findings. In: Segerstrale U, Molnar P, editors. Nonverbal communication: where nature meets culture. Mahwah, NJ: Lawrence Erlbaum Associates; 1997. pp. 27–46. [Google Scholar]
- Ekman P, Rosenberg E. What the Face Reveals. New York: Oxford University Press; 1997. [Google Scholar]
- Ekman P, Friesen WV, O’Sullivan M. Smiles when lying. In: Ekman P, Rosenberg E, editors. What the face reveals. New York: Oxford University Press; 1997. pp. 201–216. [Google Scholar]
- Ellis HD, Young AW. Faces in their biological and social context. In: Young AW, editor. Face and mind. New York: Oxford University Press; 1998. pp. 67–95. [Google Scholar]
- Endler JA. Some general comments on the evolution and design of animal communication systems. Philos Trans R Soc Lond [Biol] 1993;340:215–225. doi: 10.1098/rstb.1993.0060. [DOI] [PubMed] [Google Scholar]
- Fessler DMT. Toward an understanding of the universality of second order emotions: biocultural approaches to the emotions. New York: Cambridge University Press; 1999. pp. 75–116. [Google Scholar]
- Floyd K, Burgoon JK. Reacting to nonverbal expressions of liking: a test of interaction adaptation theory. Commun Monogr. 1999;66:219–239. [Google Scholar]
- Forbes E, Cohn JF, Lewinsohn P, Moore GA. Mother-father differences in parent and infant affect. International Conference on Infant Studies; Brighton, England. 2000. [Google Scholar]
- Frank MG, Ekman P. The ability to detect deceit generalizes across different types of high-stake lies. J Pers Soc Psychol. 1997;72:1429–1429. doi: 10.1037//0022-3514.72.6.1429. [DOI] [PubMed] [Google Scholar]
- Frank MG, Ekman P, Friesen WV. Behavioral markers and recognizability of the smile of enjoyment. J Pers Soc Psychol. 1993;64:83–93. doi: 10.1037//0022-3514.64.1.83. [DOI] [PubMed] [Google Scholar]
- Fridlund A. Human facial expression: an evolutionary view. New York: Academic Press; 1994. [Google Scholar]
- Fridlund AJ. The new ethology of human facial expressions. In: Russell J, Fernandez-Dols JM, editors. The psychology of facial expression. New York: Cambridge University Press; 1997. pp. 103–129. [Google Scholar]
- Friedman H, Miller-Herringer T. Nonverbal display of emotion in public and in private: self-monitoring, personality, and expressive cues. J Pers Soc Psychol. 1991;61:766–775. doi: 10.1037//0022-3514.61.5.766. [DOI] [PubMed] [Google Scholar]
- Galati D, Scherer KR, Ricci-Bitti PE. Voluntary facial expression of emotion comparing congenitally blind with normally sighted encoders. J Pers Soc Psychol. 1997;73:1363–1379. doi: 10.1037//0022-3514.73.6.1363. [DOI] [PubMed] [Google Scholar]
- Gangestad SW, Thornhill R. Human sexual selection and developmental stability. In: Simpson JA, Kenrick DT, editors. Evolutionary social psychology. Mahwah, NJ: Lawrence Erlbaum Associates; 1997. pp. 169–196. [Google Scholar]
- Gazzaniga MS, Smylie CS. Hemispheric mechanisms controlling voluntary and spontaneous facial expressions. J Cogn Neurosci. 1990;2:239–245. doi: 10.1162/jocn.1990.2.3.239. [DOI] [PubMed] [Google Scholar]
- Geen TR. Facial expressions in socially isolated nonhuman primates: open and closed programs for expressive behavior. J Res Pers. 1992;26:273–280. [Google Scholar]
- Godfray HCJ, Johnstone RA. Begging and bleating: the evolution of parent-offspring signaling. Philos Trans R Soc Lond [Biol] 2000;355:1581–1591. doi: 10.1098/rstb.2000.0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall J. The chimpanzees of Gombe. Cambridge, MA: Cambridge University Press; 1986. [Google Scholar]
- Goodmurphy C, Ovalle W. Morphological study of two human facial muscles: orbicularis oculi and corrugator supercilii. Clin Anat. 1999;12:1–11. doi: 10.1002/(SICI)1098-2353(1999)12:1<1::AID-CA1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Gosselin P, Kirouac G, Dore FY. Components and recognition of facial expression in the communication of emotion by actors. In: Ekman P, Rosenberg E, editors. What the face reveals. New York: Oxford University Press; 1997. pp. 243–270. [Google Scholar]
- Grafen A. Biological signals as handicaps. J Theor Biol. 1990;144:517–546. doi: 10.1016/s0022-5193(05)80088-8. [DOI] [PubMed] [Google Scholar]
- Grafen A, Johnstone RA. Why we need ESS signalling theory. Philos Trans R Soc Lond [Biol] 1993;340:245–250. doi: 10.1098/rstb.1993.0064. [DOI] [PubMed] [Google Scholar]
- Grammer K, Thornhill R. Human (Homo sapiens) facial attractiveness and sexual selection: the role of symmetry and averageness. J Comp Psychol. 1994;108:233–242. doi: 10.1037/0735-7036.108.3.233. [DOI] [PubMed] [Google Scholar]
- Grammer K, Schiefenhovel W, Schleidt M, Lorenz B, Eibl-Eibesfeldt I. Patterns on the face: the eyebrow flash in crosscultural comparison. Ethology. 1988;77:279–299. [Google Scholar]
- Grammer K, Filova V, Fieder M. The communication paradox and possible solutions: towards a radical empiricism. In: Schmitt A, Atzwanger K, Grammer K, Schaefer K, editors. New aspects of human ethology. New York: Plenum Press; 1997. pp. 91–120. [Google Scholar]
- Grammer K, Honda M, Juette A, Schmitt A. Fuzziness of nonverbal courtship communication unblurred by motion energy detection. J Pers Soc Psychol. 1999;77:487–508. doi: 10.1037//0022-3514.77.3.487. [DOI] [PubMed] [Google Scholar]
- Hager JC, Ekman P. Long-distance transmission of facial affect signals. Ethol Sociobiol. 1979;1:77–82. [Google Scholar]
- Hager JC, Ekman P. The asymmetry of facial actions is inconsistent with models of hemispheric specialization. In: Ekman P, Rosenberg E, editors. What the face reveals. New York: Oxford University Press; 1997. pp. 40–62. [Google Scholar]
- Haidt J, Keltner D. Culture and facial expression: open-ended methods find more expressions and a gradient of recognition. Cogn Emot. 1999;13:225–266. [Google Scholar]
- Hall JA. Nonverbal sex differences: communication accuracy and expressive style. Baltimore: Johns Hopkins University Press; 1984. [Google Scholar]
- Haslam N. Four grammars for primate social relations. In: Simpson JA, Kenrick DT, editors. Evolutionary social psychology. Mahwah, NJ: Lawrence Erlbaum Associates; 1997. pp. 297–316. [Google Scholar]
- Hauser MD. The evolution of communication. Cambridge, MA: MIT Press; 1996. [Google Scholar]
- Hauser MD, Akre K. Asymmetries in the timing of facial and vocal expressions by rhesus monkeys: implications for hemispheric. specialization. Anim Behav. 2001;61:391–400. [Google Scholar]
- Hess U, Banse R, Kappas A. The intensity of facial expression is determined by underlying affective state and social situation. J Pers Soc Psychol. 1995;69:280–288. [Google Scholar]
- Hietanen JK, Surakka V, Linnankoski I. Facial electromyographic responses to vocal affect expressions. Psychophysiology. 1998;35:530–536. doi: 10.1017/s0048577298970445. [DOI] [PubMed] [Google Scholar]
- Hook-Costigan MA, Rogers LJ. Lateralized use of the mouth in production of vocalizations by marmosets. Neuropsychologia. 1998;36:1265–1273. doi: 10.1016/s0028-3932(98)00037-2. [DOI] [PubMed] [Google Scholar]
- Huber E. Evolution of facial musculature and facial expression. Baltimore: The Johns Hopkins Press; 1931. [Google Scholar]
- Humphrey NB. The social function of intellect. In: Bateson PPG, Hinde RA, editors. Growing points in ethology. Cambridge: Cambridge University Press; 1976. pp. 303–317. [Google Scholar]
- Izard CE. Human emotion. New York: Plenum Press; 1977. [Google Scholar]
- Izard CE, Malatesta CZ. Perspectives on emotional development: Differential emotions theory of early emotional development. In: Osofsky JD, editor. Handbook of infant development. New York: Wiley and Sons; 1987. pp. 494–554. [Google Scholar]
- Jakobs E, Manstead ASR, Fischer AH. Social motives and emotional feelings as determinants of facial displays: the case of smiling. Pers Soc Psychol Bull. 1999;25:424–435. [Google Scholar]
- Jancke L, Kaufmann N. Facial EMG responses to odors in solitude and with an audience. Chem Senses. 1994;19:99–111. doi: 10.1093/chemse/19.2.99. [DOI] [PubMed] [Google Scholar]
- Johnstone RA. The evolution of animal signals. In: Krebs JR, Davies NB, editors. Behavioural ecology: an evolutionary approach. London: Blackwell Science, Ltd; 1997. pp. 155–178. [Google Scholar]
- Jones S. Adult recipients of infant communications: a sex difference in the salience of early “social” smiling. Infant Behav Dev. 1984;7:211–221. [Google Scholar]
- Jones SS, Raag T, Collins KL. Smiling in older infants: form and maternal response. Infant Behav Dev. 1990;13:147–165. [Google Scholar]
- Jones S, Collins K, Hong H-W. An audience effect on smile production in 10-month-old infants. Psychol Sci. 1991;2:45–49. [Google Scholar]
- Kanade T, Cohn JF, Tian Y. Comprehensive database for facial expression analysis. Proc Fourth IEEE International Conference on Automatic Face Gesture Recognition (FG’00) Grenoble; France. March 2000; 2000. pp. 46–53. [Google Scholar]
- Kanazawa S. What facial part is important for Japanese monkeys (Macaca fuscata) in recognition of smiling and sad faces of humans (Homo sapiens) J Comp Psychol. 1998;112:363–370. [Google Scholar]
- Katsikitis M, Pilowsky I, Innes JM. Encoding and decoding of facial expression. J Gen Psychol. 1997;124:357–370. [Google Scholar]
- Keltner D. Signs of appeasement: evidence for the distinct displays of embarrassment, amusement and shame. In: Ekman P, Rosenberg E, editors. What the face reveals. New York: Oxford University Press; 1997. pp. 133–160. [Google Scholar]
- Keltner D, Bonanno GA. A study of laughter and dissociation: distinct correlates of laughter and smiling during bereavement. J Pers Soc Pscyhol. 1997;73:687–702. doi: 10.1037//0022-3514.73.4.687. [DOI] [PubMed] [Google Scholar]
- Keltner D, Buswell BN. Embarrassment: its distinct form and appeasement functions. Psychol Bull. 1997;122:250–270. doi: 10.1037/0033-2909.122.3.250. [DOI] [PubMed] [Google Scholar]
- Keltner D, Gross JJ. Functional accounts of emotions. Cogn Emot. 1999;13:467–480. [Google Scholar]
- Keltner D, Haidt J. Social functions of emotions at four levels of analysis. Cogn Emot. 1999;13:505–521. [Google Scholar]
- Keltner D, Harker L. The forms and functions of the non-verbal signal of shame. In: Gilbert P, Andrews B, editors. Shame: interpersonal behavior, psychopathology, and culture. New York: Oxford University Press; 1998. pp. 78–98. [Google Scholar]
- Keltner D, Moffitt TE, Stouthamer-Loeber M. Facial expressions of emotion and psychopathology in adolescent boys. In: Ekman P, Rosenberg E, editors. What the face reveals. New York: Oxford University Press; 1997. pp. 434–452. [Google Scholar]
- Kraut RE, Johnson RE. Social and emotional messages of smiling: an ethological approach. J Pers Soc Psychol. 1979;37:1539–1553. [Google Scholar]
- Krebs JR, Dawkins R. Animal signals: mind-reading and manipulation. In: Krebs JR, Davies NB, editors. Behavioural ecology. London: Blackwell Scientific Publications; 1984. pp. 380–402. [Google Scholar]
- Kummer H, Daston L, Gigerenzer G, Silk J. The social intelligence hypothesis. In: Weingart P, Mitchell S, Richerson P, Maasen S, editors. Human by nature: between biology and the social sciences. Mahwah, NJ: Lawrence Erlbaum Associates; 1997. pp. 157–179. [Google Scholar]
- Kupperbusch C, Matsumoto D, Kooken K, Loewinger S, Uchida H, Wilson-Cohn C, Yrizarry N. Cultural influences on nonverbal expressions of emotion. In: Philippot P, Feldman RS, Coats EJ, editors. The social context of nonverbal behavior. New York: Cambridge University Press; 1999. pp. 17–44. [Google Scholar]
- LaBarre W. The cultural basis of emotions and gestures. J Pers. 1947;16:49–68. [Google Scholar]
- LaFrance M, Hecht MA. Option or obligation to smile: the effects of power and gender on facial expression. In: Philippot P, Feldman RS, Coats EJ, editors. The social context of non-verbal behavior. New York: Cambridge University Press; 1999. pp. 45–70. [Google Scholar]
- Leonard CM, Voeller KKS, Kuldau JM. When’s a smile a smile? Or how to detect a message by digitizing the signal. Psychol Sci. 1991;2:166–172. [Google Scholar]
- Levenson RW, Ekman P, Friesen W. Voluntary facial action generates emotion-specific autonomic nervous system activity. Psychophysiology. 1990;27:363–384. doi: 10.1111/j.1469-8986.1990.tb02330.x. [DOI] [PubMed] [Google Scholar]
- Lyons MJ, Campbell R, Plante A, Coleman M, Kamachi M, Akamatsu S. The Noh mask effect: vertical viewpoint dependence of facial expression perception. Proc R Soc Lond Biol. 2000;267:2239–2245. doi: 10.1098/rspb.2000.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manstead ASR. Expressiveness as an individual difference. In: Feldman RS, Rime B, editors. Fundamentals of nonverbal behavior. New York: Cambridge University Press; 1991. pp. 285–328. [Google Scholar]
- Manstead ASR, Fischer AH, Jakobs EB. The social and emotional functions of facial displays. In: Philippot P, Feldman RS, Coats EJ, editors. The social context of nonverbal behavior. New York: Cambridge University Press; 1999. pp. 287–313. [Google Scholar]
- Massaro DW. Perceiving talking faces. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Matsumoto D, Kudoh T. American-Japanese cultural differences in attributions of personality based on smiles. J Non-verb Behav. 1993;17:231–243. [Google Scholar]
- McAlister R, Harkness E, Nicoll J. An ultrasound investigation of the lip levator musculature. Eur J Orthod. 1998;20:713–720. doi: 10.1093/ejo/20.6.713. [DOI] [PubMed] [Google Scholar]
- McClure EB. A meta-analytic review of sex differences in facial expression processing and their development in infants, children, and adolescents. Psychol Bull. 2000;126:424–453. doi: 10.1037/0033-2909.126.3.424. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN. The human infant as imitative generalist: a 20-year progress report on infant imitation with implications for comparative psychology. In: Heyes CM, Galef BM, editors. Social learning in animals: the roots of culture. San Diego, CA: Academic Press, Inc; 1996. pp. 347–370. [Google Scholar]
- Messinger D, Fogel A, Dickson KL. What’s in a smile? Dev Psychol. 1999;35:701–708. doi: 10.1037//0012-1649.35.3.701. [DOI] [PubMed] [Google Scholar]
- Mitchell RW. Deception and concealment as strategic script violation in great apes and humans. In: Parker ST, Mitchell RW, Miles HL, editors. The mentalities of gorillas and orangutans. New York: Cambridge University Press; 1999. pp. 295–315. [Google Scholar]
- Morecraft RJ, Louie JL, Herrick JL, Stilwell-Morecraft KS. Cortical innervation of the facial nucleus in the non-human primate—a new interpretation of the effects of stroke and related subtotal trauma on the muscles of facial expression. Brain. 2001;124:176–208. doi: 10.1093/brain/124.1.176. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ. A differential response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Mueser KT, Valentiner DP, Agresta J. Coping with negative symptoms of schizophrenia: patient and family perspectives. Schizophr Bull. 1997;23:329–339. doi: 10.1093/schbul/23.2.329. [DOI] [PubMed] [Google Scholar]
- Nagashima K, Schellenberg JA. Situational differences in intentional smiling: a cross-cultural exploration. J Soc Psychol. 1997;137:297–301. [Google Scholar]
- Niemitz C, Loi M, Landerer S. Investigations on human laughter and its implications for the evolution of hominid visual communication. Homo. 2000;51:1–18. [Google Scholar]
- Nowicki SJ, Duke MP. Individual differences in the non-verbal communication of affect: the diagnostic analysis of non-verbal accuracy scale. J Nonverb Behav. 1994;18:9–35. [Google Scholar]
- Otta E, Lira BBP, Delevati OP, Pires CSG. The effect of smiling and of head tilting on person perception. J Psychol. 1993;128:323–331. doi: 10.1080/00223980.1994.9712736. [DOI] [PubMed] [Google Scholar]
- Otta E, Abrosio FFE, Hoshino RL. Reading a smiling face: messages conveyed by various forms of smiling. Percept Mot Skills. 1996;82:1111–1121. doi: 10.2466/pms.1996.82.3c.1111. [DOI] [PubMed] [Google Scholar]
- Parr L, deWaal FBM. Visual kin recognition in chimpanzees. Nature. 1999;399:647–648. doi: 10.1038/21345. [DOI] [PubMed] [Google Scholar]
- Parr L, Hopkins WD, deWaal FBM. The perception of facial expressions by chimpanzees, Pan troglodytes. Evol Commun. 1998;2:1–24. [Google Scholar]
- Parr L, Winslow JT, Hopkins WD, deWaal FBM. Recognizing facial cues: Individual discrimination by chimpanzees (Pan troglodytes) and rhesus monkeys (Macaca mulatta) J Comp Psychol. 2000;114:47–60. doi: 10.1037/0735-7036.114.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peburn TA, Sheridan KA, Kheck NM, Hof PR, Gasdogas J, Erwin J, Gannon PJ. Differences in the brainstem facial motor nucleus in Erythrocebus patas and Macaca fascicularis: a qualitative and morphometric analysis. Am J Phys Anthropol [Suppl] 2000;30:247–248. [Google Scholar]
- Pennock JD, Johnson PC, Manders EK, VanSwearingen JM. Relationship between muscle activity of the frontalis and the associated brow displacement. Plast Reconstr Surg. 1999;104:1789. [PubMed] [Google Scholar]
- Pessa J, Zadoo V, Adrian EJ, Yuan C, Aydelotte J, Garza J. Variability of the midfacial muscles: analysis of 50 hemifacial cadaver dissections. Plast Reconstr Surg. 1998a;102:1888–1893. doi: 10.1097/00006534-199811000-00013. [DOI] [PubMed] [Google Scholar]
- Pessa J, Zadoo V, Garza P, Adrian EJ, Dewitt A, Garza J. Double or bifid zygomaticus major muscle: anatomy, incidence, and clinical correlation. Clin Anat. 1998b;11:310–313. doi: 10.1002/(SICI)1098-2353(1998)11:5<310::AID-CA3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Pike GE, Kemp RI, Towell NA, Phillips KC. Recognizing moving faces: the relative contribution of motion and perspective information. Vis Cogn. 1997;4:409–437. [Google Scholar]
- Pinker S. The language instinct. New York: Morrow; 1994. [Google Scholar]
- Povinelli DJ, Eddy TJ. Chimpanzees: joint visual attention. Psychol Sci. 1996;7:129–135. [Google Scholar]
- Preuschoft S. Laughter and smile in Barbary macaques (Macaca sylvanus) Ethology. 1992;91:220–236. [Google Scholar]
- Preuschoft S. Primate faces and facial expressions. Soc Res. 2000;67:245–271. [Google Scholar]
- Preuschoft S, van Hooff JARAM. Homologizing primate facial displays: a critical review of methods. Folia Primatol (Basel) 1995;65:121–137. doi: 10.1159/000156878. [DOI] [PubMed] [Google Scholar]
- Provine RR. Contagious yawning and laughter: signficance for sensory feature detection, motor pattern generation, imitation, and the evolution of social behavior. In: Heyes CM, Galef BG, editors. Social learning in animals: the roots of culture. San Diego, CA: Academic Press, Inc; 1996. pp. 179–208. [Google Scholar]
- Provine RR. Yawns, laughs, smiles, tickles, and talking: naturalistic and laboratory studies of facial action and social communication. In: Russell JA, Fernandez-Dols JM, editors. The psychology of facial expression. New York: Cambridge University Press; 1997. pp. 158–175. [Google Scholar]
- Reeve HK, Sherman PW. Adaptation and the goals of evolutionary research. Q Rev Biol. 1993;68:1–32. [Google Scholar]
- Richardson C, Bowers D, Bauer R, Heilman K, Leonard CM. Digitizing the moving face during dynamic displays of emotion. Neuropsychologia. 2000;38:1028–1039. doi: 10.1016/s0028-3932(99)00151-7. [DOI] [PubMed] [Google Scholar]
- Rinn WE. The neuropsychology of facial expression: a review of the neurological and psychological mechanisms for producing facial expressions. Psychol Bull. 1984;95:52–77. [PubMed] [Google Scholar]
- Ross RT, Mathiesen R. Volitional and emotional supranu-clear facial weakness. N Engl J Med. 1998;338:1515. doi: 10.1056/NEJM199805213382105. [DOI] [PubMed] [Google Scholar]
- Russell JA, Fernandez-Dols JM. What does a facial expression mean? In: Russell JA, Fernandez-Dols JM, editors. The psychology of facial expression. New York: Cambridge University Press; 1997. pp. 3–30. [Google Scholar]
- Sakamoto S, Nameta K, Kawasaki T, Yamashita K, Shimizu A. Polygraphic evaluation of laughing and smiling in schizophrenic and depressive patients. Percept Mot Skills. 1997;85:1291–1302. doi: 10.2466/pms.1997.85.3f.1291. [DOI] [PubMed] [Google Scholar]
- Scheifenhovel W. Universals in interpersonal interactions. In: Segerstrale U, Molnar P, editors. Nonverbal communication: where nature meets culture. Mahwah, NJ: Lawrence Erlbaum Associates; 1997. pp. 61–86. [Google Scholar]
- Schmidt KL. Variation in the timing and display of the human smile. Am J Phys Anthropol [Suppl] 2000;30:272. [Google Scholar]
- Schmidt KL, Cohn JF. Dynamic modeling of human facial expression. Am J Phys Anthropol [Suppl] 2001;31:132. doi: 10.1002/ajpa.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KL, Johnson FYA, Allen JS. Cross-cultural analysis of eventfulness in the lives of people with schizophrenia. Schizophr Bull. 2000;26:825–834. doi: 10.1093/oxfordjournals.schbul.a033497. [DOI] [PubMed] [Google Scholar]
- Scriba H, Stoeckli SJ, Pollack A, Veraguth D, Fisch U. Objective evaluation of normal facial function. Ann Otol Rhinol Laryngol. 1999;108:641–644. doi: 10.1177/000348949910800703. [DOI] [PubMed] [Google Scholar]
- Shor RE. The production and judgment of smile magnitude. J Gen Psychol. 1978;98:79–96. [Google Scholar]
- Silk JB, Kaldor E, Boyd R. Cheap talk when interests conflict. Anim Behav. 2000;59:423–432. doi: 10.1006/anbe.1999.1312. [DOI] [PubMed] [Google Scholar]
- Smith WM. Hemispheric and facial asymmetry: faces of academe. J Cog Neurosci. 1998;10:663–667. doi: 10.1162/089892998563077. [DOI] [PubMed] [Google Scholar]
- Stal P, Eriksson PO, Eriksson A, Thornell LE. Enzyme-histochemical differences in fibre-type between the human major and minor zygomatic and the first dorsal interosseus muscles. Arch Oral Biol. 1987;32:833–841. doi: 10.1016/0003-9969(87)90011-2. [DOI] [PubMed] [Google Scholar]
- Strahan E, Conger AJ. Social anxiety and its effects on performance and perception. J Anx Disord. 1998;12:293–305. doi: 10.1016/s0887-6185(98)00016-4. [DOI] [PubMed] [Google Scholar]
- Sullivan DG, Masters RD. Facial displays and political leadership: some experimental findings. In: Schubert G, Masters RD, editors. Primate politics. Carbondale: Southern Illinois University Press; 1991. pp. 188–206. [Google Scholar]
- Surakka V, Hietanen JK. Facial and emotional reactions to Duchenne and non-Duchenne smiles. Int J Psychophysiol. 1998;29:23–33. doi: 10.1016/s0167-8760(97)00088-3. [DOI] [PubMed] [Google Scholar]
- Thornhill R, Gangestad S. Fluctuating asymmetry and human sexual behavior. Psychol Sci. 1994;5:297–302. [Google Scholar]
- Tian Y, Kanade T, Cohn JF. Recognizing action units for facial expression analysis. IEEE Transactions on Pattern Analysis and Machine Intelligence. 2001;23:97–115. doi: 10.1109/34.908962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello M, Call J. Primate cognition. New York: Oxford University Press; 1997. [Google Scholar]
- Trivers R. The evolution of reciprocal altruism. Q Rev Biol. 1971;46:35–57. [Google Scholar]
- Trivers R. Parent-offspring conflict. Am Zool. 1974;14:249–264. [Google Scholar]
- van Hooff JARAM. A comparative approach to the phylogeny of laughter and smiling. In: Hinde RA, editor. Non-verbal communication. New York: Oxford University Press; 1972. pp. 209–242. [Google Scholar]
- VanSwearingen JM, Cohn JF, Bajaj-Luthra A. Specific impairment of smiling increases the severity of depressive symptoms in patients with facial neuromuscular disorders. Aesthet Plast Surg. 1999;23:416–423. doi: 10.1007/s002669900312. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. Faces call for attention: evidence from patients with visual extinction. Neuropsychologia. 2000;38:693–700. doi: 10.1016/s0028-3932(99)00107-4. [DOI] [PubMed] [Google Scholar]
- Wagner H, Lee V. Facial behavior alone and in the presence of others. In: Philippot P, Feldman RS, Coats EJ, editors. The social context of nonverbal behavior. New York: Cambridge University Press; 1999. pp. 262–286. [Google Scholar]
- Yik MSM, Russell JA. Interpretation of faces: a cross-cultural study of a prediction from Fridlund’s theory. Cogn Emot. 1999;13:93–104. [Google Scholar]
- Young AW, Newcombe F, deHaan EHF, Small M, Hay DC. Dissociable deficits after brain injury. In: Young AW, editor. Face and mind. New York: Oxford University Press; 1998. pp. 181–208. [Google Scholar]
- Zahavi A. The fallacy of conventional signaling. Philos Trans R Soc Lond [Biol] 1993;340:227–230. doi: 10.1098/rstb.1993.0061. [DOI] [PubMed] [Google Scholar]
- Zahavi A, Zahavi A. The handicap principle. New York: Oxford University Press; 1997. [Google Scholar]


