Abstract
Transgenic expression of the influenza virus hemagglutinin (HA) in the pancreatic islet β cells of InsHA mice leads to peripheral tolerance of HA-specific T cells. To examine the onset of tolerance, InsHA mice were immunized with influenza virus A/PR/8 at different ages, and the presence of nontolerant T cells was determined by the induction of autoimmune diabetes. The data revealed a neonatal period wherein T cells were not tolerant and influenza virus infection led to HA-specific β cell destruction and autoimmune diabetes. The ability to induce autoimmunity gradually waned, such that adult mice were profoundly tolerant to viral HA and were protected from diabetes. Because cross-presentation of islet antigens by professional antigen-presenting cells had been reported to induce peripheral tolerance, the temporal relationship between tolerance induction and activation of HA-specific T cells in the lymph nodes draining the pancreas was examined. In tolerant adult mice, but not in 1-week-old neonates, activation and proliferation of HA-specific CD8+ T cells occurred in the pancreatic lymph nodes. Thus, lack of tolerance in the perinatal period correlated with lack of activation of antigen-specific CD8+ T cells. This work provides evidence for the developmental regulation of peripheral tolerance induction.
Although many potentially autoreactive T cells are eliminated during development in the thymus (1–3), additional mechanisms of tolerance appear to be necessary to minimize T cell responses against antigens expressed uniquely in the periphery. Approaches that have been used to study peripheral tolerance have relied on T cells from T cell antigen receptor (TCR) transgenic mice that are specific for defined epitopes, or T cells specific for superantigen to investigate the fate of mature T cells when they first encounter antigen in the periphery (4–17). In general, it has been observed that cells initially undergo activation and several rounds of division, which is soon followed by their elimination or anergy. The cells responsible for initial stimulation are not represented by parenchymal tissue, but rather, are professional bone marrow-derived antigen-presenting cells (APC). This type of abortive stimulation is true for both class I and class II restricted epitopes that are presented to CD8+ and CD4+ T cells, respectively (14, 15, 18–22).
In several models in which a transgene product is expressed in the pancreatic islets under the control of the insulin promoter, it has been demonstrated that tolerance occurs after T cells become activated in the pancreatic lymph nodes by APC that cross-present antigen (11, 12, 14). Previous studies have demonstrated that activation through cross-presentation is inefficient during the perinatal period (23, 24). If cross-presentation is necessary for tolerance induction, then neonatal would suggest neonatal mice may not exhibit tolerance of peripherally expressed antigens.
In this study we have used mice that express the influenza virus hemagglutinin (HA) as a transgene product in the pancreatic islets (InsHA mice, ref. 25) to determine when peripheral tolerance first occurs. In this transgenic model KdHA-specific thymocytes develop normally and are unaffected by the presence of the HA transgene (26). However, the adult peripheral T cell repertoire is profoundly tolerant of HA antigens, inasmuch as immunization with influenza virus does not cause autoimmune destruction of the islet β cell. The HA-specific CD8+ T cells that can be recovered from these mice demonstrate low avidity for the dominant KdHA epitope (27). It was further demonstrated that peripheral expression of HA in the pancreas was necessary and sufficient to achieve tolerance, as InsHA mice that were irradiated, thymectomized, and then reconstituted with bone marrow and a thymus from conventional nontransgenic littermates also demonstrated tolerance of HA (25). Thus, InsHA mice represent a unique model with which to explore the ontogeny of peripheral tolerance.
MATERIALS AND METHODS
Mice.
BALB/c mice were purchased from the breeding colony of The Scripps Research Institute (La Jolla, CA). InsHA transgenic mice (25) and clone-4 TCR transgenic mice (26) were generated and characterized as described, and each line was backcrossed at least eight generations with BALB/c. All mice were bred and maintained under specific pathogen-free conditions in The Scripps Research Institute vivarium. All experimental procedures were carried out in strict accordance with the guidelines laid out in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Virus.
Influenza virus A/PR/8/34 H1N1 (PR8) and recombinant equine influenza virus A/PR/8 H3N1 (EqPR8) (28) were grown in the allantoic cavity of 10- to 11-day-old hen’s eggs. After isolation, the allantoic fluid was titered for hemagglutination by using chicken RBC and later stored in 1-ml aliquots at −70°C. Wild-type vaccinia virus (Vacc-WT) and recombinant vaccinia virus expressing the H-2Kd-restricted epitope IYSTVASSL, amino acid residues 518–526, (Vacc-KdHA) were kindly provided by Jack R. Bennink and Jonathan Yewdell from the National Institutes of Health.
Immunization.
Mice were immunized i.p. with 1,200 HA units of PR8 or EqPR8 in the form of allantoic fluid or 108 plaque-forming units of Vacc-WT or Vacc-KdHA in PBS.
Preparation of Clone-4 TCR Cells.
Single-cell suspensions were prepared from the lymph nodes cells of clone-4 TCR mice. Purified CD8+ clone-4 TCR cells were prepared as follows: 108 clone-4 TCR lymph node cells were loaded on to a nylon wool column (Wako Chemicals, Richmond, VA), presoaked for 1 hr at 37°C in RPMI medium 1640 containing 10% (vol/vol) FCS, 25 mM Hepes, 2 mM glutamine, 5 × 10−5 M β-mercapto-ethanol, and 50 mg/ml of gentamycin (complete RPMI), and incubated for an additional 1 hr at 37°C in a humidified incubator with 5% (vol/vol) CO2. Cells were eluted from the column with 15 ml of complete RPMI. After centrifugation 1 ml of anti-heat stable antigen (J11D), anti-CD4 (RL172), and anti-MHC class II (CA4/A12) antibody supernatants was added per 107 cells, and the mixture was incubated on ice for 1 hr. Cells then were centrifuged, and the antibody was discarded, resuspended in Low-Tox Rabbit complement (Accurate Chemicals), and incubated for an additional 1 hr at 37°C. Cell then were washed three times in complete RPMI. The purity of CD8+ cells was determined by incubating 106 cells for 20 min on ice with FITC-conjugated and phycoerythrin-conjugated antibodies against mouse CD8 and CD4, respectively (PharMingen). Cells were washed three times in PBS containing 0.1% (wt/vol) BSA (Sigma), and 0.02% (wt/vol) sodium azide. Cells were analyzed with a FACScan and cellquest software (Becton Dickinson), and the purity of CD8+ cells was found to be greater than 85% in all cases.
5,6-Carboxy-Succinimidyl-Fluorescein-Ester (CSFE) Labeling of Clone-4 TCR CD8+ T Cells.
Purified clone-4 TCR CD8+ T cells were resuspended at 5 × 107 cells/ml of PBS. To these cells was added 2 μl of a 5 mM solution of CSFE (Molecular Probes) in DMSO (Sigma) per ml of cells and incubated for 10 min at 37°C. Cells were washed once in cold PBS, and then resuspended at 2.5 × 107 cells/ml of complete RPMI. The uptake of CSFE was determined before transfer by comparing their fluorescence in the FL1 channel with that of unstained cells. Importantly, all manipulations with CSFE were carried in such a way as to minimize exposure to light (29).
Adoptive Transfer of CSFE-Labeled Clone-4 TCR CD8+ T Cells.
Recipient mice were injected i.v. with 5 × 106 CSFE-labeled clone-4 CD8+ T cells in 200 μl of PBS. The presence of these cells in the peripheral lymphoid organs of recipient mice was determined by fluorescence-activated cell sorter analyses 3 days after transfer.
Cytometry.
CSFE-labeled clone-4 CD8+ T cells were detected by staining with phycoerythrin-conjugated anti-CD8 antibodies (PharMingen).
Immunohistochemistry.
Pancreata were excised and embedded in 10% (vol/vol) formalin solution (Sigma) and processed for paraffin embedding. Paraffin-embedded tissue was cut by using a microtome, and sections were placed onto saline-coated Superfrost slides for processing (Fisher Scientific). Tissue sections were deparaffinized in xylene and rehydrated in graded ethanol to distilled water. Nonspecific binding sites were blocked by incubating with 10% (vol/vol) goat serum in PBS. Sections were incubated for 1 hr with guinea-pig antibodies against mouse insulin (Dako). After washing for 10 min in PBS, sections were incubated with secondary biotinylated F(ab′)2 goat anti-guinea-pig IgG (Vector Laboratories), and then detected by using streptavidin-conjugated horseradish peroxidase (Jackson ImmunoResearch), together with diaminobenzidine chromagen. Separate serial sections of paraffin-embedded tissue also were stained with eosin (Sigma), and all slides were counterstained with Mayer’s hematoxylin (Sigma).
RESULTS AND DISCUSSION
To investigate the onset of peripheral tolerance induction, InsHA neonates of different ages were immunized with PR8, which expresses the homologous HA protein, or a recombinant strain of PR8 that expresses an immunologically noncross reactive HA from EqPR8 (28). Viral immunization is known to prevent peripheral tolerance and would be able to stimulate newly matured HA-specific T cells that had not yet been tolerized (16). Therefore, if nontolerized T cells were present in the periphery, the mice should develop autoimmune diabetes. Animals were monitored for the development of insulitis and incidence of diabetes (Table 1).
Table 1.
Incidence of diabetes after immunization with influenza virus (%)
| Virus | Age | Time after infection, days | Diabetes | Insulitis | Peri-Insulitis | Pristine |
|---|---|---|---|---|---|---|
| PR8 | 1 week | 9 | 0% | 0% | 0% | 100% |
| Vacc-WT | 7 | 0% | ND | ND | ND | |
| Vacc-KdHA | 7 | 100% | ND | ND | ND | |
| PR8 | 1 week | 9 | 100% | 100% | 0% | 0% |
| ” | 2 weeks | 9 | 50% | 100% | 0% | 0% |
| 21 | 0% | 36% | 24% | 40% | ||
| ” | 4 weeks | 9 | 50% | 84% | 14% | 2% |
| 21 | 0% | 11% | 21% | 68% | ||
| ” | 8 weeks | 9 | 0% | 0% | 27% | 73% |
| 21 | 0% | 0% | 10% | 90% |
Groups of between 6 and 8 InsHA mice of various ages were immunized with virus as shown. Data show the incidence of diabetes and degree of insulitis observed in pancreata take from immunized mice. Data are expressed as the percentage of the total number of islets examined from all mice in each group, 9 days and 21 days later. Mice were considered diabetic if their blood glucose was above 300 mg/dl. ND, not determined.
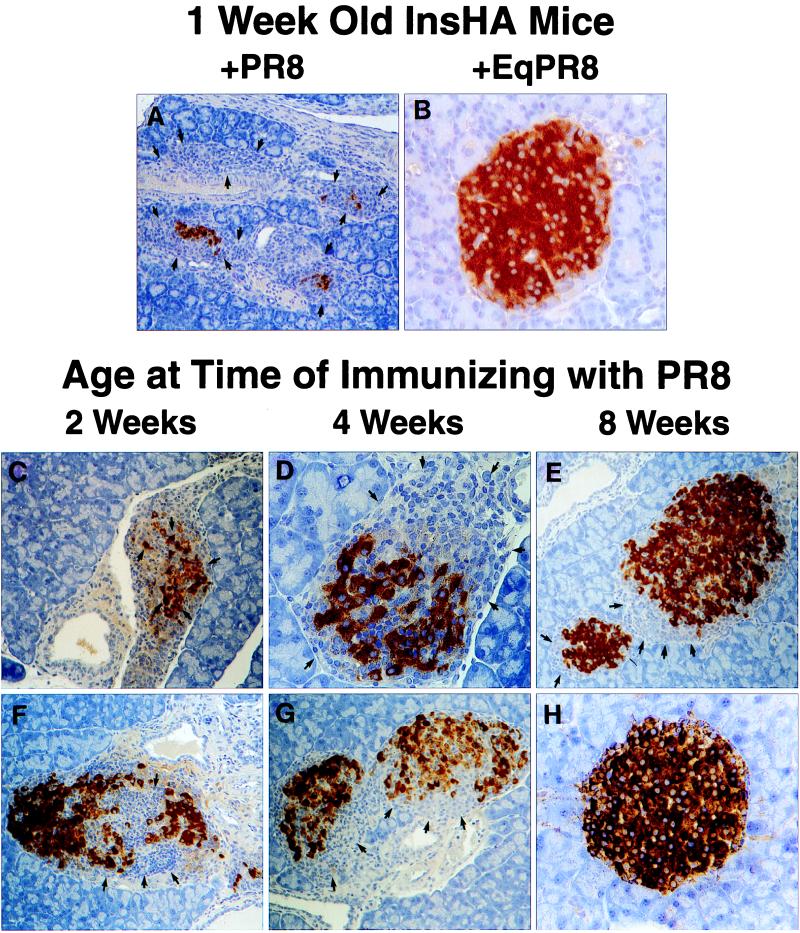
One-week-old InsHA mice immunized i.p. with PR8 developed lethal autoimmune diabetes, which was characterized by a massive cellular infiltration of the pancreas, containing large numbers of CD8+ and CD4+ lymphocytes, causing a destructive insulitis in 100% of these mice (Fig. 1A). Age-matched InsHA mice that were immunized with EqPR8 did not develop diabetes or insulitis (Fig. 1B). This finding indicated that autoimmunity occurred only if the virus-activated T cells that specifically recognized the HA molecule were present in the islets. It also confirms the effective expression of HA at this early age. Immunization of 2- and 4-week-old mice resulted in a temporary diabetic state in 50% of the mice by 9 days after infection, although extensive insulitis and destruction of pancreatic islet β cells were evident in virtually all mice at this time (Fig. 1 C and D, respectively). Of interest, by 21 days after infection, the severity of the insulitis in these mice had diminished significantly and they were no longer diabetic. Insulitis was, however, still evident in both the 2- and 4-week-old mice (Fig. 1 F and G, respectively). In contrast, PR8-immunized 8-week-old adult mice did not develop diabetes, exhibiting a mild peri-insulitis in a small proportion of the islets that was not associated with β cell destruction (Fig. 1E). By 21 days after infection the degree of insulitis had resolved such that fewer than 10% of the islets had infiltrates (Fig. 1H). Thus, newborn and 2- to 4-week-old InsHA mice demonstrated a greater potential than 8-week-old mice for developing autoimmunity toward β cell-expressed HA. Parenthetically, it is important to note that adult pancreas is susceptible to autoimmune destruction. Influenza virus infection of InsHA mice that received T cells from conventional mice, or as few as 102 KdHA-specific clone-4 TCR CD8+ T cells, results in diabetes (ref. 25, D.J.M. and L.A.S., unpublished observations). To determine whether the activation of KdHA-specific CD8+ T cells alone was sufficient to cause diabetes, we immunized 1-week-old InsHA mice with a recombinant vaccinia virus expressing the dominant KdHA-epitope IYSTVASSL, amino acids residues 518–526 (Vacc-KdHA) (27). These pups developed diabetes within 1 week, whereas 1-week-old control mice given wild-type vaccinia virus (Vacc-WT) remained healthy, (Table 1).
Figure 1.
Insulitis in neonatal InsHA mice immunized with PR8. Neonatal InsHA mice at various ages as shown were immunized i.p. with 1,200 HA units of PR8. Shown are immunohistological analyses of pancreata taken from various InsHA mice immunized with PR8. Paraffin-embedded sections are stained for insulin by using the immunoperoxidase technique with diaminobenzidine as a chromagen and counterstained with hematoxylin. (A) Tissue isolated from 1-week-old neonate 9 days after immunization with PR8. Note the overwhelming presence of mononuclear cells, arrows indicate edges of islet remnants leaving only a few insulin positive β cells. (B) One-week-old neonate 9 days after immunization with EqPR8. Islet is free from mononuclear infiltration and uniform insulin staining shows there is no β cell destruction. (C) Two-week-old and (D) 4-week-old mice 9 days after immunizing with PR8, both show considerable insulitis and β cell destruction (arrows). Mice from the same groups, 2 weeks old (F) and 4 weeks old (G), 21 days after immunization. The extent of insulitis and β cell destruction is less at this time (arrows). (E) Representative of the most severe insulitis demonstrated in 8-week-old adult mice 9 days after immunization with PR8. Only a mild peri-insulitis is observed that is not associated with any β cell destruction (arrows). (H) Representative of >90% of islets from these same adults 21 days after immunization. They are intact and express high levels of insulin. Magnifications: A, ×100; B, D, and H, ×400; C and E–G, ×200.
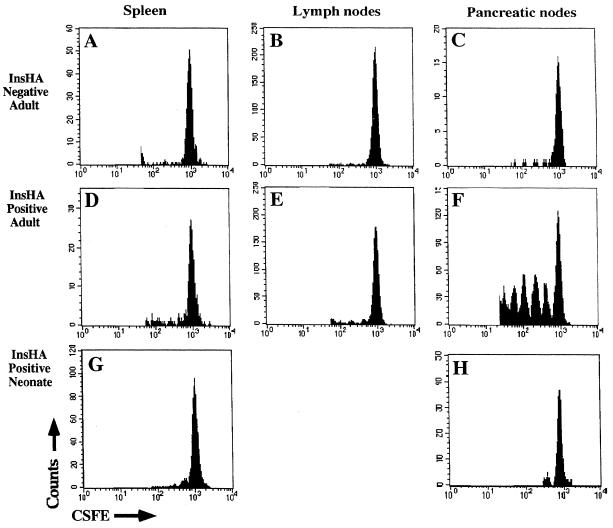
By using TCR transgenic T cells specific for transgene products expressed in the pancreatic islets, it has been shown that T cells become activated and proliferate when they encounter antigen that is cross-presented by APC in the draining nodes of the pancreas. In each case, activation was followed by deletion (11, 14), suggesting such activation was critical to tolerance induction. To determine whether a similar mechanism of activation occurred in the draining nodes of the pancreas in InsHA mice, purified clone-4 TCR transgenic CD8+ T cells were labeled with the internal fluorescent dye CSFE (12, 29), and 5 × 106 cells were transferred into both InsHA-negative and InsHA-positive mice. When CSFE-labeled cells divide, the intensity of their fluorescence is halved with each successive cell division forming a series of fluorescence peaks that correspond to the number of divisions the cells have undergone. Seventy-two hours after transfer the mice were sacrificed and populations of T cells were prepared from the peripheral lymphoid organs (Fig. 2). Examination of adult recipients revealed that only among cells recovered from the draining lymph nodes of the pancreas of InsHA-positive mice was there any evidence of proliferation (Fig. 2F). Indeed, some of the CSFE-labeled cells had undergone at least five rounds of division. Cells that had undergone division were not found in the other peripheral lymph nodes (Fig. 2E) nor in the spleen (Fig. 2D) of the InsHA-positive mice. CSFE-labeled cells did not proliferate in any of the lymphoid organs examined from recipients that did not express HA (Fig. 2 A–C), and there were fewer CSFE positive cells present in the pancreatic lymph nodes of the InsHA-negative recipients. These results indicated that at any given time a proportion of the clone-4 TCR cells that migrate to the pancreatic lymph nodes of InsHA mice become activated and proliferate. Surprisingly, histological examination of the pancreas revealed no evidence of lymphocyte infiltration, or islet cell damage at either 3 days or 9 days after adoptive transfer of clone-4 TCR cells (data not shown).
Figure 2.
CSFE-labeled, purified clone-4 TCR CD8+ T cells do not proliferate in the pancreatic lymph nodes after adoptive transfer into neonatal InsHA mice. Adult InsHA negative (A–C) and adult InsHA positive (D–F) mice were injected i.v., and 5-day-old neonatal InsHA mice (G and H) were injected i.p. with 5 × 106 CSFE-labeled, purified clone-4 TCR CD8+ T cells. Seventy-two hours later cells isolated from various peripheral lymphoid organs as shown were stained with phycoerythrin-conjugated anti-CD8 antibodies. Data show amount of CSFE label among activated CSFE-labeled CD8+ T cells obtained from pooled lymphoid tissue taken from at least three adult mice per group and seven neonates. For clarity of depiction, the unlabeled cells have been deleted from the histograms.
To determine whether such activation and proliferation of KdHA-specific CD8+ T cells also occurs in the pancreatic lymph nodes of the neonate, CSFE-labeled purified clone-4 TCR CD8+ cells were injected i.p. into 5-day-old InsHA-positive mice. Animals were sacrificed at 72 hr, and the pancreatic draining lymph node was analyzed for evidence of T cell proliferation. In contrast to the adult InsHA mice, in which CSFE-labeled clone-4 cells proliferated in the pancreatic lymph nodes, cells did not proliferate in either the spleen or the pancreatic nodes of neonatal InsHA mice (Fig. 2 G and H). These results demonstrate the inability of an islet-expressed antigen to activate naive CD8+ T cells in neonates.
Previous studies have shown that activation of islet-specific CD8+ T cells occurs in the draining lymph nodes of the pancreas as a result of cross-presentation of islet antigens by professional APCs (12, 14). We have not formally demonstrated that activation of clone-4 CD8+ T cells in the pancreatic lymph nodes of adult InsHA mice occurs because of cross-presentation rather than direct presentation of the KdHA epitope expressed by the β cells. However, we believe that the latter is unlikely, as naive T cells require antigen presentation by professional APC for their activation (30). Furthermore, we find no evidence for the presence of clone-4 CD8+ T cells in the islets of InsHA mice. Given these results, as well as previous reports that demonstrated lack of cross-presentation in neonatal mice (11, 24, 31), the most likely explanation for the lack of tolerance is a lack of cross-presentation of islet antigens in the neonatal period.
Importantly, the data indicate that there is no premium placed on the need for peripheral tolerance in the perinatal period. There may be little danger of autoimmunity until cross-presentation becomes more efficient through further development of APC function (23, 24, 31, 32) or increased levels of expression of peripheral antigens (33). In conclusion, these studies demonstrate that the early neonatal period allows for the accumulation of potentially autoreactive T cells. Depending on the availability of antigen, some of these cells are later functionally eliminated by peripheral tolerance (33). This study provides insights into how the immune system may control responses to developmentally regulated antigens such as those associated with puberty or lactation. Furthermore, these data raise the question of whether lack of cross-presentation during the juvenile period may explain vulnerability to some autoimmune diseases, such as type I diabetes.
Acknowledgments
We gratefully acknowledge the secretarial assistance of Ms. Patricia Krier. We also thank our colleagues Norman Klinman, Susan Webb, and Jonathan Sprent for critical reading of the manuscript. This work was supported by Grants AI 39664 and DK/CA50824 from the National Institutes of Health. D.J.M. is the recipient of a Senior Fellowship from the Juvenile Diabetes Foundation.
ABBREVIATIONS
- InsHA
transgenic mouse expressing the hemagglutinin from influenza virus on pancreatic islet beta cells under the control of the rat insulin promoter
- HA
hemagglutinin
- CSFE
5,6-carboxy-succinimidyl-fluorescein-ester
- PR8
influenza virus A/PR/8 H1N1
- EqPR8
recombinant equine influenza virus A/PR/8 H3N1
- APC
antigen-presenting cells
- TCR
T cell antigen receptor
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Kappler J W, Roehm N, Marrack P. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 2.Kisielow P, Bluthmann H, Staerz U D, Steinmetz M, von Boehmer H. Nature (London) 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 3.Sprent J, Schaefer M. Immunol Rev. 1990;117:213–234. doi: 10.1111/j.1600-065x.1990.tb00574.x. [DOI] [PubMed] [Google Scholar]
- 4.Webb S R, Sprent J. Science. 1990;248:1643–1646. doi: 10.1126/science.1973003. [DOI] [PubMed] [Google Scholar]
- 5.Webb S, Morris C, Sprent J. Cell. 1990;63:1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- 6.Kawabe Y, Ochi A. Nature (London) 1991;349:245–248. doi: 10.1038/349245a0. [DOI] [PubMed] [Google Scholar]
- 7.Rocha B, von Boehmer H. Science. 1991;251:1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- 8.Kearney E R, Pape K A, Loh D Y, Jenkins M K. Immunity. 1994;1:327–329. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 9.Bertolino P, Heath W R, Hardy C L, Morohan G, Miller J F A P. Eur J Immunol. 1995;25:1932–1942. doi: 10.1002/eji.1830250721. [DOI] [PubMed] [Google Scholar]
- 10.Förster I, Hirosa R, Arbeit J M, Clausen B E, Hanahan D. Immunity. 1995;2:573–585. doi: 10.1016/1074-7613(95)90002-0. [DOI] [PubMed] [Google Scholar]
- 11.Förster I, Lieberam I. Eur J Immunol. 1996;26:3194–3202. doi: 10.1002/eji.1830261253. [DOI] [PubMed] [Google Scholar]
- 12.Kurts C, Heath W R, Carbone F R, Allison J, Miller J A F P, Kosaka H. J Exp Med. 1996;184:923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L. Eur J Immunol. 1996;26:2208–2214. doi: 10.1002/eji.1830260937. [DOI] [PubMed] [Google Scholar]
- 14.Kurts C, Kosaka H, Carbone F R, Miller J F A P, Heath W R. J Exp Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adler A J, Marsh D W, Yochum G S, Guzzo J L, Nigam A, Nelson W G, Pardoll D M. J Exp Med. 1998;187:1555–1564. doi: 10.1084/jem.187.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehl S, Hombach J, Aichele P, Rulicke T, Odermatt B, Hengartner H, Zinkernagel R, Pircher H. J Exp Med. 1998;187:763–774. doi: 10.1084/jem.187.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Parijs L, Peterson D A, Abbas A K. Immunity. 1998;8:265–275. doi: 10.1016/s1074-7613(00)80478-1. [DOI] [PubMed] [Google Scholar]
- 18.Bevan M J. J Exp Med. 1987;182:639–641. doi: 10.1084/jem.182.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carbone F R, Bevan M J. J Exp Med. 1989;169:603–612. doi: 10.1084/jem.169.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lo D, Reilly C, Scott B, Liblau R, McDevitt H O, Burkly L. Eur J Immunol. 1993;23:1693–1698. doi: 10.1002/eji.1830230744. [DOI] [PubMed] [Google Scholar]
- 21.Rock K L. Immunol Today. 1996;17:131–171. doi: 10.1016/0167-5699(96)80605-0. [DOI] [PubMed] [Google Scholar]
- 22.Moore M W, Carbone F R, Bevan M J. J Exp Med. 1998;171:377–387. doi: 10.1084/jem.171.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rafii-Tabar E, Czitrom A A. Eur J Immunol. 1986;16:1025–1027. doi: 10.1002/eji.1830160827. [DOI] [PubMed] [Google Scholar]
- 24.Hoglund P, Mintern J, Heath W, Benoist C, Mathis D. J Exp Med. 1999;18:331–339. doi: 10.1084/jem.189.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo D, Freedman J, Hesse S, Palmiter R D, Brinster R L, Sherman L A. Eur J Immunol. 1992;22:1013–1019. doi: 10.1002/eji.1830220421. [DOI] [PubMed] [Google Scholar]
- 26.Morgan D J, Liblau R, Scott B, Fleck S, McDevitt H O, Sarvetnick N, Lo D, Sherman L A. J Immunol. 1996;157:978–983. [PubMed] [Google Scholar]
- 27.Morgan D J, Kreuwel H T C, Fleck S, Levitsky H I, Pardoll D M, Sherman L A. J Immunol. 1998;160:643–651. [PubMed] [Google Scholar]
- 28.Webster R. Virology. 1970;42:633–642. doi: 10.1016/0042-6822(70)90309-0. [DOI] [PubMed] [Google Scholar]
- 29.Lyons A B, Parish C R. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 30.Steinman R M. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 31.Lu C Y, Calamai E G, Unanue E R. Nature (London) 1979;282:327–329. doi: 10.1038/282327a0. [DOI] [PubMed] [Google Scholar]
- 32.Nadler P I, Lingenstein R J, Hodes R J. J Immunol. 1980;125:914–920. [PubMed] [Google Scholar]
- 33.Heath W R, Kurts C, Miller J F A P, Carbone F R. J Exp Med. 1998;187:1549–1553. doi: 10.1084/jem.187.10.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]