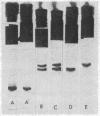
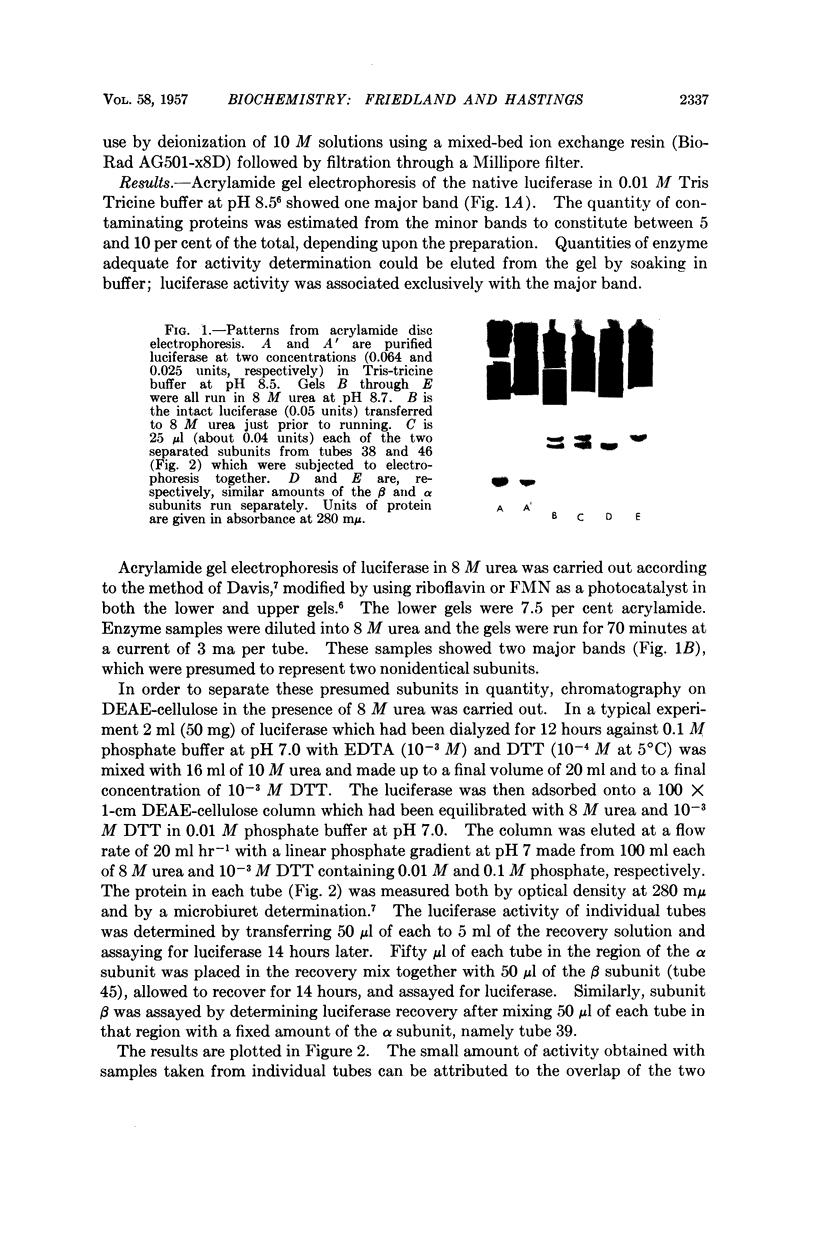
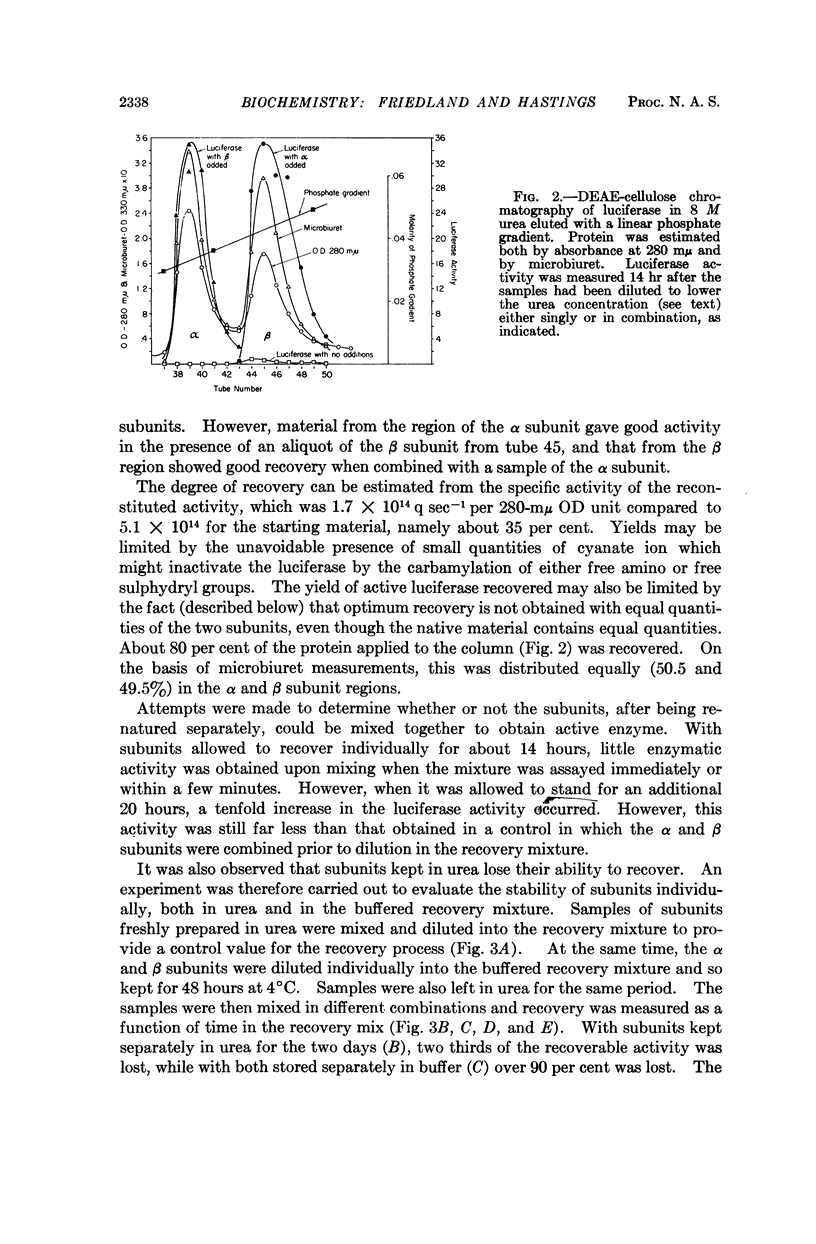
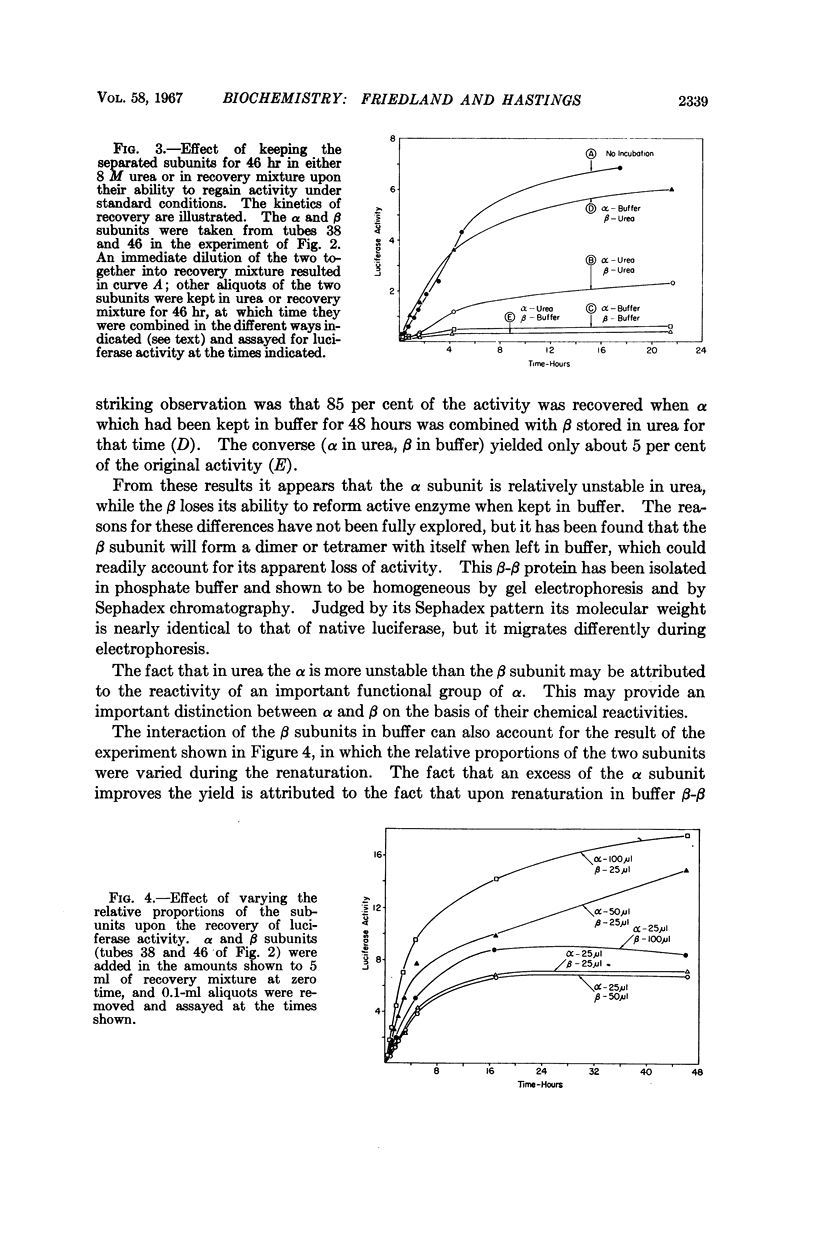
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonini E., Bucci E., Fronticelli C., Chiancone E., Wyman J., Rossi-Fanelli A. The properties and interactions of the isolated alpha- and beta-chains of human haemoglobin. V. The reaction of alpha- and beta-chains. J Mol Biol. 1966 May;17(1):29–46. doi: 10.1016/s0022-2836(66)80092-x. [DOI] [PubMed] [Google Scholar]
- Brewer J. M. Artifact produced in disc electrophoresis by ammonium persulfate. Science. 1967 Apr 14;156(3772):256–257. doi: 10.1126/science.156.3772.256. [DOI] [PubMed] [Google Scholar]
- Chilson O. P., Kitto G. B., Kaplan N. O. Factors affecting the reversible dissociation of dehydrogenases. Proc Natl Acad Sci U S A. 1965 May;53(5):1006–1014. doi: 10.1073/pnas.53.5.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPSTEIN C. J., CARTER M. M., GOLDBERGER R. F. REVERSIBLE DENATURATION OF RABBIT-MUSCLE LACTATE DEHYDROGENASE. Biochim Biophys Acta. 1964 Nov 22;92:391–394. doi: 10.1016/0926-6569(64)90198-1. [DOI] [PubMed] [Google Scholar]
- Friedland J., Hastings J. W. The reversibility of the denaturation of bacterial luciferase. Biochemistry. 1967 Sep;6(9):2893–2900. doi: 10.1021/bi00861a033. [DOI] [PubMed] [Google Scholar]
- GOA J. A micro biuret method for protein determination; determination of total protein in cerebrospinal fluid. Scand J Clin Lab Invest. 1953;5(3):218–222. doi: 10.3109/00365515309094189. [DOI] [PubMed] [Google Scholar]
- Gerhart J. C., Schachman H. K. Distinct subunits for the regulation and catalytic activity of aspartate transcarbamylase. Biochemistry. 1965 Jun;4(6):1054–1062. doi: 10.1021/bi00882a012. [DOI] [PubMed] [Google Scholar]
- HASTINGS J. W., GIBSON Q. H., GREENWOOD C. ON THE MOLECULAR MECHANISM OF BIOLUMINESCENCE. I. THE ROLE OF LONG-CHAIN ALDEHYDE. Proc Natl Acad Sci U S A. 1964 Dec;52:1529–1535. doi: 10.1073/pnas.52.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASTINGS J. W., GIBSON Q. H. Intermediates in the bioluminescent oxidation of reduced flavin mononucleotide. J Biol Chem. 1963 Jul;238:2537–2554. [PubMed] [Google Scholar]
- HASTINGS J. W., RILEY W. H., MASSA J. THE PURIFICATION PROPERTIES, AND CHEMILUMINESCENT QUANTUM YIELD OF BACTERIAL LUCIFERASE. J Biol Chem. 1965 Mar;240:1473–1481. [PubMed] [Google Scholar]
- KUWABARA S., CORMIER M. J., DURE L. S., KREISS P., PFUDERER P. CRYSTALLINE BACTERIAL LUCIFERASE FROM PHOTOBERTERIUM FISCHERI. Proc Natl Acad Sci U S A. 1965 Apr;53:822–828. doi: 10.1073/pnas.53.4.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D. E., Chan W., Horecker B. L. The subunit structure and carboxy-terminal sequence of rabbit muscle aldolase. Proc Natl Acad Sci U S A. 1967 Aug;58(2):628–634. doi: 10.1073/pnas.58.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penhoet E., Kochman M., Valentine R., Rutter W. J. The subunit structure of mammalian fructose diphosphate aldolase. Biochemistry. 1967 Sep;6(9):2940–2949. doi: 10.1021/bi00861a039. [DOI] [PubMed] [Google Scholar]
- STARK G. R. ON THE REVERSIBLE REACTION OF CYANATE WITH SULFHYDRYL GROUPS AND THE DETERMINATION OF NH2-TERMINAL CYSTEINE AND CYSTINE IN PROTEINS. J Biol Chem. 1964 May;239:1411–1414. [PubMed] [Google Scholar]