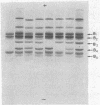
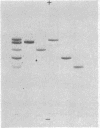
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson J. V., Jr, Jones R. T., Cormick J., Patterson J. A. Accelerated automatic chromatographic analysis of peptides on a spherical resin. Anal Biochem. 1966 Jul;16(1):91–106. doi: 10.1016/0003-2697(66)90084-4. [DOI] [PubMed] [Google Scholar]
- Gesteland R. F., Staehelin T. Electrophoretic analysis of proteins from normal and cesium chloride-treated Escherichia coli ribosomes. J Mol Biol. 1967 Mar 14;24(2):149–155. doi: 10.1016/0022-2836(67)90323-3. [DOI] [PubMed] [Google Scholar]
- Hosokawa K., Fujimura R. K., Nomura M. Reconstitution of functionally active ribosomes from inactive subparticles and proteins. Proc Natl Acad Sci U S A. 1966 Jan;55(1):198–204. doi: 10.1073/pnas.55.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEBOY P. S., COX E. C., FLAKS J. G. THE CHROMOSOMAL SITE SPECIFYING A RIBOSOMAL PROTEIN IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Dec;52:1367–1374. doi: 10.1073/pnas.52.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MATTHAEI J. H., NIRENBERG M. W. Characteristics and stabilization of DNAase-sensitive protein synthesis in E. coli extracts. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1580–1588. doi: 10.1073/pnas.47.10.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MESELSON M., NOMURA M., BRENNER S., DAVERN C., SCHLESSINGER D. CONSERVATION OF RIBOSOMES DURING BACTERIAL GROWTH. J Mol Biol. 1964 Sep;9:696–711. doi: 10.1016/s0022-2836(64)80176-5. [DOI] [PubMed] [Google Scholar]
- NATHANS D., NOTANI G., SCHWARTZ J. H., ZINDER N. D. Biosynthesis of the coat protein of coliphage f2 by E. coli extracts. Proc Natl Acad Sci U S A. 1962 Aug;48:1424–1431. doi: 10.1073/pnas.48.8.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Lowry C. V., Guthrie C. The initiation of protein synthesis: joining of the 50S ribosomal subunit to the initiation complex. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1487–1493. doi: 10.1073/pnas.58.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Lowry C. V. PHAGE f2 RNA-DIRECTED BINDING OF FORMYLMETHIONYL-TRNA TO RIBOSOMES AND THE ROLE OF 30S RIBOSOMAL SUBUNITS IN INITIATION OF PROTEIN SYNTHESIS. Proc Natl Acad Sci U S A. 1967 Sep;58(3):946–953. doi: 10.1073/pnas.58.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPITNIK-ELSON P. FRACTIONATION OF THE RIBOSOMAL PROTEIN OF ESCHERICHIA COLI BY ION-EXCHANGE CHROMATOGRAPHY ON CARBOXYMETHYL CELLULOSE. Biochim Biophys Acta. 1964 Apr 27;80:594–600. doi: 10.1016/0926-6550(64)90304-4. [DOI] [PubMed] [Google Scholar]
- SPITNIK-ELSON P. Fractionation of ribosomal protein from Escherichia coli by ammonium sulfate precipitation. Biochim Biophys Acta. 1963 Jul 2;74:105–112. doi: 10.1016/0006-3002(63)91334-9. [DOI] [PubMed] [Google Scholar]
- Spirin A. S., Belitsina N. V. Biological activity of the re-assembled ribosome-like particles. J Mol Biol. 1966 Jan;15(1):282–283. doi: 10.1016/s0022-2836(66)80227-9. [DOI] [PubMed] [Google Scholar]
- Staehelin T., Meselson M. In vitro recovery o ribosomes and of synthetic activity from synthetically inactive ribosomal subunits. J Mol Biol. 1966 Mar;16(1):245–249. doi: 10.1016/s0022-2836(66)80277-2. [DOI] [PubMed] [Google Scholar]
- Stanley W. M., Jr, Salas M., Wahba A. J., Ochoa S. Translation of the genetic message: factors involved in the initiation of protein synthesis. Proc Natl Acad Sci U S A. 1966 Jul;56(1):290–295. doi: 10.1073/pnas.56.1.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuka I., Kaji H., Kaji A. Binding of specific sRNA to 30S subunits--comparison with the binding to 70S ribosomes. Biochem Biophys Res Commun. 1965 Oct 26;21(2):187–193. doi: 10.1016/0006-291x(65)90107-5. [DOI] [PubMed] [Google Scholar]
- Traub P., Nomura M., Tu L. Physical and functional heterogeneity of ribosomal proteins. J Mol Biol. 1966 Aug;19(1):215–218. doi: 10.1016/s0022-2836(66)80064-5. [DOI] [PubMed] [Google Scholar]
- Traut R. R., Moore P. B., Delius H., Noller H., Tissières A. Ribosomal proteins of Escherichia coli. I. Demonstration of different primary structures. Proc Natl Acad Sci U S A. 1967 May;57(5):1294–1301. doi: 10.1073/pnas.57.5.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLER J. P. FRACTIONATION OF THE RIBOSOMAL PROTEIN FROM ESCHERICHIA COLI. J Mol Biol. 1964 Nov;10:319–336. doi: 10.1016/s0022-2836(64)80050-4. [DOI] [PubMed] [Google Scholar]
- WALLER J. P., HARRIS J. I. Studies on the composition of the protein from Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1961 Jan 15;47:18–23. doi: 10.1073/pnas.47.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]