Abstract
Excessive fluoride (F) can lead to abnormal bone biology. Numerous studies have focused on the anabolic action of F yet little is known regarding any action on osteoclastogenesis. Little is known regarding the influence of an individual’s genetic background on the responses of bone cells to F. Four-week old C57BL/6J (B6) and C3H/HeJ (C3H) female mice were treated with NaF in the drinking water (0ppm, 50ppm and 100ppm F ion) for 3 weeks. Bone marrow cells were harvested for osteoclastogenesis and hematopoietic colony-forming cell assays. Sera were analyzed for biochemical and bone markers. Femurs, tibiae and lumbar vertebrae were subjected to microCT analysis. Tibiae and femurs were subjected to histology and biomechanical testing, respectively. The results demonstrated new actions of F on osteoclastogenesis and hematopoietic cell differentiation. Strain specific responses were observed. The anabolic action of F was favored in B6 mice exhibiting dose dependent increases in serum ALP activity (p < 0.001); in proximal tibia trabecular and vertebral BMD (tibia at 50&100ppm, p = 0.001; vertebrae at 50&100ppm, p = 0.023&0.019, respectively); and decrease in intact PTH and sRANKL (p = 0.045 and p < 0.001, respectively). F treatment in B6 mice also resulted in increased numbers of CFU-GEMM colonies (p = 0.025). Strain specific accumulations in bone [F] were observed. For C3H mice, dose dependent increases were observed in osteoclast potential (p < 0.001), in situ trabecular osteoclast number (p = 0.007), hematopoietic colony forming units (CFU-GEMM: p < 0.001, CFU-GM: p = 0.006, CFU-M: p < 0.001), and serum markers for osteoclastogenesis (intact PTH: p = 0.004, RANKL: p = 0.022, TRAP5b: p < 0.001). A concordant decrease in serum OPG (p = 0.005) was also observed. Fluoride treatment had no significant effects on bone morphology, BMD and serum PYD crosslinks in C3H suggesting a lack of significant bone resorption. Mechanical properties were also unaltered in C3H. In conclusion, short term F treatment at physiological levels has strain specific effects in mice. The expected anabolic effects were observed in B6 and novel actions hallmarked by enhanced osteoclastogenesis shifts in hematopoietic cell differentiation in the C3H strain.
Keywords: F, osteoclastogenesis, hematopoietic colony-forming cell (CFC) assays, bone resorption markers, inbred mouse strains
Introduction
Fluoride (F) is an important micronutrient that accumulates within mineralized tissues such as teeth and bone [1,2]. In addition to dental/enamel fluorosis, excessive systemic F can lead to skeletal fluorosis a condition hallmarked by osteosclerosis, ligament calcifications and often accompanying osteoporosis, osteomalacia, or osteopenia [3,4]. F’s known actions on bone can be mediated through direct physicochemical interactions [5,6] and as an anabolic agent capable of affecting osteoblasts in vitro [2] and in vivo [7]. As an anabolic agent F is capable of increasing bone mass through an undetermined mechanism on osteoblasts [8]. Due to the anabolic action of F, its potential use as an agent for the treatment of postmenopausal osteoporosis was explored with mixed results [9,10]. While NaF may increase bone mass, the new bone lacks normal structure and strength [11,12]. These observations in humans have been extended in rodents [13,14]. The role of genetics/genetic background in F responses has been demonstrated in dental fluorosis [15,16]. In order to investigate more deeply F’s effects on bone and bone cells, we chose to utilize C57BL/6J (B6) and C3H/HeJ (C3H) mice that have been extensively characterized for their bone and bone cell properties [17–20]. These two genetically distinct inbred strains of mice are commonly known as B6 with low bone mass and C3H with high bone mass. Compared with B6 mice, C3H mice have higher peak bone density [21,22], lower rate of bone resorption [17,23] and higher serum alkaline phosphatase (ALP) activity [23]. The current study was undertaken to test the hypotheses that F responsive variations in bone metabolism are different between B6 and C3H mice; to assess bone resorption by the osteoclasts that developed in vitro in the difference.
Materials and Methods
Animals
Female B6 and C3H inbred mice were obtained from the Jackson Laboratory (Bar Harbor, ME, U.S.A.) at 3 weeks of age and were acclimated for one week prior to treatment with NaF. NaF was provided in the drinking water at concentrations of 0ppm, 50ppm and 100ppm F ion for 3 weeks. Each treatment/control group consisted of 6 mice. All animals were housed in the Division of Lab Animal Medicine facility within the Dental Research Center a fully AAALAC accredited unit and were maintained on a 12:12 hr light/dark cycle with an ambient temperature of 21°C. Mice were fed a constant nutrition LabDiet® 5001 (PMI® Nutrition International), which contained 0.95% calcium, 0.67% phosphorous, 4.5 IU/gm vitamin D3 and an average [F] of 6.56 ± 0.28 μg/gm. All experimental procedures were approved by the Institutional Animal Care and Use Committee at The University of North Carolina at Chapel Hill.
Sample collecting
Serum was collected from each mouse and then frozen at −80°C until used. Femurs, tibiae, and lumbar vertebrae from each mouse were dissected free of soft tissues. The right femurs were stored at −20°C prior to μCT and biomechanical testing. The tibiae were fixed in ice cold 10% neutral buffered formalin (10%NBF), analyzed by μCT, decalcified with buffered 0.25M EDTA (pH 7.5) for 1 week, and processed for standard paraffin embedding and sectioning. Lumbar vertebrae were stored at −20°C prior to μCT. Bone marrow cells were flushed from the left femurs and left tibiae with α-MEM and the erythrocytes lysed with 0.8% ammonium chloride.
Bone fluoride content
Femur samples were cleaned by removed the soft tissues, split open and cleaned of all marrow with cold PBS. The specimens were dried at room temperature for 30min, and then were defatted for 24h treatment of chloroform, another 24h treatment of ethyl ether with three changes, respectively. Prior to ashing the dried/defatted bones were weighed. The bones were ashed at 600°C for 8h and weighed again, dissolved in 1N HCl, neutralized by 1N NaOH and F concentration in each specimen were measured by the ion-specific F electrode, Orion 720A+ advanced ISE/pH/mV/ORP meter (Thermo Electron Corporation, USA, 2003).
Serum ELISA/EIA and chemistry assays
Sera from each animal was subjected to several immunoassays that assessed surrogate bone metabolism markers including intact parathyroid hormone (PTH) (Mouse) EIA (ALPCO Diagnostics, Salem NH); soluble receptor activator of nuclear factor (NF)-κ B ligand (sRANKL, the main stimulatory factor for the formation of mature osteoclasts and essential for their survival) mouse/rat EIA (ALPCO Diagnostics, Salem NH); mouse/rat osteoprotegerin (OPG, also called osteoclast inhibitory factor, a decoy receptor for RANKL) EIA (ALPCO Diagnostics, Salem NH), osteoclast-derived tartrate-resistant acid phosphatase form 5b (TRAP5b, a useful marker of bone resorption rate) MouseTRAP™ assay EIA (Immunodiagnostic Systems, Inc., Fountain Hills, AZ and SBA Sciences, Turku, Finland), mouse osteocalcin EIA (Biomedical Technologies, Inc., Stoughton, MA), and serum pyridinoline crosslink (PYD)(Metra Serum PYD, Quidel Corp., San Diego, CA). Each assay included adequate controls with known levels of target markers for quality control. The intra- and inter-assay precision (coefficient of variation, %) for these EIA/ELISA assays were 3.2% and 8.4% (PTH intra- and interassay, respectively); 4.2% and 9.0% (sRANKL intra- and inter-assay, respectively); 7.5% and 9.0% (OPG intra- and inter-assay, respectively); < 6.5% and < 8.0% (TRAP5b intra- and inter-assay, respectively), 6% and 8% (osteocalcin intra- and inter-assay, respectively), and 6–15% and 9–12% (PYD intra- and inter-assay, respectively). Sera from each animal was subjected to a suite of serum clinical chemistry tests were performed that included albumin, total alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), BUN, creatinine, Na, K, Cl, total Ca, PO4, Mg, and F. Microdirect analysis of serum F was performed according to the method of Vogel et al [24].
In situ bone osteoclast numbers
Quantitation of osteoclasts in demineralized bone sections was based on previously described procedures [17,25]. Fixed, demineralized, and paraffin embedded tibiae were sectioned and subjected to staining for tartrate resistant acid phosphatase (TRAP) with 0.1 mg/ml naphthol AS-MX phosphate and 0.6 mg/ml fast red violet LB salt in 0.1 M sodium acetate buffer, pH 5.0, containing 50mM sodium tartrate [26]. Osteoclasts were identified as TRAP positive cells on the trabecular bone surfaces. The numbers of osteoclasts on the sections were counted and the fractions of bone surface occupied by osteoclasts were measured in the proximal tibia trabeculae using a color camera microscopy imaging system (Nikon ECLIPSE 50i and Nikon Digital Camera DXM1200F, Japan) and the software of ImageJ (1.35s, NIH).
Osteoclast potential
The bone marrow cells were cultured (1.0 × 105 cells/0.5ml per well in a 48-well plate) for 6 days in α-MEM containing 10% FBS, 100U/ml penicillin, and 100μg/ml streptomycin. Cultures were fed every 3 days with medium containing rmM-CSF (20 ng/ml)(R&D, Minneapolis, MN), rhsRANKL (60 ng/ml)(PeproTech Inc., Rocky Hill, NJ) and maintained at 37°C in a humidified atmosphere of 5% CO2. On day 6, cells were fixed in 10% formalin and stained for TRAP with 0.1 mg/ml naphthol AS-MX phosphate and 0.6 mg/ml fast red violet LB salt in 0.1 M sodium acetate buffer, pH 5.0, containing 50mM sodium tartrate [26]. TRAP-positive cells with three or more nuclei were counted using phase-contrast microscopy (Nikon ECLIPSE TS100, Japan).
Hematopoietic colony forming cell (CFC) assays
Hematopoietic colonies were obtained by growing bone marrow cells in Methocult GF+ media (StemCell Technologies, Vancouver) consisting of 1% Methylcellulose in Iscove’s MDM, 15% Fetal Bovine Serum, 1% Bovine Serum Albumin, 10μg/ml rh Insulin, 200 μg/ml Human Transferrin (Iron saturated), 10−4mM 2-Mercaptoethanol, 2mM L-glutamine, 50 ng/ml rm Stem Cell Factor, 10ng/ml rm IL-3, 10ng/ml rh IL-6, 3 units/ml rh Erythropoietin. The bone marrow cell populations were aliquoted at 2×104 per 35 mm dish and cultures were maintained at 37°C in a humidified atmosphere of 5% CO2. After 14 days, the dishes were scored for colony-forming units (CFUs) according to standard criteria. In some cases colonies were plucked and subjected to cytospin and stained with Giemsa to confirm cellular composition. In situ staining of methylcellulose colonies with benzidine was used to identify colonies containing erythroid cells. The numbers of different colonies (CFU-GEMM: Colony-Forming Unit-Granulocyte, Erythrocyte, Macrophage, and Megakaryocyte. CFU-GM: Colony-Forming Unit-Granulocyte, Macrophage. CFU-G: Colony-Forming Unit-Granulocyte. CFU-M: Colony-Forming Unit-Macrophage. BFU-E: Burst-Forming Unit-Erythrocyte) were counted in each dish using phase-contrast microscopy (Nikon ECLIPSE TS100, Japan).
μCT analysis
Femurs rehydrated in 0.9% NaCl and tibiae fixed in 10% NBF were scanned using the Skyscan 1074HR microCT (Skyscan, Aartselaar, Belgium) at the resolution of 20.5 μm/pixel. Standardized scanning and image reconstruction settings were used. Hydroxyapatite phantoms (250mg/cc and 750mg/cc) (CIRS, Inc., Norfolk, VA) were used in order to determine bone mineral densities within regions and volumes of interest. A variety of static histomorphometric parameters were calculated either using 3D based volume models or 2D cross-sectional images. The instrument chosen incorporates algorithms to yield quantitative bone parameters accepted by the American Society of Bone and Mineral Research. Trabecular regions of the proximal tibiae and L4/L5 vertebrae were used to determine bone mineral density (BMD); bone volume fraction (BV/TV); bone specific surface (BS/BV), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), trabecular number density (Tb.N), and structure model index (SMI). One mm cortical regions within the mid-diaphysis of the femurs were used to determine, mean total cross sectional bone area (B.Ar), mean total cross sectional bone perimeter (B.Pm), mean polar moment of inertia (MMI), and cortical BMD.
Biomechanical testing
Rehydrated femurs were mechanically tested in a custom three-point bending jig. The bones were loaded in an anterior to posterior direction to failure on an EnduraTEC 3200 ELF (EnduraTEC Systems Group Bose Corporation, Minnetonka, MN), using a 225N load cell with force resolution of 0.01N. The two lower supports were set 10.0mm apart for the femurs. The crosshead speed during testing was 0.2 mm/s, and force-displacement data were collected every 0.01s. From the data, force versus displacement graphs were created and test curves were analyzed to determine standard mechanical properties, the ultimate force (Fu; N), the yield force (Fy; N), stiffness (S; N/mm), and postyield energy to failure (U; mJ). The yield point was defined using a 0.015mm offset parallel to the stiffness. Fu reflects the strength of the bone, while S reflects the rigidity, and U is the energy necessary to cause a fracture.
Statistic analysis
Data were reported as mean ± SD. Statistical analyses were performed using One-way ANOVA followed by Bonferroni as a post hoc test. The extent to which values of two variables were proportional to each other was determined using two-tailed Pearson Correlation. Adjusted p-values ≤ 0.05 were considered significant.
Results
Serum chemistries
F treatment had no significant effects on serum Na, Cl, Ca, PO4, Mg, BUN, creatinine, albumin, AST, or ALT in either mouse strain. Serum [F] increased in a dose dependent manner and were highly correlated with F exposure (r = 0.887, p < 0.001). Comparisons of serum [F] between strains at each F treatment were not statistically significant (0ppm p = 0.179, 50ppm p = 0.090, and 100ppm p = 0.546) (Table 1).
Table 1.
Serum Fluoride Analysis & Bone Fluoride Content
| Fluoride Treatment (ppm)a | Strain B6 | Strain C3H | |
|---|---|---|---|
| 0 | 3.15 ±2.08 | 1.62 ± 0.289 | |
| Serum Fluorideb (μM/L ± SD) | 50 | 6.36 ±1.58 | 8.31 ± 2.29 |
| 100 | 13.33 ± 3.32 | 14.46 ± 2.09 | |
| 0 | 699.49 ± 52.63 | 492.19 ±58.59 | |
| Bone Fluoride Contentc (μg/g ashed bone +/− SD) | 50 | 3299.72 ± 286.52* | 1941.90 ± 342.81* |
| 100 | 5255.49 ± 319.55* | 3236.34 ± 1006.73* |
4-week-old female mice were treated for 3 weeks and provided food and water ad libitum.
20μl serum assayed in triplicate according to method of Vogel et al. [24], n = 6/treatment/control group. Comparisons of serum [F] between strains at each F treatment were statistically NS
Bone fluoride content assay, n = 6/treatment/control group.
significant difference (p < 0.01) between F treatment group and control (0ppm) group.
Bone F content
The data (Table 1) showed that both strains significantly increased bone fluoride content with increasing fluoride exposure (both strains p < 0.001) with a greater increase in B6. B6 mice started with baseline bone [F] 142% greater than C3H and increased 5-fold at 50ppm and 7.5-fold at 100ppm over baseline concentration. Within each strain and between each treatment group bones [F] were significantly different (p < 001 for B6 and p < 0.01 for C3H). These increases in both strains highly correlated to increasing fluoride exposure (B6: r = 0.988 p < 001 and C3H r = 0.894, p < 001).
Bone Metabolism Markers
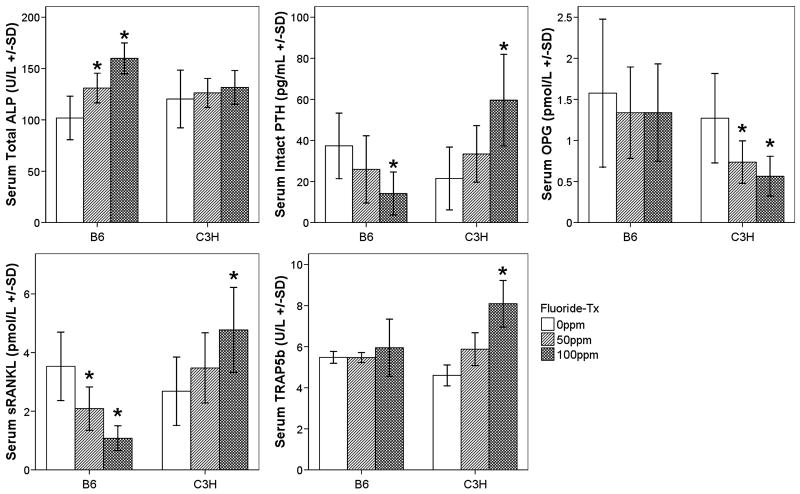
Total ALP activity increased in a dose dependent manner in the B6 strain only (p < 0.001), while intact PTH and sRANKL declined (p = 0.045, p < 0.001, respectively) (Fig. 1). C3H mice demonstrated increases in serum TRAP5b (p < 0.001, Fig. 1), intact PTH (p = 0.004, Fig. 1), sRANKL (p = 0.022, Fig. 1) and a decrease in serum OPG (p = 0.005, Fig. 1) in response to F exposure. These strain specific responses correlated with increased F exposure (B6, PTH: r = −0.582, p = 0.011; sRANKL: r = −0.791, p < 0.001; ALP: r = 0.836, p < 0.001 and C3H, PTH: r = 0.692, p = 0.001; sRANKL: r = 0.581, p = 0.006; OPG: r = −0.627, p = 0.002; TRAP5b: r = 0.876, p < 0.001). Within each strain and between each group serum osteocalcin levels did not significantly change. Additionally no significant trends were observed. Similarly for C3H PYD levels were not significantly different between treatment and control groups. B6 mice were not analyzed for serum PYD.
Figure 1.
Serum ALP (U/L) (Colorimetric method), intact PTH (pg/L), sRANKL (pmol/L), OPG (pmol/L), and TRAP5b (U/L) (ELISAs) mean ± SD, n = 6 per treatment/control group. * = significant difference (p < 0.05) between F treatment group and control (0ppm) group. White boxes (0ppm), light grey (50ppm), and dark grey (100ppm)
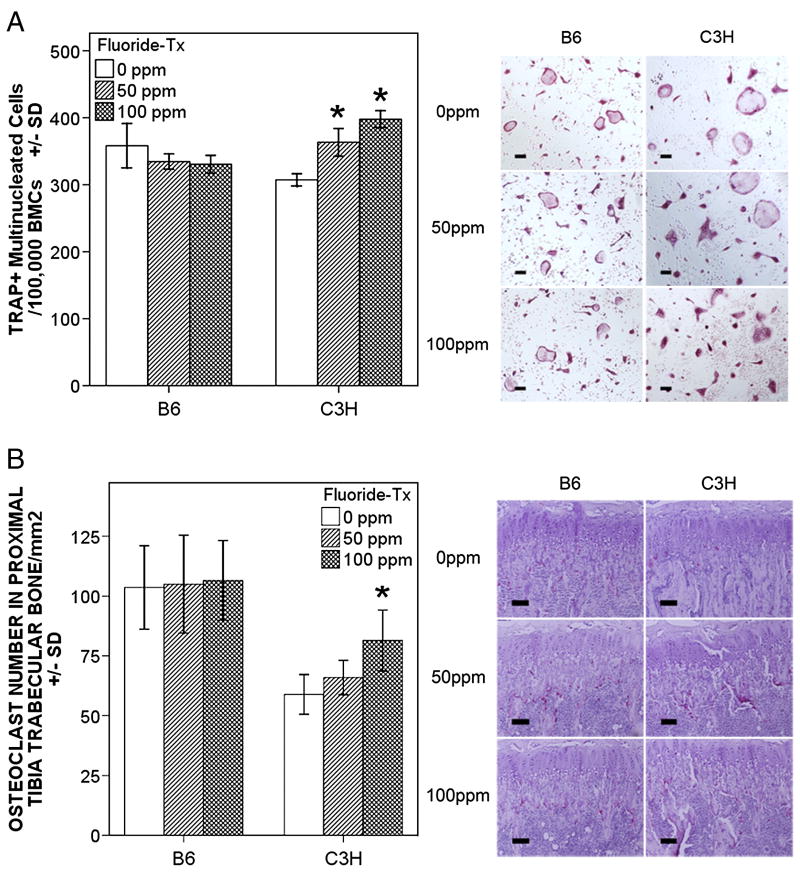
Osteoclast potential
At baseline (0ppm [F] control) osteoclast potential was greater for the B6 strain (p = 0.028) compared to C3H. Following F treatment there was a significant (p < 0.001) dose response increase in osteoclast potential for the C3H strain only. At 50ppm [F] osteoclast potential was 118% of control and at 100ppm [F] osteoclast potential was 130% of control (Fig. 2A). The increase in osteoclast potential in C3H mice was also highly correlated with: increased F exposure (r = 0.936, P < 0.001); serum PTH (r = 0.667, p = 0.002); serum RANKL (r = 0.565, p = 0.008); serum OPG (r = −0.648, p = 0.001); serum TRAP5b (r = 0.853, p < 0.001); and bone osteoclast numbers (r = 0.688, p = 0.019).
Figure 2.
Panel A mCSF and RANKL induced osteoclastogenesis from bone marrow cells. Data are shown as mean ± SD, n = 4 per treatment/control group, experiments were repeated in duplicate or triplicate. Only for C3H a significant difference ( * p < 0.01) between F treatment (50 and 100ppm) groups and control (0ppm) group was observed. Right side of panel illustrates TRAP(+) osteoclasts in bone marrow culture. Panel B. Osteoclasts in situ. Data are shown as mean ± SD, n = 6 per treatment/control group. Only for C3H a significant difference ( * p < 0.01) between control 0ppm and 100ppm F was observed. Right panel illustrates TRAP(+) cells along the trabecular bone surfaces, scale bar = 100 μm. White boxes (0ppm), light grey (50ppm), and dark grey (100ppm)
In situ Osteoclasts
Significant differences were seen between B6 and C3H control groups (p = 0.001) (Fig. 2B). B6 mice demonstrated higher osteoclast numbers compared to C3H. Following F exposure C3H mice showed increases in osteoclast numbers per total trabecular bone area in a dose dependent manner (p = 0.007)(Fig. 2B). These increases were highly correlated with: increased F exposure (r = 0.710, p = 0.002); serum PTH (r = 0.728, p = 0.001); serum RANKL (r = 0.726, p = 0.001); serum OPG (r = −0.628, p = 0.009); and serum TRAP5b (r = 0.727, p = 0.001).
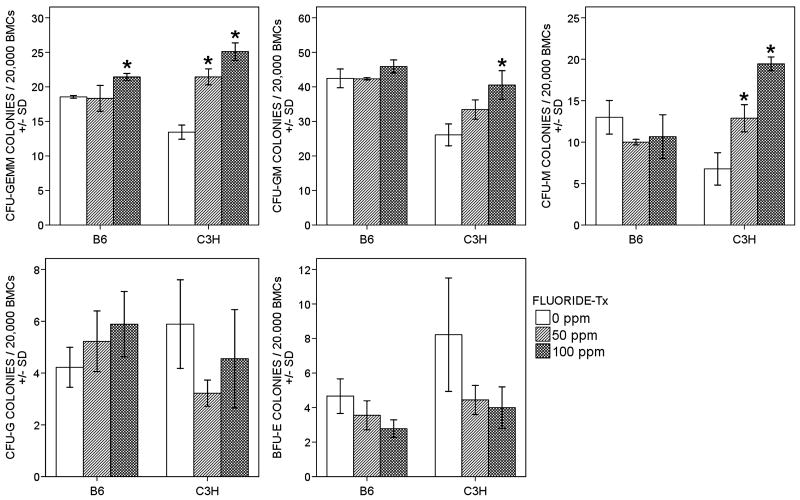
CFC assays
F exposure had very little effect on CFUs in B6 mice with an increase in CFU-GEMMs (p = 0.025, 15.52% at 100ppm). While in C3H, significant increases were observed in CFU-GEMM (p < 0.001, 162% at 50ppm and 192% at 100ppm), CFU-GM (p = 0.006, 127% at 50ppm and 158% at 100ppm), and CFU-M (p < 0.001, 186% at 50ppm and 271% at 100ppm) (Fig. 3). Changes in CFUs found in both strains highly correlated with increased F (For B6, CFU-GEMM: r = 0.700, p = 0.036; for C3H, CFU-GEMM: r = 0.960, p < 0.001; CFU-GM: r = 0.904, p = 0.001; CFU-M: r = 0.971, p < 0.001). CFU-G were not significantly changed with F in either strain (B6: p = 0.247; C3H: p = 0.175). In both strains, there was a trend for BFU-E to decline (Fig. 3), for B6 (24% at 50ppm and 40% at 100ppm) compared to the control correlating with increased F (r = −0.757, p = 0.018), and for C3H (46% at 50ppm and 51% at 100ppm) that correlated with increased F (r = −0.678, p = 0.045) (Fig. 3)
Figure 3.
Hematopoietic Colony-forming Cell Assays (CFC Assays) were used to quantitate multi-potential progenitors and single lineage-restricted progenitors of the erythroid, granulocytic, monocyte-macrophage and megakaryocytic pathways. Graphs show the number of colonies/35mm dish. Data are shown as mean ± SD, n = 3 per treatment/control group, experiments were repeated in duplicate or triplicate. * = significant difference (p < 0.01) between F treatment group and control (0ppm) group. White boxes (0ppm), light grey (50ppm), and dark grey (100ppm)
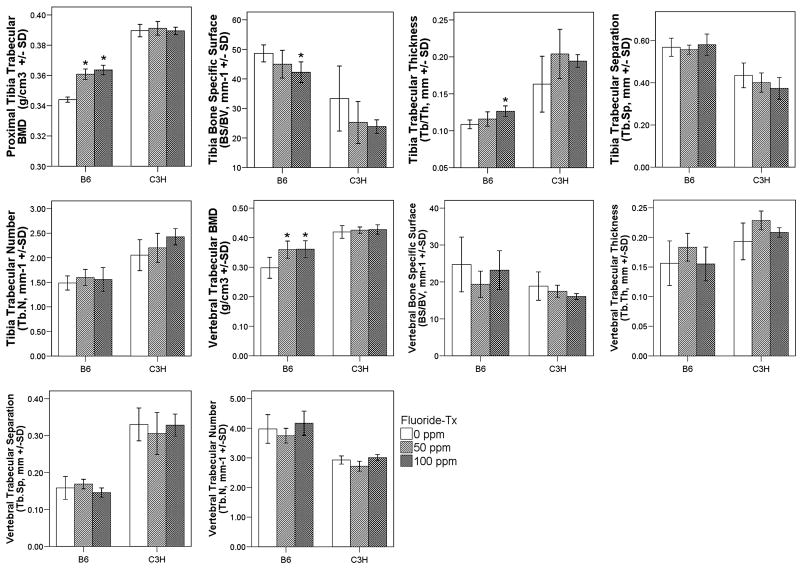
Bone Morphology and Mechanical Properties
Tibia trabecular bone quantity and architecture were significantly different between the different F treatment groups for B6 mice. Dose dependent increases in tibia trabecular BMD (p = 0.001), were associated with decreased BS/BV (p = 0.047), increased Tb.Th (p = 0.007), and decreased SMI (p = 0.046) (Fig. 4). For C3H, however there were no significant differences between the F treatment groups in tibia trabecular bone quantity, some trends were observed in tibia trabecular bone architecture (Fig. 4). Vertebral BMD was increased in B6 mice and only significant between the control and the high fluoride treatment group (0ppm and 100ppm, p = 0.011), the other parameters (BS/BV, Tb.Th, Tb.Sp, and Tb.N.) were not significantly different between control and fluoride treatment in either mouse strain (Fig. 4).
Figure 4.
Trabecular bone morphology and BMD in the regions of the proximal tibia and lumbar vertebrae from different F treatment groups of C3H and B6 mice. Values are means ± SD, n = 6 per treatment group/control. *: significant difference (p < 0.05) between current F treatment group and control (0ppm) group. White boxes (0ppm), light grey (50ppm), and dark grey (100ppm)
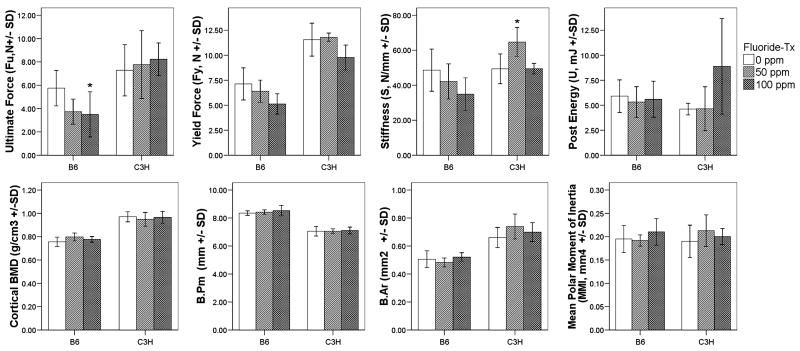
No significant changes in femur cortical bone were observed between the F treatment groups for either mouse strain,. Both strains only demonstrated changing trends in femur mechanical properties (Fig. 5). Small but significant decreases in ultimate force (Fu; N) (p < 0.05) were observed for the B6 strain and only at the high (100ppm) F treatment.
Figure 5.
Cortical bone BMD, morphology, and mechanical properties in the region of the mid-diaphysis of femur from different F treatment groups of C3H and B6 mice. Values are means ± SD, n = 6 per treatment/control group. *: significant difference (p < 0.05) between current F treatment group and control (0ppm) group. White boxes (0ppm), light grey (50ppm), and dark grey (100ppm)
Discussion
The B6 and C3H inbred mouse strains have been extensively investigated for their contrasting bone properties, hallmarked by differences in peak bone mass [22]. When compared to C3H B6 mice respond differently to stimuli affecting bone formation or resorption. These differences are particularly evident in disuse studies [18]. The low bone mass B6 mice appear to have enhanced responsiveness to anabolic signals whereas the high bone mass C3H mice more refractory [27]. In this study, the majority of F intake came from the drinking water. Monitoring serum [F] allowed us to obtain in mice F exposure levels (~8μM at 50ppm drinking water and ~15μM at 100ppm drinking water) that are relevant and typical of human exposures [8,28]. Serum F remained consistent between B6 and C3H yet bone F varied. B6 mice began with higher bone [F] and accumulated more F in the bone than C3H. Similar differences between strains were observed in a study involving A/J, 129P3/J, and SWR/J mice [29]. B6 and C3H mice contrasted with regards to fluoride’s actions. For the B6 strain, the anabolic action of F was favored and was consistent with reports that F stimulates bone formation [12,30–32], including increases in serum ALP activity [2], and increasing mineralized bone volume and trabecular thickness [12,33]. These changes were not site specific since trabecular bone in the proximal tibia was comparable to trabecular bone in the vertebrae. Small but significant decreases in femur mechanical properties were also observed, but only at the highest fluoride treatment. We could not separate the contribution of direct fluoride accumulation in bone from the quality of new bone resulting from the anabolic action of fluoride. However, for C3H strain fluoride’s action favored osteoclastogenesis. While this was supported by a suite of serum biomarkers, increased bone marrow osteoclast potential, and the increase in the number of osteoclasts residing in the trabecular bone we saw no significant evidence of bone resorption (loss of bone mass or increase in pyridoxine crosslinks). It is possible that these mice have not reached a threshold for detectable bone loss due to the short treatment period or that the osteoclasts along the bone surfaces are impaired. Regarding the later, it is possible that the accumulation of fluoride within the mineral interferes with normal osteoclast function [34] Historically such inhibition of osteooclast function has been shown when the [F] is quite high [35]. So this possible mechanism remains speculative. Finally bone quality was not altered following fluoride treatment in the C3H strain. We observed effects on the hematopoietic compartment of the bone marrow. F shifted hematopoietic differentiation along the monocyte/macrophage lineage with increases in CFU-GEMM, CFU-GM, and CFU-M in the C3H strain and a modest increase in CFU-GEMM in the B6 strain only at the highest F dose. Fluoride’s effects on hematopoiesis have been previously observed. At modest concentrations (<500 μM) NaF has been shown to promote differentiation of the human promyelocytic tumor cells (HL-60) to granulocyte-like cells and induce differentiation of mouse bone marrows cells along the granulocytic pathway in vitro [36,37]. The dramatic effect observed in the C3H strain may serve as a mechanism to provide an increased pool of cells capable of becoming osteoclasts. It has been established that both CFU-GM and CFU-GEMM have high osteoclastic potential whereas osteoclastic potential of CFU-M is considerably less [38,39].
More interestingly, our data have shown that PTH may play an important role in effect of F on bone in C3H mice. In addition to its action as an anabolic agent in bone, PTH is an important factor in osteoclastogenesis. This later effect is mediated via osteoblasts which in turn produce factors like RANKL and thereby stimulating osteoclastogenesis [40–42]. Also in humans there have been repeated associations between increased F intake and concomitant increases in circulating PTH and in the development of pseudohyperparathyroidism [43–45]. Elevated PTH associated with some cases of skeletal fluorosis may be a factor in the accompanying osteoporosis. Our data demonstrated in C3H mice PTH was highly correlated with not only increased F exposure but also the changes of bone resorption serum markers (RANKL, OPG and TRAP) and osteoclast potential (osteoclastogenesis and osteoclast progenitor recruitment). There is ample support that PTH is capable of driving hematopoiesis [46,47]. Whether PTH is directly implicated in the shift of hematopoietic differentiation observed remains to be determined.
In summary, increasing F doses at physiological levels has strain specific effects in mice. Prominent among these strain-specific changes in bone physiology is the increase in intact PTH observed in C3H/HeJ mice. Parallel changes in osteoclastogenesis biomarkers were observed in C3H/HeJ but not C57BL/6J mice. This dose dependent effect of F may have direct or indirect effects on osteoclastic or lineage specific cells. In C3H/HeJ specific increases in CFU-M (monocyte/macrophage), CFU-GM (granulocyte and macrophage) and CFU-GEMM (multipotential) suggest a role for F in early stage of osteoclastogenesis.
Acknowledgments
Drs. Angeles Martinez-Mier and Armando Soto are gratefully acknowledged for their technical support with serum F analyses, Dr. Sadaaki Takeyama for his critically reviewing the manuscript, and Mr. David Holland for his technical support with the microCT. This works was supported by the NIH/NIDCR DE14853 (ETE).
Footnotes
Conflict of Interest Page
All authors have no conflicts of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Palmer C, Wolfe SH. American Dietetic Association. Position of the american dietetic association: The impact of fluoride on health. J Am Diet Assoc. 2005;105:1620–8. doi: 10.1016/j.jada.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Farley JR, Wergedal JE, Baylink DJ. Fluoride directly stimulates proliferation and alkaline phosphatase activity of bone-forming cells. Science. 1983;222:330–2. doi: 10.1126/science.6623079. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Yin Y, Gilula LA, Wilson AJ. Endemic fluorosis of the skeleton: Radiographic features in 127 patients. AJR Am J Roentgenol. 1994;162:93–8. doi: 10.2214/ajr.162.1.8273699. [DOI] [PubMed] [Google Scholar]
- 4.Christie DP. The spectrum of radiographic bone changes in children with fluorosis. Radiology. 1980;136:85–90. doi: 10.1148/radiology.136.1.7384528. [DOI] [PubMed] [Google Scholar]
- 5.Chachra D, Turner CH, Dunipace AJ, Grynpas MD. The effect of fluoride treatment on bone mineral in rabbits. Calcif Tissue Int. 1999;64:345–51. doi: 10.1007/s002239900630. [DOI] [PubMed] [Google Scholar]
- 6.Grynpas MD. Fluoride effects on bone crystals. J Bone Miner Res. 1990;(5 Suppl 1):S169–75. doi: 10.1002/jbmr.5650051362. [DOI] [PubMed] [Google Scholar]
- 7.Turner CH, Garetto LP, Dunipace AJ, et al. Fluoride treatment increased serum IGF-1, bone turnover, and bone mass, but not bone strength, in rabbits. Calcif Tissue Int. 1997;61:77–83. doi: 10.1007/s002239900299. [DOI] [PubMed] [Google Scholar]
- 8.Lau KH, Baylink DJ. Molecular mechanism of action of fluoride on bone cells. J Bone Miner Res. 1998;13:1660–7. doi: 10.1359/jbmr.1998.13.11.1660. [DOI] [PubMed] [Google Scholar]
- 9.Haguenauer D, Welch V, Shea B, Tugwell P, Adachi JD, Wells G. Fluoride for the treatment of postmenopausal osteoporotic fractures: A meta-analysis. Osteoporos Int. 2000;11:727–38. doi: 10.1007/s001980070051. [DOI] [PubMed] [Google Scholar]
- 10.Cranney A, Guyatt G, Griffith L, et al. Meta-analyses of therapies for postmenopausal osteoporosis. IX: Summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocr Rev. 2002;23:570–8. doi: 10.1210/er.2001-9002. [DOI] [PubMed] [Google Scholar]
- 11.Riggs BL, Hodgson SF, O’Fallon WM, et al. Effect of fluoride treatment on the fracture rate in postmenopausal women with osteoporosis. N Engl J Med. 1990;322:802–9. doi: 10.1056/NEJM199003223221203. [DOI] [PubMed] [Google Scholar]
- 12.Carter DR, Beaupre GS. Effects of fluoride treatment on bone strength. J Bone Miner Res. 1990;5(Suppl 1):S177–84. doi: 10.1002/jbmr.5650051372. [DOI] [PubMed] [Google Scholar]
- 13.Sogaard CH, Mosekilde L, Schwartz W, Leidig G, Minne HW, Ziegler R. Effects of fluoride on rat vertebral body biomechanical competence and bone mass. Bone. 1995;16:163–9. doi: 10.1016/s8756-3282(94)00025-5. [DOI] [PubMed] [Google Scholar]
- 14.Turner CH, Hasegawa K, Zhang W, Wilson M, Li Y, Dunipace AJ. Fluoride reduces bone strength in older rats. J Dent Res. 1995;74:1475–81. doi: 10.1177/00220345950740080701. [DOI] [PubMed] [Google Scholar]
- 15.Everett ET, McHenry MA, Reynolds N, et al. Dental fluorosis: Variability among different inbred mouse strains. J Dent Res. 2002;81:794–8. doi: 10.1177/0810794. [DOI] [PubMed] [Google Scholar]
- 16.Vieira AP, Hanocock R, Eggertsson H, Everett ET, Grynpas MD. Tooth quality in dental fluorosis genetic and environmental factors. Calcif Tissue Int. 2005;76:17–25. doi: 10.1007/s00223-004-0075-3. [DOI] [PubMed] [Google Scholar]
- 17.Linkhart TA, Linkhart SG, Kodama Y, et al. Osteoclast formation in bone marrow cultures from two inbred strains of mice with different bone densities. J Bone Miner Res. 1999;14:39–46. doi: 10.1359/jbmr.1999.14.1.39. [DOI] [PubMed] [Google Scholar]
- 18.Judex S, Garman R, Squire M, Donahue LR, Rubin C. Genetically based influences on the site-specific regulation of trabecular and cortical bone morphology. J Bone Miner Res. 2004;19:600–6. doi: 10.1359/JBMR.040101. [DOI] [PubMed] [Google Scholar]
- 19.Turner CH, Hsieh YF, Muller R, et al. Genetic regulation of cortical and trabecular bone strength and microstructure in inbred strains of mice. J Bone Miner Res. 2000;15:1126–31. doi: 10.1359/jbmr.2000.15.6.1126. [DOI] [PubMed] [Google Scholar]
- 20.Turner CH, Hsieh YF, Muller R, et al. Variation in bone biomechanical properties, microstructure, and density in BXH recombinant inbred mice. J Bone Miner Res. 2001;16:206–13. doi: 10.1359/jbmr.2001.16.2.206. [DOI] [PubMed] [Google Scholar]
- 21.Beamer WG, Donahue LR, Rosen CJ. Genetics and bone. using the mouse to understand man. J Musculoskelet Neuronal Interact. 2002;2:225–31. [PubMed] [Google Scholar]
- 22.Beamer WG, Donahue LR, Rosen CJ, Baylink DJ. Genetic variability in adult bone density among inbred strains of mice. Bone. 1996;18:397–403. doi: 10.1016/8756-3282(96)00047-6. [DOI] [PubMed] [Google Scholar]
- 23.Dimai HP, Linkhart TA, Linkhart SG, et al. Alkaline phosphatase levels and osteoprogenitor cell numbers suggest bone formation may contribute to peak bone density differences between two inbred strains of mice. Bone. 1998;22:211–6. doi: 10.1016/s8756-3282(97)00268-8. [DOI] [PubMed] [Google Scholar]
- 24.Vogel GL, Carey CM, Chow LC, Ekstrand J. Fluoride analysis in nanoliter- and microliter-size fluid samples. J Dent Res. 1990;(69 Spec No 522):8. doi: 10.1177/00220345900690S106. discussion 556–7. [DOI] [PubMed] [Google Scholar]
- 25.Boyce BF, Wright K, Reddy SV, et al. Targeting simian virus 40 T antigen to the osteoclast in transgenic mice causes osteoclast tumors and transformation and apoptosis of osteoclasts. Endocrinology. 1995;136:5751–9. doi: 10.1210/endo.136.12.7588333. [DOI] [PubMed] [Google Scholar]
- 26.Hirotani H, Tuohy NA, Woo JT, Stern PH, Clipstone NA. The calcineurin/nuclear factor of activated T cells signaling pathway regulates osteoclastogenesis in RAW264.7 cells. J Biol Chem. 2004;279:13984–92. doi: 10.1074/jbc.M213067200. [DOI] [PubMed] [Google Scholar]
- 27.Judex S, Donahue LR, Rubin C. Genetic predisposition to low bone mass is paralleled by an enhanced sensitivity to signals anabolic to the skeleton. FASEB J. 2002;16:1280–2. doi: 10.1096/fj.01-0913fje. [DOI] [PubMed] [Google Scholar]
- 28.Pak CY. Fluoride and osteoporosis. Proc Soc Exp Biol Med. 1989;191:278–86. doi: 10.3181/00379727-191-42921. [DOI] [PubMed] [Google Scholar]
- 29.Mousny M, Banse X, Wise L, et al. The genetic influence on bone susceptibility to fluoride. Bone. 2006;39:1283–9. doi: 10.1016/j.bone.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Caverzasio J, Palmer G, Bonjour JP. Fluoride: Mode of action. Bone. 1998;22:585–9. doi: 10.1016/s8756-3282(98)00058-1. [DOI] [PubMed] [Google Scholar]
- 31.Pak CY, Zerwekh JE, Antich P. Anabolic effects of fluoride on bone. Trends Endocrinol Metab. 1995;6:229–34. doi: 10.1016/1043-2760(95)00111-t. [DOI] [PubMed] [Google Scholar]
- 32.Battmann A, Resch H, Libanati CR, et al. Serum fluoride and serum osteocalcin levels in response to a novel sustained-release monofluorophosphate preparation: Comparison with plain monofluorophosphate. Osteoporos Int. 1997;7:48–51. doi: 10.1007/BF01623460. [DOI] [PubMed] [Google Scholar]
- 33.Balena R, Kleerekoper M, Foldes JA, et al. Effects of different regimens of sodium fluoride treatment for osteoporosis on the structure, remodeling and mineralization of bone. Osteoporos Int. 1998;8:428–35. doi: 10.1007/s001980050087. [DOI] [PubMed] [Google Scholar]
- 34.Inoue M, Nagatsuka H, Tsujigiwa H, et al. In vivo effect of fluoride-substituted apatite on rat bone. Dent Mater J. 2005;24:398–402. doi: 10.4012/dmj.24.398. [DOI] [PubMed] [Google Scholar]
- 35.Taylor ML, Maconnachie E, Frank K, Boyde A, Jones SJ. The effect of fluoride on the resorption of dentine by osteoclasts in vitro. J Bone Miner Res. 1990;(5 Suppl 1):S121–30. doi: 10.1002/jbmr.5650051391. [DOI] [PubMed] [Google Scholar]
- 36.Kawase T, Oguro A, Orikasa M, Burns DM. Characteristics of NaF-induced differentiation of HL-60 cells. J Bone Miner Res. 1996;11:1676–87. doi: 10.1002/jbmr.5650111111. [DOI] [PubMed] [Google Scholar]
- 37.Oguro A, Kawase T, Orikasa M. NaF induces early differentiation of murine bone marrow cells along the granulocytic pathway but not the monocytic or preosteoclastic pathway in vitro. In Vitro Cell Dev Biol Anim. 2003;39:243–8. doi: 10.1290/1543-706X(2003)039<0243:NIEDOM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 38.Hodge JM, Kirkland MA, Aitken CJ, et al. Osteoclastic potential of human CFU-GM: Biphasic effect of GM-CSF. J Bone Miner Res. 2004;19:190–9. doi: 10.1359/JBMR.0301232. [DOI] [PubMed] [Google Scholar]
- 39.Menaa C, Kurihara N, Roodman GD. CFU-GM-derived cells form osteoclasts at a very high efficiency. Biochem Biophys Res Commun. 2000;267:943–6. doi: 10.1006/bbrc.1999.2042. [DOI] [PubMed] [Google Scholar]
- 40.Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20:345–57. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 41.Ma YL, Cain RL, Halladay DL, et al. Catabolic effects of continuous human PTH (1--38) in vivo is associated with sustained stimulation of RANKL and inhibition of osteoprotegerin and gene-associated bone formation. Endocrinology. 2001;142:4047–54. doi: 10.1210/endo.142.9.8356. [DOI] [PubMed] [Google Scholar]
- 42.Potts JT. Parathyroid hormone: Past and present. J Endocrinol. 2005;187:311–25. doi: 10.1677/joe.1.06057. [DOI] [PubMed] [Google Scholar]
- 43.Chadha M, Kumar S. Fluorosis-induced hyperparathyroidism mimicking a giant-cell tumour of the femur. J Bone Joint Surg Br. 2004;86:594–6. [PubMed] [Google Scholar]
- 44.Gupta SK, Khan TI, Gupta RC, et al. Compensatory hyperparathyroidism following high fluoride ingestion - a clinico - biochemical correlation. Indian Pediatr. 2001;38:139–46. [PubMed] [Google Scholar]
- 45.Sivakumar B, Krishnamachari KA. Circulating levels of immunoreactive parathyroid hormone in endemic genu valgum. Horm Metab Res. 1976;8:317–9. doi: 10.1055/s-0028-1093624. [DOI] [PubMed] [Google Scholar]
- 46.Whitfield JF. Parathyroid hormone: A novel tool for treating bone marrow depletion in cancer patients caused by chemotherapeutic drugs and ionizing radiation. Cancer Lett. 2006 doi: 10.1016/j.canlet.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Whitfield JF. Parathyroid hormone (PTH) and hematopoiesis: New support for some old observations. J Cell Biochem. 2005;96:278–84. doi: 10.1002/jcb.20526. [DOI] [PubMed] [Google Scholar]