Abstract
The mesolimbic dopaminergic (ML-DA) system has been recognized for its central role in motivated behaviors, various types of reward, and, more recently, in cognitive processes. Functional theories have emphasized DA's involvement in the orchestration of goal-directed behaviors, and in the promotion and reinforcement of learning. The affective neuroethological perspective presented here, views the ML-DA system in terms of its ability to activate an instinctual emotional appetitive state (SEEKING) evolved to induce organisms to search for all varieties of life-supporting stimuli and to avoid harms.
A description of the anatomical framework in which the ML system is embedded is followed by the argument that the SEEKING disposition emerges through functional integration of ventral basal ganglia (BG) into thalamocortical activities. Filtering cortical and limbic input that spread into BG, DA transmission promotes the “release” of neural activity patterns that induce active SEEKING behaviors when expressed at the motor level. Reverberation of these patterns constitutes a neurodynamic process for the inclusion of cognitive and perceptual representations within the extended networks of the SEEKING urge. In this way, the SEEKING disposition influences attention, incentive salience, associative learning, and anticipatory predictions.
In our view, the rewarding properties of drugs of abuse are, in part, caused by the activation of the SEEKING disposition, ranging from appetitive drive to persistent craving depending on the intensity of the affect. The implications of such a view for understanding addiction are considered, with particular emphasis on factors predisposing individuals to develop compulsive drug seeking behaviors.
Keywords: Mesolimbic, Dopamine, Motivation, Reward, Accumbens, Addiction
1. INTRODUCTION
1.1. The mesolimbic dopamine (ML-DA) system
The ML-DA system (Fig.1) has received considerable attention due to its involvement in a range of psychological processes and neuropsychiatric diseases. In fact, after the development of a DA theory of schizophrenia (Carlsson, 1974; 1978; Snyder, 1972; Meltzer & Stahl, 1976), additional ML-DA hypotheses have been proposed to explain addiction (Wise & Bozarth, 1981; 1987; Koob, 1992), attention deficit hyperactivity disorder (ADHD) (Oades, 1987; Levy, 1991; Russel, 2000), depression (Willner, 1983a, 1983b, Dailly et al., 2004) as well as global behavioral activation (Gray, 1995) ranging from response persistence to behavioral compulsions (Salamone & Correa, 2002; Everitt & Robbins, 2005).
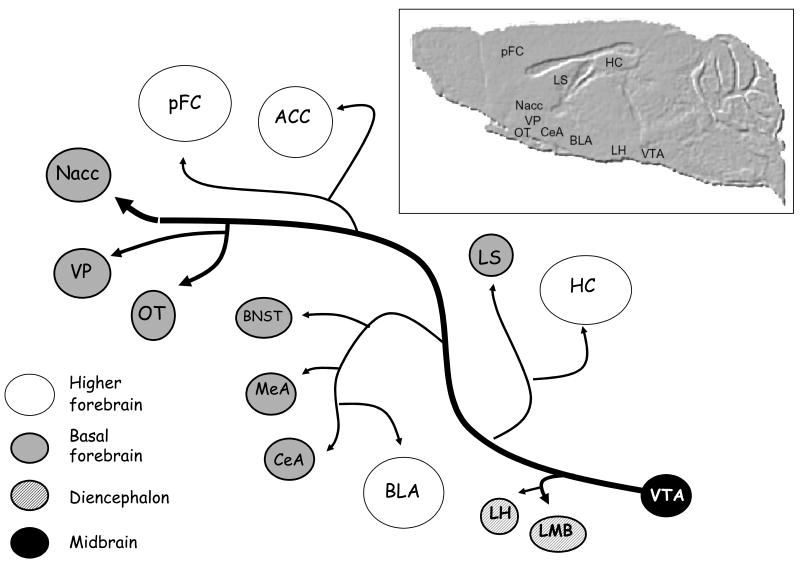
Figure 1. The ML-DA system.
The figure shows a schematic representation of the main forebrain areas reached by the mesolimbic DA system (Swanson, 1982; German & Manaye, 1993; Haber & Fudge, 1997). According to anatomical and evolutionistic criteria (Swanson 2000), the structures innervated by ML-DA have been divided in dienchephalic, basal forebrain, and higher forebrain areas.
Midbrain: VTA = ventral tegmental area
Diencephalon: LH = lateral hypothalamus, LMB = lateral mammillary body
Basal forebrain: Nacc = nucleus accumbens, VP = ventral pallidum, OT = olfactory tubercle, CeA = central nucleus of amygdala, MeA = medial nucleus of the amygdala, BNST = bed nucleus of stria terminalis, LS = lateral septum.
Higher forebrain: pFC = prefrontal cortex, ACC = anterior cingulated cortex, BLA = basolateral amygdala, HC = hippocampal complex.
Localized electrical brain stimulation studies (Olds & Milner, 1954; Heath, 1964, Olds, 1977; Wauquier & Rolls, 1976) have implicated the ML-DA in positive rewarding states (Wise, 1978; 1981; Wise & Rompre, 1989) as well as in appetitive motivated behaviors (Panksepp, 1971, 1981a, 1982; 1986, 1998; Blackburn et al., 1987; 1989; Berridge & Robinson, 1998; Ikemoto & Panksepp, 1999). Since DA is also released in response to aversive stimuli and stress (Abercombie et al., 1989; Puglisi-Allegra et al., 1991; Rouge-Pont et al., 1993; Pruessner et al., 2004), it appears to promote generalized behavioral arousal under both positive as well as negative emotional conditions, perhaps in terms best conceptualized as the seeking of safety (Ikemoto & Panksepp, 1999). Moreover, the ML-DA system has recently been recognized for its role in the determination of personality traits, including “novelty” or “sensation” seeking (Bardo et al., 1996; Zuckerman, 1990), “extraversion” (Depue & Collins, 1999), and “impulsivity” (Cardinal et al., 2004).
Current interpretations of ML-DA functions diverge with respect to emphasis on unconditioned or behavioral priming effects (motivational theories) versus conditioned effects (learning theories). The “psychomotor activation” hypothesis (Wise & Bozarth, 1987), the “behavioral activation system” hypothesis (Gray, 1995), the “behavioral facilitation” hypothesis (Depue & Collins, 1999), the “SEEKING system hypothesis” (Panksepp, 1981; 1998; Ikemoto & Panksepp, 1999), the “wanting” hypothesis (Berridge & Robinson, 1998), and the “effort-regulation” hypothesis (Salamone & Correa, 2002; Salamone et al., 2003) all acknowledge a motivational interpretation of ML-DA functioning. They share a common perspective based on the classic distinction between appetitive and consummatory phases of motivated behaviors (Sherrington, 1906; Craig, 1918), and with relatively minor differences, consider the DA system as a fundamental drive for the expression of appetitive-approach behaviors.
The “reinforcement” (Fibiger, 1978; White & Milner, 1992; Everitt & Robbins, 2005) and the “reward” hypotheses (Wise, 1978; Wise & Rompre, 1989; Schultz, 1997; 1998; 2001; Spanagel & Weiss, 1999; Di Chiara, 2002; Wise, 2004), on the other hand, have largely focused on DA as a learning mediator. While motivational theories are interested in the proactive actions of DA transmission on future behaviors, learning theories tend to consider retroactive effects on strengthened associations among past events. Although modern incentive motivation concepts view rewards as promoters of motivational arousal and increased behavioral readiness (Bolles, 1972; Binda, 1974; Toates, 1986; Berridge & Robinson, 1998), learning theories consider that the “most important role of DA in incentive motivation is historical; it is the stamping-in of stimulus-reward association that has established incentive motivational value for previously neutral stimuli” (Wise, 2004).
Multiple attempts to integrate motivational and learning perspectives of ML-DA transmission have been pursued (e.g., Berridge, 2004; Toates, 2004; Koob, 2004), but a coherent evolutionary-ethological view of how brain DA promotes certain types of unconditional psychobehavioral tendencies is typically missing in most formulations. Therefore, a comprehensive hypothesis integrating new findings with earlier literature on rewarding electric brain stimulation has yet to emerge. In our opinion, such needed integration may be achieved by postulating a role of ML-DA in modifying primary-process emotional behaviors1 and internal affective states (Panksepp, 1998, 2005)2. In fact, emotions and affects have repercussion both on the way animals act in the world and learn through experience. As extensively described in previous works (Panksepp, 1981; 1998; Ikemoto & Panksepp, 1999), ML-DA promotes the emergence of the SEEKING emotional disposition3, which we envision as an affective urge that characterizes all motivated behaviors. This view has been around as long as the more recent incentive-salience and reinforcement-type theories, but has been typically ignored by those committed to behaviorist learning paradigm.
1.2. Functional anatomy of the mesencephalic DA projections
In mammals, most DA-containing neurons clustered within three major mesencephalic groups: A8 cells in the retrorubral field, A9 cells in the substantia nigra (SN) and A10 cells in the ventral tegmental area (VTA) (Dahlstrom & Fuxe, 1964; Ungerstedt, 1971; Lindvall & Bjorklund, 1974; Fallon & Moore, 1978; German et al., 1983; Arsenault et al., 1988; German & Manaje, 1993). Similar organizations of DA cell bodies have been demonstrated in reptiles (Smeets et al., 1987; Smeets, 1988; Gonzalez et al., 1994) and birds4 (Smits et al., 1990; Durstewitz et al., 1999). In addition, less dense aggregations of DA neurons inhabit the supramammillary region of the hypothalamus, the dorsal raphe and the periaqueductal gray (Swanson, 1982; Gaspar et al., 1983). Morphological characteristics, anatomical locations, ascending projections and their associations with arousal functions, have led many to assign DA neurons to the classical “reticular formation” (Moruzzi & Magoun, 1949; Schiebel & Schiebel, 1958; Leontovich & Zhukova, 1963). Placed within the context of the reticular activating system (Parvizi & Damasio, 2001), DA neurons are sensitive to various global states of organisms, and their ascending projections modulate brain arousal in accordance with those states (Geisler & Zahm, 2005).
The mesencephalic DA cell groups (A8; A9 and A10) lack clear anatomical boundaries, develop in parallel from common embryonic tissues (Olson & Seiger, 1972; Fallon & Moore, 1978; Hu et al., 2004), and partly overlap in their projection fields (Nauta et al., 1978). Their axons project largely to structures located in the anterior part of the forebrain, and modulate the activity of cognitive-executive reentrant circuits between the cortical mantle and the BG (Alexander et al., 1986; Kalivas et al., 1999) (Fig. 2). Such circuits are involved in the organization of practically all motivated behaviors, both highly flexible and more automatic. It is thought that BG-thalamocortical circuits produce adaptive behavioral flexibility, while their dysregulation underlies a whole plethora of neuropsychiatric diseases, from depression to obsessive-compulsive disorders, from addiction to Parkinson, etc. (Swerdlow & Koob, 1987; Robbins, 1990; Deutch, 1993; Kropotov & Etlinger, 1999; Jentsch et al., 2000; Graybiel & Rauch, 2000; Joel, 2001; Groenewegen, 2003). Resembling a spiraling, functional organization (Zahm and Brog, 1992), a special type of “state” process, information flow appears to exist between different loops of such circuitries with feed-forward processing from limbic regions (especially medial frontal areas), to executive and motor circuits (Heimer & Van Horsen, 2006). DA neurons thereby act as an intermediary of limbic-emotional and motivational action outflow (Haber et al., 2000; Joel & Weiner, 2000; Mogenson, et al., 1980b).
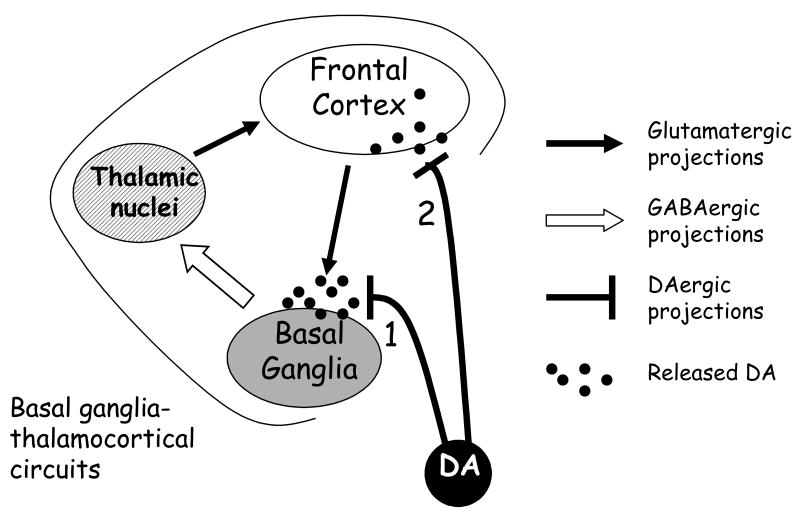
Figure 2. DA innervation of BG-thalamocortical circuits.
All ascending mesencephalic DA projections innervate the BG rather widely, while only the ML-DA system projects to the frontal cortex. Although the DA transmission in frontal cortex has received an increasing interest, our paper is mainly focused on the role of DA release in BG. In particular, DA transmission in ventral and dorsal striatal areas (the input areas of BG) modulates the communication between glutamatergic projections arriving from frontal cortex and GABAergic neurons located inside the striatum. In such a way, DA regulates the diffusion of neural activity patterns within basal ganglia-thalamocortical circuits. The figure doesn't show the segregation of BG-thalamocortical circuits described by Alexander and coll. (1986), but the schematic representation can be applied to limbic, associative or motor loops of those circuits.
Although DA cell groups form an anatomical continuum, the ML-DA system has been differentiated from the nigrostriatal (NS) DA system on the basis of anatomical, and functional criteria (Bernheimer et al., 1973; Ungerstedt et al., 1974). The ML-DA system (Fig.1), situated more medially in the brain, is more ancient in brain evolution than the more laterally situated NS-DA circuitry, and it has been more clearly implicated in the regulation of intentional, motivated movements, in flexible-emotive behaviors, and in the process of “reward” than the laterally situated NS-DA fields (Papp & Bal, 1987; Wise & Bozarth, 1987; Blackburn et al., 1989; Berridge & Robinson, 1998; Ikemoto & Panksepp, 1999). The NS-DA system, in contrast, controls procedural aspects of movements and motivated behaviors as it reaches more dorsal areas of BG, where behavioral and cognitive habits are learned, stored and expressed (Hornykiewicz, 1979; Carli et al., 1985; 1989; Graybiel, 1997; Jog et al., 1999; Haber, 2003).
1.3. How can DA affect behavioral and psychological processes?
DA-receptor activated molecular pathways have been partially unraveled (Greengard et al., 1999; Greengard, 2001a), but the precise mechanisms by which DA influences behavioral and psychological phenomena, remains unclear. As a modulator of neural activity, DA interacts with fast synaptic transmission (Greengard, 2001b) and thereby influences the way specific external information is processed within the brain (Mesulam, 1998). One hypothesis posits that DA regulatory function increases the signal-to-noise ratio and enhances the efficacy of neural networks in elaborating biologically significant signals (Rolls et al., 1984; DeFrance et al., 1985; Kiyatkin & Rebec, 1996; Nicola et al., 2000). Based on in vivo and in vitro single-cell studies, the signal-to-noise ratio hypothesis explains how behavioral and motivational arousal processes may be linked to specific cognitive or perceptual representations. However, for understanding how behavioral and psychological arousal is processed in the nervous system, large-scale energetic states of the brain, instead of electrical activity of single neurons, need to be considered (Steriade, 1996; 2000; Ciompi & Panksepp, 2004; Llinas et al., 2005; Freeman, 2005). DA modulates global-field dynamics, desynchronizes cortical-derived oscillatory rhythms and promotes high-frequency waves along the gamma band within BG-thalamocortical circuits (Brown & Mardsen, 1998; Brown, 2003; Magill et al., 2004; Lee et al., 2004). In our view, these rhythms may be accompanied by the release of neurodynamic instinctual sequences, which are essential infrastructures for intentional behaviors5. Neurodynamic sequences are repetitive sequential activity patterns reverberating across specific areas and circuits of the brain. Recently, they have been called “avalanches” (Begs & Plenz, 2003, 2004), and their influence on brain activity may be described with the concept of “dynamic attractors” (Freeman, 2000; 2001; 2003).
The sequential patterns favored by DA in ventral BG-thalamocortical circuits may relate to an instinctual drive to seek life-supportive aspects of the environment and to actively escape those aspects that could be destructive. These neurodynamic sequences are evolutionarily intrinsic, but epigenetically refined, procedural patterns associated with the expressions of exploring and approach behaviors (locomotion, sniffing, head movements, saccades, i.e.). The reverberation of such sequential patterns within brain circuits change the individual's attitude towards the environment, promoting the SEEKING disposition to dominate the motivational landscape of the organism (Panksepp, 1998). This establishes a variety of expectancy states that energize and coordinate the anticipation of life supporting events with characteristic reward seeking behavioral tendencies (Panksepp, 1981; 1986). In this way, primary-process “intentions in action” get transformed into learning and thought-related “intentions to act” (Panksepp, 2003).
1.4. Cardinal feature of the affective neuroethological perspective
Our interpretation of the behavioral functions of the ML-DA system is based on a theoretical perspective we have called the affective neuroethological view. Such a perspective has characteristic features that diverge from current dominant theoretical models and that focus on a series of currently neglected elements.
1) Energy
Modern brain research often fails to account for the energetic and dynamic aspects of neural, behavioral and mental activities. We should ask why animals perceive the world as they do and are spontaneously active in globally energetic ways. How can cognitive computations arise in the brain without the support of global dynamic states that channel an organism's needs via large-scale brain network functions? Where do such global states arise, and how do they interact with informational processes?
New neurodynamic approaches, that grant organisms intrinsic behavioral urges, are needed to make sense of why organisms do what they do (Panksepp, 1998; Kandel, 1999; Freeman, 2000; 2003; Solms & Turnbull, 2002; Ciompi & Panksepp, 2004). It is time to introduce such concepts into the discussion of brain DA functions since mesencephalic DA and ascending reticular activating system (ARAS) are fundamental energetic sources for many types of neural activity6 (Moruzzi & Magoun, 1949; Lindsley et al., 1949; 1950; Jones, 2003). In particular, behavioral activating properties of DA may depend on its capacity to influence global field dynamics in the forebrain, as reflected in DA facilitation of the emergence of fast-wave oscillatory rhythms in BG and cortical areas (Brown & Marsden, 1998; Levy et al., 2000; Tseng et al., 2001; Brown, 2003; Magill et al., 2004; Sharot et al., 2005).
2) Internal procedural sequences
Behavior is not limited to learning and associative processes; neuro-behavioral instinctual processes, shaped by evolution, are essential for almost aspects of goal-directed learning. Neurocognitive behaviorism denies (or at least ignores) an organism's intrinsic behavioral identity and thus neglects certain inborn adaptive capacities as fundamental determinants of learning (Lorenz, 1965). In addition to neural plasticity and top-down hierarchical brain processes, we must harness ethological traditions in order to better understand intrinsic capacities of organisms and thereby emphasize the importance to evolutionary constraints on learning (Tinbergen, 1951; Lorenz, 1965; Burkhardt, 2005). In vertebrates, such constraints emerge substantially from the influences that subcortical brain structures exert over neocortical functions (MacLean, 1990; Panksepp, 1998).
In particular, basal forebrain, and BG are involved in the expression of sequential, species-specific movements, such as instinctive and unlearned sequential grooming movements in rodents (Cromwell & Berridge, 1996), which are the Fixed Action Patterns (FAPs) of ethologists7 (Lorenz, 1950; Tinbergen, 1951; MacLean, 1990). Moreover, the BG influence learning, especially when different sequences of actions are linked into a single functional unit (Knowlton et al., 1996; Graybiel, 1998; Jog et al., 1999; Packard & Knowlton, 2002; Bayley et al., 2005). Basal forebrain areas, including BG, extended amygdala, septum, and nucleus of Meynert (Heimer & Van Hoesen, 2006) represent the deep, subcortical parts of the cerebral hemispheres (Swanson, 2000), and they are essential foundations for higher information processing regions of neocortex to operate. Housing abundant GABA inhibitory neurons, they form reciprocal networks and send inhibitory outputs to thalamic, hypothalamic and midbrain nuclei (Kitai et a., 1981; Berardelli et al., 1998; Kropotov & Etlinger, 1999). Situated between the cortex, the diencephalon and the brainstem, the basal forebrain is viewed as largely inhibitory with tonical suppression of behavioral actions (Swanson, 2000). Nevertheless, when something perturbs its intrinsic equilibrium, particular sequences of activity are released. Therefore, basal forebrain nuclei have been considered “doors that, when unlocked, may release into action large functions outside them” (Llinas, 2002).
3) Emotions
Dorsal BG areas control habitual behaviors, whereas other basal forebrain nuclei (ventral BG, extended amygdala, and septum) are involved in emotional behaviors (Koob, 1999; Swanson, 2000; Alheid, 2003; Heimer & Van Hoesen, 2006). Emotions comprise sequences of FAPs that characterize their expressive and communicative aspects (Darwin 1872; MacLean, 1990; Llinas, 2002), but one main characteristic of emotion is to regulate the organism's behavioral repertoire in flexible ways. Behavioral plasticity arises when each emotional operating system orchestrates a wide range of potential responses in accordance with environmental conditions (Panksepp 1998). When an emotion is activated, the organism's attention is focused largely on a particular set of stimuli, memories and responses. For example, an animal does not eat while experiencing intense fear; food is transiently excluded from its interests. Diffusion of basal forebrain/BG characteristic patterns communicates an emotional disposition within the brain. Such patterns represent the basic action tendencies characteristic of various primary-process emotions, whose neural representations influence the activity of many different brain regions and help match perceptual and cognitive representations into a global action tendency. In such a way, basal forebrain changes intentional states and orients behavior in specific directions.
From this perspective, it is inadequate to try to explain motivations, intentions and emotions simply from top-down cognitive or representational perspective. Intentions-in-action, as intrinsic impulses to act, may best be viewed as neural dynamic sequences, which, once activated, constitute internal procedural drives8 (Llinas, 2002). In our model, such neurodynamic sequences emerge from within basal forebrain and BG areas (Knowlton et al., 1996; Graybiel, 1998), and associated medial diencephalic and mesencephyalic circuits, with parallel roles in learning and expression of motor habits and emotions (MacLean, 1990; Graybiel, 1997; Jog et al., 1999).
4) Affective feelings
Neuronal activity is not limited to the production of computational representations of the world; it also helps organize a large variety of states, among which the emotions and associated affects have been ignored for perhaps too long (Panksepp, 1998, 2005). Removing affectivity from neuroscience may lead to a profound misunderstanding of intrinsic brain organization and functioning, and hinder scientific understanding of how brains truly operate. A recently re-introduced James-Lange type view of emotions considers affective feeling to be produced by “somatic marker” representations of body changes (Damasio, 1996; Damasio et al., 2000). However, the nature of feelings should also incorporate the intrinsic intentionality of many instinctual behaviors; emotions are not only a consequence of “what happened” (Damasio, 1999), but also ”what is happening”, “what is going to happen” and “what may happen”. Such processes are not uniquely human characteristics; an affective core underlying subjectivity appears to have emerged early in vertebrate brain evolution (Panksepp, 1981; 1998, 2005), derived from brain systems that regulate the inner states of the organisms (MacLean, 1990; Damasio, 1999; Craig, 2003; Thompson & Swanson, 2003; Schulkin et al., 2003; Bernston et al., 2003; Porges, 2003; Sewards & Sewards, 2003; Alheid, 2003; Denton, 2006). The core affective substrate of every emotional feeling seems to be generated, in part, inform hierarchically related neural networks that include, most prominently, the periaqueductal gray, the hypothalamus, and the extended amygdala (Panksepp, 1998). Indeed, accumulating evidence for some kind of primary-process psychological experiences arising from such primitive subcortical circuits is becoming substantial (Panksepp, 2005, Merker, 2007). In our view, the core affective states are communicated to higher brain levels through the emergence of specific neurodynamic sequences, so that the cognitive-evaluative aspects of emotion can be elaborated in a coordinated fashion by various forebrain areas, especially orbitofrontal and medial frontal regions.
2. EMPIRICAL STUDIES
2.1. Electrical self-stimulation of the brain (ESSB)
The discovery of ESSB by Olds and Milner (1954) represented a major breakthrough in understanding the neurobiological bases of reward. Electrical stimulation of various brain sites in association with specific behaviors increased the probability that animals would repeat those behaviors. These studies led to the recognition of reward areas in the brain (Olds et al., 1971; Wise, 1996; 2005; Chau et al., 2004) with the medial forebrain bundle (MFB) being a primary neural pathway interconnecting many relevant brain regions (see Wise 2002 for a review). Olds (1977) extensively analyzed the pervasive neuronal learning during appetitive conditioning that occurred along the trans-hypothalamic self-stimulation continuum (for review, see Figure 8.3 in Panksepp, 1998). Further it was demonstrated that with fixed-interval stimulation of this substrate, animals would exhibit spontaneous conditioning characteristics of fixed-interval instrumental behavior (Clark & Trowill, 1971; Burgdorf, et al., 2000)
It was also observed that electric stimulations of the MFB not only reinforce instrumental actions, but they also arouse a variety of consummatory behaviors such as drinking, feeding, gnawing and predation (Glickman & Schiff, 1967; Valenstein et al., 1969; 1970; Panksepp, 1971; 1981). Such stimulations also induced generalized arousal, leading to exploratory behaviors not strictly related to any biological needs (Gallistel, 1974, Panksepp, 1981). Thus, it was suggested that ESSB foster a general incentive-based disposition to approach environmental stimuli (Glickman & Schiff, 1967; Trowill et al., 1969; Panksepp, 1981). With the characterization of brain DA circuitry (Ungerstedt, 1971), it was further recognized that the ML-DA system is an important ascending and activating component of the MFB involved in the learning as well as in the motivational effects of electric brain stimulation (see Wise & Rompre, 1989 for a review). Moreover, increasing DA levels into the Nacc with psychostimulants enhances the rewarding properties of self-stimulation itself (Wise, 1996). The ML-DA system is now generally considered a key circuitry involved in promoting aroused states concerned with appetitive motivations, attention to rewards and behavioral persistence, and by some, the avoidance of punishement—namely the seeking of safety (Ikemoto & Panksepp, 1999).
2.2. Psychomotor activating effects of DA drugs across vertebrates and invertebrates
Drugs that enhance DA functions mediate the emergence of unconditional, behaviorally aroused state in many species. Facilitators of DA release, such as cocaine or amphetamine, and agonists of DA receptors promote waking and behavioral activation in all mammals (Randrup & Munkvad 1972; Wise & Bozarth, 1987; Trampus et al., 1991; Nishino et al., 1998; Wisor et al., 2001). Rats and mice increase locomotor activity in response to such drugs and, if high doses are used, they show stereotypical behaviors (Wise & Bozarth, 1987). In contrast, decreased DA receptor stimulation is associated with hypoactivity and catalepsy (Fog, 1972; Johnels, 1982; Monti et al., 1990). Similarly to mammals, injection of cocaine increase locomotion in birds (Levens & Akins, 2001) and DA promotes locomotor and behavioral activity in amphibians (Matsunaga et al., 2004; Endepols et al., 2004).
DA induces hyperactivity and exploration also in adult fruit flies (McClung & Hirsh, 1998; Pendleton et al., 2002; Lima & Miesenbock, 2005; Kume et al., 2005) and other invertebrate species (Torres & Horowitz, 1998; Sawin et al., 2000; Hills et al., 2004), suggesting a remarkable evolutionary conservation of function. However, pro-DA drugs may also reduce locomotor activity in invertebrates, perhaps acting peripherally (Martinez et al., 1988; Pavlova, 2001; Panksepp & Huber, 2004; Chase et al., 2004; Jorgensen, 2004). Although effects of DA on invertebrate locomotion are not uniform, the rewarding properties for pro-DA drugs seem to be conserved across invertebrates (Bellen, 1998; Wolf, 1999; Kusayama & Watanabe, 2000; Bainton et al., 2000; Brembs et al., 2002; Panksepp & Huber, 2004; Reyes et al., 2005).
2.3. Microinjections and lesion studies
Starting with the work of Ungerstedt, et al. (1974), pharmacological and lesion studies of areas with ML system cell bodies (VTA) and projections have clarified the behavioral functions of the DA transmission in mammals. Microinjections of DA drugs into the Nacc increase locomotor activity and exploratory behaviors (Jackson et al., 1975; Pijnenburg et al., 1976; Carr & White, 1997; Swanson et al., 1997; Schildein et al., 1998), conditioned approach responses (Taylors & Robbins, 1986; Kelley & Delfs, 1991; Burns et al., 1993; Wolterink et al., 1993; Parkinson et al., 1999; Wyvell and Berridge, 2000), and anticipatory sexual behaviors (Everitt et al., 1989; Everitt, 1990). DA enhancing microinjections are also associated with rewarding properties. Animals readily self-administer DA agonists or drugs that directly increase DA transmission in the Nacc (Hoebel et al., 1983; Phillips et al., 1994; Carlezon et al., 1995; Ikemoto et al., 1997). In the conditioned place preference (CPP) paradigm, animals spend more time in environments associated with Nacc injections of psychostimulants and DA agonists (Carr & White, 1986; White et al., 1991; Liao et al., 1998). Experimental modulation of DA transmission in ventral pallidum (VP) and olfactory tubercle has similar, often even more intense, effects than in the Nacc (Ikemoto, 2003; Ikemoto et al., 2005). In fact, microinjections of various DA drugs in the VP elicit locomotion and reward-related behaviors (Gong et al., 1996; 1999; Fletcher et al., 1998) whereas VP lesions reduce responses to natural and artificial rewards (Hiroi & White, 1993; Gong et al., 1997). Microinjections of GABA-A receptor antagonists (e.g., picrotoxin, bicuculline) into the VTA increases locomotion by disinhibiting DA neurons (Arnt & Scheel-Kruger, 1979; Mogenson et al., 1980b; Stinus et al., 1982), and rodents will learn to self-administer GABA-A receptor antagonists (David et al., 1997; Ikemoto et al., 1997a) or NMDA agonist (Ikemoto, 2004) into the VTA.
Experimentally enhanced DA function increases behavioral activity, whereas lesions of the ML-DA system reduce or eliminate exploratory and appetitive-approach behaviors (Koob et al., 1978; Fink & Smith, 1980; Robbins & Everitt, 1982; Evenden & Carli, 1985; Taghzouti, 1985; Robbins et al., 1989; Pierce et al., 1990; Pfaus & Phillips, 1991; Jones & Robbins, 1992; Liu et al., 1998). Pharmacological reduction of Nacc DA transmission inhibits seeking-approach behaviors in response to reward-associated cues (Blackburn et al., 1992; Di Ciano et al., 2001; Parkinson et al., 2002; Wakabayashi et al., 2004). Interestingly, ML-DA depletion or inhibition disrupts active-avoidance behaviors (Jackson et al., 1977; Koob et al., 1984; McCullogh et al., 1993), suggesting that ML-DA also participates in the seeking of safety (Ikemoto & Panksepp, 1999).
The functions of DA projections to the pFC are less clear. On one hand, intra-medial pFC injections of amphetamine produce moderate increases in open-field activity (Carr & White, 1987; Kelley et al., 1989) and DA transmission in the pFC is involved in the reinstatement of cocaine seeking-behaviors in rats (McFarland & Kalivas, 2001; Park et al., 2002; McFarland et al., 2004; Sun & Rebec, 2005). On the other hand, microinjections of DA agonists in the pFC decrease spontaneous, novelty- and psychostimulants-induced locomotor activity (Radcliffe & Erwin, 1996; Broersen et al., 1999; Lacroix et al., 2000; Beyer & Steketee, 2000). A significant negative correlation also exists between mesocortical DA transmission and locomotor activity (Hedou et al., 1999). Consistent with these findings, pFC DA lesions produce hyperactivity (Tassin et al., 1978) and have anti-depressive effects9 (Espejo & Minano, 1999; Ventura et al, 2002). Additional dilemmas exist concerning the role of mesocortical DA transmission in mediation of reward. Whereas rats self-administer cocaine directly into pFC and cocaine injected in the medial pFC induces CPP (Hemby et al., 1990), amphetamine in the medial pFC is not self-administrated (Goeders et al., 1986) nor does it induce CPP (Carr & White, 1986; Schildein et al., 1998). It has also been shown that lesion of mesocortical projections do not reduce reward learning (Isaac et al., 1989; Hemby et al., 1992; Shippenberg et al., 1993; Burns et al., 1993) or self-administration of intravenous cocaine (Martin-Iverson et al., 1986; Schenk et al., 1991; McGregor et al., 1996).
In contrast to the role of DA in ventral BG and prefrontal areas, ML-DA transmission within the amygdala (in basolateral as well as in medial and central nuclei) has been implicated in the expression and learning of fear (Pezze & Feldom, 2004). For example, inhibition of DA transmission within the amygdala reduces fear-potentiated startle (Greba & Kokkinidis, 2000), the retrieval of conditioned-fear associations (Nader & LeDoux, 1999), and has a general anxiolytic effect (de la Mora et al., 2005). On the other hands, rats self-administer d-amphetamine directly in the central nucleus of the amygdala (Chevrette et al., 2002), while DA transmission in the basolateral amygdala contribute to the establishment and reinstatement of instrumental and associative reward learning (Zarrindast et al., 2003; Andrzejewski et al., 2005; Alleweireldt et al., 2006). In sum, both positive and negative emotional behavioral dispositions appear to be stimulated by DA in the amygdala. However, since DA elicits active but not passive avoidance behaviors, it may be argued that central amygdaloid DA is still involved in promoting energized “approach towards safety” (Ikemoto & Panksepp, 1998). We would argue that in the absence of negative incentive stimuli, the ML-DA system largely promotes positive affective states, and that only in the presence of various concurrent negative emotional states or stimuli might it contribute to aversive feelings. However, we do not know whether this contribution is to directly facilitate aversive feelings or alternatively, perhaps to dampen those feelings, even though not to the point of affective neutrality. Much more work is needed on such aversion related affective issues.
2.4. The Nacc core/shell distinction
The Nacc consists of two anatomical and functional subdivisions, the shell and core (Zahm & Brog, 1992; Heimer et al., 1997; Zahm, 1999; Kelley, 1999; Di Chiara, 2002; Ikemoto, et al., 2005). DA projections to the shell are more sensitive to a great variety of stimuli, including drugs of abuse (Pontieri et al., 1995), restraint and pharmacological stress (Deutch & Cameron, 1992; Horger et al., 1995; Kalivas & Duffy, 1995; King et al., 1997), food, (Bassareo & Di Chiara, 1999) and novel stimuli or environments (Rebec et al., 1997; Rebec, 1998; Barrot et al., 2000). Moreover, microinjections of DA drugs into the medial shell, but not the core, support instrumental behaviors and CPP (Carlezon & Wise, 1996; Ikemoto et al., 1997; Chevrette et al., 2002; Sellings & Clarke, 2003). It is generally accepted that the shell is involved in mediating the rewarding effects of psychostimulants (Parkinson et al., 1999; Rodd-Henricks et al., 2002; Ito et al., 2004), but there is less agreement concerning the psychomotor activating effects of these drugs. For example, the behavioral activating property has been attributed to an action of psychostimulants in the core (Weiner et al., 1996; West et al., 1999; Boye et al., 2001; Sellings & Clarke, 2003), in the shell (Heidbreder & Feldon, 1998; Parkinson et al., 1999; Ito et al., 2004), and in both structures (Pierce & Kalivas, 1995; Ikemoto, 2002). However, a recent experiment indicated that the locomotor activating properties of cocaine depend upon DA transmission into the core, while rewarding effects of the psychostimulant depend upon DA transmission in the shell and into the olfactory tubercle (Sellings et al., 2006). It has also been shown that rats learn to self-administer the psychostimulant in the medial shell and in the medial tubercle, but not in the core, ventral shell and lateral tubercle (Ikemoto et al., 2006). Although these findings indicate that rewarding effects of psychostimulants are mediated by Nacc shell and olfactory tubercle, while the locomotor activating effects are mediated by the Nacc core, previous findings demonstrated that DA transmission in the core is necessary for some associative processes, for instance, the establishment of Pavlovian or instrumental conditioning (Parkinson et al., 1999; 2000; Hall et al., 2001; Hutcheson et al., 2001; Di Ciano et al., 2001).
Interestingly, DA transmission in the shell of the Nacc has different characteristics when compared with the transmission in the core. Basal extracellular DA levels are greater in the core and ventral medial pFC than the shell (King & Finlay, 1997; Hedou et al., 1999). However, studies in postmortem tissue punches revealed that basal DA levels are greater in the shell than the core, while the DOPAC/DA ratio is greater in core (Deutch & Cameron, 1992). Although the total amount of DA (extracellular + intracellular) could be higher in the shell, the amount of extracellular DA could be greater in the core due to a faster rate of release and uptake. In fact, in vitro voltammetric studies show that the values of DA release and uptake in the shell Nacc are approximately one-third of those measured in the core region. Moreover, the density of [3H]mazindol binding sites in the Nacc was examined by autoradiography and the shell was found to have an average of half the number of DA uptake sites than those measured in the core region (Jones et al., 1996). Together, these findings suggest that DA transmission in the shell of the Nacc presents the characteristic of so-called slow (Greengard et al., 1999), non-synaptic (Vizi, 2003) or volume transmission (Sykova, 2004; Bach-Y-Rita, 2005). Conversely, DA transmission in the core seems to be more confined to the synaptic clefts.
Besides the neurochemical differences between the core and the shell of the Nacc, important functional differences appear to be associated with these subregions. The DA volume transmission in the shell of the Nacc may be involved in the generation and the maintenance of an aroused and positive affective state. On the other hand, the DA transmission in the core may be involved in the expression of this emotion in the BG-thalamocortical circuits and then in the “control of goal-directed behavior by associative process” (Ito et al., 2004). Indeed, excitotoxic lesions of Nacc core disrupt Pavlovian approach behavior (Parkinson et al., 2000), conditioned reinforcement (Parkinson et al., 1999) and Pavlovian to instrumental transfer (Hall et al., 2001), while coincident activations of D1 receptors and NMDA receptors in the Nacc core are necessary for associative learning (Smith-Roe et al., 2000; Wickens et al., 2003; Hernandez et al., 2005).
2.5. Electric activity of DA cells: phasic and tonic DA transmission
Phasic DA transmission is the short-lasting and impulse-dependent release that appears as a consequence of neural burst firing (Gonon, 1988; Suaud-Chagny et al., 1992). Following such bursts, high levels of DA molecules are released into the synaptic cleft at up to mM concentration (Garris et al., 1994), and then rapidly removed via a re-uptake system (Floresco et al. 2003). To the contrary, tonic DA levels are diffused in the extracellular space outside the synaptic clefts, but exist in very small concentrations (in the nM range), and change relatively slowly (Grace, 2000).
It has recently been proposed that phasic DA in the Nacc is the key component in the process of reward (Grace, 1993; 2000; Wightman & Robinson, 2002; Self, 2003) and that the rewarding effect of electrical stimulation of the MFB is mediated, at least partially, by transient DA release (Wise, 2005). The role of phasic DA in reward processes is envisioned to reflect the fact that phasic DA is a time- and space-specific event, necessary for associative learning, and acts as a detector of coincidence when coupled with glutamatergic inputs directed into the Nacc (O'Donnell, 2003; Dalley et al., 2005). Since DA is transiently released before the execution of goal-directed movements (Phillips et al., 2003; Roitman et al., 2004), phasic DA may promote not only reward-related learning (Reynolds et al., 2001) but also motivated behaviors (Phillips et al., 2003; Ghitza et al., 2004; 2006).
The presence of unpredicted salient, novel and rewarding stimuli induce transient DA cell bursts (Miller et al., 1981; Freeman et al., 1985; Steinfels et al., 1983; Schultz et al., 1993; Mirenowicz & Schultz, 1996; Schultz et al., 1997; Horvitz et al., 1997; Schultz, 1998; Horvitz et al., 2000; Cooper, 2002), suggesting a role of phasic DA in the salience attribution process or the attentional-exploratory behavior that always follows such waking events. However, the overall mean DA cell bursting (and firing) appear independent from the tonic arousal state of the organism, since DA neurons do not alter firing rates with waking and sleep (Trulson et al., 1981; Steinels et al., 1983; Miller et al., 1983; Trulson & Preussler, 1984; Hyland et al., 2002). Effects of stress on DA cell bursting is also not clear with some reports of a reduction in bursts or no effect (Ungless, 2004), with increases in burst firing observed by others (Anstrom & Woodward, 2005).
In contrast, increased amounts of tonic extracellular DA levels exist during emotional arousal, either in aversive and appetitive conditions, or when organisms are actively engaged with the environment (Thierry et al., 1976; Roth et al., 1988; Cousins et al., 1999; Di Chiara et al., 1999). Evidence from voltammetry (Trulson et al., 1985) and microdialysis (Smith et al., 1992; Feenstra et al., 2000; Lena et al., 2005) illustrates that tonic DA is sensitive to fluctuations in sleep-wake states, and there is also enhanced release during REM-dream episodes (Miller et al., 1983; Solms, 2000; Maloney et al., 2002; Gottesman, 2002). Activating the D2-type inhibitory postsynaptic and presynaptic receptors, tonic DA generally reduces the influence that descending glutamatergic projections exert over neurons in the BG and VTA (Nicola et al, 2000; Schmitz et al., 2003). In such a way, tonic DA activity may block the cortical and limbic top-down control, favoring the expression of behaviorally aroused states generated subcortically (see Sect. 4).
It has been demonstrated that tonic DA reduces the firing of DA neurons and phasic DA release via D2 autoreceptor activation in terminal projections and soma (Fig. 3A) (Grace, 2000; Schmitz et al., 2003). However, long-lasting elevations of tonic DA levels may also increase the quanta of DA molecules released per single burst (Fig. 3B). Two lines of evidence suggests this hypothesis:
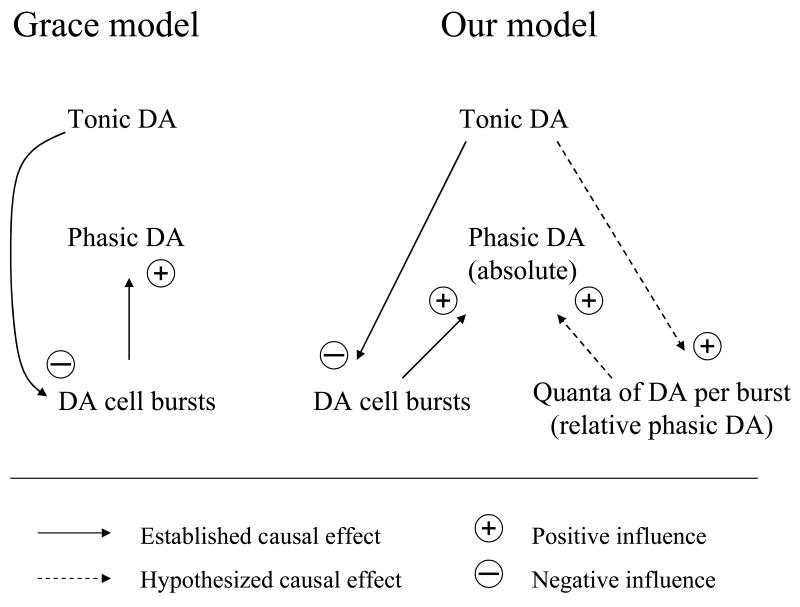
Figure 3. Functional feedbacks between tonic and phasic DA transmission.
In the Grace model (Grace 1991; 2000), tonic DA levels were indicated to inhibit phasic DA release, since D2 autoreceptors activation decreases bursting (and firing) activity of DA neurons. Without questioning the validity of the Grace theory, our alternative model considers the existence of two different feedback loops between tonic and phasic DA transmission. The first one is well experimentally demonstrated, it acts in short-time periods, and consists of the negative influences that tonic DA exerts over DA cell bursting (as in the Grace model). However, in our alternative model, a positive feedback loop has been hypothesized (but not demonstrated yet), since its existence may help in explaining some important empirical evidence. The supposed positive feedback loop should act in longer time frames and consist in tonic DA increasing the amount (or quanta) of DA released per single burst. We called this component the relative phasic DA transmission, to distinguish it from the absolute phasic DA transmission, which is dependent upon the relative phasic DA, plus the mean bursting activity of DA neurons. In our model, tonic DA transmission increases the relative phasic DA (potentiating the efficiency of each burst), and inhibits the mean bursting activity of DA neurons, without strongly modifying the absolute phasic DA. In sum, the Grace model emphasizes the existence of a negative interaction between tonic and phasic DA, whereas our model individuates the existence of a positive feedback loop.
Psychostimulants increase tonic DA levels into the Nacc, and thereby enhance the rewarding properties of self-stimulation (Wise, 1996), by presumably potentiating the amount of phasic DA released after each stimulation. Moreover, amphetamine produces an impulse-dependent DA release into the Nacc (Ventura et al., 2004; Ventura & Puglisi-Allegra, 2005), which may be associated with its rewarding effect. Since amphetamine generally suppresses the electrical activity of DA neurons (Westerink et al., 1987), the impulse-dependent DA release may arise from an increased amount of molecules released per impulse.
Continuous electrical stimulations of DA cells progressively decrease impulse-released DA quanta (Garris et al., 1999). Therefore, investigators need to consider that if an electrically overactive system promotes blunted phasic DA release, a less excitable system may be characterized by the fact that each action potential now has a greater power of each impulse.
Therefore, although the inhibitory action of tonic DA over phasic DA has been emphasized (Grace, 2000), the possibility of positive reciprocal feedbacks should also been considered (Fig. 3). In particular, we suggest that high levels of tonic DA do not decrease the total amount of phasic DA per se, but reduce the excitability of DA cells to descending excitatory glutamatergic inputs, acting either indirectly via D2 receptors located on DA neurons or directly on glutamatergic terminals reaching the VTA. However, high levels of tonic DA will increase the quanta of DA released per single impulse, potentiating the effect that each impulse will produce in term of extracellular DA release. In conclusion, we are tempted to hypothesize that high tonic DA levels will predispose to a less excitable but more powerful ML-DA network influences.
3. THEORETICAL INTERPRETATIONS
Complex relationships among neural, behavioral and psychological levels guarantee the presence of substantial gaps in our understanding that remain to be filled. The adoption of novel integrative hypotheses may be essential for promoting empirical predictions that can help fill the remaining gaps.
3.1. Neurocognitive behaviorism
Much of today's experimental work is driven by a common theoretical perspective, here termed “neurocognitive behaviorism”. It is characterized by two main assumptions. (1) Animal (and human) behaviors are the product of associative memories stored in the brain (Watson, 1913; Skinner, 1938; Martin & Levey, 1988; Resler, 2004; Rolls, 2004; Pickens & Holland, 2004). (2) Cognitive processes, mediated by higher cortical functions, can be conceptualized as computations for unconscious control of behavior and modeled in accordance with information processing theories (Kihlstrom, 1987; Gerstner et al., 1997; Fuster, 2002; Miyashita, 2004; Vogel, 2005). Behavioristic and cognitive approaches have melded together since associative learning is considered the process through which organisms acquire and modify their predictive cognitions (Sutton & Barto 1981).
Within this context, the principal focus of research is to clarify how DA modulates learning by sustained alterations of intracellular molecular mechanisms (Greengard et al., 1999; Hyman & Malenka, 2001; Barrot et al., 2002; Nestler, 2004), enhanced synaptic plasticity (Centonze et al., 2001; Li et al., 2003; Huang et al., 2004), and facilitated neural communication (White, 1996b; Robinson & Kolb, 1999; Reynolds et al., 2001; Nestler, 2001a; Wickens et al., 2003; Centonze et al., 2003). Considering the motivational properties of ML-DA transmission, neurocognitive behaviorism is characterized by a top-down, incentive salience orientation of brain functioning rather than a bottom-up view that envisions brain DA to facilitate ingrained psychobehavioral subroutines necessary for survival. Motivations are viewed as cognitive representations of future goals elaborated in cortical structures, which thereby control the activities of motor circuitries. Within this worldview, DA regulates the communication between cortico-limbic inputs and Nacc neurons, and then manages information flow from cognitive representations (neocortical and higher limbic areas) to movements (BG areas) (Cepeda et al., 1998; Kalivas & Nakamura, 1999; Nicola et al., 2000; Schultz & Dickinson, 2000; Joel et al., 2002; Dayan & Balleine, 2002; Murer et al., 2002; West et al., 2003; O'Donnell, 2003; Carelli, 2004).
The neurocognitive behaviorist perspective has advanced hypotheses about the etiology of DA-related psychiatric diseases. Drug abuse, for example, is viewed as a product of abnormal learning, occurring when the associations between external predictors of the drug's presence and behaviors directed towards its acquisition and consumption progressively consolidate (Robbins & Everitt, 1999; Robinson & Berridge, 2000) (see Sect. 5). In the establishment of compulsive seeking behaviors, the critical step is the cortico-striatal circuits fueling by drug-induced DA release (Pierce & Kalivas, 1997; Di Chiara, 1998; Di Chiara et al. 1999; Berke & Hyman, 2000; Nestler, 2001b, Everitt et al., 2001; Wolf, 2002; Kelley, 2004; Self, 2004). Despite such theoretical successes, it remains difficult for such models to explain how increased ML-DA transmission also promotes certain kinds of unconditional responses, such as behavioral activation expressed in exploratory-investigatory behaviors (Panksepp, 1981; Wise & Bozarth, 1987) the generation of positive affective states (Drevets et al., 2001; Burgdorf & Panksepp, 2006). It is also unresolved why individuals show differences in dispositional vulnerability toward addiction (True et al., 1999; Uhl, 1999; 2004; Vanyukov & Tarter, 2000). If addiction is a learned process, what predisposes an individual to be a good or bad learner?
3.2. Formal models of DA functioning
Electrophysiological recordings from DA neurons generally demonstrate that these cells burst when a reward value is better than expected (Schultz, 1997; 2002). Phasic (or transient) DA transmission is thus viewed as key for organisms to change their internal cognitive schemata in relation to what happened around them (Grace, 2000; Waelti et al., 2001; Reynolds et al., 2001; Wightman & Robinson, 2002; Cooper, 2002; Ungless, 2004). DA transmission is thereby conceptualized as a teaching signal, which reorganizes cognitive representations by indicating prediction errors (Redgrave et al., 1999; Schultz & Dickinson, 2000).
The new data on DA transmission seem congruent with temporal difference (TD) models for reward learning in animals (Sutton & Barto, 1981). TD models, just like some ethological models (Panksepp, 1981), view learned behavior as the product of anticipatory expectations processed within the brain. These expectations are modeled in algorithmic computations capable of predicting the reward value of stimuli which are dynamically modified by experience. Only recently, have such models been utilized to explain DA functions within the brain (Schultz et al. 1997; Waelti et al., 2001; Dayan & Balleine, 2002; Montague et al., 2004).
TD models describe “the function of reward according to the behavior elicited. For example, appetitive or rewarding stimuli induce approach behavior that permits an animal to consume” (Schultz et al. 1997). Such formal models predict that each collection of sensory cues represents a specific reward value, and that animals tend to seek out those that offer the greatest reward. A movement may be defined as activity leading to a sequence of perceptual configurations, whose rewarding value is measured by how strongly it entices the organism to approach or proceed with a sequence of learned configurations. A core problem of TD models concerns a stimulus' temporal representation (Schultz et al., 1997), which is essential for associating sensory cues with future rewards along a number of intermediate time points. Yet it remains unclear, in such formal models, how a representation of reward value is translated into concrete actions and how the animal behaves in novel situations, where no reward value has been solidified by previous learning.
These problems may be well addressed by considering that sensorial configurations are embedded into pre-motor sequences leading organisms to move within and between these configurations. In well-learned situations, past experiences determine the succession of perceptual configurations embedding them within the organism motor-cognitive habits. In such cases, initial presentations of reward-predicting stimuli transiently stimulate the DA system, and phasic DA transmission activates the sequences leading to the predicted outcome. However, in novel situations (or when the reward delivery is maximally uncertain), fixed sequences of movements across sensorial configurations have not yet been established. The persistent increase of DA cell firing in such unpredictable conditions (Fiorillo et al. 2003) may promote the emergence of an unstable state, characterized by the release of instinctual behavioral arousal patterns, which drive organism to explore external stimuli and to cope with life-challenging events in unpredictable environments (Panksepp, 1981; 1998).
In sum, formal neurocognitive behaviorist models of DA functions are built upon a disconnection between brain information-processing modules responsible for the cognitive prediction of reward and those intrinsic brain circuits responsible for the natural behavioral patterns exhibited during reward seeking. In our view, these two aspects are part of the same integrated process: an intrinsic instinctual action tendency to move across perceptual/cognitive landscapes so as to approach towards specific outcomes within environments. In novel and unpredictable contexts, the reward value of a stimulus is the product of the sustained emotional tendency to unconditionally move towards certain objects within the environment. In learned situations, on the other hand, a series of configurations is evoked by previously acquired knowledge so the SEEKING urge is manifested in the tendency to run along the entire sequence until the final configuration is reached. It is possible that the neural circuitry that subsumes the SEEKING response is the only “ground state” in the brain upon which effective information processing can proceed. In other words, all emotional systems control sensory input gating, as well as selective responses to those stimuli. Thus incentive salience may be as much a reflection of changing action readiness as any changing properties of the perceptual field.
3.3. The incentive salience hypothesis
Recognition of a direct involvement of the ML-DA system in the behavioral effects of ESSB (see Wise & Rompre, 1989 for a review) led to a provocative and for a while seminal hypothesis to explain both motivational and learning effects of the ESSB (Wise et al, 1978). Stimulation of the ML-DA system induced a positive hedonic state and enhanced the pleasure derived from consummatory behaviors. Criticism of the hedonic hypothesis emerged from the demonstration that more intense activation of ML-DA occurs during the appetitive phase, than during the consummatory phase of motivated behaviors (Blackburn et al., 1987; 1989; Panksepp, 1981a, 1982; 1986). ML-DA thus appears more concerned with “wanting” and less with “liking” (Berridge & Robinson, 1998). This idea is consistent with evidence from pharmacological manipulations of the ML-DA system in the context of instrumental behaviors. Blocking DA activity in the Nacc strongly diminishes maze-running speed, even though consumation of available rewards is unaffected (Ikemoto & Panksepp, 1996). Reduced DA activity diminishes the appetitive urge more than consummatory pleasure10. Likewise, by facilitating arousal of this system with amphetamine in instrumentally conditioned rats, those animals exhibit more directed appetitive behavior toward stimuli associated with rewards in the past (Wywell & Berridge, 2000; 2001).
According to Berridge, DA is a promoter of the motivational salience of external stimuli, without implying any conscious experience of affective quality. “Liking” has been considered independent from DA transmission, as DA does not seem to promote hedonic taste reactions (Berridge & Robinson, 1998). However, it is important to emphasize that taste pleasure may not exhaust the range of possible positive affects that may be facilitated by brain DA arousal. Moreover, many experiments have pointed to the involvement of ML-DA transmission in the consummatory phase of motivated behaviors, such as feeding (see MacDonald et al., 2004 for a review), while a recent study demonstrated that strongly valenced tastes, both pleasant and unpleasant, may promote DA arousal (Roitman, et al., 2005).
Since animals self-stimulate the ML system, which is strongly controlled by brain DA availability, it needs also to be explained why the activation of an appetitive “wanting” state has its own rewarding properties despite being considered an unconscious process. Otherwise, it is unclear why animals would seek to self-activate their own general purpose, appetitive states. Focusing on this aspect, Berridge (2004) concluded that problems in the field arise when we wrongly believe that appetitive behaviors are direct expressions of what used to be called “drives”. Indeed, in drive-reduction theories, only the reduction of a drive was originally related to the reward, while the drive itself was deemed to be aversive (Hull, 1943; Spence, 1956; Mowrer, 1960). As a solution to the dilemma, Berridge proposed that appetitive behaviors arise from the attribution of incentive properties to external stimuli (pursuant to the views of Bolles, 1972; Bindra, 1974; Toates, 1986), rather than from internal drives. Therefore, “when incentive salience is attributed to a stimulus representation, it makes the stimulus attractive [and] attention grabbing” (Berridge, 2004 p, 195). Since ML-DA transmission presumably helps an external stimulus to acquire incentive salience (Berridge & Robinson, 1998), it also influences the learning of stimulus-related contingencies and appetitive motivations to approach the stimulus.
3.4. The affective neuroethological perspective
With a focus on the unconscious attributions of salience to external representations, Berridge's perspective attempted to explain the role of DA transmission in the absence of any pleasure (specifically sensory “liking”). Berridge claims that motivations are commonly activated by the presence (or anticipatory representation) of external stimuli and not necessarily by internal drives nor affective states. Nevertheless, such a behavioristic shift of focus from the organism to the environment can be misleading. Although the role of external stimuli for guiding motivational processes are undeniable, an excessive reliance on how perceptual stimuli guide behavior could obscure an intrinsic, initially objectless, appetitive motivation as a real process within organisms. Indeed, the manner in which ML-DA transmission may increase the incentive salience of external stimuli is by changing the self-referential attitude of the organism towards those stimuli. In this “active-organism” view, that acknowledges the existence of experienced affect, an internally generated action tendency (i.e., the SEEKING instinct) lies at the very center of information processing.
Thus, in our estimation, ML-DA transmission subcortically promotes the emergence of the emotional SEEKING disposition, an intrinsic psychobehavioral function of the brain, that evolved to cope with all varieties of life-challenging events in unpredictable environments (Panksepp, 1981; 1998, 2005). This disposition consists of instinctual behavioral tendencies that help organism to move accross sensorial configurations and to approach specific sources of stimulations, including salient non-reward events (Horvitz, 2000). The SEEKING disposition is manifested in energized behaviors such as forward locomotion, orienting movements, sniffing, investigating, and ultrasonic 50-KHz vocalizations in rats (Ikemoto & Panksepp, 1994; Panksepp, 1998; Burgdorf & Panksepp, 2006). The SEEKING disposition, independent of world events, would also have its own hedonic properties, not the “pleasure of satisfaction”, but “enthusiastic positive excitement”, “interest”, “desire”, and “euphoria”11 (for relevant subjective human data, see Drevets et al., 2001; Jönsson, et al., 1971; Newton, et al., 2001; Romach, et al., 1999; Volkow & Swanson, 2003). Moreover, promoting the urge to project oneself forewaord in space and time, the SEEKING disposition, manifested at the cortical level (e.g., medial frontal cortex), may facilitate the generation of higher-order “forethought”, positive expectancies and anticipatory states (Panksepp, 1981, Wise, 2005).
It is well-established that emotions affect memory consolidation and retrieval (Cahil, 1997; McGaugh, 2000; Packard & Cahill, 2001; Roozendaal et al., 2001; 2002; Bernston et al., 2003; Richter-Levin, 2004). By promoting the expression of the SEEKING disposition, ML-DA transmission may then facilitate learning, both through attentive processes as well as favoring the recollection of past events related to the arousal of the SEEKING state. The SEEKING disposition may be viewed as an affect-centered instinctual structure binding together perceptual and motor configurations. Indeed, associations between perceptual and motor representations may follow the connections that each of them has established with the SEEKING state. Such an automatic, associative process relates to temporal- and cue-predictability of rewards. The role of the SEEKING disposition in learning is evident in the shaping of spontaneous sniffing behavior in rats during the free, fixed-interval delivery of rewards (Clark & Trowill, 1971; Panksepp, 1981a). Similarly, this phenomenon is also evident in 50 kHz chirping of rats (Burgdorf et al., 2000), an unconditioned component of ML-DA network activity (Burgdorf & Panksepp, 2006).
Additional evidence supports our view. In classical conditioning, novel or unusual stimuli can be associated with unconditioned stimuli whereas habitual stimuli in familiar environments do not condition readily (Rescorla & Wagner, 1972). It is noteworthy, that neutral cues initially provoke sniffing, a DA energized response, but this effect habituates rapidly (Clark, Panksepp & Trowill, 1970). Moreover, it has been demonstrated that operant responses for electrical brain stimulation are always preceded by some exploratory or investigative behaviors (Ikemoto & Panksepp, 1996). Unconditioned rewards may thus promote associative learning to the degree the SEEKING disposition has been aroused. In such a way, when the reward arrives and animals begin to exhibit consummatory behavior, the changing neurodynamic of the SEEKING state (e.g., diminished foraging) or perhaps those associated with the pleasurable interaction with the reward, solidifies the previously related appetitive activity.
The activation of the emotional SEEKING disposition by particular environmental stimuli facilitates instrumental responding within other contexts. For example, the presentation of a conditioned stimulus enhances instrumental response also for unconditioned stimuli different from the one the conditioned stimulus had previously been paired with (Corbit & Balleine, 2005). Moreover, an environment associated with food delivery enhances the locomotor activating effects of amphetamine as well as an environment associated with the amphetamine (Yetnikoff & Arvantogiannis, 2005). In these two cases, the effects of the stimulus (or the environment) on the animal's performance cannot be explained by direct stimulus-response associations simply because these associations have never occurred. On the other hand, it is very probable that associations have been established between the SEEKING disposition and the operant responses, so they are released whenever the SEEKING state is again activated (independently of the stimuli that were originally involved in the generation of that state).
In sum, the affective neuroethological perspective of the ML-DA system is centered on the SEEKING disposition concept, whose ability to explain both motivational and rewarding function of DA transmission is unique among existing scientific scenarios. Such perspective can easily incorporate most of the other views, including variants of enhanced incentive salience and the maintenance of effortful behaviors (Salmone, et al., 2005). The core of the SEEKING affective state may be generated in midbrain and hypothalamic areas (Panksepp 1998; Damasio, 2000; Parvizi & Damasio, 2000) and communicated, in part, to BG-thalamocortical circuits via midbrain DA neurons. As many empirical findings demonstrated (see section 2), ventral BG-DA transmission is essential to the behavioral and mental expression of the SEEKING disposition. In contrast, DA projections to pFC may facilitate information processing without activating the affective-emotional, euphoric aspects of the SEEKING urge. In our view, the attentive and executive functions controlled by mesocortical DA projections (Goldman-Rakic et al., 2000; Nieoullon, 2002; Castner et al., 2004; Arnsten & Li, 2005) may constitute more sophisticated cognitive processes related to the SEEKING disposition. Since under stressful conditions DA transmission in the pFC inhibits DA release in the Nacc (Deutch et al., 1990; Karreman & Moghaddam, 1996; King et al., 1997; Wilkinson, 1997; Jentsh et al., 1998; Ventura et al., 2002), it is also likely that DA-promoted pFC functions may hinder the overt expression of the SEEKING disposition in such highly aroused situations, and may potentially inhibit positive affective states.
4. NEW INROADS OF THE AFFECTIVE NEUROETHOLOGICAL PERSPECTIVE
In the previous section, we described how the behavioral functions of ML-DA emerge from its ability to activate the SEEKING emotional disposition. It is now important to provide new hypotheses describing how this disposition is processed in the brain. Obviously, this proposal needs an elucidation of the role of DA in modulating neural activity across brain circuitries. Indeed, correlative neurophysiological observations obtained from recording DA neurons (which tell us much about what DA cells are listening to, but not necessarily what message they are passing on; see Panksepp, 2005), as is common in the otherwise excellent electrophysiology work of W. Schultz and colleagues, should be integrated with neurophysiological findings about the effects of DA in its projections areas (which better informs us about what DA doing as it is being released downstream of the inputs).
4.1. DA modulation of neural activity
Binding to its receptors, DA activates a cascade of intracellular processes with many diverse neural influences (Missale et al., 1998; Greengard et al., 1999), from changing the activity of ion-channels to altering the functionality of different membrane receptors. DA transmission also regulates gene expression, and leads to permanent synaptic changes (Greengard, 2001; Wolf et al., 2003; Nestler, 2004). Along with many other G-protein–coupled receptors (Hille, 1994), DA receptors alter neuronal excitability via modulation of voltage-dependent ion channels, and influences behavioral processes by modulating large scale neural activity in widespread neural networks.
DA release generally depresses spontaneous and evoked cell firing (Siggins, 1978; Dray, 1980; Rowlands & Roberts, 1980; Yim & Mogenson, 1982; 1986; Brown & Arbuthnott, 1983; Johnson et al., 1983; Yang & Mogenson, 1984; DeFrance et al, 1985; Chiodo & Berger, 1986; Hu & Wang, 1988; Nisenbaum et al, 1988; Hu et al, 1990; Pennartz et al., 1992; Harvey & Lacey, 1996; 1997; Nicola et al., 1996; Peoples & West, 1996; Peoples et al., 1998; Nicola & Deadwyler, 2000; Zhang et al., 2002). It has been argued that behavioral arousal emerges from a DA disinhibitory role obtained by the block of an inhibitory pathway. Indeed, the main targets of DA neurons are BG GABA inhibitory neurons (Graybiel, 2001; Groenewegen, 2003), and DA decreases firing in the globus pallidus and the substantia nigra, the two main BG output nuclei (Alexander et al., 1986; Albin et al., 1989; Gerfen et al., 1990; Bergman et al., 1994; Nini et al., 1995; Brown & Marsden, 1998; Gerfen, 2000; Gurney et al., 2001; Brown et al. 2001).
Despite a predominantly inhibitory role, DA also enhances spontaneous and evoked neural activity in striatal as well in cortical neurons12 (Gonon & Sundrstom, 1996; Hernandez-Lopez et al., 1997; Hu & White, 1997; Gonon, 1997; Cepeda et al., 1998; Lewis & O'Donnell, 2000; West & Grace, 2002; Charara & Grace, 2003; Chen et al., 2004; Bandyopadhyay et al., 2005). The general interpretation of such bidirectional effects is that DA, in a manner similar to NE, enhances the signal-to-noise ratio in neural networks. In other words, DA may filter spurious activity and suppress background noise, while facilitating and enhancing neural activities related to significant incoming signals (Rolls et al., 1984; De France et al., 1985; Kiyatkin & Rebec, 1996; O'Donnell & Grace, 1996; Nicola et al., 2000; West & Grace, 2002; West et al., 2003; Brady & O'Donnell, 2004). The signal-to-noise ratio hypothesis is a computational theory based on the idea that DA facilitates the selection of Nacc competing neuronal ensembles (Pennartz et al., 1994; Redgrave et al., 1999), that receive multiple converging inputs from pFC, hippocampus, and amygdala (Pennartz et al., 1994; O'Donnell & Grace, 1995; Groenewegen et al., 1999; French & Totterdell, 2002). DA then modulates synaptic communication (West et al., 2003) and gates information to the Nacc, favoring the entrance of salient signals in BG-thalamocortical executive circuits (Mogenson et al. 1980a; Pennartz et al., 1994; Groenewegen et al., 1999; West et al., 2003; O'Donnell, 2003), and translating motivational representations into executive motor plans (Mogenson et al. 1980a, Wilner & Sheel-Kruger, 1991; O'Donnell 2003). ML-DA also strengthens synaptic associations between descending glutamatergic projections and BG neural ensembles, influencing long-term memory processes (Wise, 2004).
4.2. DA modulation of global field dynamics
It is remarkable that cognitive, top-down perspectives of ML-DA system are largely built on the observation of DA effects on single neuron firing (Schultz, 1997, 1998, 2001, 2002, 2004, 2006). Based on information from large-scale populations of neurons, an alternative picture is now emerging. DA transmission desynchronizes slow rhythms and induces fast-wave oscillations within the BG-thalamocortical circuits (Brown & Marsdan, 1998; Brown, 2003; Lee et al., 2004; Sharott et al., 2005). It also promotes a greater autonomy of BG neural patterns from a strict cortical control, blocking the spread of cortical synchronous oscillations into the BG (Marsden et al., 2001; Brown, 2001; 2003; Priori et al., 2002; Williams et al., 2002; Heimer et al., 2002; Cassidy et al., 2002; Goldberg et al., 2002; Magill et al., 2004; Sharot et al., 2005) (Fig. 4A). Such network effects may offer the best overall explanation of DA induced psychobehavioral arousal (Steriade, 1996; 2000). Collectively, local field potential studies support the hypothesis that DA promotes the emergence of characteristic rhythms and their diffusion in the brain:
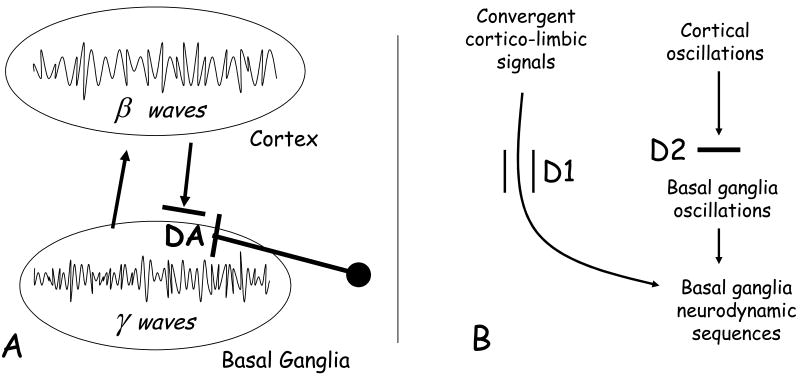
Figure 4. DA-promoted BG activity patterns.
Much evidence has shown that the release of DA into BG blocks the spreading of cortical rhythms in BG structures (A). For example, DA inhibits cortically-derived beta oscillatory patterns and promotes the emergence of BG characteristic oscillatory patterns (in the gamma range) in BG-thalamocortical circuits (Brown & Mardsen, 1998; Brown, 2003; Countermanche et al., 2003; Magill et al., 2004; Lee et al., 2004; Sharrot et al., 2005).
The inhibitory function of DA transmission on the spreading of cortical rhythms is mainly mediated by the activation of D2-type receptors (D2), since they have an inhibitory role over descending glutamatergic transmission into BG areas (Nicola et al., 2000; West et al., 2003; O'Donnell, 2003) (B). The consequent emergence of gamma and other BG rhythms may favors the release of neurodynamic sequences and their diffusion in BG-thalamocortical circuits. On the other hand, transient activation of D1-type receptors (D1) may have an excitatory function and seems to favor the entrance of specific and highly convergent cortical and limbic information into BG (West et al., 2003; O'Donnell, 2003) (B). Those signals may control the release of neurodynamic sequences in accordance with the representation of the organism-environment relationship. The global function of DA may then be conceptualized as a widespread modulation favoring the elaboration of relevant corticolimbic information into a BG intentional code.
DA decreases the power and coherence of cortically derived beta-frequency oscillations (∼15 Hz), and promotes the emergence of high-frequency gamma oscillations (>60 Hz). The prevalence of beta rhythm in BG-thalamocortical circuits is associated with motor impairments characteristic of Parkinson disease (Deuschl et al., 2000; Vitek & Giroux, 2000; Brown, 2003; Dostrovski & Bergman, 2003; Hutchison et al., 2004).
DA suppresses slow firing oscillations and regular bursting of BG neurons (∼ 1 Hz) in anaesthesized and sleeping rats (Pan & Walters, 1988; MacLeod et al., 1990; Murer et al., 1997; Tseng et al., 2000; 2001). Since rhythmic bursts have been interpreted as the result of spreading of cortical activity into BG nuclei, these changes may reflect a barrier between cortex and BG.
DA increases the multisecond temporal oscillatory patterns (from ∼30 sec to ∼ 10 sec) of BG nuclei's spike trains, and increases the spectral power of these oscillations (Ruskin et al., 1999; 2001; 2003).
The DA capacity to promote gamma rhythms needs specific attention, since these oscillatory waves are involved in diverse behavioral and psychological processes, while their alteration has been observed in neuropsychiatric disorders (Herman & Demiralp, 2005). The generation of gamma rhythms is essential for synaptic plasticity and memory processes (Paulsen and Sejnowski 2000; Buzsáki and Draguhn 2004; Sederberg et al., 2006), voluntary movement execution (Cassidy et al., 2002; Countermanche et al., 2003; Kuhn et al., 2004; Sharot et al., 2005), attentive functions (Brown, 2003), and “binding of sensory object features into a coherent conscious percept” (Engel and Singer 2001). It has also been suggested that gamma waves preside over the emergence of active intentional brain states (Freeman, 2003), which underlie all of the above mentioned functions.
In sum, the behavioral arousal function of ML-DA transmission may be explained on the basis of a DA-promoted emergence of high-frequency oscillations in BG-thalamocortical circuits. According to this view, motivated behaviors do not arise from cognitive signals activating executive motor plans, but from instinctual behavioral and emotional drives originating in midbrain and hypothalamic areas and communicated through DA within BG-thalamocortical circuits. We will next explore the possibility that gamma rhythms favor the release of specific neural activity patterns expressing intentional behavioral dispositions.
4.3. DA effects on sequential neural activity patterns
It has been shown that GABA neural networks are involved in the desynchronization of slow-wave oscillations (Slovite, 1987) and in the promotion of high-frequency rhythmic oscillations in the gamma band (Llinas et al., 1991; Steriade 2000). GABAergic neurons also preside over the release of repetitive sequential patterns (or neurodynamic sequences) (Laurent, 2002; Lagier et al., 2004; Beggs & Plenz 2003; 2004). Capturing brain activity within dynamic attractors (Freeman 2000; 2001; 2003; Lewis, 2005), the GABAergic basal forebrain neurodynamic sequences direct activity consistent with the sequence, and constitute the intrinsic structure of intentional behaviors and cognitions. Viewed as impulses to act, they translate neural activity into the intentional code13 necessary for active movements.
It is not known how GABAergic networks produce fast-wave rhythms and sequential neural activity patterns or the exact relationship between gamma rhythms and the release of neurodynamic sequences. However, it is reasonable that ML-DA favors the release of basal forebrain neurodynamic sequences reflected within fast-wave oscillatory gamma rhythms. As demonstrated for gamma rhythms (Brown, 2003), optimal levels of DA are important also for the release of neurodynamic sequences14 (Stewart & Plenz, 2006).
In classic theory of BG functions (Alexander et al., 1986; Albin et al., 1989; Gerfen et al., 1990; Gerfen, 2000; Gurney et al., 2001), DA transmission relieves thalamic and brainstem nuclei from chronic inhibition by BG output nuclei. DA arousal is supposed to emerge from a global increase in thalamocortical activity, while the activity of BG output nuclei is considered antikinetic. This view may be contradicted by evidence where electrical stimulation of BG output nuclei relieves Parkinsonian symptoms (Hamani et al., 2006). BG output nuclei may rather exert an antikinetic effect primarily when they oscillate at low frequencies, but not when normal BG oscillatory activity is restored through DA-facilitating medications or electrical stimulation15 (Garcia et al., 2005). Rather than conceiving DA behavioral effects as a consequence of BG output nuclei inhibition, we propose that DA transmission promotes high-frequency oscillatory patterns (Fig. 4A), and the release of BG neurodynamic sequences. The overall DA inhibition of excitatory input, mainly mediated by D2-type receptors of the indirect pathway of BG16 (Fig. 4B), reduces the diffusion of cortical rhythms and promotes BG characteristic rhythms17. On the other hand, acting upon D1 receptors of depolarized striatal neurons belonging to the direct pathway, phasic DA may increase their responsiveness to convergent descending excitatory influences (Gerfen, 2000; Nicola et al., 2000; Murer et al. 2002; West et al., 2003). This may promote the release of neurodynamic sequences in accordance with specific information coming from corticolimbic structures (Fig. 4B). Convergent glutamatergic input may thus form a switching signal (Redgrave et al., 1999), allowing new information to enter basal forebrain/BG areas and new sequential activity patterns to be generated in BG-thalamocortical circuits. Consistent with this view, an imbalance between phasic and tonic DA transmission may promote attention deficit hyperactivity disorders (Levy, 2004) and probably also Tourette's syndrome. BG-thalamocortical circuits of these subjects may be overcharged by switching signals, as external stimuli continuously release new neurodynamic sequences. Conversely, excesses of BG tonic DA transmissions may promote stereotypical behaviors and obsessive-compulsive disorders (Korff & Harvey, 2006). In these cases, the abnormal presence of tonic DA may completely suppress the influence that cortical and limbic areas exert over subcortical nuclei, leading neurodynamic sequences to be produced autonomously and without any input from the external environment.
4.4. ML-DA and the SEEKING neurodynamic sequences
Limbic neurodynamics in the ventral BG serve as vectors for the expression of the SEEKING emotional disposition, translating a general arousal state into active exploration. They are the neural bases of instinctual internalized movements or action tendencies directed to actively investigate elements of the external and in humans perhaps the internal (mental) environment. SEEKING tendencies are comprised of specific types of locomotor activities, associated autonomic changes, and other responses directed to attain perceptual information and to progressively orient the organism toward affectively enticing and eventually desired sources of stimulation (e.g., via whole body exploratory sequences, eye and head movements, sensory-information sampling with continuous sniffing).
The SEEKING neurodynamic sequences presumably drive motor-action pattern generators via connections from ventral BG output to brainstem motor nuclei. By integrating incoming perceptual information into SEEKING action tendencies, the organism may coordinate its relationship with the environment in flexible ways. Perceptual information from both external and internal sources receive a preliminary evaluation of its survival value as it enters the NAc through limbic structure like olfactory bulb, pFC, amygdala, hippocampus. The diffusion of SEEKING sequences in the BG-thalamocortical circuits brings about exploration and approach to the most prominent sources of positive affective stimulation. Going beyond formal models (Schultz & Dickinson, 2000; Waelti et al., 2001; Dickinson & Balleine, 2002; Schultz, 2004; Niv et al., 2005), we think that the SEEKING neurodynamic sequences are the procedural structures that concretely lead organisms to move across landscapes of perceptual configurations. Instead of being processed in abstract algorithmic computations, the rewarding value of external stimuli depends on the ability to activate such instinctual psychobehavioral sequences. Raw emotional feeling may be highly linked to the neurodynamics that generate instinctual emotional behaviors. From this perspective, it is likely that positive emotional affects, such a DA facilitated euphoria, emerge relatively directly from instinctual SEEKING dynamics (Panksepp, 2005).
The SEEKING neurodynamic sequences in the limbic BG-thalamocortical circuit interfaces continuously with other neural activities. Therefore, the role of ML-DA transmission in learning emerges when such neurodynamics intermesh with other cognitive and perceptual representations (See Lewis (2006) for another elaboration of this type of view in emotion theory). This forms a tight linkage between external stimulus configurations and the SEEKING urge, where external environmental configurations gain the ability to activate SEEKING sequences, acquiring incentive motivational value18 (via classical conditioning). When unexpected positive outcomes (sensory pleasures) emerge for a behavior in a novel environment, motor sequences that were stimulated by the presence of rewards and reward related stimuli become linked to the SEEKING sequences. Discrete operant behavior thereby becomes embedded progressively into ever narrowing SEEKING sequences, connecting the original configurations of stimuli to final reward configurations. Such behaviors eventually become habitual, and perhaps largely affectively unconscious, when ML-DA arousal is no longer necessary to activate appetitite SEEKING urges (Choi, et al., 2005).
In sum, the neurodynamics of SEEKING sequences within BG-thalamocortical circuits should be viewed as essential neural integrative substrates for associative and operant learning processes. As described in the next section, considering the SEEKING disposition as the affective substrate for appetitive learning could have profound implications in understanding addictions.
5. THE ML-DA SYSTEM IN DRUG ADDICTION
5.1. Current theories
Drug abuse has been defined as a chronically relapsing disorder, in which the addict experiences uncontrollable compulsion to take drugs, while the repertoire of behaviors not related to drug seeking, taking, and recovery, declines dramatically (White, 2002). The development of addiction is attributed to the action of drugs in the brain (Leshner, 1997). Chronic drug use causes permanent neural changes at many levels of analysis, from molecular and cellular levels to neural circuits (Hyman & Malenka, 2001; Everitt & Wolf, 2002; White, 2002; Nestler, 2004; Koob et al., 2004; Robinson & Kolb, 2004). Activity of the ML-DA system represents a key aspect of the chain of events that leads from a molecular action of drugs to the establishment of compulsive habits. In fact, most common drugs of abuse stimulate the release of DA, which modulates both their rewarding and the psychomotor arousal effects (Wise & Bozarth, 1987; Di Chiara & Imperato, 1988; White, 1996b; Di Chiara 1998). Permanent functional changes in the ML system and in BG-thalamocortcal circuits, arising from repetitive DA stimulation, are involved in the development of compulsive drug-taking behaviors (Berke et al., 1998; Robinson & Kolb, 1999; Nestler, 2001a, 2004; Hyman & Malenka, 2001; Koob & LeMoal, 2001; Li et al., 2003; Kalivas et al., 2003). Through the complex reorganization of brain circuits, drugs gradually acquire a tremendous motivational power, as organisms become captivated by drug-related activities.
Initial studies of drug abuse in the 1960-1970s considered dependence as the cardinal feature of the disease. Dependence is the physiological state of organisms necessitating continuous drug intake to avoid withdrawal symptoms. The “opponent process theory,” Solomon (1977) proposed that drug abuse arises substantially from homeostatic imbalance caused by compensatory adaptations to chronic drug usage. Concurrently, Panksepp and colleagues (1978, 1980) envisioned that the natural negative emotional processes that sustain drug addictions is related psychologically to the separation-distress process that young animals exhibit when isolated from their caretakers. In other words, endogenous opioids mediate the rewards of social reunion, which is a powerful evolutionary force for creating social bonds, and hence addictive tendencies. Thus, much of drug abuse may reflect self-medication to alleviate aversive feelings, partly engendered by drug withdrawal (see Khantzian, 2003, with commentaries). This perspective has also been adopted by Koob and his coworkers who have sought to identify the neurochemical processes directly involved in generating dependence (Koob & LeMoal, 1997; 2001; 2005; Koob, 2003). As a “hedonic homeostatic dysregulation”, drug abuse has a cyclic and progressive nature and is characterized by a pathological alteration of the reward state. As a result of ML-DA hypofunctionality, the deficit in reward functioning throws organisms into a “spiraling distress cycle” and drugs become necessary to restore the normal homeostatic state (Koob & LeMoal, 2001).
Criticism of the affective theory of drug abuse relates to the presence of relapse episodes. Specifically, the affective-homeostatic perspective fails to explain why “after prolonged drug-free periods, well after the last withdrawal symptom has receded, the risk of relapse, often precipitated by drug associated cues, remains very high” (Hyman, 2005 p1414). Moreover, in animal models, re-exposure to drugs or drug-related stimuli reinstates drug-seeking behaviors more strongly than withdrawal (Stewart & Wise, 1992). Relapse is then interpreted as the result of unconscious associative memories that, once activated, drive mechanistically the behaviors of addicts without the involvement of any hedonic-homeostatic process (Shaham et al. 2003). Such a conclusion is not probably from the Panksepp, et al (1978, 1980) analysis, where the neurological substrates of drug addiction are strongly linked to the natural social-emotional reward processes of animals that are always experienced at the affective, if not cognitive, level.
In the neurocognitive behavioristic perspective, drugs act on the neurochemical processes involved in the formation of associative and procedural memories (Di Chiara, 1999; Berke & Hyman, 2000; Nestler, 2002; Robbins & Everitt, 2002). Addiction is thus viewed as a “pathological usurpation of the mechanisms of reward-related learning” (Hyman, 2005). This interpretation has received support from work showing many common molecular pathways in addiction and memory processes19 (Nestler, 2002; Hyman et al., 2006).
A widely heralded attempt to integrate this approach with a motivational perspective argues that repetitive drug usage causes a sensitization of the ML-DA system (see next paragraph), which is involved in mediating the incentive salience of external stimuli (Robinson & Berridge, 1993; 2000; 2003). The attractiveness of drugs and drug-associated cues depends on the capacity of those cues to activate a motivational appetite (“wanting”) through the stimulation of the ML-DA system. This theoretical perspective focuses on the influence of the sensory and perceptual processes that regulate the SEEKING urge, and has had little to say about the emotional characteristics of brain states. Moreover, a pure incentive sensitization view might wrongly predict that addicts consume less drugs as their system gets sensitized to it. Namely, they are getting more effect from a smaller amount of drug.
5.2. The addiction cycle
One of the big problems in addiction studies concerns how compulsive habits get established from the occasional use of drugs. The process of sensitization is now considered a key step in the addiction development cycle where repetitive drug intake further enhances the desire to consume drug and further lead to uncontrollable urges. It has been shown that previous drug use, especially that of psychostimulants, increases locomotion, stereotypic responses (“behavioral sensitization”), or the ML-DA response (“biochemical sensitization”) to a subsequent acute dose of the same drug (Vandeschuren & Kalivas, 2000; Sax & Strakowsky, 2001; Ungless et al., 2001). And this happens not just for drug rewards, but a variety of natural rewards (Nocjar & Panksepp, 2002), especially social ones (Nocjar & Panksepp, 2007).
The concept of sensitization was originally utilized to describe the fact that the application of electrical stimuli induces a “progressively excitable neuronal locus” showing an enhanced sensitivity to subsequent application of the original stimulus or associated cues (Goddard et al., 1969; Janowski et al., 1980). Since enhanced behavioral and ML-DA responses to drugs correspond to the enhancement of rewarding properties, a study of sensitization should foster our understanding of why drugs and drug-related stimuli acquire an increasing motivational and incentive value (Robinson & Berridge, 1993; 2000; Morgan & Roberts, 2004).
Sensitized responsiveness to drugs often depends on particular stimuli and environmental conditions previously associated with drug intake (Robinson & Berridge, 2000; Weiss et al., 1989). “Context-dependent sensitization” can thus be used to explore how drug-associated stimuli acquire their incentive value. It also provides an explanation for the phenomenon of relapse, where drug-associated memories maintain the ability to activate the ML-DA system long after the withdrawal has subsided (Shaham et al., 2003). On the other hand, “context-independent sensitization” may reflect the increasing ability of drugs to activate the ML-DA system, without contributions from external stimuli (Patridge & Schenk, 1999). In such cases, it is possible that the specific response to the pharmacological action of drugs is potentiated in some way or that the activity of the ML-DA system is globally increased after drug use.
It has been shown that repetitive administration of psychostimulants causes an increased activity of midbrain DA neurons (White & Wang, 1984; Henry et al., 1989; Wolf et al., 1993; Kalivas 1995). Furthermore, molecular and cellular adaptations responsible for a sensitized DA activity have been found in the VTA (Vanderschuren & Kalivas, 2000; Kalivas et al., 2003; Vezina, 2004; Borgland et al., 2004) or along DA projections. A subsensitivity of D2 autoreceptors, which inhibit DA cell firing, also exists after repeated drug usage (White & Wang, 1984; Volkow et al., 2002). Although a general enhancement of ML-DA functions after chronic drug treatment has been postulated (Robinson & Berridge, 2000; Vezina et al., 2004), adequate evidence of enhanced ML-DA release under basal testing conditions in chronically drugged animals is missing. On the contrary, as predicted by the hedonic homeostatic dysregulation hypothesis (Koob & LeMoal, 1997; 2001; 2005; Koob, 2003), a deficiency in ML-DA transmission and consequent motivational changes have been observed after repetitive drug use (Parsons et al., 1991; Weiss et al., 1992; Koob & LeMoal, 1997; 2005; Nestler, 2004). Moreover, as already noted, it is difficult for ML-DA sensitization theories to explain why the rewarding power of drugs is enhanced, while natural rewards are commonly ignored by human addicts.
In sum, two different and opposite molecular pathways activated by drugs have been discovered (Nestler, 2004), which are being related to the experience-dependent motivational power of drugs. On one hand, compensatory adaptations responsible for a decreased ML-DA functioning induce motivational impairments and loss of interest in activities not associated with drug consumption (Koob & LeMoal, 1997; 2001; Nestler, 2001b, Volkow, 2002; Barrot et al., 2002; Aston-Jones & Harris, 2004). On the other hand, changes responsible for a sensitized DA responsiveness to drug and drug-related stimuli (Vanderschuren & Kalivas, 1999; Nestler, 2002; 2004) may lead drug-related memories to acquire an increasing motivational value (Robinson & Berridge, 2000).
5.3. The affective neuroethological perspective of addiction
Like the affective-homeostatic perspective of Koob and his coworkers, our view is centered on naturally occurring internal affective states. We have envisioned how natural “social reward” chemicals, such as endogenous opioids, participate in addictive urges (Panksepp, 1981b; Panksepp, et al., 2004). However, affectivity in our view is conceptualized not only as a result of homeostatic self-regulatory processes, but also of basic intention-in-action type emotional dispositions (Panksepp, 1998, 2003, 2005). Compared with the “psychomotor stimulant theory” (Wise & Bozarth, 1987) and with the “incentive-sensitization theory” of addiction (Robinson & Berridge, 2000), our perspective attempts to specify that the appetitive motivational component stimulated by drugs is an ancestral emotional urge (the SEEKING disposition) regulated by DA transmission and characterized by specific neurodynamic patterns along ventral striatum and ventral BG-thalamocortical circuits. Moreover, this emotion is characterized by neural, behavioral and affective components linked together in complex and synchronized ways.
According to this perspective, drugs of abuse, especially psychostimulants, provide an artificial way to stimulate the emergence of the SEEKING disposition, through which motivated behavior are normally expressed and certain positive affective feelings, such as the euphoria and exhileration of exploration and reward pursuit, arise. The role of the SEEKING disposition in mediating drug-reward is indicated by the similarity between the unconditioned effects of drugs and those of novelty. Novelty may be considered the unconditioned stimulus to which the SEEKING system is naturally predisposed to react (explaining why novelty promotes exploration), while drugs activate the same system in a pharmacological way. Interestingly, novel environments enhance the rewarding and psychomotor activating properties of drugs, leading to environment specific sensitization (Badiani et al., 1995; 1998; Badiani & Robinson, 2004). From our point of view, the disposition to seek and explore, already active in the presence of novelty, is further activated by drugs, creating an amplified effect20.
Strong associative memories between the SEEKING disposition and drug-related stimuli create the neural conditions for drugs to progressively increase their incentive value. Indeed, in this view, drug-related memories push organisms to consume drugs primarily by activating the SEEKING emotional disposition (at least at the first stages of the addiction process). The involvement of the SEEKING disposition in the first stages of addiction is consistent with evidence that sensitization arising from repeated drug injections not only promotes the establishment of drug-seeking behaviors, but also increase the vigor of normal motivational, non drug-related activities, such as a pursuit of sexual and food rewards in rats (Nocjar & Panksepp, 2002, 2006; Panksepp et al., 2004).
Molecular, cellular and synaptic learning processes stimulated by drugs could be related to the emergence of the SEEKING disposition, in the way we think this disposition is manifested at the whole brain/mind level (as neurodynamic patterns emerging into ventral BG and spreading into BG-thalamocortical circuits). It seems unlikely to us that molecular and cellular adaptations observed after drug use correspond to the storage of specific information into a linear input-to-output way of processing (Fig. 5A). To the contrary, we think that those brain changes more likely affect the way global reverberatory activity patterns within BG-thalamocortical circuits are generated, how they are supported by ML-DA transmission, and how they are related to incoming activity elaborated through the rest of the brain. We envision the SEEKING neurodynamics being the affective-action centered functional structures, whereby drug-related memories and drug-seeking behaviors become linked together (Fig. 5B).
Figure 5. The process of drugs addiction development.
In the neurocognitive behavioristic perspective, addiction has been explained as the consequence of drug-induced brain adaptations “stamping” specific associative memories in neural circuits (A). The over-representation of drug-related memories should be caused by synaptic modifications connecting cortico-limbic areas (involved in the representation of motivationally relevant stimuli) to BG areas (involved in the expression of motivated and intentional behaviors). The flow of activity through which compulsive memories are expressed is a linear input-output way of processing, while the ML-DA transmission (especially into the Nacc) is supposed to be particularly important in the drug-induced reinforcement process. The affective neuroethological perspective advanced here diverges from the previous one in considering the drug-induced activation of the SEEKING emotional disposition as the cardinal element in the formation of those memories that make drugs and drug-related stimuli always more attractive (B). In particular, we think that ML-DA release after drug intake facilitates the emergence of specific neurodynamic sequences along the BG-thalamocortical circuits, which constitute the patterns through which the SEEKING disposition is expressed at the neural level. Once generated, these sequences match the representations of specific information about the environment (which are elaborated in BG-thalamocortical circuits and related structures). In line with the “Hebbian” dynamic conception of synaptic plasticity, we think that the match between SEEKING sequences and drug-related memories permanently modify the functional organization of the brain (from the molecular to the systemic level). Therefore, the cascade of neuroadaptations observed after drug use (from molecular to cellular level) represents the tendency of the SEEKING disposition to be activated by drug-related memories and expressed through drug-seeking behaviors.
The abnormal and continuous activation of the SEEKING disposition by drugs is also responsible for the consolidation of compulsive habits, when behavioral routines to find and consume drugs become part of epigenetic changes in the SEEKING dispositions (Ikemoto & Panksepp, 1999). We can imagine that SEEKING neurodynamics activated in ventral BG by drug-associated memories are progressively transformed into behavioral sequences associated with compulsive habits and expressed habitually in dorsal BG circuitry. In such cases, addicts may no longer seek drugs just because of subjectively experienced elevated desire and euphoria but because of the power of automatically expressed habitual stereotypical compulsive behaviors (that are also well suited to effectively alleviate withdrawal distress).
A novel feature of this model is that it offers some unique unconditional indicators of SEEKING urges for monitoring drug desire and craving independently of formal conditioning paradigms (Panksepp et al., 2002; 2004). For instance, rat vocalizations may serve as an instinctual “self-report” of appetitive drug desire or aversion, since rats exhibit more 50kHz ultrasonic vocalizations (USVs) when returned to environments in which they received rewarding drugs, and more 22kHz USVs when returned to environments in which they received aversive drugs (Burdorf et al., 2001a). Indeed, the 50kHz USV system is intimately related to ascending brain DA networks (Burgdorf & Panksepp, 2006; Burgdorf, et al., 2007), and the placement of amphetamine directly into the Nacc, especially the shell region, is effectively promotes 50 kHz USVs (Burgdorf et al., 2001b, Thompson et al., 2006). Such affective vocalizations may be capable of being used to track fluctuating affective changes during various phases of the addiction cycle (Panksepp, et al., 2002, 2004).
As highlighted in the next paragraph, the affective neuroethological perspective provides a new way of envisioning individual vulnerability to psychostimulant addictions and perhaps other drugs as well. An ethological description of normal SEEKING behavior, together with the knowledge of the neural circuits involved in other emotions (Panksepp, 1998), especially negative ones such as social separation distress (Panksepp, 1981) permits a conceptualization of addiction vulnerability as the consequence of the cascade of natural but specific emotional-affective liabilities. In particular, a deficit in the ML-DA may lead individuals to become compulsive drug consumers, by promoting an enhanced ML-DA responsiveness to drugs. In other words, drugs will acquire an enhanced euphoria-producing (rewarding) power since the hypofunctional DA system is characterized by a deficient development of self-inhibitory mechanisms that usually counteract the neurochemical effects of drugs.
5.4. Individual vulnerability
An important issue in drug abuse research concerns why some individuals develop vigorous compulsive drug use after modest consumption of drugs. Human family studies demonstrate that addiction's vulnerability is influenced both by genes and environmental conditions (Uhl, 1999; 2002; True et a., 1999; Vanyukov & Tarter, 2000). Similarly, individual vulnerability to drug abuse in animal models depends on both genetic (Carney et al., 1991; Belknap et al., 1993a, 1993b, Meliska et al., 1995) and environmental risk factors for addiction (Bowling et al., 1993; Bowling & Bardo, 1994; Cabib et al., 2000; de Jong & Kloet, 2004; Nader & Czoty, 2005).
It has been demonstrated that vulnerable animals show higher locomotor and exploratory activity in novel environments (Piazza et al., 1989; Rouge-Pont et al., 1993; Deroche et al., 1995; Grimm and See, 1997; Pierre and Vezina, 1997; Kabbaj et al., 2000; Marinelli & White, 2000; Shimosato & Watanaba, 2003; Orsini et al., 2004). Because of their preference for novel environments (Dellu et al., 1996; Stansfield et al., 2004), they have been described as novelty-seekers (Bardo et al., 1996; Klebaur & Bardo, 1999) and compared to human sensation-seekers, namely individuals characterized by lower levels of internal arousal who are strongly attracted to intense sources of stimulation (Zuckerman, 1990; Dellu et al., 1996). In accordance with such views, vulnerability to addiction has been seen as the result of an endogenous deficiency in the reward state, and, more specifically, in the ML-DA functioning. Indeed, in laboratory animals, low basal levels of ML-DA are related to drug-seeking behaviors, either in individuals with genetic- and history-induced vulnerabilities (Kellogg, 1976; Kempf, 1976; Nestler, 1993; George et al., 1995; Gardner, 1999; Misra & Pandey, 2003) or in acute withdrawal from drugs (Parsons et al., 1991; Weiss et al., 1992). In an attempt to maintain “optimal levels of arousal” (Hebb, 1955), individuals with a lower endogenous DA transmission may be preferentially attracted to the hedonic effects of drug-promoted arousal of the ML-DA system, since drugs may constitute a way to compensate for endogenous arousal deficits and to pharmacologically increase internal levels of activation. On the other hand, since positive affective states are influenced by arousal following an inverted-U shaped function, drugs of abuse may constitute an excessive source of stimulation for individuals with higher basal levels of arousal, generating unpleasant states in them. Therefore, the “self-medication hypothesis” (Markou et al., 1998; Khantzian, 2003) as well as the “reward deficiency hypothesis” (Commings & Blum, 2000) look at drug-taking behaviors as instruments of self-regulation and thereby emphasize the relevance of affective feelings as signals of addiction relevant internal states.
Criticism against these theories of vulnerability came from studies showing that novelty- and drug-seeking rats are characterized by overactive ML-DA neurons (Marinelli & White, 2000; Vezina, 2004). Indeed, rats selected for high responsiveness to novelty and psychostimulants (high responders, HR) present an increased firing and bursting activity of ML-DA neurons in basal conditions (Marinelli & White, 2000). These findings have been considered strong evidence for an endogenous sensitization of the ML-DA system. Such endogenous sensitization has been attributed to a potentiation of synapses connecting glutamatergic excitatory projections and DA neurons in the VTA, and has been suggested as the cause for increased activating and rewarding properties of novelty and drugs. Indeed, animals that are more vulnerable to developing drug self-administration show higher levels of behavioral activation after drug intake (Piazza et al., 1989). This effect is explained by a greater drug response in the ML-DA system of these individuals (Bradberry et al., 1991; Hooks et al., 1992b; Rouge-Pont et al., 1993; Piazza & LeMoal, 1996; Zocchi et al., 1998; Robinson & Berridge, 2000).
A challenge to the endogenous sensitization hypothesis has emerged from experiments in which high responding rats have a slower rate of DA release and uptake in the Nacc compared with low responders (Chefer et al., 2003). The greater electrical activity of DA neurons (Marinelli & White, 2000) thus correlates with a less rapid DA transmission in projection areas21 (Chefer et al., 2003). Since DA influences the responsiveness of ML cells to external input, low DA levels should be accompanied by a prevalence of glutamatergic transmission and a hyper-excitability of DA neurons to glutamate. Indeed, DA usually reduces the amount of glutamate released or the intensity of glutamate-evoked cell firing (Siggins, 1978; Dray, 1980; Yim & Mogenson, 1982; 1986; Bradley et al., 1987; Maura et al., 1988; Harsing & Vizi, 1991). The prevalence of glutamatergic transmission in the VTA and higher ML-related regions may also cause the spreading of slow-wave cortical rhythms into the midbrain and BG. The increased bursting activity of DA neurons (Marinelli & White, 2000) may then be caused by a deficiency in DA transmission and may arise from the diffusion of cortical synchronized activity, as manifested in animals treated with chloral hydrate (Steinfels et al., 1981) and in BG output nuclei of Parkinsonian patients22 (Wichmann & De Long, 2003).
If the ML-DA deficiency is one predisposing factors in addiction vulnerability23, it is also true that sensitivity to the rewarding effects of drugs forms a key component (de Wit et al., 1986; Seale & Carney, 1991; O'Brien et al., 1996; Brunelle et al., 2004; Uhl, 2004). Therefore, it remains to be established why individuals with a blunted ML-DA transmission should present an enhanced ML-DA response to drugs and novelty. An important consequence of endogenous DA hypofunctionality is the reduced expression of neuronal self-inhibitory mechanisms in the ML system. Vulnerable individuals, after drug experiences, show fewer or less functional D2 autoreceptors (White & Wang, 1984; Cabib et al., 2002; Volkow et al., 2002; Nader & Czoty, 2005). Mice of the C57 strain (the addiction vulnerable phenotype) not only show lower levels of D2-autoreceptors in the VTA (Puglisi-Allegra & Cabib, 1997), but also a reduced concentration of DA transporter proteins (DAT) responsible for the re-uptake of extracellular DA in ventral striatal areas (Janowski et al., 2001). Maternally separated rats, which are more vulnerable to addiction, exhibit lower levels of DAT in adulthood compared with controls with direct implications for greater responsiveness to drugs and stress (Meaney et al., 2002). On the other hand, socially dominant monkeys present higher levels of D2 receptors, protecting them against the rewarding effects of cocaine (Morgan et al., 2002). It has also been shown that the pFC DA response to amphetamine in the C57 “vulnerable” mice strain is considerably lower compared with that of the DBA addiction “resistant” mice strain (Ventura et al. 2004), and prefrontal DA transmission exerts an inhibitory control over DA release in ventral striatal areas (Deutch et al., 1990; Karreman & Moghaddam, 1996; King et al., 1997; Wilkinson, 1997; Jentsh et al., 1998; Ventura et al., 2002).
In sum, the lower expression or functionality of self-inhibitory processes in the ML system may compensate for the endogenous hypofunctionality of ML-DA transmission. Although basal levels of DA are restored, the ML-DA system will became less capable of self-regulating its own activity. In situations where unusual stimuli, such as drugs of abuse or novel environments, induce a consistent release of DA into the Nacc and related basal forebrain regions, the deficiencies in the inhibitory mechanisms in the ML system will cause abnormally elevated DA responses. Therefore, vulnerable individuals may experience greater rewarding effects of drugs, partly, we would propose, due to a higher activation of the SEEKING emotional disposition.
When the system “crashes” because effective reward-seeking is thwarted, animals exhibit depressive responses partly because of the emerging dysphoria producing dominance of dynorphinergic tone over the whole ML-DA SEEKING apparatus (Nestler & Carlezon, 2006). Although we have not focused on this aspect of the ML-DA seeking urge, it would be predicted that kappa-receptor antagonists might not only be excellent antidepressants but they will tend to restore SEEKING urges in the behaviorally dysfunctional syndrome of clinical depression. Most other theoretical perspectives of the ML-DA functions, especially the neurocognitive “teaching signal” views, might have difficulty generating comparably straightforward predictions.
6. CONCLUSION
The analysis of ML-DA functions has become an enormous field of inquiry, and new findings and theoretical interpretations are emerging at a steady pace. As this paper was completed, a whole issue of the journal “Psychopharmacology” (2007, vol. 191, issue 3) appeared that was dedicated to the topic. There is no need to modify our position with respect to the cornucopia of these additional perspectives, which are mostly elaborations of previous positions. We would simply highlight that the view advanced here is one of the earliest and most holistic attempts to conceptualize how transhypothalamic reward circuitry, energized by the ML-DA system energizes a coherent organismic response to the world ((Panksepp, 1981 to Panksepp & Moskal, 2007). It can readily accommodate and be synergistic with many of the more specific views that exist in abundance in the literature.
Many theories of ML-DA still envision this system participating in goal-directed behaviours in relatively passive cognitive ways, such as “reward prediction error” which do not clearly envision or recognize the energetic psychobehavioural states this system mediates. Those alternative views remain encumbered by the failure to sift correlates from causes. Most eletrophysiological studies have been characterizing what DA neurons are listening to, truly a wide array of information, rather than what these systems are passing on in the global regulation of behavioural states (Panksepp, 2005). In our affective neuroethological perspective, the ML DA is part of a general purpose appetitive foraging system (the SEEKING system) that allows animals to become acquainted with the diverse configurations and reward of their environments, and thereby establish realistic and adaptive expectations. This system, perhaps some subcomponents more than others, also participates in protecting animals against the vicissitudes of their world (punishing contingencies) by promoting the seeking of safety.
Our view openly acknowledges affective psychological changes, which emerge from related, but poorly understood, emotional network functions (Panksepp, 2005). In its primal form the ML-DA-SEEKING system can generate a special kind of positive affect that is characterized by a euphoric engagement with the world. To the extent that we can define the normal range of arousal of this system, we would suggest that it routinely tends to promote an affectively positive engagement with the world, even though it may not be able to completely counteract a negative affective state that has been concurrently aroused by various punishing events that require the seeking of safety. It is also likely that excessive arousal of this system may be experienced as affectively extreme, leading to feelings such a cravings and excessive feelings of urgency.
We have hardly touched upon the human brain imaging data that is beginning to highlight how important this system is in all varieties of appetitive human motivation, from the excitement of anticipating monetary rewards (Breiter, et al., 2001; Knutson, et al., 2001), to the delights of love (Fisher, et al., 2006) and music (Blood & Zattore, 2001). These issues have been well reviewed elsewhere (Knutson & Wimmer, 2007), and generally support the long-standing thesis that has been updated and mechanistically developed here. Indeed, some of the new wave of “neuroeconomic” brain imaging goes back to animal work affirming the appetitive nature of some of the spontaneous signs of ML-DA arousal, such a 50 kHz ultrasonic vocalizations (USVs) in rats (Knutson, et al., 2002). This vocal index of positive social engagement, especially the “frequency modulated” (FM) variety is strongly affetced by ML-DA dynamics (Burgdorf, et al., 2001, 2007). Another, putative direct index of the arousal of this SEEKING system in rats is the appetitive invigoration of sniffing (Clark & Trowill, 1971) and this measure exhibits spontaneous temporal conditioning that helps explain why animals behave the way they do (i.e., exhibit scalloped, expectancy-type, operant responding) on fixed interval schedules of reinforcement (Panksepp, 1981, 1998). Thus, we have at least three measures of spontaneous arousability of the SEEKING urge in rodents: i) sniffing, ii) 50 kHz FM USVs, and iii) general exploratory-foraging activities. Such unconditional indices, above and beyond DA release, should help us better characterize how the SEEKING disposition helps various behavior patterns become part of the learned repertoires of animals —both “realistic” and “delusional” --as the brains of organisms try to make causal sense of the correlated events to which they are exposed.
Many modern theories of ML-DA function still reflect the old battles between behaviorists and ethologists (Burkhardt, 2005). Obviously, the two views must work together, and they need to be integrated into a seamless whole. However, it needs to be reaffirmed that, as an initial step, organisms do have certain complex behavioural abilities before those abilities get re-structured and channelled by learning. In its primal form, the ML-DA energized brain SEEKING system provides a “goad without a goal” (Panksepp, 1971), promoting the emergence of specific neurodynamic sequences first associated with instinctual exploratory and with learning, appetitive approach patterns. Thereby DA transmission rapidly becomes enmeshed in all varieties of object relations that allow animals to effectively pursue all exteroceptively detectable resources needed for survival.
Several recent publications exhibit a growing interest in integrating dorsal BG DA and ventral BG DA behavioural functions (Robbins & Everitt, 2007; Nicola, 2007). Unfortunately, only single-neuron electrophysiological findings are presented as new empirical evidence without consideration of global-field dynamics studies that first revealed their usefulness in understanding Parkinson's disease. Most of the work in the field is still motivated by computational views of ML-DA functions (see Phillips et al., 2007; Nicola, 2007; Phillips et al., 2007), focusing largely “on a role of phasic dopamine in controlling the discrete selection between different actions” (Niv et al., 2007). Moreover, such views have difficulty specifing which kinds of actions are modulated by ML DA, since there is no evidence that “stimulus-evoked firing of DA neurons encodes specific movements” (Nicola, 2007). Such important questions recur in many of the most recent theoretical papers. How the behavioral activating effect of DA may be translated into specific motor patterns? Which kinds of actions are represented in the Nacc and other ventral BG areas? How are such actions adaptive in novel environments?
In our affective neuroethological perspective, the activating effects of DA is translated into instinctual (i.e., unconditioned) action tendencies, psychobehaviorally represented in ventral BG-thalamocortical circuits, since DA-promoted high-frequency rhythms facilitate the release of SEEKING neurodynamic sequences. Such sequences lead to explicit orienting, seeking and approaching movements when coupled with various external stimulus representations that have been experienced in the context of reward aquistions. Our model integrates dorsal and ventral BG DA functions in a new way, since we considered the procedural routines represented in dorsal BG as learned subsequences of the SEEKING disposition that have become habitual (also see discussion in Ikemoto & Panksepp, 1999). Therefore, in novel and unpredictable environments, instinctual actions of exploration and approach to previously uninvestigated stimuli prevail, while in well-learned situations those patterns are no longer needed (i.e., functional) and instinctual habitual sequences, reflecting more predictable and linear input-output relations, elaborated by dorsal BG circuits, prevail.24 It is noteworthy that the latter pattern are more unconscious that the affectively rich SEEKING patterns elaborated by the more medial, and hence evolutionarily more ancient, ML-DA circuits.
Acknowledgments
Studies reported in this paper were supported by a grant to R.H. and J.P. (NIH/NIDA 1R21DA016435-01A1) and by the help of Hope for Depression Research Foundation to J.P. We would also like to thank the Department of Biological Sciences and the J.P. Scott Center for Neuroscience (Bowling Green State University), for their support.
Abbreviations
- ARAS
Ascending reticular activating system
- BG
basal ganglia
- CPP
Conditioned Place Preference
- DA
dopamine
- ESSB
Electric self-stimulation of the brain
- FAPs
Fixed Action Patterns
- GABA
Gamma aminobutyric acid
- MFB
Medial Forebrain Bundle
- ML
mesolimbic
- ML-DA system
mesolimbic dopamine system
- NS-DA system
nigrostriatal dopamine system
- Nacc
nucleus accumbens
- pFC
Prefrontal Cortex
- TD
Temporal Difference models
- VP
ventral pallidum
- VTA
Ventral Tegmental Area
Footnotes
An emotional behavior is a flexible and coherent adaptive response to biologically relevant stimuli. It has an instinctual and inherited basis, but is different from other instincts because of its plastic nature and its strong subjective—affective aspects. All the emotional behaviors are constituted by a wide array of behavioral and autonomic responses coordinated as an emotional operating system (or emotional command system) constituted of specific neural circuits within the brain (Panksepp, 1998).
An affective state is the basic subjective feeling characteristic of primary-process homeostatic drives, emotions and the resulting sustained moods.
In this paper we will continue to use the convention of capitalizing the SEEKING disposition indicating that a specific neurodynamic state is activated and the SEEKING system to help highlight that a functional neural system is being discussed. Please also note that capitalizations are used to i) avoid part-whole confusions, ii) to alert readers to the claim that these may be necessary brain systems for those types of emotional behaviors and feelings although by no means sufficient for all the emotional manifestations.
Comparative studies in vertebrates have demonstrated the loss of some dopamine (and noradrenaline) cell groups in amniotes compared with anamniotes, especially in the hypothalamic periventricular region (Smeets & Gonzales, 2000).
Intentions literally mean to tend to something. In their primary-process form, they are endogenously produced instinctual activities that naturally predispose generalized (initially objectless) action urges to evolve behaviorally towards more specific goal-directed responses. Here, we generally choose to use the concept of intentional behaviors instead of goal-directed behaviors, because goal-directed behaviors presuppose the explicit representation of the goal. On the contrary, intentional behaviors are intrinsically driven by impulses of neural activity organizing a specific type of behavioral sequence even before specific objects come to be represented as the final goals. In sum, intentional behaviors are sustained initially by the unconditional tendency of basal forebrain/BG circuits to complete a neurodynamic sequence once it has been activated.
The ARAS represents an endogenous system for regulating brain activity and responding to environmental stimuli and it includes interconnected neural nuclei in the brainstem, the diencephalon and the basal forebrain. The ARAS has also been called the “isodendridic core” of the brain, consisting in a “neural continuum with overlapping dendritic fields stretching from spinal cord to telencephalon” (Geisler & Zahm, 2005, p287). As the main source of the basic sleep-wake cycle, it promotes waking arousal as well as behavioral inhibition (Jones, 2003). Placed within the context of the reticular activating system (Parvizi & Damasio, 2001), DA neurons are sensitive to various global states of the organism and their ascending projections modulate brain arousal in accordance with those states. Moreover, since most areas innervated by DA projections sends feedback to DA neurons via direct and indirect pathways, the ascending DA systems forms re-entrant loops with the reticular formation.
In rodents, for example, the BG control instinctive and unlearned sequential grooming movements (Cromwell & Berridge, 1996). The homologues of BG in birds produce highly stereotyped behaviors, such as those used in song learning (Brainard, 2004; Kao et al., 2005), while the striatum in reptiles is involved in regulation of social behaviors (Greenberg, 2003). In primates and other mammals, BG control movements and cognitive executive processes (DeLong, 1990; Graybiel, 1995, Gerfen & Wilson, 1996), especially in initiation and expression of its automatic procedural component (Graybiel, 1998, Jog et al., 1999).
As better described in the section 4, internal procedural drives are sequential neural activity patterns spreading within neural circuits and exerting a strong influence on brain activity. They push neural activity to evolve along specific directions, in accordance with the sequence specified by the pattern.
In our opinion, the frontal cortex control and inhibit primary-process emotional processes such as those that may be disinhibited in attention deficit, hyperactivity disorders (ADHD), leading to heightened levels of emotional acting out (Panksepp, 2001).
Nevertheless, every consummatory behavior also has an appetitive component (animals fluctuate between approaching/manipulating and consuming the food), and hence it is not surprising that DA transmission is enhanced during feeding, and partially controls food intake (Hernandez & Hoebel, 1988; Hoebel et al., 1989; Martel & Fantino, 1996; Ragnauth et al., 2000; Kelley & Berridge, 2002; MacDonald et al., 2004).
This does not mean that DA arousal might not contribute to coping with aversive situations; we would simply predict that it generally tends to counteract negative feelings, even though it may not eliminate them.
The impact of DA transmission on neural activity seems to depend on three main factors: (1) DA receptors: D2-type receptors are inhibitory, while D1-type receptors feature both excitatory and inhibitory roles (Hernandez-Lopez et al., 1997, Reynolds et al., 2001, Floresco et al., 2001a, b, Chao et al., 2002, West & Grace, 2002); (2) Steady-state membrane potentials: DA inhibits hyperpolarized neurons (down-state), and excites depolarized ones (up-state) (Cepeda et al., 1998; Nicola et al., 2000; West & Grace, 2002); and (3) Concentration: evoked concentrations of DA in the range of 600 nanomolar (nM) elicit excitation (Gonon, 1997) while higher concentrations inhibit firing rates (Williams & Millar, 1990).
We refer to intentional code as the dynamic structure of the neural activity produced in basal forebrain and basal ganglia areas. The intrinsic organization of these areas evolved to favor the emergence of sequential activity patterns that may be easily translated in movements because of their procedural shape. In other words those areas have been predisposed to release coherent sequences of movements.
Massive, cortical, glutamatergic input to basal forebrain and BG nuclei blocks neurodynamic sequences through the reciprocal GABAergic connections characteristic of basal forebrain ensembles. With a metaphor taken from Dante's Inferno, basal forebrain neurons are like the damned souls of envious kept in a cauldron. They can not escape because when “one does manage to escape, the others pull him/her back in! And so the cauldron closes itself” (Llinas 2002, p.138). However, when only a subset of basal forebrain neurons receives excitation (and this effect may be potentiated by DA transmission), a behavioral coherent neurodynamic sequence is properly released.
The current interpretation of the therapeutic effects of deep brain stimulation is that such electric currents disorganize and blocks the activity of BG output nuclei. However, it is interesting to note that the frequencies of such stimulations are around the gamma range (∼100Hz) (Garcia et al., 2005). Why not hypothesize then that the deep brain stimulation is effective because it restores basal ganglia characteristic oscillatory rhythms?
But partially also by D1-type receptors belonging to hyperpolarized neurons of the direct pathway (Nicola et al., 2000).
DA transmission tonically inhibits the entrance of glutamatergic descending input in BG areas either via D2-type receptors of striatal neurons belonging to the indirect pathway, or via D1-type receptors of down-state, striatal neurons from the direct pathway (Nicola et al., 2000).
SEEKING neurodynamic sequences may simply promote approach or operant behaviors via activation of motor routines. By activating these sequences, external stimuli may acquire an unconscious incentive value (Berridge 2004). However, it is also possible that SEEKING sequences actively contribute to the emergence ofpositive hedonic state – not sensory pleasure but euphoria. Indeed, hypothalamic and midbrain nuclei receive abundant direct and indirect connections from the NAc shell, the ventral pallidum, and the pFC, and empirical data, such as conditioned place preferences, indicate that all these brain regions contribute to affective experiences (Panksepp, 2005).
The relevance given to associative learning overlaps the emphasis on overt behavioral expression as the only appropriate level of analysis. Drug addiction is now diagnosed exclusively on the basis of observable “behavioral abnormalities”, and is defined “as a loss of control over drug use, or compulsive drug seeking and taking despite adverse consequences” (Nestler, 2001b, p119). The emotional aspects of addiction are typically underemphasized.
Commonalities between novelty and drug reward explain why addiction is so pervasive and difficult to stop. Indeed, if natural rewards activate the ML-DA system in unpredictable and novel situations, a DA-induced activation of the SEEKING urge will help the animal to both achieve its goal and to learn from its current experiences. As environments become increasingly familiar, the SEEKING disposition is not activated as intensely. However, drugs of abuse will continue to activate the ML-DA system pharmacologically even in familiar situations, bringing about the experience of novelty and of its associated euphoric effects. This process will cause repetitive and abnormal learning until the motivational and behavioral repertoire of organism becomes thoroughly captivated by drug-related activities.
It is interesting to note that the same paradoxical correlation is present in animals chronically treated with drugs.
On the other side, GABA projections into the VTA exert a general inhibition on DA cell firing (Hyland et al., 2002). Keeping the DA neurons in a hyperpolarized state, GABA inputs permit the progressive accumulation of DA molecules in the presynaptic vescicles and the increase of quanta of DA released per impulse. Moreover, GABA transmission promotes the emergence and the diffusion of basal forebrain and BG oscillatory rhythms. Under GABA control, the ML system may then be regulated by those neurodynamic patterns forming the procedural structure of intentional behaviors.
Although the existence of an endogenous hypofunctionality of ML-DA transmission is considered the first link in the chain, it is not clear where this deficit arises. It is easy to speculate that it may have developmental origins, based either upon genetic or environmental factors.
It has been interestingly hypothesized that the key difference between dorsal and ventral BG is that dorsal BG control action selection in response to predictable stimuli, while the latter in response to unpredictable stimuli (Nicola, 2007). However, the maintenance of a strict associationistic perspective prevents Nicola from drawing what we believe is the right conclusion from this evidence. Indeed, to explain the role of ML-DA in novel and unpredictable situations, he postulates that stimulus-response associations may be formed also in absence of a pairing between stimuli and action. But why should ML-DA affect behavior only by acting through the auspices of stimulus-response associations? Why are so many investigators resistant to envisioning that ML-DA arousal can liberate some instinctual action tendencies, evolutionary tools for living in the world, independently of previous learning? We suspect that this reflects the implicit assumption, by many investigators interested in this system, that the battle between behaviorists and ethologists during the 1950s (see Burghardt, 2005) was, or should have been, won by the behaviorists. That is a deeply flawed, and evolutionarily improbable, view of animal life. If anything, both sides of that debate provided half the solution to the problem that still needs to be solved. This paper is dedicated to the neuro-ethological view that still needs to be recognized by neurobehaviorists if we are going to construct a more complete and coherent picture of how evolutionarily designed brain emotional and motivational systems actually operate.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alcaro A, Cabib S, Ventura R, Puglisi-Allegra S. Genotype- and experience-dependent susceptibility to depressive-like responses in the forced-swimming test. Psychopharmacology (Berl) 2002;164(2):138–43. doi: 10.1007/s00213-002-1161-8. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Alheid GF. Extended amygdala and basal forebrain. Ann N Y Acad Sci. 2003;985:185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- Anstrom KK, Woodward DJ. Restraint increases dopaminergic burst firing in awake rats. Neuropsychopharmacology. 2005;30(10):1832–1840. doi: 10.1038/sj.npp.1300730. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57(11):1377–84. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Arnt J, Scheel-Kruger J. GABA in the ventral tegmental area: differential regional effects on locomotion, aggression and food intake after microinjection of GABA agonists and antagonists. Life Sci. 1979;25(15):1351–1360. doi: 10.1016/0024-3205(79)90402-8. [DOI] [PubMed] [Google Scholar]
- Arsenault MY, Parent A, Seguela P, Descarries L. Distribution and morphological characteristics of dopamine-immunoreactive neurons in the midbrain of the squirrel monkey (Saimiri sciureus) J Comp Neurol. 1988;267(4):489–506. doi: 10.1002/cne.902670404. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Harris GC. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology. 2004;47(1):167–179. doi: 10.1016/j.neuropharm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Bach-Y-Rita P. Emerging concepts of brain function. J Integr Neurosci. 2005;4(2):183–205. doi: 10.1142/s0219635205000768. [DOI] [PubMed] [Google Scholar]
- Badiani A, Anagnostaras S, Robinson TE. The development of sensitization to the psychomotor stimulant effects of amphetamine is enhanced in a novel environment. Psychopharmacology. 1995a;117:443–452. doi: 10.1007/BF02246217. [DOI] [PubMed] [Google Scholar]
- Badiani A, Oates MM, Day HE, Watson SJ, Akil H, Robinson TE. Amphetamine-induced behavior, dopamine release, and c-fos mRNA expression: modulation by environmental novelty. J Neurosci. 1998;18(24):10579–10593. doi: 10.1523/JNEUROSCI.18-24-10579.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A, Robinson TE. Drug-induced neurobehavioral plasticity: the role of environmental context. Behav Pharmacol. 2004;15(56):327–339. doi: 10.1097/00008877-200409000-00004. [DOI] [PubMed] [Google Scholar]
- Bainton RJ, Tsai LT, Singh CM, Moore MS, Neckameyer WS, Heberlein U. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr Biol. 2000;10(4):187–194. doi: 10.1016/s0960-9822(00)00336-5. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, Gonzalez-Islas C, Hablitz JJ. Dopamine enhances spatiotemporal spread of activity in rat prefrontal cortex. J Neurophysiol. 2005;93(2):864–872. doi: 10.1152/jn.00922.2004. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Donohew RL, Harrington NG. Psychobiology of novelty seeking and drug seeking behavior. Behav Brain Res. 1996;77(12):23–43. doi: 10.1016/0166-4328(95)00203-0. [DOI] [PubMed] [Google Scholar]
- Barrot M, Marinelli M, Abrous DN, Rouge-Pont F, Le Moal M, Piazza PV. The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur J Neurosci. 2000;12(3):973–979. doi: 10.1046/j.1460-9568.2000.00996.x. [DOI] [PubMed] [Google Scholar]
- Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, Impey S, Storm DR, Neve RL, Yin JC, Zachariou V, Nestler EJ. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci U S A. 2002;99(17):11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience. 1999;89(3):637–641. doi: 10.1016/s0306-4522(98)00583-1. [DOI] [PubMed] [Google Scholar]
- Bastiaansen M, Hagoort P. Event-induced theta responses as a window on the dynamics of memory. Cortex. 2003;39(45):967–992. doi: 10.1016/s0010-9452(08)70873-6. [DOI] [PubMed] [Google Scholar]
- Bayley PJ, Frascino JC, Squire LR. Robust habit learning in the absence of awareness and independent of the medial temporal lobe. Nature. 2005;436(7050):550–3. doi: 10.1038/nature03857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs JM, Plenz D. Neuronal avalanches in neocortical circuits. J Neurosci. 2003;23(35):11167–11177. doi: 10.1523/JNEUROSCI.23-35-11167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs JM, Plenz D. Neuronal avalanches are diverse and precise activity patterns that are stable for many hours in cortical slice cultures. J Neurosci. 2004;24(22):5216–5229. doi: 10.1523/JNEUROSCI.0540-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Riggan J, O'Toole LA. Voluntary consumption of morphine in 15 inbred mouse strains. Psychopharmacology (Berl) 1993b;112(23):352–358. doi: 10.1007/BF02244932. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl) 1993a;112(4):503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Bellen HJ. The fruit fly: a model organism to study the genetics of alcohol abuse and addiction? Cell. 1998;93(6):909–912. doi: 10.1016/s0092-8674(00)81195-2. [DOI] [PubMed] [Google Scholar]
- Bennett MR, Hacker PMS. Philosophical foundations of neuroscience. Blackwell; Malden MA: 2003. [Google Scholar]
- Berardelli A, Rothwell JC, Hallett M, Thompson PD, Manfredi M, Marsden CD. The pathophysiology of primary dystonia. Brain. 1998;121(7):1195–1212. doi: 10.1093/brain/121.7.1195. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72(2):507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25(3):515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Berke JD, Paletzki RF, Aronson GJ, Hyman SE, Gerfen CR. A complex program of striatal gene expression induced by dopaminergic stimulation. J Neurosci. 1998;18(14):5301–5310. doi: 10.1523/JNEUROSCI.18-14-05301.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20(4):415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Sarter M, Cacioppo JT. Ascending visceral regulation of cortical affective information processing. Eur J Neurosci. 2003;18(8):2103–9. doi: 10.1046/j.1460-9568.2003.02967.x. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81(2):179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28(3):309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Beyer CE, Steketee JD. Intra-medial prefrontal cortex injection of quinpirole, but not SKF 38393, blocks the acute motor-stimulant response to cocaine in the rat. Psychopharmacology (Berl) 2000;151(23):211–218. doi: 10.1007/s002139900345. [DOI] [PubMed] [Google Scholar]
- Bindra D. A motivational view of learning, performance, and behavior modification. Psychol Rev. 1974;81:199–213. doi: 10.1037/h0036330. [DOI] [PubMed] [Google Scholar]
- Blackburn JR, Pfaus JG, Phillips AG. Dopamine functions in appetitive and defensive behaviours. Prog Neurobiol. 1992;39(3):247–79. doi: 10.1016/0301-0082(92)90018-a. [DOI] [PubMed] [Google Scholar]
- Blackburn JR, Phillips AG, Fibiger HC. Dopamine and preparatory behavior: I. Effects of pimozide. Behav Neurosci. 1987;101(3):352–60. doi: 10.1037//0735-7044.101.3.352. [DOI] [PubMed] [Google Scholar]
- Blackburn JR, Phillips AG, Jakubovic A, Fibiger HC. Dopamine and preparatory behavior: II. A neurochemical analysis. Behav Neurosci. 1989;103(1):15–23. doi: 10.1037//0735-7044.103.1.15. [DOI] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proce Nat Acad Sci. 2001;98:11818–11823. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles RC. Reinforcement, expectancy, and learning. Psychological Review. 1972;79:394–409. [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci. 2004;24(34):7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling SL, Bardo MT. Locomotor and rewarding effects of amphetamine in enriched, social, and isolate reared rats. Pharmacol Biochem Behav. 1994;48(2):459–464. doi: 10.1016/0091-3057(94)90553-3. [DOI] [PubMed] [Google Scholar]
- Bowling SL, Rowlett JK, Bardo MT. The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology. 1993;32(9):885–893. doi: 10.1016/0028-3908(93)90144-r. [DOI] [PubMed] [Google Scholar]
- Boye SM, Grant RJ, Clarke PB. Disruption of dopaminergic neurotransmission in nucleus accumbens core inhibits the locomotor stimulant effects of nicotine and D-amphetamine in rats. Neuropharmacology. 2001;40(6):792–805. doi: 10.1016/s0028-3908(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Bradberry CW, Gruen RJ, Berridge CW, Roth RH. Individual differences in behavioral measures: correlations with nucleus accumbens dopamine measured by microdialysis. Pharmacol Biochem Behav. 1991;39(4):877–882. doi: 10.1016/0091-3057(91)90047-6. [DOI] [PubMed] [Google Scholar]
- Bradley DG, Standaert KJ, Rhodes HD, Rees CM, Testa JM, Crowder HF, Bradford Inhibitory effects of noradrenaline and dopamine on calcium influx and neurotransmitter glutamate release in mammalian brain slices. Eur J Pharmacol. 1987;143:343–352. doi: 10.1016/0014-2999(87)90458-4. [DOI] [PubMed] [Google Scholar]
- Brady AM, O'Donnell P. Dopaminergic modulation of prefrontal cortical input to nucleus accumbens neurons in vivo. J Neurosci. 2004;24(5):1040–1049. doi: 10.1523/JNEUROSCI.4178-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard MS. Contributions of the anterior forebrain pathway to vocal plasticity. Ann N Y Acad Sci. 2004;1016:377–394. doi: 10.1196/annals.1298.042. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–639. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Brembs B, Lorenzetti FD, Reyes FD, Baxter DA, Byrne JH. Operant reward learning in Aplysia: neuronal correlates and mechanisms. Science. 2002;296(5573):1706–1709. doi: 10.1126/science.1069434. [DOI] [PubMed] [Google Scholar]
- Broersen LM, Feldon J, Weiner I. Dissociative effects of apomorphine infusions into the medial prefrontal cortex of rats on latent inhibition, prepulse inhibition and amphetamine-induced locomotion. Neuroscience. 1999;94(1):39–46. doi: 10.1016/s0306-4522(99)00287-0. [DOI] [PubMed] [Google Scholar]
- Brown JR, Arbuthnott GW. The electrophysiology of dopamine (D2) receptors: a study of the actions of dopamine on corticostriatal transmission. Neuroscience. 1983;10(2):349–355. doi: 10.1016/0306-4522(83)90138-0. [DOI] [PubMed] [Google Scholar]
- Brown P. Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson's disease. Mov Disord. 2003;18(4):357–363. doi: 10.1002/mds.10358. [DOI] [PubMed] [Google Scholar]
- Brown P, Marsden CD. What do the basal ganglia do? Lancet. 1998;351(9118):1801–1804. doi: 10.1016/s0140-6736(97)11225-9. [DOI] [PubMed] [Google Scholar]
- Brown P, Mazzone P, Oliviero A, Altibrandi MG, Pilato F, Tonali PA, Di Lazzaro V. Effects of stimulation of the subthalamic area on oscillatory pallidal activity in Parkinson's disease. Exp Neurol. 2004;188(2):480–490. doi: 10.1016/j.expneurol.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Di Lazzaro V. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson's disease. J Neurosci. 2001;21(3):1033–1038. doi: 10.1523/JNEUROSCI.21-03-01033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelle C, Assaad JM, Barrett SP, Avila C, Conrod PJ, Tremblay RE, Pihl RO. Heightened heart rate response to alcohol intoxication is associated with a reward-seekingpersonality profile. Alcohol Clin Exp Res. 2004;28(3):394–401. doi: 10.1097/01.alc.0000117859.23567.2e. [DOI] [PubMed] [Google Scholar]
- Bunney BS, Chiodo LA, Grace AA. Midbrain dopamine system electrophysiological functioning: a review and new hypothesis. Synapse. 1991;9(2):79–94. doi: 10.1002/syn.890090202. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J. Anticipation of rewarding electrical brain stimulation evokes ultrasonic vocalizations in rats. Behav Neurosci. 2000;114:320–27. [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J, Ikemoto S. Nucleus accumbens amphetamine microinjections unconditionally elicit 50 kHz ultrasonic vocalizations in rats. Behavioral Neuroscience. 2001b;115:940–944. doi: 10.1037//0735-7044.115.4.940. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J, Shippenberg T. Evaluation of rat ultrasonic vocalizations as predictors of the conditioned aversive effects of drugs. Psychopharmacology. 2001a;155:35–42. doi: 10.1007/s002130100685. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J. The neurobiology of positive emotions. Neurosci Biobehav Rev. 2006;30(2):173–187. doi: 10.1016/j.neubiorev.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Wood PL, Kroes RA, Moskal JR, Panksepp J. Neurobiology of 50-kHz ultrasonic vocalizations in rats: electrode mapping, lesion, and pharmacological studies. Behav Brain Res. 2007 doi: 10.1016/j.bbr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Burkhardt RW., Jr . Patterns of behavior: Konrad Lorenz, Niko Tinbergen, and the founding of ethology. Chicago: University of Chicago Press; 2005. [Google Scholar]
- Burns LH, Robbins TW, Everitt BJ. Differential effects of excitotoxic lesions of the basolateral amygdala, ventral subiculum and medial prefrontal cortex on responding with conditioned reinforcement and locomotor activity potentiated by intra-accumbens infusions of D-amphetamine. Behav Brain Res. 1993;55(2):167–183. doi: 10.1016/0166-4328(93)90113-5. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Cabib S, Puglisi-Allegra S. Stress, depression and the mesolimbic dopamine system. Psychopharmacology (Berl) 1996;128(4):331–42. doi: 10.1007/s002130050142. [DOI] [PubMed] [Google Scholar]
- Cabib S, Orsini C, Le Moal M, Piazza PV. Abolition and reversal of strain differences in behavioral responses to drugs of abuse after a brief experience. Science. 2000;289(5478):463–465. doi: 10.1126/science.289.5478.463. [DOI] [PubMed] [Google Scholar]
- Cabib S, Puglisi-Allegra S, Ventura R. The contribution of comparative studies in inbred strains of mice to the understanding of the hyperactive phenotype. Behav Brain Res. 2002;130(12):103–9. doi: 10.1016/s0166-4328(01)00422-3. 2002. [DOI] [PubMed] [Google Scholar]
- Cahill L. The neurobiology of emotionally influenced memory. Implications for understanding traumatic memory. Ann N Y Acad Sci. 1997;821:238–46. doi: 10.1111/j.1749-6632.1997.tb48283.x. [DOI] [PubMed] [Google Scholar]
- Cardenas L, Tremblay LK, Naranjo CA, Herrmann N, Zack M, Busto UE. Brain reward system activity in major depression and comorbid nicotine dependence. J Pharmacol Exp Ther. 2002;302(3):1265–1271. doi: 10.1124/jpet.302.3.1265. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Winstanley CA, Robbins TW, Everitt BJ. Limbic corticostriatal systems and delayed reinforcement. Ann N Y Acad Sci. 2004;1021:33–50. doi: 10.1196/annals.1308.004. [DOI] [PubMed] [Google Scholar]
- Carelli RM. Nucleus accumbens cell firing and rapid dopamine signaling during goal-directed behaviors in rats. Neuropharmacology. 2004;47(1):180–189. doi: 10.1016/j.neuropharm.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Devine DP, Wise RA. Habit-forming actions of nomifensine in nucleus accumbens. Psychopharmacology (Berl) 1995;122(2):194–7. doi: 10.1007/BF02246095. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Wise RA. Microinjections of phencyclidine (PCP) and related drugs into nucleus accumbens shell potentiate medial forebrain bundle brain stimulation reward. Psychopharmacology (Berl) 1996;128(4):413–420. doi: 10.1007/s002130050151. [DOI] [PubMed] [Google Scholar]
- Carli M, Evenden JL, Robbins TW. Depletion of unilateral striatal dopamine impairs initiation of contralateral actions and not sensory attention. Nature. 1985;313:679–682. doi: 10.1038/313679a0. [DOI] [PubMed] [Google Scholar]
- Carli M, Jones GH, Robbins TW. Effects of unilateral dorsal and ventral striatal dopamine depletion on visual neglect in the rat: a neural and behavioural analysis. Neuroscience. 1989;29(2):309–27. doi: 10.1016/0306-4522(89)90059-6. [DOI] [PubMed] [Google Scholar]
- Carlsson A. Antipsychotic drugs and catecholamine synapses. J Psychiatr Res. 1974;11:57–64. doi: 10.1016/0022-3956(74)90070-3. [DOI] [PubMed] [Google Scholar]
- Carlsson A. Does dopamine have a role in schizophrenia? Biol Psychiatry. 1978;13(1):3–21. [PubMed] [Google Scholar]
- Carney JM, Landrum RW, Cheng MS, Seale TW. Establishment of chronic intravenous drug self-administration in the C57BL/6J mouse. Neuroreport. 1991;2(8):477–480. doi: 10.1097/00001756-199108000-00017. [DOI] [PubMed] [Google Scholar]
- Carr GD, White NM. Anatomical disassociation of amphetamine's rewarding and aversive effects: an intracranial microinjection study. Psychopharmacology (Berl) 1986;89(3):340–346. doi: 10.1007/BF00174372. [DOI] [PubMed] [Google Scholar]
- Carr GD, White NM. Effects of systemic and intracranial amphetamine injections on behavior in the open field: a detailed analysis. Pharmacol Biochem Behav. 1987;27(1):113–122. doi: 10.1016/0091-3057(87)90485-0. [DOI] [PubMed] [Google Scholar]
- Cassidy M, Mazzone P, Oliviero A, Insola A, Tonali P, Di Lazzaro V, Brown P. Movement-related changes in synchronization in the human basal ganglia. Brain. 2002;125(6):1235–1246. doi: 10.1093/brain/awf135. [DOI] [PubMed] [Google Scholar]
- Castner SA, Goldman-Rakic PS, Williams GV. Animal models of working memory: insights for targeting cognitive dysfunction in schizophrenia. Psychopharmacology (Berl) 2004;174(1):111–25. doi: 10.1007/s00213-003-1710-9. [DOI] [PubMed] [Google Scholar]
- Centonze D, Gubellini P, Pisani A, Bernardi G, Calabresi P. Dopamine, acetylcholine and nitric oxide systems interact to induce corticostriatal synaptic plasticity. Rev Neurosci. 2003;14(3):207–16. doi: 10.1515/revneuro.2003.14.3.207. [DOI] [PubMed] [Google Scholar]
- Centonze D, Picconi B, Gubellini P, Bernardi G, Calabresi P. Dopaminergic control of synaptic plasticity in the dorsal striatum. Eur J Neurosci. 2001;13(6):1071–1077. doi: 10.1046/j.0953-816x.2001.01485.x. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Colwell CS, Itri JN, Chandler SH, Levine MS. Dopaminergic modulation of NMDA-induced whole cell currents in neostriatal neurons in slices: contribution of calcium conductances. J Neurophysiol. 1998;79(1):82–94. doi: 10.1152/jn.1998.79.1.82. [DOI] [PubMed] [Google Scholar]
- Chao SZ, Lu W, Lee HK, Huganir RL, Wolf ME. D(1) dopamine receptor stimulation increases GluR1 phosphorylation in postnatal nucleus accumbens cultures. J Neurochem. 2002;81(5):984–992. doi: 10.1046/j.1471-4159.2002.00877.x. [DOI] [PubMed] [Google Scholar]
- Charara A, Grace AA. Dopamine receptor subtypes selectively modulate excitatory afferents from the hippocampus and amygdala to rat nucleus accumbens neurons. Neuropsychopharmacology. 2003;28(8):1412–1421. doi: 10.1038/sj.npp.1300220. [DOI] [PubMed] [Google Scholar]
- Chase DL, Pepper JS, Koelle MR. Mechanism of extrasynaptic dopamine signaling in Caenorhabditis elegans. Nat Neurosci. 2004;7(10):1096–1103. doi: 10.1038/nn1316. [DOI] [PubMed] [Google Scholar]
- Chau DT, Roth RM, Green AI. The neural circuitry of reward and its relevance to psychiatric disorders. Curr Psychiatry Rep. 2004;6(5):391–9. doi: 10.1007/s11920-004-0026-8. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Zakharova I, Shippenberg TS. Enhanced responsiveness to novelty and cocaine is associated with decreased basal dopamine uptake and release in the nucleus accumbens: quantitative microdialysis in rats under transient conditions. J Neurosci. 2003;23(7):3076–3084. doi: 10.1523/JNEUROSCI.23-07-03076.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Greengard P, Yan Z. Potentiation of NMDA receptor currents by dopamine D1 receptors in prefrontal cortex. Proc Natl Acad Sci U S A. 2004;101(8):2596–600. doi: 10.1073/pnas.0308618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrette J, Stellar JR, Hesse GW, Markou A. Both the shell of the nucleus accumbens and the central nucleus of the amygdala support amphetamine self-administration in rats. Pharmacol Biochem Behav. 2002;71(3):501–507. doi: 10.1016/s0091-3057(01)00686-4. [DOI] [PubMed] [Google Scholar]
- Chiodo LA, Berger TW. Interactions between dopamine and amino acid-induced excitation and inhibition in the striatum. Brain Res. 1986;375:198–203. doi: 10.1016/0006-8993(86)90976-5. [DOI] [PubMed] [Google Scholar]
- Choi WY, Balsam PD, Horvitz JC. Extended habit training reduces dopamine mediation of appetitive response expression. Journal of Neuroscience. 2005;25:6729–6733. doi: 10.1523/JNEUROSCI.1498-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciompi L, Panksepp J. Energetic effects of emotions on cognitions-complementary psychobiological and psychosocial finding. In: Ellis R, Newton N, editors. Consciousness & Emotions. Vol. 1. John Benjamins; Amsterdam: 2004. pp. 23–55. [Google Scholar]
- Clark S, Panksepp J, Trowill JA. A method for recording sniffing in the free-moving rat. Physiol Behav. 1970;5:125–126. doi: 10.1016/0031-9384(70)90024-7. [DOI] [PubMed] [Google Scholar]
- Clark S, Trowill JA. Sniffing and motivated behavior in the rat. Physiol Behav. 1971;6:49–52. doi: 10.1016/0031-9384(71)90013-8. [DOI] [PubMed] [Google Scholar]
- Comings DE, Blum K. Reward deficiency syndrome: genetic aspects of behavioral disorders. Prog Brain Res. 2000;126:325–341. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- Cooper DC. The significance of action potential bursting in the brain reward circuit. Neurochem Int. 2002;41(5):333–340. doi: 10.1016/s0197-0186(02)00068-2. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer. J Neurosci. 2005;25(4):962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtemanche R, Fujii N, Graybiel AM. Synchronous, focally modulated beta-band oscillations characterize local field potential activity in the striatum of awake behaving monkeys. J Neurosci. 2003;23(37):11741–11752. doi: 10.1523/JNEUROSCI.23-37-11741.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins MS, Trevitt J, Atherton A, Salamone JD. Different behavioral functions of dopamine in the nucleus accumbens and ventrolateral striatum: a microdialysis and behavioral investigation. Neuroscience. 1999;91:925–934. doi: 10.1016/s0306-4522(98)00617-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13(4):500–5. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig W. Appetites and aversions as constituents of instincts. Biology Bull Woods Hole. 1918;34:91–107. [Google Scholar]
- Cromwell HC, Berridge KC. Implementation of action sequences by a neostriatal site: a lesion mapping study of grooming syntax. J Neurosci. 1996;16(10):3444–3458. doi: 10.1523/JNEUROSCI.16-10-03444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Localization of monoamines in the lower brain stem. Experientia. 1964;20(7):398–399. doi: 10.1007/BF02147990. [DOI] [PubMed] [Google Scholar]
- Dailly E, Chenu F, Renard CE, Bourin M. Dopamine, depression and antidepressants. Fundam Clin Pharmacol. 2004;18(6):601–7. doi: 10.1111/j.1472-8206.2004.00287.x. [DOI] [PubMed] [Google Scholar]
- Dalley W, Laane K, Theobald DEH, Armstrong HC, Corlett PR, Chudasama Y, Robbins TW. Time-limited modulation of appetitive Pavlovian memory by D1 and NMDA receptors in the nucleus accumbens. PNAS. 2005;102(17):6189–6194. doi: 10.1073/pnas.0502080102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. Harcourt Brace; New York: 1999. [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, Hichwa RD. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3(10):1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Darwin C. The expression of the emotions in man and animals. John Murray; London: 1872. [Google Scholar]
- David V, Durkin TP, Cazala P. Self-administration of the GABAA antagonist bicuculline into the ventral tegmental area in mice: dependence on D2 dopaminergic mechanisms. Psychopharmacology (Berl) 1997;130(2):85–90. doi: 10.1007/s002130050214. [DOI] [PubMed] [Google Scholar]
- Dayan P, Balleine BW. Reward, motivation and reinforcement learning. Neuron. 2002;36:285–298. doi: 10.1016/s0896-6273(02)00963-7. [DOI] [PubMed] [Google Scholar]
- De Jong IE, de Kloet ER. Glucocorticoids and vulnerability to psychostimulant drugs: toward substrate and mechanism. Ann N Y Acad Sci. 2004;1018:192–198. doi: 10.1196/annals.1296.022. [DOI] [PubMed] [Google Scholar]
- De Wit H, Uhlenhuth EH, Johanson CE. Individual differences in the reinforcing and subjective effects of amphetamine and diazepam. Drug Alcohol Depend. 1986;16:341–360. doi: 10.1016/0376-8716(86)90068-2. [DOI] [PubMed] [Google Scholar]
- DeFrance JF, Sikes RW, Chronister RB. Dopamine action in the nucleus accumbens. J Neurophysiol. 1985;54:1568–1577. doi: 10.1152/jn.1985.54.6.1568. [DOI] [PubMed] [Google Scholar]
- Dellu F, Piazza PV, Mayo W, Le Moal M, Simon H. Novelty-seekingin rats--biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology. 1996;34(3):136–145. doi: 10.1159/000119305. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Denton D. The primordial emotions: The dawning of consciousness. New York: Oxford University Press; 2006. [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: dopamine, facilitation of incentive motivation, and extraversion. Behav Brain Sci. 1999;22(3):491–517. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- Deroche V, Rouge-Pont F, LeMoal M, Piazza PV. Individual differences in cocaine self-administration: dose-response and ratio-response study. Soc Neurosci. 1995;Abstr 21:767–769. [Google Scholar]
- Deuschl G, Raethjen J, Baron R, Lindemann M, Wilms H, Krack P. The pathophysiology of parkinsonian tremor: a review. J Neurol. 2000;247(5):33–48. doi: 10.1007/pl00007781. [DOI] [PubMed] [Google Scholar]
- Deutch AY. Prefrontal cortical dopamine systems and the elaboration of functional corticostriatal circuits: implications for schizophrenia and Parkinson's disease. J Neural Transm Gen Sect. 1993;91(23):197–221. doi: 10.1007/BF01245232. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Cameron DS. Pharmacological characterization of dopamine systems in the nucleus accumbens core and shell. Neuroscience. 1992;46(1):49–56. doi: 10.1016/0306-4522(92)90007-o. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Clark WA, Roth RH. Prefrontal cortical dopamine depletion enhances the responsiveness of mesolimbic dopamine neurons to stress. Brain Res. 1990;521(12):311–315. doi: 10.1016/0006-8993(90)91557-w. [DOI] [PubMed] [Google Scholar]
- Devos D, Labyt E, Derambure P, Bourriez JL, Cassim F, Guieu JD, Destee A, Defebvre L. Effect of L-Dopa on the pattern of movement-related (de)synchronisation in advanced Parkinson's disease. Neurophysiol Clin. 2003;33(5):203–212. doi: 10.1016/j.neucli.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. A motivational learning hypothesis of the role of mesolimbic dopamine in compulsive drug use. J Psychopharmacol. 1998;12(1):54–67. doi: 10.1177/026988119801200108. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. Eur J Pharmacol. 1999;375(13):13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137(12):75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Loddo P, Tanda G. Reciprocal changes in prefrontal and limbic dopamine responsiveness to aversive and rewarding stimuli after chronic mild stress: implications for the psychobiology of depression. Biol Psychiatry. 1999;46:1624–1633. doi: 10.1016/s0006-3223(99)00236-x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Bassareo V, Pontieri F, Acquas E, Fenu S, Cadoni C, Carboni E. Drug addiction as a disorder of associative learning. Role of nucleus accumbens shell/extended amygdala dopamine. Ann N Y Acad Sci. 1999;877:461–485. doi: 10.1111/j.1749-6632.1999.tb09283.x. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ. Differential involvement of NMDA, AMPA/Kainate, and dopamine receptors in the nucleus accumbens Core in the acquisition and performance of pavlovian approach behavior. J Neurosci. 2001;21(23):9471–9477. doi: 10.1523/JNEUROSCI.21-23-09471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A, Balleine BW. The role of learning in motivation. In: Gallistel CR, editor. Steven's handbook of experimental psychology. Learning, motivation and emotion. Vol. 3. Wiley; New York: 2002. pp. 497–533. [Google Scholar]
- Donzanti BA, Hite JF, Yamamoto BK. Extracellular glutamate levels increase with age in the lateral striatum: potential involvement of presynaptic D-2 receptors. Synapse. 1993;13:376–382. doi: 10.1002/syn.890130410. [DOI] [PubMed] [Google Scholar]
- Dostrovsky J, Bergman H. Oscillatory activity in the basal ganglia--relationship to normal physiology and pathophysiology. Brain. 2004;127(4):721–722. doi: 10.1093/brain/awh164. [DOI] [PubMed] [Google Scholar]
- Dray A. DocSumThe physiology and pharmacology of mammalian basal ganglia. Prog Neurobiol. 1980;14(4):221–335. doi: 10.1016/0301-0082(80)90017-9. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, Price JL, Mathis CA. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry. 2001;49(2):81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Kroner S, Gunturkun O. The dopaminergic innervation of the avian telencephalon. Prog Neurobiol. 1999;59(2):161–195. doi: 10.1016/s0301-0082(98)00100-2. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK. The computational role of dopamine D1 receptors in working memory. Neural Netw. 2002;15(46):561–72. doi: 10.1016/s0893-6080(02)00049-7. [DOI] [PubMed] [Google Scholar]
- Endepols H, Schul J, Gerhardt HC, Walkowiak W. 6-hydroxydopamin lesions in anuran amphibians: a new model system for Parkinson's disease? J Neurobiol. 2004;60(4):395–410. doi: 10.1002/neu.20047. [DOI] [PubMed] [Google Scholar]
- Engel AK, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends Neurosci. 2001;5:16–25. doi: 10.1016/s1364-6613(00)01568-0. [DOI] [PubMed] [Google Scholar]
- Espejo EF, Minano FJ. Prefrontocortical dopamine depletion induces antidepressant-like effects in rats and alters the profile of desipramine during Porsolt's test. Neuroscience. 1999;88(2):609–615. doi: 10.1016/s0306-4522(98)00258-9. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Carli M. The effects of 6-hydroxydopamine lesions of the nucleus accumbens and caudate nucleus of rats on feeding in a novel environment. Behav Brain Res. 1985;15(1):63–70. doi: 10.1016/0166-4328(85)90018-x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev. 1990;14(2):217–32. doi: 10.1016/s0149-7634(05)80222-2. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Cador M, Robbins TW. Interactions between the amygdala and ventral striatum in stimulus-reward associations: studies using a second-order schedule of sexual reinforcement. Neuroscience. 1989;30(1):63–75. doi: 10.1016/0306-4522(89)90353-9. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36(23):129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcment for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22(9):3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J comp Neurol. 1978;180:545–580. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- Feenstra MG, Botterblom MH, Mastenbroek S. Dopamine and noradrenaline efflux in the prefrontal cortex in the light and dark period: effects of novelty and handling and comparison to the nucleus accumbens. Neuroscience. 2000;100(4):741–748. doi: 10.1016/s0306-4522(00)00319-5. [DOI] [PubMed] [Google Scholar]
- Fibiger HC. Drugs and reinforcement mechanisms: a critical review of the catecholamine theory. Annu Rev Pharmacol Toxicol. 1978;18:37–56. doi: 10.1146/annurev.pa.18.040178.000345. [DOI] [PubMed] [Google Scholar]
- Filion M, Tremblay L. Abnormal spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res. 1991;547(1):142–51. [PubMed] [Google Scholar]
- Fink JS, Smith GP. Mesolimbicocortical dopamine terminal fields are necessary for normal locomotor and investigatory exploration in rats. Brain Res. 1980;199(2):359–84. doi: 10.1016/0006-8993(80)90695-2. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299(5614):1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Korth KM, Sabijan MS, DeSousa NJ. Injections of D-amphetamine into the ventral pallidum increase locomotor activity and responding for conditioned reward: a comparison with injections into the nucleus accumbens. Brain Res. 1998;805(12):29–40. doi: 10.1016/s0006-8993(98)00633-7. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG. Modulation of hippocampal and amygdalar-evoked activity of nucleus accumbens neurons by dopamine: cellular mechanisms of input selection. J Neurosci. 2001a;21(8):2851–2860. doi: 10.1523/JNEUROSCI.21-08-02851.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG. Dopamine D1 and NMDA receptors mediate potentiation of basolateral amygdala-evoked firing of nucleus accumbens neurons. J Neurosci. 2001b;21(16):6370–6376. doi: 10.1523/JNEUROSCI.21-16-06370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology. 2006;188(4):567–85. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6(9):968–7. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Fog R. On stereotypy and catalepsy: studies on the effect of amphetamines and neuroleptics in rats. Acta Neurol Scand Suppl. 1972;50:3–66. [PubMed] [Google Scholar]
- Freeman AS, Meltzer LT, Bunney BS. Firing properties of substantia nigra dopaminergic neurons in freely moving rats. Life Sci. 1985;36(20):1983–1994. doi: 10.1016/0024-3205(85)90448-5. [DOI] [PubMed] [Google Scholar]
- Freeman WJ. Mesoscopic neurodynamics: from neuron to brain. J Physiol Paris. 2000;94(56):303–22. doi: 10.1016/s0928-4257(00)01090-1. [DOI] [PubMed] [Google Scholar]
- Freeman WJ. How brains make up their minds. Columbia University Press; New York: 2001. [Google Scholar]
- Freeman WJ. Neurodynamic models of brain in psychiatry. Neuropsychopharmacology. 2003;28 1:S54–63. doi: 10.1038/sj.npp.1300147. [DOI] [PubMed] [Google Scholar]
- Freeman WJ. Ndn, volume transmission, and self-organization in brain dynamics. J Integr Neurosci. 2005;4(4):407–21. doi: 10.1142/s0219635205000963. [DOI] [PubMed] [Google Scholar]
- French SJ, Totterdell S. Hippocampal and prefrontal cortical inputs monosynaptically converge with individual projection neurons of the nucleus accumbens. J Comp Neurol. 2002;446(2):151–65. doi: 10.1002/cne.10191. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Prefrontal Cortex in Temporal Organization of Action. In: Arbib MA, editor. The Handbook of Brain Theory and Neural Network. Second. MIT Press; Cambridge: 2002. pp. 905–910. [Google Scholar]
- Gallistel CR. Note on temporal summation in the reward system. J Comp Physiol Psychol. 1974;87(5):870–5. doi: 10.1037/h0037219. [DOI] [PubMed] [Google Scholar]
- Garcia L, D'Alessandro G, Bioulac B, Hammond C. High-frequency stimulation in Parkinson's disease: more or less? Trends Neurosci. 2005;28(4):209–16. doi: 10.1016/j.tins.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Gardner EL. The neurobiology and genetics of addiction: Implications of the “reward deficiency syndrome” for therapeutic strategies in chemical dependency. In: Elster J, editor. Addiction: Entries and Exits. Russell Sage Foundation; New York: 1999. pp. 57–119. [Google Scholar]
- Garris PA, Ciolkowski EL, Pastore P, Wightman RM. Efflux of dopamine from the synaptic cleft in the nucleus accumbens of the rat brain. J Neurosci. 1994;14(10):6084–6093. doi: 10.1523/JNEUROSCI.14-10-06084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris PA, Kilpatrick M, Bunin MA, Michael D, Walker QD, Wightman RM. Dissociation of dopamine release in the nucleus accumbens from intracranial self-stimulation. Nature. 1999;398(6722):67–69. doi: 10.1038/18019. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Berger B, Gay M, Hamon M, Cesselin F, Vigny A, Javoy-Agid F, Agid Y. Tyrosine hydroxylase and methionine-enkephalin in the human mesencephalon. Immunocytochemical localization and relationships. J Neurol Sci. 1983;58(2):247–67. doi: 10.1016/0022-510x(83)90221-6. [DOI] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. J Comp Neurol. 2005;490(3):270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- George SR, Fan T, Ng GY, Jung SY, O'Dowd BF, Naranjo CA. Low endogenous dopamine function in brain predisposes to high alcohol preference and consumption: reversal by increasing synaptic dopamine. J Pharmacol Exp Ther. 1995;273(1):373–379. [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Wilson CJ. The basal ganglia. In: Swanson LW, Björklund A, Hökfelt T, editors. Handbook of Chemical Neuroanatomy Integrated Systems of the CNS. Part III. Vol. 12. Elsevier; Amsterdam: 1996. pp. 371–468. [Google Scholar]
- Gerfen CR. Molecular effects of dopamine on striatal-projection pathways. Trends Neurosci. 2000;23 10:S64–70. doi: 10.1016/s1471-1931(00)00019-7. [DOI] [PubMed] [Google Scholar]
- German DC, Manaye KF. Midbrain dopaminergic neurons (nuclei A8, A9, and A10): three-dimensional reconstruction in the rat. J Comp Neurol. 1993;331(3):297–309. doi: 10.1002/cne.903310302. [DOI] [PubMed] [Google Scholar]
- German DC, Schlusselberg DS, Woodward DJ. Three-dimensional computer reconstruction of midbrain dopaminergic neuronal populations: from mouse to man. J Neural Transm. 1983;57(4):243–254. doi: 10.1007/BF01248996. [DOI] [PubMed] [Google Scholar]
- Gerstner W, Kreiter AK, Markram H, Herz AV. Neural codes: firing rates and beyond. Proc Natl Acad Sci U S A. 1997;94(24):12740–12741. doi: 10.1073/pnas.94.24.12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Fabbricatore AT, Prokopenko VF, West MO. Differences between accumbens core and shell neurons exhibiting phasic firing patterns related to drug-seeking behavior during a discriminative-stimulus task. J Neurophysiol. 2004;92(3):1608–14. doi: 10.1152/jn.00268.2004. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Prokopenko VF, West MO, Fabbricatore AT. Higher magnitude accumbal phasic firing changes among core neurons exhibiting tonic firing increases during cocaine self-administration. Neuroscience. 2006;137(3):1075–1085. doi: 10.1016/j.neuroscience.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Glickman SE, Schiff BB. A biological theory of reinforcement. Psychol Rev. 1967;74(2):81–109. doi: 10.1037/h0024290. [DOI] [PubMed] [Google Scholar]
- Goddard GV, McIntyre DC, Leech CK. A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol. 1969;25:295–330. doi: 10.1016/0014-4886(69)90128-9. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Dworkin SI, Smith JE. Neuropharmacological assessment of cocaine self-administration into the medial prefrontal cortex. Pharmacol Biochem Behav. 1986;24(5):1429–1440. doi: 10.1016/0091-3057(86)90206-6. [DOI] [PubMed] [Google Scholar]
- Goldberg JA, Boraud T, Maraton S, Haber SN, Vaadia E, Bergman H. Enhanced synchrony among primary motor cortex neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine primate model of Parkinson's disease. J Neurosci. 2002;22(11):4639–4653. doi: 10.1523/JNEUROSCI.22-11-04639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The physiological approach: functional architecture of working memory and disordered cognition in schizophrenia. Biol Psychiatry. 1999;46(5):650–61. doi: 10.1016/s0006-3223(99)00130-4. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Muly EC, 3rd, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev. 2000;31(23):295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Gong W, Neill D, Justice JB., Jr Conditioned place preference and locomotor activation produced by injection of psychostimulants into ventral pallidum. Brain Res. 1996;707(1):64–74. doi: 10.1016/0006-8993(95)01222-2. 22. [DOI] [PubMed] [Google Scholar]
- Gong W, Neill DB, Lynn M, Justice JB., Jr Dopamine D1/D2 agonists injected into nucleus accumbens and ventral pallidum differentially affect locomotor activity depending on site. Neuroscience. 1999;93(4):1349–58. doi: 10.1016/s0306-4522(99)00235-3. [DOI] [PubMed] [Google Scholar]
- Gonon FG. Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neuroscience. 1988;24(1):19–28. doi: 10.1016/0306-4522(88)90307-7. [DOI] [PubMed] [Google Scholar]
- Gonon F. Prolonged and extrasynaptic excitatory action of dopamine mediated by D1 receptors in the rat striatum in vivo. J Neurosci. 1997;17(15):5972–5978. doi: 10.1523/JNEUROSCI.17-15-05972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonon F, Sundstrom L. Excitatory effects of dopamine released by impulse flow in the rat nucleus accumbens in vivo. Neuroscience. 1996;75(1):13–18. doi: 10.1016/0306-4522(96)00320-x. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Munoz M, Munoz A, Marin O, Smeets WJ. On the basal ganglia of amphibians: dopaminergic mesostriatal projections. Eur J Morphol. 1994;32(24):271–274. [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Kroener S, Seamans JK, Lewis DA, Barrionuevo G. Dopaminergic modulation of short-term synaptic plasticity in fast-spiking interneurons of primate dorsolateral prefrontal cortex. J Neurophysiol. 2005;94(6):4168–77. doi: 10.1152/jn.00698.2005. [DOI] [PubMed] [Google Scholar]
- Gottesmann C. The neurochemistry of waking and sleeping mental activity: the disinhibition-dopamine hypothesis. Psychiatry Clin Neurosci. 2002;56(4):345–354. doi: 10.1046/j.1440-1819.2002.01022.x. [DOI] [PubMed] [Google Scholar]
- Grace AA. Cortical regulation of subcortical dopamine systems and its possible relevance to schizophrenia. J Neural Transm Gen Sect. 1993;91(23):111–134. doi: 10.1007/BF01245228. [DOI] [PubMed] [Google Scholar]
- Grace AA. Gating of information flow within the limbic system and the pathophysiology of schizophrenia. Brain Res Rev. 2000;31(23):330–41. doi: 10.1016/s0165-0173(99)00049-1. [DOI] [PubMed] [Google Scholar]
- Gray JA. Dopamine release in the nucleus accumbens: the perspective from aberrations of consciousness in schizophrenia. Neuropsychologia. 1995;33(9):1143–53. doi: 10.1016/0028-3932(95)00054-7. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Building action repertoires: memory and learning functions of the basal ganglia. Curr Opin Neurobiol. 1995;5(6):733–741. doi: 10.1016/0959-4388(95)80100-6. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia and cognitive pattern generators. Schizophr Bull. 1997;23(3):459–469. doi: 10.1093/schbul/23.3.459. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia and chunking of action repertoires. Neurobiol Learn Mem. 1998;70(12):119–136. doi: 10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Neural networks: neural systems V: basal ganglia. Am J Psychiatry. 2001;158(1):21. doi: 10.1176/appi.ajp.158.1.21. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28(2):343–347. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- Greenberg N. Sociality, stress, and the corpus striatum of the green anolis lizard. Physiol Behav. 2003;79(3):429–440. doi: 10.1016/s0031-9384(03)00162-8. [DOI] [PubMed] [Google Scholar]
- Greengard P. The neurobiology of dopamine signaling. Biosci Rep. 2001a;21(3):247–69. doi: 10.1023/a:1013205230142. [DOI] [PubMed] [Google Scholar]
- Greengard P. The neurobiology of slow synaptic transmission. Science. 2001b;294(5544):1024–30. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23(3):435–47. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Grimm JW, See RE. Cocaine self-administration in ovariectomized rats is predicted by response to novelty, attenuated by 17-beta estradiol, and associated with abnormal vaginal cytology. Physiol Behav. 1997;61(5):755–761. doi: 10.1016/s0031-9384(96)00532-x. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ. The basal ganglia and motor control. Neural Plast. 2003;10(12):107–20. doi: 10.1155/NP.2003.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV. The nucleus accumbens: gateway for limbic structures to reach the motor system? Prog Brain Res. 1996;107:485–511. doi: 10.1016/s0079-6123(08)61883-x. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Gurney K, Prescott TJ, Redgrave P. A computational model of action selection in the basal ganglia. I. A new functional anatomy. Biol Cybern. 2001;84(6):401–410. doi: 10.1007/PL00007984. [DOI] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26(4):317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL. The primate substantia nigra and VTA: integrative circuitry and function. Crit Rev Neurobiol. 1997;11(4):323–42. doi: 10.1615/critrevneurobiol.v11.i4.40. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20(6):2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Parkinson JA, Connor TM, Dickinson A, Everitt BJ. Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. Eur J Neurosci. 2001;13(10):1984–1992. doi: 10.1046/j.0953-816x.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- Hallworth NE, Bland BH. Basal ganglia--hippocampal interactions support the role of the hippocampal formation in sensorimotor integration. Exp Neurol. 2004;188(2):430–443. doi: 10.1016/j.expneurol.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Hamani C, Neimat J, Lozano AM. Deep brain stimulation for the treatment of Parkinson's disease. J Neural Transm Suppl. 2006;70:393–9. doi: 10.1007/978-3-211-45295-0_59. [DOI] [PubMed] [Google Scholar]
- Harsing LG, Jr, Vizi ES. Alpha 2-adrenoceptors are not involved in the regulation of striatal glutamate release: comparison to dopaminergic inhibition. J Neurosci Res. 1991;28(3):376–381. doi: 10.1002/jnr.490280309. [DOI] [PubMed] [Google Scholar]
- Harvey J, Lacey MG. Endogenous and exogenous dopamine depress EPSCs in rat nucleus accumbens in vitro via D1 receptors activation. J Physiol. 1996;492(1):143–154. doi: 10.1113/jphysiol.1996.sp021296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J, Lacey MG. A postsynaptic interaction between dopamine D1 and NMDA receptors promotes presynaptic inhibition in the rat nucleus accumbens via adenosine release. J Neurosci. 1997;17:5271–5280. doi: 10.1523/JNEUROSCI.17-14-05271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RG. Pleasure response of human subjects to direct stimulation of the brain: Physiologic and psychodynamic considerations. In: Heath RG, editor. The Role of Pleasure in Human Behavior. Hoeber; New York: 1964. pp. 219–243. [Google Scholar]
- Hebb DO. The Organization of Behavior. John Wiley; New York: 1949. [Google Scholar]
- Hebb DO. Drives and the C.N.S. (Conceptual Nervous System) Psychological Review. 1955;62:243–245. doi: 10.1037/h0041823. [DOI] [PubMed] [Google Scholar]
- Hedou G, Feldon J, Heidbreder CA. Effects of cocaine on dopamine in subregions of the rat prefrontal cortex and their efferents to subterritories of the nucleus accumbens. Eur J Pharmacol. 1999;372(2):143–155. doi: 10.1016/s0014-2999(99)00218-6. [DOI] [PubMed] [Google Scholar]
- Heidbreder C, Feldon J. Amphetamine-induced neurochemical and locomotor responses are expressed differentially across the anteroposterior axis of the core and shell subterritories of the nucleus accumbens. Synapse. 1998;29(4):310–322. doi: 10.1002/(SICI)1098-2396(199808)29:4<310::AID-SYN3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Heimer G, Bar-Gad I, Goldberg JA, Bergman H. Dopamine replacement therapy reverses abnormal synchronization of pallidal neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine primate model of parkinsonism. J Neurosci. 2002;22(18):7850–7855. doi: 10.1523/JNEUROSCI.22-18-07850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L, Alheid GF, de Olmos JS, Groenewegen HJ, Haber SN, Harlan RE, Zahm DS. The accumbens: beyond the core-shell dichotomy. J Neuropsychiatry Clin Neurosci. 1997;9(3):354–8. doi: 10.1176/jnp.9.3.354. [DOI] [PubMed] [Google Scholar]
- Heimer L, Van Hoesen GW. The limbic lobe and its output channels: Implications for emotional functions and adaptive behavior. Neurosci Biobehav Rev. 2006;30(2):126–47. doi: 10.1016/j.neubiorev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Helmus TC, Downey KK, Wang LM, Rhodes GL, Schuster CR. The relationship between self-reported cocaine withdrawal symptoms and history of depression. Addict Behav. 2001;26(3):461–467. doi: 10.1016/s0306-4603(00)00105-2. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Jones GH, Justice JB, Neill DB. Neuropharmacological assessment of cocaine-induced conditioned place preference using intra-cranial microinjections. Soc Neuorosci. 1990;Abstract 16:243–6. [Google Scholar]
- Hemby SE, Jones GH, Neill DB, Justice JB., Jr 6-Hydroxydopamine lesions of the medial prefrontal cortex fail to influence cocaine-induced place conditioning. Behav Brain Res. 1992;49(2):225–230. doi: 10.1016/s0166-4328(05)80168-8. [DOI] [PubMed] [Google Scholar]
- Henry DJ, Greene MA, White FJ. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: repeated administration. J Pharmacol Exp Ther. 1989;251(3):833–839. [PubMed] [Google Scholar]
- Herrmann CS, Demiralp T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin Neurophysiol. 2005;116(12):2719–33. doi: 10.1016/j.clinph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Hernandez PJ, Andrzejewski ME, Sadeghian K, Panksepp JB, Kelley AE. AMPA/kainate, NMDA, and dopamine D1 receptor function in the nucleus accumbens core: A context-limited role in the encoding and consolidation of instrumental memory. Learn Mem. 2005;12(3):285–295. doi: 10.1101/lm.93105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Feeding and hypothalamic stimulation increase dopamine turnover in the accumbens. Physiol Behav. 1988;44(45):599–606. doi: 10.1016/0031-9384(88)90324-1. [DOI] [PubMed] [Google Scholar]
- Hernandez-Lopez S, Bargas J, Surmeier DJ, Reyes A, Galarraga E. D1 receptor activation enhances evoked discharge in neostriatal medium spiny neurons by modulating an L-type Ca 2 conductance. J Neurosci. 1997;17:3334–3342. doi: 10.1523/JNEUROSCI.17-09-03334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 1994;17(12):531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Hills T, Brockie PJ, Maricq AV. Dopamine and glutamate control area-restricted search behavior in Caenorhabditis elegans. J Neurosci. 2004;24(5):1217–1225. doi: 10.1523/JNEUROSCI.1569-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi N, White NM. The ventral pallidum area is involved in the acquisition but not expression of the amphetamine conditioned place preference. Neurosci Lett. 1993;156(12):9–12. doi: 10.1016/0304-3940(93)90426-l. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Hernandez L, Schwartz DH, Mark GP, Hunter GA. Microdialysis studies of brain norepinephrine, serotonin, and dopamine release during ingestive behavior. Theoretical and clinical implications. Ann N Y Acad Sci. 1989;575:171–191. doi: 10.1111/j.1749-6632.1989.tb53242.x. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Monaco AP, Hernandez L, Aulisi EF, Stanley BG, Lenard L. Self-injection of amphetamine directly into the brain. Psychopharmacology (Berl) 1983;81(2):158–63. doi: 10.1007/BF00429012. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Liem BJ, Justice JB., Jr Sensitization and individual differences to IP amphetamine, cocaine, or caffeine following repeated intracranial amphetamine infusions. Pharmacol Biochem Behav. 1992b;43(3):815–823. doi: 10.1016/0091-3057(92)90413-a. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Kalivas PW. The role of mesoaccumbens--pallidal circuitry in novelty-induced behavioral activation. Neuroscience. 1995;64(3):587–97. doi: 10.1016/0306-4522(94)00409-x. 1995 Feb. [DOI] [PubMed] [Google Scholar]
- Horger BA, Elsworth JD, Roth RH. Selective increase in dopamine utilization in the shell subdivision of the nucleus accumbens by the benzodiazepine inverse agonist FG 7142. J Neurochem. 1995;65(2):770–774. doi: 10.1046/j.1471-4159.1995.65020770.x. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Brain dopamine in Parkinson's disease and other neurological disturbances. In: Horn AS, Korf J, Westerning BHC, editors. The Neurobiology of Dopamine. Academic; London: 1979. pp. 633–654. [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96(4):651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Horvitz JC, Stewart T, Jacobs BL. Burst activity of ventral tegmental dopamine neurons is elicited by sensory stimuli in the awake cat. Brain Res. 1997;759(2):251–258. doi: 10.1016/s0006-8993(97)00265-5. [DOI] [PubMed] [Google Scholar]
- Hu XT, Wachtel SR, Galloway MP, White FJ. Lesions of the nigrostriatal dopamine projection increase the inhibitory effects of D1 and D2 dopamine agonists on caudateputamen neurons and relieve D2 receptors from the necessity of D1 receptor stimulation. J Neurosci. 1990;10:2318–2329. doi: 10.1523/JNEUROSCI.10-07-02318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XT, Wang RY. Comparison of effects of D-1 and D-2 dopamine receptor agonists on neurons in the rat caudate putamen: an electrophysiological study. J Neurosci. 1988;8:4340–4348. doi: 10.1523/JNEUROSCI.08-11-04340.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XT, White FJ. Dopamine enhances glutamate-induced excitation of rat striatal neurons by cooperative activation of D1 and D2 class receptors. Neurosci Lett. 1997;224:61–65. doi: 10.1016/s0304-3940(97)13443-7. [DOI] [PubMed] [Google Scholar]
- Hu Z, Cooper M, Crockett DP, Zhou R. Differentiation of the midbrain dopaminergic pathways during mouse development. J Comp Neurol. 2004;476(3):301–311. doi: 10.1002/cne.20230. [DOI] [PubMed] [Google Scholar]
- Huang YY, Simpson E, Kellendonk C, Kandel ER. Genetic evidence for the bidirectional modulation of synaptic plasticity in the prefrontal cortex by D1 receptors. Proc Natl Acad Sci U S A. 2004;101(9):3236–3241. doi: 10.1073/pnas.0308280101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C. Principles of Behavior. Appleton-Century-Crofts; New York: 1943. [Google Scholar]
- Hutcheson DM, Parkinson JA, Robbins TW, Everitt BJ. The effects of nucleus accumbens core and shell lesions on intravenous heroin self-administration and the acquisition of drug-seeking behaviour under a second-order schedule of heroin reinforcement. Psychopharmacology (Berl) 2001;153(4):464–472. doi: 10.1007/s002130000635. [DOI] [PubMed] [Google Scholar]
- Hutchison WD, Dostrovsky JO, Walters JR, Courtemanche R, Boraud T, Goldberg J, Brown P. Neuronal oscillations in the basal ganglia and movement disorders: evidence from whole animal and human recordings. J Neurosci. 2004;24(42):9240–9243. doi: 10.1523/JNEUROSCI.3366-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114(2):475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162(8):1414–22. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2(10):695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–98. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Ventral striatal anatomy of locomotor activity induced by cocaine, D-amphetamine, dopamine and D1/D2 agonists. Neuroscience. 2002;113(4):939–955. doi: 10.1016/s0306-4522(02)00247-6. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Involvement of the olfactory tubercle in cocaine reward: intracranial self-administration studies. J Neurosci. 2003;23(28):9305–11. doi: 10.1523/JNEUROSCI.23-28-09305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Unconditional hyperactivity and transient reinforcing effects of NMDA administration into the ventral tegmental area in rats. Psychopharmacology (Berl) 2004;172(2):202–10. doi: 10.1007/s00213-003-1651-3. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Murphy JM, McBride WJ. Self-infusion of GABA(A) antagonists directly into the ventral tegmental area and adjacent regions. Behav Neurosci. 1997a;111(2):369–80. doi: 10.1037//0735-7044.111.2.369. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Glazier BS, Murphy JM, McBride WJ. Role of dopamine D1 and D2 receptors in the nucleus accumbens in mediating reward. J Neurosci. 1997b;17(21):8580–7. doi: 10.1523/JNEUROSCI.17-21-08580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The relationship between self-stimulation and sniffing in rats: does a common brain system mediate these behaviors? Behav Brain Res. 1994;61(2):143–62. doi: 10.1016/0166-4328(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. Dissociations between appetitive and consummatory responses by pharmacological manipulations of reward-relevant brain regions. Behav Neurosci. 1996;110(2):331–345. doi: 10.1037//0735-7044.110.2.331. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The relationship between self-stimulation and sniffing in rats: does a common brain system mediate these behaviors? Behav Brain Res. 1999;61(2):143–62. doi: 10.1016/0166-4328(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Quin M, Liu ZH. The functional divide for primary reinforcment of D-ampheatmine lies between the medial and lateral ventral striatum: is the division of accumbens core, shell, and olfactory tubercle valid? J Neurosci. 2005;25:5061–5. doi: 10.1523/JNEUROSCI.0892-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac WL, Nonneman AJ, Neisewander J, Landers T, Bardo MT. Prefrontal cortex lesions differentially disrupt cocaine-reinforced conditioned place preference but not conditioned taste aversion. Behav Neurosci. 1989;103(2):345–355. doi: 10.1037//0735-7044.103.2.345. [DOI] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7(4):389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- Jackson DM, Ahlenius S, Anden NE, Engel J. Antagonism by locally applied dopamine into the nucleus accumbens or the corpus striatum of alpha-methyltyrosine-induced disruption of conditioned avoidance behaviour. J Neural Transm. 1977;41(4):231–239. doi: 10.1007/BF01252018. [DOI] [PubMed] [Google Scholar]
- Jackson DM, Anden NE, Dahlstrom A. A functional effect of dopamine in the nucleus accumbens and in some other dopamine-rich parts of the rat brain. Psychopharmacologia. 1975;45(2):139–49. doi: 10.1007/BF00429052. [DOI] [PubMed] [Google Scholar]
- Janowsky A, Mah C, Johnson RA, Cunningham CL, Phillips TJ, Crabbe JC, Eshleman AJ, Belknap JK. Mapping genes that regulate density of dopamine transporters and correlated behaviors in recombinant inbred mice. J Pharmacol Exp Ther. 2001;298(2):634–643. [PubMed] [Google Scholar]
- Janowsky JS, Laxer KD, Rushmer DS. Classical conditioning of kindled seizures. Epilepsia. 21:393–8. doi: 10.1111/j.1528-1157.1980.tb04087.x. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Tran A, Taylor JR, Roth RH. Prefrontal cortical involvement in phencyclidine-induced activation of the mesolimbic dopamine system: behavioral and neurochemical evidence. Psychopharmacology (Berl) 1988;138(1):89–95. doi: 10.1007/s002130050649. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH, Taylor JR. Role for dopamine in the behavioral functions of the prefrontal corticostriatal system: implications for mental disorders and psychotropic drug action. Prog Brain Res. 2000;126:433–5. doi: 10.1016/S0079-6123(00)26028-7. [DOI] [PubMed] [Google Scholar]
- Joel D. Open interconnected model of basal ganglia-thalamocortical circuitry and its relevance to the clinical syndrome of Huntington's disease. Mov Disord. 2001;16(3):407–423. doi: 10.1002/mds.1096. [DOI] [PubMed] [Google Scholar]
- Joel D, Niv Y, Ruppin E. Actor-critic models of the basal ganglia: new anatomical and computational perspectives. Neural Netw. 2002;15(46):535–47. doi: 10.1016/s0893-6080(02)00047-3. [DOI] [PubMed] [Google Scholar]
- Joel D, Weiner I. The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience. 2000;96(3):451–474. doi: 10.1016/s0306-4522(99)00575-8. [DOI] [PubMed] [Google Scholar]
- Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. Building neural representations of habits. Science. 1999;286(5445):1745–9. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- Johnels B. Locomotor hypokinesia in the reserpine-treated rat: drug effects from the corpus striatum and nucleus accumbens. Pharmacol Biochem Behav. 1982;17(2):283–289. doi: 10.1016/0091-3057(82)90082-x. [DOI] [PubMed] [Google Scholar]
- Johnson SW, Palmer MR, Freedman R. Effects of dopamine on spontaneous and evoked activity of caudate neurons. Neuropharmacology. 1983;22(7):843–851. doi: 10.1016/0028-3908(83)90130-2. [DOI] [PubMed] [Google Scholar]
- Jones BE. Arousal systems. Front Biosci. 2003;8:438–451. doi: 10.2741/1074. [DOI] [PubMed] [Google Scholar]
- Jones GH, Robbins TW. Differential effects of mesocortical, mesolimbic, and mesostriatal dopamine depletion on spontaneous, conditioned, and drug-induced locomotor activity. Pharmacol Biochem Behav. 1992;43(3):887–895. doi: 10.1016/0091-3057(92)90422-c. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998;18(6):1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, O'Dell SJ, Marshall JF, Wightman RM. Functional and natomical evidence for different dopamine dynamics in the core and shell of the nucleus accumbens in slices of rat brain. Synapse. 1996;23(3):224–231. doi: 10.1002/(SICI)1098-2396(199607)23:3<224::AID-SYN12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Jorgensen EM. Dopamine: should I stay or should I go now? Nat Neurosci. 2004;7(10):1019–1021. doi: 10.1038/nn1004-1019. [DOI] [PubMed] [Google Scholar]
- Jönsson LE, Anggard E, Gunne LM. Blockade of intravenous amphetamine euphoria in man. Clinic Pharmacol Therap. 1971;12:889–896. doi: 10.1002/cpt1971126889. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000;20(18):6983–6988. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. Interactions between dopamine and excitatory amino acids in behavioral sensitization to psychostimulants. Drug Alcohol Depend. 1995;37(2):95–100. doi: 10.1016/0376-8716(94)01063-q. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Churchill L, Romanides A. Involvement of the pallidal-thalamocortical circuit in adaptive behavior. Ann N Y Acad Sci. 1999;877:64–70. doi: 10.1111/j.1749-6632.1999.tb09261.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res. 1995;675(12):325–328. doi: 10.1016/0006-8993(95)00013-g. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Nakamura M. Neural systems for behavioral activation and reward. Curr Opin Neurobiol. 1999;9(2):223–227. doi: 10.1016/s0959-4388(99)80031-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Toda S, Bowers MS, Baker DA, Ghasemzadeh MB. The temporal sequence of changes in gene expression by drugs of abuse. Methods Mol Med. 2003;79:3–11. doi: 10.1385/1-59259-358-5:03. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kandel ER. Biology and the future of psychoanalysis: a new intellectual framework for psychiatry revisited. Am J Psychiatry. 1999;156(4):505–24. doi: 10.1176/ajp.156.4.505. [DOI] [PubMed] [Google Scholar]
- Kao MH, Doupe AJ, Brainard MS. Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature. 2005;433(7026):638–643. doi: 10.1038/nature03127. [DOI] [PubMed] [Google Scholar]
- Karreman M, Moghaddam B. The prefrontal cortex regulates the basal release of dopamine in the limbic striatum: an effect mediated by ventral tegmental area. J Neurochem. 1996;66(2):589–598. doi: 10.1046/j.1471-4159.1996.66020589.x. [DOI] [PubMed] [Google Scholar]
- Kay LM. Theta oscillations and sensorimotor performance. Proc Natl Acad Sci U S A. 2005;102(10):3863–3868. doi: 10.1073/pnas.0407920102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE. Functional specificity of ventral striatal compartments in appetitive behaviors. Ann N Y Acad Sci. 1999;877:71–90. doi: 10.1111/j.1749-6632.1999.tb09262.x. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27(8):765–76. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22(9):3306–11. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Delfs JM. Dopamine and conditioned reinforcement. I. Differential effects of amphetamine microinjections into striatal subregions. Psychopharmacology (Berl) 1991;103(2):187–96. doi: 10.1007/BF02244202. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Gauthier AM, Lang CG. Amphetamine microinjections into distinct striatal subregions cause dissociable effects on motor and ingestive behavior. Behav Brain Res. 1989;35(1):27–39. doi: 10.1016/s0166-4328(89)80005-1. [DOI] [PubMed] [Google Scholar]
- Kellogg C. Audiogenic seizures: relation to age and mechanisms of monoamine neurotransmission. Brain Res. 1976;106(1):87–103. doi: 10.1016/0006-8993(76)90075-5. [DOI] [PubMed] [Google Scholar]
- Kempf E, Gill M, Mack G, Mandel P. Effects of acute morphine administration on the catecholamine metabolism of three strains of mice. Psychopharmacol Commun. 1976;2(3):241–250. [PubMed] [Google Scholar]
- Khantzian EJ. Understanding addictive vulnerability: An evolving psychodynamic perspective. Neuro-psychoanalysis. 2003;5:5–56. (with commentaries by B. Johnson, G.F. Koob, V. Morrison, J. Panksepp & C. Yorke) [Google Scholar]
- Kihlstrom JF. The cognitive unconscious. Science. 1987;237(4821):1445–1452. doi: 10.1126/science.3629249. [DOI] [PubMed] [Google Scholar]
- King D, Finlay JM. Loss of dopamine terminals in the medial prefrontal cortex increased the ratio of DOPAC to DA in tissue of the nucleus accumbens shell: role of stress. Brain Res. 1997;767(2):192–200. doi: 10.1016/s0006-8993(97)00534-9. [DOI] [PubMed] [Google Scholar]
- King D, Zigmond MJ, Finlay JM. Effects of dopamine depletion in the medial prefrontal cortex on the stress-induced increase in extracellular dopamine in the nucleus accumbens core and shell. Neuroscience. 1997;77(1):141–153. doi: 10.1016/s0306-4522(96)00421-6. [DOI] [PubMed] [Google Scholar]
- Kitai ST. Anatomy and physiology of the neostriatum. In: Di Chiara G, Gessa GL, editors. GABA and the Basal Ganglia. Adv Biochem Psychopharmacol. Vol. 30. Raven Press; New York: 1981. pp. 1–21. [PubMed] [Google Scholar]
- Kiyatkin EA, Rebec GV. Dopaminergic modulation of glutamate-induced excitations of neurons in the neostriatum and nucleus accumbens of awake, unrestrained rats. J Neurophysiol. 1996;75(1):142–53. doi: 10.1152/jn.1996.75.1.142. [DOI] [PubMed] [Google Scholar]
- Klebaur JE, Bardo MT. Individual differences in novelty seeking on the playground maze predict amphetamine conditioned place preference. Pharmacol Biochem Behav. 1999;63(1):131–136. doi: 10.1016/s0091-3057(98)00258-5. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273(5280):1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, fong gW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rat. Psych Bull. 2002;128:961–977. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- Knutson B, Wimmer GE. Reward: Neural circuitry for social valuation. In: Harmon-Jones E, Winkelman P, editors. Social Neuroscience: Integrating biological and psychological explanations of social behavior. Guilford; New York: 2007. pp. 157–175. [Google Scholar]
- Koob GF. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci. 1992;654:171–91. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. The role of the striatopallidal and extended amygdala systems in drug addiction. Ann N Y Acad Sci. 1999;877:445–60. doi: 10.1111/j.1749-6632.1999.tb09282.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol. 2003;13(6):442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Koob GF. Allostatic view of motivation: implications for psychopathology. Nebr Symp Motiv. 2004;50:1–18. [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O'Dell LE, Parsons LH, Sanna PP. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27(8):739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278(5335):52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. The neurobiology of addiction. Academic Press; New York: 2005. [Google Scholar]
- Koob GF, Riley SJ, Smith SC, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi and olfactory tubercle on feeding, locomotor activity, and amphetamine anorexia in the rat. J Comp Physiol Psychol. 1978;92(5):917–927. doi: 10.1037/h0077542. [DOI] [PubMed] [Google Scholar]
- Koob GF, Simon H, Herman JP, Le Moal M. Neuroleptic-like disruption of the conditioned avoidance response requires destruction of both the mesolimbic and nigrostriatal dopamine systems. Brain Res. 1984;303(2):319–329. doi: 10.1016/0006-8993(84)91218-6. [DOI] [PubMed] [Google Scholar]
- Korff S, Harvey BH. Animal models of obsessive-compulsive disorder: rationale to understanding psychobiology and pharmacology. Psychiatr Clin North Am. 2006;29(2):371–90. doi: 10.1016/j.psc.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Kriegstein AR, Noctor SC. Patterns of neuronal migration in the embryonic cortex. Trends Neurosci. 2004;27(7):392–399. doi: 10.1016/j.tins.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Kropotov JD, Etlinger SC. Selection of actions in the basal ganglia-thalamocortical circuits: review and model. Int J Psychophysiol. 1999;31(3):197–217. doi: 10.1016/s0167-8760(98)00051-8. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Williams D, Kupsch A, Limousin P, Hariz M, Schneider GH, Yarrow K, Brown P. Event-related beta desynchronization in human subthalmic nucleus correlates with motor performance. Brain. 2004;127:735–746. doi: 10.1093/brain/awh106. [DOI] [PubMed] [Google Scholar]
- Kume K, Kume S, Park SK, Hirsh J, Jackson FR. Dopamine is a regulator of arousal in the fruit fly. J Neurosci. 2005;25(32):7377–7384. doi: 10.1523/JNEUROSCI.2048-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusayama T, Watanabe S. Reinforcing effects of methamphetamine in planarians. Neuroreport. 2000;11(11):2511–2513. doi: 10.1097/00001756-200008030-00033. [DOI] [PubMed] [Google Scholar]
- Lacroix L, Broersen LM, Feldon J, Weiner I. Effects of local infusions of dopaminergic drugs into the medial prefrontal cortex of rats on latent inhibition, prepulse inhibition and amphetamine induced activity. Behav Brain Res. 2000;107(12):111–121. doi: 10.1016/s0166-4328(99)00118-7. [DOI] [PubMed] [Google Scholar]
- Lagier S, Carleton A, Lledo PM. Interplay between local GABAergic interneurons and relay neurons generates γ oscillations in the rat olfactory bulb. J Neurosci. 2004;24(18):4382–4392. doi: 10.1523/JNEUROSCI.5570-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G. Olfactory network dynamics and the coding of multidimensional signals. Nat Rev Neurosci. 2002;3:884–895. doi: 10.1038/nrn964. [DOI] [PubMed] [Google Scholar]
- Lee KM, Ahn TB, Jeon BS, Kim DG. Change in phase synchronization of local field potentials in anesthetized rats after chronic dopamine depletion. Neurosci Res. 2004;49(2):179–184. doi: 10.1016/j.neures.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Lena I, Parrot S, Deschaux O, Muffat-Joly S, Sauvinet V, Renaud B, Suaud-Chagny MF, Gottesmann C. Variations in extracellular levels of dopamine, noradrenaline, glutamate, and aspartate across the sleep--wake cycle in the medial prefrontal cortex and nucleus accumbens of freely moving rats. J Neurosci Res. 2005;81(6):891–899. doi: 10.1002/jnr.20602. [DOI] [PubMed] [Google Scholar]
- Leontovich TA, Zhukova GP. The specificity of the neuronal structure and the topography of the reticular formation in the brain and spinal cord of carnivora. J Comp Neurol. 1963;121:347–379. doi: 10.1002/cne.901210305. [DOI] [PubMed] [Google Scholar]
- Lepish CC, Kroener S, Durstewitz D, Lavin A, Seamans JK. The ability of the mesocortical dopamine system to operate in distinct temporal modes. Psychopharmacology. doi: 10.1007/s00213-006-0527-8. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278(5335):45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- Levens N, Akins CK. Cocaine induces conditioned place preference and increases locomotor activity in male Japanese quail. Pharmacol Biochem Behav. 2001;68(1):71–80. doi: 10.1016/s0091-3057(00)00439-1. [DOI] [PubMed] [Google Scholar]
- Levy F. The dopamine theory of attention deficit hyperactivity disorder (ADHD) Aust N Z J Psychiatry. 1991;25(2):277–83. doi: 10.3109/00048679109077746. [DOI] [PubMed] [Google Scholar]
- Levy F. Synaptic gating and ADHD: a biological theory of comorbidity of ADHD and anxiety. Neuropsychopharmacology. 2004;29(9):1589–96. doi: 10.1038/sj.npp.1300469. [DOI] [PubMed] [Google Scholar]
- Levy R, Hutchison WD, Lozano AM, Dostrovsky JO. High-frequency synchronization of neuronal activity in the subthalamic nucleus of parkinsonian patients with limb tremor. J Neurosci. 2000;20(20):7766–7775. doi: 10.1523/JNEUROSCI.20-20-07766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BL, O'Donnell P. Ventral tegmental area afferents to the prefrontal cortex maintain membrane potential ‘up’ states in pyramidal neurons via D(1) dopamine receptors. Cereb Cortex. 2000;10(12):1168–1175. doi: 10.1093/cercor/10.12.1168. [DOI] [PubMed] [Google Scholar]
- Lewis MD. Bridging emotion theory and neurobiology through dynamic systems modeling. Behav Brain Sci. 2005;28(2):169–245. doi: 10.1017/s0140525x0500004x. [DOI] [PubMed] [Google Scholar]
- Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 2003;6(5):526–531. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- Liao RM, Chang YH, Wang SH. Influence of SCH23390 and spiperone on the expression of conditioned place preference induced by d-amphetamine or cocaine in the rat. Chin J Physiol. 1998;41(2):85–92. 30. [PubMed] [Google Scholar]
- Lima SQ, Miesenbock G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell. 2005;121(1):141–152. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Lindsley DB, Bowden J, Magoun HW. Effect upon the EEG of acure injury to the brain stem activating system. Electroencephalogr Clin Neurol. 1949;1:475–486. [PubMed] [Google Scholar]
- Lindsley DB, Schreiner LH, Knowles WB, Magoun W. Behavioral and EEG Changes following chronic Brain Stem Lesions in the Cat. Electroencephalogr Clin Neurophysiol. 1950;2:483–498. doi: 10.1016/0013-4694(50)90086-1. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Bjorklund A. The organization of the ascending catecholamine neuron systems in the rat brain as revealed by the glyoxylic acid fluorescence method. Acta Physiol Scand Suppl. 1974;412:1–48. [PubMed] [Google Scholar]
- Liu YC, Sachs BD, Salamone JD. Sexual behavior in male rats after radiofrequency or dopamine-depleting lesions in nucleus accumbens. Pharmacol Biochem Behav. 1998;60(2):585–592. doi: 10.1016/s0091-3057(98)00022-7. [DOI] [PubMed] [Google Scholar]
- Llinas R. I of the Vortex: From Neurons to Self. MIT Press; Cambridge, Massachusetts: 2002. [Google Scholar]
- Llinas RR, Grace AA, Yarom Y. In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-Hz frequency range. Proc Natl Acad Sci. 1991;88(3):897–901. doi: 10.1073/pnas.88.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Urbano FJ, Leznik E, Ramirez RR, van Marle HJ. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci. 2005;28(6):325–33. doi: 10.1016/j.tins.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Lorenz K. The comparative method in studying innate behavior patterns. Symp Soc Exp Biol. 1950;4:221–68. [Google Scholar]
- Lorenz K. Evolution and modification of behavior. Metheuen: London; 1965. [Google Scholar]
- MacDonald AF, Billington CJ, Levine AS. Alterations in food intake by opioid and dopamine signaling pathways between the ventral tegmental area and the shell of the nucleus accumbens. Brain Res. 2004;1018(1):78–85. doi: 10.1016/j.brainres.2004.05.043. [DOI] [PubMed] [Google Scholar]
- MacLean PD. The triune brain in evolution: role in paleocerebral functions. Plenum Press; New York: 1990. [DOI] [PubMed] [Google Scholar]
- MacLeod NK, Ryman A, Arbuthnott GW. Electrophysiological properties of nigrothalamic neurons after 6-hydroxydopamine lesions in the rat. Neuroscience. 1990;38(2):447–56. doi: 10.1016/0306-4522(90)90041-2. [DOI] [PubMed] [Google Scholar]
- Magill PJ, Sharott A, Bolam JP, Brown P. Brain state-dependency of coherent oscillatory activity in the cerebral cortex and basal ganglia of the rat. J Neurophysiol. 2004;92(4):2122–2136. doi: 10.1152/jn.00333.2004. [DOI] [PubMed] [Google Scholar]
- Mallet N, Ballion B, Le Moine C, Gonon F. Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of parkinsonian rats. J Neurosci. 26(14):3875–84. doi: 10.1523/JNEUROSCI.4439-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney KJ, Mainville L, Jones BE. c-Fos expression in dopaminergic and GABAergic neurons of the ventral mesencephalic tegmentum after paradoxical sleep deprivation and recovery. Eur J Neurosci. 2002;15(4):774–778. doi: 10.1046/j.1460-9568.2002.01907.x. [DOI] [PubMed] [Google Scholar]
- Marinelli M, White FJ. Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons. J Neurosci. 2000;20(23):8876–8885. doi: 10.1523/JNEUROSCI.20-23-08876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18(3):135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- Marsden JF, Limousin-Dowsey P, Ashby P, Pollak P, Brown P. Subthalamic nucleus, sensorimotor cortex and muscle interrelationships in Parkinson's disease. Brain. 2001;124(2):378–388. doi: 10.1093/brain/124.2.378. [DOI] [PubMed] [Google Scholar]
- Martel P, Fantino M. Influence of the amount of food ingested on mesolimbic dopaminergic system activity: a microdialysis study. Pharmacol Biochem Behav. 1996;55(2):297–302. doi: 10.1016/s0091-3057(96)00087-1. [DOI] [PubMed] [Google Scholar]
- Martin I, Levey AB. Classical conditioning in a cognitive era. Biol Psychol. 1988;27(2):153–66. doi: 10.1016/0301-0511(88)90047-6. [DOI] [PubMed] [Google Scholar]
- Martinez EA, Murray M, Leung MK, Stefano GB. Evidence for dopaminergic and opioid involvement in the regulation of locomotor activity in the land crab Gecarcinus lateralis. Comp Biochem Physiol C. 1988;90(1):89–93. doi: 10.1016/0742-8413(88)90103-x. [DOI] [PubMed] [Google Scholar]
- Martin-Iverson MT, Szostak C, Fibiger HC. 6-Hydroxydopamine lesions of the medial prefrontal cortex fail to influence intravenous self-administration of cocaine. Psychopharmacology (Berl) 1986;88(3):310–314. doi: 10.1007/BF00180830. [DOI] [PubMed] [Google Scholar]
- Matsunaga M, Ukena K, Baulieu EE, Tsutsui K. 7alpha-Hydroxypregnenolone acts as a neuronal activator to stimulate locomotor activity of breeding newts by means of the dopaminergic system. Proc Natl Acad Sci U S A. 2004;101(49):17282–7. doi: 10.1073/pnas.0407176101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maura G, Giardi A, Raiteri M. Release-regulating D-2 dopamine receptors are located on striatal glutamatergic nerve terminals. J Pharmacol Exp Ther. 1988;247(2):680–684. [PubMed] [Google Scholar]
- McClung C, Hirsh J. Stereotypic behavioral responses to free-base cocaine and the development of behavioral sensitization in Drosophila. Curr Biol. 1998;8(2):109–112. doi: 10.1016/s0960-9822(98)70041-7. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Salamone JD. Involvement of nucleus accumbens dopamine in the motor activity induced by periodic food presentation: a microdialysis and behavioral study. Brain Research. 1992;592:29–36. doi: 10.1016/0006-8993(92)91654-w. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Sokolowski JD, Salamone JD. A neurochemical and behavioral investigation of the involvement of nucleus accumbens dopamine in instrumental avoidance. Neuroscience. 1993;52(4):919–925. doi: 10.1016/0306-4522(93)90538-q. [DOI] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24(7):1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21(21):8655–63. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a Century of Consolidation. Science. 2000;287(5451):248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGregor A, Baker G, Roberts DC. Effect of 6-hydroxydopamine lesions of the medial prefrontal cortex on intravenous cocaine self-administration under a progressive ratio schedule of reinforcement. Pharmacol Biochem Behav. 1996;53(1):5–9. doi: 10.1016/0091-3057(95)00192-1. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Brake W, Gratton A. Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology. 2002;27(12):127–138. doi: 10.1016/s0306-4530(01)00040-3. [DOI] [PubMed] [Google Scholar]
- Meliska CJ, Bartke A, McGlacken G, Jensen RA. Ethanol, nicotine, amphetamine, and aspartame consumption and preferences in C57BL/6 and DBA/2 mice. Pharmacol Biochem Behav. 1995;50(4):619–626. doi: 10.1016/0091-3057(94)00354-8. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Stahl SM. The dopamine hypothesis of schizophrenia: a review. Schizophr Bull. 1976;2(1):19–76. doi: 10.1093/schbul/2.1.19. [DOI] [PubMed] [Google Scholar]
- Merker B. Consciousness without a cerebral cortex: A challenge for neuroscience and medicine. Behav Brain Sci. 2007;30:63–134. doi: 10.1017/S0140525X07000891. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121(6):1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Miller JD, Farber J, Gatz P, Roffwarg H, German DC. Activity of mesencephalic dopamine and non-dopamine neurons across stages of sleep and walking in the rat. Brain Res. 1983;273(1):133–141. doi: 10.1016/0006-8993(83)91101-0. [DOI] [PubMed] [Google Scholar]
- Miller JD, Sanghera MK, German DC. Mesencephalic dopaminergic unit activity in the behaviorally conditioned rat. Life Sci. 1981;29(12):1255–1263. doi: 10.1016/0024-3205(81)90231-9. [DOI] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379(6564):449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- Misra K, Pandey SC. Differences in basal levels of CREB and NPY in nucleus accumbens regions between C57BL/6 and DBA/2 mice differing in inborn alcohol drinking behavior. J Neurosci Res. 2003;74(6):967–975. doi: 10.1002/jnr.10831. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78(1):189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Miyashita Y. Cognitive memory: cellular and network machineries and their top-down control. 2004;306(5695):435–40. doi: 10.1126/science.1101864. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980a;14(23):69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Wu M, Jones DL. Locomotor activity elicited by injections of picrotoxin into the ventral tegmental area is attenuated by injections of GABA into the globus pallidus. Brain Res. 1980b;191(2):569–571. doi: 10.1016/0006-8993(80)91308-6. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Yang CR, Yim CY. Influence of dopamine on limbic inputs to the nucleus accumbens. Ann N Y Acad Sci. 1988;537:86–100. doi: 10.1111/j.1749-6632.1988.tb42098.x. [DOI] [PubMed] [Google Scholar]
- Montague PR, Hyman SE, Cohen JD. Computational roles for dopamine in behavioural control. Nature. 2004;431(7010):760–7. doi: 10.1038/nature03015. [DOI] [PubMed] [Google Scholar]
- Monti JM, Fernandez M, Jantos H. Sleep during acute dopamine D1 agonist SKF 38393 or D1 antagonist SCH 23390 administration in rats. Neuropsychopharmacology. 1990;3(3):153–162. [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5(2):169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Morgan D, Roberts DC. Sensitization to the reinforcing effects of cocaine following binge-abstinent self-administration. Neurosci Biobehav Rev. 2004;27(8):803–12. doi: 10.1016/j.neubiorev.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- Mowrer OH. Learning Theory and Behavior. Wiley; New York: 1960. [Google Scholar]
- Murer MG, Riquelme LA, Tseng KY, Pazo JH. Substantia nigra pars reticulata single unit activity in normal and 60HDA-lesioned rats: effects of intrastriatal apomorphine and subthalamic lesions. Synapse. 1997;27(4):278–293. doi: 10.1002/(SICI)1098-2396(199712)27:4<278::AID-SYN2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Murer MG, Tseng KY, Kasanetz F, Belluscio M, Riquelme LA. Brain oscillations, medium spiny neurons, and dopamine. Cell Mol Neurobiol. 2002;22(56):611–632. doi: 10.1023/A:1021840504342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Czoty PW. PET imaging of dopamine D2 receptors in monkey models of cocaine abuse: genetic predisposition versus environmental modulation. Am J Psychiatry. 2005;162(8):1473–1482. doi: 10.1176/appi.ajp.162.8.1473. [DOI] [PubMed] [Google Scholar]
- Nauta WJ, Smith GP, Faull RL, Domesick VB. Efferent connections and nigral afferents of the nucleus accumbens septi in the rat. Neuroscience. 1978;3(45):385–401. doi: 10.1016/0306-4522(78)90041-6. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Cellular responses to chronic treatment with drugs of abuse. Crit Rev Neurobiol. 1993;7(1):23–39. [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001a;2(2):119–28. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular neurobiology of addiction. Am J Addict. 2001b;10(3):201–217. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Common molecular and cellular substrates of addiction and memory. Neurobiol Learn Mem. 2002;78(3):637–47. doi: 10.1006/nlme.2002.4084. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular mechanisms of drug addiction. Neuropharmacology. 2004;47 1:24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8(11):1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA. The mesolimbic dopamine reward circuit in depression. Biol Psychiat. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Newton TF, Ling W, Kalechstein AD, Uslaner J, Tervo K. Risperidone pre-treatment reduces the euphoric effects of experimentally administered cocaine. Psychiat Res. 2001;102:227–233. doi: 10.1016/s0165-1781(01)00255-4. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Deadwyler SA. Firing rate of nucleus accumbens neurons is dopamine-dependent and reflects the timing of cocaine-seeking behavior in rats on a progressive ratio schedule of reinforcement. J Neurosci. 2000;20(14):5526–5537. doi: 10.1523/JNEUROSCI.20-14-05526.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Kombian SB, Malenka RC. Psychostimulants depress excitatory synaptic transmission in the nucleus accumbens via presynaptic D1-like dopamine receptors. J Neurosci. 1996;16(5):1591–1604. doi: 10.1523/JNEUROSCI.16-05-01591.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Malenka RC. Dopamine depresses excitatory and inhibitory synaptic transmission by distinct mechanisms in the nucleus accumbens. J Neurosci. 1997;17(15):5697–7510. doi: 10.1523/JNEUROSCI.17-15-05697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- Nieoullon A. Dopamine and the regulation of cognition and attention. Prog Neurobiol. 2002;67(1):53–83. doi: 10.1016/s0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- Nini A, Feingold A, Slovin H, Bergman H. Neurons in the globus pallidus do not show correlated activity in the normal monkey, but phase-locked oscillations appear in the MPTP model of parkinsonism. J Neurophysiol. 1995;74(4):1800–1805. doi: 10.1152/jn.1995.74.4.1800. [DOI] [PubMed] [Google Scholar]
- Nisenbaum ES, Orr WB, Berger TW. Evidence for two functionally distinct subpopulations of neurons in the rat striatum. J Neurosci. 1988;8:4138–4150. doi: 10.1523/JNEUROSCI.08-11-04138.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino S, Mao J, Sampathkumaran R, Shelton J. Increased dopaminergic transmission mediates the wake-promoting effects of CNS stimulants. Sleep Res Online. 1998;1(1):49–61. [PubMed] [Google Scholar]
- Niv Y, Duff MO, Dayan P. Dopamine, uncertainty and TD learning. Behav Brain Funct. 2005;1:6. doi: 10.1186/1744-9081-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocjar C, Panksepp J. Chronic intermittent amphetamine pretreatment enhances future appetitive behavior for drug-, food- and sexual-reward: Interaction with environmental variables. Behav Brain Res. 2002;128:189–203. doi: 10.1016/s0166-4328(01)00321-7. [DOI] [PubMed] [Google Scholar]
- Nocjar C, Panksepp J. Prior morphine experiene induces long-term increases in social interest and in appetitive behavior for natural reward. Behav Brain Res. 2007;181:191–199. doi: 10.1016/j.bbr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Oades RD. Attention deficit disorder with hyperactivity (ADDH): the contribution of catecholaminergic activity. Prog Neurobiol. 1987;29(4):365–91. doi: 10.1016/0301-0082(87)90019-0. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Ehrman R, Ternes J. Classical conditioning in human opioid dependence. In: Goldberg S, Stolerman I, editors. Behavioral Analysis of Drug Dependence. Academic Press; San Diego: 1986. pp. 329–356. [Google Scholar]
- O'Donnell P. Dopamine gating of forebrain neural ensembles. Eur J Neurosci. 2003;17(3):429–435. doi: 10.1046/j.1460-9568.2003.02463.x. [DOI] [PubMed] [Google Scholar]
- O'Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15(5 Pt 1):3622–39. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P, Grace AA. Dopaminergic reduction of excitability in nucleus accumbens neurons recorded in vitro. Neuropsychopharmacology. 1996;15(1):87–97. doi: 10.1016/0893-133X(95)00177-F. [DOI] [PubMed] [Google Scholar]
- Olds J. Drives and reinforcements: Behavioral studies of hypothalamic functions. Raven Press; New York: 1977. [Google Scholar]
- Olds J, Allan WS, Briese E. Differentiation of hypothalamic drive and reward centers. Am J Physiol. 1971;221(1):368–75. doi: 10.1152/ajplegacy.1971.221.1.368. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47(6):419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Olson L, Seiger A. Early prenatal ontogeny of central monoamine neurons in the rat: fluorescence histochemical observations. Z Anat Entwicklungsgesch. 1972;137(3):301–316. doi: 10.1007/BF00519099. [DOI] [PubMed] [Google Scholar]
- Orsini C, Buchini F, Piazza PV, Puglisi-Allegra S, Cabib S. Susceptibility to amphetamine-induced place preference is predicted by locomotor response to novelty and amphetamine in the mouse. Psychopharmacology (Berl) 2004;172(3):264–270. doi: 10.1007/s00213-003-1647-z. [DOI] [PubMed] [Google Scholar]
- Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res Brain Res Rev. 1997;25(3):312–334. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- Packard MG, Cahill L. Affective modulation of multiple memory systems. Curr Opin Neurobiol. 2001;11(6):752–6. doi: 10.1016/s0959-4388(01)00280-x. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annu Rev Neurosci. 2002;25:563–93. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Pan HS, Walters JR. Unilateral lesion of the nigrostriatal pathway decreases the firing rate and alters the firing pattern of globus pallidus neurons in the rat. Synapse. 1988;2(6):650–656. doi: 10.1002/syn.890020612. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Aggression elicited by electrical stimulation of the hypothalamus in albino rats. Physiology & Behavior. 1971;6:311–16. doi: 10.1016/0031-9384(71)90163-6. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Hypothalamic integration of behavior: Rewards, punishments, and related psycho-biological process. In: Morgane PJ, Panksepp J, editors. Handbook of the Hypothalamus, Vol. 3, Part A. Behavioral Studies of the Hypothalamus. Marcel Dekker; New York: 1981a. pp. 289–487. [Google Scholar]
- Panksepp J. Brain opioids: a neurochemical substrate for narcotic and social dependence. In: Cooper S, editor. Progress in theory in psychopharmacology. Vol. 1. Academic Press; London: 1981b. pp. 149–175. [Google Scholar]
- Panksepp J. Toward a general psychobiological theory of emotions. Behav Brain Sci. 1982;5:407–67. [Google Scholar]
- Panksepp J. The anatomy of emotions. In: Plutchik R, editor. Emotion: Theory, Research and Experience Vol. III. Biological Foundations of Emotions. Academic Press; Orlando: 1986. pp. 91–124. [Google Scholar]
- Panksepp J. Affective Neuroscience. The foundation of human and animal emotions. Oxford University Press; New York: 1998. [Google Scholar]
- Panksepp J. The long-term psychobiological consequences of infant emotions: prescriptions for the 21st century. Infant Mental Health Journal. 2001;22:132–173. [Google Scholar]
- Panksepp J. At the interface between the affective, behavioral and cognitive neurosciences: Decoding the emotional feelings of the brain. Brain Cognit. 2003;52:4–14. doi: 10.1016/s0278-2626(03)00003-4. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Affective consciousness: Core emotional feelings in animals and humans. Conscious Cogn. 2005;14(1):30–80. doi: 10.1016/j.concog.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Herman B, Conner R, Bishop P, Scott JP. The biology of social attachments: Opiates alleviate separation distress. Biol Psychiat. 1978;9:213–220. [PubMed] [Google Scholar]
- Panksepp J, Herman BH, Vilberg T, Bishop P, DeEskinazi FG. Endogenous opioids and social behavior. Neurosci Biobehav Revs. 1980;4:473–487. doi: 10.1016/0149-7634(80)90036-6. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Knutson B, Burgdorf J, Panksepp J. The role of brain emotional systems in addictions: a neuro-evolutionary perspective and new ‘self-report’ animal model. Addiction. 2002;97(4):459–69. doi: 10.1046/j.1360-0443.2002.00025.x. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Moskal J. Dopamine, and SEEKING: Subcortical “reward” systems and appetitive urges. In: Elliot A, editor. Handbook of approach and avoidance motivation. Lawrence Erlbaum Associates; Mahwah, NJ: 2007. in press. [Google Scholar]
- Panksepp J, Nocjar C, Burgdorf J, Panksepp JB, Huber R. The role of emotional systems in addiction: a neuroethological perspective. Nebr Symp Motiv. 2004;50:85–126. [PubMed] [Google Scholar]
- Panksepp JB, Huber R. Ethological analyses of crayfish behavior: a new invertebrate system for measuring the rewarding properties of psychostimulants. Behav Brain Res. 2004;153(1):171–180. doi: 10.1016/j.bbr.2003.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowill JA, Panksepp J, Gandelman R. An incentive model of rewarding brain stimulation. Psychological Review. 1969;76:264–281. doi: 10.1037/h0027295. [DOI] [PubMed] [Google Scholar]
- Papp M, Bal A. Separation of the motivational and motor consequences of 6-hydroxydopamine lesions of the mesolimbic or nigrostriatal system in rats. Behav Brain Res. 1987;23:221–229. doi: 10.1016/0166-4328(87)90022-2. [DOI] [PubMed] [Google Scholar]
- Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, Pierce RC. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci. 2002;22(7):2916–25. doi: 10.1523/JNEUROSCI.22-07-02916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-amphetamine. J Neurosci. 1999;19(6):2401–2411. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Willoughby PJ, Robbins TW, Everitt BJ. Disconnection of the anterior cingulate cortex and nucleus accumbens core impairs Pavlovian approach behavior: further evidence for limbic cortical-ventral striatopallidal systems. Behav Neurosci. 2000;114(1):42–63. [PubMed] [Google Scholar]
- Parsons LH, Smith AD, Justice JB., Jr Basal extracellular dopamine is decreased in the rat nucleus accumbens during abstinence from chronic cocaine. Synapse. 1991;9(1):60–65. doi: 10.1002/syn.890090109. [DOI] [PubMed] [Google Scholar]
- Patridge B, Schenk S. Context-independent sensitization to the locomotor-activating effects of cocaine. Pharmacol Biochem Behav. 1999;63(4):543–8. doi: 10.1016/s0091-3057(99)00013-1. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Dalley JW, Cardinal RN, Bamford A, Fehnert B, Lachenal G, Rudarakanchana N, Halkerston KM, Robbins TW, Everitt BJ. Nucleus accumbens dopamine depletion impairs both acquisition and performance of appetitive Pavlovian approach behaviour: implications for mesoaccumbens dopamine function. Behav Brain Res. 2002;137(12):149–63. doi: 10.1016/s0166-4328(02)00291-7. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Damasio A. Consciousness and the brainstem. Cognition. 2001;79(12):1. doi: 10.1016/s0010-0277(00)00127-x. [DOI] [PubMed] [Google Scholar]
- Paulsen O, Sejnoski TJ. Natural patterns of activity and long-term synaptic plasticity. Curr Opin Neurobiol. 2000;10:172–179. doi: 10.1016/s0959-4388(00)00076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlova GA. Effects of serotonin, dopamine and ergometrine on locomotion in the pulmonate mollusc Helix lucorum. J Exp Biol. 2001;204(9):1625–1633. doi: 10.1242/jeb.204.9.1625. [DOI] [PubMed] [Google Scholar]
- Paz JT, Deniau JM, Charpier S. Rhythmic bursting in the cortico-subthalamo-pallidal network during spontaneous genetically determined spike and wave discharges. J Neurosci. 2005;25(8):2092–101. doi: 10.1523/JNEUROSCI.4689-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendleton RG, Rasheed A, Sardina T, Tully T, Hillman R. Effects of tyrosine hydroxylase mutants on locomotor activity in Drosophila: a study in functional genomics. Behav Genet. 2002;32(2):89–94. doi: 10.1023/a:1015279221600. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Dolleman-Van der Weel MJ, Kitai ST, Lopes da Silva FH. Presynaptic dopamine D1 receptors attenuate excitatory and inhibitory limbic inputs to the shell region of the rat nucleus accumbens studied in vitro. J Neurophysiol. 1992;67(5):1325–1334. doi: 10.1152/jn.1992.67.5.1325. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol. 1994;42(6):719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Peoples LL, Gee F, Bibi R, West MO. Phasic firing time locked to cocaine self-infusion and locomotion: dissociable firing patterns of single nucleus accumbens neurons in the rat. J Neurosci. 1998;18(18):7588–7598. doi: 10.1523/JNEUROSCI.18-18-07588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples LL, West MO. Phasic firing of single neurons in the rat nucleus accumbens correlated with the timing of intravenous cocaine self-administration. J Neurosci. 1996;16(10):3459–3473. doi: 10.1523/JNEUROSCI.16-10-03459.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaus JG, Phillips AG. Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat. Behav Neurosci. 1991;105(5):727–743. doi: 10.1037//0735-7044.105.5.727. [DOI] [PubMed] [Google Scholar]
- Phillips GD, Robbins TW, Everitt BJ. Bilateral intra-accumbens self-administration of d-amphetamine: antagonism with intra-accumbens SCH-23390 and sulpiride. Psychopharmacology (Berl) 1994;114(3):477–85. doi: 10.1007/BF02249339. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422(6932):614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Phillipson OT. The cytoarchitecture of the interfascicular nucleus and ventral tegmental area of Tsai in the rat. J Comp Neurol. 1979;187(1):85–98. doi: 10.1002/cne.901870106. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245(4925):1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal ML. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol. 1996;36:359–378. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Holland PC. Conditioning and cognition. Neurosci Biobehav Rev. 2004;28(7):651–61. doi: 10.1016/j.neubiorev.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Crawford CA, Nonneman AJ, Mattingly BA, Bardo MT. Effect of forebrain dopamine depletion on novelty-induced place preference behavior in rats. Pharmacol Biochem Behav. 1990;36(2):321–325. doi: 10.1016/0091-3057(90)90411-a. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. Amphetamine produces sensitized increases in locomotion and extracellular dopamine preferentially in the nucleus accumbens shell of rats administered repeated cocaine. J Pharmacol Exp Ther. 1995;275(2):1019–29. [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Rev. 1997;25(2):192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Pijnenburg AJ, Honig WM, Van der Heyden JA, Van Rossum JM. Effects of chemical stimulation of the mesolimbic dopamine system upon locomotor activity. Eur J Pharmacol. 1976;35(1):45–58. doi: 10.1016/0014-2999(76)90299-5. [DOI] [PubMed] [Google Scholar]
- Plutchick R. Emotion: A Psychoevolutionary synthesis. Harper & Row; New York: 1980. [Google Scholar]
- Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci U S A. 1995;92(26):12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. The Polyvagal Theory: phylogenetic contributions to social behavior. Physiol Behav. 2003;79(3):503–13. doi: 10.1016/s0031-9384(03)00156-2. [DOI] [PubMed] [Google Scholar]
- Priori A, Foffani G, Pesenti A, Bianchi A, Chiesa V, Baselli G, Caputo E, Tamma F, Rampini P, Egidi M, Locatelli M, Barbieri S, Scarlato G. Movement-related modulation of neural activity in human basal ganglia and its L-DOPA dependency: recordings from deep brain stimulation electrodes in patients with Parkinson's disease. Neurol Sci. 2002;23(2):101–102. [Google Scholar]
- Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci. 2004;24(11):2825–31. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglisi-Allegra S, Cabib S. Psychopharmacology of dopamine: the contribution of comparative studies in inbred strains of mice. Prog Neurobiol. 1997;51(6):637–661. doi: 10.1016/s0301-0082(97)00008-7. [DOI] [PubMed] [Google Scholar]
- Puglisi-Allegra S, Imperato A, Angelucci L, Cabib S. Acute stress induces time-dependent responses in dopamine mesolimbic system. Brain Res. 1991;554(12):217–22. doi: 10.1016/0006-8993(91)90192-x. [DOI] [PubMed] [Google Scholar]
- Randrup A, Munkvad I. Influence of amphetamines on animal behaviour: stereotypy, functional impairment and possible animal-human correlations. Psychiatr Neurol Neurochir. 1972;75:193–202. [PubMed] [Google Scholar]
- Radcliffe RA, Erwin VG. Alterations in locomotor activity after microinjections of GBR-12909, selective dopamine antagonists or neurotensin into the medial prefrontal cortex. J Pharmacol Exp Ther. 1996;277(3):1467–1476. [PubMed] [Google Scholar]
- Ragnauth A, Znamensky V, Moroz M, Bodnar RJ. Analysis of dopamine receptor antagonism upon feeding elicited by mu and delta opioid agonists in the shell region of the nucleus accumbens. Brain Res. 2000;877(1):65–72. doi: 10.1016/s0006-8993(00)02674-3. [DOI] [PubMed] [Google Scholar]
- Rebec GV. Real-time assessments of dopamine function during behavior: single-unit recording, iontophoresis, and fast-scan cyclic voltammetry in awake, unrestrained rats. Alcohol Clin Exp Res. 1998;22(1):32–40. doi: 10.1111/j.1530-0277.1998.tb03614.x. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Grabner CP, Johnson M, Pierce RC, Bardo MT. Transient increases in catecholaminergic activity in medial prefrontal cortex and nucleus accumbens shell during novelty. Neuroscience. 1997;76(3):707–714. doi: 10.1016/s0306-4522(96)00382-x. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K. Is the short-latency dopamine response too short to signal reward error? Trends Neurosci. 1999;22(4):146–151. doi: 10.1016/s0166-2236(98)01373-3. [DOI] [PubMed] [Google Scholar]
- Rescorla R, Wagner A. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black A, Prokasy W, editors. Classical conditioning II: Current research and theory. AppletonCentury -Crofts: New York; 1972. pp. 64–99. [Google Scholar]
- Ressler N. Rewards and punishments, goal-directed behavior and consciousness. Neurosci Biobehav Rev. 2004;28(1):27–39. doi: 10.1016/j.neubiorev.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Reuter M, Schmitz A, Corr P, Hennig J. Molecular genetics support Gray's personality theory: the interaction of COMT and DRD2 polymorphisms predicts the behavioural approach system. Int J Neuropsychopharmacol. 2006;9(2):155–66. doi: 10.1017/S1461145705005419. [DOI] [PubMed] [Google Scholar]
- Reyes FD, Mozzachiodi R, Baxter DA, Byrne JH. Reinforcement in an in vitro analog of appetitive classical conditioning of feeding behavior in Aplysia: blockade by a dopamine antagonist. Learn Mem. 2005;12(3):216–220. doi: 10.1101/lm.92905. [DOI] [PubMed] [Google Scholar]
- Reynolds JN, Hyland BI, Wickens JR. A cellular mechanism of reward-related learning. Nature. 2001;413(6851):67–70. doi: 10.1038/35092560. [DOI] [PubMed] [Google Scholar]
- Richter-Levin G. The amygdala, the hippocampus, and emotional modulation of memory. Neuroscientist. 2004;10(1):31–9. doi: 10.1177/1073858403259955. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24(6):1265–71. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. The case of frontostriatal dysfunction in schizophrenia. Schizophr Bull. 1990;16(3):391–402. doi: 10.1093/schbul/16.3.391. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Cador M, Taylor JR, Everitt BJ. Limbic-striatal interactions in reward-related processes. Neurosci Biobehav Rev. 1989;13(23):155–162. doi: 10.1016/s0149-7634(89)80025-9. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Functional studies of the central catecholamines. Int Rev Neurobiol. 1989;23:303–65. doi: 10.1016/s0074-7742(08)60628-5. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Comparative functions of the central noradrenergic, dopaminergic and cholinergic systems. Neuropharmacology. 1987;26:893–901. doi: 10.1016/0028-3908(87)90067-0. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Drug addiction: bad habits add up. Nature. 1999;398(6728):567–70. doi: 10.1038/19208. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem. 2002;78(3):625–636. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(2):S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11(5):1598–604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Li TK, Murphy JM, McBride WJ. Cocaine is self-administered into the shell but not the core of the nucleus accumbens of Wistar rats. J Pharmacol Exp Ther. 2002;303(3):1216–1226. doi: 10.1124/jpet.102.038950. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24(6):1265–71. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman M, Wheeler R, Carelli R. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45:587–597. doi: 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55(1):11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Thorpe SJ, Boytim M, Szabo I, Perrett DI. Responses of striatal neurons in the behaving monkey. 3. Effects of iontophoretically applied dopamine on normal responsiveness. Neuroscience. 1984;12(4):1201. doi: 10.1016/0306-4522(84)90014-9. [DOI] [PubMed] [Google Scholar]
- Romach MK, Glue P, Kampman K, Kaplan HL, Somer GR, Poole S, Clarke L, Coffin V, Cornish J, O'Brien CP, Sellers EM. Attenuation of the euphoric effects of cocaine by the dopamine D1/D5 antagonist ecopipam (SCH 39166) Arc Gen Psychiat. 1999;56:1107–1108. doi: 10.1001/archpsyc.56.12.1101. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ. Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. PNAS. 2002;99(21):13908–13913. doi: 10.1073/pnas.212504599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, de Quervain DJF, Ferry B, Setlow B, McGaugh JL. Basolateral Amygdala-Nucleus Accumbens Interactions in Mediating Glucocorticoid Enhancement of Memory Consolidation. J Neurosci. 2001;21(7):2518–2525. doi: 10.1523/JNEUROSCI.21-07-02518.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth RH, Tam SY, Ida Y, Yang JX, Deutch AY. Stress and the mesocorticolimbic dopamine systems. Ann NY Acad Sci. 1988;537:138–147. doi: 10.1111/j.1749-6632.1988.tb42102.x. [DOI] [PubMed] [Google Scholar]
- Rouge-Pont F, Piazza PV, Kharouby M, Le Moal M, Simon H. Higher and longer stress-induced increase in dopamine concentrations in the nucleus accumbens of animals predisposed to amphetamine self-administration. A microdialysis study. Brain Res. 1993;602(1):169–74. doi: 10.1016/0006-8993(93)90260-t. [DOI] [PubMed] [Google Scholar]
- Rowlands GF, Roberts PJ. Activation of dopamine receptors inhibits calcium-dependent glutamate release from cortico-striatal terminals in vitro. Eur J Pharmacol. 1980;62:241–242. doi: 10.1016/0014-2999(80)90285-x. [DOI] [PubMed] [Google Scholar]
- Ruskin DN, Bergstrom DA, Tierney PL, Walters JR. Correlated multisecond oscillations in firing rate in the basal ganglia: modulation by dopamine and the subthalamic nucleus. Neuroscience. 2003;117(2):427–438. doi: 10.1016/s0306-4522(02)00921-1. [DOI] [PubMed] [Google Scholar]
- Ruskin DN, Bergstrom DA, Baek D, Freeman LE, Walters JR. Cocaine or selective block of dopamine transporters influences multisecond oscillations in firing rate in the globus pallidus. Neuropsychopharmacology. 2001;25(1):28–40. doi: 10.1016/S0893-133X(00)00241-4. [DOI] [PubMed] [Google Scholar]
- Ruskin DN, Bergstrom DA, Walters JR. Multisecond oscillations in firing rate in the globus pallidus: synergistic modulation by D1 and D2 dopamine receptors. J Pharmacol Exp Ther. 1999;290(3):1493–1501. [PubMed] [Google Scholar]
- Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137(12):3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote S, Weber SM. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. J Pharmacol Exp Ther. 2003;305(1):1–8. doi: 10.1124/jpet.102.035063. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Current Opinions in Pharmacology. 2005;5:34–45. doi: 10.1016/j.coph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Salenius S, Avikainen S, Kaakkola S, Hari R, Brown P. Defective cortical drive to muscle in Parkinson's disease and its improvement with levodopa. Brain. 2002;125(3):491–500. doi: 10.1093/brain/awf042. [DOI] [PubMed] [Google Scholar]
- Sanderson P, Mavoungou R, Albe-Fessard D. Changes in substantia nigra pars reticulata activity following lesions of the substantia nigra pars compacta. Neurosci Lett. 1986;67(1):25–30. doi: 10.1016/0304-3940(86)90202-8. [DOI] [PubMed] [Google Scholar]
- Sawin ER, Ranganathan R, Horvitz HR. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26(3):619–631. doi: 10.1016/s0896-6273(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Sax KW, Strakowski SM. Behavioral sensitization in humans. J Addict Dis. 2001;20(3):55–65. doi: 10.1300/J069v20n03_06. [DOI] [PubMed] [Google Scholar]
- Schenk S, Horger BA, Peltier R, Shelton K. Supersensitivity to the reinforcing effects of cocaine following 6-hydroxydopamine lesions to the medial prefrontal cortex in rats. Brain Res. 1991;543(2):227–235. doi: 10.1016/0006-8993(91)90032-q. [DOI] [PubMed] [Google Scholar]
- Schiebel ME, Scheibel AB. Structural substrates for integrative patterns in the brain stem reticular core. In: Jasper HH, Proctor LD, Knighton RS, Noshay WC, Costello RT, editors. The Reticular Formation of the Brain. Little, Brown & Co.; Boston, Massachusetts: 1958. pp. 31–55. [Google Scholar]
- Schildein S, Agmo A, Huston JP, Schwarting RK. Intraaccumbens injections of substance P, morphine and amphetamine: effects on conditioned place preference and behavioral activity. Brain Res. 1998;790(12):185–94. doi: 10.1016/s0006-8993(98)00062-6. [DOI] [PubMed] [Google Scholar]
- Schmitz Y, Benoit-Marand M, Gonon F, Sulzer D. Presynaptic regulation of dopaminergic neurotransmission. J Neurochem. 2003;87(2):273–289. doi: 10.1046/j.1471-4159.2003.02050.x. [DOI] [PubMed] [Google Scholar]
- Schulkin J, Thompson BL, Rosen JB. Demythologizing the emotions: adaptation, cognition, and visceral representations of emotion in the nervous system. Brain Cogn. 2003;52(1):15–23. doi: 10.1016/s0278-2626(03)00004-6. [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol. 1997;7(2):191–197. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- Schultz W. The phasic reward signal of primate dopamine neurons. Adv Pharmacol. 1998;42:686–690. doi: 10.1016/s1054-3589(08)60841-8. [DOI] [PubMed] [Google Scholar]
- Schultz W. Reward signaling by dopamine neurons. Neuroscientist. 2001;7(4):293–302. doi: 10.1177/107385840100700406. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36(2):241–63. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schultz W. Neural coding of basic reward terms of animal learning theory, game theory, microeconomics and behavioural ecology. Curr Opin Neurobiol. 2004;14(2):139–47. doi: 10.1016/j.conb.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral Theories and the Neurophysiology of Reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J Neurosci. 1993;13(3):900–913. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu Rev Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]; Science. 235:73–76. [Google Scholar]
- Seale TW, Carney JM. Genetic determinants of susceptibility to the rewarding and other behavioral actions of cocaine. J Addict Dis. 1991;10(12):141–162. doi: 10.1300/J069v10n01_10. [DOI] [PubMed] [Google Scholar]
- Sederberg PB, Schulze-Bonhage A, Madsen JR, Bromfield EB, McCarthy DC, Brandt A, Tully MS, Kahana MJ. Hippocampal and Neocortical Gamma Oscillations Predict Memory Formation in Humans. Cereb Cortex. 2006 doi: 10.1093/cercor/bhl030. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Self D. Neurobiology: Dopamine as chicken and egg. Nature. 2003;422(6932):573–574. doi: 10.1038/422573a. [DOI] [PubMed] [Google Scholar]
- Self DW. Regulation of drug-taking and -seeking behaviors by neuroadaptations in the mesolimbic dopamine system. Neuropharmacology. 2004;47(1):242–255. doi: 10.1016/j.neuropharm.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Sellings LH, Clarke PB. Segregation of amphetamine reward and locomotor stimulation between nucleus accumbens medial shell and core. J Neurosci. 2003;23(15):6295–303. doi: 10.1523/JNEUROSCI.23-15-06295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellings LH, McQuade LE, Clarke PB. Evidence for multiple sites within rat ventral striatum mediating cocaine-conditioned place preference and locomotor activation. J Pharmacol Exp Ther. 2006;317(3):1178–87. doi: 10.1124/jpet.105.100339. [DOI] [PubMed] [Google Scholar]
- Sewards TV, Sewards MA. Representations of motivational drives in mesial cortex, medial thalamus, hypothalamus and midbrain. Brain Res Bull. 2003;61(1):25–49. doi: 10.1016/s0361-9230(03)00069-8. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168(12):3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Sharott A, Magill PJ, HarNacck D, Kupsch A, Meissner W, Brown P. Dopamine depletion increases the power and coherence of beta-oscillations in the cerebral cortex and subthalamic nucleus of the awake rat. Eur J Neurosci. 2005;21(5):1413–1422. doi: 10.1111/j.1460-9568.2005.03973.x. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. Integrated Action of the Nervous System. Cambridge University Press; Cambridge: 1906. [Google Scholar]
- Shimosato K, Watanabe S. Concurrent evaluation of locomotor response to novelty and propensity toward cocaine conditioned place preference in mice. J Neurosci Methods. 2003;128(12):103–110. doi: 10.1016/s0165-0270(03)00153-5. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Bals-Kubik R, Herz A. Examination of the neurochemical substrates mediating the motivational effects of opioids: role of the mesolimbic dopamine system and D-1 vs. D-2 dopamine receptors. J Pharmacol Exp Ther. 1993;265(1):53–59. [PubMed] [Google Scholar]
- Siggins GR. Electrophysiological role of dopamine in the striatum: excitatory or inhibitory? In: Lipton MA, Killam KF, editors. Psychopharmacology: A Generation of Progress. Raven; New York: 1978. pp. 143–157. [Google Scholar]
- Siggins GR, Hoffer BJ, Bloom FE, Ungerstedt U. Cytochemical and electrophysiological studies of dopamine in the caudate nucleus. Res Publ Assoc Res Nerv Ment Dis. 1976;55:227–248. [PubMed] [Google Scholar]
- Skinner BF. The behavior of organisms. Appleton-Century-Crofts; New York: 1938. [Google Scholar]
- Sloviter RS. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. 1987 doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- Smeets WJ. Distribution of dopamine immunoreactivity in the forebrain and midbrain of the snake Python regius: a study with antibodies against dopamine. J Comp Neurol. 1988;271(1):115–129. doi: 10.1002/cne.902710112. [DOI] [PubMed] [Google Scholar]
- Smeets WJ, Gonzalez A. Catecholamine systems in the brain of vertebrates: new perspectives through a comparative approach. Brain Res Rev. 2000;33(23):308–79. doi: 10.1016/s0165-0173(00)00034-5. [DOI] [PubMed] [Google Scholar]
- Smeets WJ, Jonker AJ, Hoogland PV. Distribution of dopamine in the forebrain and midbrain of the red-eared turtle, Pseudemys scripta elegans, reinvestigated using antibodies against dopamine. Brain Behav Evol. 1987;30(34):121–142. doi: 10.1159/000118642. [DOI] [PubMed] [Google Scholar]
- Smith AD, Olson RJ, Justice JB., Jr Quantitative microdialysis of dopamine in the striatum: effect of circadian variation. J Neurosci Methods. 1992;44(1):33–41. doi: 10.1016/0165-0270(92)90111-p. [DOI] [PubMed] [Google Scholar]
- Smith-Roe SL, Kelley AE. Coincident activation of NMDA and dopamine D1 receptors within the nucleus accumbens core is required for appetitive instrumental learning. J Neurosci. 2000;20(20):7737–42. doi: 10.1523/JNEUROSCI.20-20-07737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits RP, Steinbusch HW, Mulder AH. Distribution of dopamine-immunoreactive cell bodies in the guinea-pig brain. J Chem Neuroanat. 1990;3(2):101–123. [PubMed] [Google Scholar]
- Snyder SH. Catecholamines in the brain as mediators of amphetamine psychosis. Arch Gen Psychiatry. 1972;27(2):169–79. doi: 10.1001/archpsyc.1972.01750260021004. [DOI] [PubMed] [Google Scholar]
- Solms M. Dreaming and REM sleep are controlled by different brain mechanisms. Behav Brain Sci. 2000;23(6):843–50. doi: 10.1017/s0140525x00003988. [DOI] [PubMed] [Google Scholar]
- Solms M, Turnbull O. The Brain and the Inner World: An Introduction to the Neuroscience of Subjective Experience. Other Press; New York: 2002. [Google Scholar]
- Solomon RL. An opponent-process theory of acquired motivation: The affective dynamics of addiction. In: Maser JD, Seligman MEP, editors. Psychopathology: Experimental models. San Francisco: W. H. Freeman; 1977. pp. 66–103. [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends Neurosci. 1999;22(11):521–7. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Spence KW. Behavior theory and conditioning. Yale University Press; New Haven, Connecticut: 1956. [Google Scholar]
- Stansfield KH, Philpot RM, Kirstein CL. An animal model of sensation seeking: the adolescent rat. Ann N Y Acad Sci. 2004;1021:453–458. doi: 10.1196/annals.1308.063. [DOI] [PubMed] [Google Scholar]
- Steinfels GF, Heym J, Jacobs BL. Single unit activity of dopaminergic neurons in freely moving cuts. Life Sci. 1981;29(14):1435–1442. doi: 10.1016/0024-3205(81)90007-2. [DOI] [PubMed] [Google Scholar]
- Steinfels GF, Heym J, Strecker RE, Jacobs BL. Behavioral correlates of dopaminergic unit activity in freely moving cats. Brain Res. 1983;258(2):217–228. doi: 10.1016/0006-8993(83)91145-9. [DOI] [PubMed] [Google Scholar]
- Steriade M. Arousal: revisiting the reticular activating system. Science. 1996;272(5259):225–226. doi: 10.1126/science.272.5259.225. [DOI] [PubMed] [Google Scholar]
- Steriade M. Corticothalamic resonance, states of vigilance and mentation. Neuroscience. 2000;101(2):243–76. doi: 10.1016/s0306-4522(00)00353-5. [DOI] [PubMed] [Google Scholar]
- Stewart CV, Plenz D. Inverted-U profile of dopamine-NMDA-mediated spontaneous avalanche recurrence in superficial layers of rat prefrontal cortex. J Neurosci. 2006;26(31):8148–59. doi: 10.1523/JNEUROSCI.0723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J, Wise RA. Environment makes amphetamine-induced dopamine release in the nucleus accumbens totally impulse-dependent. Synapse. 1992;58(3):211–4. doi: 10.1002/syn.20197. [DOI] [PubMed] [Google Scholar]
- Stinus L, Herman JP, Le Moal M. GABAergic mechanisms within the ventral tegmental area: involvement of dopaminergic (A 10) and non-dopaminergic neurones. Psychopharmacology (Berl) 1982;77(2):186–192. doi: 10.1007/BF00431946. [DOI] [PubMed] [Google Scholar]
- Suaud-Chagny MF, Chergui K, Chouvet G, Gonon F. Relationship between dopamine release in the rat nucleus accumbens and the discharge activity of dopaminergic neurons during local in vivo application of amino acids in the ventral tegmental area. Neuroscience. 1992;49(1):63–72. doi: 10.1016/0306-4522(92)90076-e. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. The role of prefrontal cortex D1-like and D2-like receptors in cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2005;177(3):315–23. doi: 10.1007/s00213-004-1956-x. [DOI] [PubMed] [Google Scholar]
- Sutton RS, Barto AG. Toward a modern theory of adaptive networks: expectation and prediction. Psychol Rev. 1981;1981;88(2):135–70. [PubMed] [Google Scholar]
- Swanson CJ, Heath S, Stratford TR, Kelley AE. Differential behavioral responses to dopaminergic stimulation of nucleus accumbens subregions in the rat. Pharmacol Biochem Behav. 1997;58(4):933–45. doi: 10.1016/s0091-3057(97)00043-9. [DOI] [PubMed] [Google Scholar]
- Swanson L. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9(16):321–53. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886(12):113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Koob GF. Dopamine, schizophrenia, mania and depression: toward a unified hypthesis of cortico-striato-pallido-thalamic function. Behav Brais Sci. 1987;10:197–245. [Google Scholar]
- Sykova E. Extrasynaptic volume transmission and diffusion parameters of the extracellular space. Neuroscience. 2004;129(4):861–76. doi: 10.1016/j.neuroscience.2004.06.077. [DOI] [PubMed] [Google Scholar]
- Taghzouti K, Simon H, Louilot A, Herman JP, Le Moal M. Behavioral study after local injection of 6-hydroxydopamine into the nucleus accumbens in the rat. Brain Res. 1985;344(1):9–20. doi: 10.1016/0006-8993(85)91184-9. [DOI] [PubMed] [Google Scholar]
- Tanaka S. Dopaminergic control of working memory and its relevance to schizophrenia: a circuit dynamics perspective. Neuroscience. 2006;139(1):153–71. doi: 10.1016/j.neuroscience.2005.08.070. [DOI] [PubMed] [Google Scholar]
- Tassin JP, Stinus L, Simon H, Blanc G, Thierry AM, Le Moal M, Cardo B, Glowinski J. Relationship between the locomotor hyperactivity induced by A10 lesions and the destruction of the fronto-cortical dopaminergic innervation in the rat. Brain Res. 1978;141(2):267–281. doi: 10.1016/0006-8993(78)90197-x. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Robbins TW. 6-Hydroxydopamine lesions of the nucleus accumbens, but not of the caudate nucleus, attenuate enhanced responding with reward-related stimuli produced by intra-accumbens d-amphetamine. Psychopharmacology (Berl) 1986;90(3):390–7. doi: 10.1007/BF00179197. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Tassin JP, Blanc G, Glowinski J. Selective activation of mesocortical DA system by stress. Nature. 1976;263(5574):242–244. doi: 10.1038/263242a0. [DOI] [PubMed] [Google Scholar]
- Thompson B, Leonard KC, Brudzynski SM. Amphetamine-induced 50 kHz calls from rat nucleus accumbens: A quantitative mapping study and acoustic analysis. Behav Brain Res. 2006;268:64–73. doi: 10.1016/j.bbr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Structural characterization of a hypothalamic visceromotor pattern generator network. Brain Res Brain Res Rev. 2003;41(23):153–202. doi: 10.1016/s0165-0173(02)00232-1. [DOI] [PubMed] [Google Scholar]
- Tinbergen N. The Study of Instinct. Oxford University Press; Oxford: 1951. [Google Scholar]
- Toates F. Cognition, motivation, emotion and action: a dynamic and vulnerable interdependence. Applied Animal Behaviour Science. 2004;86:173–204. [Google Scholar]
- Toates FM. Motivational Systems. Cambridge University Press; Cambridge: 1986. [Google Scholar]
- Torres G, Horowitz JM. Activating properties of cocaine and cocaethylene in a behavioral preparation of Drosophila melanogaster. Synapse. 1998;29(2):148–161. doi: 10.1002/(SICI)1098-2396(199806)29:2<148::AID-SYN6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Trampus M, Ferri N, Monopoli A, Ongini E. The dopamine D1 receptor is involved in the regulation of REM sleep in the rat. Eur J Pharmacol. 1991;194:189–194. doi: 10.1016/0014-2999(91)90104-x. [DOI] [PubMed] [Google Scholar]
- Treiman DM. GABAergic mechanisms in epilepsy. Epilepsia. 2001;42(S3):8–12. doi: 10.1046/j.1528-1157.2001.042suppl.3008.x. [DOI] [PubMed] [Google Scholar]
- Trowill JA, Panksepp J, Gandelman R. An incentive model of rewarding brain stimulation. Psychological Review. 1969;76:264–81. doi: 10.1037/h0027295. [DOI] [PubMed] [Google Scholar]
- True WR, Xian H, Scherrer JF, Madden PA, Bucholz KK, Heath AC, Eisen SA, Lyons MJ, Goldberg J, Tsuang M. Common genetic vulnerability for nicotine and alcohol dependence in men. Arch Gen Psychiatry. 1999;56(7):655–61. doi: 10.1001/archpsyc.56.7.655. [DOI] [PubMed] [Google Scholar]
- Trulson ME. Simultaneous recording of substantia nigra neurons and voltammetric release of dopamine in the caudate of behaving cats. Brain Res Bull. 1985;15(2):221–223. doi: 10.1016/0361-9230(85)90140-6. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Preussler DW. Dopamine-containing ventral tegmental area neurons in freely moving cats-activity during the sleep-waking cycle and effects of stress. Exp Neurol. 1984;83:367–377. doi: 10.1016/S0014-4886(84)90105-5. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Preussler DW, Howell GA. Activity of substantia nigra units across the sleep-waking cycle in freely moving cats. Neurosci Lett. 1981;26(2):183–188. doi: 10.1016/0304-3940(81)90346-3. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Kasanetz F, Kargieman L, Riquelme LA, Murer MG. Cortical slow oscillatory activity is reflected in the membrane potential and spike trains of striatal neurons in rats with chronic nigrostriatal lesions. J Neurosci. 2001;21(16):6430–6439. doi: 10.1523/JNEUROSCI.21-16-06430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Riquelme LA, Belforte JE, Pazo JH, Murer MG. Substantia nigra pars reticulata units in 6-hydroxydopamine-lesioned rats: responses to striatal D2 dopamine receptor stimulation and subthalamic lesions. Eur J Neurosci. 2000;12(1):247–256. doi: 10.1046/j.1460-9568.2000.00910.x. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Riquelme LA, Murer MG. Impact of D1-class dopamine receptor on striatal processing of cortical input in experimental parkinsonism in vivo. Neuroscience. 2004;123(2):293–8. doi: 10.1016/j.neuroscience.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Pharmacology and behavioral pharmacology of the mesocortical dopamine system. Prog Neurobiol. 2001;63(3):241–320. doi: 10.1016/s0301-0082(00)00033-2. [DOI] [PubMed] [Google Scholar]
- Uhl GR. Molecular genetics of substance abuse vulnerability: a current approach. Neuropsychopharmacology. 1999;20(1):3–9. doi: 10.1016/S0893-133X(98)00061-X. [DOI] [PubMed] [Google Scholar]
- Uhl GR. Molecular genetics of substance abuse vulnerability: remarkable recent convergence of genome scan results. Ann N Y Acad Sci. 2004;1025:1–13. doi: 10.1196/annals.1316.001. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Liu QR, Naiman D. Substance abuse vulnerability loci: converging genome scanning data. Trends Genet. 2002;18(8):420–425. doi: 10.1016/s0168-9525(02)02719-1. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand Suppl. 1971;367:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U, Ljungberg T, Steg G. Behavioral, physiological, and neurochemical changes after 6-hydroxydopamine-induced degeneration of the nigro-striatal dopamine neurons. Adv Neurol. 1974;5:421–426. [PubMed] [Google Scholar]
- Ungless MA. Dopamine: the salient issue. Trends Neurosci. 2004;27(12):702–6. doi: 10.1016/j.tins.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Valenstein ES, Cox VC, Kakolewski JW. Hypothalamic motivational systems: fixed or plastic neural circuits? Science. 1969;163(871):1084. doi: 10.1126/science.163.3871.1084. [DOI] [PubMed] [Google Scholar]
- Valenstein ES, Cox VC, Kakolewski JW. Reexamination of the role of the hypothalamus in motivation. Psychol Rev. 1970;77(1):16–31. doi: 10.1037/h0028581. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151(23):99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Tarter RE. Genetic studies of substance abuse. Drug Alcohol Depend. 2000;59(2):101–23. doi: 10.1016/s0376-8716(99)00109-x. [DOI] [PubMed] [Google Scholar]
- Ventura R, Cabib S, Puglisi-Allegra S. Genetic susceptibility of mesocortical dopamine to stress determines liability to inhibition of mesoaccumbens dopamine and to behavioral ‘despair’ in a mouse model of depression. Neuroscience. 2002;115(4):999–1007. doi: 10.1016/s0306-4522(02)00581-x. [DOI] [PubMed] [Google Scholar]
- Ventura R, Alcaro A, Mandolesi L, Puglisi-Allegra S. In vivo evidence that genetic background controls impulse-dependent dopamine release induced by amphetamine in the nucleus accumbens. J Neurochem. 2004;89(2):494–502. doi: 10.1111/j.1471-4159.2004.02342.x. [DOI] [PubMed] [Google Scholar]
- Ventura R, Puglisi-Allegra S. Environment makes amphetamine-induced dopamine release in the nucleus accumbens totally impulse-dependent. Synapse. 2005 2005 Dec 1;58(3):211–4. doi: 10.1002/syn.20197. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Hippocampal theta rhythm: a tag for short-term memory. Hippocampus. 2005;15(7):923–935. doi: 10.1002/hipo.20118. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Viana Di Prisco G. Theta rhythm of the hippocampus: subcortical control and functional significance. Behav Cogn Neurosci Rev. 2004;3(3):173–200. doi: 10.1177/1534582304273594. [DOI] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27(8):827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Vitek JL, Giroux M. Physiology of hypokinetic and hyperkinetic movement disorders: model for dyskinesia. Ann Neurol. 2000;47(4) 1:S131–S140. [PubMed] [Google Scholar]
- Vizi ES. Non-synaptic interaction between neurons in the brain, an analog system: far from Cajal-Sherringtons's galaxy. Bull Mem Acad R Med Belg. 2003;158(1012):373–379. [PubMed] [Google Scholar]
- Vogel DD. A neural network model of memory and higher cognitive functions. Int J Psychophysiol. 2005;55(1):3–21. doi: 10.1016/j.ijpsycho.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2002;158(12):2015–21. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. Role of dopamine in drug reinforcement and addiction in humans: results from imaging studies. Behav Pharmacol. 2002;13(56):355–366. doi: 10.1097/00008877-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM. Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Amer J Psychiat. 2003;160:1909–1918. doi: 10.1176/appi.ajp.160.11.1909. [DOI] [PubMed] [Google Scholar]
- Waelti P, Dickinson A, Schultz W. Dopamine responses comply with basic assumptions of formal learning theory. Nature. 2001;412(6842):43–48. doi: 10.1038/35083500. [DOI] [PubMed] [Google Scholar]
- Wakabayashi KT, Fields HL, Nicola SM. Dissociation of the role of nucleus accumbens dopamine in responding to reward-predictive cues and waiting for reward. Behav Brain Res. 2004;154(1):19–30. doi: 10.1016/j.bbr.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Watson JB. Psychology as the behaviorist views it. Psychol Review. 1913;20:158–177. [Google Scholar]
- Wauquier A, Rolls ET, editors. Brain-stimulation Reward. North Holland Pub. Co./Elsevier; Amsterdam & New York: 1976. [Google Scholar]
- Weiner I, Gal G, Rawlins JN, Feldon J. Differential involvement of the shell and core subterritories of the nucleus accumbens in latent inhibition and amphetamine-induced activity. Behav Brain Res. 1996;81(12):123–133. doi: 10.1016/s0166-4328(96)00051-4. [DOI] [PubMed] [Google Scholar]
- Weiss F, Markou A, Lorang MT, Koob GF. Basal extracellular dopamine levels in the nucleus accumbens are decreased during cocaine withdrawal after unlimited-access self-administration. Brain Res. 1992;593(2):314–318. doi: 10.1016/0006-8993(92)91327-b. [DOI] [PubMed] [Google Scholar]
- Weiss SR, Post RM, Pert A, Woodward R, Murman D. Context-dependent cocaine sensitization: differential effect of haloperidol on development versus expression. Pharmacol Biochem Behav. 1989;34(3):655–61. [PubMed] [Google Scholar]
- West AR, Floresco SB, Charara A, Rosenkranz JA, Grace AA. Electrophysiological interactions between striatal glutamatergic and dopaminergic systems. Ann N Y Acad Sci. 2003;1003:53–74. doi: 10.1196/annals.1300.004. [DOI] [PubMed] [Google Scholar]
- West AR, Grace AA. Opposite influences of endogenous dopamine D1 and D2 receptor activation on activity states and electrophysiological properties of striatal neurons: studies combining in vivo intracellular recordings and reverse microdialysis. J Neurosci. 2002;22(1):294–304. doi: 10.1523/JNEUROSCI.22-01-00294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West CH, Boss-Williams KA, Weiss JM. Motor activation by amphetamine infusion into nucleus accumbens core and shell subregions of rats differentially sensitive to dopaminergic drugs. Behav Brain Res. 1999;98(1):155–165. doi: 10.1016/s0166-4328(98)00064-3. [DOI] [PubMed] [Google Scholar]
- Westerink BH, Tuntler J, Damsma G, Rollema H, de Vries JB. The use of tetrodotoxin for the characterization of drug-enhanced dopamine release in conscious rats studied by brain dialysis. Naunyn Schmiedebergs Arch Pharmacol. 1987;336(5):502–507. doi: 10.1007/BF00169306. [DOI] [PubMed] [Google Scholar]
- White NM, Packard MG, Hiroi N. Place conditioning with dopamine D1 and D2 agonists injected peripherally or into nucleus accumbens. Psychopharmacology (Berl) 1991;103(2):271–6. doi: 10.1007/BF02244216. [DOI] [PubMed] [Google Scholar]
- White FJ. A behavioral/systems approach to the neuroscience of drug addiction. J Neurosci. 22(9):3303–3305. doi: 10.1523/JNEUROSCI.22-09-03303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FJ, Wang RY. Electrophysiological evidence for A10 dopamine autoreceptor subsensitivity following chronic D-amphetamine treatment. Brain Res. 1984;309(2):283–292. doi: 10.1016/0006-8993(84)90594-8. [DOI] [PubMed] [Google Scholar]
- White FJ, Wang RY. Electrophysiological evidence for the existence of both D-1 and D-2 dopamine receptors in the rat nucleus accumbens. J Neurosci. 1986;6(1):274–280. doi: 10.1523/JNEUROSCI.06-01-00274.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NM. Mnemonic functions of the basal ganglia. Curr Opin Neurobiol. 1996a;7(2):164–9. doi: 10.1016/s0959-4388(97)80004-9. [DOI] [PubMed] [Google Scholar]
- White NM. Addictive drugs as reinforcers: multiple partial actions on memory systems. Addiction. 1996b;91(7):921–949. 951–965. [PubMed] [Google Scholar]
- White NM, Milner PM. The psychobiology of reinforcers. Ann Rev Psychol. 1992;43:443–471. doi: 10.1146/annurev.ps.43.020192.002303. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, Starr PA, Subramanian T, Watts RL, DeLong MR. Comparison of MPTP-induced changes in spontaneous neuronal discharge in the internal pallidal segment and in the substantia nigra pars reticulata in primates. Exp Brain Res. 1999;125(4):397–409. doi: 10.1007/s002210050696. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Pathophysiology of Parkinson's disease: the MPTP primate model of the human disorder. Ann N Y Acad Sci. 2003;991:199–213. doi: 10.1111/j.1749-6632.2003.tb07477.x. [DOI] [PubMed] [Google Scholar]
- Wickens JR, Reynolds JN, Hyland BI. Neural mechanisms of reward-related motor learning. Curr Opin Neurobiol. 2003;13(6):685–90. doi: 10.1016/j.conb.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Robinson DL. Transient changes in mesolimbic dopamine and their association with ‘reward’. J Neurochem. 2002;82(4):721–35. doi: 10.1046/j.1471-4159.2002.01005.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson LS. The nature of interactions involving prefrontal and striatal dopamine systems. J Psychopharmacol. 1997;11(2):143–150. doi: 10.1177/026988119701100207. [DOI] [PubMed] [Google Scholar]
- Williams D, Tijssen M, Van Bruggen G, Bosch A, Insola A, Di Lazzaro V, Mazzone P, Oliviero A, Quartarone A, Speelman H, Brown P. Dopamine-dependent changes in the functional connectivity between basal ganglia and cerebral cortex in humans. Brain. 2002;125(7):1558–1569. doi: 10.1093/brain/awf156. [DOI] [PubMed] [Google Scholar]
- Williams GV, Millar J. Differential Actions of Endogenous and Iontophoretic Dopamine in Rat Striatum. Eur J Neurosci. 1990;2(7):658–661. doi: 10.1111/j.1460-9568.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Widespread origin of the primate mesofrontal dopamine system. Cereb Cortex. 1998;8(4):321–45. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]
- Willner P. Dopamine and depression: a review of recent evidence. I. Empirical studies. Brain Res. 1983a;287(3):211–24. doi: 10.1016/0165-0173(83)90005-x. [DOI] [PubMed] [Google Scholar]
- Willner P. Dopamine and depression: a review of recent evidence. II. Theoretical approaches. Brain Res. 1983b;287(3):225–36. doi: 10.1016/0165-0173(83)90006-1. [DOI] [PubMed] [Google Scholar]
- Willner P, Sheel-Krüger J. The mesolimbic dopamine system: from motivation to action. John Wiley & Sons; Lodon: 1991. pp. 225–250. [Google Scholar]
- Winterer G. Cortical microcircuits in schizophrenia--the dopamine hypothesis revisited. Pharmacopsychiatry. 2006;39:S68–71. doi: 10.1055/s-2006-931498. [DOI] [PubMed] [Google Scholar]
- Wise RA. Catecholamine theories of reward: a critical review. Brain Res. 1978;152(2):215–247. doi: 10.1016/0006-8993(78)90253-6. [DOI] [PubMed] [Google Scholar]
- Wise RA. Intracranial self-stimulation: mapping against the lateral boundaries of the dopaminergic cells of the substantia nigra. Brain Res. 1981;213(1):190–4. doi: 10.1016/0006-8993(81)91260-9. [DOI] [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36(2):229–40. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5(6):483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wise RA. Forebrain substrates of reward and motivation. J Comp Neurol. 2005;493(1):115–21. doi: 10.1002/cne.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. Brain substrates for reinforcement and drug self-administration. Prog Neuropsychopharmacol. 1981;5(56):467–74. doi: 10.1016/0364-7722(81)90028-x. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94(4):469–92. [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Wise RA, Spindler J, Dewit H, Gerberg GJ. Neuroleptic-induced “anhedonia” in rats: pimozide blocks reward quality of food. Science. 1978;201(4352):262–4. doi: 10.1126/science.566469. [DOI] [PubMed] [Google Scholar]
- Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001;21(5):1787–1794. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. Addiction: making the connection between behavioral changes and neuronal plasticity in specific pathways. Mol Interv. 2002;2(3):146–157. doi: 10.1124/mi.2.3.146. [DOI] [PubMed] [Google Scholar]
- Wolf ME. Cocaine addiction: clues from Drosophila on drugs. Curr Biol. 2003;9(20):R770–R772. doi: 10.1016/S0960-9822(00)80009-3. [DOI] [PubMed] [Google Scholar]
- Wolf ME, White FJ, Nassar R, Brooderson RJ, Khansa MR. Differential development of autoreceptor subsensitivity and enhanced dopamine release during amphetamine sensitization. J Pharmacol Exp Ther. 1993;264(1):249–55. [PubMed] [Google Scholar]
- Wolf ME, Mangiavacchi S, Sun X. Mechanisms by which dopamine receptors may influence synaptic plasticity. Ann N Y Acad Sci. 2003;1003:241–9. doi: 10.1196/annals.1300.015. [DOI] [PubMed] [Google Scholar]
- Wolterink G, Phillips G, Cador M, Donselaar-Wolterink I, Robbins TW, Everitt BJ. Relative roles of ventral striatal D1 and D2 dopamine receptors in responding with conditioned reinforcement. Psychopharmacology (Berl) 1993;110(3):355–64. doi: 10.1007/BF02251293. [DOI] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of surcrose reward: enhancement of reward ‘wanting’ without enhanced ‘liking’ or response reinforcment. J Neurosci. 2000;20:8122–30. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Incentive-sensitization by previous ampehtamine exposure: Increased cue-triggered ‘wanting’ for sucrose reward. J Neurosci. 2001;21:7831–40. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CR, Mogenson GJ. Electrophysiological responses of neurones in the nucleus accumbens to hippocampal stimulation and the attenuation of the excitatory responses by the mesolimbic dopaminergic system. Brain Res. 1984;324(1):69–84. doi: 10.1016/0006-8993(84)90623-1. [DOI] [PubMed] [Google Scholar]
- Yang CR, Seamans JK, Gorelova N. Developing a neuronal model for the pathophysiology of schizophrenia based on the nature of electrophysiological actions of dopamine in the prefrontal cortex. Neuropsychopharmacology. 1999;21(2):161–94. doi: 10.1016/S0893-133X(98)00112-2. [DOI] [PubMed] [Google Scholar]
- Yetnikoff L, Arvanitogiannis A. A role for affect in context-dependent sensitization to amphetamine. Behav Neurosci. 2005 2005 Dec;119(6):1678–81. doi: 10.1037/0735-7044.119.6.1678. [DOI] [PubMed] [Google Scholar]
- Yim CY, Mogenson GJ. Response of nucleus accumbens neurons to amygdala stimulation and its modification by dopamine. Brain Res. 1982;239(2):401–415. doi: 10.1016/0006-8993(82)90518-2. [DOI] [PubMed] [Google Scholar]
- Yim CY, Mogenson GJ. Mesolimbic dopamine projection modulates amygdala-evoked EPSP in nucleus accumbens neurons: an in vivo study. Brain Res. 1986;369(12):347–352. doi: 10.1016/0006-8993(86)90548-2. [DOI] [PubMed] [Google Scholar]
- Zahm DS. Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Ann N Y Acad Sci. 1999;877:113–128. doi: 10.1111/j.1749-6632.1999.tb09264.x. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Brog JS. On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience. 1992;50(4):751–767. doi: 10.1016/0306-4522(92)90202-d. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Cooper DC, White FJ. Repeated cocaine treatment decreases whole-cell calcium current in rat nucleus accumbens neurons. J Pharmacol Exp Ther. 2002;301(3):1119–1125. doi: 10.1124/jpet.301.3.1119. [DOI] [PubMed] [Google Scholar]
- Zocchi A, Orsini C, Cabib S, Puglisi-Allegra S. Parallel strain-dependent effect of amphetamine on locomotor activity and dopamine release in the nucleus accumbens: an in vivo study in mice. Neuroscience. 1998;82(2):521–528. doi: 10.1016/s0306-4522(97)00276-5. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. The psychophysiology of sensation seeking. J Pers. 1990;58(1):313–345. doi: 10.1111/j.1467-6494.1990.tb00918.x. [DOI] [PubMed] [Google Scholar]