Abstract
The receptor 2B4 belongs to the Ig superfamily and is found on the surface of all murine natural killer (NK) cells as well as T cells displaying non-MHC-restricted cytotoxicity. Previous studies have suggested that 2B4 is an activating molecule because cross-linking of this receptor results in increased cytotoxicity and γ-interferon secretion as well as granule exocytosis. However, it was recently shown that the gene for 2B4 encodes two different products that arise by alternative splicing. These gene products differ solely in their cytoplasmic domains. One form has a cytoplasmic tail of 150 amino acids (2B4L) and the other has a tail of 93 amino acids (2B4S). To determine the function of each receptor, cDNAs for 2B4S and 2B4L were transfected into the rat NK cell line RNK-16. Interestingly, the two forms of 2B4 had opposing functions. 2B4S was able to mediate redirected lysis of P815 tumor targets, suggesting that this form represents an activating receptor. However, 2B4L expression led to an inhibition of redirected lysis of P815 targets when the mAb 3.2.3 (specific for rat NKRP1) was used. In addition, 2B4L constitutively inhibits lysis of YAC-1 tumor targets. 2B4L is a tyrosine phosphoprotein, and removal of domains containing these residues abrogates its inhibitory function. Like other inhibitory receptors, 2B4L associates with the tyrosine phosphatase SHP-2. Thus, 2B4L is an inhibitory receptor belonging to the Ig superfamily.
Natural killer (NK) cells are large, granular lymphocytes that are able to exhibit non-MHC-restricted lysis (1). They mediate the lysis of certain tumors and virally infected cells and are also responsible for the acute rejection of non-MHC-matched bone marrow transplants (2, 3). NK cell functions are regulated by a dynamic balance between positive signaling receptors (resulting in lysis) and negative signaling receptors (preventing lysis) (4–6). NK cells possess a family of MHC class I receptors that transmit inhibitory signals, thereby preventing lysis of cells that express adequate levels of MHC class I and allowing the lysis of cells with diminished surface levels of MHC class I (5, 7). However, noninhibitory receptors that also recognize MHC class I have recently been identified (8, 9). Human NK cells possess MHC class I receptors of the Ig superfamily that perform both inhibitory and stimulatory functions. These receptors have been termed KIRs and KARs, respectively (10). However, rodent NK cells seem to possess MHC class I receptors of the C-type lectin superfamily termed Ly49s and are represented by both inhibitory and stimulatory members as well (11). In addition, both rodent and human NK cells have been shown to possess another group of inhibitory/stimulatory MHC class I receptor pairs represented by heterodimers of the CD94/NKG2 proteins (12–16). Other receptors present on NK cells have also been shown to be represented by inhibitory/noninhibitory pairs such as the LIR or ILT family of receptors (16, 17). Therefore, a common theme among NK cell receptors is the presence of functionally opposite pairs of receptors for a particular ligand.
To date, murine homologs of the KIR/KAR family of Ig domain receptors have not been identified. However, orphan receptors of the Ig superfamily have been identified on murine NK cells (18–21). One of these receptors, 2B4, is found on all NK and T cells that exhibit non-MHC-restricted cytotoxicity (19, 22). Recently the ligand for 2B4 was identified as the previously defined CD2 ligand CD48 (23). Previous studies have implicated 2B4 as a positive signaling molecule because cross-linking of surface 2B4 by specific antibodies resulted in a stimulation of target lysis, granule exocytosis and γ-IFN secretion (19). Recent evidence indicates that the gene for murine 2B4 encodes two distinct polypeptides, 2B4L and 2B4S, that are identical except in their intracellular domains (S.E.S. and P.A.M., unpublished work). The cytoplasmic region of 2B4L contains five unique potential tyrosine phosphorylation sites that are similar in context to those described previously for various immunoregulatory tyrosine-based inhibitory motifs (ITIM) (24). To define the functions of the two forms of the 2B4 receptor, each isoform was expressed separately in the rat NK cell line RNK-16. A variety of lytic assays were used to establish that 2B4L and 2B4S represent inhibitory and stimulatory receptors, respectively.
MATERIALS AND METHODS
Cells and Tissue Culture.
RNK-16, a spontaneous NK cell leukemia from F344 rats, was grown in RPMI 1640 medium supplemented with 10% fetal calf serum, 2 mM l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin (25). P815 and YAC-1 tumor cell lines used as targets were also cultured in complete RPMI 1640 medium. Lymphokine-activated killer cell cultures were established as described (26). Various RNK-16 transfectants were grown in complete RPMI 1640 medium supplemented with 0.5 mg/ml G418.
Antibodies and Flow Cytometry.
Except where noted, all antibodies were purchased from PharMingen. The 3.2.3 ascites (anti-rat NKR-P1A) and the anti-P815 rabbit polyclonal antisera were a kind gift of W. Chambers (University of Pittsburgh). Fab′ and F(ab′)2 fragments of the 2B4 mAb were prepared as described (27). Purity of these preparations was determined by SDS/PAGE. For fluorescent cell staining, mAbs were used at 2 μg per 106 cells. Routine analysis was performed with a fluorescence-activated cell sorter scan.
Cytotoxicity Assays.
Specific lysis of targets was determined by using a standard 4-hr 51Cr release assay as described (26). Redirected lysis assays were performed by adding antibodies at the indicated concentrations to the effectors in the 96-well plates and incubating for 15 min at 37°C before the addition of labeled targets. Plates were then incubated at 37°C for 4 hr and then centrifuged for 5 min, and the radioactivity in 100 μl of the supernatant was measured by scintillation counting. Percent specific lysis was determined as described (26). Cytotoxicity assays were repeated at least three times using multiple clonal isolates of each transfectant.
Vector Constructs and Transfections.
cDNAs corresponding to each of the 2B4 isoforms or the various deletion mutants were subcloned into the SalI and XbaI sites of the expression vector pEMCVαEN (a kind gift of A. Shaw and M. Olszowy, Washington University, St. Louis, MO). Constructs were confirmed by sequencing before transfection. The 2B4 deletion constructs were generated by PCR mutagenesis using 3′ 2B4-specific primers containing stop codons at the indicated positions. The 3′ primers were as follows: 2B4D1, CTT AGT TCA CAC ACA GAA ACA;, 2B4D2, CAA TTC TCT TTA TCC CCT CCC; and 2B4D3, GGA AGG CTG TCA TAC TGA ATA. Transfections were performed using 10 μg of ClaI linearized plasmids that had been prepared by using the Maxi-Prep system (Qiagen, Chatsworth, CA) as described (27).
Immunoprecipitations and Western Blot Analysis.
Where indicated, transfectants and wild-type RNK-16 cells were incubated with 0.03% H2O2 and 100 μM orthovanadate (pervanadate) for 10 min at 37°C. Approximately 2 × 107 cells per sample were used at a concentration of 1 × 106 cells per ml. Cells were harvested and washed in PBS; cell pellets were then lysed at 4°C in lysis buffer (50 mM Hepes, pH 7.4/150 mM NaCl/1 mM EDTA/1 mM MgCl2/10% glycerol) containing 1% Triton X-100 (27). Immunoprecipitations and Western blot analyses were performed as described (27) using antibodies purchased from Santa Cruz Biotechnology, and blots were developed with the enhanced chemiluminescence system (Amersham).
RESULTS
Expression of the Two Forms of Murine 2B4 in RNK-16 Cells and Evaluation of Their Signaling Potential.
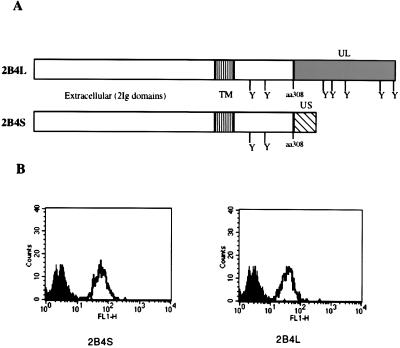
Previous studies have shown that cross-linking of surface 2B4 by a specific mAb resulted in stimulation of NK cells and γδ dendritic epidermal T cells, thereby increasing cytolytic activity, γ-IFN secretion, and granule exocytosis (19, 28). However, we have recently found that activated NK cells (LAKs) express two forms of this receptor, 2B4L and 2B4S, that arise by alternative splicing (S.E.S. and P.A.M., unpublished work; GenBank accession nos. L19057 for 2B4L and AF082803 for 2B4S). To assess the function of each receptor individually, the rat NK cell line RNK-16 was stably transfected with cDNAs encoding each form of the receptor. Fig. 1A shows the predicted ORFs of each 2B4 receptor. The forms are identical up to amino acid 308, from which they diverge because of alternative splicing of the 2B4 transcript. There are two potential tyrosine phosphorylation sites, located at amino acids 267 and 269, that are present in both forms of 2B4. In addition, there are five potential tyrosine phosphorylation sites that are found exclusively in the cytoplasmic region of 2B4L. The tyrosine residues are flanked by amino acids that are similar to those found in ITIMs of various inhibitory receptors (29, 30). Fig. 1B shows 2B4 expression of representative transfectants. Clones expressing similar levels of surface 2B4 were selected for further studies.
Figure 1.
Expression of 2B4 isoforms in RNK-16 cells. (A) Diagram of the gene products (2B4L or 2B4S) that arise by alternative splicing of the murine 2B4 gene. The two forms are identical up to amino acid 308, at which point sequences unique to each form are represented (░⃞ or ▧). The potential tyrosine phosphorylation sites are represented by (Y). cDNAs for each form were cloned into expression vectors and transfected into the rat NK cell line RNK-16. (B) The staining profiles of stable transfectants of RNK-16 cells are shown. Cells were transfected with expression constructs as indicated and stained with FITC-conjugated isotype control antibodies (■) or FITC-conjugated anti-2B4 (□).
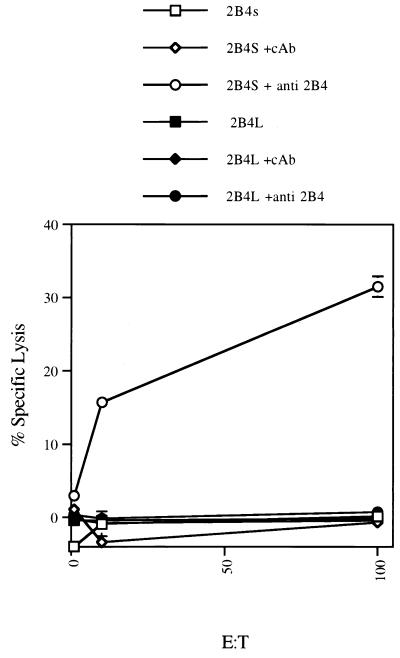
To assess the ability of 2B4L or 2B4S to stimulate cytotoxicity, a redirected lysis assay was employed. RNK-16 cells are unable to lyse the Fc receptor-positive (FcR+) tumor cell line P815. However, it has been shown that addition of antibodies to positive signaling receptors found on RNK-16 cells, such as NKR-P1A, is able to stimulate lysis of P815 targets (31). Similar studies conducted with the 2B4L or 2B4S transfectants showed that addition of mAb to 2B4 stimulated cytotoxicity of P815 targets by 2B4S-expressing transfectants only (Fig. 2). Untreated 2B4S or 2B4L transfectants as well as RNK-16 mock transfectants exhibited no appreciable lysis of P815 targets at all effector-to-target ratios tested (Fig. 2 and data not shown). Additions of isotype control antibody as well as Fab′ or F(ab′)2 fragments of mAb to 2B4 were unable to stimulate lysis by any of the RNK-16 cell lines tested (Fig. 2 and data not shown), indicating that cross-linking of surface 2B4S is required to stimulate lysis of P815 targets. These studies were reproduced three to five times with independently derived 2B4-isoform-expressing clones. These data indicate that whereas 2B4S can transmit stimulatory signals, 2B4L fails to do so.
Figure 2.
Redirected lysis of P815 targets by 2B4L or 2B4S transfectants. RNK-16 cells transfected with empty vector (RNK-16) or with 2B4 expression constructs (2B4L or 2B4S) were used as effectors in a standard chromium release assay against the murine FcR+ target P815. The effectors used are shown on the top of the figure. Effectors were preincubated with 25 μg/ml of isotype control antibody (cAb) or with anti-2B4 antibody before the addition of targets as indicated.
Deletion Mutants Map Functional Domains of 2B4.
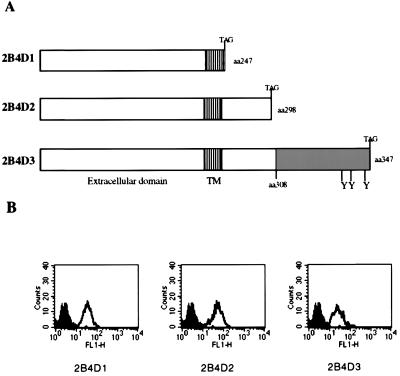
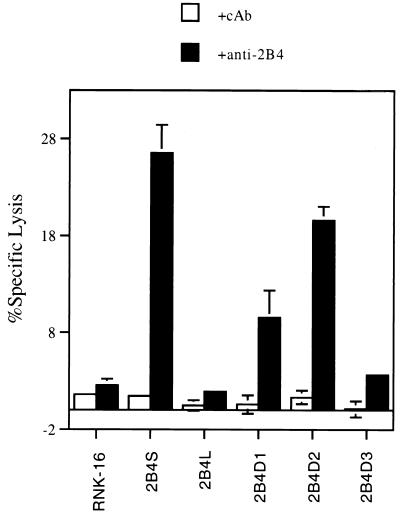
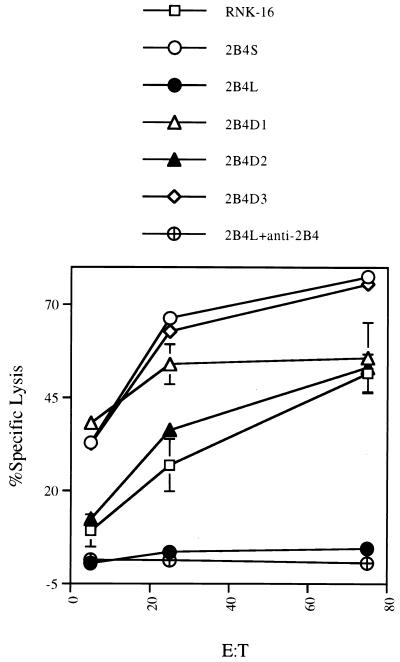
A series of C-terminal deletion mutants of 2B4 were constructed by PCR mutagenesis to define domains that are important in signal transduction. Fig. 3A is a diagram of the various deletion constructs generated. 2B4D1 encodes a protein truncated at amino acid 247, thereby removing all intracellular domains; 2B4D2 is truncated at amino acid 298, resulting in removal of all residues unique to either 2B4L or 2B4S; and 2B4D3 is truncated at amino acid 347 of 2B4L, thereby removing three of the C-terminal, potential tyrosine phosphorylation sites unique to the intracellular domain of 2B4L. Fig. 3B shows a representative FACS profile of stable transfectants of RNK-16 expressing each of the deletion mutants of 2B4 (open histograms). Clones expressing similar levels of surface 2B4 were selected for further studies. Fig. 4 shows a redirected lysis assay using RNK-16 transfectants expressing the 2B4 deletion mutants as effectors against the tumor target, P815 as described for Fig. 2. Once again, mock transfectants or transfectants expressing 2B4L were unable to lyse P815 targets, whereas transfectants expressing 2B4S showed increased target lysis in the presence of mAb to 2B4. Removal of the entire cytoplasmic domain of 2B4 (2B4D1) did not abolish its ability to stimulate redirected lysis, indicating that this region is not necessary for positive signaling. However, redirected lysis was diminished compared with 2B4S, indicating that although the cytoplasmic tail of 2B4S is not essential to stimulate lysis, it is needed for maximum effectiveness. Interestingly, transfectants expressing 2B4D2 were able to mediate redirected lysis upon cross-linking. Because 2B4D2 contains the shared cytoplasmic residues of 2B4L and 2B4S, but lacks the residues unique to the cytoplasmic domain of 2B4L and 2B4S, it follows that residues located in the shared cytoplasmic tail of 2B4L or 2B4S are sufficient for maximal lytic activation. In contrast, transfectants expressing 2B4D3 (in which only a small region of the C-terminal segment of 2B4L was deleted) were similar to 2B4L transfectants in that they were unable to exhibit redirected lysis of P815 cells in the presence of mAb to 2B4. This indicates that removal of amino acids 347–398 of 2B4L was insufficient to convert this receptor into a stimulatory molecule. In addition, these data also show that residues unique to the cytoplasmic portion of 2B4L (amino acids 308–398) confer inactivity to the 2B4 receptor because the unique region of 2B4S is not needed for stimulation. The nonresponsive phenotype conferred by 2B4L cannot be relieved by removal of a portion of the cytoplasmic tail of 2B4L (2B4D3) but requires deletion of all residues unique to 2B4L (2B4D2). These studies indicate that 2B4L is either a null receptor or in fact may represent an inhibitory form of the receptor.
Figure 3.
Expression of 2B4 deletion constructs in RNK-16 cells. (A) Various 2B4 deletion constructs used to map functional domains of 2B4 are depicted. Residues unique to 2B4L are represented by shaded regions. Stop codons were added at the desired positions by PCR mutagenesis, and the resulting products were placed into expression vectors and used to transfect RNK-16 cells. (B) The staining profiles of stable transfectants of RNK-16 cells are shown. Transfected cell lines were stained with FITC-conjugated isotype control antibody (■) or with FITC-conjugated anti-2B4 antibody (□).
Figure 4.
Redirected lysis of P815 targets by RNK-16 clones expressing the 2B4 deletion mutants. Various transfected RNK-16 cell lines were used as effectors (indicated on x axis) at an effector-to-target ratio of 100:1 in a standard chromium release assay. As indicated, effectors were preincubated with control antibody (cAb) or with anti-2B4 as described for Fig. 2.
2B4L Possesses Inhibitory Functions.
The studies described above along with the fact that 2B4L contains multiple, potential tyrosine phosphorylation sites similar to those described for other inhibitory receptors prompted us to examine the ability of 2B4L to function in an inhibitory fashion. To examine this possibility, we evaluated the ability of RNK-16 transfectants to lyse YAC-1 tumor targets. Fig. 5 shows that mock-transfected RNK-16 cells and transfectants expressing 2B4S are able to effectively lyse YAC-1 cells. However, expression of 2B4L inhibited the ability of RNK-16 cells to lyse these targets. Addition of either whole mAb to 2B4 or Fab′ or F(ab′)2 fragments was unable to relieve the inhibition of lysis by 2B4L (Fig. 5 and data not shown). As described for the redirected lysis assays, transfectants expressing the 2B4 deletions 2B4D2 or 2B4D1 were also able to lyse these targets. Interestingly, transfectants expressing 2B4D3, though unable to mediate redirected lysis of P815 targets in the presence of mAb to 2B4, were able to lyse YAC-1 cells much like parental RNK-16 cells. Therefore, removal of the three most C-terminal, potential tyrosine phosphorylation sites on 2B4L abolished the inhibitory effect of this receptor on YAC-1 lysis.
Figure 5.
2B4L expression inhibits tumor lysis by RNK-16. A typical chromium release assay using YAC-1 tumor targets is shown. Various RNK-16 transfectants (described on top of the figure) were used as effectors at the indicated effector-to-target (E:T) ratios. Where indicated, effectors were preincubated with anti-2B4 antibody as described for Fig. 2.
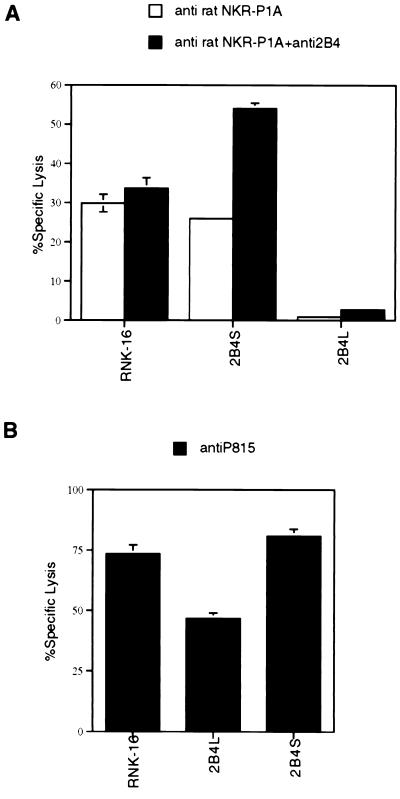
To evaluate further the ability of 2B4L to inhibit cytotoxicity, we determined the effect of 2B4L expression on redirected lysis by a stimulatory receptor unrelated to 2B4. Both mock-transfected RNK-16 cells and transfectants expressing 2B4S were able to mediate redirected lysis of the FcR+ target P815 in the presence of the mAb 3.2.3 (which recognizes the rat NK receptor NKR-P1A) (Fig. 6A). As described for Fig. 2, the baseline lysis of P815 cells was negligible. However, RNK-16 transfectants expressing 2B4L were unable to mediate redirected lysis of P815 targets, and this inhibition was not relieved by the addition of mAb to 2B4 (Fig. 6A). Addition of mAb to both 2B4 and NKR-P1A resulted in an even greater level of redirected lysis when 2B4S transfectants were used as effectors than was observed in the presence of either antibody alone (Fig. 6A). However, no increase in lysis was observed when both antibodies were added to mock-transfected RNK-16 cells. These data indicate that both 2B4S and NKR-P1A can independently activate cytotoxicity. Interestingly, redirected lysis in the presence of mAb 3.2.3 was similar for transfectants expressing 2B4S, 2B4D1, 2B4D2, and 2B4D3 (data not shown), further indicating that 2B4D3 removes inhibitory properties of 2B4L under certain conditions.
Figure 6.
2B4L expression inhibits redirected lysis by NKR-P1A but not antibody-dependent cellular cytotoxicity in RNK-16 cells. (A) A redirected lysis assay using P815 targets is shown. Various RNK-16 transfectants were used at an effector-to-target ratio of 100:1. Where indicated, effectors were preincubated with anti-rat NKR-P1A (3.2.3 antibody) or with anti-2B4 antibody as described in Fig. 2. As shown for Fig. 2, baseline lysis of P815 targets by all transfectants was consistently <2%. (B) An antibody-dependent cellular cytotoxicity assay using P815 targets is shown. Various RNK-16 transfectants were used as effectors as described for Fig. 6. Where indicated, effectors were preincubated with anti-P815 (rabbit polyclonal antisera) or anti-2B4 antibody as described for Fig. 2. As shown for Fig. 2, baseline lysis of P815 targets by all transfectants was consistently <2%.
It is possible that expression of high levels of 2B4L results in a null phenotype rendering RNK-16 cells ineffective in all forms of cytotoxicity. To test this possibility, we employed a more potent mechanism of stimulating lytic function of RNK-16 cells. Because RNK-16 cells express FcR, they are able to mediate antibody-dependent cellular cytotoxicity. This was accomplished by using RNK-16 transfectants as effectors against P815 targets in the presence of a rabbit polyclonal anti-P815 antiserum. This antiserum was used to coat the P815 targets with antibody, thereby exposing the Fc portion of the antibody for interaction with the FcRs of the RNK-16 effectors. Under these conditions, RNK-16 cells transfected with 2B4L lysed P815 targets quite effectively (Fig. 6B). Although lysis was lower for 2B4L transfectants compared with mock transfectants and those expressing 2B4S, the killing was still substantial, indicating that expression of 2B4L does not result in a null phenotype and that the inhibitory signals mediated by 2B4L can be overcome by potent positive signaling mechanisms.
Phosphorylation Profile of 2B4 and Association with Tyrosine Phosphatases.
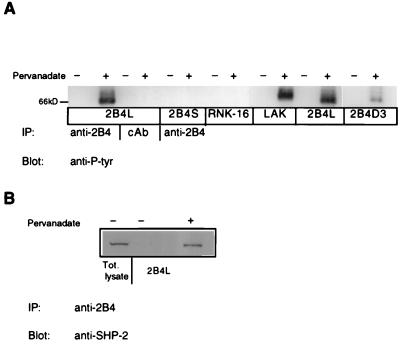
Like other NK inhibitory receptors, 2B4L contains tyrosine residues in its cytoplasmic domain that could be phosphorylated. The five tyrosine residues unique to 2B4L are contained within the following sequence stretches: TMYSMI, TVYSVV, QNYSLS, TVYEEV, and DVYS, none of which exactly follow the ITIM consensus sequence, VXYXXV (32, 33). Therefore, to determine whether 2B4L is indeed a tyrosine phosphoprotein, RNK-16 transfectants were treated with pervanadate, which results in an inhibition of tyrosine phosphatases and increases tyrosine kinase activity (34). Transfectants treated with pervanadate were then subjected to immunoprecipitation with mAb to 2B4 followed by Western blot analysis with anti-phosphotyrosine antibodies. Anti-phosphotyrosine immunoblots of 2B4 immunoprecipitates of mock-transfected RNK-16 or transfectants expressing 2B4S failed to show any tyrosine phosphorylation of 2B4 (Fig. 7A). However, similar experiments using transfectants expressing 2B4L did show a tyrosine-phosphorylated protein of the correct molecular mass of 2B4 (66 kDa) in pervanadate-treated cell lysates (Fig. 7A). Immunoprecipitation with isotype control antibody failed to show a band corresponding to 2B4. A similar tyrosine phosphoprotein was immunoprecipitated from lysates of pervanadate-treated B6 LAK cells (Fig. 7A), indicating that phosphorylation of 2B4L was not unique to expression in RNK-16 cells. The molecular weights of 2B4 immunoprecipitated from LAK cells and RNK-16 transfectants were slightly different because of differences in glycosylation patterns (data not shown). In addition, immunoprecipitation of pervanadate-treated lysates obtained from RNK-16 transfectants expressing 2B4D3 showed a tyrosine phosphoprotein of the correct molecular weight for 2B4 but the level of phosphorylation appeared to be less than that of 2B4L (Fig. 7A). This apparent decrease in tyrosine phosphorylation observed for 2B4D3 compared with 2B4L could not be attributed to decreased surface expression of 2B4D3 because surface staining of transfectants expressing 2B4L and 2B4D3 (Figs. 1B and 3B) was quite similar. These data suggest that removal of the three most C-terminal tyrosine residues of 2B4L may result in decreased phosphorylation of the 2B4 protein, implicating these as phosphorylation sites. In addition, because removal of these residues relieved the inhibition of YAC-1 lysis (Fig. 5), the level of phosphorylation may affect the inhibitory function of this receptor. However, specific point mutations will be required to fully resolve this question.
Figure 7.
2B4L tyrosine phosphorylation and SHP-2 association. (A) Approximately 2 × 107 cells of various RNK-16 transfectants or C57/Bl6 LAKs were incubated at 37°C for 10 min in the presence or absence of 1 mM pervanadate. Cells were lysed and immunoprecipitated with 5 μg of anti-2B4 antibody or control antibody (cAb) as indicated. Immunoprecipitates were resolved by nonreducing SDS/PAGE and immunoblotted with horseradish peroxidase-conjugated anti-phosphotyrosine antibody and analyzed by enhanced chemiluminescence. Bands corresponding to 2B4 are located near the 66-kDa marker. (B) Approximately 1 × 108 cells of 2B4L expressing RNK-16 cells were incubated with or without pervanadate as described above. Cells were then lysed and subjected to immunoprecipitation with anti-2B4 antibody. Immunoprecipitates were then resolved by nondenaturing SDS/PAGE and subjected to immunoblotting with anti-SHP-2 antibody followed by horseradish peroxidase-conjugated secondary antibody and analyzed by enhanced chemiluminescence. The lane marked total lysate represents 1 × 106 cell equivalents loaded onto the gel.
Because a common feature of inhibitory immune receptors is their ability to associate with various cellular tyrosine phosphatases, we examined the ability of 2B4L to bind to similar proteins. Immunoprecipitation of 2B4L from lysates of RNK-16 transfectants after pervanadate treatment failed to show an association of 2B4L with the tyrosine phosphatase SHP-1 (data not shown). However, an association between 2B4L and the tyrosine phosphatase SHP-2 was observed (Fig. 7B). No association of SHP-2 and 2B4S was observed (data not shown). Therefore, the inhibitory form of 2B4 (2B4L) is able to associate with at least one of the tyrosine phosphatases described previously to function in the signaling pathways of other inhibitory receptors.
DISCUSSION
The murine receptor 2B4 is a member of the Ig superfamily and is expressed on the surface of all NK cells and those T cells that exhibit non-MHC-restricted cytotoxicity. Interpretation of previous studies describing the stimulatory nature of this receptor have been complicated by the recent description of alternatively spliced forms of the transcript of the 2B4 gene that encode two distinct polypeptides that differ solely in their cytoplasmic domains. Studies reported here indicate that 2B4S is a stimulatory receptor and 2B4L functions in an inhibitory capacity. This conclusion is supported by the fact that (i) RNK-16 transfectants of 2B4S but not 2B4L could be triggered by whole anti-2B4 mAb to lyse the FcR+ tumor target P815; (ii) 2B4S but not 2B4L transfectants could be triggered via the cell surface signaling receptor NKR-P1A; and (iii) RNK-16 transfectants of 2B4S but not 2B4L lysed the NK-sensitive target YAC-1. However, 2B4L does not completely impair lytic function, because RNK-16 cells transfected with 2B4L could lyse target cells when their FcRs were cross-linked in an antibody-dependent cellular cytotoxicity assay.
Analysis of the functional signaling domains of 2B4 by deletion mutagenesis showed that removal of sequences unique to either 2B4L or 2B4S resulted in a receptor form (2B4D2) that could still function in a stimulatory fashion (Figs. 3A and 4). It follows that cytoplasmic regions shared by 2B4L and 2B4S are sufficient for transmitting activating signals. Furthermore, because 2B4L is an inhibitory receptor and 2B4D2 is not, the regions conferring inhibitory function can be mapped to those domains unique to 2B4L. This domain of 2B4L was shown to contain tyrosine residues that were phosphorylated in response to pervanadate treatment. In addition, 2B4L was shown to associate with the cellular tyrosine phosphatase SHP-2, which has been implicated in the inhibitory mechanisms of other ITIM-containing receptors (34, 35). However, unlike most NK inhibitory receptors, 2B4L was not able to associate with SHP-1. The fact that 2B4L does not contain a consensus ITIM may explain the lack of association with SHP-1. This is a property shared with CTLA4, another inhibitory molecule that does not possess a canonical ITIM (35). Further experiments are needed to define the residues responsible for SHP-2 association and elucidate the downstream signal transduction pathways for 2B4L.
The nature of the stimulatory signal transmitted by 2B4S is still unknown. Because 2B4S does not contain any known activation domains and truncation of the cytoplasmic tail of 2B4 did not completely eliminate its signaling potential, we postulate that 2B4S signaling occurs through some adaptor molecule. However, unlike other NK stimulatory receptors, 2B4S does not contain a charged residue in its transmembrane domain that would allow for association with the DAP-12 signaling molecule (36). We were unable to demonstrate association of 2B4S with DAP-12 or CD3 ζ chain by coimmunoprecipitation studies (data not shown). Therefore, 2B4S may transmit stimulatory signals through molecular association with other signaling intermediates; it may be possible to identify these by coimmunoprecipitation and the yeast two-hybrid system.
The observation that 2B4 exists as two isoforms with opposing functions places this receptor among the ever-growing list of NK cell receptors with similar ligand-binding domains but divergent signaling mechanisms. Given that 2B4 exists in at least two isoforms, further questions arise: are both isoforms expressed in each NK cell and if so, which of the two isoforms dominates functionally? Although definitive data regarding expression of these isoforms at the clonal level are lacking, preliminary evidence suggests that both 2B4L and 2B4S are present within populations of LAK cells (S.E.S. and P.A.M., unpublished work). In addition, both forms of spliced mRNA are present in LAK cells as well (18) (J.D.S. and V.K., unpublished results). Functional studies with populations of LAK effectors and a variety of targets indicate that Fab′ fragments of anti-2B4 antibody are as effective as whole antibody in stimulating lysis. However, populations of LAK effectors are also able to mediate redirected lysis and exhibit granule release and γ-IFN secretion in the presence of anti-2B4 antibody. Therefore, defining the exact role of 2B4 in NK function will require clonal analysis as well as further analysis of transfectants expressing only one isoform of 2B4. Future studies aimed at defining the effect of 2B4–CD48 interactions, regulation of the alternative splicing event, and targeted gene disruptions to functionally incapacitate the 2B4 receptor should elucidate its role in NK function and development.
Acknowledgments
The authors thank Dr. James Ryan (University of California, San Francisco) for the kind gift of the RNK-16 cell line and for helpful discussion regarding the use of these cells in transfection and lytic assays. We also thank Dr. William Chambers (University of Pittsburgh, Pittsburgh, PA) for the kind gift of the 3.2.3 mAb and the polyclonal anti-P815 sera and Dr. John Ortaldo (National Cancer Institute–Frederick Cancer Research and Development Center, Frederick, MD) for helpful discussions regarding Western blot analysis. We also thank members of the Bennet/Kumar laboratory for technical assistance (especially Deming Zhou for antibody purification) and provocative discussion. This work was supported by National Institutes of Health Grants AI 25041, AI 38938, CA 36922, and CA 70143.
ABBREVIATIONS
- NK
natural killer
- ITIM
immunoregulatory tyrosine-based inhibitory motif
- FcR
Fc receptor
References
- 1.Trinchieri G. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trinchieri G, Murphy M, Perussia B. Crit Rev Oncol Hematol. 1987;7:219–265. doi: 10.1016/s1040-8428(87)80009-4. [DOI] [PubMed] [Google Scholar]
- 3.Yu Y Y, Kumar V, Bennett M. Annu Rev Immunol. 1992;10:189–213. doi: 10.1146/annurev.iy.10.040192.001201. [DOI] [PubMed] [Google Scholar]
- 4.Karre K. Immunol Rev. 1997;155:5–9. doi: 10.1111/j.1600-065x.1997.tb00935.x. [DOI] [PubMed] [Google Scholar]
- 5.Moretta L, Ciccone E, Mingari M C, Biassoni R, Moretta A. Adv Immunol. 1994;55:341–380. doi: 10.1016/s0065-2776(08)60513-1. [DOI] [PubMed] [Google Scholar]
- 6.Ljunggren H G, Karre K. Immunol Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 7.Karlhofer F M, Ribaudo R K, Yokoyama W M. Nature (London) 1992;358:66–70. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- 8.Vely F, Vivier E. J Immunol. 1997;159:2075–2077. [PubMed] [Google Scholar]
- 9.Mason L H, Anderson S K, Yokoyama W M, Smith H R, Winkler-Pickett R, Ortaldo J R. J Exp Med. 1996;184:2119–2128. doi: 10.1084/jem.184.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biassoni R, Cantoni C, Falco M, Verdiani S, Bottino C, Vitale M, Conte R, Poggi A, Moretta A, Moretta L. J Exp Med. 1996;183:645–650. doi: 10.1084/jem.183.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raulet D H, Held W. Cell. 1995;82:697–700. doi: 10.1016/0092-8674(95)90466-2. [DOI] [PubMed] [Google Scholar]
- 12.Vance R E, Tanamachi D M, Hanke T, Raulet D H. Eur J Immunol. 1997;27:3236–3241. doi: 10.1002/eji.1830271222. [DOI] [PubMed] [Google Scholar]
- 13.Moretta A, Vitale M, Sivori S, Bottino C, Morelli L, Augugliaro R, Barbaresi M, Pende D, Ciccone E, Lopez-Botet M, et al. J Exp Med. 1994;180:545–555. doi: 10.1084/jem.180.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips J H, Chang C, Mattson J, Gumperz J E, Parham P, Lanier L L. Immunity. 1996;5:163–172. doi: 10.1016/s1074-7613(00)80492-6. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Botet M, Carretero M, Bellon T, Perez-Villar J J, Llano M, Navarro F. Res Immunol. 1997;148:155–159. doi: 10.1016/s0923-2494(97)84217-4. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Botet M, Carretero M, Perez-Villar J, Bellon T, Llano M, Navarro F. Immunol Res. 1997;16:175–185. doi: 10.1007/BF02786361. [DOI] [PubMed] [Google Scholar]
- 17.Samaridis J, Colonna M. Eur J Immunol. 1997;27:660–665. doi: 10.1002/eji.1830270313. [DOI] [PubMed] [Google Scholar]
- 18.Mathew P A, Garni-Wagner B A, Land K, Takashima A, Stoneman E, Bennett M, Kumar V. J Immunol. 1993;151:5328–5337. [PubMed] [Google Scholar]
- 19.Garni-Wagner B A, Purohit A, Mathew P A, Bennett M, Kumar V. J Immunol. 1993;151:60–70. [PubMed] [Google Scholar]
- 20.Kuroiwa A, Yamashita Y, Inui M, Yuasa T, Ono M, Nagabukuro A, Matsuda Y, Takai T. J Biol Chem. 1998;273:1070–1074. doi: 10.1074/jbc.273.2.1070. [DOI] [PubMed] [Google Scholar]
- 21.Arm J P, Nwankwo C, Austen K F. J Immunol. 1997;159:2342–2349. [PubMed] [Google Scholar]
- 22.Schuhmachers G, Ariizumi K, Mathew P A, Bennett M, Kumar V, Takashima A. J Invest Dermatol. 1995;105:592–596. doi: 10.1111/1523-1747.ep12323533. [DOI] [PubMed] [Google Scholar]
- 23.Brown M H, Boles K, Anton Van der Merwe P, Kumar V, Mathew P A, Barclay A N. J Exp Med. 1998;188:2083–2090. doi: 10.1084/jem.188.11.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vivier E, Daeron M. Immunol Today. 1997;18:286–291. doi: 10.1016/s0167-5699(97)80025-4. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds C W, Bere E W, Jr, Ward J M. J Immunol. 1984;132:534–540. [PubMed] [Google Scholar]
- 26.Yu Y Y, George T, Dorfman J R, Roland J, Kumar V, Bennett M. Immunity. 1996;4:67–76. doi: 10.1016/s1074-7613(00)80299-x. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura M C, Niemi E C, Fisher M J, Shultz L D, Seaman W E, Ryan J C. J Exp Med. 1997;185:673–684. doi: 10.1084/jem.185.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuhmachers G, Ariizumi K, Mathew P A, Bennett M, Kumar V, Takashima A. Eur J Immunol. 1995;25:1117–1120. doi: 10.1002/eji.1830250440. [DOI] [PubMed] [Google Scholar]
- 29.Daeron M, Latour S, Malbec O, Espinosa E, Pina P, Pasmans S, Fridman W H. Immunity. 1995;3:635–646. doi: 10.1016/1074-7613(95)90134-5. [DOI] [PubMed] [Google Scholar]
- 30.D’Ambrosio D, Hippen K L, Minskoff S A, Mellman I, Pani G, Siminovitch K A, Cambier J C. Science. 1995;268:293–297. doi: 10.1126/science.7716523. [DOI] [PubMed] [Google Scholar]
- 31.Ryan J C, Niemi E C, Goldfien R D, Hiserodt J C, Seaman W E. J Immunol. 1991;147:3244–3250. [PubMed] [Google Scholar]
- 32.Burshtyn D N, Yang W, Yi T, Long E O. J Biol Chem. 1997;272:13066–13072. doi: 10.1074/jbc.272.20.13066. [DOI] [PubMed] [Google Scholar]
- 33.Olcese L, Lang P, Vely F, Cambiaggi A, Marguet D, Blery M, Hippen K L, Biassoni R, Moretta A, Moretta L, Cambier J C, Vivier E. J Immunol. 1996;156:4531–4534. [PubMed] [Google Scholar]
- 34.O’Shea J J, McVicar D W, Bailey T L, Burns C, Smyth M J. Proc Natl Acad Sci USA. 1992;89:10306–10310. doi: 10.1073/pnas.89.21.10306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vely F, Olivero S, Olcese L, Moretta A, Damen J E, Liu L, Krystal G, Cambier J C, Daeron M, Vivier E. Eur J Immunol. 1997;27:1994–2000. doi: 10.1002/eji.1830270825. [DOI] [PubMed] [Google Scholar]
- 36.Lanier L L, Corliss B C, Wu J, Leong C, Phillips J H. Nature (London) 1998;391:703–707. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]