Abstract
Neural cells differentiated in vitro from human embryonic stem cells (hESC) exhibit broad cellular heterogeneity with respect to developmental stage and lineage specification. Here, we describe standard conditions for the use and discovery of markers for analysis and cell selection of hESC undergoing neuronal differentiation. To generate better-defined cell populations, we established a working protocol for sorting heterogeneous hESC-derived neural cell populations by fluorescence-activated cell sorting (FACS). Using genetically labeled synapsin-green fluorescent protein-positive hESC-derived neurons as a proof of principle, we enriched viable differentiated neurons by FACS. Cell sorting methodology using surface markers was developed, and a comprehensive profiling of surface antigens was obtained for immature embryonic stem cell types (such as stage-specific embryonic antigen [SSEA]-3, -4, TRA-1-81, TRA-1-60), neural stem and precursor cells (such as CD133, SSEA-1 [CD15], A2B5, forebrain surface embryonic antigen-1, CD29, CD146, p75 [CD271]), and differentiated neurons (such as CD24 or neural cell adhesion molecule [NCAM; CD56]). At later stages of neural differentiation, NCAM (CD56) was used to isolate hESC-derived neurons by FACS. Such FACS-sorted hESC-derived neurons survived in vivo after transplantation into rodent brain. These results and concepts provide (a) a feasible approach for experimental cell sorting of differentiated neurons, (b) an initial survey of surface antigens present during neural differentiation of hESC, and (c) a framework for developing cell selection strategies for neural cell-based therapies.
Keywords: Human embryonic stem cells, Neurons, Surface antigens, Fluorescence-activated cell sorting, Immunomagnetic cell separation, Cell therapy, Parkinson disease, Neural lineage, Neural development
Introduction
Current neuronal differentiation protocols of human embryonic stem cells (hESC) are able to enrich for particular cell subtypes [1–3]. However, such protocols are currently not able to synchronize the birth and development of cell populations to the extent seen in normal development, and consequently cells at different stages of maturation are present in such cultures, causing a cellular heterogeneity that impedes experimental and clinical utility [4–7] (Fig. 1). New cell selection methods are needed to realize the possible scientific and clinical benefits of using hESC.
Figure 1.
Differentiating hESC are a heterogeneous cell population. (A): Differentiation of hESC in a neuronal induction protocol. In brief, hESC (H1, H7, H9) were neurally induced on Wnt1-MS5 stromal feeder cells with the addition of 300 ng/ml Noggin [2]. Neuroectodermal precursors were then harvested at div 21 and further differentiated using patterning factors such as bFGF, FGF-8, and Shh. For details, see supplemental online methods. (B): During neuronal differentiation in vitro (div 37), continuously proliferative neural precursor cells, in this dopaminergic differentiation paradigm positive for the midbrain-marker Otx-2+, differentiated neuronal cells (TuJ1+), and remaining clusters of immature SSEA-4+stem cells are present. Scale bar: 50 μm. (C): This cellular heterogeneity of hESC differentiation can be illustrated schematically. The developmental potency of immature hESC (t1) may lead to heterogeneity with regard to developmental stage and to cell lineage. The overall population at any given time is therefore composed of different subpopulations, progeny of cells A, B, and C. Remaining immature pluripotent stem and precursor cells may proliferate and spin off progeny at later times (t1–6) and thus increase the anisochronicity of the differentiating cultures. Additionally, non-neural cells, which escape the in vitro patterning factors, develop (supplemental online material 2). Restricting the cultured cells to the population of interest would increase homogeneity and isochronicity of its derivatives for in vitro and in vivo studies. A population purified at an early developmental stage would differentiate free of contamination with unwanted cells more homogenously and synchronize toward the population of interest. Abbreviations: AA, ascorbic acid; BDNF, brain-derived neurotrophic factor; bFGF, basic fibroblast growth factor; cAMP, dibutyryl cyclic adenosine 5′ monophosphate; div, days in vitro; FGF8, fibroblast growth factor 8β; GDNF, glial cell line-derived neurotrophic factor; hESC, human embryonic stem cells; Shh, sonic hedgehog; SSEA, stage-specific embryonic antigen; β3; TuJ1, t1–6, stages of in vitro development; TGF-beta3, tumor growth factor β-III-tubulin.
Cell selection strategies have been developed, explored and refined in hematological research, diagnosis, and treatment [8, 9]. Flow cytometric analysis and fluorescence-activated cell sorting (FACS) provide separation of cellular populations based on fluorescent labeling, for example according to surface antigens [10, 11]. Several adaptations are required to translate this methodology from human hematopoietic to neural cells in the context of stem cell-based regenerative medicine. First, markers must be identified that define developmental maturity and specification, for example distinguishing among glial and neuronal precursor cells or neural subtype-progenitors. A neural cell marker profile similar to the lineage specification charts for hematopoiesis [12] using cluster of differentiation (CD) antigens [13] is needed. Furthermore, the cell selection methodology needs to be adjusted to allow efficient isolation of viable neural and neuronal subpopulations. After such work has been accomplished, defined combinations of surface markers can be used to identify and to isolate specific neural subpopulations by FACS or by immunomagnetic cell separation (MACS) [14]. Such neural cell selection procedures and marker sets will enable the analysis, characterization, and separation of distinct subpopulations of neural cells for basic studies of stem cell biology, neural development, and potential therapeutic application. For example, in neurotransplantation experiments, FACS analysis and cell purification can describe and define the exact composition of cells prior to transplantation into animal models [15, 16]. We believe that for translation of cell-based therapies to the clinical level, cell selection steps will be required for reasons of safety and reproducibility. To help achieve these goals, this article documents the development of new protocols to initiate, promote, and facilitate surface marker discovery, flow cytometric profiling, and cell selection of hESC-derived neural and neuronal cell populations.
Materials and Methods
Human Embryonic Stem Cell Culture and In Vitro Differentiation
The work with hESC was approved by the Partners Embryonic Stem Cell Research Oversight Committee. Human embryonic stem (ES) cell lines H1 (WA-01, XY), H7 (WA-07, XX), and H9 (WA-09, XX) were propagated on Mitomycin-C-treated human fibro-blasts (D551, ATCC) in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum according to standard protocols [2]. A stromal feeder-based hESC differentiation protocol was used as recently described [2, 17]; after neural induction on murine stromal feeder cells in combination with the bone morphogenic protein antagonist Noggin, cells were patterned by fibroblast growth factor-8 and sonic hedgehog and differentiated using dibutyryl cyclic adenosine 5′ monophosphate and other factors as indicated (Fig. 1A; supplemental online Fig. 1).
Immunocytochemistry
After fixation in 4% paraformaldehyde, cells were analyzed by standard procedures for immunofluorescence staining as previously described [2] and examined using an LSM510 Meta confocal microscope equipped with ultraviolet, argon, and helium-neon lasers (Carl Zeiss, Jena, Germany, http://www.zeiss.com). The following primary antibodies were used: rabbit polyclonal anti-β-III-tubulin (TuJ1, 1:2,000; Covance, Princeton, NJ, http://www.covance.com); sheep anti-tyrosine hydroxylase (TH, 1:300; Pel-Freez, Rogers, AK, http://www.invitrogen.com); mouse IgG1 anti-CD133 (1:15; Miltenyi Biotec, Bergisch Gladbach, Germany, http://www.miltenyibiotec.com); rabbit polyclonal anti-brain factor-1 (FoxG1B, 1:500; L. Studer, New York, http://www.mskcc.org/mskcc/html/10920.cfm); rabbit anti-ki67 (1:3,000; Novocastra Ltd., Newcastle upon Tyne, U.K., http://www.novocastra.co.uk); rabbit anti-Synaptotagmin-1 (1:1,000; Synaptic Systems, Goettingen, Germany, http://www.sysy.com); rabbit anti-enhanced green fluorescent protein (eGFP, 1:1,000), mouse IgG1 anti-CD146 (1:500; both from Abcam, Cambridge, U.K., http://www.abcam.com); mouse IgG1 anti-neural cell adhesion molecule (NCAM) (2 μg/ml, Eric-1; Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com); rabbit polyclonal anti-Sox1 (1:300), mouse IgM anti-Tra-1-81 (15 μg/ml), mouse anti-microtubule-associated protein-2 (1:500), rabbit polyclonal anti-p75 (1:100), mouse IgM anti-A2B5 (1:500), mouse anti-Syntaxin (1:100), and guinea pig anti-doublecortin (1:500; all from Chemicon, Temecula, CA, http://www.chemicon.com); mouse anti-Nestin (1:1,000), goat polyclonal anti-Otx2 (1:1,000), IgG1 goat anti-Oct-3/-4 (1:1,000; all from Neuromics, Northfield, MN, http://www.neuromics.com); mouse IgM anti-forebrain surface embryonic (FORSE) antigen-1 (1:80), mouse IgM anti-stage-specific embryonic antigen (SSEA)-1 (0.4 μg/ml, clone MC-480), mouse IgM anti-SSEA-3 (3 μg/ml), mouse IgG3 anti-SSEA-4 (3 μg/ml, clone MC-813; all from Developmental Studies Hybridoma Bank, Iowa City, IA, http://www.uiowa.edu/~dshbwww); and rat anti-CD29 (1:500), mouse IgG anti-CD24 (1:500; both from BD Pharmingen, San Diego, http://www.bdbiosciences.com/index_us.shtml). Antibody concentrations for detection of surface antigens were determined by titration assays using flow cytometric analysis and immunocytochemistry, including staining of viable, attached hESC culture (supplemental online material 5). The appropriate fluorescence-labeled secondary antibodies (Alexa Fluor goat or donkey anti-rabbit, -mouse, or -sheep 488, 568, 594, 647; Molecular Probes, Eugene, OR, http://probes.invitrogen.com; 1:500) were applied for visualization, and nuclei were counter-stained with Hoechst 33342 (Molecular Probes; 5 μg/ml). On selected samples, the primary antibody was omitted to verify specificity of staining.
FACS Analysis and Purification
Cells were harvested at the immature stage by mechanical selection and at later stages using 0.05% Trypsin/EDTA or TrypLE Express (Gibco, Grand Island, NY, http://www.invitrogen.com). Gentle trituration was used, and cells were filtered through cell strainer caps (35-μm mesh) to obtain a single cell suspension (approximately 106 cells per milliliter for analysis, 0.5–2 × 107 cells per milliliter for sorting). Surface antigens were labeled by incubating with the primary antibodies described above for 30 minutes in the dark at 4°C to prevent internalization of antibodies, followed by incubation for 20–30 minutes with the appropriate Alexafluor-488 or Alexa-fluor-647 fluorescent secondary antibodies. All washing steps were performed in phenol-free, Ca2+-free, Mg2+-free Hank’s buffered saline solution (Gibco) containing penicillin-streptomycin, 20 mM D-glucose, and 2% fetal bovine serum (supplemental online material 3). The stained cells were analyzed and sorted on a fluorescence-activated cell sorter FACSAria (BD Biosciences, San Diego, http://www.bdbiosciences.com) using FACSDiva software (BD Biosciences); data were additionally analyzed and presented using FlowJo software (Tree Star, Ashland, OR, http://www.treestar.com). The fluorochromes were excited with this instrument’s standard 488-nm and 633-nm lasers, and green fluorescence was detected using 490 LP and 510/20 filters and far red fluorescence using 660/20 filters. All analyses and sorts were repeated at least three times, and purity of sorted fractions was checked visually and by FACS reanalysis as described before [15]. Prior to sorting, the nozzle, sheath, and sample lines were sterilized with 70% ethanol or 2% hydrogen peroxide for 15 minutes, followed by washes with sterile water to remove remaining decontaminant. A 100-μm ceramic nozzle (BD Biosciences), sheath pressure of 20–25 pounds per square inch (PSI), and an acquisition rate of 1,000–3,000 events per second were used as conditions optimized for neuronal cell sorting (“gentle FACS”).
Immunomagnetic Cell Separation
After harvesting cells as described above, cells were incubated for 30 minutes with primary antibodies as described, followed by a washing step and incubation with the corresponding magnetically labeled secondary antibody (Miltenyi Biotec) for 15 minutes at 4°C. Positive and negative fractions were separated using MiniMACS LC cell columns (Miltenyi Biotec) according to the manufacturer’s protocols [14]. After fluorescent labeling, samples of the obtained positive and negative fractions were reanalyzed by FACS to assess purity.
Combined Caspase-3 and 7-Aminoactinomycin D Viability Assay
After the respective cell sorting procedures, cells were incubated at 37°C for 60–90 minutes according to the manufacturer’s protocol with a fluorogenic caspase-3 substrate (PhiPhiLux-G1D2; OncoImmunin Inc., Gaithersburg, MD, http://www.phiphilux.com) as a marker for cell damage/apoptosis [18]. After washing, cells were immediately analyzed by flow cytometry, and green fluorescence was detected as described above. For bivariate analysis, 5 μg/ml 7-Aminoactinomycin D (7-AAD) (Sigma-Aldrich, St. Louis, http://www.sigmaaldrich.com) was added to detect cell death/membrane permeability [18]. The functionality of the caspase-3 assay was determined by incubation of cells with or without puromycin (1 μg/ml). Statistical analyses of these experiments (n = 3) were done by Tukey-Kramer multiple comparison tests using InStat software (GraphPad, San Diego, http://www.graphpad.com).
Lentiviral Transduction with Synapsin-Green Fluorescent Protein
After neuroectodermal induction, hESC (H9) were transduced at days in vitro (div) 42 with pHIV7/synapsin-eGFP (kindly provided by Dr. Atsushi Miyanohara, University of California, San Diego) according to published protocols [19] and further differentiated. On div 47, cells were harvested, dissociated, and FACS-purified against nontransduced controls as described above. Green fluorescent protein (GFP)+, GFP− fractions, and nonsorted controls were replated and differentiated for a further three div before immunocytochemistry for GFP (purified rabbit anti-eGFP antibody; Abcam), TuJ1, Syntaxin, and TH was performed.
Transplantation into the Striatum of 6-Hydroxydopamine Treated Rats
Animal studies were approved by the Institutional Animal Care and Use Committee at McLean Hospital and HMS. Unilateral 6-hy-droxydopamine (OHDA)-lesioned adult female Sprague-Dawley rats (200–250 g) were purchased from Taconic (Germantown, NY, http://www.taconic.com). After FACS purification (see above), differentiated H1 hESC were counted and resuspended at ~25,000 viable cells per μl in the final differentiation medium. Four μl were slowly injected into the lesioned striatum of the rats (anterior-posterior = 0; lateral = −2.8 from bregma and from −5.5 to −4.5 mm ventral from dura, with the tooth bar set at −3.3). Injections were performed as previously described [2]. Rats were immunosup-pressed with cyclosporin A (15 mg/kg per day; Sandimmune; Sandoz, East Hannover, NJ, http://www.sandoz.com) starting 1 day prior to surgery. Four weeks after transplantation, animals were terminally anesthetized by an intraperitoneal overdose of pentobarbital (150 mg/kg) and perfused intracardially with 70 ml of heparinized saline (0.1% heparin in 0.9% saline) followed by 100 ml of paraformaldehyde (4% in phosphate-buffered saline [PBS]). Brains were removed, postfixed for 4 hours in 4% paraformaldehyde, equilibrated in sucrose (20% in PBS), and sectioned on a freezing microtome in 40-μm slices that were serially collected. To identify human cells in the rodent brain, we used the human specific antibody against human nuclear (HuN) antigen (1:50; Chemicon) and immunohistochemical characterization for human NCAM (Eric-1; Santa Cruz) and TH (1:250; Pel-Freez). The sections were permeabilized with 0.1% Triton X-100 and incubated with primary antibodies in 2% normal donkey serum overnight at 4°C. After rinsing, sections were incubated with appropriate fluorescence labeled secondary antibodies (Molecular Probes; 1:500) for 1 hour at room temperature, rinsed, and incubated with Hoechst 33342 (5 μg/ml). Confocal analysis was performed using a Zeiss LSM510/Meta station. For identification of signal colocalization within a cell, optical sections were kept to a minimal thickness, and orthogonal reconstructions were analyzed.
Results
Neuronal Differentiation Protocols of Human Embryonic Stem Cells Result in the Development of Heterogeneous Cell Populations
Obtaining new protocols for the efficient derivation of functional neuronal cells from hESC is a major focus in stem cell biology [5, 20]. Current neural induction protocols lead to the effective generation of Nestin-positive (Nestin+) neuroectodermal cells [1, 2]. However, when using a multistep induction protocol [2, 17] (Fig. 1A; supplemental online material 1), it is evident that hESC develop into multiple cell types that mature at different rates, resulting in heterogeneity of cell types and lack of synchronized development (Fig. 1). The heterogeneity of cell lineage specification and developmental stage is exemplified by the coexistence of immature SSEA-4+ cells, Otx-2+ neural precursor cells, and process-bearing TuJ1+ neuronal cells (Fig. 1B). Even at later stages of hESC differentiation, clusters of differentiated neuronal subtypes coexist with comparably more immature Nestin+ proliferative cells, and recent in vivo data confirm this phenomenon after transplantation of neural cell suspensions [2, 7]. A conceptual model for these findings suggests that the presence of a proliferative pluripotent population at a given time point leads to heterogeneity of cell fate and of developmental stage (anisochronicity), which compromises cell culture and transplantation studies (Fig. 1C, supplemental online material 2). Attempts to instruct all subsets of a heterogeneous population by specific developmental signals and growth factors are also difficult; although the major fraction of the cultured cells may be in the desired stage of differentiation, minor fractions escape the patterning signals and remain at a more immature stage (Fig. 1C).
Methodological Adaptations to Enable Sorting of Mixed hESC-Derived Neural Cell Populations
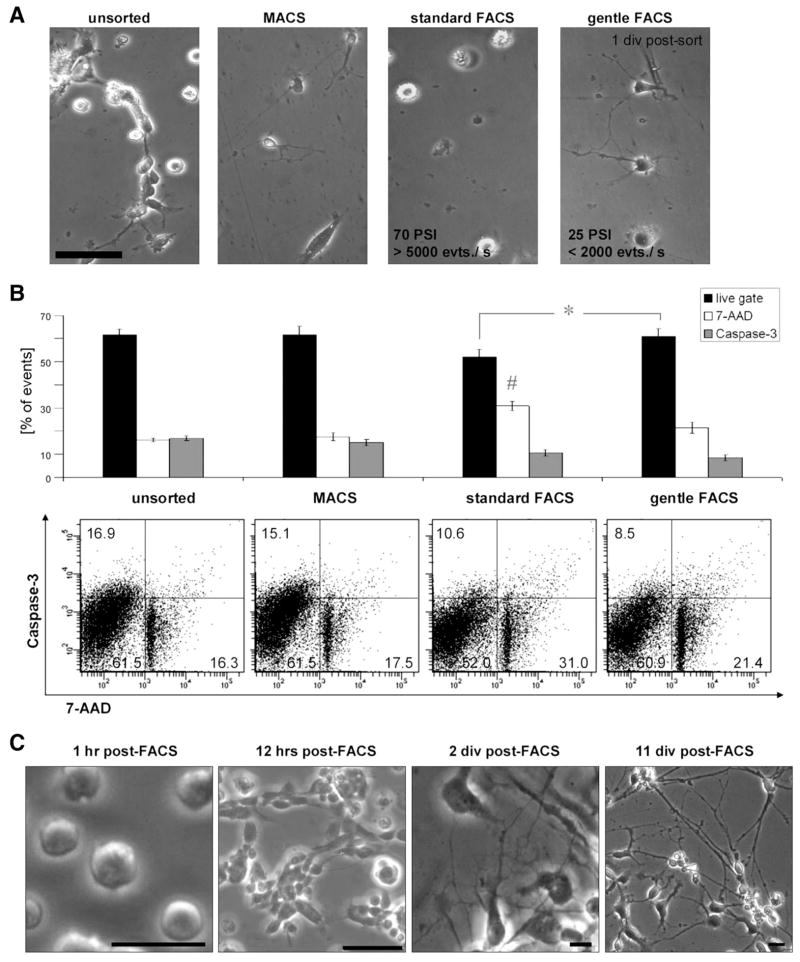
Compared with hematopoietic stem cells, which have been extensively purified in research and clinical applications, neuronal cells are less amenable to sorting with the same technical settings [21, 22]. Whereas blood cells are round and adapted to floating through circulation, neuronal cells typically bear axonal and dendritic processes, which form a highly interwoven network. However, fetal neural tissue can be dissociated and has successfully been used as cell suspensions in clinical trials of neural cell therapy [23]. This proves that, at least at certain developmental stages, postmitotic neuronal cells can indeed be harvested, resulting in a suspension of round-shaped cells without neural processes that are able to regain neuronal morphology and functionality after reculture in vitro and after implantation into brain [20, 24–26]. When adding a sorting step, such as the FACS procedure, cells are subjected to a variety of additional physical stressors, such as shearing forces, laser damage, and osmotic stress. We found that the purity of cell types in a population has to be carefully weighed against the goals of yield and cell survival when optimizing FACS parameters such as duration, speed, and width of the output nozzle. In our hands, adjusting the FACS setting toward a low sheath pressure (20–25 PSI), larger nozzle size (100 μm), and reduced sorting speed (approximately 1,000–3,000 events per second) helped to reduce mechanical stress and enabled the sorting of viable neural cells, including mature neurons for experiments in vitro and in vivo (supplemental online material 3). A comparison of cell selection methods (Fig. 2A, 2B) showed that cells isolated with such modified gentle FACS conditions were comparable to unsorted controls and cells selected by immunomagnetic cell selection with regard to cell viability as determined by caspase-3 activation and 7-AAD nuclear dye uptake [18] but superior compared with FACS standard conditions (70 PSI, 70-μm nozzle) (Fig. 2B). Testing a pneumatic cell sorting system (BioSorter; Union Biometrica, Holliston, MA, http://www.unionbio.com) did not further improve outcomes in terms of viability, and a sorting speed of up to 50 events per second for sorting single neural cells made it difficult to yield sufficient cells (data not shown). Using the described FACS protocol optimized for the efficient selection of neurons, cells plated after sorting attached within 1–2 hours, began to re-extend processes within 12 hours after FACS, and displayed an extensive neuronal network after 2 days (Fig. 2C).
Figure 2.
Selection methods for mixed human embryonic stem cell (hESC)-derived neural cell populations. (A): After gentle dissociation using enzymatic digestion (trypsin replacement; TrypLE), single cell suspensions from hESC-derived neural cell cultures (div 40–50) were subjected to cell selection procedures as indicated and replated. Representative images of unsorted control, cells after immunomagnetic selection, standard FACS conditions, and optimized “gentle” FACS conditions are shown 1 div postsort. Scale bar: ~100 μm. (B): Sorted cells from all conditions were analyzed in a flow cytometric viability assay utilizing a caspase-3 fluorescent substrate as an early apoptotic marker and DNA labeling by 7-Aminoactinomycin D as an indicator of cell death/membrane permeability. FACS conditions optimized for neuronal cell sorting (“FACS gentle”) yielded significantly more “live” (7-AAD−/caspase-3−) cells compared with “standard FACS” conditions (70 PSI, 70-μm nozzle);* = p < .05 (n = 3). Although caspase activity did not differ between both FACS groups, 7-AAD positivity was significantly increased in the standard FACS condition compared with the other groups; # = p < .05 (n = 3). Data from three independent experiments are shown; error bars indicate standard error. (C): Using the neuronal cell sorting conditions described here, FACS-sorted neurons attach to the substrate within 1 hour, begin to re-extend processes within 12 hours post-FACS, and further mature in vitro, forming an elaborate network of neuronal processes. Scale bars: 50 μm. Abbreviations: 7-AAD, 7-Aminoactinomycin D; div, days in vitro; evts./s, events per second; FACS, fluorescence-activated cell sorting; hr(s), hour(s); MACS, immunomagnetic cell separation; PSI, pounds per square inch.
Labeling of Differentiated Neurons for Cell Selection and Transplantation
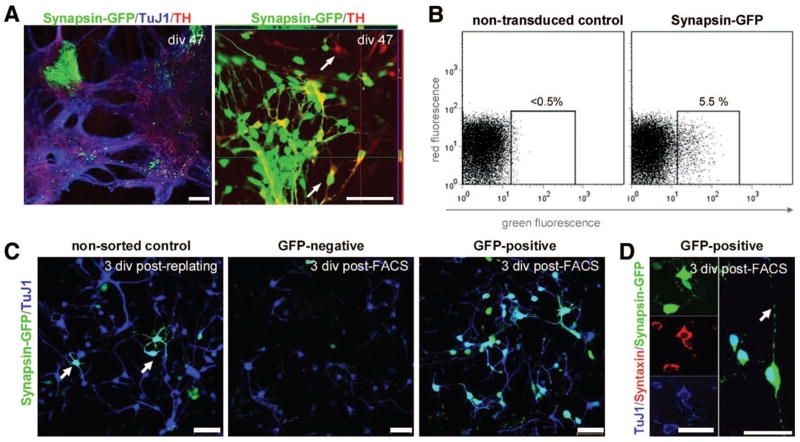
One strategy to specifically label cell subsets is the use of promoter-driven fluorescent proteins [15, 26–29]. As a proof of principle, and to optimize parameters for cell sorting of mature hESC-derived neurons, we transduced hESC at div 42 with a lentivirus expressing eGFP from the synapsin promoter and selected GFP+ cells at div 49 by FACS (Fig. 3A–3C). As confirmed by FACS reanalysis and fluorescent microscopy after reculturing, there was a high enrichment of GFP-labeled neurons (Fig. 3D). The synapsin-GFP+ sorted cells extended processes, displayed axonal varicosities, and coexpressed TuJ1 and the synaptic marker Syntaxin (Fig. 3D, 3E). In summary, this showed how differentiated mature neuronal cells could be efficiently isolated using FACS. We have also used the optimized FACS parameters and protocols described here for the isolation of primary neurons from embryonic and postnatal rodent central nervous system (data not shown).
Figure 3.
Proof-of-principle selection of mature neuronal cell populations from human embryonic stem cells (hESC) using synapsin-GFP as a genetic marker. (A): After lentiviral transduction, Synapsin-GFP was strongly expressed in clusters of neuronally differentiated hESC. Colabeling of TH and synapsin-GFP was present, although single-labeled cells of each type were common (arrows). (B): Synapsin-GFP was detected by flow cytometry compared with nontransduced hESC (H9) at the same stage of differentiation; approximately 5% of the gated cells displayed GFP positivity. (C): The conditions of nonsorted control cells (left panel), the GFP−selected population (mid panel), and the GFP+ population are shown 3 div after FACS purification. The sorting step enriched for viable neuronal TuJ1+/GFP+ cells (D), which costained for synaptic markers such as Syntaxin and extended neuronal processes bearing varicosities (arrow). Scale bars: 100 μm (A), 50 μm (C), and 25 μm (D). Abbreviations: div, days in vitro; FACS, fluorescence-activated cell sorting; GFP, green fluorescent protein; TH, tyrosine hydroxylase; TuJ1, β-III-tubulin.
Toward a Neural Cell Surface Antigen Profile
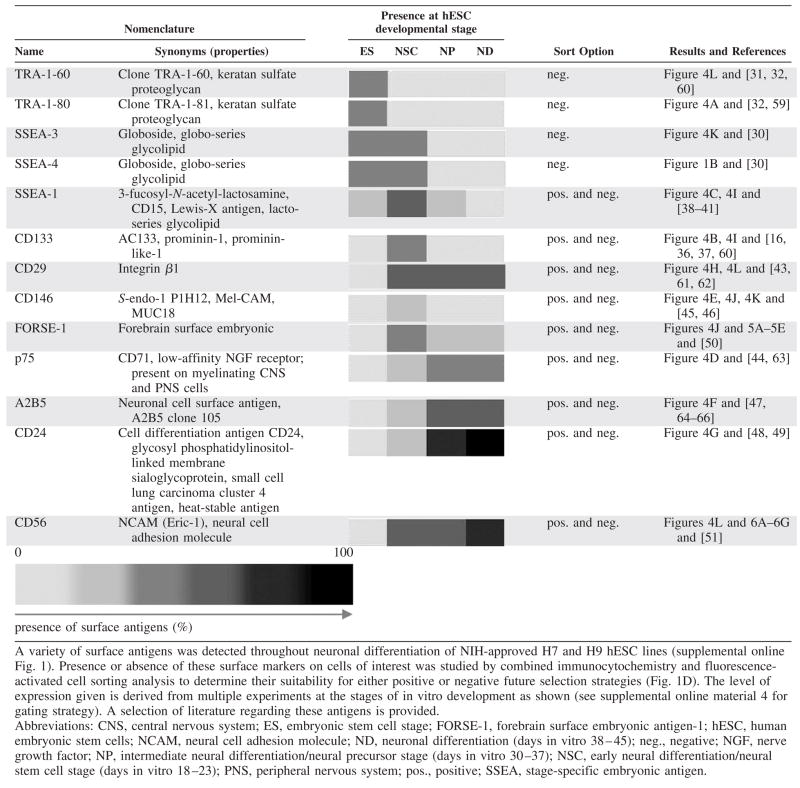
Using such protocols, various surface antigens were detected throughout neuronal differentiation of NIH-approved H7 and H9 hESC lines to determine their suitability for either positive or negative flow cytometric and immunomagnetic (MACS) selection strategies (Table 1 and data below). Surface markers present on immature hESC were used for negative selection to eliminate unwanted cells (supplemental online material 2). hESC at the most immature stage are characterized by expression of the globoseries glycolipids SSEA-3 and SSEA-4 [4, 30] and the keratin sulfate-related antigens TRA-1-60 and TRA-1-81 [31, 32] (Fig. 4A, 4L; Table 1). Immature hESC were FACS-purified according to these antigens and recultured in vitro, but low efficiency was observed for subculture postsort, similar to other reports [4, 33]. Upon differentiation, expression of the embryonic antigens is downregulated [2, 34] (Table 1). Consequently, such markers could be used to remove remaining undifferentiated stem cells and potentially eliminate these cells responsible for teratoma formation in vivo. Indeed, using mouse ES cell-derived Sox1-GFP+ neural precursors, we [15] and others [35] have recently shown that tumor formation in vivo can be attenuated by eliminating immature embryonic stem cells using an early FACS purification step (supplemental online Fig. 2). The pentaspan membrane glycoprotein CD133 (AC133; human prominin-1 ortholog), a known somatic stem-cell marker, was present on cells within neuroepithelial rosette-like structures, mainly toward the lumen of the rosette, similar to the location of prominin-positive neuroepithelial cells during development (Fig. 4B; [16, 22, 36, 37]).
Table 1.
Surface markers present during neuronal differentiation of hESC in vitro
|
Figure 4.

Surface antigens detected during neural differentiation of human embryonic stem cells (hESC). (A): Expression of embryonic stem cell markers such as SSEA-4 and Tra-1-60 is downregulated upon neural differentiation of hESC in vitro. Nonetheless, nests of immature hESC, here a cell cluster labeled with the surface antigen Tra-1-81, remain present after neural induction. (B): Presence of the CD133 antigen as a somatic stem cell marker was found to be localized on clusters of proliferative neuroepithelial cells, most prominently on the apical side of the neural precursors, toward the lumen (*) of the neural rosette structures (dotted line). Scale bar: 20 μm. (C): Early intermediate surface markers such as SSEA-1 are not present on immature hESC (in contrast to mouse embryonic stem cells, which are SSEA-1+ [34, 42]). During neural induction, presence of SSEA-1 emerges on Sox1+ neuroepithelial rosette cells, which may allow for positive selection at the earlier stage of the differentiation protocol. More differentiated process-bearing DCX-positive cells are SSEA-1 negative. Scale bars: 50 μm; far right panel: 20 μm. (D–H): Fluorescence-activated cell sorting (FACS) profile of neural surface markers present during neuronal differentiation of hESC at the late neural precursor stage (div 30–37). Flow cytometric analysis and corresponding immunocytochemistry for the p75 (CD271), A2B5, CD24, and CD29 surface antigens are shown. (I): Combinatorial FACS analysis for presumptive neural stem cell markers: a subset of CD29+ and SSEA-1+ cells coexpresses the CD133 antigen. (J): Cells positive for the CD146 antigen do not coexpress FORSE-1, suggesting the presence of distinct neural precursor cell populations. (K, L): Such neural markers could be used to select against unwanted immature stem cells: CD146+ and NCAM+ cell populations do not coexpress the immature hESC markers SSEA-3 or Tra-1-61. Scale bars: 50 μm. FACS plots: black line, control; green line, stained sample. Abbreviations: DCX, doublecortin; div, days in vitro; FORSE1, forebrain surface embryonic antigen-1; max., maximum; NCAM, neural cell adhesion molecule; SSEA, stage-specific embryonic antigen; TuJ1, β-III-tubulin.
Early intermediate surface markers could be used for negative or positive selection, depending on the stage of in vitro differentiation. For example, we found that the lacto-series glycolipid SSEA-1 (Lewis-X Antigen, SSEA-1, CD15) [38–41] was present on early neuroectodermal precursor cells (Fig. 4C) and can therefore be used for positive selection. At later stages of differentiation, the same marker can be used for negative selection, eliminating the SSEA-1+ dividing clusters while preserving the SSEA-1− differentiated neurons [42].
Neural differentiation antigens were identified as surface markers for application in positive cell selection. A number of antigens were tested at different developmental stages of human ESC-derived cells (Table 1). The β-1 integrin CD29 [43], the low-affinity nerve growth factor receptor p75 [44], and the presumptive endothelial marker CD146 [45, 46] were present on rosette structures and on other proliferative cells. The surface antigen A2B5, recognizing a sialoganglioside/sulfatide epitope on various neuronal and glial subtypes [47], was present on proliferative hESC-derived neural cells and was also found to be present on “flat” cells and cells of the glial lineage. On the other hand, the surface molecule CD24 (heat-stable antigen) [16, 48, 49] was clearly upregulated during neuronal differentiation and appeared to be more highly expressed on TuJ1+ neuronal cells (Fig. 4G, Table 1). Combinatorial flow cytometric analysis revealed that a multitude of subpopulations are present in the differentiation of hESC, opening a vast field for further detailed studies and refinement of hESC differentiation protocols. Consistently, cells positive for the proliferative neural markers CD29 [43] and SSEA-1 coexpressed the CD133 antigen on a subset of cells (Fig. 4I). This confirms the immunocytochemical finding of CD29- and SSEA-1-positivity (Fig. 4C, 4H), with a restriction of CD133 staining to the apical subset (Fig. 4B) on these neuroepithelial rosette structures. Further combinatorial FACS analysis also suggests that cell selection strategies for differentially expressed neural cell surface antigens, for example, for CD146 or NCAM, can be applied to eliminate unwanted proliferative cell populations (Fig. 4J–4L). In addition, this profiling enables studies elucidating the functional significance of the identified surface marker molecules in the neural context.
Applications of Neural Cell Surface Markers for Cell Sorting
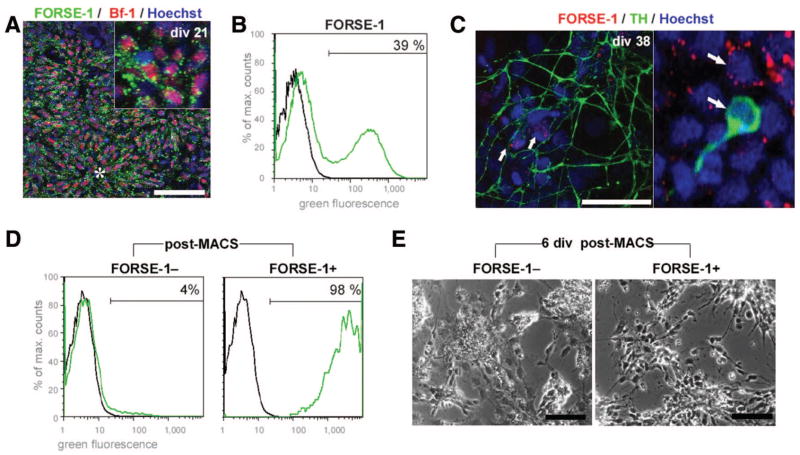
The novel marker forebrain-surface-embryonic antigen FORSE-1 is structurally related to SSEA-1 and was used for either negative or positive selection. FORSE-1 is expressed in the developing mouse brain, where it labels proliferative cells in the embryonic forebrain and in proximity to the central canal [50]. We found that in in vitro cultures of hESC, FORSE-1 was robustly expressed at the early neuroectodermal stage, mostly coexpressing the forebrain-precursor marker brain factor-1 (Bf-1 or FoxG1; Fig. 5A; Table 1), and also at later stages in remaining nests of less differentiated cells (Fig. 5C). FACS allowed for separation of FORSE-1 positive and negative populations (Fig. 5B). We determined that TH+ dopamine neurons were not stained by FORSE-1 (Fig. 3C). This could enable negative selection strategies necessary to decrease the unwanted fraction of proliferative, possibly forebrain-bound, FORSE-1+ precursors in dopaminergic differentiation paradigms. As an alternative to FACS, MACS [14] was also used to separate FORSE-1+ and FORSE-1− subsets (Fig. 5D, 5E). When compared with cell suspensions purified by FACS, MACS-purified cells were of lower purity as determined by postsort reanalysis (Fig. 5D). Based on the observation that there was good survival of cells after immunomagnetic cell separation (Fig. 2A, 2B), this method may thus be useful to enrich specific subpopulations, whereas FACS purification would be applied when a higher degree of purity is required, to exclude rare proliferative or tumorigenic cells prior to further procedures, and for applications using genetic labeling (Fig. 3) or combinatorial surface antigen staining (Fig. 4I–4L).
Figure 5.
Applying methods and neural markers for sorting of human embryonic stem cell (hESC)-derived early neural cell populations: Immunomagnetic cell selection for FORSE-1 [50]. (A): In hESC cultures at early stages cells (div 21), FORSE-1 was present in vitro on neuroectodermal precursor cells coexpressing the forebrain marker Bf-1. (B): Fluorescence-activated cell sorting (FACS) allowed for separation of FORSE-1-positive and -negative populations. (C): At later stages (div 37), clusters of FORSE-1+ cells could be detected next to TH+ dopamine neurons, which were not stained by FORSE-1 (arrows). (D): Enrichment of FORSE-1+ and FORSE-1− populations after immunomagnetic cell separation is shown by FACS reanalysis. (E): The selected populations exhibit different growth patterns and morphology, enriching for neural precursor cells in the FORSE-1+ fraction. Scale bars: 50 μm. FACS plots: black line, control; green line, stained sample. Abbreviations: Bf-1, brain factor-1; div, days in vitro; FORSE-1, forebrain surface embryonic antigen-1; MACS, immunomagnetic cell separation; max., maximum; TH, tyrosine hydroxylase.
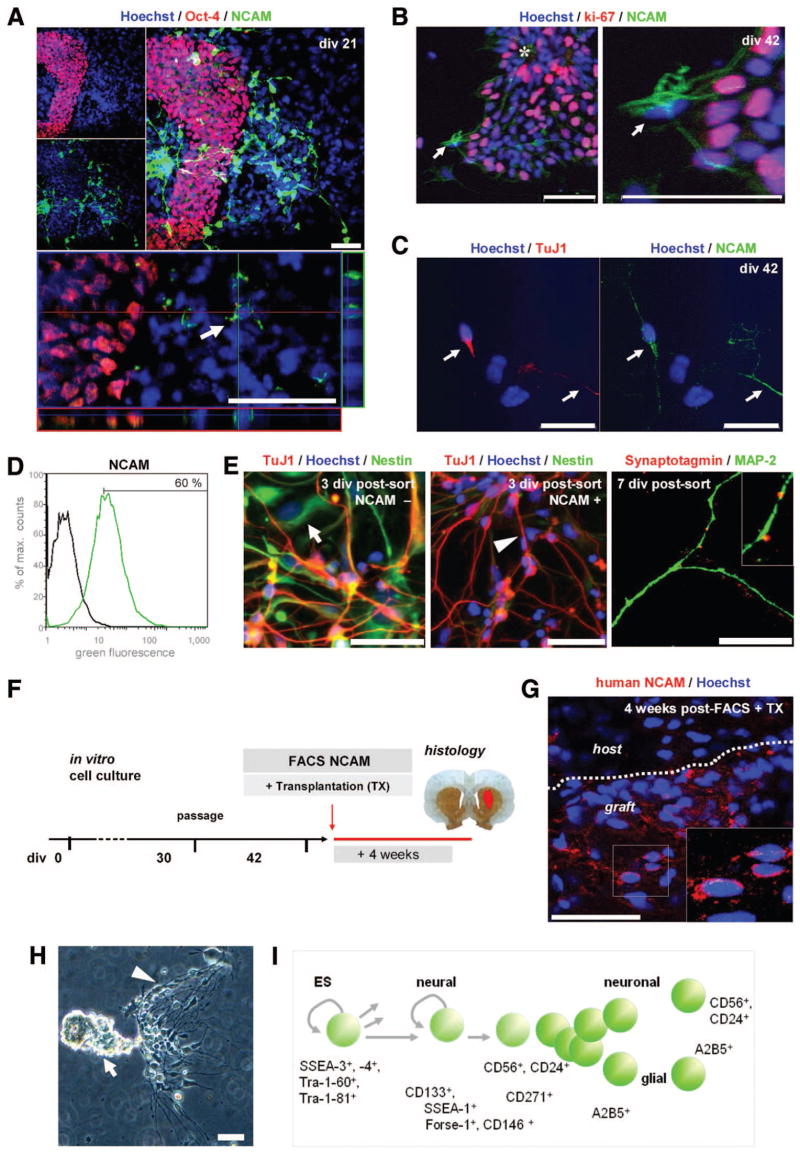
For positive selection according to a surface marker at the late stage of differentiation (supplemental online Fig. 2), the neural cell adhesion molecule NCAM (CD56, human/primate specific clone Eric-1) [51] was used. At early stages (div 21), neuronally differentiating NCAM+ cells were distinct from clusters of immature Oct-4+ hESC (Fig. 6A), making NCAM an appropriate surface antigen candidate for proof-of-principle transplantation experiments. Prior to sorting at div 42, presence of the NCAM-antigen was confirmed on differentiated neurons in vitro (Fig. 6C), whereas remaining less mature proliferative ki67+ cells were negative for NCAM (Fig. 6B; Table 1). We chose FACS and not immunomagnetic cell separation for these experiments, considering the high purity required for neural transplantation paradigms. At the late stage of differentiation (div 42), the NCAM+ population was isolated by FACS from hESC-derived neuronal cultures (Fig. 6D), replated, and further analyzed.
Figure 6.
Applying methods and neural markers for sorting of human embryonic stem cell (hESC)-derived differentiated neuronal cell types: FACS for NCAM for in vitro and in vivo proof-of-principle studies. (A): NCAM was upregulated upon neural differentiation in vitro (Table 1), and clusters of immature, Oct-4+ cells did not express NCAM, allowing for positive selection strategies to eliminate unwanted immature stem cells (compare Fig. 4L). (B): At div 42, NCAM+ cells were negative for the proliferative marker ki-67 (arrows), whereas neural precursors in close proximity were highly proliferative. (C): NCAM-positivity was present on differentiated TuJ1+ neurons in vitro. (D): At the late stage of differentiation (div 42), the NCAM− population was isolated by FACS and replated. (E): FACS-purified NCAM+ cells were distinct from the NCAM− fraction, showed fewer “Nestin-flat” cells [2] and more neuronal morphology postsort, and contained TuJ1, tyrosine hydroxylase, and Nestin-positive cells in vitro. Cells in the NCAM+ fraction also expressed other neuronal markers such as MAP-2 and synaptic markers such as Synaptotagmin (far right panel). (F): Differentiated hESC-derived neurons were FACS-purified on div 42 for NCAM and transplanted into 6-hydroxydopamine-lesioned rats. Animals were sacrificed after 4 weeks, and brain sections were analyzed by immunohistochemistry (n = 4). (G): The grafts consisted of neural cells positive for a human specific NCAM antibody (Eric-1), confirming survival in the brain of FACS-sorted, hESC-derived neuronal cells after transplantation. (H): Outlook: A cluster of hESC-derived neural cells in culture (div 30) displaying the cellular heterogeneity described above, and (I) a schematic summary of the presented surface antigens for future study and selection of distinct neural cell types—toward a surface antigen chart for neural lineages. Scale bars: 50 μm. FACS plots: black line, control; green line, stained sample. Abbreviations: div, days in vitro; ES, embryonic stem; FACS, fluorescence-activated cell sorting; Forse-1, forebrain surface embryonic antigen-1; MAP-2, microtubule-associated protein-2; max., maximum; NCAM, neural cell adhesion molecule; SSEA, stage-specific embryonic antigen; TuJ1, β-III-tubulin; TX, transplantation.
Compared with the NCAM− fraction, FACS-purified NCAM+ cells displayed more neuronal morphology in vitro and stained for neural (Nestin) and neuronal markers including β-III-tubulin and microtubule-associated protein and synaptic markers such as Synaptotagmin (Fig. 4E). Consequently, NCAM+ cells were isolated by FACS and subsequently transplanted into 6-OHDA-lesioned rats (n = 4; Fig. 6F). Animals were sacrificed after 4 weeks and no tumor formation was observed, whereas control animals receiving unsorted cell suspensions developed tumors within this period [2, 7]. Immunohistochemical analysis of brain sections showed cells positive for a human-/primate-specific NCAM (Eric-1) (Fig. 6G) and for the HuN antigen (data not shown) in the grafts, demonstrating survival of FACS-purified human neural cells in the brain after transplantation.
Discussion
Here, we describe the development of standard conditions that allow for the use and discovery of surface antigens as markers for analysis and cell selection of hESC undergoing neuronal differentiation. Although most opinions to date discuss the use of cell sorting to eliminate tumors or enrich neural precursors [6, 52], we believe that such methods are also critical with respect to developmental stage (time) and the heterogeneity of cell populations typical of hESC-derived cultures. An additional advantage illustrated by our studies is also the ability to isolate hESC-differentiated neurons for analysis in science and biomedicine. Using immunocytochemical and flow cytometric analysis of differentiating hESC, we characterized the temporal profile of a variety of surface antigens expressed throughout a neuronal cell differentiation protocol. In parallel, we optimized the conditions of FACS to enable growth and analysis of purified hESC-derived neural cells and mature neurons postsort. We found that identification of and selection according to cell surface antigens during neuronal differentiation of hESC (a) is feasible with the protocols described, (b) can be applied for developmental studies in neurobiology, and (c) might facilitate the development of cell therapeutic studies in human embryonic and neural stem cell research.
In the scientific and clinical contexts, the potential of embryonic stem cells for self-renewal and differentiation into any given tissue of the human body is both an opportunity for cell restoration and a concern, when noncontrolled growth occurs [5]. Given the cellular heterogeneity observed during differentiation of hESC in vitro, eliminating the unwanted cell populations from further cultivation steps, prior to transplantation, would increase the purity of the graft and allow for a better defined cell composition to be transferred. The characterization of putatively therapeutic cell suspensions according to surface markers is likely a prerequisite to future applications in a clinical setting. Flow cytometry has previously been applied in the analysis of neural cells from fetal neural tissue [16, 28, 53] and more recently in the prospective isolation of neural stem cells from the adult nervous system [54, 55]. Our studies show now that the principles of flow cytometric methodology can be applied in the analysis and purification of hESC-derived neural cells. By utilizing these advantages and by reducing physical stressors, we were thus able to purify viable neuronal cells for extended in vitro and in vivo characterization.
Analytically, FACS technology was successfully used here in a hESC differentiation paradigm to elucidate the characteristics of specific cellular subpopulations, thereby allowing further studies of critical steps in neural development. Additionally, the increasing necessity for cell selection is exemplified by the heterogeneity observed in this study (Fig. 1B, 1C) and others [2, 7]. Nonpurified embryonic stem cell populations have a broader developmental spectrum compared with fetal primary cultures. Although the surface antigens documented here represent an initial subset of immature, neural, and neuronal markers expressed during hESC differentiation, further characterization is needed.
The profiling of neural surface antigens initiated here forms the basis for exploring other markers and for investigating their mechanisms and function in the neural context. This profiling of surface marker antigens needs to be extended so that a more complete picture of neural cell-cell interaction can be achieved. Such studies are critical for further understanding of human neural development, the potential application of cell-based therapies of neurological diseases, and may also prove particularly useful in the diagnosis of neural tumors, where the same markers may have similar relevance.
In the studies described, we demonstrate that FACS can be used to monitor the presence of immature hESC during neuronal differentiation protocols (SSEA-3, -4, Tra-1-60, Tra-1-81) and, concluding from previous studies, may therefore be applied to avert tumor formation in neurotransplantation of ES-derived neural cells. By identifying a number of neural markers (SSEA-1, FORSE-1, CD29, CD146, A2B5, p75) present at neural stem and precursor stages of hESC differentiation, our methods allow for the separation of cell populations committed to specific lineages of neural differentiation. Such isolated cells could provide a major advantage both in experimental and potential cell therapy settings. Although the full development from a hESC to a differentiated postmitotic neuron in this protocol takes in the order of 30–40 days [2, 17], using an intermediate population, frozen or expanded, provides a shorter cell culture protocol [52]. Moreover, differential expression of these antigens on neural precursor populations will allow for detailed studies, for example, of lineage restriction at this stage. For the later stages of hESC differentiation, an antibody targeting human specific NCAM (CD56) was used to monitor neural specification in vitro and in vivo. Such experiments indicated that hESC-derived neuronal cells can be FACS-purified, transplanted, and survive in the brain of a rodent model.
How can functional integration into synaptic circuitry, and ultimately the reconstitution of function in models of neurological disease, be demonstrated? For such situations, genetic approaches using transduction with fluorescence-labeled specific constructs such as synapsin-eGFP, or using fluorogenic substrates for enzymes such as aldehyde dehydrogenase, may be applied to isolate neural cell subpopulations. The successful cell selection of synapsin-eGFP+ cells demonstrates that even mature, neuronally differentiated cells can be FACS-purified. Synapsin expression correlates well with other synaptic markers such as Syntaxin (Fig. 4E). An advantage of using gene-engineered cells is the avoidance of several washing and centrifugation steps during cell preparation prior to FACS, which can increase cell loss when using surface marker staining. When aiming for potential clinical applications though, the antibody-based fluorescent labeling will advance current approaches and make neural transplantation methods more feasible, as proven by clinical routines in hematological medicine [8]. Although FACS for neuronal cells has been used in studies of animal models, so far it has not been widely applied in neurobiology, probably due to the specific inherent problems in using these fragile neuronal cell types. However, we demonstrate here that, with appropriate methodological adaptations, FACS can be used as a tool with great advantage in the studies of neuronal development and function. One advantage of using embryonic stem cell-derived neuronal cells is the large number of cells that can be generated in vitro. For example, in clinical trials of cellular transplantation therapy for Parkinson disease, approximately 1.1–1.3 × 106 cells are obtained per human fetal ventral midbrain dissected (sixth to ninth week of gestation). Using hESC derived neuronal cells at late stage of differentiation, in our hands more than 3 × 107 cells are obtained from one 10-cm dish, an amount of cells that allows the use of FACS to sort out rare subpopulations relevant to transplantation in neurological disease. The neuronal cell selection procedures described here enable researchers from different fields of neurobiology to use FACS as a method to isolate viable neuronal cell populations derived from hESC. Obviously this methodology can also be applied to mouse ES or primary neural cells. In the future, alternative cell selection procedures using microfluidics and optical switches [56, 57] may be used to facilitate the isolation and yield of hESC-derived cells for clinical applications.
Beyond the sorting aspect itself, flow cytometric methodology offers analytical options such as quantification according to intracellular stains of fixed cells [58], viability assays using fluorescent DNA-binding dyes or caspase substrates (Fig. 2), cell cycle analysis, or 5-bromo-2′-deoxyuridine studies, which can be applied in neurobiological research. Multiparametric analysis using a combination of surface markers [11], as well as the detection of phosphorylation states of intracellular proteins for the elucidation of cellular signaling networks [8], provides further powerful analytical tools for studies of the nervous system. Testing drugs or factors on a defined population of primary or ESC-derived cells allows for a more precise analysis and understanding of their effects. The isolation of distinct neural cell subpopulations can thus promote our understanding of biology and cell-cell interactions, as in previous immunological and hematological research during the last decade [9]. We believe that broader application of the methods presented here will enable scientific investigations of neural cell subsets derived from hESC to enhance our understanding of cellular developmental neurobiology and eventually lead to its translation to the treatment of neurological diseases.
Supplementary Material
See www.StemCells.com for supplemental material available online.
Acknowledgments
We are grateful to Dr. Howard Shapiro for valuable comments on the manuscript. We thank Shreeya Karki and Wesley Ludwig for excellent technical assistance. This work was supported by NS-39793, the Stern Foundation, Orchard Foundation, Consolidated Anti-Aging Foundation, and the Harvard Stem Cell Institute.
Footnotes
Disclosure of potential conflicts of interest
The authors indicate no potential conflicts of interest.
References
- 1.Perrier AL, Tabar V, Barberi T, et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A. 2004;101:12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sonntag KC, Pruszak J, Yoshizaki T, et al. Enhanced yield of neuroepithelial precursors and midbrain-like dopaminergic neurons from human embryonic stem cells using the bone morphogenic protein antagonist noggin. STEM CELLS. 2007;25:411–418. doi: 10.1634/stemcells.2006-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan Y, Yang D, Zarnowska ED, et al. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. STEM CELLS. 2005;23:781–790. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart MH, Bosse M, Chadwick K, et al. Clonal isolation of hESCs reveals heterogeneity within the pluripotent stem cell compartment. Nat Methods. 2006;3:807–815. doi: 10.1038/nmeth939. [DOI] [PubMed] [Google Scholar]
- 5.Isacson O, Bjorklund LM, Schumacher JM. Toward full restoration of synaptic and terminal function of the dopaminergic system in Parkinson’s disease by stem cells. Ann Neurol. 2003;53(suppl 3):S135–S146. doi: 10.1002/ana.10482. [DOI] [PubMed] [Google Scholar]
- 6.Carson CT, Aigner S, Gage FH. Stem cells: The good, bad and barely in control. Nat Med. 2006;12:1237–1238. doi: 10.1038/nm1106-1237. [DOI] [PubMed] [Google Scholar]
- 7.Roy NS, Cleren C, Singh SK, et al. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- 8.Perez OD, Nolan GP. Phospho-proteomic immune analysis by flow cytometry: From mechanism to translational medicine at the single-cell level. Immunol Rev. 2006;210:208–228. doi: 10.1111/j.0105-2896.2006.00364.x. [DOI] [PubMed] [Google Scholar]
- 9.Herzenberg LA, Parks D, Sahaf B, et al. The history and future of the fluorescence activated cell sorter and flow cytometry: A view from Stanford. Clin Chem. 2002;48:1819–1827. [PubMed] [Google Scholar]
- 10.Kantor AB, Roederer M. FACS analysis of leukocytes. In: Herzenberg L, Herzenberg L, Blackwell C, et al., editors. Handbook of Experimental Immunology. Boston: Blackwell Science; 1996. pp. 43.1–49.13. [Google Scholar]
- 11.Baumgarth N, Roederer M. A practical approach to multicolor flow cytometry for immunophenotyping. J Immunol Methods. 2000;243:77–97. doi: 10.1016/s0022-1759(00)00229-5. [DOI] [PubMed] [Google Scholar]
- 12.Morrison SJ, Uchida N, Weissman IL. The biology of hematopoietic stem cells. Annu Rev Cell Dev Biol. 1995;11:35–71. doi: 10.1146/annurev.cb.11.110195.000343. [DOI] [PubMed] [Google Scholar]
- 13.Zola H. Medical applications of leukocyte surface molecules—the CD molecules. Mol Med. 2006;12:312–316. doi: 10.2119/2006-00081.Zola. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miltenyi S, Muller W, Weichel W, et al. High gradient magnetic cell separation with MACS. Cytometry. 1990;11:231–238. doi: 10.1002/cyto.990110203. [DOI] [PubMed] [Google Scholar]
- 15.Chung S, Shin BS, Hedlund E, et al. Genetic selection of sox1GFP-expressing neural precursors removes residual tumorigenic pluripotent stem cells and attenuates tumor formation after transplantation. J Neurochem. 2006;97:1467–1480. doi: 10.1111/j.1471-4159.2006.03841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uchida N, Buck DW, He D, et al. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pruszak J, Isacson O. Directed differentiation of human embryonic stem cells into dopaminergic neurons. In: Sullivan S, Cowan CA, Eggan K, editors. Human Embryonic Stem Cells: The Practical Handbook. Chichester: John Wiley & Sons Ltd; 2007. pp. 337–348. [Google Scholar]
- 18.Telford WG, Komoriya A, Packard BZ. Multiparametric analysis of apoptosis by flow and image cytometry. Methods Mol Biol. 2004;263:141–160. doi: 10.1385/1-59259-773-4:141. [DOI] [PubMed] [Google Scholar]
- 19.Seo H, Sonntag KC, Isacson O. Generalized brain and skin proteasome inhibition in Huntington’s disease. Ann Neurol. 2004;56:319–328. doi: 10.1002/ana.20207. [DOI] [PubMed] [Google Scholar]
- 20.Isacson O. The production and use of cells as therapeutic agents in neurodegenerative diseases. Lancet Neurol. 2003;2:417–424. doi: 10.1016/s1474-4422(03)00437-x. [DOI] [PubMed] [Google Scholar]
- 21.Scheel JR, Ray J, Gage FH, et al. Quantitative analysis of gene expression in living adult neural stem cells by gene trapping. Nat Methods. 2005;2:363–370. doi: 10.1038/nmeth755. [DOI] [PubMed] [Google Scholar]
- 22.Panchision DM, Chen HL, Pistollato F, et al. Optimized flow cytometric analysis of CNS tissue reveals novel functional relationships between CD133, CD15 and CD24 expressing cells. STEM CELLS. 2007;25:1560–1570. doi: 10.1634/stemcells.2006-0260. [DOI] [PubMed] [Google Scholar]
- 23.Mendez I, Sanchez-Pernaute R, Cooper O, et al. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson’s disease. Brain. 2005;128:1498–1510. doi: 10.1093/brain/awh510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozdinler PH, Macklis JD. IGF-I specifically enhances axon outgrowth of corticospinal motor neurons. Nat Neurosci. 2006;9:1371–1381. doi: 10.1038/nn1789. [DOI] [PubMed] [Google Scholar]
- 25.Sinclair SR, Fawcett JW, Dunnett SB. Dopamine cells in nigral grafts differentiate prior to implantation. Eur J Neurosci. 1999;11:4341–4348. doi: 10.1046/j.1460-9568.1999.00867.x. [DOI] [PubMed] [Google Scholar]
- 26.Sawamoto K, Nakao N, Kobayashi K, et al. Visualization, direct isolation, and transplantation of midbrain dopaminergic neurons. Proc Natl Acad Sci U S A. 2001;98:6423–6428. doi: 10.1073/pnas.111152398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh RN, Nakano T, Xuing L, et al. Enhancer-specified GFP-based FACS purification of human spinal motor neurons from embryonic stem cells. Exp Neurol. 2005;196:224–234. doi: 10.1016/j.expneurol.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 28.Keyoung HM, Roy NS, Benraiss A, et al. High-yield selection and extraction of two promoter-defined phenotypes of neural stem cells from the fetal human brain. Nat Biotechnol. 2001;19:843–850. doi: 10.1038/nbt0901-843. [DOI] [PubMed] [Google Scholar]
- 29.Yoshizaki T, Inaji M, Kouike H, et al. Isolation and transplantation of dopaminergic neurons generated from mouse embryonic stem cells. Neurosci Lett. 2004;363:33–37. doi: 10.1016/j.neulet.2004.03.074. [DOI] [PubMed] [Google Scholar]
- 30.Kannagi R, Cochran NA, Ishigami F, et al. Stage-specific embryonic antigens (SSEA-3 and -4) are epitopes of a unique globo-series ganglioside isolated from human teratocarcinoma cells. EMBO J. 1983;2:2355–2361. doi: 10.1002/j.1460-2075.1983.tb01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Badcock G, Pigott C, Goepel J, et al. The human embryonal carcinoma marker antigen TRA-1–60 is a sialylated keratan sulfate proteoglycan. Cancer Res. 1999;59:4715–4719. [PubMed] [Google Scholar]
- 32.Andrews PW, Banting G, Damjanov I, et al. Three monoclonal antibodies defining distinct differentiation antigens associated with different high molecular weight polypeptides on the surface of human embryonal carcinoma cells. Hybridoma. 1984;3:347–361. doi: 10.1089/hyb.1984.3.347. [DOI] [PubMed] [Google Scholar]
- 33.Draper JS, Moore HD, Ruban LN, et al. Culture and characterization of human embryonic stem cells. Stem Cells Dev. 2004;13:325–336. doi: 10.1089/scd.2004.13.325. [DOI] [PubMed] [Google Scholar]
- 34.Draper JS, Pigott C, Thomson JA, et al. Surface antigens of human embryonic stem cells: Changes upon differentiation in culture. J Anat. 2002;200:249–258. doi: 10.1046/j.1469-7580.2002.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukuda H, Takahashi J, Watanabe K, et al. Fluorescence-activated cell sorting-based purification of embryonic stem cell-derived neural precursors averts tumor formation after transplantation. STEM CELLS. 2006;24:763–771. doi: 10.1634/stemcells.2005-0137. [DOI] [PubMed] [Google Scholar]
- 36.Marzesco AM, Janich P, Wilsch-Brauninger M, et al. Release of extra-cellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J Cell Sci. 2005;118:2849–2858. doi: 10.1242/jcs.02439. [DOI] [PubMed] [Google Scholar]
- 37.Dubreuil V, Marzesco AM, Corbeil D, et al. Midbody and primary cilium of neural progenitors release extracellular membrane particles enriched in the stem cell marker prominin-1. J Cell Biol. 2007;176:483–495. doi: 10.1083/jcb.200608137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cui L, Johkura K, Yue F, et al. Spatial distribution and initial changes of SSEA-1 and other cell adhesion-related molecules on mouse embryonic stem cells before and during differentiation. J Histochem Cytochem. 2004;52:1447–1457. doi: 10.1369/jhc.3A6241.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fox N, Damjanov I, Martinez-Hernandez A, et al. Immunohistochemical localization of the early embryonic antigen (SSEA-1) in postimplantation mouse embryos and fetal and adult tissues. Dev Biol. 1981;83:391–398. doi: 10.1016/0012-1606(81)90487-5. [DOI] [PubMed] [Google Scholar]
- 40.Solter D, Knowles BB. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1) Proc Natl Acad Sci U S A. 1978;75:5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Capela A, Temple S. LeX is expressed by principle progenitor cells in the embryonic nervous system, is secreted into their environment and binds Wnt-1. Dev Biol. 2006;291:300–313. doi: 10.1016/j.ydbio.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 42.Hedlund E, Pruszak J, Ferree A, et al. Selection of embryonic stem cell derived eGFP+ dopamine neurons using the tyrosine hydroxylase promoter is confounded by reporter gene expression in immature cell populations. STEM CELLS. 2007;25:1126–1135. doi: 10.1634/stemcells.2006-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall PE, Lathia JD, Miller NG, et al. Integrins are markers of human neural stem cells. STEM CELLS. 2006;24:2078–2084. doi: 10.1634/stemcells.2005-0595. [DOI] [PubMed] [Google Scholar]
- 44.Barnett SC, Alexander CL, Iwashita Y, et al. Identification of a human olfactory ensheathing cell that can effect transplant-mediated remyelination of demyelinated CNS axons. Brain. 2000;123:1581–1588. doi: 10.1093/brain/123.8.1581. [DOI] [PubMed] [Google Scholar]
- 45.Wurmser AE, Nakashima K, Summers RG, et al. Cell fusion-independent differentiation of neural stem cells to the endothelial lineage. Nature. 2004;430:350–356. doi: 10.1038/nature02604. [DOI] [PubMed] [Google Scholar]
- 46.Bardin N, Frances V, Lesaule G, et al. Identification of the S-Endo 1 endothelial-associated antigen. Biochem Biophys Res Commun. 1996;218:210–216. doi: 10.1006/bbrc.1996.0037. [DOI] [PubMed] [Google Scholar]
- 47.Schnitzer J, Schachner M. Cell type specificity of a neural cell surface antigen recognized by the monoclonal antibody A2B5. Cell Tissue Res. 1982;224:625–636. doi: 10.1007/BF00213757. [DOI] [PubMed] [Google Scholar]
- 48.Nieoullon V, Belvindrah R, Rougon G, et al. mCD24 regulates proliferation of neuronal committed precursors in the subventricular zone. Mol Cell Neurosci. 2005;28:462–474. doi: 10.1016/j.mcn.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 49.Calaora V, Chazal G, Nielsen PJ, et al. mCD24 expression in the developing mouse brain and in zones of secondary neurogenesis in the adult. Neuroscience. 1996;73:581–594. doi: 10.1016/0306-4522(96)00042-5. [DOI] [PubMed] [Google Scholar]
- 50.Tole S, Kaprielian Z, Ou SK, et al. FORSE-1: A positionally regulated epitope in the developing rat central nervous system. J Neurosci. 1995;15:957–969. doi: 10.1523/JNEUROSCI.15-02-00957.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phimister E, Kiely F, Kemshead JT, et al. Expression of neural cell adhesion molecule (NCAM) isoforms in neuroblastoma. J Clin Pathol. 1991;44:580–585. doi: 10.1136/jcp.44.7.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chung S, Shin BS, Hwang M, et al. Neural precursors derived from embryonic stem cells, but not those from fetal ventral mesencephalon, maintain the potential to differentiate into dopaminergic neurons after expansion in vitro. STEM CELLS. 2006;24:1583–1593. doi: 10.1634/stemcells.2005-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.St John PA, Kell WM, Mazzetta JS, et al. Analysis and isolation of embryonic mammalian neurons by fluorescence-activated cell sorting. J Neurosci. 1986;6:1492–1512. doi: 10.1523/JNEUROSCI.06-05-01492.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roy NS, Wang S, Jiang L, et al. In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus. Nat Med. 2000;6:271–277. doi: 10.1038/73119. [DOI] [PubMed] [Google Scholar]
- 55.Roy NS, Benraiss A, Wang S, et al. Promoter-targeted selection and isolation of neural progenitor cells from the adult human ventricular zone. J Neurosci Res. 2000;59:321–331. doi: 10.1002/(sici)1097-4547(20000201)59:3<321::aid-jnr5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 56.Leary JF. Ultra high-speed sorting. Cytometry A. 2005;67:76–85. doi: 10.1002/cyto.a.20160. [DOI] [PubMed] [Google Scholar]
- 57.Wang MM, Tu E, Raymond DE, et al. Microfluidic sorting of mammalian cells by optical force switching. Nat Biotechnol. 2005;23:83–87. doi: 10.1038/nbt1050. [DOI] [PubMed] [Google Scholar]
- 58.Krichevsky AM, Sonntag KC, Isacson O, et al. Specific microRNAs modulate embryonic stem cell-derived neurogenesis. STEM CELLS. 2006;24:857–864. doi: 10.1634/stemcells.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Henderson JK, Draper JS, Baillie HS, et al. Preimplantation human embryos and embryonic stem cells show comparable expression of stage-specific embryonic antigens. STEM CELLS. 2002;20:329–337. doi: 10.1634/stemcells.20-4-329. [DOI] [PubMed] [Google Scholar]
- 60.Piccirillo SG, Reynolds BA, Zanetti N, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 61.Campos LS, Leone DP, Relvas JB, et al. Beta1 integrins activate a MAPK signalling pathway in neural stem cells that contributes to their maintenance. Development. 2004;131:3433–3444. doi: 10.1242/dev.01199. [DOI] [PubMed] [Google Scholar]
- 62.Nagato M, Heike T, Kato T, et al. Prospective characterization of neural stem cells by flow cytometry analysis using a combination of surface markers. J Neurosci Res. 2005;80:456–466. doi: 10.1002/jnr.20442. [DOI] [PubMed] [Google Scholar]
- 63.Camu W, Henderson CE. Purification of embryonic rat motoneurons by panning on a monoclonal antibody to the low-affinity NGF receptor. J Neurosci Methods. 1992;44:59–70. doi: 10.1016/0165-0270(92)90114-s. [DOI] [PubMed] [Google Scholar]
- 64.Dietrich J, Noble M, Mayer-Proschel M. Characterization of A2B5+ glial precursor cells from cryopreserved human fetal brain progenitor cells. Glia. 2002;40:65–77. doi: 10.1002/glia.10116. [DOI] [PubMed] [Google Scholar]
- 65.Rao MS, Noble M, Mayer-Proschel M. A tripotential glial precursor cell is present in the developing spinal cord. Proc Natl Acad Sci U S A. 1998;95:3996–4001. doi: 10.1073/pnas.95.7.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raff MC, Abney ER, Cohen J, et al. Two types of astrocytes in cultures of developing rat white matter: Differences in morphology, surface gangliosides, and growth characteristics. J Neurosci. 1983;3:1289–1300. doi: 10.1523/JNEUROSCI.03-06-01289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See www.StemCells.com for supplemental material available online.