Abstract
In principle, there is agreement about the clinical diagnostic criteria for dental erosion, basically defined as cupping and grooving of the occlusal/incisal surfaces, shallow defects on smooth surfaces located coronal from the enamel–cementum junction with an intact cervical enamel rim and restorations rising above the adjacent tooth surface. This lesion characteristic was established from clinical experience and from observations in a small group of subjects with known exposure to acids rather than from systematic research. Their prevalence is higher in risk groups for dental erosion compared to subjects not particularly exposed to acids, but analytical epidemiological studies on random or cluster samples often fail to find a relation between occurrence or severity of lesions and any aetiological factor. Besides other aspects, this finding might be due to lack of validity with respect to diagnostic criteria. In particular, cupping and grooving might be an effect of abrasion as well as of erosion and their value for the specific diagnosis of erosion must be doubted. Knowledge about the validity of current diagnostic criteria of different forms of tooth wear is incomplete, therefore further research is needed.
Keywords: Epidemiology, Erosion, Diagnosis, Criteria, Validity
The process of diagnosis and current criteria for dental erosion
From the chemical view, the aetiology of dental erosion can be defined as the chronic exposure of the teeth to extrinsic or intrinsic acids under the condition that the oral fluids are undersaturated with respect to tooth mineral [23, 26]. Under in vitro conditions without physical impact, teeth demineralise centripetally (Fig. 1), a feature of substance loss which is normally not observed in the mouth. In fact, the multitudes of physical and chemical assaults occurring during a lifetime result in a more or less characteristic pattern of tooth wear. The classification of wear is therefore made from morphological features which are frequently seen clinically. The tooth morphology as apparent after eruption is the idealised status, deviations of which, if not caries or trauma, are diagnosed as (erosive) tooth wear. Various forms of wear including dental erosion are listed in the International Classification of Diseases [52] thus defining them as a disease (for critical discussion of this notion see [12]).
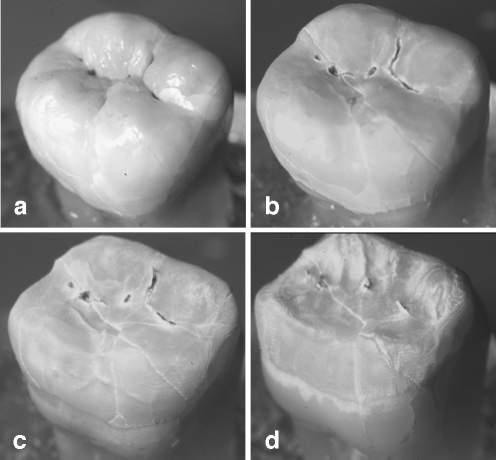
Fig. 1.
Effect of the continuous exposure of a human third molar to 10% citric acid. The amorphous, centripetal tissue loss is obvious (a unaffected tooth, b tissue loss after 4, c 8, and d 12 h immersion time)
Looking at what “diagnosis” is, one will find mostly definitions like “identification of disease from signs or symptoms”, implicating that the physician or scientist “reads off” from the patient thus detecting the disease. Health and disease, however, is not a given condition, but is constituted by the theoretical concepts and the discursive practice the physician or scientist is subjected to [12]. That is to say, rather than reading off the disease from the patient, a pattern of criteria is projected onto the diagnosed subject which determines the diagnostic procedure and outcome. Following this approach, the diagnostic process, in a first step, is a theoretical concept ordering signs and symptoms to diseases which takes place within the configurative power of the discourses. In whatever discourse the physician acts, the act of clinical diagnosis, in a second step, is classification. The conclusion reached through this process is called “a diagnosis”. From this background, it becomes obvious that the diagnostic process must be accompanied by a recurrent reconsideration of its theoretical concepts and the reevaluation of the criteria used. The latter is what the paper aims to achieve.
The diagnosis of tooth wear in general and erosion in particular is made from its lesion characteristics, from the results of nutritional, medical and occupational analysis, and from dietary records. The diagnostic process can be more differentiated with the individual patient, whereas in field trials it is restricted to the classification of lesion shape.
Throughout the literature, there is more or less consensus about the aetiology of the various forms of tooth wear, the clinical criteria for dental erosion and its differential diagnosis [12, 14].
The early signs of erosive tooth wear appear as changes of the optical properties of enamel resulting in a smooth silky–shining glazed surface. When the tissue loss continues, changes in the original morphology occur. On smooth surfaces, convex areas flatten or concavities develop, the width of which clearly exceeds the depth. Lesions are located coronal from the enamel–cementum junction (CEJ) with an intact enamel rim along the gingival margin. On occlusal and incisal surfaces, rounding and cupping of the cusps and grooving of the incisal edges occur, and restorations may rise above the level of the adjacent tooth surfaces. In advanced cases, the whole occlusal morphology disappears.
The validity of these clinical criteria for erosion, however, has not been under critical consideration. Validity, in general terms, means the degree, to which a measurement measures what it purports to measure. For the issue addressed here, criterion validity is the relevant term, meaning the extent to which the measurement correlates with an external criterion under study. A further aspect of validity is concurrent validity. Concurrent validity means to which extent the measurement and the criterion refer to the same time [24]. In the case of erosion this would address the question if (a) the diagnostic criteria reflect lesions being an effect of an exposure to acids and (b) if the presence of characteristic signs is concurrent with an acid exposure.
The implementation of current diagnostic criteria
Considering how the diagnostic criteria for erosion were established, the literature reveals that it was individual clinical experience and case reports rather than systematic research. One of the first publications on the characteristics of acid induced tissue loss was in 1946 from Robinson [43] and in 1947 from Stafne and Lovestedt [47]. Robinson, whilst being in doubt about the aetiology of dental erosion, described the lesions as located on smooth surfaces and as wedge shaped, cup shaped, disk shaped, irregular in form, L- or U-shaped, or simply as small round depressions or larger surface lesions. It is not clear how these descriptions were established, but Robinson referred to a work from McClure and Ruzicka [31] describing the morphology of lesions of rats teeth after being fed with lactate and citrate drinking fluid.
Stafne and Lovestedt presented their observations in subjects with known acid exposure, amongst them 50 patients with frequent consumption of lemon juice. They did not give a concrete description of lesion shape, but attributed hypersensitivity, absence of stain and defects with rounded margins as effect of the action of the acids. Their most important sign of diagnostic value was the presence of fillings projecting above the surface of the tooth. In their publication, a number of clinical images were included, presenting lesions clearly matching current erosion criteria.
More than twenty years later, in 1970, it was Pindborg [40] who gave the often cited definition of erosion as being superficial loss of dental hard tissue by a chemical process which does not involve bacteria. He described the clinical signs of chemically-induced tissue loss as usually located to the gingival third of the facial surfaces, possibly also located at proximal surfaces, lesions to appear shallow, disc-shaped, smooth, polished, or scooped out. In contrast to abrasion, he attributed erosive lesions to be located evenly on the left and right side.
It appears noteworthy, that Pindborg, as well as Robinson, ascribed cupping of the cusps, loss of the occlusal morphology or loss of crown height, and incisal grooving to attrition which in their publications was defined as result of mastication.
It is probably Eccles and Jenkins [8, 10] who were the first to give a detailed and systematic description of the lesion characteristics and also suggested a system for classification. The basis of the development of the clinical criteria was a sample of 72 patients seen in the dental hospital over a period of 9 years [8]. All cases were thoroughly documented with respect to their medical and dietary history, that is, it was a group of subjects with known exposure to intrinsic or dietary acids. The findings derived from this group of subjects (Table 1) have been retained nearly unchanged until now. The main aspects are in general terms loss of surface contour, shallow concavities on smooth surfaces, cupping and grooving on occlusal/incisal surfaces and restorations rising above the level of the adjacent tooth surface.
Table 1.
| Diagnostic criteria for dental erosion | |
|---|---|
| Initial | Absence of developmental ridges of the enamel, smooth glazed surface |
| Advanced | |
| Facial/oral surfaces | Concavities whose breadth greatly exceeds their depth |
| Lesion ovoid or crescentic in outline, concave in cross section or | |
| Lesion entirely in the crown, irregular in outline, punched out appearance | |
| Occlusal/incisal surfaces | Surfaces appear flattened, depression of the cusps (cupping) and on the incisal edges (grooving), edges of restorations raising above the level of the adjacent tooth surface |
Conclusions from epidemiological studies using current diagnostic criteria
Current criteria, derived from a relatively small sample, have been applied in case reports [8, 9, 16, 32, 39, 50] and studies with risk groups [15, 17, 21, 25, 34, 38, 44, 48, 51]. Indeed, when used in subjects with known or strongly assumed exposure to acids, most studies revealed a higher prevalence of lesions in the exposed groups compared to the control groups (Table 2).
Table 2.
Prevalence of lesions in risk groups deriving from the use of current diagnostic criteria for dental erosion
| Index | Group size | Prevalence risk group | Prevalence control group | |
|---|---|---|---|---|
| Intrinsic acid exposure | ||||
| Meurman et al. [34] reflux disease | Eccles and Jenkins index | n = 117 | 28/117 = 24% | No control group |
| Rytömaa et al. [44] eating disorders | Eccles and Jenkins index | n = 140 | 22/35 = 63% | 12/105 = 11% |
| Öhrn et al. [38] eating disorders | Lussi index | n = 133 | 79/81 = 98% | Minor, less severe |
| Incisal/occlusal | Incisal/occlusal | |||
| Grade 1: 93% | Grade 1: 73% | |||
| Grade 2: 52% | Grade 2: 23% | |||
| Buccal | Buccal | |||
| Grade 1: 30% | Grade 1: 19% | |||
| Grade 2: 9% | Grade 2: 6% | |||
| Palatal | Palatal | |||
| Grade 1: 21% | Grade 1: 10% | |||
| Grade 2: 5% | Grade 2: 0% | |||
| Extrinsic acid exposure | ||||
| Linkosalo and Markkanen [25] vegetarians | Eccles and Jenkins index | n = 52 | 16/26 = 60% | 0/26 = 0% |
| Wiktorsson et al. [51] wine tasters | Eccles and Jenkins index | n = 19 | 14/19 = 74% | No control group |
| Ganss et al. [15] raw food diet | Lussi index | n = 206 | 127/130 = 98% | 66/76 = 87% |
These observations support the finding that subjects with continuous exposure to acids have a higher rate of lesions with a specific characterisation. This is, however, not enough support for the assumption that, vice versa, subjects presenting with such defects must be exposed to acids.
Analytical epidemiological studies on random or cluster samples attempted to relate the occurrence of lesions with any of the known aetiological factors for erosion, but only few studies of this type have been published. Most of them include children or adolescents, but there is lack of studies on older population groups. Furthermore, off the few studies, some used the Tooth Wear Index (TWI [46]) which is not designed to assess dental erosion specifically. The overall findings (Table 3) are controversial since some authors found no or only a partial relation between aetiological factors and the occurrence or severity of lesions [2, 3, 5, 7, 20, 30, 35, 37, 49], whereas others revealed strong relationships [22]. In addition, a relation to the intake of yoghurt or other foodstuff which certainly has no erosive potential was mentioned [1, 27, 35].
Table 3.
Analytical epidemiological studies attempting to relate the occurrence of (erosive) wear to aetiological factors
| Index, group size, age and prevalence | Conclusion | |
|---|---|---|
| Järvinen et al. [22] | Eccles & Jenkins index | Citrus fruits: odds ratio (OR) 2 |
| Case-control, n = 100 each | Soft drinks: OR 4 | |
| 13–83-year-olds | ||
| Lussi et al. [27] | Lussi index | Significant relation to the consumption of fruit, acidic drinks, yoghurt, vomiting |
| n = 417 | ||
| 26–30- and 46–50-year-olds at least 36 and 43% resp. with any erosion | ||
| Bartlett et al. [5] | TWI (Smith and Knight) | No significant relation to drinks or other acidic food |
| n = 210 | Significant relation to heart burn | |
| 11–14-year-olds | ||
| 57% had wear in enamel on more than 10 teeth | ||
| Jaeggi et al. [20] | Lussi index | No relation to any aetiological factor |
| n = 417 | ||
| 19–25-year-olds at least 82% with erosion | ||
| Al-Dlaigan et al. [1] | TWI (Smith and Knight) | Significant relation to drinks and fruit, but also to milk, yoghurt and beer |
| n = 418 | ||
| 14-year-olds | ||
| 48% low, 51% moderate 1% severe lesions | ||
| Al-Majed at al. [2] | TWI (Smith and Knight) modified for erosion | No association to erosive drinks for the total sample |
| n = 862 | Significant association to frequency of drinks at night and duration of drinks retained in the mouth only in advanced cases (n = 95) | |
| 12–14-year-olds | ||
| 95% with erosion | ||
| Mathew et al. [30] | Lussi index | No relation to the intake of sport drinks |
| n = 304 | ||
| 18–28-year-olds | ||
| 37% with erosion | ||
| Van Rijkom et al. [49] | Modified Lussi index | No relation to acidic drinks and fruits |
| n = 400 | ||
| 15–16-year-olds | ||
| 30% with visible smooth wear | ||
| Arnadottir et al. [3] | Modified Lussi index | No significant association to risk factors |
| n = 278 | ||
| 15-year-olds | ||
| 72% grade 1 | ||
| 24% grade 2 | ||
| 5% grade 3 | ||
| Nunn et al. [37] | TWI (Smith and Knight) modified for erosion | No significant association with dietary factors |
| n = 1726 | Significant relationship with gastro-oesophageal symptoms | |
| 4–18-year-olds | ||
| 36, 56 and 34% with any erosion on buccal and palatal surfaces of the incisors, and first permanent molars resp. | ||
| Dugmore and Rock [7] | TWI (Smith and Knight) modified for erosion | Drinking fizzy pop: odds ratio 1.59–2.52 depending on amount and frequency |
| n = 1149 | ||
| 12-year-olds | No relation to eating apples, citrus fruit | |
| 56% with erosion | ||
| Milosevic et al. [35] | TWI (Smith and Knight) on labial and lingual surfaces in front teeth, occlusal surfaces of first molars | No association to apples, fresh oranges |
| n = 2385 | Weak association (OR 1–1.4) to yoghurt, grapefruit, salad dressing, vinegar, fruit juice, fizzy drinks | |
| 14-year-olds | Strong association to herbal/lemon tea (OR 3.97) | |
| 53.5% with exposed dentine |
There are many points of discussion for explanation. At first, (erosive) wear is the effect of various concurrent or past chemical exposures from different sources, the variety of which can hardly be covered by simple questionnaires, and especially in younger people the dietary intake and lifestyle might be open to variations. Furthermore, questionnaires may reflect only in part the dietary habits due to interpretation by the responder or seasonal changes in daily life habits. When the young age of the groups studied is considered, the determinants for dental erosion may not have acted for long enough, and, in addition, there might be considerable differences in the individual susceptibility to erosive demineralisation [15].
It must, however, also be taken into consideration that current diagnostic criteria might not be valid enough to really reflect the effect of a chronic acid exposure.
Taking into account that tooth wear is the result of various factors, the occlusal and incisal surfaces of teeth are not only exposed to acids but are particularly prone to physical impacts from mastication. From this background, the finding that many studies using erosion indices reveal that occlusal lesions are the most prevalent and that it is the first lower molar which is most affected [19], is of particular importance. Occlusal surfaces might be the only location exhibiting a relation between lesion severity and prevalence, and age [28] thus indicating a significant contribution of abrasion from mastication. Furthermore, particularly the teeth from ancient remains exhibit defects being strikingly of the same shape as those attributed to erosion today (Fig. 2).
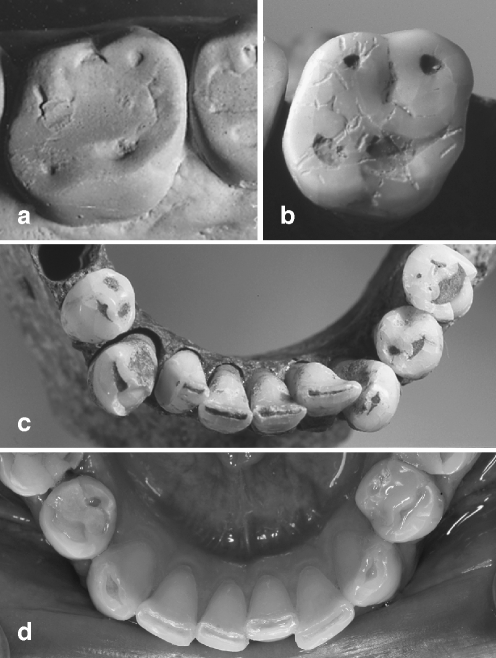
Fig. 2.
a Occlusal aspect of a subject living on a raw food diet with multiple acid impacts, and a medieval subject b with an assumed abrasive diet (images a, b, and c samples from [13, 15]). Occlusal/incisal defects in a subject with chronic vomiting d and in a medieval subject c. The shape of lesions from predominantly erosive and predominantly abrasive aetiology is strikingly similar
Comparative studies on lesion characteristics of wear
Attempts have been made to characterise the shape of wear resulting from abrasion and erosion [6, 13].
A comparison of subjects with known exposure to a non-acidic but coarse diet and subjects with an acidic, but refined diet using silicon impressions revealed that in the former, the scooped out dentine was significantly shallower than in the latter. Furthermore, the deepest lesion region in teeth from subjects with abrasive diet was located at the functional cusps, whereas in teeth from subjects with an erosive diet, the deepest region of the defects was located more centrally. The authors concluded that in cases when the dentine is severely scooped out, the causative agent is most likely of erosive origin whereas scooping tends to be shallower when wear is caused by abrasion. The conclusion of the study was to suggest a quantitative diagnostic procedure by calculating the depth/breadth ratio for clinical differential diagnosis [6].
Another approach was to compare the shape of defects occurring in subjects with substantially different nutrition patterns [13]. The study included three groups of age matched individuals — randomly selected contemporary subjects, medieval remains and a group of subjects living on raw food diet. From the latter group, extensive information was available on their intake of acid food qualifying their nutrition as rather erosive. For the medieval group, an abrasive nutrition was assumed from knowledge about the general nature of the early medieval diet.
As to the smooth surfaces, the most interesting finding was that in the medieval group, no lesions were observed (Table 4). Even in cases with severe wear, the lingual and buccal aspects of the teeth appeared undisturbed and, in many cases, even developmental ridges were present. The lack of cervical defects is also reported by Aubry et al. [4] and Kaidonis (in this issue) and also inherently supported by the fact that the overwhelming number of anthropological studies on tooth wear only deal with occlusal and proximal wear and that the indices used there do not include criteria for cervical wear [11, 36]. There are, however, few studies reporting wear on lingual surfaces in archaeological remains [18, 41] and there is one publication discussing regurgitation as a possible aetiological factor [42]. In this study, 151 adult pre-Conquest British skulls were examined using the TWI. Indeed, buccal and lingual wear was observed to the same degree as in a contemporary comparison group, but cervical wear did not develop beyond criterion 0 in most individuals. In 20% of the skulls, however, a cervical wear to TWI score of 2–3 occurred. No information was given about the shape of the lesions, and from the images included, it appears that their characteristics would not match the current criteria for dental erosion.
Table 4.
Prevalence of lesions of defined shape in three groups (n = 100 each) of subjects with substantially different nutrition patterns [13]
| Abrasive diet (medieval group) | Acidic diet (raw food group) | Average western diet | ||
|---|---|---|---|---|
| Incisal/occlusal surfaces | ||||
| Incisors/canines | ||||
| Grooving | 93% | 96% | 90% | n.s. |
| Molars/premolars | ||||
| Shallow cupping (<0.5 mm) | 87% | 59% | 47% | p < 0.001 |
| Deep cupping (>0.5 mm) | 78% | 45% | 4% | p < 0.0001 |
| Smooth surfaces (all teeth) | ||||
| Concavity coronal to the CEJ | 0% | 63% | 8% | p < 0.0001 |
| V-shaped defects | 0% | 38% | 10% | p < 0.0001 |
In contrast to the findings in the medieval group, shallow defects located coronal from the CEJ were considerably prevalent in the raw food group (Table 4, Fig. 3b), whereas in the western diet group, the prevalence of this kind of lesions was much lower and corresponds to its general prevalence in Germany [45].
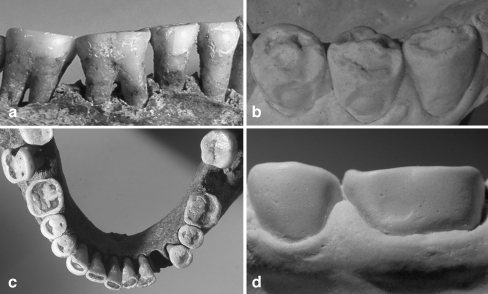
Fig. 3.
a Buccal aspect of teeth 44–47 with significant loss of crown height, but without any lesion in a medieval remain [13] with severe generalised occlusal wear c. b Occlusal defects in a subject living on a raw food diet with a high intake of acidic food [15]. The shape of the occlusal lesions is similar to c, but combined with shallow lesions with intact cervical rim lesions. d Same subject with Fig. 4b with an initial buccal lesion
As to the occlusal/incisal surfaces, grooving and cupping was common in all groups even though most often in the medieval group, followed by the raw food group, and the western diet group.
The conclusion from this study was that shallow defects on smooth surfaces might be a valid criterion for dental erosion, whereas cupping, and especially incisal grooving, was common in all groups and therefore not valid for a differential diagnosis.
The similarity of occlusal/incisal defects in all groups might be explained from a tribological view [29]. Wear, as a result of abrasion, occurs as three body wear that means the intervention of an abrasive slurry or bolus. During mastication, the abrasive tends to hollow out softer surface regions. Cupping or grooving can occur when deeper enamel regions with lower microhardness [33] are exposed or when the dentine is reached. In the case of erosion, tissue loss is on one hand caused by direct dissolution of mineral but also due to an increased susceptibility of acid softened surfaces to physical wear. In these cases, also a non- or less abrasive bolus could cause similar defects when acting on acid weakened surfaces.
Hence, the occlusal/incisal substance loss observed in individuals prone to dietary acids may be explained as pronounced abrasion/demastication of acid softened surfaces. Therefore, it is questionable if the occlusal morphological criteria used for the diagnosis of occlusal erosion per se are valid.
Even though there are often striking similarities between lesions of predominantly abrasive or predominantly erosive origin, there is, on the other hand, often also considerable variation in lesion shape in cases of erosion. The morphology of occlusal lesions in subjects with known exposure to acids varies from deep hollowing out of the cusps to totally amorphous loss of the occlusal structure even in subjects with a similar dietary history (Fig. 4).
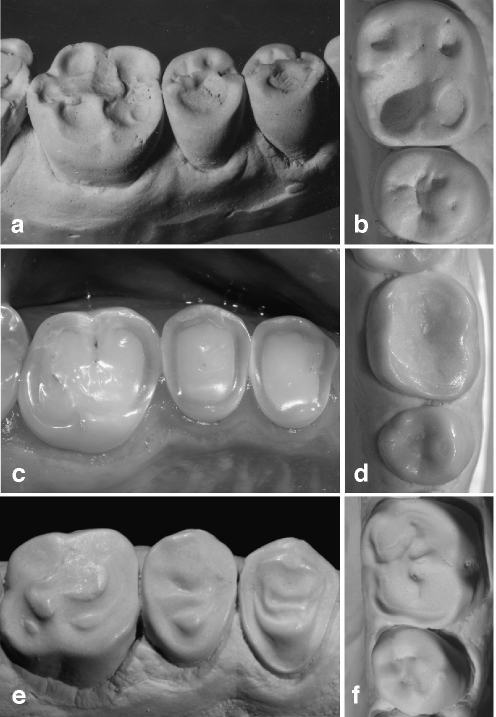
Fig. 4.
Occlusal tissue loss from erosive aetiology can also be of strikingly different shape either presenting as deeply hollowed out lesions (a subject with raw food diet [15], b subject with excessive consumption of orange juice) or as amorphous generalised tissue loss affecting the entire surface (c, d subjects with excessive consumption of erosive drinks). An interesting feature is seen in an adolescent with a history of severe anterior open bite with only the molars being in function e Substance loss occurred from excessive consumption of a cola type drink. In the premolars, dentine is proud of the surface. f Hollowing out the entire occlusal surface with enamel remnants in the centre, aetiology is the excessive consumption of sport drinks
Conclusion
The validity of current diagnostic criteria for dental erosion has not been systematically studied, even though there is consensus about their definition.
The following conclusions can be drawn:
Grooving of incisal surfaces is a common phenomenon and possibly the effect of any physical or chemical impact. It should be considered to abandon grooving of anterior teeth and canines as a clinical criterion for dental erosion.
Shallow defects located coronal from the CEJ may predominantly occur as effect of chronic acid exposure and might be pathogonomic for dental erosion. This assumption is supported by the finding that these types of lesions are not present in ancient remains even in cases of severe wear.
Cupping of cusps is the most uncertain criterion because it can be an effect of abrasion as well as of erosion. In industrialised countries, abrasion is not expected to be a significant factor in young people. Cupping occurring at younger ages can therefore be an effect of erosion. At older ages, however, physical and chemical impacts add up increasingly and cupping will therefore be of little diagnostic value in adults.
These conclusions are drawn from very few studies; therefore systematic research on this issue is needed. Nevertheless, there is enough support for a criticism of current diagnostic criteria particularly in the light of the development of a new index.
Acknowledgement
Conflict of interest statement The author declares that there is no conflict of interest.
References
- 1.Al-Dlaigan YH, Shaw L, Smith A (2001) Dental erosion in a group of British 14-year-old school children. Part II: influence of dietary intake. Br Dent J 190:258–261 [DOI] [PubMed]
- 2.Al-Majed I, Maguire A, Murray JJ (2002) Risk factors for dental erosion in 5–6 year old and 12–14 year old boys in Saudi Arabia. Community Dent Oral Epidemiol 30:38–46 [DOI] [PubMed]
- 3.Arnadottir IB, Saemundsson SR, Holbrook WP (2003) Dental erosion in Icelandic teenagers in relation to dietary and lifestyle factors. Acta Odontol Scand 61:25–28 [DOI] [PubMed]
- 4.Aubry M, Mafart B, Donat B, Brau JJ (2003) Brief communication: study of noncarious cervical tooth lesions in samples of prehistoric, historic, and modern populations from the South of France. Am J Phys Anthropol 121:10–14 [DOI] [PubMed]
- 5.Bartlett DW, Coward PY, Nikkah C, Wilson RF (1998) The prevalence of tooth wear in a cluster sample of adolescent schoolchildren and its relationship with potential explanatory factors. Br Dent J 184:125–129 [DOI] [PubMed]
- 6.Bell EJ, Kaidonis J, Townsend G, Richards L (1998) Comparison of exposed dentinal surfaces resulting from abrasion and erosion. Aust Dent J 43:362–366 [DOI] [PubMed]
- 7.Dugmore CR, Rock WP (2004) A multifactorial analysis of factors associated with dental erosion. Br Dent J 196:283–286 [DOI] [PubMed]
- 8.Eccles JD (1979) Dental erosion of nonindustrial origin. A clinical survey and classification. J Prosthet Dent 42:649–653 [DOI] [PubMed]
- 9.Eccles JD (1982) Erosion affecting the palatal surfaces of upper anterior teeth in young people. A report of 19 cases. Br Dent J 152:375–378 [DOI] [PubMed]
- 10.Eccles JD, Jenkins WG (1974) Dental erosion and diet. J Dent 2:153–159 [DOI] [PubMed]
- 11.Eshed V, Gopher A, Hershkovitz I (2006) Tooth wear and dental pathology at the advent of agriculture: new evidence from the Levant. Am J Phys Anthropol 130:145–159 [DOI] [PubMed]
- 12.Ganss C (2006) Definition of erosion and links to tooth wear. Monogr Oral Sci 20:9–16 [DOI] [PubMed]
- 13.Ganss C, Klimek J, Borkowski N (2002) Characteristics of tooth wear in relation to different nutritional patterns including contemporary and medieval subjects. Eur J Oral Sci 110:54–60 [DOI] [PubMed]
- 14.Ganss C, Lussi A (2006) Diagnosis of erosive tooth wear. Monogr Oral Sci 20:32–43 [DOI] [PubMed]
- 15.Ganss C, Schlechtriemen M, Klimek J (1999) Dental erosions in subjects living on a raw food diet. Caries Res 33:74–80 [DOI] [PubMed]
- 16.Geurtsen W (2000) Rapid general dental erosion by gas-chlorinated swimming pool water. Review of the literature and case report. Am J Dent 13:291–293 [PubMed]
- 17.Imirzalioglu P, Onay EO, Agca E, Ogus E (2007) Dental erosion in chronic renal failure. Clin Oral Invest 11:175–180 [DOI] [PubMed]
- 18.Irish JD, Turner CG (1987) More lingual surface attrition of the maxillary anterior teeth in American Indians: prehistoric Panamanians. Am J Phys Anthropol 73:209–213 [DOI] [PubMed]
- 19.Jaeggi T, Lussi A (2006) Prevalence, incidence and distribution of erosion. Monogr Oral Sci 20:44–65 [DOI] [PubMed]
- 20.Jaeggi T, Schaffner M, Bürgin W, Lussi A (1999) Erosionen und keilförmige Defekte bei Rekruten der Schweitzer Armee. Schweiz Monatsschr Zahnmed 109:1171–1178 [PubMed]
- 21.Järvinen V, Meurman JH, Hyvarinen H, Rytömaa I, Murtomaa H (1988) Dental erosion and upper gastrointestinal disorders. Oral Surg Oral Med Oral Pathol 65:298–303 [DOI] [PubMed]
- 22.Järvinen VK, Rytömaa I, Heinonen OP (1991) Risk factors in dental erosion. J Dent Res 70:942–947 [DOI] [PubMed]
- 23.Larsen MJ (1990) Chemical events during tooth dissolution. J Dent Res 69:575–580 [DOI] [PubMed]
- 24.Last JM (2001) A dictionary of epidemiology. Oxford University Press, New York
- 25.Linkosalo E, Markkanen H (1985) Dental erosions in relation to lactovegetarian diet. Scand J Dent Res 93:436–441 [DOI] [PubMed]
- 26.Lussi A, Featherstone JD (2006) Understanding the chemistry of dental erosion. Monogr Oral Sci 20:66–76 [DOI] [PubMed]
- 27.Lussi A, Schaffner M, Hotz P, Suter P (1991) Dental erosion in a population of Swiss adults. Community Dent Oral Epidemiol 19:286–290 [DOI] [PubMed]
- 28.Lussi A, Schaffner M, Hotz P, Suter P (1993) Epidemiology and risk factors of wedge-shaped defects in a Swiss population. Schweiz Monatsschr Zahnmed 103:276–280 [PubMed]
- 29.Mair LH (2000) Wear in the mouth: the tribological dimension. In: Addy M, Embery G, Edgar WM, Orchardson R (eds) Tooth wear and sensitivity. Clinical advances in restorative dentistry. Martin Dunitz, London, pp 181–188
- 30.Mathew T, Casamassimo PS, Hayes JR (2002) Relationship between sports drinks and dental erosion in 304 university athletes in Columbus, Ohio, USA. Caries Res 36:281–287 [DOI] [PubMed]
- 31.McClure F, Ruzicka SJ (1946) The destructive effect of citrate vs lactate ions on rats’ molar tooth surfaces, in vivo. J Dent Res 25:1–12 [DOI] [PubMed]
- 32.McCracken M, O’Neal SJ (2000) Dental erosion and aspirin headache powders: a clinical report. J Prosthodont 9:95–98 [DOI] [PubMed]
- 33.Meredith N, Sherriff M, Setchell DJ, Swanson SA (1996) Measurement of the microhardness and Young’s modulus of human enamel and dentine using an indentation technique. Arch Oral Biol 41:539–545 [DOI] [PubMed]
- 34.Meurman JH, Toskala J, Nuutinen P, Klemetti E (1994) Oral and dental manifestations in gastroesophageal reflux disease. Oral Surg Oral Med Oral Pathol 78:583–589 [DOI] [PubMed]
- 35.Milosevic A, Bardsley PF, Taylor S (2004) Epidemiological studies of tooth wear and dental erosion in 14-year old children in North West England. Part 2: the association of diet and habits. Br Dent J 197:479–483 [DOI] [PubMed]
- 36.Molnar S (1971) Human tooth wear, tooth function and cultural variability. Am J Phys Anthropol 34:175–190 [DOI] [PubMed]
- 37.Nunn JH, Gordon PH, Morris AJ, Pine CM, Walker A (2003) Dental erosion-changing prevalence? A review of British National childrens’ surveys. Int J Paediatr Dent 13:98–105 [DOI] [PubMed]
- 38.Öhrn R, Enzell K, Angmar-Månsson B (1999) Oral status of 81 subjects with eating disorders. Eur J Oral Sci 107:157–163 [DOI] [PubMed]
- 39.O’Sullivan EA, Curzon ME (1998) Dental erosion associated with the use of ‘alcopop’—a case report. Br Dent J 184:594–596 [DOI] [PubMed]
- 40.Pindborg JJ (1970) Pathology of the dental hard tissues. Munksgaard, Copenhagen
- 41.Robb ND, Cruwys E, Smith BG (1991) Is “lingual surface attrition of the maxillary teeth (LSAMAT)” caused by dental erosion? Am J Phys Anthropol 85:345–347 [DOI] [PubMed]
- 42.Robb ND, Cruwys E, Smith BG (1991) Regurgitation erosion as possible cause of tooth wear in ancient British populations. Arch Oral Biol 36:595–602 [DOI] [PubMed]
- 43.Robinson HBG (1946) A clinic on the differential diagnosis of oral lesions. Am J Orthod Oral Surg 32:729–762 [DOI] [PubMed]
- 44.Rytömaa I, Järvinen V, Kanerva R, Heinonen OP (1998) Bulimia and tooth erosion. Acta Odontol Scand 56:36–40 [DOI] [PubMed]
- 45.Schiffner U, Micheelis W, Reich E (2002) Erosionen und keilförmige Defekte bei deutschen Erwachsenen und Senioren. Dtsch Zahnärztl Z 57:102–106
- 46.Smith BG, Knight JK (1984) An index for measuring the wear of teeth. Br Dent J 156:435–438 [DOI] [PubMed]
- 47.Stafne ECLA, Lovestedt SA (1947) Dissolution of tooth substance by lemon juice, acid beverages and acids from other sources. J Am Dent Assoc 34:586–592 [DOI] [PubMed]
- 48.Tuominen ML, Tuominen RJ, Fubusa F, Mgalula N (1991) Tooth surface loss and exposure to organic and inorganic acid fumes in workplace air. Community Dent Oral Epidemiol 19:217–220 [DOI] [PubMed]
- 49.van Rijkom HM, Truin GJ, Frencken JE, Konig KG, van’t Hof MA, Bronkhorst EM, Roeters FJ (2002) Prevalence, distribution and background variables of smooth-bordered tooth wear in teenagers in the Hague, the Netherlands. Caries Res 36:147–154 [DOI] [PubMed]
- 50.Westergaard J, Moe D, Pallesen U, Holmen L (1993) Exaggerated abrasion/erosion of human dental enamel surfaces: a case report. Scand J Dent Res 101:265–269 [DOI] [PubMed]
- 51.Wiktorsson AM, Zimmerman M, Angmar-Månsson B (1997) Erosive tooth wear: prevalence and severity in Swedish winetasters. Eur J Oral Sci 105:544–550 [DOI] [PubMed]
- 52.World Health Organisation (2003) ICD-10 Online Version. International statistical classification of diseases and related health problems.