Summary
The bone marrow (BM) milieu confers drug resistance in multiple myeloma (MM) cells to conventional therapies. Therefore novel biologically-based therapies are needed. Preclinical studies have identified and validated molecular targeted therapeutics in MM. In particular, recognition of the biologic significance of the BM microenvironment both in MM pathogenesis and as a potential target for novel therapeutics has already derived several promising approaches. Thalidomide, lenalidomide (Revlimid®) and bortezomib (Velcade®) are directed not only at MM cells, but also BM milieu, and have rapidly from the bench to the bedside and FDA approval to treat MM.
Introduction
Despite advances in systemic and supportive therapies, MM remains incurable due to intrinsic or acquired chemotherapeutic resistance. High-dose chemotherapy with stem cell transplantation has significantly extended progression-free and overall survival, but cures few, if any, patients. Novel therapeutic approaches overcoming drug-resistance are therefore urgently needed in MM. The interaction of MM cells with extracellular matrix (ECM) proteins and BM stromal cells (SCs), as well as other components in the BM milieu (ie, osteoblast, osteoclast, vascular endothelial cells), plays a crucial role in MM cell pathogenesis and drug resistance. Importantly, novel biologically-based treatments which target not only the MM cell, but also the MM cell interaction with other accessory cells and cytokines/growth factors in the BM milieu, can overcome resistance to conventional therapies in both preclinical and clinical studies, and have great promise to improve patient outcome in MM.
The role of the BM microenvironment in MM
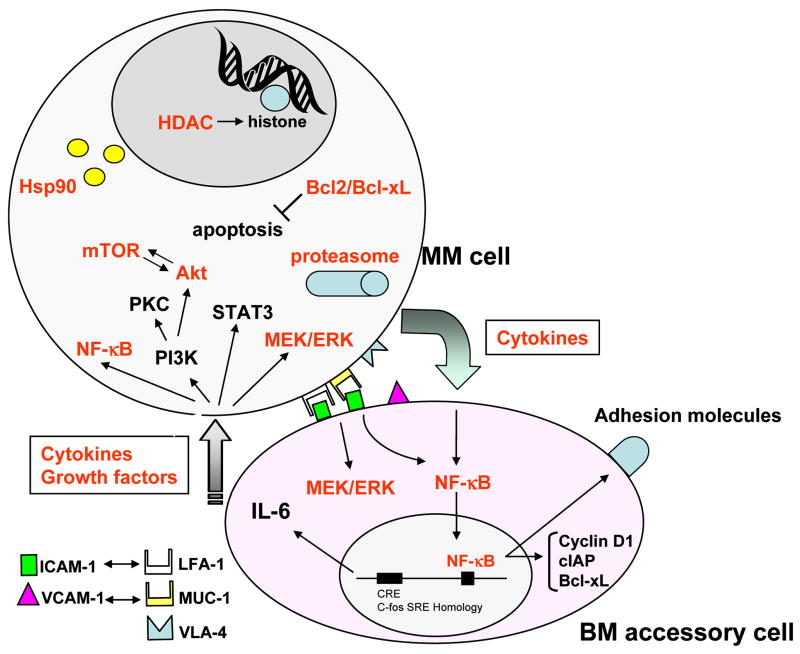
The BM microenvironment promotes MM cell growth, survival, migration and drug resistance. It is composed of different types of cellular component: including: hematopoietic stem cells; progenitor and precursor cells; immune cells; erythrocytes; BMSCs; BM endothelial cells (ECs); as well as osteoclasts and osteoblasts. These cells not only physically interact with MM cells, but also secrete growth and/or anti-apoptotic factors, such as interleukin (IL)-6, insulin-like growth factor (IGF)-1, vascular endothelial growth factor (VEGF), and tumor necrosis factor (TNF)-α, stromal cell-derived factor (SDF) 1α, and B-cell activating factor (BAFF). The interaction of these cellular components with growth/anti-apoptotic factors, several proliferative/anti-apoptotic signaling cascades in MM cells: phosphatidylinositol-3 kinase (PI3K)/Akt; Ras/Raf/mitogen-activated protein kinase (MAPK) kinase (MEK)/extracellular signal-related kinase (ERK); Janus kinase (JAK) 2/signal transducers and activators of transcription (STAT)-3; and nuclear factor (NF)-κB. These signaling cascades activate downstream target kinases and/or transcription factors which in turn regulate MM cell cycle progression, proliferation, and anti-apoptosis. Importantly, cytokines secreted from MM cells and BMSCs in turn further augment these signaling pathways 1–3. Therefore, cytokines, their receptors, transcription factors and protein kinases represent potential targets for novel therapies (Figure 1).
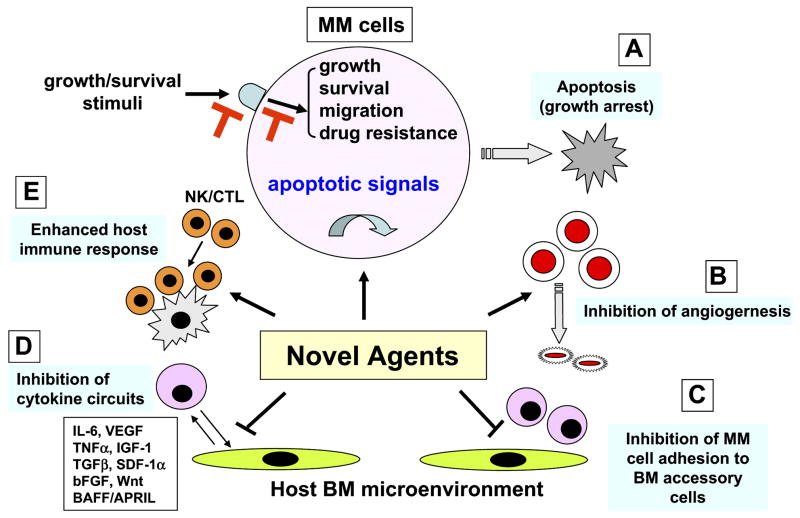
Figure 1.
Novel biologically-based therapies targeting MM cells and the BM microenvironment. Novel agents A. directly inhibit MM cell growth; B. inhibit angiogenesis; C. inhibit MM cell adhesion to BM accessory cells; D. decrease cytokine production and sequelae in the BM microenvironment; and E. enhance host anti-MM immunity.
Targeting growth factors and their receptors
1. IL-6
IL-6 mediates autocrine and paracrine growth of MM cells within the BM milieu (Figure 1). Specifically, some MM cells spontaneously secrete IL-6, and IL-6 secretion can be induced by CD 40 activation of tumor cells 4 or by cytokines (TNFα, VEGF, IL-1) within the BM microenvironment 5,6. Most IL-6 in the BM milieu is secreted by BMSCs; importantly, transcription and secretion of IL-6 in BMSCs is upregulated both by binding of MM cells to BMSCs 7,8 and by secretion of cytokines (VEGF, TGF-β, TNFα) from MM cells 9–11. IL-6-induced proliferation is associated with activation of Ras/Raf/mitogen-activated protein kinase kinase (MEK)/p42/44 MAPK signaling cascade 12,13, and can be abrogated by either MAPK antisense oligonucleotide or by the ERK or MEK inhibitor 14. Survival of MM cells triggered by IL-6 is conferred via Janus kinase2 (JAK2)/signal transducers and activators of transcription (STAT) 3 signaling and downstream induction of Bcl-xL 15 and Mcl-1 expression 16,17. IL-6 triggered drug (dexamethasone, Dex) resistance is mediated via phosphatidylinositol-3 kinase (PI3-K)/Akt signaling cascade, which can be neutralized by PI3K inhibitors (ie, wartmannin or LY294002). Specifically, Dex-mediated MM apoptosis is not associated with mitochondrial cytochrome c release 18, but is mediated by Second mitochondria activator of caspase (Smac) release, from mitochondria 19; cytosolic Smac disrupts the inhibitor of apoptosis XIAP/caspase-9 complex, thereby allowing activation of caspase-9, caspase-3 cleavage, and apoptosis. IL-6 inhibits apoptosis triggered by Dex via PI3-K/Akt signaling 20. We have used gene microarray profiling both to further delineate these cytokine-induced growth and anti-apoptotic pathways, and to derive targeted therapeutic strategies to overcome drug resistance based upon interrupting growth or triggering apoptotic signaling cascades 21. For example, these studies have demonstrated that IL-6 induces the XBP-1 transcription factor 22, which is implicated in differentiation of normal B cells to plasma cells 23,24 and is markedly upregulated in freshly isolated MM patient samples.
Clinically, serum IL-6 and IL-6 receptors are prognostic factors which reflect the proliferative fraction of MM cells 25–27. IL-6 or CRP, either alone or coupled with serum β2 microglobulin (β2m) as a measure of MM cell mass 28, provide one example of a biologically-based staging system in MM. Attempts to target IL-6 in treatment strategies to date have included antibodies to IL-6 and IL-6 receptor as well as IL-6 superantagonists (ie, Sant7) 29,30 which bind to IL-6R but do not trigger downstream signaling; although in vivo anti-MM activities have been observed, to date responses have only been transient.
2. IGF1
Insulin-like growth factor-1 is a multifunctional peptide that regulates cell proliferation, differentiation, and apoptosis 31,32. In the circulation, IGF-1 binds mainly to the main IGF binding protein (IGFBP-3). Several studies suggest that high concentrations of circulating IGF-1 are associated with an increased risk of prostate, breast, lung, and colorectal cancer, whereas high IGFBP-3 concentrations are associated with a decreased risk 32. However, the direct relationship of serum IGF-1 level and prognosis in MM has not yet been clarified. Standal et al reported that the mean IGF-1 level did not differ between MM patients and controls. However, IGF-1 was a strong indicator of prognosis: median survival of patients with low levels (<13 nmol/l) of serum IGF-1 had not been reached at 80 months 33. Previous studies have delineated the biological sequelae of IGF-1 in MM cells. Specifically, IGF-1 augments the proliferative and anti-apoptotic effects of IL-6 34. In contrast to IL-6, IGF-1 activates only Ras/Raf/MAPK kinase/ERK and PI3K/Akt signalling, but not JAK2/STAT3 pathways, via type1 IGF receptor (IGF1R) 35.
IGF-1 stimulates sustained activation of PI3K/Akt and NFjB; induces phosphorylation of FKHR (forkhead) transcription factor; upregulates a series of intracellular anti-apoptotic proteins including FLIP, survivin, cIAP-2, A1/Bfl-1, and XIAP; as well as decreases drug sensitivity of MM cells 36. IGF-1 primes MM cell responsiveness to IL-6 and stimulates production of angiogenic cytokines 37. Importantly, it is more potent than IL-6 in mediating these effects, setting the stage for novel MM treatments targeting IGF-1. IGF-1 also mediates MM cell migration via activation of PI3K/Akt signalling cascade 38. The anti-apoptotic effect of IGF-1 has also been studied using an in vitro model system of MM cells in the BM milieu. Specifically, IGF-1 inhibits Dex-induced apoptosis in MM cell lines, without altering Bcl-2 or Bcl-XL proteins, associated with activation of ERK and PI3K/Akt signalling pathways 36. IGF-1 mediates MM cell growth and survival in MM cells both in vitro 34 and in vivo 31. Recently, we showed that caveolin-1, which is usually absent in blood cells, is expressed in MM cells and plays a crucial role in both IL-6 and IGF-1-mediated signalling cascades 39. Preclinical studies of IGF1R targeted strategies have shown efficacy comparable with that of other antineoplastic strategies, i.e. proteasome inhibitors and IMiDs, which have proven to be clinically useful 32. Small-molecule IGF1R kinase inhibitor NVP-ADW742 31, anti-IGF1R antibodies, or anti-IGF-1 ligand antibodies, will be evaluated in clinical trials in several cancers, including MM 40.
3. VEGF
VEGF is a known angiogenic factor in both solid tumors and haematological malignancies 41. In MM, VEGF is produced both by MM cells and BMSCs and may account, at least in part, for the increased angiogenesis in MM patient BM. Our recent studies show that VEGF triggers ERK activation, proliferation, and migration of MM cells 42,43, which can be neutralised by VEGF receptor tyrosine kinase inhibitors PTK787 44 and GW654652 45. VEGF also triggers Src-dependent phosphorylation of caveolin-1, which is required for p130Cas phosphorylation and MM cell migration 46. Recently, we have shown that VEGF upregulates Mcl-1 expression in MM cell lines and MM patient cells; conversely, pan-VEGF inhibitor GW654652 inhibits VEGF-induced upregulation of Mcl-1, associated with decreased proliferation and induction of apoptosis 47.
We have also shown that a VEGF receptor inhibitor pazopanib (GW786034B) inhibits VEGF-triggered signaling pathways in both tumor and endothelial cells 48. Humanized monoclonal antibody against VEGF Bevacizumab (Avastin) was recently approved by the FDA for the therapy of metastatic colorectal cancer, and ongoing studies in MM are evaluating the efficacy of bevacizumab, with or without thalidomide, in patients with relapsed or refractory MM 41.
4. FGF
MM cells express and secrete bFGF, which contributes to the increased angiogenic potential of BM plasma cells in progressive MM 49. BMSCs from MM patients and control subjects express high-affinity FGF receptors R1–R4. Importantly, stimulation of BMSCs with bFGF induces a time- and dose-dependent increase in IL-6 secretion; conversely, stimulation with IL-6 enhances bFGF expression and secretion by MM cell lines, as well as MM patient cells 50. In MM, dysregulation of fibroblast growth factor receptor 3 (FGFR3) by the t(4;14) translocation is a primary event in 10–20% MM patients and confers poor prognosis 51–54. As a surface receptor, FGFR3 can be targeted by monoclonal antibodies 55,56 or be inhibited by selective tyrosine kinase inhibitors (SU5402, SU10991, PD173074, or PKC412) 57,58. Preclinical studies have validate FGFR3 as a therapeutic target in t(4;14) MM, and FGFR3 inhibitors are currently under clinical evaluation to improve prognosis of this patient subgroup.
5. BAFF
B-lymphocyte stimulating factor (Blys) is a TNF family member, which plays a critical role for maintenance of normal B-cell development and homeostasis. B-cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL), another TNF family members sharing significant homology, are both expressed on MM cells 59,60. Three receptors for BAFF have been identified: B-cell maturation antigen (BCMA), transmembrane activator and calcium-modulating cyclophilin ligand interactor (TACI), and BAFF-receptor. TACI and BCMA can also bind to APRIL, whereas BAFF-R is specific for BAFF. It has been shown that the serum levels of BAFF and APRIL are increased in patients with MM 61. BAFF and APRIL promote MM cell growth and activate NF-κB, PI3K/Akt, and Ras/Raf/MAPK pathways with upregulation of Mcl-1 and Bcl- 2 anti-apoptotic proteins, leading to protection of MM cells against Dex-induced apoptosis 59. Therefore blockade of BAFF/BAFR axis represents a potential therapeutic target.
6. Wnt
Wnt signalling regulates various developmental processes and can lead to malignant transformation. Wnts are a family of secreted cysteine-rich glycoproteins that act as short-range ligands locally and bind to frizzled transmembrane receptors. Intracellularly, a canonical Wnt/β-catenin signaling cascade inhibits GSK-3β activity, thereby blocking β-catenin phosphorylation and degradation by proteasomes. In MM, Wnt/β-catenin pathway is activated following treatment with Wnt-3a. MM cells highly express β-catenin, which is consistent with active β-catenin/T-cell factor (TCF)-mediated transcription 62. Further accumulation and nuclear localisation of β-catenin, and/or increased cell proliferation, is achieved by stimulation of Wnt signaling with either the Wnt-3a or the constitutively active mutant of β-catenin 62. Recent studies have shown that inhibition of β-catenin and TCF-4 interaction by PKF115-584 induces cytotoxicity in both patient MM cells and MM cell lines and mouse xenograft models of human MM 63.
In the BM microenvironment, Wnt signaling is involved in osteblastogenesis. MM cells in patient BM-biopsy specimens express dickkopf 1 (DKK1), a negative regulator of the Wnt/β-catenin signaling cascade 64. Moreover, elevated DKK1 levels in BM plasma and peripheral blood from patients with MM correlate with DKK1 gene expression patterns and were associated with focal bone lytic lesions 65. Importantly, recent studies have shown that anti-DKK1 neutralizing Ab increases numbers of osteocalcin-expressing osteoblasts and bone mineral density of implanted bone in SCID mice 66.
7. CD40
CD40 is a TNFα super family member. CD40 ligand (L) triggers p53-dependent MM cell proliferation, as well as PI3K/Akt/NFκB-dependent migration in MM cells 67,68. In BMSCs, CD40 triggers secretion of IL-6 and VEGF, which further promotes MM cell growth in the BM milieu. Therefore, inhibition of CD40-CD40L interaction is a possible therapeutic strategy in MM. Indeed, anti-CD40 antibodies (SGN-40, CHIR-12.12) modestly inhibit MM cell proliferation 69. Importantly, these antibodies can induce antibody-dependent cell-mediated cytotoxicity (ADCC) against CD40-positive MM cells, which can be further enhanced by lenalidomide 70,71.
8. Others
Serotherapy directed against CD20 targets only a minority of MM patient tumor cells, since CD20 expression is not common in MM (20% CD20+). The anti-CD20 monoclonal antibody (Rituximab) achieved response in 32% previously treated MM patients, all of whom had CD20+ tumor cells 72. CS1 (CD2 subset 1) is a member of the CD2 family of cell surface glycoproteins and highly expresses on myeloma cells. Recent studies have shown that a novel humanized anti-CS1 mAb, HuLuc63, induces significant ADCC against MM cells including drug-resistant cells, and inhibited their interaction with BMSCs 73
Targeting intracellular molecules
1. Proteasome
Ubiquitin-proteasome pathway is a protein degradation system which maintains intracellular protein homeostasis. It plays a central role in the targeted degradation of cellular proteins, including cell cycle regulatory proteins and apoptosis associated proteins. Ubiquitin is a small protein (76 amino acids). The C-terminus of ubiquitin forms an isopeptide bond with the amino group of a lysine side chain in a target protein. After attaching multiple copies of ubiquitin to target proteins, the protein will be degraded by 26S proteasome, which consists of a proteolytic core, the 20S proteasome, sandwiched between two 19S regulatory complexes. The 20S proteasome has multiple active sites, including caspase-like, trypsin-like, and chymotrypsin-like sites. Since Ubiquitin-proteasome pathway is crucial for survival of cancer cells, its inhibition represents a novel therapeutic strategy in cancer. The proteasome inhibitors are classified as reversible and irreversible according to their inhibition of chymotrypsin-like, trypsin-like, and/or caspase-like activities. Bortezomib is a reversible inhibitor of chymotrypsin-like activity, and has demonstrated significant anti-tumor activity in preclinical and clinical studies in MM.
a. Bortezomib (Velcade®)
Bortezomib (N-pyrazinecarbonyl-L-phenylalanine-L-leucine boronicacid) is a boronic acid dipeptide which inhibits β1, β1i, and β5 subunits of the 20S proteasome core in the 26S proteasome complex 74. The initial rationale to use bortezomib in MM is its inhibitory effect of NF-κB, which plays a crucial role in the pathogenesis in cancer cells including MM. The NF-κB complex is a dimer of different combinations of Rel family proteins, including p65 (RelA), RelB, c-Rel, p50 (NF-κB1), and p52 (NF-κB2). Recent studies have revealed that NF-κB activity is mediated via two distinct pathways. In the canonical pathway, NF-κB is typically a heterodimer composed of p50 and p65 subunits 75, and its activity is regulated by association with IκB family proteins 76. Following stimulation by various factor, including cytokines (ie, TNFα, IL-1β, IGF-1), IκB protein is phosphorylated by IκB kinase (IKK), typically IKKβ. Phosphorylated IκB is subsequently poly-ubiquitinated and degraded by the 26S proteasome 77,78, which allows p50/p65 NF-κB nuclear translocation. Bortezomib inhibits degradation of IκB and blocks NF-κB activity.
Although NF-κB is a major target of bortezomib, it also has other target molecules. First, it directly induces apoptosis of human MM cell lines and freshly isolated patient MM cells despite induction of p53-independent p21Cip1 and p27Kip1. Second, it triggers apoptosis even in drug resistant cells, and adds to the anti-MM activity of Dex. Importantly, IL-6 and other growth factors do not overcome bortezomib -induced apoptosis, which is triggered by activation of caspase-3 via caspase-8/9 79,80. Third, bortezomib cleaves DNA repair enzymes (DNA-PKcs, ATM) 81,82, and enhances sensitivity of MM cells to conventional chemotherapeutic agents, especially to DNA damaging agents (ie, doxorubicin, melphalan) 81. Forth, previous studies have also shown that normal plasma cells, as well as MM cells, produce and secrete abundant immunoglobulins, which require a highly developed endoplasmic reticulum (ER) and chaperone proteins (ie, heat shock proteins (Hsps)) that effect proper translation and folding. The unfolded protein response ensures that the plasma cells can catabolize immunoglobulins, therefore proteasome inhibition is an ideal novel therapeutic strategy for MM 83. Fifth, bortezomib induces a stress response in MM cells. For example, bortezomib upregulates HSPs and c-Jun NH2-terminal kinase (JNK) which mediate apoptosis triggered by unfolded proteins. While bortezomib directly induces caspase-dependent apoptosis, it also targets the BM microenvironment. Specifically, in MM cells, it triggers downregulation of gp130 84, which is phosphorylated after IL-6 binding to its receptor, thereby inhibiting phosphorylation of ERK, STAT3, and Akt induced by either IL-6 or by binding of MM cells to BMSCs. Sixth, bortezomib also inhibits VEGF-triggered caveolin-1 phosphorylation and markedly decreases caveolin-1 expression, thereby inhibiting VEGF-induced MM cell migration 46. Seventh, expression of adhesion molecules (ie, ICAM-1, VCAM-1) on both MM cells and BMSCs is also regulated by NF-κB, inhibition of NF-κB by bortezomib decreases adhesion and thereby enhances susceptibility of MM cells to therapeutic agents 85,86 (Figure 2). Importantly, bortezomib also inhibits the paracrine growth of human MM cells in the BM milieu by decreasing their adherence to BMSCs and related NF-κB dependent induction of IL-6 secretion in BMSCs (Figure 1).
Figure 2.
Cell surface and intracellular targets of novel therapeutic agents. Novel agents block signaling cascade triggered by MM cell-BM accessory cell interaction and induce growth inhibition in the BM microenvironment. Novel agents; inhibit interaction of cytokines/growth factors and their receptors expressed on MM cell; inhibit receptor tyrosine kinase activity; intracellular molecules (kinases, anti-apoptotic proteins, molecular chaperons, transcription factors).
Most recently, the effects of bortezomib in bone remodeling, specifically on osteoblasts and osteoclasts, have been reported 87,88. Bortezomib significantly induced a stimulatory effect on osteoblast markers in human mesenchymal cells without affecting the number of osteoblast progenitors in bone marrow cultures or the viability of mature osteoblasts, associated with upregulated Runx2/Cbfa1 activity in human osteoblast progenitors and osteoblasts. Importantly, numbers of osteoblastic cells was significantly increased by bortezomib. Specifically, Runx2/Cbfa1-positive osteoblastic cells was observed in MM patients responded to bortezomib treatment 88. Moreover, bortezomib inhibited osteoclast differentiation and bone resorption activity. The mechanisms of action targeting early osteoclast differentiation was related to the inhibition of p38 MAPK pathways, whereas targeting the later phase of differentiation and activation was due to inhibition of p38 MAPK, AP-1 and NF-κB activation 89.
Other proteasome inhibitors
NPI-0052 is a novel proteasome inhibitor from Salinospora tropica, a marine actinomycete. Although bortezomib only blocks chymotryptic activity, NPI-0052 inhibits chymotryptic, trypsin-like and caspase-like activities. NPI-0052-induced cytotoxicity is predominantly triggered by caspase-dependent apoptosis. It induces cytotoxicity in MM cells resistant to conventional agents. Importantly, it is also able to overcome bortezomib resistance in vitro 90. NPI-0052 triggers reactive oxygen species/caspase-8-dependent apoptosis, which can be enhancd by histone deacetylase inhibitor in ALL cells 91.
PR-171 is another novel epoxyketone-based irreversible proteasome inhibitor, which primarily inhibits chymotriptic activity of 20S proteasome. It triggers JNK/caspase-dependent apoptosis. In comparison to bortezomib, PR-171 exhibits equal potency but greater selectivity for the chymotrypsin-like activity of the proteasome. In cell culture, PR-171 is more cytotoxic than bortezomib following brief treatments that mimic the in vivo pharmacokinetics of both molecules.92. Multicenter phase I studies to evaluate the safety, tolerability, and clinical response to intensive dosing with PR-171 in patients with relapsed or refractory hematological malignancies has already been reported. In this study, 51 patients are enrolled, and 17 out of 21 myeloma patients were previously treated with bortezomib; importantly, 4 myeloma patients responded to PR-171 treatment (PR, 19%) 93.
2. Lenalidomide (Revlimid®)
Although lenalidomide, an immunomodulatory derivative of thalidomide has multiple mechanisms of anti-MM activities: including directly inducing G1 growth arrest or apoptosis; inhibits MM cell adherence to BMSCs; decreasing production of cytokines; inhibiting BM angiogenesis which is increased in MM patients; and enhancing anti-MM immunity with stimulation of T cell and natural killer cell responses. We and others have recently studied the mechanism of anti-MM activity of thalidomide derivatives known as immunomodulatory drugs (IMiDs), which have significantly higher potency at inducing apoptosis or growth arrest in MM cells resistant to melphalan, doxorubicin and dexamethasone 94. The IMiDs reduce the secretion of IL-6 and VEGF triggered by the binding of MM cells to BMSCs, and inhibit angiogenesis 11. We and others demonstrated that the IMiDs stimulated T-cell proliferation via T cell co-stimulatory mechanism. Specifically, IMiDs trigger tyrosine phosphorylation of CD28 on T cells, followed by activation of nuclear factor of activated T cell 2 (NFAT2) and production of IL-2 70,95. Moreover, IMiDs induce NK cell cytotoxicity, since both NK cell proliferation and antibody-dependent cell-mediated cytotoxicity (ADCC) activity were enhanced by IL-2 production from T cells triggered by IMiDs 70,71,96 (Figure 2). These data provide the cellular and molecular basis for use of IMiDs as an adjuvant in immunotherapeutic treatment strategies for MM.
3. Histone deacetylase (HDAC)
HDAC inhibitors are members of novel class of anti-tumor agents for malignancies, and a large number of structurally diverse HDAC inhibitors have been purified from natural sources or synthetically developed. HDAC inhibitors can be divided into six classes based on their chemical structure. These classes are short-chain fatty acid, hydroxamate, benzamide, cyclic tetrapeptide, electrophilic ketone and the others 97. Accumulated histone acetylation by HDAC inhibitors attenuates their electrostatic interaction with the negatively charged DNA backbone, promoting the unfolding of histone–DNA complex, thereby modulating access of transcription factors to their binding sites of action and transcription of their target genes 98–100 (Figure 1). Previous studies have shown that deletions or inactivating mutations of HATs which decrease histone acetylation are involved in development of human neoplasms 101,102. In contrast, inhibition of HDAC activity triggers growth arrest and/or apoptosis of tumor cells. Possible mechanisms of anti-tumor activities of HDAC inhibitors have recently been comprehensively described 97; however, their mechanisms of growth inhibitory effects in MM cells have not yet been fully characterized.
a. Suberoylanilide hydroxamic acid (SAHA)
SAHA is prototype class I, II HDAC inhibitor which directly interacts with the catalytic site of HDAC like protein and inhibits its enzymatic activity. Inhibition of HDAC activity by SAHA therefore results in alteration of gene expression in various cell types including MM 103. Like other HDAC inhibitors, SAHA upregulates p21WAF1 expression 104,105, thereby inhibiting tumor cell growth. In MM, SAHA: modulates gene expression and inhibits of tumor cell growth 103,106; induces upregulation of p21WAF1; upregulates p53 protein expression; and dephosphorylates Rb, followed by apoptosis. Importantly, upregulation of p21WAF1 occurs prior to p53 induction, suggesting that p21WAF1 upregulation is independent of p53 activity 103. SAHA-induced apoptosis in MM cells is associated with Bcl-2 interacting protein Bid; conversely, overexpression of Bcl-2 blocks SAHA-induced apoptosis, suggesting that Bcl-2 plays a crucial role regulating SAHA-induced apoptosis in MM cells. Interestingly, SAHA does not trigger caspase activation, and the caspase inhibitor does not protect against SAHA-induced cytotoxicity. However, poly (ADP) ribose polymerase (PARP) is significantly cleaved by SAHA, suggesting that SAHA triggers atypical PARP cleavage in MM cells 103. Importantly, SAHA suppresses expression and activity of the proteasome and its subunits, providing the rationale for its use in combination with bortezomib to enhance its cytotoxicity 106. It has also shown that SAHA enhances tumour necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced cytotoxicity, associated with upregulation of the proapoptotic proteins (Bim, Bak, Bax, Noxa, and PUMA) and downregulation of anti-apoptotic proteins (Bcl-2 and Bcl-xL) 107.
b. MVP-LAQ824 (LAQ824)
LAQ824 is a member of hydroxamate HDAC inhibitor which blocks class I and II HDAC activity. LAQ824 inhibits proliferation of cancer cell lines with IC50s of 10–150nM ranges in vitro, indicating that anti-proliferative potency of LAQ824 is up to 200-fold higher than that of SAHA. 108,109. Anti-tumor activity of LAQ824 has been extensively studied in leukemia cells 110–114. In MM, LAQ824 induces apoptosis at IC50 of 100 nM at 24 hour in most MM cell lines and patient tumor cells. Importantly, LAQ824 is effective in cells which are resistant to conventional therapies (dexamethasone, doxorubicin, melphalan). Moreover, LAQ824 inhibits cell growth in vivo in a preclinical murine myeloma model. Unlike SAHA, LAQ824-induced apoptosis is associated with caspase activation 115.
c. LBH589
LBH589 is a hydroxamic acid analog which blocks class I and II HDAC activity. LBH589 has been studied in many malignancies as a single agent, as well as combined with other anticancer agents 116–119. LBH589 has also been shown to inhibit angiogenesis in vitro 120. In MM, LBH589 blocks cell cycle progression, associated with upregulation of p21WAF1, p53, and p57, and induces cytotoxicity through an increase in mitochondrial outer membrane permeability 121. The IC50 of LBH589 is 40–80 nM in most MM cell lines 121,122. LBH589-induced cytotoxicity is associated with caspase/PARP cleavage; however, interestingly, LBH589 also triggers a caspase-independent apoptotic pathway through the release of apoptosis-inducing factor (AIF) from mitochondria 121. Synergistic cytotoxicity against MM cells is observed with LBH589 in combination with bortezomib 122. Phase II clinical trials of LBH589 are ongoing in MM, and a clinical trial of bortezomib with LBH589 to block proteasomal and aggresomal breakdown of protein, respectively, is soon to begin.
e. Other HDAC inhibitors
Tubacin is a hydroxamic acid HDAC inhibitor and inhibits only HDAC6 activity 123. Previous studies have characterized the aggresome as an alternative system to the proteasome for degradation of polyubiquitinated proteins. The aggresome pathway therefore likely provides a novel system for delivery of aggregated proteins from the cytoplasm to lysosomes for degradation 124. In this aggresomal protein degradation pathway, HDAC6 has an essential role, since it can bind both polyubiquitinated proteins and dynein motors, thereby acting to recruit protein cargo to dynein motors for transport to aggresomes 125. We have demonstrated that blockade of both proteasomal and aggresomal protein degradation by bortezomib and tubacin, respectively, synergistically enhances cytotoxicity in MM cells in vitro 126. Depsipeptide (FR901228, FK228) is a class of cyclic tetrapeptide and inhibits only class I HDAC activity 127. Depsipeptide induces apoptosis in MM cell lines and in primary patient tumor cells, associated with downregulation of Bcl-2, BCL-xL and Mcl-1 expression 128. PXD101 is a hydroxamate class HDAC inhibitor 129 which has antiproliferative activity in MM cell lines, and shows additive and/or synergistic effects with conventional agents used in MM. MS-275 belongs to the benzamide class and inhibits class I and II HDACs. KD5170 is non-hydroxamate, orally bioavailable HDAC inhibitor which significantly inhibits osteoclast formation at lower μM range and triggers apoptosis in MM cells 130.
4. Heat shock protein (Hsp) 90
Hsp90 is a molecular chaperone which facilitates intracellular protein trafficking, conformational maturation, and 3-dimensional folding required for protein function. Intracellular overexpression of Hsp90 proteins are observed in most MM tumor cells, but not in monoclonal gammopathy of undetermined significance (MGUS) or in normal plasma cells 131. The ansamycin antibiotic geldanamycin (GA) and its analogs bind to the critical ATP-binding site of Hsp90, thereby abrogating its chaperoning activity in the MM BM milieu; decreasing IGF-1R and IL-6R expression on MM cells; depleting growth kinases (e.g., Akt, IKK, Raf) and anti-apoptotic proteins (FLIP, XIAP, cIAP, telomerase); as well as inhibiting both constitutive and cytokine-induced activation of NF-κB and telomerase (hTERT) in the BM milieu 132. GA and other Hsp90 inhibitors induce apoptosis of MM cell lines and patient cells which are resistant to Dex, anthracyclines, Thal or IMiDs, TRAIL/Apo2L, and bortezomib. Moreover, a geldanamycin analog 17-AAG suppresses in MM cells the expression and/or function of multiple levels of insulin-like growth factor receptor (IGF-1R) and interleukin-6 receptor (IL-6R) signaling (eg, IKK/NF-κB, PI-3K/Akt, and Raf/MAPK) and downstream effectors (eg, proteasome, telomerase, and HIF-1α activities)in MM cells 132. Most recently, Hsp90 inhibitors have been reported to induce myeloma cell death, at least in part, via ER stress and the unfolded protein response death pathway 133.
IPI-504 is a hydroquinone hydrochloride derivative of 17-AAG. In MM, IPI-504 inhibits MM cell growth in vitro and in mouse models. Like other Hsp90 inhibitors, IPI-504 synergistically enhances cytotoxicity of bortezomib 134. 17-dimethylaminoethylamino-17-demethoxygeldanamycin hydrochloride (17-DMAG) is also a water soluble novel Hsp90 inhibitor 17-DMAG, which attenuates the levels of STAT3 and phospho-ERK, as well as decreases the viability of MM cells 131.
5. Akt (protein kinase B)
Akt signaling mediates MM cell resistance to conventional therapeutics 20,36,135, therefore, biologically-based treatments targeting Akt are a promising therapeutic strategy in MM. Perifosine is a synthetic novel alkylphospholipid which inhibits Akt activation. In MM cells, we have shown that Perifosine inhibits both baseline and cytokine (IL-6, IGF-1)-triggered Akt activation. Importantly, Perifosine triggers significant cytotoxicity even of MM cells adherent to BM stromal cells (SCs) and therefore overcomes CAM-DR. Furthermore, Perifosine augments both conventional agent- and bortezomib-induced MM cell cytotoxicity. Importantly, we have also demonstrated in vivo anti-MM activity of Perifosine in a human plasmacytoma mouse model, associated with downregulation of Akt phosphorylation in tumor cells 136. Perifosine has been shown to induce selective apoptosis in MM cells by recruitment of death receptors, such as TNF-related apoptosis-inducing ligand (TRAIL)-R1/DR4 and TRAIL-R2/DR5 137. Most recently, we have shown that Perifosine-indiced cytotoxicity is strongly associated with downregulation of survivin 138.
6. Mammalian target of rapamycin (mTOR)
mTOR is a serine/threonine protein kinase that regulates transcription, cell proliferation, and survival. Inhibition of mTOR by its inhibitors therefore induces potent cytotoxicity in MM cells 139,140. Specifically, rapamycin induced G0/G1 arrest, associated with an increase of the cyclin-dependent kinase inhibitor p27 and a decrease of cyclins D2 and D3 in MM cells 141.. Interestingly, PTEN-negative myeloma cells are more sensitive to mTOR inhibition that PTEN-positive cells 142. Rapamycin shows synergistic cytotoxicity in combination with dexamethasone Stromberg, 2004 #5217} and lenalidomide 143. CCI-779 is a rapamycin analog which demonstrates inhibition of proliferation and induction of apoptosis, associated with cyclin D1 and c-myc downregulation and up-regulation of p27Kip1 in OPM-2 cells 144. Moreover, CCI-779 downregulates VEGF translation, and ultimately blocks angiogenesis 145.
7. MAPK kinase (MEK)
MEK/ERK pathway is one of the major signaling cascades which can be activated by many cytokines (ie, IL-6, IGF-1, SDF1α, BAFF) in MM cells. We have shown that inhibition of ERK by antisense oligonucleotide blocks MM cell proliferation 12,13. Therefore inhibition of MEK/ERK signaling is a promising therapeutic strategy.
Recent studies have shown that clinical grade novel MEK1/2 inhibitor AZD6244 (ARRY-142886) induces apoptosis in MM cell lines and patient MM cells, associated with caspase-3 activation. Importantly, AZD6244 down-regulates the expression/secretion of osteoclast (OC)-activating factors from MM cells and inhibits in vitro differentiation of MM patient PBMCs to OCs 14.
8. Bcl2 and Bcl-xL
Bcl2 family members have a crucial role in protecting cells from apoptotic stimuli. In MM, Bcl-2 antisense oligonucleotide (G3139) 146,147, and Bcl2/Bcl-XL inhibitor (ABT-737) 148,149 induce strong anti-MM activities as single agents and in combination with Dex 150.
Future directions
Although each of novel agent demonstrates significant preclinical anti-MM activity in vitro and using an in vivo mouse model of human MM, treatment with single agents may not achieve sufficient clinical efficacy. Therefore, treatments combining novel agents with conventional and/or novel agents to overcome clinical drug resistance are required. Among these combination therapies, thalidomide with dexamethasone, bortezomib with dexamethasone, and bortezomib with doxorubicin have shown promising results in clinical studies based upon our preclinical studies. Our recent preclinical studies indicate that other novel agents enhance cytotoxicity induced by conventional agents. For example, bortezomib induces stress response-related proteins such as heat shock proteins hsp27, hsp70, and hsp90. Blockade of Hsp90 or Hsp27 by their inhibitors restores sensitivity to bortezomib. Recent studies have demonstrated that unfolded and ubiquitinated proteins are degraded not only by proteasomes, but also by aggresomes dependent on HDAC6 activity. Inhibition of both proteasome and aggresome mechanisms using bortezomib and HDAC6 specific inhibitor tubacin induces accumulation of ubiquitinated proteins, followed by significant cell stress and cytotoxicity in MM cells. Most recently, we demonstrated that the potent Akt inhibitor perifosine augments bortezomib-induced cytotoxicity in MM. These preclinical studies of combination therapies of bortezomib with novel agents provide the rational framework for clinical evaluation of these treatment options.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hideshima T, Anderson KC. Molecular mechanisms of novel therapeutic approaches for multiple myeloma. Nat Rev Cancer. 2002;2:927–937. doi: 10.1038/nrc952. [DOI] [PubMed] [Google Scholar]
- 2.Hideshima T, Bergsagel PL, Kuehl WM, Anderson KC. Advances in biology of multiple myeloma: clinical applications. Blood. 2004;104:607–618. doi: 10.1182/blood-2004-01-0037. [DOI] [PubMed] [Google Scholar]
- 3.Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7:585–598. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- 4.Chauhan D, Kharbanda S, Ogata A, Urashima M, Frank D, Malik N, et al. Oncostatin M induces association of Grb2 with Janus Kinase JAK2 in multiple myeloma cells. Journal of Experimental Medicine. 1995;182:1801–1806. doi: 10.1084/jem.182.6.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costes V, Portier M, Lu ZY, Rossi JF, Bataille R, Klein B. Interleukin-1 in multiple myeloma: producer cells and their role in the control of IL-6 production. Br J Haematol. 1998;103:1152–1160. doi: 10.1046/j.1365-2141.1998.01101.x. [DOI] [PubMed] [Google Scholar]
- 6.Lust JA, Donovan KA. The role of interleukin-1 beta in the pathogenesis of multiple myeloma. Hematol Oncol Clin North Am. 1999;13:1117–1125. doi: 10.1016/s0889-8588(05)70115-5. [DOI] [PubMed] [Google Scholar]
- 7.Uchiyama H, Barut BA, Mohrbacher AF, Chauhan D, Anderson KC. Adhesion of human myeloma-derived cell lines to bone marrow stromal cells stimulates IL-6 secretion. Blood. 1993;82:3712–3720. [PubMed] [Google Scholar]
- 8.Chauhan D, Uchiyama H, Akbarali Y, Urashima M, Yamamoto K, Libermann TA, et al. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kB. Blood. 1996;87:1104–1112. [PubMed] [Google Scholar]
- 9.Urashima M, Ogata A, Chauhan D, Hatziyanni M, Vidriales MB, Dedera DA, et al. Transforming growth factor b1: Differential effects on multiple myeloma versus normal B cells. Blood. 1996;87:1928–1938. [PubMed] [Google Scholar]
- 10.Dankbar B, Padro T, Leo R, Feldman B, Kropff M, Mesters RM, et al. Vascular endothelial growth factor and interleukin-6 in paracrine tumor-stromal cell interactions in multiple myeloma. Blood. 2000;95:2630–2636. [PubMed] [Google Scholar]
- 11.Gupta D, Treon SP, Shima Y, Hideshima T, Podar K, Tai YT, et al. Adherence of multiple myeloma cells to bone marrow stromal cells upregulates vascular endothelial growth factor secretion: therapeutic applications. Leukemia. 2001;15:1950–1961. doi: 10.1038/sj.leu.2402295. [DOI] [PubMed] [Google Scholar]
- 12.Ogata A, Chauhan D, Teoh G, Treon SP, Urashima M, Schlossman RL, et al. Interleukin-6 triggers cell growth via the ras-dependent mitogen-activated protein kinase cascade. J Immunol. 1997;159:2212–2221. [PubMed] [Google Scholar]
- 13.Ogata A, Chauhan D, Urashima M, Teoh G, Treon SP, Anderson KC. Blockade of mitogen-activated protein kinase cascade signaling in interleukin-6 independent multiple myeloma cells. Clin Cancer Res. 1997;3:1017–1022. [PubMed] [Google Scholar]
- 14.Tai YT, Fulciniti M, Hideshima T, Song W, Leiba M, Li XF, et al. Targeting MEK induces myeloma cell cytotoxicity and inhibits osteoclastogenesis. Blood. 2007 doi: 10.1182/blood-2007-03-081240. in press. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, et al. Constitutive activation of STAT-3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- 16.Puthier D, Bataille R, Amiot M. IL-6 up-regulates mcl-1 in human myeloma cells through JAK/STAT rather than ras/MAP kinase pathway. Eur J Immunol. 1999;29:3945–3950. doi: 10.1002/(SICI)1521-4141(199912)29:12<3945::AID-IMMU3945>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 17.Epling-Burnette PK, Liu JH, Catlett-Falcone R, Turkson J, Oshiro M, Kothapalli R, et al. Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J Clin Invest. 2001;107:351–362. doi: 10.1172/JCI9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chauhan D, Pandey P, Ogata A, Teoh G, Krett N, Halgren R, et al. Cytochrome-c dependent and independent induction of apoptosis in multiple myeloma cells. J Biol Chem. 1997;272:29995–299957. doi: 10.1074/jbc.272.48.29995. [DOI] [PubMed] [Google Scholar]
- 19.Chauhan D, Hideshima T, Rosen S, Reed JC, Kharbanda S, Anderson KC. Apaf-1/cytochrome c independent and Smac dependent induction of apoptosis in multiple myeloma cells. J Biol Chem. 2001;276:24453–24456. doi: 10.1074/jbc.C100074200. [DOI] [PubMed] [Google Scholar]
- 20.Hideshima T, Nakamura N, Chauhan D, Anderson KC. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene. 2001;20:5991–6000. doi: 10.1038/sj.onc.1204833. [DOI] [PubMed] [Google Scholar]
- 21.Chauhan D, Auclair D, Robinson EK, Hideshima T, Li G, Podar K, et al. Identification of genes regulated by dexamethasone in multiple myeloma cells using oligonucleotide arrays. Oncogene. 2002;21:1346–1358. doi: 10.1038/sj.onc.1205205. [DOI] [PubMed] [Google Scholar]
- 22.Chauhan D, Li G, Auclair D, Hideshima T, Richardson P, Podar K, et al. Identification of genes regulated by 2-methoxyestradiol (2ME2) in multiple myeloma cells using oligonucleotide arrays. Blood. 2003;101:3606–3614. doi: 10.1182/blood-2002-10-3146. [DOI] [PubMed] [Google Scholar]
- 23.Iwakoshi NN, Lee AH, Vallabhajosyula P, Otipoby KL, Rajewsky K, Glimcher LH. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat Immunol. 2003;4:321–329. doi: 10.1038/ni907. [DOI] [PubMed] [Google Scholar]
- 24.Carrasco DR, Sukhdeo K, Protopopov M, Sinha R, Enos M, Carrasco DE, et al. The differentiation and stress response factor XBP-1 drives multiple myeloma pathogenesis. Cancer Cell. 2007;11:349–360. doi: 10.1016/j.ccr.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pulkki K, Pelliniemi TT, Rajamaki A, Tienhaara A, Laakso M, Lahtinen R. Soluble interleukin-6 receptor as a prognostic factor in multiple myeloma. Br J Haematol. 1996;92:370–374. doi: 10.1046/j.1365-2141.1996.d01-1470.x. [DOI] [PubMed] [Google Scholar]
- 26.Kyrtsonis MC, Dedoussis G, Zervas C, Perifanis V, Baxevanis C, Stamatelou M, et al. Soluble interleukin-6 receptor (sIL-6R), a new prognostic factor in multiple myeloma. Br J Haematol. 1996;93:398–400. doi: 10.1046/j.1365-2141.1996.4721018.x. [DOI] [PubMed] [Google Scholar]
- 27.Stasi R, Brunetti M, Parma A, Di Giulio C, Terzoli E, Pagano A. The prognostic value of soluble interleukin-6 receptor in patients with multiple myeloma. Cancer. 1998;82:1860–1866. [PubMed] [Google Scholar]
- 28.Tricot G, Spencer T, Sawyer J, Spoon D, Desikan R, Fassas A, et al. Predicting long-term (> or = 5 years) event-free survival in multiple myeloma patients following planned tandem autotransplants. Br J Haematol. 2002;116:211–217. doi: 10.1046/j.1365-2141.2002.03231.x. [DOI] [PubMed] [Google Scholar]
- 29.Tassone P, Galea E, Forciniti S, Tagliaferri P, Venuta S. The IL-6 receptor super-antagonist Sant7 enhances antiproliferative and apoptotic effects induced by dexamethasone and zoledronic acid on multiple myeloma cells. Int J Oncol. 2002;21:867–873. [PubMed] [Google Scholar]
- 30.Tassone P, Neri P, Burger R, Savino R, Shammas M, Catley L, et al. Combination therapy with IL-6 receptor super-antagonist Sant7 and dexamethasone induces antitumor effects in a novel SCID-hu in vivo model of human multiple myeloma. Clin Cancer Res. 2005;11:4251–4258. doi: 10.1158/1078-0432.CCR-04-2611. [DOI] [PubMed] [Google Scholar]
- 31.Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Shringarpure R, Akiyama M, et al. Inhibition of the insulin-like growth factor receptor-1 tyrosine kinase activity as a therapeutic strategy for multiple myeloma, other hematologic malignancies, and solid tumors. Cancer Cell. 2004;5:221–230. doi: 10.1016/s1535-6108(04)00050-9. [DOI] [PubMed] [Google Scholar]
- 32.Pollak MN. Insulin-like growth factors and neoplasia. Novartis Found Symp. 2004;262:84–98. [PubMed] [Google Scholar]
- 33.Standal T, Borset M, Lenhoff S, Wisloff F, Stordal B, Sundan A, et al. Serum insulinlike growth factor is not elevated in patients with multiple myeloma but is still a prognostic factor. Blood. 2002;100:3925–3929. doi: 10.1182/blood-2002-05-1406. [DOI] [PubMed] [Google Scholar]
- 34.Jelinek DF, Witzig TE, Arendt BK. A role for insulin-like growth factor in the regulation of IL-6-responsive human myeloma cell line growth. J Immunol. 1997;159:487–496. [PubMed] [Google Scholar]
- 35.Qiang YW, Kopantzev E, Rudikoff S. Insulinlike growth factor-I signaling in multiple myeloma: downstream elements, functional correlates, and pathway cross-talk. Blood. 2002;99:4138–4146. doi: 10.1182/blood.v99.11.4138. [DOI] [PubMed] [Google Scholar]
- 36.Mitsiades CS, Mitsiades N, Poulaki V, Schlossman R, Akiyama M, Chauhan D, et al. Activation of NF-kB and upregulation of intracellular anti-apoptotic proteins via the IGF-1/Akt signaling in human multiple myeloma cells: therapeutic implications. Oncogene. 2002;21:5673–5683. doi: 10.1038/sj.onc.1205664. [DOI] [PubMed] [Google Scholar]
- 37.Mitsiades CS, Mitsiades N, Kung AL, Shringapurne R, Poulaki V, Richardson PG, et al. The IGF/IGF-1R system is a major therapeutic target for multiple myeloma, other hematologic malignancies and solid tumors. Blood. 2002;100:170a. [Google Scholar]
- 38.Tai YT, Podar K, Catley L, Tseng YH, Akiyama M, Shringarpure R, et al. Insulin-like growth factor-1 induces adhesion and migration in human multiple myeloma cells via activation of beta1-integrin and phosphatidylinositol 3′-kinase/AKT signaling. Cancer Res. 2003;63:5850–5858. [PubMed] [Google Scholar]
- 39.Podar K, Tai YT, Cole CE, Hideshima T, Sattler M, Hamblin A, et al. Essential role of caveolae in interleukin-6- and insulin-like growth factor I-triggered Akt-1-mediated survival of multiple myeloma cells. J Biol Chem. 2003;278:5794–5801. doi: 10.1074/jbc.M208636200. [DOI] [PubMed] [Google Scholar]
- 40.Mitsiades CS, Mitsiades N, Munshi NC, Anderson KC. Focus on multiple myeloma. Cancer Cell. 2004;6:439–444. doi: 10.1016/j.ccr.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 41.Podar K, Anderson KC. The pathophysiological role of VEGF in hematological malignancies: therapeutic implications. Blood. 2005;105:1383–1395. doi: 10.1182/blood-2004-07-2909. [DOI] [PubMed] [Google Scholar]
- 42.Podar K, Tai YT, Davies FE, Lentzsch S, Sattler M, Hideshima T, et al. Vascular endothelial growth factor triggers signaling cascades mediating multiple myeloma cell growth and migration. Blood. 2001;98:428–435. doi: 10.1182/blood.v98.2.428. [DOI] [PubMed] [Google Scholar]
- 43.Podar K, Tai YT, Lin BK, Narsimhan RP, Sattler M, Kijima T, et al. Vascular endothelial growth factor-induced migration of multiple myeloma cells is associated with beta 1 integrin- and phosphatidylinositol 3-kinase-dependent PKC alpha activation. J Biol Chem. 2002;277:7875–7881. doi: 10.1074/jbc.M109068200. [DOI] [PubMed] [Google Scholar]
- 44.Lin B, Podar K, Gupta D, Tai YT, Li S, Weller E, et al. The vascular endothelial growth factor receptor tyrosine kinase inhibitor PTK787/ZK222584 inhibits growth and migration of multiple myeloma cells in the bone marrow microenvironment. Cancer Res. 2002;62:5019–5026. [PubMed] [Google Scholar]
- 45.Podar K, Catley LP, Tai YT, Shringarpure R, Carvalho P, Hayashi T, et al. GW654652, the pan-inhibitor of VEGF receptors, blocks the growth and migration of multiple myeloma cells in the bone marrow microenvironment. Blood. 2004;103:3474–3479. doi: 10.1182/blood-2003-10-3527. [DOI] [PubMed] [Google Scholar]
- 46.Podar K, Shringarpure R, Tai YT, Simoncini M, Sattler M, Ishitsuka K, et al. Caveolin-1 is required for vascular endothelial growth factor-triggered multiple myeloma cell migration and is targeted by bortezomib. Cancer Res. 2004;64:7500–7506. doi: 10.1158/0008-5472.CAN-04-0124. [DOI] [PubMed] [Google Scholar]
- 47.Le Gouill S, Podar K, Amiot M, Hideshima T, Chauhan D, Itshitsuka K, et al. VEGF induces MCL-1 upregulation and protects multiple myeloma cells against apoptosis. Blood. 2004;104:2886–28892. doi: 10.1182/blood-2004-05-1760. [DOI] [PubMed] [Google Scholar]
- 48.Podar K, Tonon G, Sattler M, Tai YT, Legouill S, Yasui H, et al. The small-molecule VEGF receptor inhibitor pazopanib (GW786034B) targets both tumor and endothelial cells in multiple myeloma. Proc Natl Acad Sci U S A. 2006;103:19478–19483. doi: 10.1073/pnas.0609329103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vacca A, Ribatti D, Presta M, Minischetti M, Iurlaro M, Ria R, et al. Bone marrow neovascularization, plasma cell angiogenic potential, and matrix metalloproteinase-2 secretion parallel progression of human multiple myeloma. Blood. 1999;93:3064–3073. [PubMed] [Google Scholar]
- 50.Bisping G, Leo R, Wenning D, Dankbar B, Padro T, Kropff M, et al. Paracrine interactions of basic fibroblast growth factor and interleukin-6 in multiple myeloma. Blood. 2003;101:2775–2783. doi: 10.1182/blood-2002-09-2907. [DOI] [PubMed] [Google Scholar]
- 51.Chesi M, Nardini E, Lim RSC, Smith KD, Kuehl WM, Bergsagel PL. The t(4;14) translocation in myeloma dysregulates both FGFR3 and a novel gene, MMSET, resulting in IgH/MMSET hybrid transcripts. Blood. 1998;92:3025–3034. [PubMed] [Google Scholar]
- 52.Chesi M, Nardini E, Brents LA, Schrock E, Ried T, Kuehl WM, et al. Frequent translocation t(4;14)(p16.3;q32.3) in multiple myeloma: association with increased expression and activating mutations of fibroblast growth factor receptor 3. Nature Genet. 1997;16:260–264. doi: 10.1038/ng0797-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keats JJ, Reiman T, Maxwell CA, Taylor BJ, Larratt LM, Mant MJ, et al. In multiple myeloma, t(4;14)(p16;q32) is an adverse prognostic factor irrespective of FGFR3 expression. Blood. 2003;101:1520–1529. doi: 10.1182/blood-2002-06-1675. [DOI] [PubMed] [Google Scholar]
- 54.Chang H, Stewart AK, Qi XY, Li ZH, Yi QL, Trudel S. Immunohistochemistry accurately predicts FGFR3 aberrant expression and t(4;14) in multiple myeloma. Blood. 2005;106:353–355. doi: 10.1182/blood-2005-01-0033. [DOI] [PubMed] [Google Scholar]
- 55.Trudel S, Li ZH, Wei E, Wiesmann M, Chang H, Chen C, et al. CHIR-258, a novel, multitargeted tyrosine kinase inhibitor for the potential treatment of t(4;14) multiple myeloma. Blood. 2005;105:2941–2948. doi: 10.1182/blood-2004-10-3913. [DOI] [PubMed] [Google Scholar]
- 56.Trudel S, Stewart AK, Rom E, Wei E, Li ZH, Kotzer S, et al. The inhibitory anti-FGFR3 antibody, PRO-001, is cytotoxic to t(4;14) multiple myeloma cells. Blood. 2006;107:4039–4046. doi: 10.1182/blood-2005-10-4179. [DOI] [PubMed] [Google Scholar]
- 57.Trudel S, Ely S, Farooqi Y, Affer M, Robbiani DF, Chesi M, et al. Inhibition of fibroblast growth factor receptor 3 induces differentiation and apoptosis in t(4;14) myeloma. Blood. 2004;103:3521–3528. doi: 10.1182/blood-2003-10-3650. [DOI] [PubMed] [Google Scholar]
- 58.Chen J, Lee BH, Williams IR, Kutok JL, Mitsiades CS, Duclos N, et al. FGFR3 as a therapeutic target of the small molecule inhibitor PKC412 in hematopoietic malignancies. Oncogene. 2005;24:8259–8267. doi: 10.1038/sj.onc.1208989. [DOI] [PubMed] [Google Scholar]
- 59.Moreaux J, Legouffe E, Jourdan E, Quittet P, Reme T, Lugagne C, et al. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood. 2004;103:3148–3157. doi: 10.1182/blood-2003-06-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Novak AJ, Darce JR, Arendt BK, Harder B, Henderson K, Kindsvogel W, et al. Expression of BCMA, TACI, and BAFF-R in multiple myeloma: a mechanism for growth and survival. Blood. 2004;103:689–694. doi: 10.1182/blood-2003-06-2043. [DOI] [PubMed] [Google Scholar]
- 61.Tai YT, Li XF, Breitkreutz I, Song W, Neri P, Catley L, et al. Role of B-cell-activating factor in adhesion and growth of human multiple myeloma cells in the bone marrow microenvironment. Cancer Res. 2006;66:6675–6682. doi: 10.1158/0008-5472.CAN-06-0190. [DOI] [PubMed] [Google Scholar]
- 62.Derksen PW, Tjin E, Meijer HP, Klok MD, MacGillavry HD, van Oers MH, et al. Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proc Natl Acad Sci U S A. 2004;101:6122–6127. doi: 10.1073/pnas.0305855101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sukhdeo K, Mani M, Zhang Y, Dutta J, Yasui H, Rooney MD, et al. Targeting the beta-catenin/TCF transcriptional complex in the treatment of multiple myeloma. Proc Natl Acad Sci U S A. 2007;104:7516–7521. doi: 10.1073/pnas.0610299104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, et al. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene. 2004 doi: 10.1038/sj.onc.1207892. in press. [DOI] [PubMed] [Google Scholar]
- 65.Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 66.Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD., Jr Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2007;109:2106–2111. doi: 10.1182/blood-2006-09-047712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tai YT, Podar K, Gupta D, Lin B, Young G, Akiyama M, et al. CD40 activation induces p53-dependent vascular endothelial growth factor secretion in human multiple myeloma cells. Blood. 2002;99:1419–1427. doi: 10.1182/blood.v99.4.1419. [DOI] [PubMed] [Google Scholar]
- 68.Tai YT, Podar K, Mitsiades N, Lin B, Mitsiades C, Gupta D, et al. CD40 induces human multiple myeloma cell migration via phosphatidylinositol 3-kinase/AKT/NF-kappa B signaling. Blood. 2003;101:2762–2769. doi: 10.1182/blood-2002-09-2813. [DOI] [PubMed] [Google Scholar]
- 69.Tai YT, Catley LP, Mitsiades CS, Burger R, Podar K, Shringpaure R, et al. Mechanisms by which SGN-40, a humanized anti-CD40 antibody, induces cytotoxicity in human multiple myeloma cells: clinical implications. Cancer Res. 2004;64:2846–2852. doi: 10.1158/0008-5472.can-03-3630. [DOI] [PubMed] [Google Scholar]
- 70.Hayashi T, Hideshima T, Akiyama M, Podar K, Yasui H, Raje N, et al. Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: clinical application. Br J Haematol. 2005;128:192–203. doi: 10.1111/j.1365-2141.2004.05286.x. [DOI] [PubMed] [Google Scholar]
- 71.Tai YT, Li XF, Catley L, Coffey R, Breitkreutz I, Bae J, et al. Immunomodulatory drug lenalidomide (CC-5013, IMiD3) augments anti-CD40 SGN-40-induced cytotoxicity in human multiple myeloma: clinical implications. Cancer Res. 2005;65:11712–11720. doi: 10.1158/0008-5472.CAN-05-1657. [DOI] [PubMed] [Google Scholar]
- 72.Treon SP, Pilarski LM, Belch AR, Kelliher A, Preffer FI, Shima Y, et al. CD20-directed serotherapy in patients with multiple myeloma: biologic considerations and therapeutic applications. J Immunother. 2002;25:72–81. doi: 10.1097/00002371-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 73.Tai Y-T, Song W, Li X-F, Burger P, Schlossman R, Rice A, et al. Killing of drug-sensitive and resistant myeloma cells and disruption of their bone marrow stromal interaction by HuLuc63, a novel humanized anti-CS1 monoclonal antibody. Blood. 2006;108:990a. [Google Scholar]
- 74.Berkers CR, Verdoes M, Lichtman E, Fiebiger E, Kessler BM, Anderson KC, et al. Activity probe for in vivo profiling of the specificity of proteasome inhibitor bortezomib. Nat Methods. 2005;2:357–362. doi: 10.1038/nmeth759. [DOI] [PubMed] [Google Scholar]
- 75.Baldwin AS., Jr The NF-kB and I kB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 76.Beg AA, Baldwin AS., Jr The IkB proteins: multifunctional regulators of Rel NF-kB transcription factors. Genes Dev. 1993;7:2064–2070. doi: 10.1101/gad.7.11.2064. [DOI] [PubMed] [Google Scholar]
- 77.Zandi E, Chen Y, Karin M. Direct phosphorylation of IkappaB by IKKalpha and IKKbeta: discrimination between free and NF-kappaB-bound substrate. Science. 1998;281:1360–1363. doi: 10.1126/science.281.5381.1360. [DOI] [PubMed] [Google Scholar]
- 78.Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M. The IkB kinase complex (IKK) contains two kinase subunits, IKKa and IKKb, necessary for IkB phosphorylation and NF-kB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 79.Hideshima T, Richardson P, Chauhan D, Palombella V, Elliott P, Adams J, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–3076. [PubMed] [Google Scholar]
- 80.Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Gu X, Bailey C, et al. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proc Natl Acad Sci U S A. 2002;99:14374–14379. doi: 10.1073/pnas.202445099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mitsiades N, Mitsiades CS, Richardson PG, Poulaki V, Tai YT, Chauhan D, et al. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood. 2003;101:2377–2380. doi: 10.1182/blood-2002-06-1768. [DOI] [PubMed] [Google Scholar]
- 82.Hideshima T, Mitsiades C, Akiyama M, Hayashi T, Chauhan D, Richardson P, et al. Molecular mechanisms mediating antimyeloma activity of proteasome inhibitor PS-341. Blood. 2003;101:1530–1534. doi: 10.1182/blood-2002-08-2543. [DOI] [PubMed] [Google Scholar]
- 83.Lee AH, Iwakoshi NN, Anderson KC, Glimcher LH. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc Natl Acad Sci U S A. 2003;100:9946–9951. doi: 10.1073/pnas.1334037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hideshima T, Chauhan D, Hayashi T, Akiyama M, Mitsiades N, Mitsiades C, et al. Proteasome Inhibitor PS-341 abrogates IL-6 triggered signaling cascades via caspase-dependent downregulation of gp130 in multiple myeloma. Oncogene. 2003;22:8386–8393. doi: 10.1038/sj.onc.1207170. [DOI] [PubMed] [Google Scholar]
- 85.Hideshima T, Chauhan D, Schlossman RL, Richardson PR, Anderson KC. Role of TNF-a in the pathophysiology of human multiple myeloma: therapeutic applications. Oncogene. 2001;20:4519–4527. doi: 10.1038/sj.onc.1204623. [DOI] [PubMed] [Google Scholar]
- 86.Hideshima T, Chauhan D, Richardson P, Mitsiades C, Mitsiades N, Hayashi T, et al. NF-kB as a therapeutic target in multiple myeloma. J Biol Chem. 2002;277:16639–16647. doi: 10.1074/jbc.M200360200. [DOI] [PubMed] [Google Scholar]
- 87.Heider U, Kaiser M, Muller C, Jakob C, Zavrski I, Schulz CO, et al. Bortezomib increases osteoblast activity in myeloma patients irrespective of response to treatment. Eur J Haematol. 2006;77:233–238. doi: 10.1111/j.1600-0609.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- 88.Giuliani N, Morandi F, Tagliaferri S, Lazzaretti M, Bonomini S, Crugnola M, et al. The proteasome inhibitor bortezomib affects osteoblast differentiation in vitro and in vivo in multiple myeloma patients. Blood. 2007;110:334–338. doi: 10.1182/blood-2006-11-059188. [DOI] [PubMed] [Google Scholar]
- 89.von Metzler I, Krebbel H, Hecht M, Manz RA, Fleissner C, Mieth M, et al. Bortezomib inhibits human osteoclastogenesis. Leukemia. 2007;21:2025–2034. doi: 10.1038/sj.leu.2404806. [DOI] [PubMed] [Google Scholar]
- 90.Chauhan D, Catley L, Li G, Podar K, Hideshima T, Velankar M, et al. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from Bortezomib. Cancer Cell. 2005;8:407–419. doi: 10.1016/j.ccr.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 91.Miller CP, Ban K, Dujka ME, McConkey DJ, Munsell M, Palladino M, et al. NPI-0052, a novel proteasome inhibitor, induces caspase-8 and ROS-dependent apoptosis alone and in combination with HDAC inhibitors in leukemia cells. Blood. 2007 doi: 10.1182/blood-2006-03-013128. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Demo SD, Kirk CJ, Aujay MA, Buchholz TJ, Dajee M, Ho MN, et al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 2007;67:6383–6391. doi: 10.1158/0008-5472.CAN-06-4086. [DOI] [PubMed] [Google Scholar]
- 93.O’Connor OA, Orlowski RZ, Alsina M, Stewart K, Trudel S, Vallone MK, et al. Multicenter phase I studies to evaluate the safety, tolerability, and clinical response to intensive dosing with the proteasome Inhibitor PR-171 in patients with relapsed or refractory hematological malignancies. Blood. 2006;108:687a. [Google Scholar]
- 94.Hideshima T, Chauhan D, Shima Y, Raje N, Davies FE, Tai Y-T, et al. Thalidomide and its analogues overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood. 2000;96:2943–2950. [PubMed] [Google Scholar]
- 95.LeBlanc R, Hideshima T, Catley LP, Shringarpure R, Burger R, Mitsiades N, et al. Immunomodulatory drug costimulates T cells via the B7-CD28 pathway. Blood. 2004;103:1787–1790. doi: 10.1182/blood-2003-02-0361. [DOI] [PubMed] [Google Scholar]
- 96.Davies FE, Raje N, Hideshima T, Lentzsch S, Young G, Tai YT, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98:210–216. doi: 10.1182/blood.v98.1.210. [DOI] [PubMed] [Google Scholar]
- 97.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 98.Finnin MS, Donigian JR, Cohen A, Richon VM, Rifkind RA, Marks PA, et al. Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature. 1999;401:188–1893. doi: 10.1038/43710. [DOI] [PubMed] [Google Scholar]
- 99.Marks PA, Jiang X. Histone deacetylase inhibitors in programmed cell death and cancer therapy. Cell Cycle. 2005;4:549–551. doi: 10.4161/cc.4.4.1564. [DOI] [PubMed] [Google Scholar]
- 100.Marks PA, Dokmanovic M. Histone deacetylase inhibitors: discovery and development as anticancer agents. Expert Opin Investig Drugs. 2005;14:1497–1511. doi: 10.1517/13543784.14.12.1497. [DOI] [PubMed] [Google Scholar]
- 101.Lin RJ, Nagy L, Inoue S, Shao W, Miller WH, Jr, Evans RM. Role of the histone deacetylase complex in acute promyelocytic leukaemia. Nature. 1998;391:811–814. doi: 10.1038/35895. [DOI] [PubMed] [Google Scholar]
- 102.Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1:194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 103.Mitsiades N, Mitsiades CS, Richardson PG, McMullan C, Poulaki V, Fanourakis G, et al. Molecular sequelae of histone deacetylase inhibition in human malignant B cells. Blood. 2003;101:4055–4062. doi: 10.1182/blood-2002-11-3514. [DOI] [PubMed] [Google Scholar]
- 104.Huang L, Sowa Y, Sakai T, Pardee AB. Activation of the p21WAF1/CIP1 promoter independent of p53 by the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) through the Sp1 sites. Oncogene. 2000;19:5712–5719. doi: 10.1038/sj.onc.1203963. [DOI] [PubMed] [Google Scholar]
- 105.Richon VM, Sandhoff TW, Rifkind RA, Marks PA. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc Natl Acad Sci U S A. 2000;97:10014–10019. doi: 10.1073/pnas.180316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Shringarpure R, Hideshima T, et al. Transcriptional signature of histone deacetylase inhibition in multiple myeloma: biological and clinical implications. Proc Natl Acad Sci U S A. 2004;101:540–545. doi: 10.1073/pnas.2536759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fandy TE, Shankar S, Ross DD, Sausville E, Srivastava RK. Interactive effects of HDAC inhibitors and TRAIL on apoptosis are associated with changes in mitochondrial functions and expressions of cell cycle regulatory genes in multiple myeloma. Neoplasia. 2005;7:646–657. doi: 10.1593/neo.04655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Remiszewski SW, Sambucetti LC, Bair KW, Bontempo J, Cesarz D, Chandramouli N, et al. N-hydroxy-3-phenyl-2-propenamides as novel inhibitors of human histone deacetylase with in vivo antitumor activity: discovery of (2E)-N-hydroxy-3-[4-[[(2-hydroxyethyl)[2-(1H-indol-3-yl)ethyl]amino]methyl ]phenyl]-2-prop enamide (NVP-LAQ824) J Med Chem. 2003;46:4609–4624. doi: 10.1021/jm030235w. [DOI] [PubMed] [Google Scholar]
- 109.Atadja P, Gao L, Kwon P, Trogani N, Walker H, Hsu M, et al. Selective growth inhibition of tumor cells by a novel histone deacetylase inhibitor, NVP-LAQ824. Cancer Res. 2004;64:689–695. doi: 10.1158/0008-5472.can-03-2043. [DOI] [PubMed] [Google Scholar]
- 110.Fiskus W, Pranpat M, Balasis M, Herger B, Rao R, Chinnaiyan A, et al. Histone deacetylase inhibitors deplete enhancer of zeste 2 and associated polycomb repressive complex 2 proteins in human acute leukemia cells. Mol Cancer Ther. 2006;5:3096–3104. doi: 10.1158/1535-7163.MCT-06-0418. [DOI] [PubMed] [Google Scholar]
- 111.Guo F, Sigua C, Tao J, Bali P, George P, Li Y, et al. Cotreatment with histone deacetylase inhibitor LAQ824 enhances Apo-2L/tumor necrosis factor-related apoptosis inducing ligand-induced death inducing signaling complex activity and apoptosis of human acute leukemia cells. Cancer Res. 2004;64:2580–2589. doi: 10.1158/0008-5472.can-03-2629. [DOI] [PubMed] [Google Scholar]
- 112.Rosato RR, Maggio SC, Almenara JA, Payne SG, Atadja P, Spiegel S, et al. The histone deacetylase inhibitor LAQ824 induces human leukemia cell death through a process involving XIAP down-regulation, oxidative injury, and the acid sphingomyelinase-dependent generation of ceramide. Mol Pharmacol. 2006;69:216–225. doi: 10.1124/mol.105.017145. [DOI] [PubMed] [Google Scholar]
- 113.Weisberg E, Catley L, Kujawa J, Atadja P, Remiszewski S, Fuerst P, et al. Histone deacetylase inhibitor NVP-LAQ824 has significant activity against myeloid leukemia cells in vitro and in vivo. Leukemia. 2004;18:1951–1963. doi: 10.1038/sj.leu.2403519. [DOI] [PubMed] [Google Scholar]
- 114.Qian DZ, Wang X, Kachhap SK, Kato Y, Wei Y, Zhang L, et al. The histone deacetylase inhibitor NVP-LAQ824 inhibits angiogenesis and has a greater antitumor effect in combination with the vascular endothelial growth factor receptor tyrosine kinase inhibitor PTK787/ZK222584. Cancer Res. 2004;64:6626–6634. doi: 10.1158/0008-5472.CAN-04-0540. [DOI] [PubMed] [Google Scholar]
- 115.Catley L, Weisberg E, Tai YT, Atadja P, Remiszewski S, Hideshima T, et al. NVP-LAQ824 is a potent novel histone deacetylase inhibitor with significant activity against multiple myeloma. Blood. 2003;102:2615–2622. doi: 10.1182/blood-2003-01-0233. [DOI] [PubMed] [Google Scholar]
- 116.George P, Bali P, Annavarapu S, Scuto A, Fiskus W, Guo F, et al. Combination of the histone deacetylase inhibitor LBH589 and the hsp90 inhibitor 17-AAG is highly active against human CML-BC cells and AML cells with activating mutation of FLT-3. Blood. 2005;105:1768–1776. doi: 10.1182/blood-2004-09-3413. [DOI] [PubMed] [Google Scholar]
- 117.Fiskus W, Pranpat M, Bali P, Balasis M, Kumaraswamy S, Boyapalle S, et al. Combined effects of novel tyrosine kinase inhibitor AMN107 and histone deacetylase inhibitor LBH589 against Bcr-Abl-expressing human leukemia cells. Blood. 2006;108:645–652. doi: 10.1182/blood-2005-11-4639. [DOI] [PubMed] [Google Scholar]
- 118.Geng L, Cuneo KC, Fu A, Tu T, Atadja PW, Hallahan DE. Histone deacetylase (HDAC) inhibitor LBH589 increases duration of gamma-H2AX foci and confines HDAC4 to the cytoplasm in irradiated non-small cell lung cancer. Cancer Res. 2006;66:11298–11304. doi: 10.1158/0008-5472.CAN-06-0049. [DOI] [PubMed] [Google Scholar]
- 119.Yu C, Friday BB, Lai JP, McCollum A, Atadja P, Roberts LR, et al. Abrogation of MAPK and Akt signaling by AEE788 synergistically potentiates histone deacetylase inhibitor-induced apoptosis through reactive oxygen species generation. Clin Cancer Res. 2007;13:1140–1148. doi: 10.1158/1078-0432.CCR-06-1751. [DOI] [PubMed] [Google Scholar]
- 120.Qian DZ, Kato Y, Shabbeer S, Wei Y, Verheul HM, Salumbides B, et al. Targeting tumor angiogenesis with histone deacetylase inhibitors: the hydroxamic acid derivative LBH589. Clin Cancer Res. 2006;12:634–642. doi: 10.1158/1078-0432.CCR-05-1132. [DOI] [PubMed] [Google Scholar]
- 121.Maiso P, Carvajal-Vergara X, Ocio EM, Lopez-Perez R, Mateo G, Gutierrez N, et al. The histone deacetylase inhibitor LBH589 is a potent antimyeloma agent that overcomes drug resistance. Cancer Res. 2006;66:5781–5789. doi: 10.1158/0008-5472.CAN-05-4186. [DOI] [PubMed] [Google Scholar]
- 122.Catley L, Weisberg E, Kiziltepe T, Tai YT, Hideshima T, Neri P, et al. Aggresome induction by proteasome inhibitor bortezomib and alpha-tubulin hyperacetylation by tubulin deacetylase (TDAC) inhibitor LBH589 are synergistic in myeloma cells. Blood. 2006;108:3441–3449. doi: 10.1182/blood-2006-04-016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci U S A. 2003;100:4389–4394. doi: 10.1073/pnas.0430973100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Garcia-Mata R, Gao YS, Sztul E. Hassles with taking out the garbage: aggravating aggresomes. Traffic. 2002;3:388–396. doi: 10.1034/j.1600-0854.2002.30602.x. [DOI] [PubMed] [Google Scholar]
- 125.Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell. 2003;115:727–738. doi: 10.1016/s0092-8674(03)00939-5. [DOI] [PubMed] [Google Scholar]
- 126.Hideshima H, Bradner JE, Wong JDC, Richardson P, Schreiber SL, et al. Small molecule inhibition of proteasome and aggresome function induces synergistic anti-tumor activity in multiple myeloma. Proc Natl Acad Sci USA. 2005;102:8567–8572. doi: 10.1073/pnas.0503221102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Furumai R, Matsuyama A, Kobashi N, Lee KH, Nishiyama M, Nakajima H, et al. FK228 (depsipeptide) as a natural prodrug that inhibits class I histone deacetylases. Cancer Res. 2002;62:4916–4921. [PubMed] [Google Scholar]
- 128.Khan SB, Maududi T, Barton K, Ayers J, Alkan S. Analysis of histone deacetylase inhibitor, depsipeptide ( FR901228), effect on multiple myeloma. Br J Haematol. 2004;125:156–161. doi: 10.1111/j.1365-2141.2004.04882.x. [DOI] [PubMed] [Google Scholar]
- 129.Plumb JA, Finn PW, Williams RJ, Bandara MJ, Romero MR, Watkins CJ, et al. Pharmacodynamic response and inhibition of growth of human tumor xenografts by the novel histone deacetylase inhibitor PXD101. Mol Cancer Ther. 2003;2:721–728. [PubMed] [Google Scholar]
- 130.Feng R, Hager JH, Hassig CA, Scranton SA, Payne JE, Mapara MY, et al. A novel, mercaptoketone-based HDAC inhibitor, KD5170 exerts marked inhibition of osteoclast formation and anti-Myeloma activity in vitro. Blood. 2006;108:991a. [Google Scholar]
- 131.Chatterjee M, Jain S, Stuhmer T, Andrulis M, Ungethum U, Kuban RJ, et al. STAT3 and MAPK signaling maintain overexpression of heat shock proteins 90alpha and beta in multiple myeloma cells, which critically contribute to tumor-cell survival. Blood. 2007;109:720–728. doi: 10.1182/blood-2006-05-024372. [DOI] [PubMed] [Google Scholar]
- 132.Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Kung AL, Davies FE, et al. Antimyeloma activity of heat shock protein-90 inhibition. Blood. 2006;107:1092–1100. doi: 10.1182/blood-2005-03-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Davenport EL, Moore HE, Dunlop AS, Sharp SY, Workman P, Morgan GJ, et al. Heat shock protein inhibition is associated with activation of the unfolded protein response (UPR) pathway in myeloma plasma cells. Blood. 2007 doi: 10.1182/blood-2006-11-053728. in press. [DOI] [PubMed] [Google Scholar]
- 134.Sydor JR, Normant E, Pien CS, Porter JR, Ge J, Grenier L, et al. Development of 17-allylamino-17-demethoxygeldanamycin hydroquinone hydrochloride (IPI-504), an anti-cancer agent directed against Hsp90. Proc Natl Acad Sci U S A. 2006;103:17408–17413. doi: 10.1073/pnas.0608372103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tu Y, Gardner A, Lichtenstein A. The phosphatidylinositol 3-kinase/AKT kinase pathway in multiple myeloma plasma cells: roles in cytokine-dependent survival and proliferative responses. Cancer Res. 2000;60:6763–6770. [PubMed] [Google Scholar]
- 136.Hideshima T, Catley L, Yasui H, Ishitsuka K, Raje N, Mitsiades C, et al. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood. 2006;107:4053–4062. doi: 10.1182/blood-2005-08-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gajate C, Mollinedo F. Edelfosine and perifosine induce selective apoptosis in multiple myeloma by recruitment of death receptors and downstream signaling molecules into lipid rafts. Blood. 2007;109:711–719. doi: 10.1182/blood-2006-04-016824. [DOI] [PubMed] [Google Scholar]
- 138.Hideshima T, Catley L, Raje N, Chauhan D, Podar K, Mitsiades C, et al. Inhibition of Akt induces significant downregulation of survivin and cytotoxicity in human multiple myeloma cells. Br J Haematol. 2007;138:783–791. doi: 10.1111/j.1365-2141.2007.06714.x. [DOI] [PubMed] [Google Scholar]
- 139.Shi Y, Hsu JH, Hu L, Gera J, Lichtenstein A. Signal pathways involved in activation of p70S6K and phosphorylation of 4E-BP1 following exposure of multiple myeloma tumor cells to interleukin-6. J Biol Chem. 2002;277:15712–15720. doi: 10.1074/jbc.M200043200. [DOI] [PubMed] [Google Scholar]
- 140.Pene F, Claessens YE, Muller O, Viguie F, Mayeux P, Dreyfus F, et al. Role of the phosphatidylinositol 3-kinase/Akt and mTOR/P70S6-kinase pathways in the proliferation and apoptosis in multiple myeloma. Oncogene. 2002;21:6587–6597. doi: 10.1038/sj.onc.1205923. [DOI] [PubMed] [Google Scholar]
- 141.Stromberg T, Dimberg A, Hammarberg A, Carlson K, Osterborg A, Nilsson K, et al. Rapamycin sensitizes multiple myeloma cells to apoptosis induced by dexamethasone. Blood. 2004;103:3138–3147. doi: 10.1182/blood-2003-05-1543. [DOI] [PubMed] [Google Scholar]
- 142.Shi Y, Gera J, Hu L, Hsu JH, Bookstein R, Li W, et al. Enhanced sensitivity of multiple myeloma cells containing PTEN mutations to CCI-779. Cancer Res. 2002;62:5027–5034. [PubMed] [Google Scholar]
- 143.Raje N, Kumar S, Hideshima T, Ishitsuka K, Chauhan D, Mitsiades C, et al. Combination of the mTOR inhibitor Rapamycin and RevlimidTM(CC-5013) has synergistic activity in multiple myeloma. Blood. 2004;104:4188–4193. doi: 10.1182/blood-2004-06-2281. [DOI] [PubMed] [Google Scholar]
- 144.Frost P, Moatamed F, Hoang B, Shi Y, Gera J, Yan H, et al. In vivo antitumor effects of the mTOR inhibitor CCI-779 against human multiple myeloma cells in a xenograft model. Blood. 2004;104:4181–4187. doi: 10.1182/blood-2004-03-1153. [DOI] [PubMed] [Google Scholar]
- 145.Frost P, Shi Y, Hoang B, Lichtenstein A. AKT activity regulates the ability of mTOR inhibitors to prevent angiogenesis and VEGF expression in multiple myeloma cells. Oncogene. 2007;26:2255–2262. doi: 10.1038/sj.onc.1210019. [DOI] [PubMed] [Google Scholar]
- 146.van de Donk NW, de Weerdt O, Veth G, Eurelings M, van Stralen E, Frankel SR, et al. G3139, a Bcl-2 antisense oligodeoxynucleotide, induces clinical responses in VAD refractory myeloma. Leukemia. 2004;18:1078–1084. doi: 10.1038/sj.leu.2403363. [DOI] [PubMed] [Google Scholar]
- 147.Badros AZ, Goloubeva O, Rapoport AP, Ratterree B, Gahres N, Meisenberg B, et al. Phase II study of G3139, a Bcl-2 antisense oligonucleotide, in combination with dexamethasone and thalidomide in relapsed multiple myeloma patients. J Clin Oncol. 2005;23:4089–4099. doi: 10.1200/JCO.2005.14.381. [DOI] [PubMed] [Google Scholar]
- 148.Kline MP, Rajkumar SV, Timm MM, Kimlinger TK, Haug JL, Lust JA, et al. ABT-737, an inhibitor of Bcl-2 family proteins, is a potent inducer of apoptosis in multiple myeloma cells. Leukemia. 2007;21:1549–1560. doi: 10.1038/sj.leu.2404719. [DOI] [PubMed] [Google Scholar]
- 149.Chauhan D, Velankar M, Brahmandam M, Hideshima T, Podar K, Richardson P, et al. A novel Bcl-2/Bcl-X(L)/Bcl-w inhibitor ABT-737 as therapy in multiple myeloma. Oncogene. 2007;26:2374–2380. doi: 10.1038/sj.onc.1210028. [DOI] [PubMed] [Google Scholar]
- 150.Trudel S, Stewart AK, Li Z, Shu Y, Liang SB, Trieu Y, et al. The Bcl-2 family protein inhibitor, ABT-737, has substantial antimyeloma activity and shows synergistic effect with dexamethasone and melphalan. Clin Cancer Res. 2007;13:621–629. doi: 10.1158/1078-0432.CCR-06-1526. [DOI] [PubMed] [Google Scholar]