Abstract
ETS1 is a cellular homologue of the product of the viral ets oncogene of the E26 virus, and it functions as a tissue-specific transcription factor. It plays an important role in cell proliferation, differentiation, lymphoid cell development, transformation, angiogenesis, and apoptosis. ETS1 controls the expression of critical genes involved in these processes by binding to ets binding sites present in the transcriptional regulatory regions. The ETS1 gene generates two proteins, p51 and a spliced variant, p42, lacking exon VII. In this paper we show that p42-ETS1 expression bypasses the damaged Fas-induced apoptotic pathway in DLD1 colon carcinoma cells by up-regulating interleukin 1β-converting enzyme (ICE)/caspase-1 and causes these cancer cells to become susceptible to the effects of the normal apoptosis activation system. ICE/caspase-1 is a redundant system in many cells and tissues, and here we demonstrate that it is important in activating apoptosis in cells where the normal apoptosis pathway is blocked. Blocking ICE/caspase-1 activity by using specific inhibitors of this protease prevents the p42-ETS1-induced apoptosis from occurring, indicating that the induced ICE/caspase-1 enzyme is responsible for killing the cancer cells. p42-ETS1 activates a critical alternative apoptosis pathway in cancer cells that are resistant to normal immune attack, and thus it may be useful as an anticancer therapeutic.
Keywords: interleukin 1β-converting enzyme, caspase-1
Apoptosis, or programmed cell death, is a process essential for normal development and homeostasis in multicellular organisms. It provides a defense against cell immortalization caused by oncogenesis or viral infection (1–4). It is well known that Apo-1/Fas is an important death signaling molecule that initiates apoptosis (5). Although Fas is ubiquitously expressed in various tissues, its ligand, Fas-L, is predominantly expressed in cytotoxic T lymphocytes and natural killer cells. The interaction of Fas with Fas-L initiates a death signal that is responsible for eliminating virally infected lymphoid cells and/or tumor cells through T cell-mediated cytotoxicity (6). However, it is known that many tumor cells escape from immune cytolysis and become resistant to Fas-L-induced apoptosis, including cells that express the Fas receptor (7, 8). The molecular mechanisms for this process are still unknown.
The identification and characterization of the FLICE-inhibitory protein (FLIP; refs. 9–11) and X-chromosome-linked IAP (XIAP, ref. 12) provide a clue as to how those cells become resistant to Fas-mediated apoptosis. Both of these proteins inhibit the activation of apoptosis-related proteases [caspases/interleukin 1β-converting enzyme (ICE)-like proteases]. The FLIP protein inhibits the activation of FLICE/caspase-8 and is expressed at high levels in melanoma cell lines and tumors. The XIAP protein inhibits the activity of the executioner proteases CCP32/caspase-3.
It is important, therefore, to be able to reconstitute the Fas-mediated apoptotic pathway, which is defective in tumor cells, to cause them to undergo programmed cell death.
Caspase/ICE-like proteases are key components of the apoptotic pathway. At present, several ICE/caspase family members have been isolated (13). Activation of the caspase/ICE-like proteolytic activity is initiated upon the interaction of the Fas receptor (Fas-R) with its ligand Fas-L. MACH/FLICE/caspase-8 is an upstream component in the Fas/APO-1-induced cell death pathway (14–16). Caspase-3 is thought to act as an executioner protease (17). ICE/caspase-1, a member of the mammalian caspase family, is involved in the Fas-induced pathway (18). The role of caspase-1/ICE is not integrated into the current model of sequential ICE protease activation. Furthermore, an ICE/caspase-1-dependent apoptotic pathway has been identified in some cells after activation by cytotoxic T lymphocyte granzymes (19). Although the ICE/caspase-1 protease is independent of the activation of the other caspases in the Fas-induced apoptotic pathway, ICE/caspase-1 mutant mice develop normally even in the absence of interleukin 1 (20), whereas mice lacking functional caspase-3 show hyperplasia and disorganized cells (21). These results suggest that ICE/caspase-1 provides an alternative, redundant caspase enzyme system for the execution of apoptosis in certain cell types. Although the CPP32/caspase-3(-like) proteases and not ICE/caspase-1 play the dominant role in Fas-mediated apoptosis in some cell lines (22), overexpression of ICE induces apoptosis (23). The detailed mechanism of action of ICE/caspase-1 in the Fas-induced apoptotic pathway remains unknown.
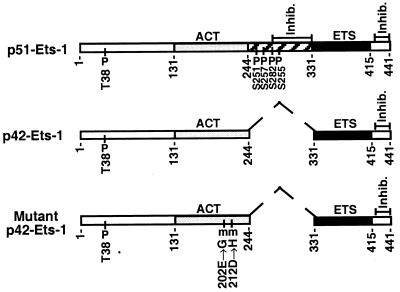
The ETS1 gene encodes two distinct proteins, a p51 protein encoded by full-length mRNA, and a p42 protein encoded by an alternatively spliced mRNA lacking exon VII (24, 25). The protein diagrams for p51-ETS1, p42-ETS1, and mutant p42-ETS1 are shown in Fig. 1. ETS1 proteins are nuclear, acidic, and rapidly turned over (t1/2 ≈ 90 min), and they function as sequence-specific (GGAA) transcription factors. ETS1 has been shown to bind to the GGAA sequences in the promoter and enhancer regions of a number of cellular and viral genes and thus to control their expression (26, 30).
Figure 1.
Schematic representation of the protein structure for p51-ETS1, p42-ETS1, and mutant p42-ETS1. The DNA-binding domains of ETS1 protein (ETS) are indicated by solid regions. The transcriptional activation domains (ACT) are indicated by lightly shaded regions (26). Hatched boxes indicate domains (amino acids 244–331) encoded by exon VII (25), which is deleted in p42-ETS1 and indicated by dashed line. Amino acid numbers are indicated below each protein. The domains inhibiting DNA binding are indicated by “inhib.” (27). Phosphorylated threonine and serine residues are indicated by a “P” (28), and mutation sites in mutant p42-ETS1 are indicated by an “m” (29).
We have previously established a model system (33, 39) in epithelial cancer cells showing that the two major ETS1 isotypes have overlapping (both repress the tumorigenic phenotype of epithelial cells) and certain different (only the p42-ETS1 protein promotes apoptosis) biological properties. Understanding the mechanism of p42-ETS1-induced apoptosis in epithelial cells will provide information about its function in T cells in which it is normally expressed. This can have a profound medical significance in that it may open new insights into the potential role of the p42-ETS1 variant in the induction of apoptosis in epithelial cancers and may provide a rationale for its use for potential gene therapy experiments to initiate cell death in cancer cells.
In this study, we have examined the potential role of the p42-ETS1 protein in Fas-mediated apoptotic cell death in the colon carcinoma cell DLD1. Our results show that the p42-ETS1 protein rescues a defect of Fas-induced apoptosis in colon carcinoma through the up-regulation of ICE/caspase-1 gene expression.
MATERIALS AND METHODS
Cell Line and Cell Culture.
The human colon carcinoma cell line DLD1 was transfected with the indicated expression vector as described (31), and stable transfectants expressing the p42-ETS1, p51-ETS1, and a mutant p42-ETS1 were isolated. In addition, stable transfectants expressing both p42-ETS1 and Bcl2 were isolated. Clones were maintained in RPMI 1640 medium with 15% fetal bovine serum in the presence of G418 (400 μg/ml), tetracycline (2 μg/ml), and hygromycin (100 μg/ml).
Cell Cycle Analysis and Viability Assays.
The Fas mAb CH-11 was purchased (MBL, Watertown, MA) and was used for inducing apoptosis at a concentration of 100 ng/ml. Cell cycle analysis was performed by propidium iodide staining: 5 × 105 cells were harvested and fixed in 75% ethanol overnight, washed three times in PBS, and resuspended in 0.5 ml of PBS containing propidium iodide (20 μg/ml) and 0.05% Triton X-100. Cells were subjected to fluorescence-activated cell sorting analysis (Coulter EPICS) (32). Cell viability assay: 2 × 104 cells were plated in 96-well plates and incubated overnight. After Fas antibody treatments, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma), 10 μl at 5 mg/ml, was added to each well and the plates were incubated for 4 hr at 37°C; optical density at 550 nm was measured using 690 nm as a reference. The average value of samples without the addition of the anti-Fas antibody was regarded as indicating 100% survival. The percentage cell viability was then expressed as ODtreated/ODuntreated.
Reverse Transcription—PCR Analysis.
Reverse transcription–PCR assays were performed as described (33). Total RNA was extracted from 1 × 107 cells with the Trizol-reagent (GIBCO) and 5 μg of RNA was incubated with 0.1 μg of oligo(dT) at 65°C for 15 min. Reverse transcription was then performed for 1 hr at 37°C. The following primers were amplified by PCR: Fas-R, 5′ primer CAGAACTTGGAAGGCCTGCATC, 3′ primer TCTGTTCTGCTGTGTCTTGGAC; Fas-L, 5′ primer GGATTGGGCCTGGGGATGTTTCA, 3′ primer TTGTGGCTCAGGGGCAGGTTGTTG; ICE/caspase-1, 5′ primer TTGCTCCCTAGAAGAAGCTCAAAG, 3′ primer GCCTTCCCGAATACCATGAGAC; CPP32/caspase-3, 5′ primer TGGAACAAATGGACCTGTTGACC, 3′ primer AGGACTCAAATTCTGTTGCCACC; FLIP, 5′ primer, GAAGAAGCACTTGATACAGATGAG, 3′ primer TCAGGTCTATTCTGTGGATGTTC; XIAP, 5′ primer GGACATGGATATACTCAGTT, 3′ primer AGTAATGACTGTGTAGCACA; and housekeeping gene S26, 5′ primer GCTCTCCGGTCCGTGCCTCCA, 3′ primer TCTCCAGAGAATAGCCT GTC.
Caspase Activity Assays in Vitro.
Caspase activity was measured in cell extracts by using the caspase assay kit (PharMingen) as described in the manufacturer’s instruction. Cells were harvested after treatment and lysed with buffer (10 mM Tris⋅HCl, pH 7.5/10 mM NaH2PO4/Na2HPO4, pH 7.5/130 mM NaCl/1% Triton X-100/10 mM NaPPi), and centrifuged at 16,000 × g for 10 min. The supernatants were normalized to equal protein (100 μg/ml), were diluted in 2 ml of assay buffer, and were incubated at 30°C for 2 hr in 20 or 25 μM fluorescent substrate acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin (Ac-DEVD-AMC) or acetyl-Tyr-Val-Ala-Asp-7-amino-4-methylcoumarin (Ac-YVAD-AMC), respectively. The fluorescence of the cleaved substrate was measured in a spectrofluorimeter at 380-nm excitation and 440-nm emission. In assays using peptide inhibitors, the aldehyde analogue Ac-DEVD-aldehyde (Ac-DEVD-CHO) or Ac-YVAD-CHO was incubated with cells for 1 hr before the addition of the Fas mAb.
RESULTS
DLD1 Cells Expressing p42-ETS1 Undergo Fas-Induced Apoptosis.
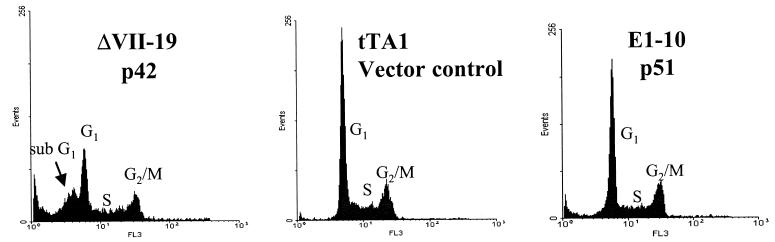
We have previously shown that p42-ETS1 suppresses tumorigenicity of DLD1 colon cancer cells and induces apoptosis in epithelial carcinomas (29). To better understand the mechanism by which p42-ETS1 induces apoptosis, we have examined the Fas-mediated apoptosis in DLD1 cells that express p42-ETS1, p51-ETS1, and a vector control, respectively. We have analyzed the susceptibility of these cells to apoptosis by treating the cells with Fas mAb and measuring the degree of apoptosis by fluorescence-activated cell sorter (Fig. 2). The apoptotic population (sub-G1) of cells transfected with the control vector (tTA1) and with p51-ETS1 were found to be 3.2% and 9.8%, respectively, after 18-hr treatment with the antibody. However, cells expressing p42-ETS1 reach an apoptotic (sub-G1) population of 38.2%. This fluorescence-activated cell sorter analysis was further confirmed by a DNA ladder analysis (data not shown). Therefore, we conclude that only the p42-ETS1-expressing cells undergo apoptosis.
Figure 2.
Fas-induced apoptosis in the DLD1 colon carcinoma cell line transfected with the variant p42-ETS1 protein. DLD1 cells transfected with p42-ETS1 (ΔVII-19), control vector (tTA1), and p51-ETS1 (E1–10) were cultured in 10% serum, in RPMI 1640 medium without tetracycline. After 18-hr treatment with anti-Fas antibody CH-11 (100 ng/ml), 5 × 105 cells were collected and fixed in 75% ethanol before staining with propidium iodide. DNA content was analyzed by fluorescence-activated cell sorting. Peak sub-G1 indicates early apoptotic cells.
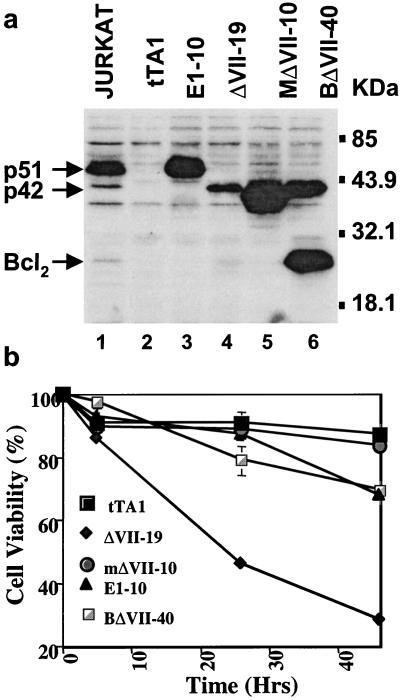
To exclude the possibility that this is due to quantitative levels of proteins, Western blot analysis was conducted measuring the amounts of p42- and p51-ETS1 in the different transfectants (Fig. 3a). The parental cell line does not express either p42-ETS1 or p51-ETS1. The transfectant cell line E1–10 expresses the p51-ETS1 protein at much higher levels (7-fold higher) than the transfectant expressing p42-ETS1 (ΔVII-19). The transfectant expressing the mutant p42-ETS1 (mΔVII-10) expresses the highest level of protein. The double transfectant of p42-ETS1 and Bcl2 (BΔVII-40) expresses higher levels of p42-ETS1 than the stable transfectant (ΔVII-19). The mutant p42-ETS1 transfectant (mΔVII-10) shows the highest expression of ETS1 protein. The p42-ETS1 expression level in the Bcl2 coexpressing clone is about 2 times higher than in the p42-ETS1 transfectant. This double transfectant (BΔVII-40) expresses high levels of Bcl2 as well. All three proteins are expressed in the Jurkat control cell line, with the highest being p51-ETS1. It is interesting to note that the p42-ETS1 transfectant expresses the lowest amount of ETS1-related protein. To better quantitate the susceptibility of the transfectants to undergo apoptosis, the MTT assay was adopted to measure cell viability after Fas crosslinking (Fig. 3b). Control transfected cells (tTA1) remain 87.4% viable at 45 hr. In contrast, p42-ETS1-expressing cells had undergone significant apoptosis with a viability of 28.1% at 45 hr, whereas p51-ETS1-expressing cells remained as high as 68% viable. The mutant p42-ETS1-expressing cells remained as viable as the control cells, indicating that the mutation directly affects the induction of apoptosis. The p42-ETS1/Bcl2-coexpressing cells remained nearly as viable as the control at 45 hr at 70%, indicating that Bcl2 overrides the apoptotic effect of p42-ETS1.
Figure 3.
Protein levels and cell viability of p42-ETS1, p51-ETS1, mutant p42-ETS1, and p42-ETS1/bcl2 transfectants. (a) Comparison of protein expression levels of different transfectants. DLD1 cells were transfected with control vector (tTA1, lane 2), p51-ETS1 (E1–10, line 3), p42-ETS1 (ΔVII-19, lane 4), or mutant p42-ETS1 (mΔVII-10, lane 5) and cotransfectant p42-ETS1/Bcl2 (BΔVII-40, lane 6). Approximately 2 × 105 cells were obtained from the individual clones and lysed, and the same amount of protein was electrophoresed and detected with a polyclonal antibody, C-20 to ETS1 (Santa Cruz Biotechnology) or N-19 to bcl2 (Santa Cruz Biotechnology). Jurkat cells were the positive control (lane 1). Molecular weight markers are shown on the right. (b) Cell viability after treatment with anti-Fas mAb CH-11. DLD1 cells expressing the control vector tTA1 (■), p51ETS1 (▴), p42-ETS1 (⧫), mutant p42-ETS1 (●), and p42-ETS1/Bcl2 (□) were placed in a 96-well plate and incubated with anti-Fas antibody CH-11 (100 ng/ml) at times as indicated. Cell viability was measured by MTT assay. Each data point is the mean of three independent experiments.
p42-ETS1 Up-Regulates ICE/Capase-1 Expression.
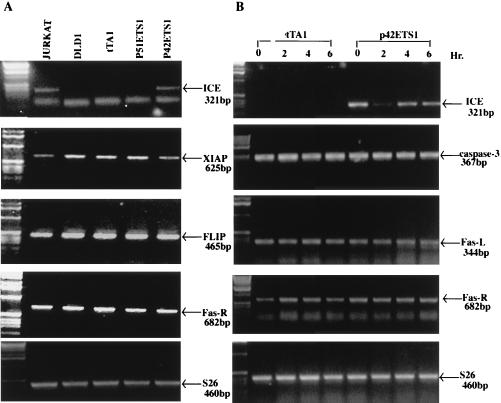
To understand which steps in the Fas-mediated apoptosis pathway are affected by the expression of p42-ETS1, we investigated possible downstream targets by evaluating the expression of the following genes: caspase-1, -3, -4, -5, -7, and -8; Fas-R; XIAP and FLIP; and the housekeeping gene S26. The expression of these genes was evaluated in p42-ETS1 and p51-ETS1 transfectants, with the Jurkat cell line as a control. Fig. 4A shows that ICE/caspase-1 mRNA is expressed only in the p42-ETS1 transfectant and not in the other cells tested, indicating that p42-ETS1 and not p51-ETS1 induces expression of ICE/caspase-1. The data also show a slight increased expression of FLIP mRNA in the DLD1 cell line as compared with the Jurkat cell line, whereas XIAP expression in all cell lines is at the same level. To further evaluate the effect of Fas crosslinking on the expression of these genes, total RNA was extracted at various times after treatment with anti-Fas antibody (Fig. 4B). The ICE/caspase-1 mRNA levels were undetectable at all time points in the control cells, whereas high expression was detected in the p42-ETS1 cells regardless of anti-Fas antibody treatment. CPP32/caspase-3, Fas-L, and Fas-R mRNA were identically expressed in both control and p42-ETS1-expressing cells. Similarly, the levels of expression of caspase-2, -4, -6, and -7 remained the same in both the control and p42-ETS1-expressing cells (data not shown).
Figure 4.
p42-ETS1 up-regulates ICE/caspase-1 expression in DLD1 cells. Expression of Fas apoptotic pathway genes by reverse transcription–PCR analysis. Total RNA was isolated from Jurkat cells, DLD1 parental cells, and various transfectant ETS1-expressing cell lines without treatment with anti-Fas-R antibody CH-11 (A) or from control cells (tTA1) and p42-ETS1-expressing cells (ΔVII-19) after incubation with anti-Fas-R antibody CH-11 (100 ng/ml) at different time points (B). Reverse transcription–PCR analysis was conducted under the following conditions: ICE/caspase-1, 56°C, 40 cycles; CPP32/caspase-3, 54°C, 34 cycles; Fas-R, 56°C, 32 cycles; Fas-L, 60°C, 34 cycles; XIAP, 48°C, 30 cycles; FLIP, 57°C, 38 cycles; and S26, 58°C, 28 cycles. Each of these genes and the size of the products are indicated. These results represent at least four independent experiments.
ICE/Caspase-1 and Not CPP32/Caspase-3 Is Involved in Fas-Mediated Apoptosis in p42-ETS1-Expressing Cells.
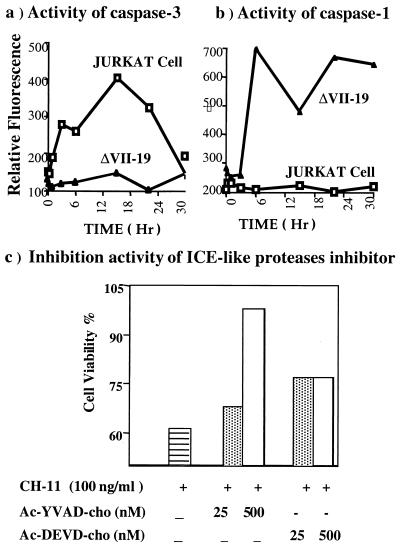
To further verify that p42-ETS1 induces ICE/caspase-1, the activities of ICE/caspase-1 and CPP32/caspase-3 were assayed after Fas mAb treatment in both p42-ETS1-expressing transfectants and Jurkat cells. The activity of CPP32/caspase-3 in Jurkat cells increases and reaches a peak at 20 hr but it does not increase significantly in p42-ETS1 cells (Fig. 5a). ICE/capase-1 activity increases in p42-ETS1 cells by 6 hr. However, we cannot detect this activity in Jurkat cells (Fig. 5b). Treatment of p42-ETS1 cells with inhibitors of ICE-like family of proteases protects them from apoptosis induced by Fas crosslinking. More cells become viable in p42-ETS1 cultures with increased concentrations of Ac-YVAD-CHO, an ICE/caspase-1-specific inhibitor (Fig. 5c). However, Ac-DEVD-CHO, a caspase-3-sepcific inhibitor, only slightly inhibits the apoptosis, because Ac-YVAD-CHO is highly specific for ICE/caspase-1 compared with CPP32/caspase-3 (34). This result strongly indicates that ICE/caspase-1 is involved in the Fas-induced apoptosis in the p42-ETS1-expressing cells.
Figure 5.
Role of ICE-like protease activity in p42-ETS1-mediated Fas-induced apoptosis. DLD1 cells expressing the p42-ETS1 gene (▴, ΔVII-19) and control Jurkat cells (□, Jurkat) were incubated with Fas antibody CH-11 (100 ng/ml). Approximately 2 × 105 cells were collected and lysed at the indicated times, and equal amounts of protein (100 μg per reaction) were incubated for 2 hr with Ac-YVAD-AMC (substrate for caspase-1; 25 μM) or Ac-DEVD-AMC (substrate for caspase-3; 20 μM). The activities of caspase-1 (a) and caspase-3-like (b) proteases were measured as described in Materials and Methods. Data presented are means derived from two independent experiments. The inhibitors of ICE-like protease Ac-YVAD-CHO (caspase-1 specific) and Ac-DEVD-CHO (caspase-3 specific) were incubated with p42-ETS1-expressing cells 2 hr before adding anti-Fas antibody. After 18 hr, cell viability was measured by MTT assay (c). These results represent two independent experiments.
DISCUSSION
ETS1 protein is needed for the maintenance and survival of T cell and for B cell proliferation (35). Many ETS1 target genes are known to be involved in lymphoid cell development and maturation, including T cell receptor α and β chains, CD4, interleukin 2 receptor β, and Igμ (26, 30). However, little is known about the role of ETS1 in other cells or tissues, especially in somatic tumor cells. ETS1 is not expressed in epithelial tissues or in tumor cell lines (36). It has been shown previously that ectopic expression of ETS1 protein in colon cancer cells, as well as other epithelial cell lines, suppresses tumorigenicity both in vitro and in vivo (29, 31). In this paper we demonstrate that that p42-ETS1 induces apoptosis in epithelial cancer cells, rescuing the damaged Fas-apoptotic pathway that exists in these tumor cells. This result is consistent with previous data showing that p42-ETS1 protein-expressing colon cancer cells undergo apoptosis only in the presence of low concentrations of serum. Fas crosslinking acts as a trigger of apoptosis in p42-ETS1-expressing colon cancer cells, as does serum starvation in p42-ETS1-expressing cells.
The ETS1 gene encodes two distinct proteins, a 51-kDa protein (p51-ETS1) encoded by full-length mRNA, and a 42-kDa protein (p42-ETS1) encoded by an alternatively spliced mRNA lacking exon VII (24, 25). We have previously shown that p42-ETS1 and not p51-ETS1 triggers apoptosis in low serum (33). We show in this paper that p42-ETS1 also induces apoptosis after the crosslinking of Fas antibodies in normal serum. Thus, regardless of the apoptotic trigger (e.g., low serum or Fas antibody), the p42-ETS1 protein is the factor that is responsible for induction of apoptosis. It seems that ETS1 may have different activity depending on the spliced isoform. Because only p42-ETS1 induces apoptosis, and not p51-ETS1, the apoptotic inducing activity must result from the absence of exon VII. Alternate splicing of p51-ETS1 brings an important change in protein structure. First, the major p51-ETS1 phosphorylation site occurs in a cluster of serine residues localized in the exon VII-encoded domain (see Fig. 1). This phosphorylation has been shown to inhibit DNA binding and to decrease the half-life of the ETS1 protein (28, 37). Lack of phosphorylation sites in p51-ETS1 results in a protein with dramatically enhanced transcriptional activity (38). Furthermore, the N-terminal inhibitory domain (amino acids 280–331, see Fig. 1) of the p51-ETS1 protein is deleted in p42-ETS1, thus enhancing its DNA-binding activity (27, 39). These properties may explain the unique transcriptional activity of p42-ETS1 with respect to ICE/caspase-1. Protein–protein interactions may also provide various functions to the p42-ETS1 protein. Intramolecular inhibition of ETS1 could be relieved by its partner(s) through an allosteric change (e.g., dephosphorylation) or a conformational change (variant form). The fact that p42-ETS1 induces apoptosis, behaving as transcriptional factor, is shown because mutations in the transactivation domain result in loss of transcriptional activity (29) as well as loss of apoptotic induction. The p42-ETS1 mutations in the transactivation domain include amino acid substitutions at codon 202 (Glu-202 to Gly) and 212 (Asp-212 to His) (see Fig. 1). It would be very interesting to determine if deletion of the p42-ETS1 DNA-binding domain has an affect on apoptosis.
Our data clearly demonstrate that the p42-ETS1 protein and not the p51-ETS1 protein restores the damage to the Fas-induced apoptosis of the epithelial cancer cells by up-regulating, directly or indirectly, the ICE/caspase-1 gene expression, and not that of the other caspases.
Interesting questions raised by the results presented in this paper include the following: (i) Does ICE/caspase-1 or its transcription-level regulatory properties provide an alternative apoptotic pathway in cancer cells? (ii) Can ICE/caspase-1 be an executioner protease?
It is known that the expression of ICE/caspase-1 is tightly controlled at the transcriptional level by other transcription factors: (i) The tissue specificity of caspase-1/ICE is more restricted than that of CPP32/caspase-3 subfamily (40), and also less ICE/caspase-1 mRNA is detected in advanced tumors than in early stage tumors (41). (ii) ICE expression is induced by DNA damage and by chemotherapeutic agents in T lymphocytes (42) in which p53 independent apoptosis depends upon the interferon regulatory factor-1 that activates ICE expression (43, 44). Also epidermal growth factor and interferon-γ induce ICE gene expression in a STAT1-dependent manner (45, 46). (iii) It has been reported that ICE gene expression is negatively regulated by basement membrane extracellular matrix in mammary epithelial cells (47). Although we do not know whether p42-ETS1 directly activates ICE/caspase-1 or acts indirectly through other transcription factor(s), two potential ETS binding sites have been identified in the ICE/caspase-1 promoter region (−465 and −810).
Regarding the role of caspase-1, it has been shown recently that ICE/caspase-1 may function as an executioner protease. This is indicated as follows: (i) Interleukin 1β is not the only substrate of ICE, which also cleaves PARP (48), Ich1(49), actin (50), and PITSLRE (51). The proteolytic modification of these proteins leads to the morphological and biochemical changes that accompany apoptosis. (ii) ICE-treated mitochondria release an apoptosis-inducing factor that causes changes in chromatin condensation and leads to oligonucleosomal DNA fragmentation in isolated nuclei in vitro (52). (iii) ICE/caspase-1 and caspase-11 in the murine system (the counterpart of human caspase-4/ich-2/TX) are members of the ICE-like superfamily and have similarities in function (53) and tissue distribution (40). Therefore, it is reasonable that this subfamily of ICE-like proteases provides an alternative apoptotic pathway for cells in which the dominant pathway has been blocked.
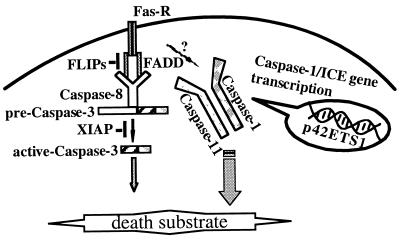
In summary, we have shown that p42-ETS1 by-passes the damaged Fas-induced apoptotic pathway in colon cancer cells by up-regulating ICE/caspase-1 and causes these cancer cells to become susceptible to the effects of the normal apoptosis activation system. ICE/caspase-1 is a redundant system in many cells and tissues, and it becomes important in activating apoptosis in cells where the normal apoptosis pathway is blocked. A hypothetical model of p42-ETS1-mediated apoptosis is shown in Fig. 6. p42-ETS1 activates a critical alternative apoptosis pathway in cancer cells that are resistant to normal immune attack, and thus it may be useful as an anticancer therapeutic.
Figure 6.
Proposed model for role of p42-ETS1 in the Fas-induced apoptotic pathway.
Acknowledgments
This work was supported in part by National Institutes of Health Grant P01CA78582 and the American Cancer Society GN-164 to T.S.P.
ABBREVIATIONS
- ICE
interleukin 1β-converting enzyme
- Fas-R
Fas receptor
- Fas-L
Fas ligand
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- Ac-DEVD
acetyl-Asp-Glu-Val-Asp
- Ac-YVAD
acetyl-Tyr-Val-Ala-Asp
- AMC
7-amino-4-methylcoumarin
- CHO
aldehyde
References
- 1.Steller H. Science. 1995;267:1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- 2.Jacobson M D, Weil M, Raff M C. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- 3.Rowan S, Fisher D E. Leukemia. 1997;11:457–465. doi: 10.1038/sj.leu.2400626. [DOI] [PubMed] [Google Scholar]
- 4.White E. Genes Dev. 1996;10:1–15. doi: 10.1101/gad.10.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, Seto Y, Nagata S. Cell. 1991;66:233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 6.Nagata S, Golstein P. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 7.Shiraki K, Tsuji N, Shioda T, Isselbacher K J, Takahashi H. Proc Natl Acad Sci USA. 1997;94:6420–6425. doi: 10.1073/pnas.94.12.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Connell J, O’Sullivan G C, Collins J K, Shanahan F. J Exp Med. 1996;184:1075–1082. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hitoshi Y, Lorens J, Kitada S I, Fisher J, LaBarge M, Ring H Z, Francke U, Reed J C, Kinoshita S, Nolan G P. Immunity. 1998;8:461–471. doi: 10.1016/s1074-7613(00)80551-8. [DOI] [PubMed] [Google Scholar]
- 10.Hu S, Vincenz C, Ni J, Gentz R, Dixit V M. J Biol Chem. 1997;272:17255–17257. doi: 10.1074/jbc.272.28.17255. [DOI] [PubMed] [Google Scholar]
- 11.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer J L, Schroter M, Burns K, Mattmann C, et al. Nature (London) 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 12.Deveraux Q L, Takahashi R, Salvesen G S, Reed J C. Nature (London) 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson D W, Thornberry N A. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 14.Muzio M, Chinnaiyan A M, Kischkel F C, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, et al. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 15.Medema J P, Scaffidi C, Kischkel F C, Shevchenko A, Mann M, Krammer P H, Peter M E. EMBO J. 1997;16:2794–2804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 17.Enari M, Talanian R V, Wong W W, Nagata S. Nature (London) 1996;380:723–726. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- 18.Enari M, Hug H, Nagata S. Nature (London) 1995;375:78–81. doi: 10.1038/375078a0. [DOI] [PubMed] [Google Scholar]
- 19.Shi L, Chen G, MacDonald G, Bergeron L, Li H, Miura M, Rotello R J, Miller D K, Li P, Seshadri T, et al. Proc Natl Acad Sci USA. 1996;93:11002–11007. doi: 10.1073/pnas.93.20.11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J, et al. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 21.Kuida K, Zheng T S, Na S, Kuan C, Yang D, Karasuyama H, Rakic P, Flavell R A. Nature (London) 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- 22.Hasegawa J, Kamada S, Kamiike W, Shimizu S, Imazu T, Matsuda H, Tsujimoto Y. Cancer Res. 1996;56:1713–1718. [PubMed] [Google Scholar]
- 23.Miura M, Zhu H, Rotello R, Hartwieg E A, Yuan J. Cell. 1993;75:653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- 24.Jorcyk C L, Watson D K, Mavrothalassitis G J, Papas T S. Oncogene. 1991;6:523–532. [PubMed] [Google Scholar]
- 25.Koizumi S, Fisher R J, Fujiwara S, Jorcyk C, Bhat N K, Seth A, Papas T S. Oncogene. 1990;5:675–681. [PubMed] [Google Scholar]
- 26.Bhat N K, Papas T S. In: Challenges of Modern Medicine. Verna R, Shamoo A, editors. Vol. 5. Rome: Aves-Sevono Symposia; 1994. pp. 63–68. [Google Scholar]
- 27.Jonsen M D, Petersen J M, Xu Q P, Graves B J. Mol Cell Biol. 1996;16:2065–2073. doi: 10.1128/mcb.16.5.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabault B, Ghysdael J. J Biol Chem. 1994;269:28143–28151. [PubMed] [Google Scholar]
- 29.Suzuki H, Romano-Spica V, Papas T S, Bhat N K. Proc Natl Acad Sci USA. 1995;92:4442–4446. doi: 10.1073/pnas.92.10.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wasylyk B, Hahn S L, Giovane A. Eur J Biochem. 1993;211:7–18. doi: 10.1007/978-3-642-78757-7_2. [DOI] [PubMed] [Google Scholar]
- 31.Huang C C, Papas T S, Bhat N K. Oncogene. 1997;15:851–856. doi: 10.1038/sj.onc.1201408. [DOI] [PubMed] [Google Scholar]
- 32.Li R, Sutphin P D, Schwartz D, Pei H, Rotter V. Oncogene. 1998;16:3269–3277. doi: 10.1038/sj.onc.1201867. [DOI] [PubMed] [Google Scholar]
- 33.Almog N, Li R, Peled A, Schwartz D, Wolkowicz R, Goldfinger N, Pei H, Rotter V. Mol Cell Biol. 1997;17:713–722. doi: 10.1128/mcb.17.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Margolin N, Raybuck S A, Wilson K P, Chen W, Fox T, Gu Y, Livingston D J. J Biol Chem. 1997;272:7223–7228. doi: 10.1074/jbc.272.11.7223. [DOI] [PubMed] [Google Scholar]
- 35.Bhat N K, Fischinger P J, Seth A, Watson D K, Papas T. Int J Oncol. 1996;8:841–846. doi: 10.3892/ijo.8.5.841. [DOI] [PubMed] [Google Scholar]
- 36.Romano-spica V S H, Georgious P, Chen S-L, Ascione R, Papas T K, Bhat N K. Int J Oncol. 1994;4:521–531. doi: 10.3892/ijo.4.3.521. [DOI] [PubMed] [Google Scholar]
- 37.Fisher R J, Fivash M, Casas-Finet J, Erickson J W, Kondoh A, Bladen S V, Fisher C, Watson D K, Papas T. Protein Sci. 1994;3:257–266. doi: 10.1002/pro.5560030210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hodge D R, Robinson L, Watson D, Lautenberger J, Zhang X K, Venanzoni M, Seth A. Oncogene. 1996;12:11–18. [PubMed] [Google Scholar]
- 39.Wasylyk C, Kerckaert J P, Wasylyk B. Genes Dev. 1992;6:965–974. doi: 10.1101/gad.6.6.965. [DOI] [PubMed] [Google Scholar]
- 40.Van de Careen M, Vandenabeele P, Declercq W, Van den Brande I, Van Loo G, Molemans F, Schotte P, Van Criekinge W, Beyaert R, Fiers W. FEBS Lett. 1997;403:61–69. doi: 10.1016/s0014-5793(97)00026-4. [DOI] [PubMed] [Google Scholar]
- 41.Ikeda H, Nakamura Y, Hiwasa T, Sakiyama S, Kuida K, Su M S, Nakagawara A. Eur J Cancer. 1997;33:2081–2083. doi: 10.1016/s0959-8049(97)00214-1. [DOI] [PubMed] [Google Scholar]
- 42.Kondo S, Barna B P, Morimura T, Takeuchi J, Yuan J, Akbasak A, Barnett G H. Cancer Res. 1995;55:6166–6171. [PubMed] [Google Scholar]
- 43.Tamura T, Ueda S, Yoshida M, Matsuzaki M, Mohri H, Okubo T. Biochem Biophys Res Commun. 1996;229:21–26. doi: 10.1006/bbrc.1996.1752. [DOI] [PubMed] [Google Scholar]
- 44.Tamura T, Ishihara M, Lamphier M S, Tanaka N, Oishi I, Aizawa S, Matsuyama T, Mak T W, Taki S, Taniguchi T. Nature (London) 1995;376:596–599. doi: 10.1038/376596a0. [DOI] [PubMed] [Google Scholar]
- 45.Kumar A, Commane M, Flickinger T W, Horvath C M, Stark G R. Science. 1997;278:1630–1632. doi: 10.1126/science.278.5343.1630. [DOI] [PubMed] [Google Scholar]
- 46.Chin Y E, Kitagawa M, Kuida K, Flavell R A, Fu X Y. Mol Cell Biol. 1997;17:5328–5337. doi: 10.1128/mcb.17.9.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boudreau N, Sympson C J, Werb Z, Bissell M J. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gu Y, Sarnecki C, Aldape R A, Livingston D J, Su M S. J Biol Chem. 1995;270:18715–18718. doi: 10.1074/jbc.270.32.18715. [DOI] [PubMed] [Google Scholar]
- 49.Harvey N L, Trapani J A, Fernandes-Alnemri T, Litwack G, Alnemri E S, Kumar S. Genes Cells. 1996;1:673–685. doi: 10.1046/j.1365-2443.1996.00255.x. [DOI] [PubMed] [Google Scholar]
- 50.Mashima T, Naito M, Fujita N, Noguchi K, Tsuruo T. Biochem Biophys Res Commun. 1995;217:1185–1192. doi: 10.1006/bbrc.1995.2894. [DOI] [PubMed] [Google Scholar]
- 51.Beyaert R, Kidd V J, Cornelis S, Van de Craen M, Denecker G, Lahti J M, Gururajan R, Vandenabeele P, Fiers W. J Biol Chem. 1997;272:11694–11697. doi: 10.1074/jbc.272.18.11694. [DOI] [PubMed] [Google Scholar]
- 52.Susin S A, Zamzami N, Castedo M, Daugas E, Wang H G, Geley S, Fassy F, Reed J C, Kroemer G. J Exp Med. 1997;186:25–37. doi: 10.1084/jem.186.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang S, Miura M, Jung Y K, Zhu H, Li E, Yuan J. Cell. 1998;92:501–509. doi: 10.1016/s0092-8674(00)80943-5. [DOI] [PubMed] [Google Scholar]