Abstract
Purpose:
Arsenic is a valuable therapeutic tool in cancer treatment. T-LAK cell-originated protein kinase (TOPK) is highly expressed in cancer cells but its specific function is still unknown. We investigated the role of TOPK in arsenic-induced apoptosis in RPMI7951 human melanoma cells.
Experimental Design:
Expression of TOPK was evaluated in different melanoma cell lines and LC-MS/MS analysis was used to identify proteins binding with TOPK. Immunofluorescence, Western blot and flow cytometry were used to assess the effect of arsenic on TOPK, histone H2AX and apoptosis in RPMI7951 cells.
Results:
Melanoma cell lines expressing high levels of TOPK were more resistant to arsenite (As3+)-induced apoptosis. As3+ treatment induced phosphorylation of TOPK and histone H2AX in RPMI7951 human melanoma cells. LC-MS/MS results indicated that TOPK could bind with histone H2AX and in vitro and in vivo assays confirmed that TOPK binds with and phosphorylates histone H2AX. As3+ treatment caused phosphorylation of TOPK, which co-localized with phosphorylated histone H2AX in the nucleus. TOPK siRNA cells exhibited a decreased phosphorylation of histone H2AX with As3+ treatment. As3+-induced apoptosis was decreased in H2AX−/− cells but increased in TOPK siRNA cells.
Conclusion:
TOPK binds with histone H2AX and inhibits As3+-induced apoptosis through phosphorylation of histone H2AX. Melanoma cell lines with high levels of TOPK are more resistant to As3+-induced apoptosis. Therefore, inhibition of TOPK activity combined with As3+ treatment may be helpful in the treatment of melanomas.
Keywords: apoptosis, histone H2AX, TOPK, phosphorylation, JB6 Cl41
TOPK (T-LAK cell-originated protein kinase) is a novel mitotic protein kinase that is highly expressed only in various cancers such as leukemia, myeloma, and lymphoma, and its expression has been correlated with the malignant potential of these tumors (1-4). TOPK is phosphorylated and active only during mitosis (2) and has been shown to phosphorylate the p38 mitogen-activated protein (MAP) kinase, but not extracellular signal-regulated kinases (ERKs) or c-Jun N-terminal kinases (JNKs) (1). Recent reports demonstrated that TOPK is up-regulated in murine myeloma cells by an IL-6 mediated protein-protein interaction between TOPK and Raf-A (4, 5). In addition, TOPK has been shown to be involved in cell cycle regulation and can be a substrate of cdc2/cyclin B (2, 6). TOPK expression has also been shown to be up-regulated during the G2 to M phase transition, where the cdc2/cyclin B complex plays an important role (6). Thr9 is an important phosphorylation site of TOPK because when Thr9 was substituted to Ala, the binding ability of TOPK to cdc2/cyclin B was decreased (6). The cell cycle-specific transcription factors E2F and CREB/ATF are critical regulators of TOPK expression during growth arrest in leukemia cells (7). Thus TOPK may be a potential target for chemotherapeutic or chemopreventive compounds.
The effectiveness of many therapeutic approaches, including γ-irradiation and chemotherapeutic drug treatment, has been proposed to be associated with the reactivation of apoptosis in cancer cells. Arsenic is a paradoxical compound that induces global changes in gene expression and cell signal transduction pathways in different types of cells (8, 9). Dose-dependent effects of arsenic in a tissue-specific manner have enabled arsenic to be used for the effective treatment of certain types of cancers, including leukemia and myeloma, through its induction of apoptosis (10, 11). Arsenic treatment has been shown to induce the up-regulation of cyclin B1 and activates the cdc2/cyclin B1 complex in mitotic cells (12), and therefore could have an effect on TOPK and its potential binding partners.
Melanomas develop through well-defined morphological and histological stages that involve the loss of cell proliferation control, the acquisition of invasiveness, and ultimately, the acquisition of metastatic potential (13). Melanoma cells generally express chromosome instability during their development (14). Histone H2AX has been shown to prevent aberrant repair of both programmed and general DNA breakage (15). H2AX deficiency decreases genomic stability and increases tumor susceptibility of normal cells and tissues (16). In addition, a lack of histone H2AX causes genomic instability in mice (17). Here, we have investigated a functional relationship and interaction of TOPK and histone H2AX in mediating arsenite-induced apoptosis in melanoma cells. Results indicated that arsenite induced phosphorylation of TOPK and TOPK directly phosphorylated histone H2AX, resulting in the inhibition of arsenite-induced apoptosis in RPMI7951 melanoma cells. This suggested that TOPK could be a potential target for chemotherapeutic treatment of melanomas.
Materials and Methods
Reagents and antibodies
Arsenite (As3+) and anti-β-actin were from Sigma (St. Louis, MO); NE-PER Nuclear and Cytoplasmic Extraction Reagents were from PIERCE Biotechnology (Rockville, IL). Stock solutions of As3+ (10 mM) in 0.1% DMSO were stored at −20°C. PBK/TOPK, phospho-PBK/TOPK (Thr9) antibodies and active GST-TOPK were from Cell Signaling Technology, Inc. (Beverly, MA); anti-histone H2AX, anti-phospho-H2AX and histone H2AX (recombinant protein expressed in E. coli) were from Upstate Biotechnology, Inc. (Lake Placid, NY); anti-HA-probe (F-7) was from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell culture
SK-MEL5 and SK-MEL28 human malignant melanoma and SK-MEL31 and RPMI7951 human malignant melanoma epithelial-like cell lines were from America Type culture collection (ATCC, Manassas, VA). Human melanoma cell lines were cultured in MEM containing 10% FBS (15% FBS for SK-MEL31), 2 mM L-glutamine, and 25 μg/ml gentamicin, 0.1 mM non-essential amino acids, 1 mM sodium pyruvate (SK-MEL31 also required 1.5 g/L sodium bicarbonate) at 37°C in humidified air with 5% CO2. Mouse epidermal JB6 promotion-sensitive Cl41 cells were cultured in MEM containing 5% FBS, 2 mM L-glutamine, and 25 μg/ml gentamicin at 37°C in humidified air with 5% CO2. H2AX+/+ and H2AX−/− cell lines were a gift from Dr. Andre Nussenzweig (Experimental Immunology Branch, National Cancer Institute, NIH, Bethesda, MD) and were cultured in DMEM supplemented with 10% FBS, 2 mM L-glutamine, and 25 μg/ml gentamicin at 37°C in humidified air with 5% CO2.
Generation of the TOPK overexpressing cell line
pc-DNA3, pcDNA3-HA-TOPK or mutant pcDNA3-HA-TOPK-T9A (gifts from Dr. J. Abe, Department of Pathology, Division of Molecular Pathology, Ehime University School of Medicine, Toh-on, Ehime, Japan) (6) was transfected into JB6 Cl41 cells using Superfect (Qiagen, Valencia, CA). Transfected cells were selected in MEM containing 5% FBS and G418 (800 μg/ml) for two weeks. Stable cell lines were maintained in MEM containing 5%FBS and 200 μg/ml G418.
siRNA preparation and vector construction
Two pairs of hairpin siRNA oligonucleotides, containing BamHI and HindIII sites, were designed as described previously (18). Hairpin siRNA template oligonucleotides were chemically synthesized, deprotected and gel-purified by Sigma Genosys (Woodlands, TX). The TOPK siRNA sequence target sequences were aligned to the genome database in a BLAST search to ensure sequences without significant homology to other genes. The sense siRNA template sequence for TOPK was 5′-GATCCGAGGTTTGTCTCATTCTCCTTCAAGAGAGGAGAATGAGACAAACCTCTTTTTTGGAAA-3′ and the antisense siRNA template sequence was 5′-AGCTTTTCCAAAAAAGAGGTTTGTCTCATTCTCCTCTCTTGAAGGAGAATGAGACAAACCTCG-3′. The sense and antisense oligonucleotides were annealed and cloned into the pSilencer 3.1-H1 neo vector (Amnion, TX) at the BamHI and HindIII sites as described by the manufacturer. A scrambled siRNA with a sequence lacking significant homology to the mouse, human, or rat genome database was used as the control or mock siRNA. The resulting pSilencer 3.1-H1-siRNA plasmids were transfected into RPMI7951 cells and the stable cell lines were obtained by G418 screening. These TOPK siRNA cell lines were cultured in MEM supplemented with 10% FBS, 2 mM L-glutamine, 25 μg/ml gentamicin, 0.1 mM non-essential amino acids, 1 mM sodium pyruvate and 200 μg/ml G418.
Identification of proteins binding with TOPK by LC-MS/MS analysis
The topk gene was amplified by PCR and then cloned into pET-46 using a pET-46 EK/LIC kit (Novagen, Madison, WI). His-TOPK was purified from BL21 (DE3) cells (Novagen, Inc., Madison, WI). His-TOPK (0.5 mg) was used for binding with 400 μl Ni-NTA-Agarose beads (QIAGEN, Hilden, Germany). Then a lysate (10 mg) of RPMI7951 cells was incubated with His-TOPK-beads at 4°C overnight. The TOPK binding proteins were eluted with 50% acetonitrile (Fisher Biotech, Fair Lawn, New Jersey). Approximately 12 μg of protein eluted from the His-TOPK beads were digested in solution with sequencing grade modified trypsin (Promega, Madison, WI) according to the manufacturer's protocol. The sample was purified using a C18 SepPak cartridge (Waters Ins., Milford, MA) according to manufacturer's directions, then speed-vacuumed to dryness. The peptide mixture was reconstituted with loading buffer (98:2, H2O:acetonirile, 0.1% formic acid) and analyzed by capillary LC-MS/MS (19, 20). Product ion mass spectra were searched against NCBI's (http://www.ncbi.nlm.nih.gov/) non-redundant database (April 20, 2005, total 1,182,676 protein sequences), interpreted using the Pro ID v 1.1 software (ABI), which used the Interrogator algorithm for scoring peptide/protein candidates (21) and results were verified by manual interpretation.
SDS-PAGE and Western blotting
Cell lines (7 × 105) were cultured in their respective medium for 12-15 h in 10-cm diameter dishes to 70-80% confluence. Cells were treated with As3+ and harvested after 24 h with 200 μl of RIPA buffer (1×PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM Na3VO4, and 1 mM aprotinin and 1 mM phenylmethylsulfonyl fluoride). The quantity of protein was determined by the Bradford method (22). The samples (30-50 μg of protein) with 5×SDS were loaded into 10%-15% SDS polyacrylamide gel for electrophoresis and subsequently transferred onto an Immobilon-P transfer membrane (Millipore, Chelmsford, MA). Antibody-bound proteins were detected by chemiluminescence (ECF, Amersham-Pharmacia Biotech, Piscataway, New Jersey) and analyzed using the Storm 840 Scanner (Molecular Dynamics, Sunnyvale, CA). Untreated cell samples were used as negative controls.
In vitro kinase assays
Samples containing recombinant histone H2AX expressed in E. coli (Upstate Biotechnology) were incubated at 30°C for 30 min with active GST-TOPK (Cell Signaling) in 10x kinase buffer A [50 mM Tris-HCl pH 7.5, 10 mM MgCl2, 1 mM EGTA, 1 mM DTT, and 0.01 % Brij 35] (Cell Signaling) containing 200 μM ATP. The reactions were stopped by adding 5×SDS sample buffer. Then phosphorylation of H2AX (Ser139) was analyzed by Western blot using a phospho-H2AX (Ser139) antibody. Phosphorylation of histone H2AX by JNK1 was used as a positive control. For some experiments, equal protein loading was verified by silver staining for histone H2AX.
Immunofluorescence assay
To determine the translocation ability of phosphorylated TOPK and phosphorylated H2AX, RPMI7951 melanoma cells (5×105) treated or not treated with 2.5 μM As3+ were incubated for 24 h. Cells were fixed in 4% paraformaldehyde and incubated with anti-phospho-TOPK and anti-phospho-H2AX and then with either FITC-conjugated secondary antibody or Texas Red-conjugated secondary antibody (Invitrogen). Samples were analyzed with a fluorescence microscope system (Leica, Mannheim, Germany).
Isolation of histone H2AX (23)
Histones were extracted from As3+ treated cells by disrupting cells with NETN buffer [150 mM NaCl, 1 mM EDTA, 20 mM Tris (pH 8.0), 0.5% nonionic detergent Igeral CA 630 (NP-40), Sigma]. The insoluble fraction was pelleted for 5 min in a microcentrifuge (8,400 rpm). Nuclei were extracted with 0.1 N HCl to isolate total histones. Samples were precipitated with 1 M Tris-HCl, pH 8.0 and then resuspended in double-distilled H2O.
Flow-cytometry analysis
Apoptosis induced by As3+ was determined using the Annexin V-FITC Apoptosis Detection Kit (Medical& Biological Laboratories, Nagoya, Japan) according to the protocol provided. Briefly, cells were trypsinized, washed once with MEM containing serum, and incubated with annexin V-conjugated FITC. Apoptosis was analyzed using a flow cytometer (FACSCalibur; Becton-Dickinson).
Statistical analysis
Comparisons were made using one-way ANOVA and data are expressed as means ± S.D. of 3-4 independent experiments. Differences were considered significant with a p value of < 0.05.
Results
TOPK expression in human melanoma cell lines
TOPK is expressed in a wide range of cancers, including leukemia, myeloma, and lymphoma (1-4, 7). However the expression of TOPK in melanoma cells is yet to be determined. Thus the expression of TOPK was compared in the mouse epidermal JB6 Cl41 skin cell line and in 4 different human malignant melanoma cell lines, SK-MEL28, SK-MEL31, SK-MEL5 and RPMI7951, which are routinely used in our laboratory. Results indicated that the expression of TOPK in the SK-MEL28 and RPMI7951 cell lines was highest compared to SK-MEL31, SK-MEL5 or JB6 Cl41 cells (Fig. 1). Based on these results, we chose the RPMI7951 melanoma cell line to study TOPK function and to identify potential binding partners.
Fig. 1.

TOPK expression in several cell lines. Mouse skin JB6 Cl41 cells and several different human melanoma cell lines were screened by Western blot to determine the total protein expression level of endogenous nonphosphorylated TOPK. β–actin was used to verify equal protein loading. These data are representative of at least three independent experiments.
Identification of TOPK-binding partners by LC-MS/MS
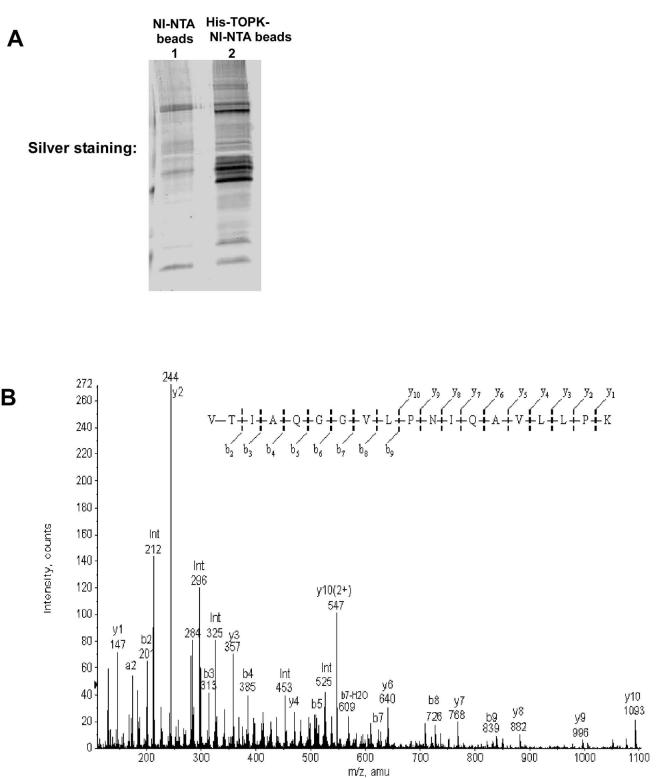
First, a His-TOPK protein was generated and was found to bind strongly with Ni-NTA-agarose beads. His-TOPK was confirmed to remain bound to the beads even after elution with 50% acetonitrile (data not shown). Therefore, His-TOPK-Ni-NTA-agarose (His-TOPK) beads could be used for immobilizing TOPK-binding proteins for subsequent identification. RPMI7951 cell lysate was incubated with Ni-NTA-agarose beads as a negative control (Fig. 2A, lane 1) or Ni-NTA-His-TOPK beads (Fig. 2A, lane 2). After washing with PBS, proteins binding with His-TOPK beads were visualized with silver staining after elution with 50% acetonitrile (Fig. 2A). LC-MS/MS analysis (24) identified 26 individual proteins that could bind with TOPK (data not shown). A fragment comprised of 19 amino acids, VTIAQGGVLPNIQAVLLPK, was identified with a Pro ID (ABI) Confidence of 99%, as a peptide from the family of H2A proteins (Fig. 2B). The human genome contains 16 genes that encode for H2A peptides classified as H2A variants (25). The identified peptide sequence was 100% homologous to the sequences of 12 genes of the 16 H2A histone family members, including the important H2A variant, H2AX. A role for H2AX phosphorylation has been demonstrated in DNA repair, cell cycle checkpoint regulation, regulation of gene recombination events, and tumor suppression (26). The LC-MS/MS result suggested that TOPK could bind with histone H2AX. Thus, we focused on elucidating the function and the physiologic significance of the interaction of TOPK and histone H2AX.
Fig. 2.
TOPK binds with and phosphorylates histone H2AX in vitro. A, TOPK-binding proteins visualized by silver staining. (1) Silver staining of gel showing RPMI7951 cell lysate binding with Ni-NTA agarose beads ; (2) Silver staining of gel showing RPMI7951 cell lysate binding with His-TOPK-Ni-NTA agarose beads. B, tandem mass spectrum of the VTIAQGGVLPNIQAVLLPK peptide. The gene sequence of this peptide was found to be 100% homologous to the sequences of 12 genes of the 16 H2A histone family members, including histone H2AX. The error in the experimental peptide MW (1930.140) was within 11 ppm of the theoretical MW. Diagnostic b- and y-type fragment ions are labeled with the value and the ion type according to the fragment ion nomenclature of Biemann (24). The amino acid sequence is displayed above the spectrum. The experimentally measured y and b ions are written above and below the sequence, respectively. C, confirmation of the binding of TOPK with histone H2AX in vitro using His-TOPK-Ni-NTA agarose beads. Histone H2AX was incubated with Ni-NTA agarose beads (as control, lane 3) or His-TOPK-Ni-NTA agarose beads (lane 5) at 30°C for 30 min. Their binding interaction was analyzed by Western blot using an H2AX antibody. H2AX only (lane 1), Ni-NTA agarose beads only (lane 2), and His-TOPK-NI-NTA agarose beads only (lane 4) were used as controls. The total levels of TOPK served to confirm the presence of TOPK bound to the beads (bottom panel). D, in vitro kinase assay to determine the ability of TOPK to phosphorylate histone H2AX visualized by autoradiography in the presence of [γ-32P]ATP. E, in vitro kinase assay to determine the ability of TOPK to phosphorylate histone H2AX visualized by Western blot using a phospho-H2AX (Ser139) antibody. The binding of JNK1 with H2AX was used as a positive control (unpublished data) and equal protein loading was verified by silver staining for H2AX. These data are representative of at least three independent experiments.
TOPK binds with and phosphorylates histone H2AX in vitro
Histone H2AX was incubated with Ni-NTA agarose beads (as a negative control) or His-TOPK-NI-NTA agarose beads at 30°C for 30 min. The binding interaction was analyzed by Western blot using an H2AX antibody. Results confirmed that TOPK binds with histone H2AX (Fig. 2C). Because TOPK is a kinase, we next determined whether TOPK can phosphorylate histone H2AX by using histone H2AX as substrate for active TOPK. The phosphorylation was visualized by autoradiography in the presence of [γ-32P]ATP (Fig. 2D) or by a phospho-H2AX (Ser139) antibody (Fig. 2E). Phosphorylation of H2AX by JNK1 was used as positive control (unpublished data). These results strongly indicated that TOPK binds with and phosphorylates H2AX at Ser139 in vitro. Silver staining of H2AX, TOPK and JNK1 was utilized as an internal control to verify equal protein loading.
Arsenite induces phosphorylation of TOPK and H2AX in RPMI7951 melanoma cells
Arsenite (As3+) is a toxin with multiple effects in animal and human populations. Low concentration doses of arsenite have been reported to induce apoptosis of human melanoma cells (10, 11). The effect of arsenite on TOPK (Thr9) or H2AX (Ser139) phosphorylation in RPMI7951 melanoma cells was investigated herein by using Western blot analysis. Results indicated that a strong phosphorylation of TOPK (Thr9) and H2AX (Ser139) was induced in a dose-dependent manner following a 24 h As3+ treatment of RPMI7951 cells (Fig. 3, top two panels). For further experiments, cells were treated with 2.5 μM As3+ and harvested 24 h after treatment. No change in the non-phosphorylated levels of TOPK or H2AX expression was observed in these cell lines under these conditions with As3+ treatment (Fig. 3, bottom two panels).
Fig. 3.

Dose-dependent phosphorylation of TOPK (Thr9) and H2AX (Ser139) is induced in As3+ treated cells. RPMI7951 cells were harvested 24 h after treatment with different doses of As3+. TOPK and H2AX proteins in the cell lysate were separated by 10% or 15% SDS-PAGE, respectively, followed by Western blot analysis with specific antibodies against phosphorylation of TOPK at Thr9 and H2AX at Ser139 or against nonphosphorylated TOPK or H2AX. Untreated samples served as negative controls. Total TOPK and H2AX were used to verify equal protein loading. These data are representative of at least three independent experiments.
Phosphorylated TOPK and phosphorylated H2AX co-localize in the nucleus after As3+ treatment
Yih, et al. (27) previously reported that phosphorylated H2AX was clearly detectable in the interface nuclei of CGL-2 cells treated for 24 h with 2 μM As3+. In the present study, RPMI7951 cells were treated with 2.5 μM As3+ for 24 h and then cytoplasmic and nuclear proteins were extracted and TOPK and phosphorylated TOPK were detected by Western blot (Fig. 4A). The results indicated that TOPK is located mostly in the cytoplasm with very little protein in the nucleus in the absence of As3+ treatment (Fig. 4A). TOPK localization to the nucleus increased dramatically with As3+ treatment (Fig. 4A, top panel) and phosphorylated TOPK was located only in the nucleus (Fig. 4A, bottom panel). Immunocytofluorescence analysis was used to further examine the nuclear localization and interaction of TOPK and histone H2AX (Fig. 4B). Results indicated that phosphorylated TOPK (green) and phosphorylated H2AX (red) were both localized in the nucleus after As3+ treatment (Fig. 4B, top and middle right panels). The merged result confirmed that TOPK co-localized with histone H2AX in the nucleus with As3+ treatment (Fig. 4B, bottom right panel).
Fig. 4.
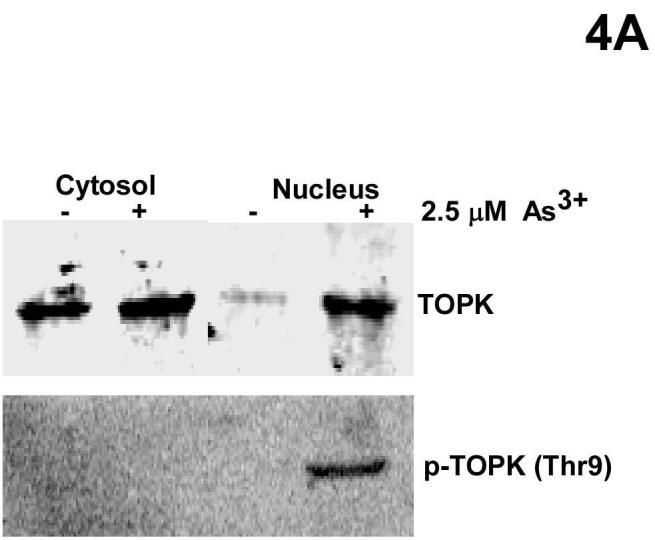

TOPK and histone H2AX co-localize in the nucleus. A, cytosolic and nuclear localization of total and phosphorylated TOPK in RPMI7951 cells after 24 h treatment with 2.5 μM As3+. Cytosolic and nuclear proteins were extracted and separated by 10% SDS-PAGE followed by Western blot analysis with specific antibodies against phosphorylated and nonphosphorylated TOPK. B, nuclear co-localization of phosphorylated TOPK and phosphorylated H2AX. RPMI7951 cells were or were not treated with 2.5 μM As3+ for 24 h. TOPK was visualized under a fluorescence microscope using an FITC-specific antibody (upper panels) and histone H2AX was visualized (middle panels) using a Texas Red-conjugated antibody. Pictures of As3+ treated (right panels) and untreated (left panel) cells represent exactly the same region for each, respectively, allowing the lower panels to demonstrate the merged staining result (x630). Scale bar, 25 μm. These data are representative of at least three independent experiments.
TOPK siRNA transfected cells inhibit the phosphorylation of histone H2AX
RPMI7951 cells were transfected with pSilencer 3.1-H1-topk-siRNA and selected by G418. The expression of TOPK was verified by Western blot using anti-TOPK. Results indicated that in control siRNA mock transfected cells, As3+ induced phosphorylation of TOPK (Thr9) and H2AX (Ser139) (Fig. 5A). On the other hand, As3+-induced phosphorylation of histone H2AX (Ser139), TOPK (Thr9) and non-phosphorylated TOPK were dramatically decreased in TOPK siRNA transfected cells (Fig. 5A). The expression of total H2AX or β-actin was not different between these cell lines (Fig. 5A, bottom two panels). Thus, these data indicated that TOPK is required for mediating As3+-stimulated phosphorylation of H2AX at Ser139.
Fig. 5.
TOPK inhibits As3+-induced apoptosis through phosphorylation of histone H2AX at Ser 139. A, effect of TOPK deficiency on H2AX phosphorylation. Control siRNA and TOPK siRNA cells were cultured and treated with 2.5 μM As3+ for 24 h. Phosphorylation of histone H2AX at Ser139 and TOPK at Thr9 was visualized by Western blotting using phospho-specific antibodies. Total TOPK, H2AX and β-actin were used as internal controls for confirmation of TOPK deficiency and equal protein loading. B, As3+ induced phosphorylation of H2AX in H2AX+/+ cells, but had no effect on phosphorylation of TOPK in H2AX+/+ or H2AX−/− cells. Total TOPK, H2AX and histone H3 were used as internal controls for confirmation of H2AX deficiency and equal protein loading. C, flow cytometry analysis of apoptosis in H2AX+/+ and H2AX−/− cells and D, flow cytometry analysis of apoptosis in control siRNA or TOPK siRNA cells. Cells were incubated with annexin V-conjugated FITC after 24 h treatment with 2.5 μM As3+. Stained cells were analyzed by flow cytometry. Lower-right panel indicates the percentage of early apoptotic cells (annexin V-stained positive cells). These data are representative of at least four independent experiments.
Phosphorylation of histone H2AX is associated with inhibition of As3+-induced apoptosis
H2AX+/+ and H2AX−/− cell lines were treated for 24 h with 2.5 μM As3+ and phosphorylation of histone H2AX (Ser139) and total histone H2AX were determined by Western blot analysis. As expected, the H2AX−/− cell line displayed a markedly reduced expression and phosphorylation of histone H2AX compared with the H2AX+/+ cells (Fig. 5B). However, As3+-induced phosphorylation of TOPK and the expression of total histone H3 or TOPK was similar in the two cell lines (Fig. 5B). Furthermore, early apoptosis was significantly less (P < 0.0001) following treatment with 2.5 μM As3+ at all time points in H2AX−/− cells compared to H2AX+/+ cells (Fig. 5C, bottom right quadrants). This suggested that phosphorylation of H2AX protects cells against As3+-induced apoptosis.
TOPK inhibits As3+-induced apoptosis
Expression of TOPK was associated with decreased As3+-induced apoptosis. At all times after As3+ treatment, a significant (P < 0.0001) increase in early apoptosis was observed in TOPK siRNA cells compared to control siRNA transfected cells (Fig. 5D). This suggested that the absence of TOPK sensitizes cells to As3+-induced apoptosis. Taken together, these results indicated that TOPK plays an important role in inhibiting As3+-induced apoptosis through its phosphorylation of histone H2AX.
As3+-induced apoptosis is dependent on TOPK status
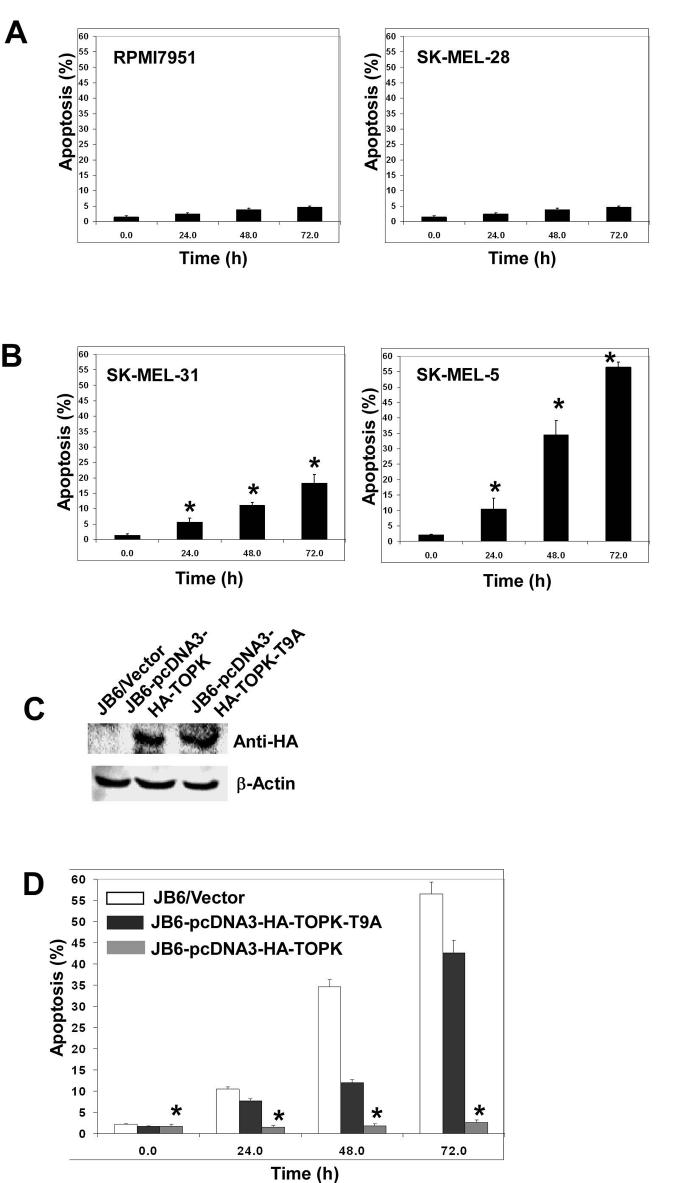
We further confirmed these results by examining the effect of As3+ treatment on apoptosis in human melanoma cells, including SK-MEL28, SK-MEL31, SK-MEL5 and RPMI7951 cells, which have varying expression levels of TOPK. RPMI7951 and SK-MEL28 cells, which both express TOPK, showed very little induction of apoptosis by As3+ at all time points tested (Fig. 6A). In contrast, apoptosis was markedly induced in SK-MEL31 and SK-MEL5, which express low levels of TOPK (Fig. 6B). To further confirm that the expression level of TOPK has a major influence on whether As3+ can induce apoptosis, JB6 Cl41 cells, which do not express TOPK (see Fig. 1), were transfected with pc-DNA3 mock vector (JB6/Vector), pcDNA3-HA-TOPK for overexpression of TOPK or pcDNA3-HA-TOPK-T9A for overexpression of a mutant TOPK (tyrosine 9 replaced with alanine). Expression of these plasmids was verified by Western blot using an HA antibody (Fig. 6C). Induction of apoptosis by As3+ in JB6/Vector cells was nearly 60% after 72 h and nearly 42% in JB6-pcDNA3-HA-TOPK-T9A cells (Fig. 6D). In contrast, very little apoptosis occurred in TOPK-overexpressing cells at any time point. This suggested that suppression of TOPK activity by the mutant TOPK-T9A cells almost totally restored the apoptotic response to As3+. Overall, these results indicated that TOPK is critical in protecting against As3+-induced apoptosis and thus could be an important target in As3+ treatment of cancers.
Fig. 6.
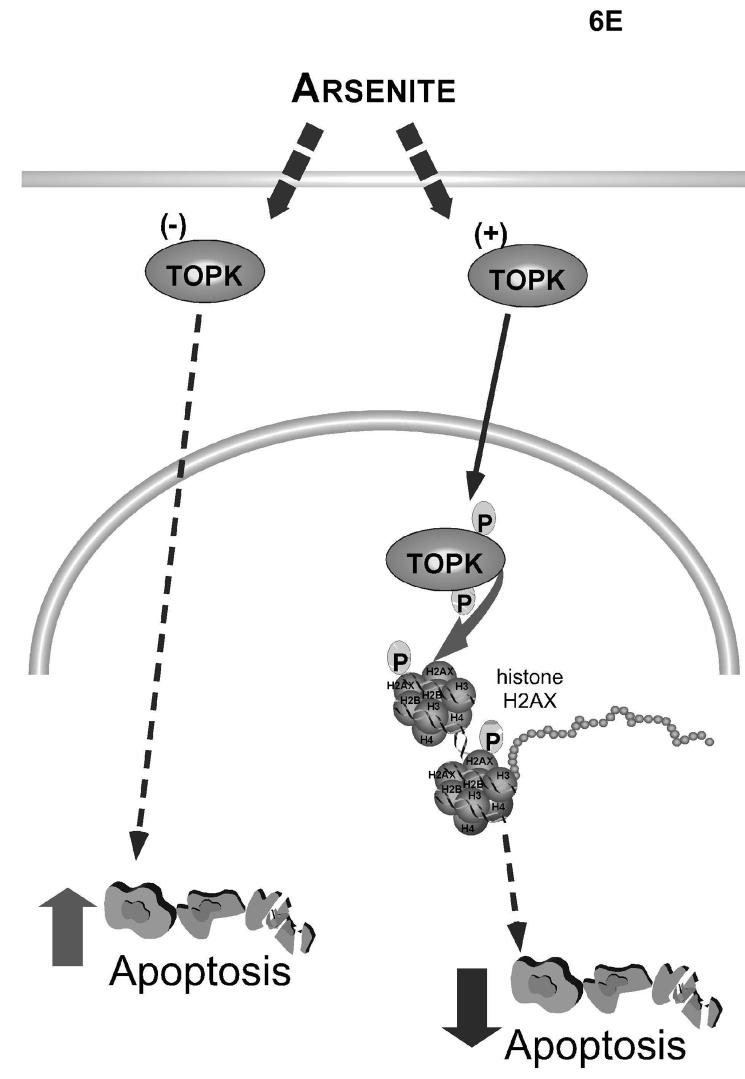
TOPK blocks As3+-induced apoptosis in RPMI7951 melanoma cells. A, As3+ does not induce apoptosis in cell lines that express TOPK compared (B) to cell lines that do not express TOPK. Data are represented as means ± S.D. of 4 independent experiments. Asterick (*) indicates a significant increase in apoptosis over time (P < 0.0001). C, JB6 cells were transfected with pcDNA3-HA-TOPK (JB6-pcDNA3-HA-TOPK), pcDNA3-HA-TOPK-T9A mutant (JB6-pcDNA3-HA-TOPK-T9A) or mock vector (JB6/Vector). Expression of HA-TOPK was detected with an HA antibody; β-actin was used as an internal control to verify equal protein loading. D, apoptosis is not induced in JB6 cells overexpressing TOPK but is restored in cells expressing mutant TOPK. JB6 cells were transfected as in C and cultured for 24, 48 or 72 h with 2.5 μM As3+. Apoptosis was determined by flow cytometry using annexin V-conjugated FITC. Data for D are represented as means ± S.D. of at least 4 independent experiments. Asterick (*) indicates significantly less apoptosis in JB6-TOPK overexpressing cells compared to either JB6/Vector or JB6-TOPK-T9A mutant cells (P < 0.0001). E, in cells expressing TOPK and stimulated with As3+, TOPK is localized in the nucleus, where it phosphorylates histone H2AX resulting in decreased induction of apoptosis.
Discussion
TOPK is highly expressed in lymphoma cells, myeloid leukemia cells and in several other highly proliferative malignant cell lines derived from sarcomas, carcinomas or myelomas of various tissue origins (3, 28). In the present study, we used LC-MS/MS analysis to confirm that His-TOPK could bind with histone H2AX. The in vitro kinase data indicated that TOPK directly interacted with and phosphorylated histone H2AX. This is significant because H2AX has been designated as the histone guardian of the genome (26). Phosphorylation of H2AX has been suggested to have two important functions in DNA repair: to promote changes in the structural configuration of chromatin and to assist in chromatin binding of repair factors. These functions may or may not be related, but both are likely to be required for efficient synapses of broken chromosome ends (26, 29). Several reports have demonstrated that arsenite can induce DNA damage (30, 31). Our laboratory has focused on the elucidation of mechanisms explaining how arsenic can act both as a carcinogen and as an effective chemotherapeutic agent (9, 32, 33). Accumulating data suggested that As3+ may specifically induce apoptosis in certain types of tumor cells, including megakaryocytic leukemia cell lines, human breast cancer cells (31), and in human myeloma cells (11, 33, 34). Furthermore, MAP kinases have been shown to have a very important role in mediating arsenite-induced apoptosis (35).
TOPK, a novel MAPKK-like protein kinase (6), is highly expressed in tumor cells but not in normal cells and the function of this kinase is not clear. In this study, we demonstrated that As3+ induced phosphorylation of both TOPK (Thr9) and histone H2AX (Ser139) and that TOPK directly phosphorylated histone H2AX (Ser139) in vitro and in vivo. Further, Western blot and immunocytofluorescence analyses clearly demonstrated that As3+ induced the accumulation of phosphorylated TOPK and phosphorylated H2AX in the nucleus of RPMI7951 cells. Another group has also shown that arsenic treatment induces the phosphorylation and nuclear accumulation of histone H2AX (27). However, our data indicated the co-localization and direct involvement of TOPK in As3+-induced apoptosis. The accumulation of phosphorylated H2AX in the nucleus suggested that DNA damage occurred in As3+-treated RPMI7951 cells. Previous data showed that after γ-irradiation, nuclei from H2AX+/+ and H2AX−/− fibroblasts exhibited 0.25% and 10% nuclear fragmentation, respectively (17). Loss of H2AX has been shown to lead to increased chromosomal abnormalities, deficiencies in gene targeting, and radiation sensitivity. Furthermore, DNA repair was observed to proceed less efficiently in the absence of H2AX, resulting in increased apoptosis (17). In the present study, we showed that apoptosis in H2AX−/− cells was increased substantially compared to H2AX+/+ cells and in TOPK siRNA transfected cells, As3+-induced apoptosis was also markedly increased. These results suggested that TOPK phosphorylation of histone H2AX is associated with an inhibition of As3+-induced apoptosis in RPMI7951 melanoma cells.
Investigations using many different experimental systems suggested that arsenic may offer significant therapeutic benefits to patients across many neoplasms, and numerous clinical trials are under way in hematopoietic malignancies and solid tumors. Miller at al. summarized reports of the activity of arsenic in animal models and in the clinic (36). Low doses of arsenic trioxide (0.06-0.2 mg/kg of body) can induce complete remission in relapsed acute promyelocytic leukemia patients and complete remissions occurred in 11 of the 12 patients (37). Low doses of As3+ induced apoptosis in some (11, 31-33), but not all cancer cell types (38-40), suggesting a mechanism exists that is responsible for resistance to As3+. Using different melanoma cell lines with different levels of TOPK expression, we found that As3+ induces apoptosis dependent on TOPK expression level. In melanoma cells without expression of TOPK, a low concentration of As3+ induces rapid apoptosis–more than 25-50% in 48 hours. In contrast, melanoma cells expressing high levels of TOPK demonstrated full resistance to As3+ following 72 h exposure, suggesting that melanoma cell lines expressing TOPK are more resistant to As3+-induced apoptosis. These various melanoma cells also display other abnormalities. For example, the p16 gene is deleted in SK-MEL5 (41) cells. Furthermore, B-raf is mutated (V599E) in SK-MEL28 and SK-MEL5 cells, but not in SK-MEL31 cells (42, 43). Interestingly, the TOPK-expressing SK-MEL28 and RPMI7951 cells each express a mutant p53 (44, 45), whereas SK-MEL5 cells express wildtype p53 (44). However, additional studies are needed in order to specifically link the genetic background of these cell lines with resistance to As3+-induced apoptosis. Our study indicated that this resistance appears to be related to the activation and phosphorylation of histone H2AX by activated TOPK (Fig. 6E). In this model, cells lacking TOPK expression readily undergo apoptosis induced by low levels of arsenite. On the other hand, in cells expressing TOPK, arsenite induces its phosphorylation, which leads to phosphorylation of histone H2AX and resistance to apoptosis. Thus, discovery of a small molecule inhibitor of TOPK may be very important in combination with low doses of As3+ in the treatment of melanomas and other TOPK-positive cancers.
Acknowledgments
We are grateful to Dr. A. Nussenzweig (Experimental Immunology Branch, National Cancer Institute, NIH, Bethesda, MD) for the H2AX+/+ and H2AX−/− murine embryonic fibroblasts and Dr. J. Abe (Department of Pathology, Division of Molecular Pathology, Ehime University School of Medicine, Toh-on, Ehime, Japan) for pcDNA3-HA-TOPK and pcDNA3-HA-TOPK-T9A. We thank Todd Schuster for flow cytometry analysis, Andria Hansen for secretarial assistance and Paul Hoversten for his help in the writing of the text for this paper.
Grant support: The Hormel Foundation and National Institutes of Health grants CA77646, CA111356, and CA111536.
Footnotes
T. Zykova and F. Zhu contributed equally to this work.
The University of Minnesota is an equal opportunity educator and employer.
References
- 1.Abe Y, Matsumoto S, Kito K, Ueda N. Cloning and expression of a novel MAPKK-like protein kinase, lymphokine-activated killer T-cell-originated protein kinase, specifically expressed in the testis and activated lymphoid cells. J Biol Chem. 2000;275:21525–31. doi: 10.1074/jbc.M909629199. [DOI] [PubMed] [Google Scholar]
- 2.Gaudet S, Branton D, Lue RA. Characterization of PDZ-binding kinase, a mitotic kinase. Proc Natl Acad Sci U S A. 2000;97:5167–72. doi: 10.1073/pnas.090102397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simons-Evelyn M, Bailey-Dell K, Toretsky JA, Ross DD, Fenton R, Kalvakolanu D, Rapoport AP. PBK/TOPK is a novel mitotic kinase which is upregulated in Burkitt's lymphoma and other highly proliferative malignant cells. Blood Cells Mol Dis. 2001;27:825–9. doi: 10.1006/bcmd.2001.0452. [DOI] [PubMed] [Google Scholar]
- 4.Cote S, Simard C, Lemieux R. Regulation of growth-related genes by interleukin-6 in murine myeloma cells. Cytokine. 2002;20:113–20. doi: 10.1006/cyto.2002.1988. [DOI] [PubMed] [Google Scholar]
- 5.Yuryev A, Wennogle LP. Novel raf kinase protein-protein interactions found by an exhaustive yeast two-hybrid analysis. Genomics. 2003;81:112–25. doi: 10.1016/s0888-7543(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto S, Abe Y, Fujibuchi T, Takeuchi T, Kito K, Ueda N, Shigemoto K, Gyo K. Characterization of a MAPKK-like protein kinase TOPK. Biochem Biophys Res Commun. 2004;325:997–1004. doi: 10.1016/j.bbrc.2004.10.133. [DOI] [PubMed] [Google Scholar]
- 7.Nandi AKRA. Expression of PDZ-binding kinase (PBK) is regulated by cell cycle-specific transcription factors E2F and CREB/ATF. Leukem Res. 2005;96:271–8. doi: 10.1016/j.leukres.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Qian Y, Castranova V, Shi X. New perspectives in arsenic-induced cell signal transduction. J Inorg Biochem. 2003;96:271–8. doi: 10.1016/s0162-0134(03)00235-6. [DOI] [PubMed] [Google Scholar]
- 9.Dong Z. The molecular mechanisms of arsenic-induced cell transformation and apoptosis. Environ Health Perspect. 2002;110(Suppl 5):757–9. doi: 10.1289/ehp.02110s5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu XH, Shen YL, Jing YK, Cai X, Jia PM, Huang Y, Tang W, Shi GY, Sun YP, Dai J, Wang ZY, Chen SJ, Zhang TD, Waxman S, Chen Z, Chen GQ. Apoptosis and growth inhibition in malignant lymphocytes after treatment with arsenic trioxide at clinically achievable concentrations. J Natl Cancer Inst. 1999;91:772–8. doi: 10.1093/jnci/91.9.772. [DOI] [PubMed] [Google Scholar]
- 11.Ivanov VN, Hei TK. Arsenite sensitizes human melanomas to apoptosis via tumor necrosis factor alpha-mediated pathway. J Biol Chem. 2004;279:22747–58. doi: 10.1074/jbc.M314131200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ling YH, Jiang JD, Holland JF, Perez-Soler R. Arsenic trioxide produces polymerization of microtubules and mitotic arrest before apoptosis in human tumor cell lines. Mol Pharmacol. 2002;62:529–38. doi: 10.1124/mol.62.3.529. [DOI] [PubMed] [Google Scholar]
- 13.Clark WH, Jr., Elder DE, Guerry Dt, Epstein MN, Greene MH, Van Horn M. A study of tumor progression: the precursor lesions of superficial spreading and nodular melanoma. Hum Pathol. 1984;15:1147–65. doi: 10.1016/s0046-8177(84)80310-x. [DOI] [PubMed] [Google Scholar]
- 14.Warters RL, Adamson PJ, Pond CD, Leachman SA. Melanoma cells express elevated levels of phosphorylated histone H2AX foci. J Invest Dermatol. 2005;124:807–17. doi: 10.1111/j.0022-202X.2005.23674.x. [DOI] [PubMed] [Google Scholar]
- 15.Bassing CH, Suh H, Ferguson DO, Chua KF, Manis J, Eckersdorff M, Gleason M, Bronson R, Lee C, Alt FW. Histone H2AX: a dosage-dependent suppressor of oncogenic translocations and tumors. Cell. 2003;114:359–70. doi: 10.1016/s0092-8674(03)00566-x. [DOI] [PubMed] [Google Scholar]
- 16.Celeste A, Difilippantonio S, Difilippantonio MJ, Fernandez-Capetillo O, Pilch DR, Sedelnikova OA, Eckhaus M, Ried T, Bonner WM, Nussenzweig A. H2AX haploinsufficiency modifies genomic stability and tumor susceptibility. Cell. 2003;114:371–83. doi: 10.1016/s0092-8674(03)00567-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Celeste A, Petersen S, Romanienko PJ, Fernandez-Capetillo O, Chen HT, Sedelnikova OA, Reina-San-Martin B, Coppola V, Meffre E, Difilippantonio MJ, Redon C, Pilch DR, Olaru A, Eckhaus M, Camerini-Otero RD, Tessarollo L, Livak F, Manova K, Bonner WM, Nussenzweig MC, Nussenzweig A. Genomic instability in mice lacking histone H2AX. Science. 2002;296:922–7. doi: 10.1126/science.1069398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–8. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 19.Moseley MA, Deterding LJ, Tomer KB, Jorgenson JW. Nanoscale packed-capillary liquid chromatography coupled with mass spectrometry using a coaxial continuous-flow fast atom bombardment interface. Anal Chem. 1991;63:1467–73. doi: 10.1021/ac00014a023. [DOI] [PubMed] [Google Scholar]
- 20.Gatlin CL, Kleemann GR, Hays LG, Link AJ, Yates JR., 3rd Protein identification at the low femtomole level from silver-stained gels using a new fritless electrospray interface for liquid chromatography-microspray and nanospray mass spectrometry. Anal Biochem. 1998;263:93–101. doi: 10.1006/abio.1998.2809. [DOI] [PubMed] [Google Scholar]
- 21.Tang WH, Halpern BR, Shilov IV, Seymour SL, Keating SP, Loboda A, Patel AA, Schaeffer DA, Nuwaysir LM. Discovering known and unanticipated protein modifications using MS/MS database searching. Anal Chem. 2005;77:3931–46. doi: 10.1021/ac0481046. [DOI] [PubMed] [Google Scholar]
- 22.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 23.Ward IM, Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem. 2001;276:47759–62. doi: 10.1074/jbc.C100569200. [DOI] [PubMed] [Google Scholar]
- 24.Biemann K. Contributions of mass spectrometry to peptide and protein structure. Biomed Environ Mass Spectrom. 1988;16:99–111. doi: 10.1002/bms.1200160119. [DOI] [PubMed] [Google Scholar]
- 25.Redon C, Pilch D, Rogakou E, Sedelnikova O, Newrock K, Bonner W. Histone H2A variants H2AX and H2AZ. Curr Opin Genet Dev. 2002;12:162–9. doi: 10.1016/s0959-437x(02)00282-4. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A. H2AX: the histone guardian of the genome. DNA Repair (Amst) 2004;3:959–67. doi: 10.1016/j.dnarep.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 27.Yih LH, Hsueh SW, Luu WS, Chiu TH, Lee TC. Arsenite induces prominent mitotic arrest via inhibition of G2 checkpoint activation in CGL-2 cells. Carcinogenesis. 2005;26:53–63. doi: 10.1093/carcin/bgh295. [DOI] [PubMed] [Google Scholar]
- 28.Nandi A, Tidwell M, Karp J, Rapoport AP. Protein expression of PDZ-binding kinase is up-regulated in hematologic malignancies and strongly down-regulated during terminal differentiation of HL-60 leukemic cells. Blood Cells Mol Dis. 2004;32:240–5. doi: 10.1016/j.bcmd.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Daniel R, Ramcharan J, Rogakou E, Taganov KD, Greger JG, Bonner W, Nussenzweig A, Katz RA, Skalka AM. Histone H2AX is phosphorylated at sites of retroviral DNA integration but is dispensable for postintegration repair. J Biol Chem. 2004;279:45810–4. doi: 10.1074/jbc.M407886200. [DOI] [PubMed] [Google Scholar]
- 30.Wang TS, Hsu TY, Chung CH, Wang AS, Bau DT, Jan KY. Arsenite induces oxidative DNA adducts and DNA-protein cross-links in mammalian cells. Free Radic Biol Med. 2001;31:321–30. doi: 10.1016/s0891-5849(01)00581-0. [DOI] [PubMed] [Google Scholar]
- 31.Schwerdtle T, Walter I, Mackiw I, Hartwig A. Induction of oxidative DNA damage by arsenite and its trivalent and pentavalent methylated metabolites in cultured human cells and isolated DNA. Carcinogenesis. 2003;24:967–74. doi: 10.1093/carcin/bgg018. [DOI] [PubMed] [Google Scholar]
- 32.Bode AM, Dong Z. The paradox of arsenic: molecular mechanisms of cell transformation and chemotherapeutic effects. Crit Rev Oncol Hematol. 2002;42:5–24. doi: 10.1016/s1040-8428(01)00215-3. [DOI] [PubMed] [Google Scholar]
- 33.Bode A, Dong Z. Apoptosis induction by arsenic: mechanisms of action and possible clinical applications for treating therapy-resistant cancers. Drug Resist Updat. 2000;3:21–29. doi: 10.1054/drup.2000.0114. [DOI] [PubMed] [Google Scholar]
- 34.Alemany M, Levin J. The effects of arsenic trioxide (As2O3) on human megakaryocytic leukemia cell lines. With a comparison of its effects on other cell lineages. Leuk Lymphoma. 2000;38:153–63. doi: 10.3109/10428190009060329. [DOI] [PubMed] [Google Scholar]
- 35.Rousselot P, Labaume S, Marolleau JP, Larghero J, Noguera MH, Brouet JC, Fermand JP. Arsenic trioxide and melarsoprol induce apoptosis in plasma cell lines and in plasma cells from myeloma patients. Cancer Res. 1999;59:1041–8. [PubMed] [Google Scholar]
- 36.Miller WH, Jr., Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62:3893–903. [PubMed] [Google Scholar]
- 37.Soignet SL, Frankel SR, Douer D, Tallman MS, Kantarjian H, Calleja E, Stone RM, Kalaycio M, Scheinberg DA, Steinherz P, Sievers EL, Coutre S, Dahlberg S, Ellison R, Warrell RP., Jr. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001;19:3852–60. doi: 10.1200/JCO.2001.19.18.3852. [DOI] [PubMed] [Google Scholar]
- 38.Yang CH, Kuo ML, Chen JC, Chen YC. Arsenic trioxide sensitivity is associated with low level of glutathione in cancer cells. Br J Cancer. 1999;81:796–9. doi: 10.1038/sj.bjc.6690766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gianni M, Koken MH, Chelbi-Alix MK, Benoit G, Lanotte M, Chen Z, de The H. Combined arsenic and retinoic acid treatment enhances differentiation and apoptosis in arsenic-resistant NB4 cells. Blood. 1998;91:4300–10. [PubMed] [Google Scholar]
- 40.Munshi NC. Arsenic trioxide: an emerging therapy for multiple myeloma. Oncologist. 2001;6(Suppl 2):17–21. doi: 10.1634/theoncologist.6-suppl_2-17. [DOI] [PubMed] [Google Scholar]
- 41.Berggren P, Kumar R, Sakano S, Hemminki L, Wada T, Steineck G, Adolfsson J, Larsson P, Norming U, Wijkstrom H, Hemminki K. Detecting homozygous deletions in the CDKN2A(p16(INK4a))/ARF(p14(ARF)) gene in urinary bladder cancer using real-time quantitative PCR. Clin Cancer Res. 2003;9:235–42. [PubMed] [Google Scholar]
- 42.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 43.Grbovic OM, Basso AD, Sawai A, Ye Q, Friedlander P, Solit D, Rosen N. V600E B-Raf requires the Hsp90 chaperone for stability and is degraded in response to Hsp90 inhibitors. Proc Natl Acad Sci U S A. 2006;103:57–62. doi: 10.1073/pnas.0609973103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Girnita L, Girnita A, Larsson O. Mdm2-dependent ubiquitination and degradation of the insulin-like growth factor 1 receptor. Proc Natl Acad Sci U S A. 2003;100:8247–52. doi: 10.1073/pnas.1431613100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haapajarvi T, Pitkanen K, Laiho M. Human melanoma cell line UV responses show independency of p53 function. Cell Growth Differ. 1999;10:163–71. [PubMed] [Google Scholar]