Abstract
Carcinogenesis is a multistage process consisting of initiation, promotion and progression stages and each stage may be a possible target for chemopreventive agents. A significant outcome of these investigations on the elucidation of molecular and cellular mechanisms is the explication of signal transduction pathways induced by tumor promoters in cancer development. The current belief today is that cancer may be prevented or treated by targeting specific cancer genes, signaling proteins and transcription factors. The molecular mechanisms explaining how normal cells undergo neoplastic transformation induced by tumor promoters are rapidly being clarified. Accumulating research evidence suggests that many of dietary factors, including tea compounds, may be used alone or in combination with traditional chemotherapeutic agents to prevent or treat cancer. The potential advantage of many natural or dietary compounds seems to focus on their potent anticancer activity combined with low toxicity and very few adverse side effects. This review summarizes some of our recent work regarding the effects of the various tea components on signal transduction pathways involved in neoplastic cell transformation and carcinogenesis.
Keywords: EGCG, catechins, activator protein-1, nuclear factor kappa B, apoptosis, theaflavins, caffeine, vimentin
Introduction
Carcinogenesis is a complex, multistage process that affects many genes and gene products, which are critical in the regulation of numerous cellular functions. A major focus of much our work has been the elucidation of molecular and cellular mechanisms in cancer development and prevention. A significant outcome of these investigations is the explication of signal transduction pathways induced by tumor promoters in cancer development. The prevailing idea today is that cancer may be prevented or treated by targeting specific cancer genes, signaling proteins and transcription factors. Each stage of cancer development could be a potential target for anticancer agents, but especially the promotion stage. Importantly, the molecular mechanisms explaining how normal cells undergo neoplastic transformation induced by tumor promoters are rapidly being clarified. In particular, the mitogen-activated protein (MAP) kinase signaling pathways are activated differentially by various tumor promoters (reviewed in [1-10]. MAP kinases are activated by translocation to the nucleus, where they phosphorylate a variety of target transcription factors important in tumor development, including activator protein-1 (AP-1) and nuclear factor kappaB (NFκB) [11-14], which in turn may activate transcription of a variety of cancer-related genes such as cyclooxygenase-2 (COX-2). AP-1 is a well-characterized transcription factor composed of homodimers and/or heterodimers of the Jun, Fos, ATF (activating transcription factor) and MAF (musculoaponeurotic fibrosarcoma) protein families [15, 16]. AP-1 plays a major role in cell transformation and is crucial in tumor promotion, progression and metastasis [17-19]. Furthermore, neoplastic transformation and TPA-induced progression are blocked by inhibiting tumor promoter-induced AP-1 activity [19-23]. The MAP kinases include the extracellular-signal-regulated protein kinases (ERKs), c-Jun N-terminal kinases/stress-activated protein kinases (JNKs/SAPKs) and p38 kinases. ERKs generally transmit signals initiated by tumor promoters such as 12-O-tetradecanoylphorbol-13-acetate (TPA), epidermal growth factor (EGF) and platelet-derived growth factor (PDGF) [24]. The JNKs/SAPKs and p38 kinases are strongly stimulated by stresses such as UV irradiation [13] and arsenic [25]. The activation of these signaling cascades can result in a multitude of cellular responses including apoptosis, proliferation, inflammation, differentiation and development (Fig. 1).
Figure 1.

General scheme of MAP kinase (MAPK) activation by tumor promoters. Tumor promoters such as TPA, UV, arsenic, or EGF induce MAP kinase pathways to varying degrees. Generally the promoter induces a MAP kinase kinase kinase (MAPKKK), which then induces a downstream MAP kinase kinase (MAPKK) followed by its induction of the respective MAPK. MAPKs translocate to the nucleus and in a process referred to as “transcriptional activation” phosphorylate a variety of target transcription factors, including AP-1 and NFκB. The final cellular response ranges from an induction of apoptosis to an increase in proliferation depending on the stimulus and the specific MAPK.
Many natural or dietary compounds are believed to have potent anticancer activity, low toxicity and cause very few adverse side effects. Accumulating research evidence suggests that many of these compounds, including tea compounds, may be used alone or in combination with traditional chemotherapeutic agents to prevent or treat cancer (reviewed in [2, 4, 6-9]. This review summarizes some of our recent work regarding the effects of the various tea components on signal transduction pathways involved in neoplastic cell transformation and carcinogenesis.
Signal transduction and tea components
Signal transduction is the process by which information from a stimulus outside the cell is transmitted from the cell membrane into the cell and along an intracellular chain of signaling molecules to stimulate a response. Signal transduction molecules induced by tumor promoters appear to be excellent targets for nutritional or dietary factors, and especially for the components of tea. Nutritional or dietary factors have attracted a great deal of interest because of their professed ability to act as highly effective anticancer agents. They are perceived as being generally safe and may have efficacy as chemopreventive agents by preventing or reversing premalignant lesions and/or reducing second primary tumor incidence [26]. Much of our recent work has focused on elucidating the effects of tea on cancer cells, discovering mechanisms to explain the effects, and identifying the specific cellular and molecular targets of tea.
Green, oolong and black teas, which are distinguished according to their level of oxidation [27], are all derived from the same plant, Camellia sinensi. Green tea is processed immediately from fresh leaves and is protected from oxidation whereas oolong tea has been partially oxidized and black tea has been fully oxidized. Green and black teas contain several active polyphenols collectively known as catechins and theaflavins, respectively. Teas also contain caffeine, which may be another important bioactive compound. Much evidence suggests that tea compounds may possess potent anticancer activity [28-31]. For example, studies have shown that topical application or oral consumption of green tea, black tea, and tea polyphenol preparations has inhibitory effects on skin, lung, esophagus, stomach, liver, duodenum and small intestine, pancreas, and colorectal cancers in rodent models [28-31]. Unfortunately, results are somewhat less conclusive in humans mainly due to insufficient information regarding bioavailability and tissue distribution of tea polyphenols in humans compared to animal models. However, more research data are continually being produced, which report possible mechanisms explaining the chemopreventive effects of tea.
Green tea catechins
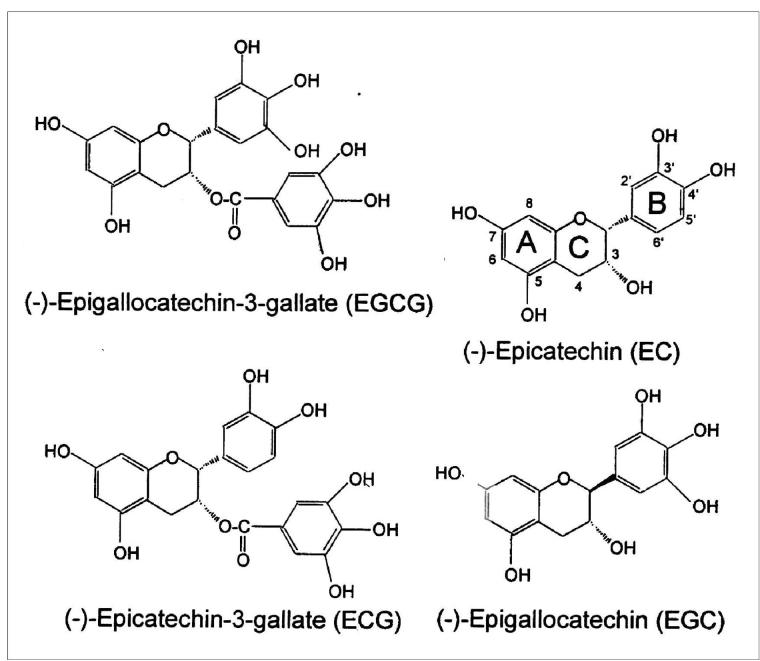
The green tea catechins (Fig. 2) include (−)-epigallocatechin-3-gallate (EGCG), (−)-epigallocatechin (EGC), (−)-epicatechin-3-gallate (ECG), and (−)-epicatechin (EC) [32]. EGCG is the major active polyphenol in green tea and may account for 50-80% of the total catechins found in tea [2, 4, 29, 33]. A cup of green tea (2.5 g of dried green tea leaves brewed in 200 ml of water) usually contains about 90 mg of EGCG. In addition, it contains a similar or slightly smaller amount (65 mg) of (−)-epigallocatechin (EGC), about 20 mg each of (−)-epigallocatechin 3-gallate (ECG) and (−)-epigallocatechin (EC), and about 50 mg of caffeine [29, 34]. However, the achievable tissue concentrations of tea polyphenols are in the low micromolar range. Thus, results observed with much higher concentrations in vitro may not be relevant to the anti-carcinogenic process [35, 36]. We have reported that EGCG or theaflavins inhibit tumor promoter induced activator protein-1 (AP-1) and MAP kinase activation at a concentration range (1-20 μM) that is effective for inhibition of cell transformation [37, 38].
Figure 2.
Structure and nomenclature of the green tea polyphenols.
EGCG suppresses AP-1 activation
A substantial body of evidence suggests that EGCG and theaflavins inhibit tumor promoter- or growth factor-induced cell transformation and AP-1 activation. Others have shown that EGCG inhibited cell transformation in A172 and NIH 3T3 cells [39]. In our work, we used the JB6 mouse epidermal cell line, which is a well-developed cell culture system for studying genetic susceptibility to neoplastic transformation, promotion and progression at the molecular level. The promotion-sensitive (P+), promotion-resistant (P−), and transformed (Tx) variants are a series of cell lines representing “earlier-to-later” stages of preneoplastic-to-neoplastic progression. TPA or EGF induce the formation of large, tumorigenic, anchorage-independent [40, 41] colonies in soft agar at a high frequency in the P+ JB6 cells but are less effective in the P− cells [42, 43]. One major difference between P+ and P− variants is the AP-1 transcription factor, which activates gene expression in response to tumor promoters in P+ but not in P− JB6 cells and P+ cells revert to the P− phenotype when AP-1 activity is blocked [18, 19, 44, 45]. Using this model, we found that EGCG or theaflavins inhibited EGF- or TPA-induced cell transformation, c-Jun phosphorylation, JNKs (c-Jun N-terminal kinases) activation, AP-1-dependent transcriptional activity and AP-1 DNA binding activity at similar doses [37]. The Ras pathway is also important in the activation of AP-1. Mutations of the Ras gene occur frequently in many cancers and are associated with uncontrolled growth. Chung, et al. [46] found that the H-Ras-activated AP-1 pathway was a major growth stimulant in transformed mutant H-ras JB6 cells. Treatment of cells with green or black tea polyphenols strongly inhibited cell growth, ERKs (extracellular signal-regulated protein kinases) phosphorylation, c-Jun and Fra-1 phosphorylation and protein levels and AP-1 activity [46]. In addition, our collaborators and we have shown that pretreatment of JB6 cells with EGCG or theaflavins inhibited UVB-induced AP-1 activity [47, 48]. Barthelman, et al. [47] found that in mouse skin epidermis, UVB irradiation induced a nearly 40-fold increase in AP-1 activity, as compared with acetone-treated controls. Treatment with topical EGCG reduced the UVB-induction of AP-1 transactivation activity by 60%. Chen, et al. [49] found that EGCG inhibited UVB-induced transcriptional activation of the c-fos gene and accumulation of the c-Fos protein in a dose-dependent manner. EGCG has also been shown to effectively block arsenite-induced ERKs activation, AP-1 transcriptional activation and subsequent DNA binding activity [49]. Thus, based on what is known about AP-1, the inhibitory activities of EGCG and theaflavins toward AP-1 transactivation may explain their effective prevention of cell transformation [37].
NF-κB activation and EGCG
Besides, AP-1, EGCG and theaflavins also suppress activation of the NFκB pathway. NFκB activation is associated with initiation or acceleration of tumorigenesis [50]. Treatment of normal human keratinocytes with EGCG resulted in an inhibition of UVB-induced IKK-α activation, phosphorylation and degradation of IκBα and activation and nuclear translocation of p65 [51]. We observed that EGCG suppressed both UVB- and TPA-induced IκBα phosphorylation and NFκB activation and DNA binding in JB6 cells [52]. The inhibition appeared to occur through a suppression of the TPA-induced phosphorylation of IκBα (Ser32). In Jurkat T cells, EGCG inhibited 20S proteasome activity, which targets IκBα for degradation, resulting in cell growth arrest and an accumulation of IκBα [53]. Others [54] found that treatment with EGCG (10-100 μM, 24 h) caused growth inhibition, G1-phase arrest, and apoptosis in human epidermoid carcinoma cells (A431) but not in the normal cells. EGCG inhibited constitutive, TNF- and lipopolysaccharide-induced NFκB DNA binding and protein expression in the cancer cells at much lower doses than were required for inhibition in normal cells. In the cancer cells, EGCG also effectively inhibited IκBα degradation resulting in repression of NFκB activation [54]. These results suggest a differential response of normal cells and tumor cells to treatment with EGCG that could be highly significant for the potential of EGCG use in chemoprevention.
Phosphatidylinositol-3 (PI-3) kinase is activated by numerous oncogenes and the PI-3/Akt pathway is deregulated in many human cancers [55]. EGCG and theaflavins have also been shown to decrease UVB-induced PI-3 kinase activity, which was associated with decreased Akt phosphorylation (Thr308, Ser473) and p70S6K (Thr389, Thr421/Ser424) [56]. Another report showed that EGCG inhibited viability of the MMTV-Her-2/neu mouse breast tumor derived cell line N639 and its growth in soft agar [57]. The effect was associated with inhibition of the PI-3 kinase/Akt kinase to NF-κB signaling pathway, which appeared to result from an inhibition of basal Her-2/neu receptor tyrosine phosphorylation [57].
NFκB activation is strongly linked to increased COX-2 expression. Recent studies showed that EGCG significantly inhibited COX-2 and iNOS activity and nitric oxide production in LPS-activated Raw 264.7 cells [58], suggesting an effect mediated by NF-κB. However, not all COX-2 activation is associated directly with increased NF-κB activation and EGCG can also stimulate COX-2 expression. In the macrophage cell line, Raw264.7, COX-2 expression and activity and prostaglandin production were increased by EGCG treatment and were associated with the activation of both the ERKs and protein-tyrosine phosphatase signaling pathways [59]. In the NMBA-induced rat esophageal tumorigenesis model, treatment with EGCG resulted in significantly less tumor development and tumor incidence. These results corresponded with decreased expression of cyclin D1 and COX-2 and decreased production of prostaglandin E2 [60].
Topical pretreatment with green tea extract has been shown to block the acute COX-2 response to UVB in mice or humans [61]. Another recent study showed that pretreatment of mice with EGCG significantly inhibited TPA-induced COX-2 expression in mouse skin and also in TPA-induced human mammary epithelial cells [62]. The inhibition was associated with a suppression of TPA-stimulated ERKs and p38 kinases activities, but surprisingly, had no effect on TPA-induced AP-1 DNA binding [62]. This report further confirmed that EGCG treatment inhibited lipid peroxidation and UVB-induced COX and ornithine decarboxylase activities [51]. Taken together, these results and those above, strongly suggest that EGCG and theaflavins may be highly effective as chemopreventive agents, which act by targeting specific tumor promoter-induced transcription factors, especially AP-1 and NFκB. In addition, the propensity of these compounds for tumor cells makes them ideal candidates for chemopreventive agents.
EGCG induces and suppresses apoptosis
EGCG has been reported to induce or suppress apoptosis. EGCG treatment of human colorectal carcinoma HT-29 cells resulted in apoptosis mediated by the JNKs pathway [63]. In human prostate carcinoma LNCaP cells, treatment with EGCG induced apoptosis and was associated with stabilization of p53 and also with a down-regulation of NF-κB activity and a decreased expression of the anti-apoptotic protein Bcl-2 [64]. In liver cancer cells (HepG2), EGCG has been shown to induce apoptosis and block cell cycle progression at G1 [65]. These effects were accompanied by increased expression of p53, p21/WAF1, and pro-apoptotic Fas and Bax proteins [65]. EGCG (1-20 μM) or theaflavins (1-20 μM) may also inhibit apoptosis under certain conditions, such as exposure to arsenic. Arsenic's toxicity may be due to its ability to induce abnormal apoptosis and green tea has been used in Chinese medicine for detoxification of arsenite-associated toxicity. We have shown that EGCG or theaflavins block arsenite-induced apoptosis of JB6 cells [38]. In normal human keratinocytes, EGCG also inhibited UV-induced apoptosis by two mechanisms involving phosphorylation of the Bad protein through the ERKs and Akt pathways and by changing the ratio of Bcl-2 and Bax [66].
Theaflavins
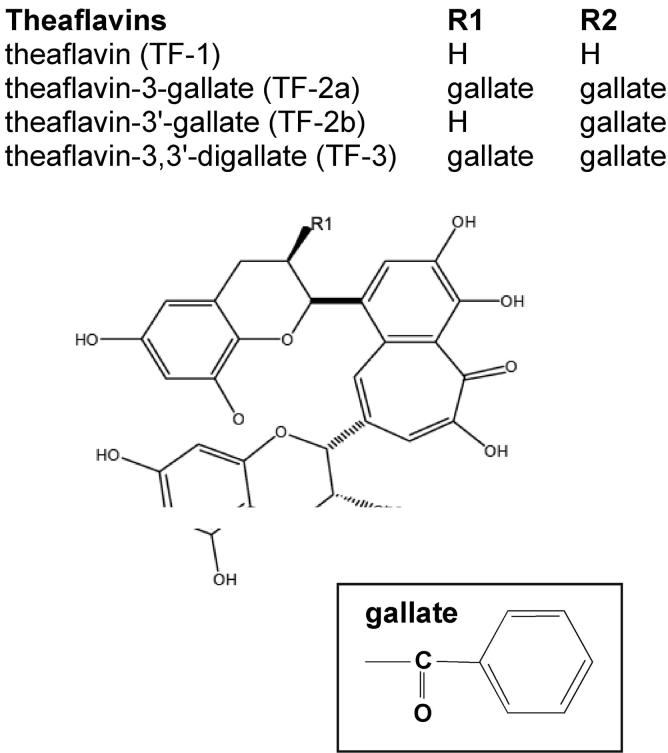
Theaflavins (Fig. 3) give black tea its characteristic color and taste and are differentiated by the gallate group. They include theaflavin (TF), theaflavin-3-gallate (TF-2a), theaflavin-3′-gallate (TF-2b), and theaflavin-3,3′-digallate (TF-3) [32]. In black tea, polyphenols are reduced to about one fourth of those in green tea, and theaflavins account for 1 to 2% of the total dry matter [29]. Research data suggest that theaflavins may have even more potent anticancer activity than EGCG and may act by different mechanisms.
Figure 3.
Structure and nomenclature of the theaflavins
EGCG and TF-3 were compared for their effects on the MAP kinase signaling pathways and both inhibited phosphorylation of c-Jun and ERKs but only TF-3 inhibited p38 kinase [46]. Further studies [67] confirmed that either EGCG or TF-3 decreased phosphorylation of ERKs and MEKs but TF-3 acted at 15 min compared to 60 min for EGCG. In addition, TF-3 decreased Raf-1 protein levels and EGCG decreased the association of Raf-1 with MEK1 [67]. We compared the effects of EGCG and theaflavins on UVB-induced AP-1 activation and found that compared to EGCG, the theaflavins were stronger inhibitors of UVB-induced AP-1 activation [48]. Others have reported that theaflavins, and especially TF-3, also inhibited IκB kinase activity, which prevented the phosphorylation and degradation of IκB and subsequent activation of NFκB [68].
Most recently, we found that theaflavins at very low concentrations (0.5 μM) inhibited UVB-induced phosphorylation of JNKs [69]. We have also very recently reported that the theaflavins (20 μM), and especially TF-3, induce EGFR down-regulation in JB6 Cl41 mouse epidermal and A431 human EGFR overexpressing epidermoid carcinoma cells [70]. TF-3 acted by inducing the internalization of EGFR and ubiquitination in A431 cells. Even though TF-3 induced tyrosine phosphorylation of the EGFR, the TF-3-induced EGFR down-regulation did not require receptor tyrosine kinase activation. The EGFR inhibitor, AG1478, blocked tyrosine phosphorylation of the EGFR but had no effect on TF-3-induced ubiquitination and down-regulation. Further, TF-3 inhibited EGFR-induced phosphorylation and downstream signaling to ERKs and AP-1. Finally, TF-3 inhibited EGF-induced cell transformation [70]. These results suggest that theaflavins may be just as effective as EGCG in suppressing cell transformation and carcinogenesis.
Caffeine
Another component of tea and coffee that may be important in cancer prevention is caffeine. Low concentrations of caffeine (50-450 μM) induced apoptosis in JB6 Cl41 cell (IC50 2.7 mM) and the apoptosis was p53-dependent because it did not occur in p53 knockout cells [71]. Caffeine induced phosphorylation of p53 (Ser15) and increased p53 activation. Bax, a p53 target protein [72], and cleaved caspase 3, a key executioner of apoptosis expression also increased in a time and dose-dependent manner. These effects did not occur in p53 knockout cells. Bax is a pro-apoptotic member of the Bcl-2 family of proteins and is known to form heterodimers with the anti-apoptotic Bcl-2 protein in vivo. The molar ratio of Bcl-2 to Bax has been shown to establish whether apoptosis is induced or inhibited in many tissues [73]. Caspase-3 is known to be one of the key executioners of apoptosis and its activation requires proteolytic processing of its inactive zymogen into activated p17 and p19 subunits [74]. Bax drives the release of cytochrome c from the mitochondria and cytochrome c release activates caspase 3 [75].
Lower levels of caffeine (0.25-1 mM) were shown to suppress cell cycle progression at the G0/G1 phase [76]. In this work, JB6 Cl41 mouse epidermal cells were synchronized in G0 phase by serum deprivation (36 h in 0.1% FBS) and then stimulated with 5% fetal bovine serum (FBS) as a mitogenic stimulus. Treatment of cells with caffeine inhibited FBS-stimulated cell proliferation (IC50 = 0.7 mM) by inducing G0/G1 arrest but without inducing apoptosis. The inhibitory effect appeared to result from the indirect suppression of cyclin D1/Cdk4 activation and subsequent inhibition of Rb phosphorylation (Ser780, Ser807, Ser811). Phosphorylation of Rb by Cdk4/6 and Cdk2 [77-79] occurs at the G0/G1 and G1/S transitions [80]. This phosphorylation leads to the disruption of the Rb/E2F transcription factor complex, resulting in the release of active E2F and its subsequent activation of many genes required for cell cycle progression [81]. Hashimoto et al. [76] also found that caffeine inhibited the time-dependent phosphorylation of protein kinase B (Akt Thr308) and its substrate, GSK-3β (Ser9). GSK-3β normally phosphorylates cyclin D1 (Thr286), which triggers cyclin D1 degradation, thus decreasing the activity of Cdk4 [82]. Because caffeine has also been reported to directly inhibit PI-3 kinase activity [83], Hashimoto, et al. [76] suggested that the inhibitory effects of caffeine on cell growth signaling through Akt/GSK-3β may result from the direct inhibition of PI-3 kinase, which is upstream of Akt/GSK-3β.
Protein targets of EGCG
Searching for the EGCG “receptor” or high affinity proteins that bind to EGCG is the first step in understanding the molecular and biochemical mechanism of the anticancer effects of tea polyphenols. A few proteins that can directly bind with EGCG have been identified, including plasma proteins: fibronectin, fibrinogen, and histidine-rich glycoprotein [84], also fatty acid synthase (Fas) [85] laminin and the 67-kDa laminin receptor [86, 87]. Plasma proteins may act as carrier proteins for EGCG and interacting with Fas might trigger a Fas-mediated apoptosis cascade. The fact that EGCG can bind and regulate biological functions of the 67-kDa laminin receptor has possible implications for prion-related diseases. However, the biologic and physiologic significance for the anticancer effects of tea polyphenols is still not clear. Identification of new EGCG-binding proteins should facilitate the design of new strategies to prevent cancer. Recently, we identified the intermediate filament protein, vimentin, as a novel EGCG-binding protein [88]. Proteins from JB6 Cl41 cell lysates were subjected to affinity chromatography using EGCG-Sepharose 4B. Fractions containing proteins binding with EGCG were analyzed by two-dimensional electrophoresis and MALDI-TOF-MS to identify vimentin. Results were verified using combination of a pull-down assay with EGCG-Sepharose 4B and an immunoprecipitation assay with protein G-Sepharose 4B and a vimentin antibody. To characterize the binding interaction, we measured the binding affinity of GST-vimentin and [3H]EGCG using a pull-down assay. Results indicated that vimentin displayed a high affinity for binding with [3H]EGCG with a Kd value for the binding of EGCG to vimentin of 3.3 nM. EGCG was also found to inhibit phosphorylation of vimentin (Ser50, Ser55). EGCG specifically interfered with the Cdc2 kinase-mediated phosphorylation of vimentin (IC50 17 □M). Besides EGCG, only EGC (20 □M) inhibited Cdc2 kinase activity. Intermediate filaments (IFs), such as vimentin, have an important functional involvement in cell division and proliferation [89]. EGCG has been reported to inhibit cell proliferation of a variety of cell lines [57, 90-94] and in the present study, when vimentin expression was suppressed with siRNA, cell growth was inhibited. We showed that proliferation was inhibited in control (GFP siRNA) cells in a dose-dependent manner compared to cells expressing vimentin siRNA, which were not affected by EGCG treatment. These results strongly suggested that EGCG binds with vimentin and the association can have a regulatory role in controlling cell proliferation.
Conclusions/summary
From animal investigations and some epidemiologic studies, tea and tea constituents have been shown to have a protective effect against the development of cancer. This effect is in part due to the anti-tumor promotion effect of tea and tea constituents. Anti-tumor promotion agents are expected to have greater utility than blocking (anti-initiation) agents in the prevention of human cancer. The molecular targets and molecular mechanisms of anti-promotion by tea and tea constituents are still not clear. Our laboratory has demonstrated strength in studying the signal transduction pathways leading to tumor promotion and we have provided important information on novel mechanisms of tea polyphenols on anti-tumor promotion. We found that:
EGCG or theaflavins suppress EGF- or TPA-induced neoplastic cell transformation
EGCG or theaflavins inhibit UVB-, arsenite, TPA- or EGF-induced phosphorylation and activation of MAP kinases and/or their downstream targets, including AP-1 and NFκB.
Theaflavins also act by inducing the down-regulation and ubiquitination of the EGFR.
Low concentrations of caffeine induce an increased p53 phosphorylation (Ser15) and total p53 protein level that is associated with an induction of p53-dependent apoptosis, and increased proapoptotic Bax and cleaved caspase-3 activation.
Caffeine also induces G0/G1 cell cycle arrest in quiescent JB6 cells stimulated with FBS (as mitogenic stimuli) that is associated with an inhibition of the cyclin D1/Cdk4 complex activation and phosphorylation of pRb and Akt.
Understanding the molecular mechanisms of tea in anti-tumor promotion may reveal key molecular targets for the development of more effective agents with fewer side effects for the chemoprevention of cancer. A continuing emphasis on obtaining rigorous research data and critical analysis of those data regarding these and other food factors is vital to determine the molecular basis and long-term effectiveness and safety of these compounds as chemopreventive agents. Large-scale animal and molecular biology studies are needed to address the bioavailability, toxicity, molecular target, signal transduction pathways, and side effects of dietary factors. Clinical trials based on clear mechanistic studies are also needed to assess the effectiveness of these dietary factors in the human population.
Acknowledgements
This work is supported by The Hormel Foundation, the Rochester Eagle's Telethon, Hormel Foods, Pediatric Pharmaceuticals, University of Minnesota Graduate School and grants from the American Institute for Cancer Research and NIH grants CA27502, CA081064, CA077646, CA88961, CA74916 and CA77451.
Citations
- 1.Bode AM, Dong Z. Apoptosis induction by arsenic: Mechanisms of actions and possible clinical applications for treating therapy-resistant cancers. Drug Resistance Updates. 2000;3:21–29. doi: 10.1054/drup.2000.0114. [DOI] [PubMed] [Google Scholar]
- 2.Bode AM, Dong Z. Signal transduction pathways: Targets for chemoprevention of skin cancer. The Lancet Oncology. 2000;1:181–188. doi: 10.1016/s1470-2045(00)00029-2. [DOI] [PubMed] [Google Scholar]
- 3.Bode AM, Dong Z. The paradox of arsenic: Molecular mechanisms of cell transformation and chemotherapeutic effects. Crit Rev Oncol Hematol. 2002;42:5–24. doi: 10.1016/s1040-8428(01)00215-3. [DOI] [PubMed] [Google Scholar]
- 4.Bode AM, Dong Z. Signal transduction pathways: Targets for green and black tea polyphenols. J Biochem Mol Biol. 2003;36:66–77. [PubMed] [Google Scholar]
- 5.Bode AM, Dong Z. Mitogen-activated protein kinase activation in uv-induced signal transduction. Sci STKE. 2003;2003:RE2. doi: 10.1126/stke.2003.167.re2. [DOI] [PubMed] [Google Scholar]
- 6.Bode AM, Dong Z. Targeting signal transduction pathways by chemopreventive agents. Mutat Res. 2004;555:33–51. doi: 10.1016/j.mrfmmm.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 7.Bode AM, Dong Z. Post-translational modification of p53 in tumorigenesis. Nature Reviews Cancer. 2004;4:793–805. doi: 10.1038/nrc1455. [DOI] [PubMed] [Google Scholar]
- 8.Bode AM, Dong Z. Cancer prevention by food factors through targeting signal transduction pathways. Nutrition. 2004;20:89–94. doi: 10.1016/j.nut.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Bode AM, Dong Z. Signal transduction pathways in cancer development and as targets for cancer prevention. Prog Nucleic Acid Res Mol Biol. 2005;79:237–297. doi: 10.1016/S0079-6603(04)79005-4. [DOI] [PubMed] [Google Scholar]
- 10.Bode AM, Dong Z. Inducible covalent posttranslational modification of histone h3. Sci STKE. 2005;2005:re4. doi: 10.1126/stke.2812005re4. [DOI] [PubMed] [Google Scholar]
- 11.Davis RJ. Mapks: New jnk expands the group. Trends Biochem Sci. 1994;19:470–473. doi: 10.1016/0968-0004(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 12.Angel P, Hattori K, Smeal T, Karin M. The jun proto-oncogene is positively autoregulated by its product, jun/ap-1. Cell. 1988;55:875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- 13.Kallunki T, Su B, Tsigelny I, Sluss HK, Derijard B, Moore G, Davis R, Karin M. Jnk2 contains a specificity-determining region responsible for efficient c-jun binding and phosphorylation. Genes Dev. 1994;8:2996–3007. doi: 10.1101/gad.8.24.2996. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez I, Hughes RT, Mayer BJ, Yee K, Woodgett JR, Avruch J, Kyriakis JM, Zon LI. Role of sapk/erk kinase-1 in the stress-activated pathway regulating transcription factor c-jun. Nature. 1994;372:794–798. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- 15.Eferl R, Wagner EF. Ap-1: A double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;3:859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 16.Angel P, Karin M. The role of jun, fos and the ap-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 17.Barthelman M, Chen W, Gensler HL, Huang C, Dong Z, Bowden GT. Inhibitory effects of perillyl alcohol on uvb-induced murine skin cancer and ap-1 transactivation. Cancer Res. 1998;58:711–716. [PubMed] [Google Scholar]
- 18.Dong Z, Watts SG, Sun Y, Colburn NH. Progressive elevation of ap-1 activity during preneoplastic-to neoplastic progression as modeled in mouse jb6 cell variants. Int J Oncol. 1995;7:359–364. doi: 10.3892/ijo.7.2.359. [DOI] [PubMed] [Google Scholar]
- 19.Dong Z, Birrer MJ, Watts RG, Matrisian LM, Colburn NH. Blocking of tumor promoter-induced ap-1 activity inhibits induced transformation in jb6 mouse epidermal cells. Proc Natl Acad Sci U S A. 1994;91:609–613. doi: 10.1073/pnas.91.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang C, Ma WY, Dawson MI, Rincon M, Flavell RA, Dong Z. Blocking activator protein-1 activity, but not activating retinoic acid response element, is required for the antitumor promotion effect of retinoic acid. Proc Natl Acad Sci U S A. 1997;94:5826–5830. doi: 10.1073/pnas.94.11.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li JJ, Dong Z, Dawson MI, Colburn NH. Inhibition of tumor promoter-induced transformation by retinoids that transrepress ap-1 without transactivating retinoic acid response element. Cancer Res. 1996;56:483–489. [PubMed] [Google Scholar]
- 22.Huang C, Ma W, Dong Z. Inhibitory effects of ascorbic acid on ap-1 activity and transformation of jb6 cells. Int J Oncol. 1996;8:389–393. doi: 10.3892/ijo.8.2.389. [DOI] [PubMed] [Google Scholar]
- 23.Dong Z, Crawford HC, Lavrovsky V, Taub D, Watts R, Matrisian LM, Colburn NH. A dominant negative mutant of jun blocking 12-o-tetradecanoylphorbol-13- acetate-induced invasion in mouse keratinocytes. Mol Carcinog. 1997;19:204–212. doi: 10.1002/(sici)1098-2744(199707)19:3<204::aid-mc8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 24.Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of map kinase kinase is necessary and sufficient for pc12 differentiation and for transformation of nih 3t3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 25.Huang C, Ma WY, Li J, Dong Z. Arsenic induces apoptosis through a c-jun nh2-terminal kinase-dependent, p53-independent pathway. Cancer Res. 1999;59:3053–3058. [PubMed] [Google Scholar]
- 26.Hong WK. General keynote: The impact of cancer chemoprevention. Gynecol Oncol. 2003;88:S56–58. doi: 10.1006/gyno.2002.6685. [DOI] [PubMed] [Google Scholar]
- 27.Trevisanato SI, Kim YI. Tea and health. Nutr Rev. 2000;58:1–10. doi: 10.1111/j.1753-4887.2000.tb01818.x. [DOI] [PubMed] [Google Scholar]
- 28.Yang CS, Yang GY, Lee ML, Chen L. Mechanistic considerations of the inhibition of carcinogenesis by tea. In: Ohigashi H, editor. Proceedings of the International Conference on Food Factor in Cancer Prevention. Springer-Verlag; Tokyo: 1997. pp. 113–117. [Google Scholar]
- 29.Yang CS, Wang ZY. Tea and cancer. J Natl Cancer Inst. 1993;85:1038–1049. doi: 10.1093/jnci/85.13.1038. [DOI] [PubMed] [Google Scholar]
- 30.Katiyar SK, Mukhtar H. Tea in chemoprevention of cancer: Epidemiologic and experimental studies. Int J Oncology. 1996;8:221–238. doi: 10.3892/ijo.8.2.221. [DOI] [PubMed] [Google Scholar]
- 31.Dreosti IE, Wargovich MJ, Yang CS. Inhibition of carcinogenesis by tea: The evidence from experimental studies. Crit Rev Food Sci Nutr. 1997;37:761–770. doi: 10.1080/10408399709527801. [DOI] [PubMed] [Google Scholar]
- 32.Yang CS, Chung JY, Yang G, Chhabra SK, Lee MJ. Tea and tea polyphenols in cancer prevention. J Nutr. 2000;130:472S–478S. doi: 10.1093/jn/130.2.472S. [DOI] [PubMed] [Google Scholar]
- 33.Graham HN. Green tea composition, consumption, and polyphenol chemistry. Prev Med. 1992;21:334–350. doi: 10.1016/0091-7435(92)90041-f. [DOI] [PubMed] [Google Scholar]
- 34.Huang MT, Ho CT, Wang ZY, Ferraro T, Finnegan-Olive T, Lou YR, Mitchell JM, Laskin JD, Newmark H, Yang CS, et al. Inhibitory effect of topical application of a green tea polyphenol fraction on tumor initiation and promotion in mouse skin. Carcinogenesis. 1992;13:947–954. doi: 10.1093/carcin/13.6.947. [DOI] [PubMed] [Google Scholar]
- 35.Yang CS. Inhibition of carcinogenesis by tea. Nature. 1997;389:134–135. doi: 10.1038/38154. [DOI] [PubMed] [Google Scholar]
- 36.Jankun J, Selman SH, Swiercz R, Skrzypczak-Jankun E. Why drinking green tea could prevent cancer. Nature. 1997;387:561. doi: 10.1038/42381. [DOI] [PubMed] [Google Scholar]
- 37.Dong Z, Ma W, Huang C, Yang CS. Inhibition of tumor promoter-induced activator protein 1 activation and cell transformation by tea polyphenols, (−)-epigallocatechin gallate, and theaflavins. Cancer Res. 1997;57:4414–4419. [PubMed] [Google Scholar]
- 38.Chen NY, Ma WY, Yang CS, Dong Z. Inhibition of arsenite-induced apoptosis and ap-1 activity by epigallocatechin-3-gallate and theaflavins. J Environ Pathol Toxicol Oncol. 2000;19:287–295. [PubMed] [Google Scholar]
- 39.Ahn HY, Hadizadeh KR, Seul C, Yun YP, Vetter H, Sachinidis A. Epigallocathechin-3 gallate selectively inhibits the pdgf-bb-induced intracellular signaling transduction pathway in vascular smooth muscle cells and inhibits transformation of sis-transfected nih 3t3 fibroblasts and human glioblastoma cells (a172) Mol Biol Cell. 1999;10:1093–1104. doi: 10.1091/mbc.10.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong Z, Cmarik JL, Wendel EJ, Colburn NH. Differential transformation efficiency but not ap-1 induction under anchorage-dependent and -independent conditions. Carcinogenesis. 1994;15:1001–1004. doi: 10.1093/carcin/15.5.1001. [DOI] [PubMed] [Google Scholar]
- 41.Dong Z, Cmarik JL. Harvesting cells under anchorage-independent cell transformation conditions for biochemical analyses. Sci STKE. 2002;2002:PL7. doi: 10.1126/stke.2002.130.pl7. [DOI] [PubMed] [Google Scholar]
- 42.Colburn NH, Former BF, Nelson KA, Yuspa SH. Tumour promoter induces anchorage independence irreversibly. Nature. 1979;281:589–591. doi: 10.1038/281589a0. [DOI] [PubMed] [Google Scholar]
- 43.Colburn NH, Wendel EJ, Abruzzo G. Dissociation of mitogenesis and late-stage promotion of tumor cell phenotype by phorbol esters: Mitogen-resistant variants are sensitive to promotion. Proc Natl Acad Sci U S A. 1981;78:6912–6916. doi: 10.1073/pnas.78.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong Z, Lavrovsky V, Colburn NH. Transformation reversion induced in jb6 rt101 cells by ap-1 inhibitors. Carcinogenesis. 1995;16:749–756. doi: 10.1093/carcin/16.4.749. [DOI] [PubMed] [Google Scholar]
- 45.Lavrovsky V, Dong Z, Ma WY, Colburn N. Drug-induced reversion of progression phenotype is accompanied by reversion of ap-1 phenotype in jb6 cells. In Vitro Cell Dev Biol Anim. 1996;32:234–237. doi: 10.1007/BF02722951. [DOI] [PubMed] [Google Scholar]
- 46.Chung JY, Huang C, Meng X, Dong Z, Yang CS. Inhibition of activator protein 1 activity and cell growth by purified green tea and black tea polyphenols in h-ras-transformed cells: Structure-activity relationship and mechanisms involved. Cancer Res. 1999;59:4610–4617. [PubMed] [Google Scholar]
- 47.Barthelman M, Bair WB, 3rd, Stickland KK, Chen W, Timmermann BN, Valcic S, Dong Z, Bowden GT. (−)-epigallocatechin-3-gallate inhibition of ultraviolet b-induced ap-1 activity. Carcinogenesis. 1998;19:2201–2204. doi: 10.1093/carcin/19.12.2201. [DOI] [PubMed] [Google Scholar]
- 48.Nomura M, Ma WY, Huang C, Yang CS, Bowden GT, Miyamoto K, Dong Z. Inhibition of ultraviolet b-induced ap-1 activation by theaflavins from black tea. Mol Carcinog. 2000;28:148–155. [PubMed] [Google Scholar]
- 49.Chen W, Dong Z, Valcic S, Timmermann BN, Bowden GT. Inhibition of ultraviolet b--induced c-fos gene expression and p38 mitogen-activated protein kinase activation by (−)-epigallocatechin gallate in a human keratinocyte cell line. Mol Carcinog. 1999;24:79–84. doi: 10.1002/(sici)1098-2744(199902)24:2<79::aid-mc1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 50.Gilmore TD. Clinically relevant findings [editorial] J Clin Invest. 1997;100:2935–2936. doi: 10.1172/JCI119843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Afaq F, Adhami VM, Ahmad N, Mukhtar H. Inhibition of ultraviolet b-mediated activation of nuclear factor kappab in normal human epidermal keratinocytes by green tea constituent (−)-epigallocatechin-3-gallate. Oncogene. 2003;22:1035–1044. doi: 10.1038/sj.onc.1206206. [DOI] [PubMed] [Google Scholar]
- 52.Nomura M, Ma W, Chen N, Bode AM, Dong Z. Inhibition of 12-o-tetradecanoylphorbol-13-acetate-induced nf-kappab activation by tea polyphenols, (−)-epigallocatechin gallate and theaflavins. Carcinogenesis. 2000;21:1885–1890. doi: 10.1093/carcin/21.10.1885. [DOI] [PubMed] [Google Scholar]
- 53.Nam S, Smith DM, Dou QP. Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J Biol Chem. 2001;276:13322–13330. doi: 10.1074/jbc.M004209200. [DOI] [PubMed] [Google Scholar]
- 54.Ahmad N, Gupta S, Mukhtar H. Green tea polyphenol epigallocatechin-3-gallate differentially modulates nuclear factor kappab in cancer cells versus normal cells. Arch Biochem Biophys. 2000;376:338–346. doi: 10.1006/abbi.2000.1742. [DOI] [PubMed] [Google Scholar]
- 55.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase akt pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 56.Nomura M, Kaji A, He Z, Ma WY, Miyamoto K, Yang CS, Dong Z. Inhibitory mechanisms of tea polyphenols on the ultraviolet b-activated phosphatidylinositol 3-kinase-dependent pathway. J Biol Chem. 2001;276:46624–46631. doi: 10.1074/jbc.M107897200. [DOI] [PubMed] [Google Scholar]
- 57.Pianetti S, Guo S, Kavanagh KT, Sonenshein GE. Green tea polyphenol epigallocatechin-3 gallate inhibits her-2/neu signaling, proliferation, and transformed phenotype of breast cancer cells. Cancer Res. 2002;62:652–655. [PubMed] [Google Scholar]
- 58.Lee SJ, Lee IS, Mar W. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 activity by 1,2,3,4,6-penta-o-galloyl-beta-d-glucose in murine macrophage cells. Arch Pharm Res. 2003;26:832–839. doi: 10.1007/BF02980029. [DOI] [PubMed] [Google Scholar]
- 59.Park JW, Choi YJ, Suh SI, Kwon TK. Involvement of erk and protein tyrosine phosphatase signaling pathways in egcg-induced cyclooxygenase-2 expression in raw 264.7 cells. Biochem Biophys Res Commun. 2001;286:721–725. doi: 10.1006/bbrc.2001.5415. [DOI] [PubMed] [Google Scholar]
- 60.Li ZG, Shimada Y, Sato F, Maeda M, Itami A, Kaganoi J, Komoto I, Kawabe A, Imamura M. Inhibitory effects of epigallocatechin-3-gallate on n-nitrosomethylbenzylamine-induced esophageal tumorigenesis in f344 rats. Int J Oncol. 2002;21:1275–1283. [PubMed] [Google Scholar]
- 61.An KP, Athar M, Tang X, Katiyar SK, Russo J, Beech J, Aszterbaum M, Kopelovich L, Epstein EH, Jr., Mukhtar H, Bickers DR. Cyclooxygenase-2 expression in murine and human nonmelanoma skin cancers: Implications for therapeutic approaches. Photochem Photobiol. 2002;76:73–80. doi: 10.1562/0031-8655(2002)076<0073:ceimah>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 62.Kundu JK, Na HK, Chun KS, Kim YK, Lee SJ, Lee SS, Lee OS, Sim YC, Surh YJ. Inhibition of phorbol ester-induced cox-2 expression by epigallocatechin gallate in mouse skin and cultured human mammary epithelial cells. J Nutr. 2003;133:3805S–3810S. doi: 10.1093/jn/133.11.3805S. [DOI] [PubMed] [Google Scholar]
- 63.Chen C, Shen G, Hebbar V, Hu R, Owuor ED, Kong AN. Epigallocatechin-3-gallate-induced stress signals in ht-29 human colon adenocarcinoma cells. Carcinogenesis. 2003;24:1369–1378. doi: 10.1093/carcin/bgg091. [DOI] [PubMed] [Google Scholar]
- 64.Hastak K, Gupta S, Ahmad N, Agarwal MK, Agarwal ML, Mukhtar H. Role of p53 and nf-kappab in epigallocatechin-3-gallate-induced apoptosis of lncap cells. Oncogene. 2003;22:4851–4859. doi: 10.1038/sj.onc.1206708. [DOI] [PubMed] [Google Scholar]
- 65.Kuo PL, Lin CC. Green tea constituent (−)-epigallocatechin-3-gallate inhibits hep g2 cell proliferation and induces apoptosis through p53-dependent and fas-mediated pathways. J Biomed Sci. 2003;10:219–227. doi: 10.1007/BF02256057. [DOI] [PubMed] [Google Scholar]
- 66.Chung JH, Han JH, Hwang EJ, Seo JY, Cho KH, Kim KH, Youn JI, Eun HC. Dual mechanisms of green tea extract (egcg)-induced cell survival in human epidermal keratinocytes. Faseb J. 2003;17:1913–1915. doi: 10.1096/fj.02-0914fje. [DOI] [PubMed] [Google Scholar]
- 67.Chung JY, Park JO, Phyu H, Dong Z, Yang CS. Mechanisms of inhibition of the ras-map kinase signaling pathway in 30.7b ras 12 cells by tea polyphenols (−)-epigallocatechin-3-gallate and theaflavin-3,3′-digallate. Faseb J. 2001;15:2022–2024. doi: 10.1096/fj.01-0031fje. [DOI] [PubMed] [Google Scholar]
- 68.Pan MH, Lin-Shiau SY, Ho CT, Lin JH, Lin JK. Suppression of lipopolysaccharide-induced nuclear factor-kappab activity by theaflavin-3,3′-digallate from black tea and other polyphenols through down-regulation of ikappab kinase activity in macrophages. Biochem Pharmacol. 2000;59:357–367. doi: 10.1016/s0006-2952(99)00335-4. [DOI] [PubMed] [Google Scholar]
- 69.Zykova TA, Zhang Y, Zhu F, Bode AM, Dong Z. The signal transduction networks required for phosphorylation of stat1 at ser727 in mouse epidermal jb6 cells in the uvb response and inhibitory mechanisms of tea polyphenols. Carcinogenesis. 2005;26:331–342. doi: 10.1093/carcin/bgh334. [DOI] [PubMed] [Google Scholar]
- 70.Mizuno H, Cho Y-Y, Zhu F, Ma W, Bode AM, Yang CS, Ho CT, Dong Z. Theaflavin-3, 3′-digallate induces epidermal growth factor receptor down-regulation. Mol Carcinog. 2005 doi: 10.1002/mc.20174. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He Z, Ma WY, Hashimoto T, Bode AM, Yang CS, Dong Z. Induction of apoptosis by caffeine is mediated by the p53, bax, and caspase 3 pathways. Cancer Res. 2003;63:4396–4401. [PubMed] [Google Scholar]
- 72.Marzo I, Brenner C, Zamzami N, Jurgensmeier JM, Susin SA, Vieira HL, Prevost MC, Xie Z, Matsuyama S, Reed JC, Kroemer G. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science. 1998;281:2027–2031. doi: 10.1126/science.281.5385.2027. [DOI] [PubMed] [Google Scholar]
- 73.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 74.Hoshi T, Sasano H, Kato K, Yabuki N, Ohara S, Konno R, Asaki S, Toyota T, Tateno H, Nagura H. Immunohistochemistry of caspase3/cpp32 in human stomach and its correlation with cell proliferation and apoptosis. Anticancer Res. 1998;18:4347–4353. [PubMed] [Google Scholar]
- 75.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- 76.Hashimoto T, He Z, Ma WY, Schmid PC, Bode AM, Yang CS, Dong Z. Caffeine inhibits cell proliferation by g0/g1 phase arrest in jb6 cells. Cancer Res. 2004;64:3344–3349. doi: 10.1158/0008-5472.can-03-3453. [DOI] [PubMed] [Google Scholar]
- 77.Stein GH, Dulic V. Origins of g1 arrest in senescent human fibroblasts. Bioessays. 1995;17:537–543. doi: 10.1002/bies.950170610. [DOI] [PubMed] [Google Scholar]
- 78.Kitagawa M, Higashi H, Jung HK, Suzuki-Takahashi I, Ikeda M, Tamai K, Kato J, Segawa K, Yoshida E, Nishimura S, Taya Y. The consensus motif for phosphorylation by cyclin d1-cdk4 is different from that for phosphorylation by cyclin a/e-cdk2. Embo J. 1996;15:7060–7069. [PMC free article] [PubMed] [Google Scholar]
- 79.Kato J, Matsushime H, Hiebert SW, Ewen ME, Sherr CJ. Direct binding of cyclin d to the retinoblastoma gene product (prb) and prb phosphorylation by the cyclin d-dependent kinase cdk4. Genes Dev. 1993;7:331–342. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- 80.Taya Y. Rb kinases and rb-binding proteins: New points of view. Trends Biochem Sci. 1997;22:14–17. doi: 10.1016/s0968-0004(96)10070-0. [DOI] [PubMed] [Google Scholar]
- 81.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 82.Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin d1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Foukas LC, Daniele N, Ktori C, Anderson KE, Jensen J, Shepherd PR. Direct effects of caffeine and theophylline on p110 delta and other phosphoinositide 3-kinases. Differential effects on lipid kinase and protein kinase activities. J Biol Chem. 2002;277:37124–37130. doi: 10.1074/jbc.M202101200. [DOI] [PubMed] [Google Scholar]
- 84.Sazuka M, Isemura M, Isemura S. Interaction between the carboxyl-terminal heparin-binding domain of fibronectin and (−)-epigallocatechin gallate. Biosci Biotechnol Biochem. 1998;62:1031–1032. doi: 10.1271/bbb.62.1031. [DOI] [PubMed] [Google Scholar]
- 85.Hayakawa S, Saeki K, Sazuka M, Suzuki Y, Shoji Y, Ohta T, Kaji K, Yuo A, Isemura M. Apoptosis induction by epigallocatechin gallate involves its binding to fas. Biochem Biophys Res Commun. 2001;285:1102–1106. doi: 10.1006/bbrc.2001.5293. [DOI] [PubMed] [Google Scholar]
- 86.Suzuki Y, Isemura M. Inhibitory effect of epigallocatechin gallate on adhesion of murine melanoma cells to laminin. Cancer Lett. 2001;173:15–20. doi: 10.1016/s0304-3835(01)00685-1. [DOI] [PubMed] [Google Scholar]
- 87.Tachibana H, Koga K, Fujimura Y, Yamada K. A receptor for green tea polyphenol egcg. Nat Struct Mol Biol. 2004;11:380–381. doi: 10.1038/nsmb743. [DOI] [PubMed] [Google Scholar]
- 88.Ermakova S, Choi BY, Choi HS, Kang BS, Bode AM, Dong Z. The intermediate filament protein vimentin is a new target for epigallocatechin gallate. J Biol Chem. 2005;280:16882–16890. doi: 10.1074/jbc.M414185200. [DOI] [PubMed] [Google Scholar]
- 89.Chou YH, Goldman RD. Intermediate filaments on the move. J Cell Biol. 2000;150:F101–106. doi: 10.1083/jcb.150.3.f101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 2002;42:25–54. doi: 10.1146/annurev.pharmtox.42.082101.154309. [DOI] [PubMed] [Google Scholar]
- 91.Liang YC, Chen YC, Lin YL, Lin-Shiau SY, Ho CT, Lin JK. Suppression of extracellular signals and cell proliferation by the black tea polyphenol, theaflavin-3,3′-digallate. Carcinogenesis. 1999;20:733–736. doi: 10.1093/carcin/20.4.733. [DOI] [PubMed] [Google Scholar]
- 92.Kavanagh KT, Hafer LJ, Kim DW, Mann KK, Sherr DH, Rogers AE, Sonenshein GE. Green tea extracts decrease carcinogen-induced mammary tumor burden in rats and rate of breast cancer cell proliferation in culture. J Cell Biochem. 2001;82:387–398. doi: 10.1002/jcb.1164. [DOI] [PubMed] [Google Scholar]
- 93.Islam S, Islam N, Kermode T, Johnstone B, Mukhtar H, Moskowitz RW, Goldberg VM, Malemud CJ, Haqqi TM. Involvement of caspase-3 in epigallocatechin-3-gallate-mediated apoptosis of human chondrosarcoma cells. Biochem Biophys Res Commun. 2000;270:793–797. doi: 10.1006/bbrc.2000.2536. [DOI] [PubMed] [Google Scholar]
- 94.Brusselmans K, De Schrijver E, Heyns W, Verhoeven G, Swinnen JV. Epigallocatechin-3-gallate is a potent natural inhibitor of fatty acid synthase in intact cells and selectively induces apoptosis in prostate cancer cells. Int J Cancer. 2003;106:856–862. doi: 10.1002/ijc.11317. [DOI] [PubMed] [Google Scholar]