Abstract
μ-Opioid agonists frequently activate output neurons in the brain via disinhibition, that is, by inhibiting “secondary cells”, which results in disinhibition of “primary cells”, considered to be output neurons. Secondary cells are generally presumed to be inhibitory interneurons that serve only to regulate the activity of the output neurons. However, studies of the opioid-sensitive neurons in the rostral ventromedial medulla, a region with a well-documented role in nociceptive modulation, indicate that the opioid-inhibited neurons in this region (termed “on-cells” when recorded in vivo) have a distinct functional role that parallels and opposes the output of the subset of RVM neurons that are activated following opioid administration, the “off-cells”.
The aim of the present study was to analyze the relative timing of on- and off-cell reflex-related firing in the rostral ventromedial medulla to help determine whether on-cells are likely to function as inhibitory interneurons in this region. On- and off-cells display complementary firing patterns during noxious-evoked withdrawal: off-cells stop firing and on-cells show a burst of activity. If on-cells are inhibitory interneurons mediating the off-cell pause, the on-cells would be expected to begin their reflex-related discharge before the off-cells cease firing. To examine this we recorded activity of on-and off-cell pairs during heat-evoked paw or tail withdrawal in lightly anesthetized rats. For each cell pair, we measured the onsets of the off-cell pause and the on-cell burst. Contrary to what would be expected if on-cells were inhibitory interneurons, off-cells typically ceased firing before on-cells began reflex-related firing, with a mean 481 (± 69) ms lag between the final off-cell spike and the first on-cell spike. This suggests that on-cells do not mediate the off-cell pause, and points instead to presynaptic mechanisms in opioid-mediated disinhibition of medullary output neurons. These data also support an independent role for on-cells in pain modulation.
Keywords: pain modulation, descending control, disinhibition, nucleus raphe magnus, ON cells, OFF cells
Introduction
μ-Opioid agonists frequently activate output neurons in the brain via disinhibition. Thus, direct inhibition of “secondary cells” disinhibits “primary cells” or output neurons, allowing them to become active (Zieglgänsberger et al., 1979, Duggan and North, 1983, Madison and Nicoll, 1988, Johnson and North, 1992, Pan et al., 2004). It is generally assumed that the secondary cells are inhibitory interneurons, serving only to regulate the activity of the output neurons.
One region in which this opioid disinhibition model has been applied is the rostral ventromedial medulla (RVM), which includes the nucleus raphe magnus and adjacent reticular formation. Consistent with the model, μ-opioid agonists hyperpolarize secondary cells in the RVM, and reduce GABA-mediated inhibition of primary cells (Pan et al., 1990). At a behavioral level, focal application of morphine or μ-opioid agonists in this region produces antinociception via activation of a class of neurons referred to as “off-cells”. Off-cells become continuously active, presumably via disinhibition, following administration of morphine or μ-opioid agonists (Fields et al., 1983b, Heinricher et al., 1994), and off-cell activation is necessary for the analgesic actions of systemically administered morphine (Heinricher et al., 1999, Heinricher et al., 2001a, Heinricher et al., 2001b). A second class of RVM neurons, referred to as “on-cells” are inhibited by μ-opioid agonists, and presumably correspond to secondary cells recorded in vitro (Barbaro et al., 1986, Heinricher et al., 1992). Although at least a subset of both on- and off-cells project to the dorsal horn (Vanegas et al., 1984, Fields et al., 1995) and anatomical evidence suggests that on-cell axons do not arborize within the RVM (Mason and Fields, 1989), on-cells have nevertheless been widely assumed to function as opioid-sensitive inhibitory interneurons in the RVM. In this model, inhibition of on-cells by opioids in turn disinhibits off-cells to produce antinociception (Fields and Heinricher, 1985, Barbaro et al., 1986, Heinricher et al., 1994).
In addition to their opposing responses to μ-opioid agonists, the firing patterns of the off- and on-cell classes are generally consistent with a role for the latter as inhibitory interneurons. The two populations show reciprocal firing in lightly anesthetized preparations, with both on-cells and off-cells alternating between phases of silence and activity. Activity within each class is in phase, and the two classes fire out of phase (Barbaro et al., 1989). Moreover, on-cells are defined by a burst of activity that begins just before nocifensor reflexes such as the tail flick, while off-cells cease firing at that time (Fields et al., 1983a). That is, on-cells show a characteristic reflex-related “burst”, and off-cells a characteristic GABA-mediated reflex-related “pause”, and it was therefore reasonable to suggest that on-cells might serve as the GABA-containing inhibitory interneurons mediating the off-cell pause (Heinricher et al., 1991).
However, despite the complementary firing patterns of the on- and off-cell classes, there is now accumulating evidence that on-cells have an independent functional role that parallels and opposes the output of off-cells. Whereas off-cells have a net antinociceptive effect, on-cells are now known to promote nociception, an effect that does not require inhibition of off-cells. Moreover, on-cell firing can be suppressed directly without producing apparent disinhibition of off-cells (Heinricher and McGaraughty, 1998, Heinricher and Neubert, 2004, Neubert et al., 2004). A more detailed analysis is therefore needed to determine the nature of the interactions between these cell classes. If on-cells inhibit off-cells directly and mediate the reflex-related off-cell pause, it would be expected that on-cells should begin reflex-related firing before off-cells cease firing. The aim of the present study was to analyze the timing of the reflex-related on-cell burst and off-cell pause to help determine whether on-cell activity could account for the off-cell pause. Using simultaneous recordings from on-cell/off-cell pairs, we now show that the reflex-related off-cell pause most often precedes the onset of the on-cell burst.
Experimental Procedures
Animals and surgical preparation
All experimental procedures followed the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Committee for Research and Ethical Issues of the International Association for the Study of Pain, and were approved by the Institutional Animal Care and Use Committee at Oregon Health & Science University. Male Sprague-Dawley rats (Sasco, 250–350 g) were anesthetized with pentobarbital (60 mg/kg, i.p.), and a catheter inserted into an external jugular vein for administration of anesthetic. The rat was placed in a stereotaxic apparatus, a hole drilled in the skull over the cerebellum, and the dura removed to allow placement of an electrode or pair of electrodes in the RVM. Body temperature was maintained at approximately 37 °C by a circulating water pad.
Following surgery, the anesthetic level was reduced until a withdrawal reflex could be elicited by application of noxious heat using a feedback-controlled projector lamp focused on the blackened ventral surface of the tail or on the plantar surface of the hindpaw. The animals were then maintained in this lightly anesthetized state using a continuous infusion of methohexital at a rate (15–30 mg/kg per hour, i.v.) that allowed a stable withdrawal latency and that prevented any signs of discomfort. The animals did not move spontaneously, nor did they vocalize or produce vigorous or prolonged withdrawal reflexes following noxious pinch. The protocol was begun after a stabilization period of at least 30 min, and infusion rate was not altered during the protocol.
Recording
RVM neurons were recorded using stainless steel microelectrodes (FHC, Bowdoinham, ME) and classified as previously described (Fields et al., 1983a, Neubert et al., 2004). Spike waveforms were monitored and stored for off-line analysis (Datawave Systems, Thornton, CO or Spike 2, CED) to ensure that the units under study were unambiguously discriminated throughout the experiment. Spike times were stored with a temporal resolution of 0.1 ms. Each reflex trial consisted of a linear increase in temperature at approximately 1.8 °C/s from a holding temperature of 34 °C until the withdrawal reflex occurred. Off-cells were characterized by an abrupt pause in ongoing activity beginning just prior to the occurrence of the withdrawal. On-cells were identified by a sudden burst of activity beginning just prior to the occurrence of the reflex. Cells of a third class, “neutral cells,” were identified by the absence of change in activity associated with withdrawal and were not studied further.
We studied pairs of neurons (one off-cell and one on-cell) fortuitously encountered with a single electrode or recorded with two independently movable electrodes (about half of the pairs were recorded with each method). Trials were separated by a minimum of 5 min, and only initiated or included in the analysis when the off-cell was active and the on-cell silent at the stimulus onset. All recording sites were verified to be in the RVM.
Population analysis
The parameters of class-specific firing patterns were determined as follows. To study the population dynamics of the two cell classes, we combined the accumulated spike data for all the cells of each class. Histograms showing total number of spikes at any time relative to either the withdrawal or heat onset were constructed using the spike times for each trial aligned with these parameters. The number of spikes per bin and the time values from the histogram then provided the x-y points to which a curve could be fit (GraphPad Prism). Interpolating from the equations describing these curves, we then determined the timing for a given percentage change in population discharge (10%, 50% and 90%) relative to withdrawal.
Single trial analysis
The focus of the single-trial analysis was on the timing of the off-cell pause and on-cell burst, measured as the time between the final action potential of an off-cell before the withdrawal and the initial spike of the on-cell as part of a reflex-related burst. A positive value indicates that the off-cell stopped firing before the on-cell started firing. A negative value arises from an overlap in firing times, that is, trials on which there was at least one spike attributed to the on-cell burst before the final off-cell spike preceding the reflex. The time of withdrawal and the beginning of the heat stimulus were also marked, and the time between these events and the beginning of the burst and pause calculated. “Pause duration” was defined for off-cells as the time between the final spike before a withdrawal and the succeeding spike after the reflex. Similarly, for on-cells the “burst duration” was the time between the first and last spike of the reflex-related burst, with the end of the burst considered a silent period lasting at least 2 s. Burst/pause parameters for the two populations were compared using Student’s t-test for independent means and an F ratio for comparison of variances.
Results
Population Analysis
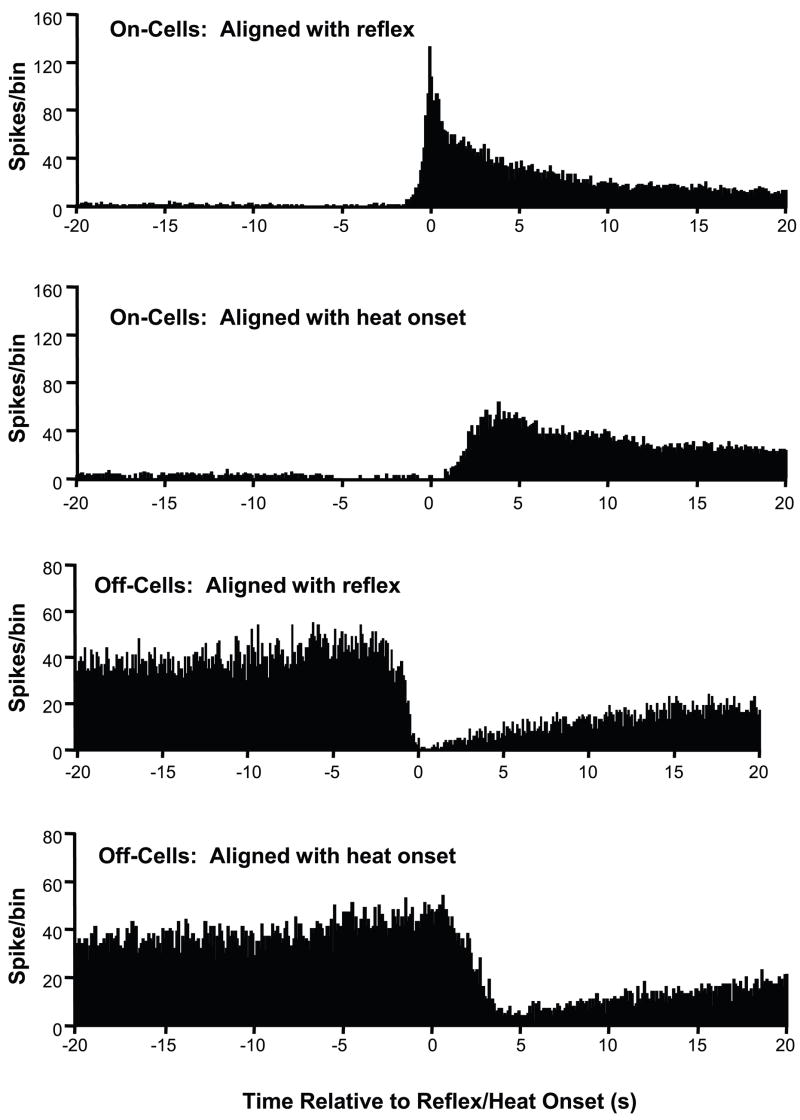
We first created a summed histogram of cell activity for a sample of 69 trials recorded from 28 on-cell/off-cell pairs, summing data obtained over all trials and aligning with either the heat onset or the reflex. As indicated in the early description of on- and off-cell firing (Fields et al., 1983a), changes in cell activity were more closely linked to the reflex than to the heat stimulus (Fig. 1). This was confirmed using single-trial analyses of the timing of the burst and pause. Variances in the timing of the burst and pause were significantly greater when calculated relative to the heat onset vs. the reflex for both on- and off-cell populations (F ratio, p < 0.0001 for both classes). The withdrawal, not the heat stimulus, is thus the relevant variable defining these cell populations and their patterns of activity.
Fig. 1. Reflex- and stimulus-related changes in on- and off-cell firing.
Population histograms show spike times aligned separately with heat-evoked withdrawal and heat onset. Upper two histograms are spike times for all on-cells aligned with the withdrawal and with heat onset. Lower two histograms are spike times for all off-cells, again aligned with withdrawal and heat onset. These histograms show that changes in cell discharge for both populations are more tightly linked to the reflex than to heat onset. (100 ms bins, alignment point at time 0). Note that trials were initiated and analyzed only when the off-cell was active and the on-cell silent.
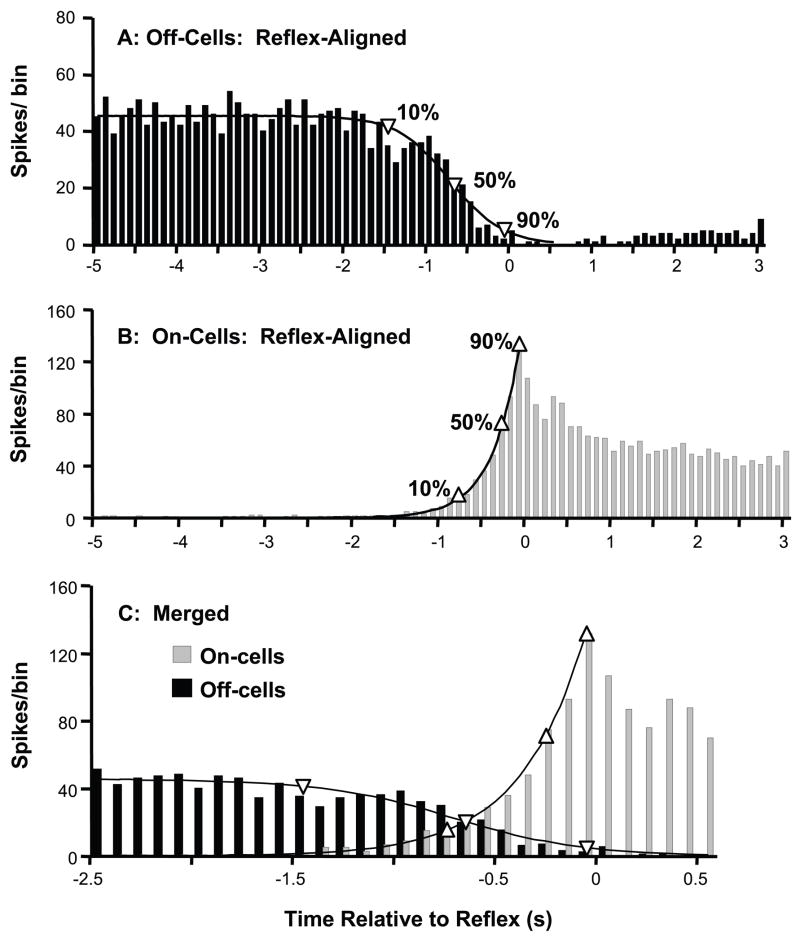
If on-cells function primarily as inhibitory interneurons, the reflex-related activation of the on-cell population should precede the inhibition of the off-cell population. With the relatively large, continuous data set provided by the summed histogram, we were able to fit curves to the on-cell and off-cell population output to determine the timing of changes in on- and off-cell firing relative to the withdrawal. Based on visual inspection of the population data, we chose an exponential function to describe the reflex-related activation of the on-cell population (R2 = 0.99), and a function with a logarithmic decay to fit the reflex-related decrease in off-cell firing (R2 = 0.96).
Because the size and values for the histograms change with number of trials and bin sizes, the exact equations that fit these curves are less important than how accurately they describe the data. The tight fit of the equations to the histogram values allows accurate determination of the timing of changes in population activity. The decline in off-cell population discharge preceded the activation of on-cells. Off-cell population firing had decreased by 10% from baseline at 1.5 s before the withdrawal, and then reached 50% of baseline by 753 ms before the withdrawal (Fig. 2, 95% CI: 846 to 589 ms). By contrast, firing of the on-cell population did not increase to 10% of its peak until 800 ms before the withdrawal, by which time off-cell firing had already fallen more than 50% from its baseline. In addition, on-cell firing did not reach 50% of the reflex-related peak until 320 ms before the reflex (95% CI: 430 to 268 ms, no overlap with off-cell 50% change point). Thus, off-cells as a population exhibited a reflex-related decline in firing that preceded the recruitment of on-cells.
Fig. 2. Inhibition of the off-cells as a population precedes activation of on-cells.
A,B: The reflex-related decrease in off-cell firing (A, fit with a logarithmic decline), and on-cell activation (B, well described by an exponential function) are shown. Change points (90%, 50% and 10%) are marked by upward pointing triangles for on-cells and downward pointing triangles for off-cells. C: Merged data from on- and off-cells plotted on an expanded time scale. Changes in the off-cell population preceded those in the on-cell population, with both an earlier onset and half-maximal change. Bin width: 100 ms.
Relationship between cessation of off-cell firing and beginning of on-cell firing associated with reflex withdrawal
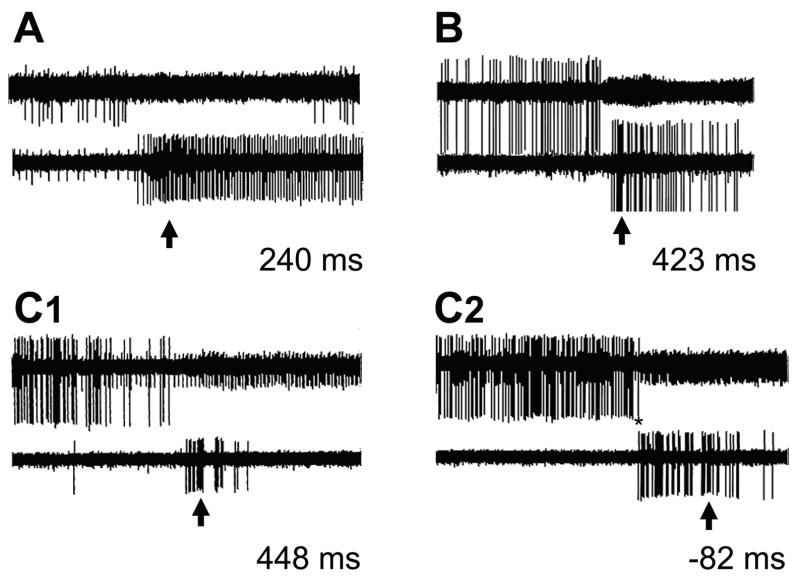
The population analysis showed that, as a class, off-cells slow their firing prior to activation of on-cells, an observation inconsistent with a primary function for on-cells as inhibitory interneurons. However, firing rates and thresholds differ within the on- and off-cell populations, and at any time, population firing rate reflects the number of cells active at that time as well as firing rate of active cells. Data from individual paired trials (79 trials/33 pairs) were therefore used to determine relative onset of the pause (final off-cell spike prior to the withdrawal) and burst (first on-cell spike prior to withdrawal). Examples of individual trials are shown in Fig. 3. The off-cell in most cases became silent before the first spike of the on-cell (pairs in Fig. 3, A, B, and C1). Some overlap in firing between cell types was seen on a minority of trials (e.g., C2 in Fig. 3), but all pairs exhibited at least one trial with no overlap, and such overlap as did occur was generally only one or a few spikes (for example, the single off-cell spike indicated by the asterisk in Fig. 3, C2 followed the first spike of the on-cell burst).
Fig. 3. Simultaneously recorded pause and burst on individual heat trials.
A, B and C are three on-cell/off-cell pairs. Oscilloscope traces (duration 10 s) show activity of an off-cell (top trace in each case) and on-cell (lower trace). The arrow indicates the exact time of the withdrawal. The trials shown for pairs A and B and the first trial for pair C (C1) are examples of the pause in off-cell discharge beginning before the on-cell started firing. Pair C also showed one trial (C2) on which the on-cell began to fire before the final off-cell spike (overlapping spike of the off-cell marked by an asterisk). Lag between final off-cell spike and first on-cell spike are given for each trial.
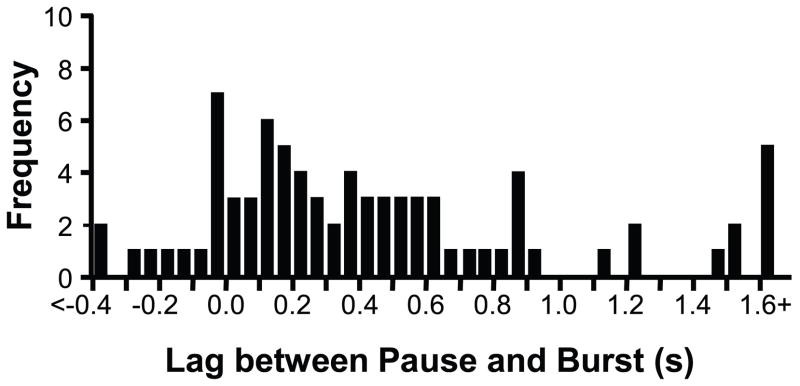
We quantified the lag between the final action potential of the off-cell and the first action potential of the on-cell. As shown in Fig. 4, there was quite a wide range from trial to trial and between pairs, ranging from −0.744 s (the on-cell began firing before the final off-cell spike) to 2.417 s (the off-cell stopped firing well before the on-cell cell began firing). However, the mean lag from the final off-cell spike to the first on-cell spike was 481 ± 69 ms, which is significantly greater than the zero lag that would be expected if there was a random relationship between the off-cell pause and on-cell burst (one-sample t-test, p < 0.0001). Moreover, the off-cell fired at least one spike after the on-cell had begun to discharge on only 17.7% of the trials examined. The 99% confidence interval (9.0% to 31.4% of trials showing overlap) does not include the 50% of trials that would have been expected with a random relationship.
Fig. 4. Latencies between last off-cell spike (pause onset) and first on-cell spike (burst onset).
A positive lag indicates that the off-cell paused before the on-cell began to fire, and a negative lag that the on-cell began to fire before the off-cell had stopped firing completely. Although there was significant variability in the lag time, the on-cell burst followed the off-cell pause in the majority (82.3%) of trials.
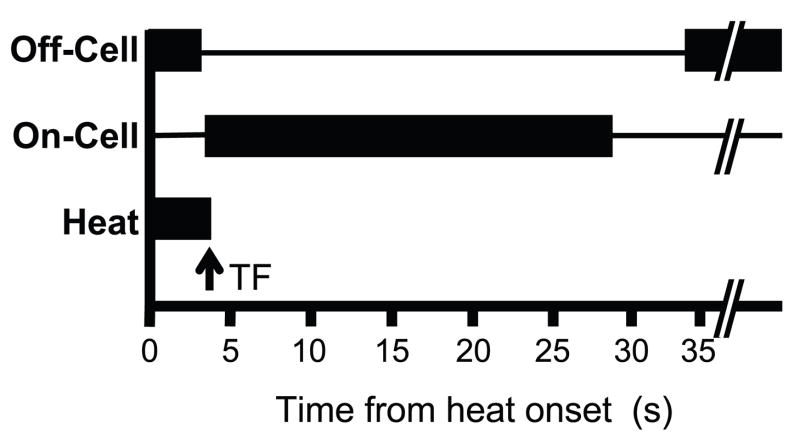
The relationships between the onset of the heat stimulus, the cell responses, and the withdrawal reflex are shown schematically in Fig. 5. Consistent with the off-cell pause preceding the on-cell burst, the mean latency from the off-cell pause to the reflex was longer than from the onset of the on-cell burst to the reflex (1172 ± 106 ms vs. 584 ± 88 ms for off-cell pause and on-cell burst, respectively, p < 0.001). The average duration of the on-cell burst, from the onset of the first spike to the time of last spike, was 28.042 ± 9.558 s, and the duration of the off-cell pause was 35.447 ± 7.729 s. These values are not significantly different (p > 0.05).
Fig. 5. Schematic illustrates average timing of off-cell pause, on-cell burst, and withdrawal for the recorded cells.
Withdrawal time is indicated by arrow. On average, the off-cell pauses before the on-cell burst begins. The duration of the pause and burst are less consistent, and there is often recovery of off-cell firing before the on-cell burst is complete.
Discussion
Relationship between on-cell and off-cell firing
The idea that on-cells have a primary role as inhibitory interneurons has been widely accepted, although based on indirect evidence. The central observation supporting this idea was that on-cells become inactive and off-cells continuously active following administration of morphine (Fields et al., 1983b, Barbaro et al., 1986). In addition, the two classes show reciprocal patterns of spontaneous and reflex-related firing. Spontaneous firing of the two populations is out of phase, while periods of spontaneous activity within each population are in phase (Barbaro et al., 1989, Heinricher et al., 1989). Similarly, on-cells exhibit reflex-related bursts of activity whereas off-cells cease firing. Based on the reciprocity between the two cell classes, it was reasonable to suggest that on-cells function as inhibitory interneurons. If so, opioids would disinhibit off-cells to produce antinociception by inhibiting on-cells (Fields and Heinricher, 1985, Barbaro et al., 1986).
There have nevertheless been several observations inconsistent with this idea of on-cells as interneurons. First, general suppression of on-cell firing does not disinhibit off-cell firing. Second, off-cell firing rate is not affected by selective pharmacological activation of the on-cell population (Heinricher and McGaraughty, 1998, Heinricher and Neubert, 2004, Neubert et al., 2004). The present data add to this evidence against a role for on-cells primarily as inhibitory interneurons. We used paired recordings to sample simultaneously the reflex-related activity of the on- and off-cell populations. If the on-cells were inhibiting the off-cells, the former should have begun firing before the off-cells slowed or stopped their firing. Such was not the case, since the reflex-related burst on most trials began only after the off-cell had stopped firing. Although it is unlikely that the individual neurons recorded were directly connected, cells within each class tend to fire in phase, so a given cell can be considered representative of the population. Moreover, the gap between the off-cell pause and on-cell burst should have centered around zero if there were no relationship between the two populations. Instead, the gap from the off-cell pause to the on-cell burst averaged almost half a second, which if anything would point to disinhibition of on-cells rather than vice versa. Occasional cases in which at least one on-cell spike occurred before the off-cell completely ceased firing could represent a subset of on-cells functioning as inhibitory interneurons, with a strong one-to-many inhibitory relationship. However, observed overlaps were sparse, with only one or a few interdigitated spikes. Moreover, overlaps were inconsistent across trials, and did not seem to mark a particular on-cell as a candidate inhibitory interneuron. These results therefore imply that on-cells are unlikely to trigger the off-cell pause.
Population Analysis
Both on- and off-cells are defined by a reflex-related change in firing, and the reflex-related coordinated firing within each cell class lends itself to a population analysis. Thus, spikes from cells within each class can be aligned with the reflex in order to study the firing patterns of that class as a whole. The points marking 90%, 50%, and 10% changes in firing (relative to pre-stimulus ongoing activity for off-cells or to peak reflex-related rate for on-cells) are an index of the timing of population changes relative to the reflex. Unlike single trial data, changes in population output around the withdrawal reflect a combination of altered firing rate of active cells with recruitment or loss from the active population. Nevertheless, the population analysis confirms the single trial data in showing that changes in off-cell population output precede changes in on-cells as a class. Notably, on-cell output was increased to only slightly more than 10% of its peak at a time when that of the off-cells had already fallen by 50% from its prestimulus level. Indeed, the off-cell population output achieved a half-maximum decrease in overall activity almost half a second before on-cells showed a half-maximal activation. The population data thus bolster the conclusion from the single-trial findings that on-cells are not inhibitory interneurons in the RVM.
Functional implications
On- and off-cell populations display opposite modulation of firing in many paradigms. This is evident in the reflex-related burst and pause, but also in spontaneous firing (Barbaro et al., 1989, Jinks et al., 2004), following administration of analgesic compounds including μ-opioids, nonsteroidal analgesics, and cannabinoids (Heinricher et al., 1994, Vanegas and Tortorici, 2002, Meng and Johansen, 2004), with chemical stimulation of the periaqueductal gray, amygdala or hypothalamus producing analgesia or hyperalgesia (Morgan et al., 1992, McGaraughty et al., 2003, Heinricher et al., 2004a, Heinricher et al., 2004b, de Novellis et al., 2005, Marabese et al., 2007), in acute inflammation (Kincaid et al., 2006), and following peripheral nerve injury (Carlson et al., 2007). The fact that firing of the two classes is altered in parallel in so many paradigms has made it difficult to discern the function of each class independent of the other. However, at least a subset of both on- and off-cells project to the dorsal horn (Vanegas et al., 1984, Fields et al., 1995), and recent work has shown that the firing of on-cells can be manipulated pharmacologically (blocked or activated) without necessarily altering the firing off-cells (Heinricher and McGaraughty, 1998, Heinricher et al., 2001a, Neubert et al., 2004). These findings indicate that on-cells have their own role in pain modulation, exerting a net pro-nociceptive action that can occur independent of altered off-cell firing.
Opioid inhibition of on-cells is thus in parallel, rather than in series, with the disinhibition of off-cells. Notably, the inhibition of on-cells seems unlikely to contribute significantly to the analgesic effects of these drugs under acute conditions (such as when using the heat-evoked withdrawal). This is because selective elimination of on-cell activity does not result in hypoalgesia in this situation (Heinricher and McGaraughty, 1998). However, on-cell activation contributes to hyperalgesia following inflammation and nerve injury (Porreca et al., 2001, Kincaid et al., 2006, Xu et al., 2007), which indicates that opioid suppression of exaggerated on-cell firing in abnormal pain states could contribute to an anti-hyperalgesic action of these drugs. The source of the off-cell pause remains to be determined, but our data suggest that it arises from outside of the RVM, since any RVM neuron active at the time of the off-cell pause would, by definition, be an on-cell. This would imply that opioids act presynaptically, on this as yet unidentified extrinsic GABAergic input.
On-cell burst and off-cell pause are reflex-related
Our data confirm earlier reports that the on-cell burst and off-cell pause are related to the nocifensor reflex rather than to the sensory stimulus (Fields et al., 1983a). This relationship is what would be expected of modulatory neurons, and is very different from sensory transmission neurons, which by contrast should code some aspect of the stimulus. Worth noting however is that this firing pattern by itself does not provide information as to what is being modulated. Identifying the target of the modulation requires other kinds of evidence, including information about connectivity and the behavioral effects of activating the modulating system. Such evidence supports the idea that on- and off-cells modulate processing of nociceptive sensory information, rather than having a direct motor effect. First, the RVM as a whole projects to the spinal gray matter, but most prominently to the dorsal horn and intermediate zones. Consistent with this idea, identified on- and off-cells are antidromically activated predominantly from laminae I, II and V (Fields et al., 1995). An identified off-cell has also been shown to project to the trigeminal sensory complex (Mason and Fields, 1989). Second, stimulation in the RVM alters responses of dorsal horn neurons, including identified spinothalamic tract cells (Willis, 1988). Third, stimulation of the RVM does not alter monosynaptic reflexes, indicating that the influence of the RVM in the spinal gray is proximal to the motoneurons (Floeter and Fields, 1991). Fourth, behavioral studies in awake behaving animals do not support a generalized motor inhibition from the RVM. Although Morgan and Whitney (2000) reported immobility in an open field with RVM stimulation, they characterized this as defensive freezing, since the animals were able to ambulate when handled. Finally, human patients that receive pain relief from stimulation of the periventricular gray report analgesia rather than motor inhibition (Hosobuchi et al., 1977, Barbaro, 1988), and activation of RVM neurons alters affective state as well as nociception (Hirakawa et al., 2000). Although the boundary between sensory and motor systems cannot be clearly demarcated, these data collectively indicate that RVM on- and off-cells influence sensory processing.
Conclusions
Contrary to what would be expected if on-cells mediated the reflex-related off-cell pause, off-cells typically stop firing before significant activation of on-cells. This implies that the GABAergic input mediating the off-cell pause originates from outside of the RVM, which is consistent with an earlier report that blocking the on-cell burst did not interfere with the off-cell pause (Heinricher et al., 1991, Heinricher and McGaraughty, 1998), with the lack of local arborizations of on-cell axons within the RVM (Mason and Fields, 1989), and the absence of GABA-synthetic enzymes in identified on-cells (Winkler et al., 2006). These data thus support the idea that on-cells can promote nociception independently of off-cells, and it seems likely that μ-opioid inhibition of on-cells contributes to the anti-hyperalgesic actions of these compounds in abnormal pain states.
Acknowledgments
This work was supported by a grant from NIDA (DA05608).
Abbreviations
- RVM
rostral ventromedial medulla
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barbaro NM. Studies of PAG/PVG stimulation for pain relief in humans. Prog Brain Res. 1988;77:165–173. doi: 10.1016/s0079-6123(08)62783-1. [DOI] [PubMed] [Google Scholar]
- Barbaro NM, Heinricher MM, Fields HL. Putative pain modulating neurons in the rostral ventral medulla: reflex-related activity predicts effects of morphine. Brain Res. 1986;366:203–210. doi: 10.1016/0006-8993(86)91296-5. [DOI] [PubMed] [Google Scholar]
- Barbaro NM, Heinricher MM, Fields HL. Putative nociceptive modulatory neurons in the rostral ventromedial medulla of the rat display highly correlated firing patterns. Somatosens Mot Res. 1989;6:413–425. doi: 10.3109/08990228909144684. [DOI] [PubMed] [Google Scholar]
- Carlson JD, Maire JJ, Heinricher MM. Sensitization of the rostral ventromedial medulla (RVM) following nerve injury. Soc Neurosci Abstr. 2007 doi: 10.1523/JNEUROSCI.3715-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Novellis V, Mariani L, Palazzo E, Vita D, Marabese I, Scafuro M, Rossi F, Maione S. Periaqueductal grey CB1 cannabinoid and metabotropic glutamate subtype 5 receptors modulate changes in rostral ventromedial medulla neuronal activities induced by subcutaneous formalin in the rat. Neuroscience. 2005;134:269. doi: 10.1016/j.neuroscience.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Duggan AW, North RA. Electrophysiology of opioids. Pharmacol Rev. 1983;35:219–281. [PubMed] [Google Scholar]
- Fields HL, Bry J, Hentall I, Zorman G. The activity of neurons in the rostral medulla of the rat during withdrawal from noxious heat. J Neurosci. 1983a;3:2545–2552. doi: 10.1523/JNEUROSCI.03-12-02545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Heinricher MM. Anatomy and physiology of a nociceptive modulatory system. Philos Trans of the R Soc Lond B Biol Sci. 1985;308:361–374. doi: 10.1098/rstb.1985.0037. [DOI] [PubMed] [Google Scholar]
- Fields HL, Malick A, Burstein R. Dorsal horn projection targets of ON and OFF cells in the rostral ventromedial medulla. J Neurophysiol. 1995;74:1742–1759. doi: 10.1152/jn.1995.74.4.1742. [DOI] [PubMed] [Google Scholar]
- Fields HL, Vanegas H, Hentall ID, Zorman G. Evidence that disinhibition of brain stem neurones contributes to morphine analgesia. Nature. 1983b;306:684–686. doi: 10.1038/306684a0. [DOI] [PubMed] [Google Scholar]
- Floeter MK, Fields HL. Evidence that inhibition of a nociceptive flexion reflex by stimulation in the rostroventromedial medulla in rats occurs at a premotoneuronal level. Brain Res. 1991;538:340–342. doi: 10.1016/0006-8993(91)90452-2. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Barbaro NM, Fields HL. Putative nociceptive modulating neurons in the rostral ventromedial medulla of the rat: firing of on- and off-cells is related to nociceptive responsiveness. Somatosens Mot Res. 1989;6:427–439. doi: 10.3109/08990228909144685. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Haws CM, Fields HL. Evidence for GABA-mediated control of putative nociceptive modulating neurons in the rostral ventromedial medulla: iontophoresis of bicuculline eliminates the off-cell pause. Somatosens Mot Res. 1991;8:215–225. doi: 10.3109/08990229109144745. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Martenson ME, Neubert MJ. Prostaglandin E2 in the midbrain periaqueductal gray produces hyperalgesia and activates pain-modulating circuitry in the rostral ventromedial medulla. Pain. 2004a;110:419–426. doi: 10.1016/j.pain.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, McGaraughty S. Analysis of excitatory amino acid transmission within the rostral ventromedial medulla: Implications for circuitry. Pain. 1998;75:247–255. doi: 10.1016/s0304-3959(97)00226-1. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, McGaraughty S, Farr DA. The role of excitatory amino acid transmission within the rostral ventromedial medulla in the antinociceptive actions of systemically administered morphine. Pain. 1999;81:57–65. doi: 10.1016/s0304-3959(98)00271-1. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, McGaraughty S, Tortorici V. Circuitry underlying antiopioid actions of cholecystokinin within the rostral ventromedial medulla. J Neurophysiol. 2001a;85:280–286. doi: 10.1152/jn.2001.85.1.280. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Morgan MM, Fields HL. Direct and indirect actions of morphine on medullary neurons that modulate nociception. Neuroscience. 1992;48:533–543. doi: 10.1016/0306-4522(92)90400-v. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Morgan MM, Tortorici V, Fields HL. Disinhibition of off-cells and antinociception produced by an opioid action within the rostral ventromedial medulla. Neuroscience. 1994;63:279–288. doi: 10.1016/0306-4522(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Neubert MJ. Neural basis for the hyperalgesic action of cholecystokinin in the rostral ventromedial medulla. J Neurophysiol. 2004;92:1982–1989. doi: 10.1152/jn.00411.2004. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Neubert MJ, Martenson ME, Gonçalves L. Prostaglandin E2 in the medial preoptic area produces hyperalgesia and activates pain-modulating circuitry in the rostral ventromedial medulla. Neuroscience. 2004b;128:389–398. doi: 10.1016/j.neuroscience.2004.06.050. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Schouten JC, Jobst EE. Activation of brainstem N-methyl-D-aspartate receptors is required for the analgesic actions of morphine given systemically. Pain. 2001b;92:129–138. doi: 10.1016/s0304-3959(00)00480-2. [DOI] [PubMed] [Google Scholar]
- Hirakawa N, Tershner SA, Fields HL, Manning BH. Bi-directional changes in affective state elicited by manipulation of medullary pain-modulatory circuitry. Neuroscience. 2000;100:861–871. doi: 10.1016/s0306-4522(00)00329-8. [DOI] [PubMed] [Google Scholar]
- Hosobuchi Y, Adams JE, Linchitz R. Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science. 1977;197:183–186. doi: 10.1126/science.301658. [DOI] [PubMed] [Google Scholar]
- Jinks SL, Carstens E, Antognini JF. Isoflurane differentially modulates medullary ON and OFF neurons while suppressing hind-limb motor withdrawals. Anesthesiology. 2004;100:1224–1234. doi: 10.1097/00000542-200405000-00026. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid W, Neubert MJ, Xu M, Kim CJ, Heinricher MM. Role for medullary pain facilitating neurons in secondary thermal hyperalgesia. J Neurophysiol. 2006;95:33–41. doi: 10.1152/jn.00449.2005. [DOI] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Enkephalin hyperpolarizes interneurones in the rat hippocampus. J Physiol. 1988;398:123–130. doi: 10.1113/jphysiol.1988.sp017033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marabese I, Rossi F, Palazzo E, de Novellis V, Starowicz K, Cristino L, Vita D, Gatta L, Guida F, Di Marzo V, Rossi F, Maione S. Periaqueductal gray metabotropic glutamate receptor subtype 7 and 8 mediate opposite effects on amino acid release, rostral ventromedial medulla cell activities, and thermal nociception. J Neurophysiol. 2007;98:43–53. doi: 10.1152/jn.00356.2007. [DOI] [PubMed] [Google Scholar]
- Mason P, Fields HL. Axonal trajectories and terminations of on- and off-cells in the cat lower brainstem. J Comp Neurol. 1989;288:185–207. doi: 10.1002/cne.902880202. [DOI] [PubMed] [Google Scholar]
- McGaraughty S, Chu KL, Bitner RS, Martino B, Kouhen RE, Han P, Nikkel AL, Burgard EC, Faltynek CR, Jarvis MF. Capsaicin infused into the PAG affects rat tail flick responses to noxious heat and alters neuronal firing in the RVM. J Neurophysiol. 2003;90:2702–2710. doi: 10.1152/jn.00433.2003. [DOI] [PubMed] [Google Scholar]
- Meng ID, Johansen JP. Antinociception and modulation of rostral ventromedial medulla neuronal activity by local microinfusion of a cannabinoid receptor agonist. Neuroscience. 2004;124:685–693. doi: 10.1016/j.neuroscience.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Heinricher MM, Fields HL. Circuitry linking opioid-sensitive nociceptive modulatory systems in periaqueductal gray and spinal cord with rostral ventromedial medulla. Neuroscience. 1992;47:863–871. doi: 10.1016/0306-4522(92)90036-2. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Whitney PK. Immobility accompanies the antinociception mediated by the rostral ventromedial medulla of the rat. Brain Res. 2000;872:276–281. doi: 10.1016/s0006-8993(00)02502-6. [DOI] [PubMed] [Google Scholar]
- Neubert MJ, Kincaid W, Heinricher MM. Nociceptive facilitating neurons in the rostral ventromedial medulla. Pain. 2004;110:158–165. doi: 10.1016/j.pain.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Pan YZ, Li DP, Chen SR, Pan HL. Activation of mu-opioid receptors excites a population of locus coeruleus-spinal neurons through presynaptic disinhibition. Brain Res. 2004;997:67–78. doi: 10.1016/j.brainres.2003.10.050. [DOI] [PubMed] [Google Scholar]
- Pan ZZ, Williams JT, Osborne PB. Opioid actions on single nucleus raphe magnus neurons from rat and guinea-pig in vitro. J Physiol. 1990;427:519–532. doi: 10.1113/jphysiol.1990.sp018185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F, Burgess SE, Gardell LR, Vanderah TW, Malan TP, Jr, Ossipov MH, Lappi DA, Lai J. Inhibition of neuropathic pain by selective ablation of brainstem medullary cells expressing the μ-opioid receptor. J Neurosci. 2001;21:5281–5288. doi: 10.1523/JNEUROSCI.21-14-05281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanegas H, Barbaro NM, Fields HL. Tail-flick related activity in medullospinal neurons. Brain Res. 1984;321:135–141. doi: 10.1016/0006-8993(84)90689-9. [DOI] [PubMed] [Google Scholar]
- Vanegas H, Tortorici V. Opioidergic effects of nonopioid analgesics on the central nervous system. Cell Mol Neurobiol. 2002;22:655–661. doi: 10.1023/A:1021896622089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis WD., Jr Anatomy and physiology of descending control of nociceptive responses of dorsal horn neurons: comprehensive review. Prog Brain Res. 1988;77:1–29. doi: 10.1016/s0079-6123(08)62776-4. [DOI] [PubMed] [Google Scholar]
- Winkler CW, Hermes SM, Chavkin CI, Drake CT, Morrison SF, Aicher SA. Kappa opioid receptor (KOR) and GAD67 immunoreactivity are found in OFF and NEUTRAL cells in the rostral ventromedial medulla. J Neurophysiol. 2006;96:3465–3473. doi: 10.1152/jn.00676.2006. [DOI] [PubMed] [Google Scholar]
- Xu M, Kim CJ, Neubert MJ, Heinricher MM. NMDA receptor-mediated activation of medullary pro-nociceptive neurons is required for secondary thermal hyperalgesia. Pain. 2007;127:253–262. doi: 10.1016/j.pain.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieglgänsberger W, French ED, Siggins GR, Bloom FE. Opioid peptides may excite hippocampal pyramidal neurons by inhibiting adjacent inhibitory interneurons. Science. 1979;205:415–417. doi: 10.1126/science.451610. [DOI] [PubMed] [Google Scholar]