Abstract
CD40/CD40 ligand interactions have a central role in the induction of both humoral and cellular immunity. In this study, we examined whether a plasmid expressing CD40 ligand/trimer (CD40LT) could enhance immune responses in vivo. BALB/c mice were injected with plasmid expressing β-galactosidase DNA with or without CD40LT DNA or IL-12 DNA, and immune responses were assessed. Mice vaccinated with β-gal DNA plus CD40LT DNA or IL-12 DNA had a striking increase in Ag-specific production of IFN-γ, cytolytic T cell activity, and IgG2a Ab. The mechanism by which CD40LT DNA enhanced these responses was further assessed by treating vaccinated mice with anti-IL-12 mAb or CTLA-4 Ig (CTLA4Ig). Production of IFN-γ and CTL activity was abrogated by these treatments, suggesting that CD40LT DNA was mediating its effects on IFN-γ and CTL activity through induction of IL-12 and enhancement of B7 expression, respectively. Physiologic relevance for the ability of CD40LT DNA to enhance immune responses by the aforementioned pathways was shown in two in vivo models. First, with regard to CTL activity, mice vaccinated with CD40LT DNA did not develop metastatic tumor following challenge with lethal dose of tumor. Moreover, in a mouse model requiring IL-12-dependent production of IFN-γ, mice vaccinated with soluble Leishmania Ag and CD40LT DNA were able to control infection with Leishmania major. These data suggest that CD40LT DNA could be a useful vaccine adjuvant for diseases requiring cellular and/or humoral immunity.
The goal in developing effective vaccines for a particular disease depends on several factors. The first and perhaps most important factor is identification of a conserved Ag capable of inducing protection in an outbred population. Because various diseases have different requirements for protective immunity, it is also important to design vaccines that can induce an appropriate qualitative and quantitative immune response. One final consideration is that some diseases may require different types of immune responses for effective primary and memory immunity. In experimental murine models for most viral infections, primary immunity appears to be mediated predominantly through humoral immunity (1), while for other intracellular infections, such as Leishmania major (2) or Mycobacterium tuberculosis (3), cell-mediated immunity appears to be the likely mechanism. It should be noted, however, that in some instances (e.g., HIV), both humoral and cellular responses may be required for primary immunity and the maintenance of an effective memory response. Thus, vaccine strategies designed to induce a broad immune response may provide Ab-based protection following primary exposure and memory/effector cellular responses capable of controlling disease on reactivation or reinfection.
DNA immunization has recently been shown to induce potent humoral and cellular responses in vivo (4). More important, protective immunity for several infectious disease and tumor models has been demonstrated using this technology (4). Among the potential advantages of using plasmid DNA for a particular Ag rather than a purified protein plus adjuvant is that DNA can induce both MHC class I (5-7) and class II (8) responses. In addition, plasmid DNA itself contains specific immunostimulatory sequences (ISS)2 containing unmethylated CpG motifs that provide potent adjuvant effects in murine in vivo models (9-14). Moreover, while in vitro data using PBMCs suggest that ISS are able to elicit inflammatory cytokines (14), it is not clear whether these ISS are as effective in inducing immune responses from human cells in vivo. Thus, because DNA vaccination still provides such an effective way to present Ag, several studies have utilized plasmid DNA encoding for cytokine or costimulatory molecules as an additional way to enhance the type and magnitude of the immune response.
The study reported in this work focuses on the ability of a novel trimeric CD40 ligand DNA (CD40LT) to enhance Ag-specific Ab, cytokine, and CTL responses following in vivo immunization. Previous work has shown the CD40L/CD40 costimulatory pathway to be a central regulator of both humoral and cellular immune responses (15, 16). CD40L is a type II membrane glycoprotein with homology to TNF and lymphotoxin-α, which are ligands known to exist as homotrimers (17, 18). The interaction of CD40L and CD40 is both responsible for activation of B cells allowing for isotype switching (19) and important in T cell activation (20) and production of type 1 cytokines (IL-12, IFN-γ) in response to protein Ags (21-25). Moreover, CD40L has been shown to play an important role in CTL memory responses following viral infection (26). The mechanism by which CD40L/CD40 regulates cellular responses is primarily through induction of IL-12 and other inflammatory mediators from APCs (22-25) and/or through enhancement of the expression of various cell surface molecules (e.g., B7-1, B7-2) (27-31). Previous work has revealed that monomeric, dimeric, and trimeric CD40L constructs all bind to CD40 (18); however, there appears to be a greater capacity for trimeric CD40L to trigger biologic responses (18). Thus, in this study, the mechanism by which a trimeric CD40L DNA (CD40LT) induces enhancement of both humoral and cellular immune responses in vivo was investigated. The data show that the ability of CD40LT DNA to enhance cytolytic T cell activity and cytokine responses is influenced by enhancement of B7 costimulation and production of IL-12, respectively. These mechanisms were shown to be biologically important using a murine tumor and infectious disease model.
Materials and Methods
Mice
Female BALB/c mice were purchased from Division of Cancer Treatment, National Cancer Institute (Frederick, MD), and kept in National Institute of Allergy and Infectious Diseases Animal Care Facility under pathogen-free conditions. Mice used were between 6 and 8 wk of age.
Media and reagents
HBSS (Biofluids, Rockville, MD) was used as wash medium. RPMI 1640 (Biofluids) supplemented with 10% FCS (Biofluids), penicillin (100 U/ml), l-glutamine (2 mM), sodium pyruvate (1 mM), and 2-ME (0.005 mM) was used to culture splenocytes and lymph node cells.
Plasmid construction and purification
A plasmid encoding the Escherichia coli LacZ gene under the control of a CMV intermediate-early promoter, designated β-gal DNA, was kindly provided by J. Haynes (PowderJect, Madison, WI). cDNA encoding murine CD40L fused to an IL-7 leader and a leucine zipper sequence (CD40LT) in plasmid vector (Bluescript11SK±) was kindly provided by M. Spriggs (Immunex, Seattle, WA). The CD40LT fusion protein insert was excised using NotI/KpnI and ligated into expression vector PcDNA-3, downstream of the CMV promoter (Invitrogen, San Diego, CA) and designated CD40 ligand/trimer DNA (CD40LT DNA). Immunoprecipitation of murine CD40LT from COS cells (transfected with the PcDNA-3 CD40LT DNA) using a mAb to the leucine zipper showed a 35-kDa band in lysates and supernatants of the transfected cells. A eukaryotic expression vector carrying IL-12 DNA was kindly provided by J. Haynes (PowderJect). IL-12 DNA was able to produce biologically active protein from supernatants of COS cells transfected with IL-12 DNA (data not shown). Plasmid DNA was purified by two cycles of cesium chloride gradient ultracentrifugation. The 260/280 ratios ranged from 1.8 to 2. The endotoxin content from purified plasmid DNA was found to be <5 endotoxin units/ml.
Immunization
Female BALB/c mice were injected s.c. in their hind footpads with 100 μg of β-gal plasmid DNA in 50 μl of sterile PBS. Additionally, in some groups, β-gal DNA was combined with 100 μg of plasmid DNA encoding for IL-12 or CD40LT DNA and injected as above. Mice were boosted 2 to 3 wk later with their initial regimen.
Treatment of mice with neutralizing Abs
Purified neutralizing mAb (1 mg/mouse i.p.) against murine IL-12 (hybridoma c17.8) was obtained from G. Trinchieri (Wistar Institute, Philadelphia, PA) and injected into mice at the time of initial vaccination and boost. CTLA4Ig (100 μg/mouse i.p.) and control L6Ig Ab (100 μg/mouse i.p.) were obtained from P. Linsley (Bristol-Meyers Squibb, Seattle, WA) and injected into mice at the time of vaccination and boost.
Cytokine production assay
Mice were euthanized, and spleens from individual mice were harvested 2 wk after the last vaccination. Single-cell preparations from spleens were plated in triplicate in a 96-well microtiter plate at 3 × 105 cells/200 μl. Recombinant β-gal protein (20 μg/ml) (Sigma, St. Louis, MO) or human serum albumin (10 μg/ml) was added to cultures, and supernatants were collected 48 h later and stored at −20°C.
Measurement of cytokine production
Measurements of IFN-γ (32) and IL-4 were assessed by specific ELISA. The lower limits of detection of IFN-γ and IL-4 were 30 and 1.5 pg/ml, respectively.
Measurement of β-gal-specific Ab responses
β-gal-specific Ab responses were measured using a sandwich ELISA. BALB/c mice were immunized as described above. Pooled serum samples (n = 5–10 mice per group) were obtained 2 wk after the last immunization and were analyzed for the presence of β-gal-specific Abs. Briefly, 96-well microtiter Immulon-4 plates (Dynatech, Chantilly, VA) were coated with β-gal protein (10 μg/ml) overnight at 4°C. Plates were blocked with 2% BSA/PBS at 37°C for 1 h to prevent nonspecific binding. Serum was added at serial fivefold dilutions (starting at 1/5) and incubated overnight at room temperature. Horseradish peroxidase-conjugated goat anti-mouse IgG1 or IgG2a (Southern Biotechnology Associates, Birmingham, AL) was added for 1 h at 37°C to detect the Ab complex immobilized to the wells. The resulting complex was detected by peroxidase substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD). Absorbance was read on an ELISA plate reader (Dynatech) using a 410-nm filter referenced to 570 nm.
Cytolytic T cell 51Cr release assay
Primary lymphocyte effector cells were generated in vivo by immunization with β-gal DNA with or without cytokine DNA. Secondary in vitro effector populations were generated by harvesting spleens 10 to 14 days after the second immunization and culturing single-cell suspension of splenocytes for 5 days in T-75 flasks (Nunc, Roskilde, Denmark) at a density of 3 × 106 cells/ml with 1 μg/ml of H-2 Ld-restricted synthetic peptide (33). A β-gal-expressing tumor cell line (CT26.CL25) or peptide-pulsed CT26 wild-type cells were used as targets. Target cells were mixed with effector cells for 6 h at 37°C at the E:T ratios indicated. A 6-h 51Cr release assay was performed as previously described (33). Unpulsed CT26 wild-type target cells (CT26.WT) were used as negative controls for each experiment. In all experiments, lysis was <10% using unpulsed target wild-type cells.
Flow-cytometry analysis
Mice from each of the vaccination groups were euthanized 2 or 7 days after a DNA boost, and single-cell suspensions of spleens from two mice were pooled. Splenocytes (106) were stained with FITC-conjugated Abs to B7.1 or B7.2 and phycoerythrin-conjugated (I-Ad) anti-class II (PharMingen, San Diego, CA) and analyzed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA). Cells displaying typical lymphocyte and macrophage scatter were gated, and two-color dot plots were generated using CellQuest software.
Infectious challenge with L. major
Female BALB/c mice were injected in their hind footpads with 100 μg of plasmid DNA (IL-12, CD40LT, control PcDNA-3) in 50 μl of sterile PBS. Two days after the above injection, plasmid DNA was combined with 25 μg of protein Ag (SLA) and injected into the hind footpad as above. As a control, mice were injected as above with 25 μg of SLA with or without 2 μg of rIL-12 (Genetics Institute, Cambridge, MA). Mice were boosted 3 wk later with their initial regimen. Two weeks after the last vaccination, mice were challenged by injection in the hind footpad opposite that in which they received vaccination with 1 × 105 metacyclic promastigotes (34). Weekly footpad swelling measurements were recorded using a caliper.
Tumor challenge studies
CT26.WT is a clone of the N-nitroso-N-methylurethane-induced BALB/c (H-2d) undifferentiated colon carcinoma (33). Following transduction with a retrovirus encoding the LacZ gene, CT26.WT was subcloned to generate the β-gal-expressing cell line CT26.CL25. CT26.CL25 was grown in complete media and 400 μg/ml of G418 (Life Technologies, Grand Island, NY). Mice were immunized with β-gal DNA with or without CD40LT DNA, as described above, and challenged i.v. with 1.75 × 106 CT26.CL25 tumor cells. Twelve days later, lungs were harvested and pulmonary metastases were enumerated in a blind manner.
Statistics
Statistical evaluation of differences between means of experimental groups was done by analyses of variance and multiple Student's t tests. A value of p < 0.05 was considered to be significant.
Results
CD40LT DNA and IL-12 DNA can influence the production of Ag-specific IFN-γ
CD40L/CD40 interactions have been shown to be important regulators in the induction of type 1 cytokine (IL-12, IFN-γ) responses (21-25, 35). Thus, it was of interest to evaluate whether CD40LT DNA, when administered together with DNA for a specific Ag, could affect the qualitative and/or quantitative immune response in vivo. In addition, the ability of CD40LT DNA to effect production of Th1 cytokines was directly compared with IL-12 DNA.
In the initial experiment, mice were injected s.c. with β-gal DNA with or without IL-12 DNA or CD40LT DNA and boosted 2 to 3 wk later with the same regimen. Production of IFN-γ and IL-4 was assessed 2 wk after the last vaccination by stimulating total splenocytes in vitro with β-gal or a control protein. As shown in Figure 1A, mice injected with β-gal DNA plus IL-12 DNA or CD40LT DNA had increased production of Ag-specific IFN-γ compared with mice injected with β-gal DNA alone. In contrast, mice vaccinated with β-gal DNA and IL-12 DNA or CD40LT DNA had a minimal increase in Ag-specific IL-4 production over mice vaccinated with β-gal DNA alone (Fig. 1B). These data suggest that IL-12 and CD40LT DNA specifically enhance Th1 cytokine production.
FIGURE 1.
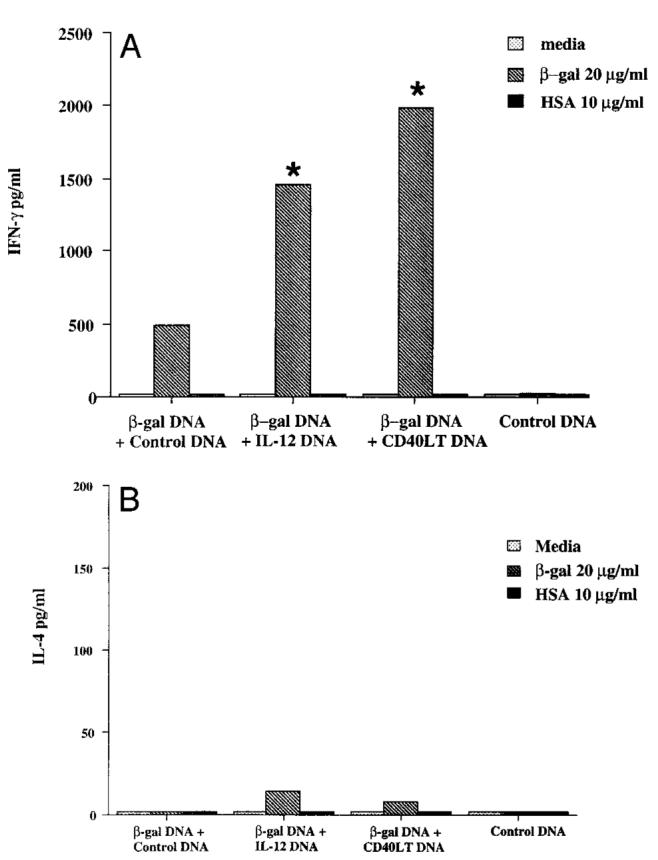
In vitro production of IL-4 and IFN-γ from spleen cells of mice vaccinated with β-gal DNA plus IL-12 DNA or CD40LT DNA. Mice (4–6 per group) were vaccinated s.c. with β-gal DNA (100 μg) in the presence or absence of 100 μg of control DNA, IL-12 DNA, or CD40LT DNA. Two to three weeks later, mice were boosted with the same regimen. Spleen cells were harvested 2 wk after the boost. Single-cell suspensions were plated in triplicate in 96-well microtiter plates at 3 × 105 cells/200 μl in media alone, β-gal protein (20 μg/ml), or human serum albumin protein (10 μg/ml). Forty-eight hours later, supernatants were harvested, and IFN-γ (A) and IL-4 (B) content were assayed by ELISA. Production of IFN-γ in media alone was <30 pg/ml. Production of IL-4 in media alone was usually 10 pg/ml or less. Data as shown represent the amount of IL-4 and IFN-γ averaged from four pooled mice. Results are representative of five independent experiments. SEM was <10% in the experiment shown. *, p < 0.005 in comparing IFN-γ produced from mice vaccinated with β-gal DNA alone.
CD40LT DNA and cytokine DNA influence the production of Ig subtypes in vivo
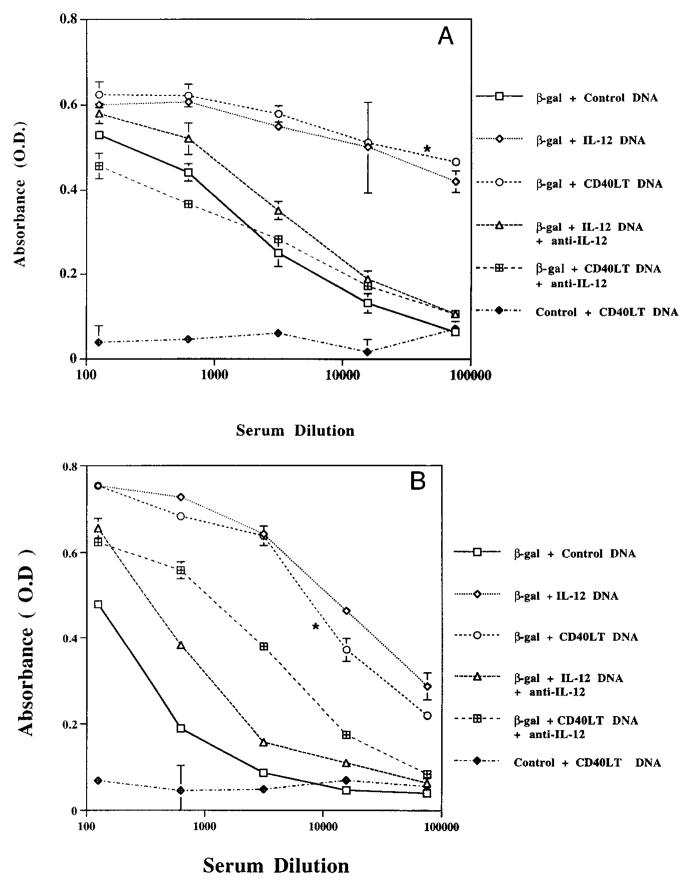
As cytokines such as IL-4 and IFN-γ influence Ab class switching, β-gal-specific production of Ab subtypes was assessed to provide an indirect but physiologic correlate of the pattern of cytokine production in vivo. As shown in Figure 2A, mice vaccinated with β-gal DNA plus CD40LT DNA or IL-12 DNA had a substantially higher (2–3 log) increase in the level of IgG2a compared with mice vaccinated with β-gal DNA alone. Furthermore, treatment of mice injected with β-gal plus CD40LT DNA or IL-12 DNA with anti-IL-12 mAb abrogated much of the increase in IgG2a, providing evidence that induction of IgG2a in vivo by these DNAs is due to induction of IL-12.
FIGURE 2.
β-gal-specific production of IgG1 and IgG2a in vaccinated mice infected with β-gal DNA plus IL-12 DNA or CD40LT DNA. Mice were vaccinated in a similar manner to that described in Figure 1. In some groups, mice were treated with anti-IL-12 (1 mg) at the time of each vaccination. Pooled sera (n = 4–6 mice per group) were collected 2 to 3 wk after the second immunization and tested for β-gal-specific Ab IgG2a (A) or IgG1 (B). Data shown are an average of duplicate absorbance (OD) values using serial fivefold dilutions with SEM. Results are representative of five independent experiments. *, p < 0.05 in comparing IgG1/IgG2a produced from mice vaccinated with β-gal plus CD40LT DNA with that from mice vaccinated with β-gal DNA alone.
With regard to IgG1 Ab production, mice vaccinated with β-gal DNA plus IL-12 or CD40LT DNA all had substantially greater titers (2–3 log) of IgG1 compared with those vaccinated with β-gal alone (Fig. 2B). Of interest, anti-IL-12 treatment reduced much of the increase in IgG1 induced by IL-12 DNA and, to a lesser extent, CD40LT DNA.
CD40LT DNA and IL-12 DNA increase Ag-specific cytotoxic T cell responses
To study the effects that CD40LT DNA and IL-12 DNA had on Ag-specific CTL responses, mice were immunized with β-gal DNA with or without IL-12 DNA or CD40LT DNA and boosted 2 to 3 wk later with the same regimen. Two weeks after the boost, CTL responses were assessed. As shown in Figure 3, cells from mice injected with β-gal DNA plus CD40LT or IL-12 DNA had enhanced CTL responses compared with those from mice injected with β-gal DNA alone. Taken together with the previous figures, these data show that CD40LT DNA enhances both humoral and cellular immune responses. The mechanism by which CD40LT DNA mediates these responses is discussed below.
FIGURE 3.
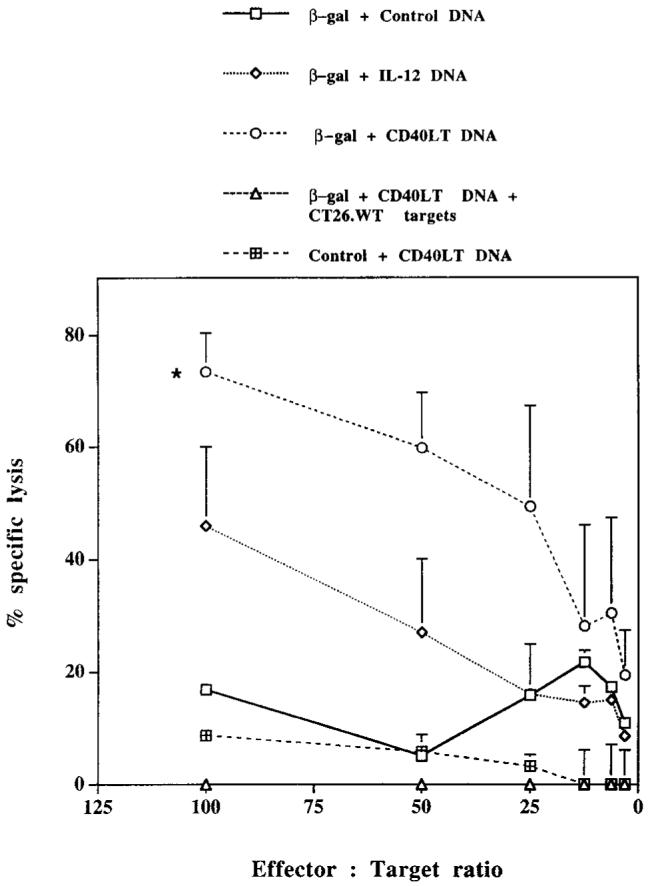
IL-12 DNA and CD40LT DNA enhance β-gal-specific CTL responses. Mice were vaccinated in a similar manner to that described in Figure 1. Two weeks after the second immunization, total splenocytes were cultured for 5 days at a density of 3 × 106 cells/ml with 1 μg/ml of H-2 Ld-restricted synthetic peptide. Target cells (a β-gal-expressing tumor cell line (CT26.CL25) or peptide-pulsed CT26 wild-type cells) were mixed with effector cells for 6 h at 37°C at E:T ratios, as indicated. A 6-h 51Cr release assay was performed; results are presented as specific lysis. Data shown represent values averaged from four pooled mice with SEM for each E:T ratio. Results are representative of three independent experiments. In all experiments, lysis was <10% using wild-type unpulsed (CT26.WT) targets. Statistical analysis was performed for E:T ratio of 100:1, 50:1, and 25:1. *, p < 0.05 in comparing CTL from mice vaccinated with β-gal plus CD40LT DNA with that from mice vaccinated with β-gal DNA alone.
CD40LT DNA enhances production of IFN-γ in an IL-12- and B7-dependent manner
The ability of CD40L/CD40 stimulation to regulate type 1 (IFN-γ, IL-12) cytokine production has been shown to occur through at least two mechanisms (35), the first through the induction of IL-12 from APCs (22-25) and the second via the ability of CD40L/CD40 stimulation to enhance expression of costimulatory cell surface molecules (e.g., B7-1, B7-2), causing increased T cell stimulation and production of IFN-γ (21, 35). To evaluate both of these mechanisms, mice were injected with β-gal DNA plus CD40LT DNA and treated with either anti-IL-12 or CTLA4Ig at the time of each vaccination. Similar to the results seen above, mice injected with β-gal plus CD40LT DNA plus L6Ig (control for CTLA4Ig) had an increase in IFN-γ compared with those injected with β-gal alone (Fig. 4). By contrast, mice injected with β-gal DNA plus CD40LT DNA and treated with anti-IL-12 had >90% inhibition of IFN-γ production. These results are consistent with the central role for CD40L/CD40 stimulation in regulating IL-12 production in response to a protein Ag in vivo (20).
FIGURE 4.

IFN-γ enhancement by CD40LT DNA is dependent on IL-12 and B7 costimulation. Mice were vaccinated in a manner similar to that described in Figure 1. In some groups, mice were treated with anti-IL-12 (1 mg), CTLA4Ig (100 μg), or L6Ig (100 μg) i.p. at the time of each vaccination. IFN-γ was assessed from spleen cells 2 wk after the second vaccination, as described in Figure 1. Data shown represent values averaged from four pooled mice. SEM was <10% in the experiment shown. *, p < 0.005 in comparing IFN-γ produced from mice vaccinated with β-gal DNA with or without CD40LT DNA and treated with anti-IL-12 or CTLA4Ig.
The role of B7/CD28 costimulation in regulating IFN-γ production following vaccination with β-gal with or without CD40LT DNA was evaluated by treating mice with CTLA4Ig or a control Ab (L6Ig) at the time of vaccination. All mice treated with CTLA4Ig had complete inhibition of IFN-γ production. In the same experiment, CTLA4Ig also inhibited β-gal-specific in vitro proliferation (data not shown). These data underscore the importance of B7/CD28 costimulation in regulating primary T cell responses and show that the enhancement of IFN-γ by CD40LT DNA is also dependent on B7 costimulation (see below).
CD40LT DNA enhances expression of costimulatory molecules B7-1 and B7-2
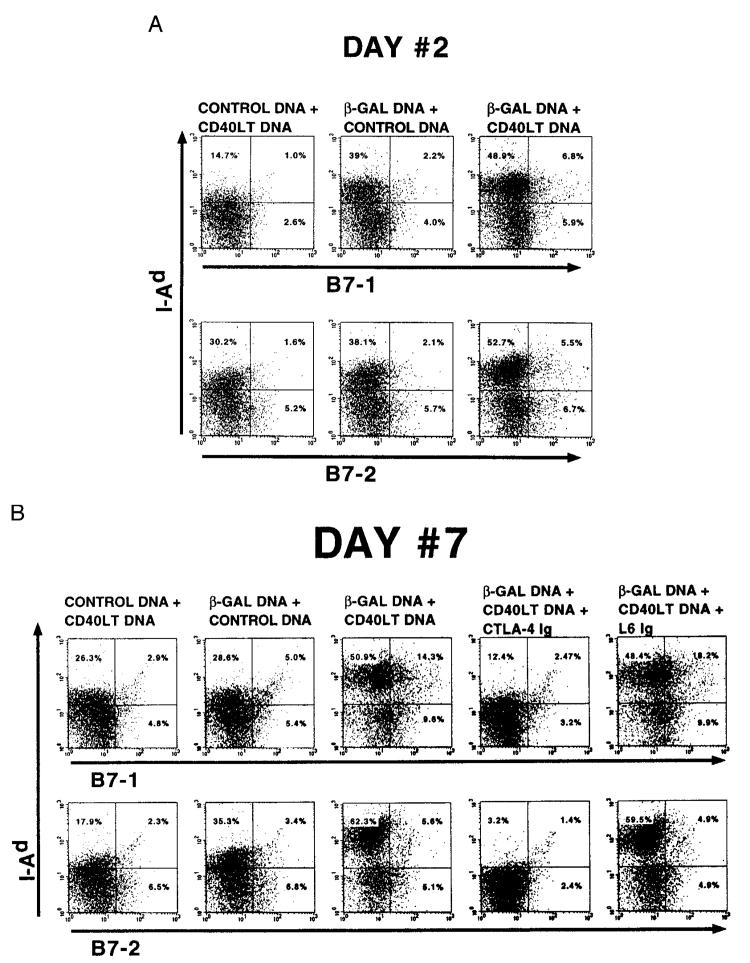
To further evaluate the mechanism by which CD40LT DNA enhances immune responses in vivo, splenocytes from mice vaccinated with β-gal DNA with or without CD40LT DNA were analyzed by FACS for the expression of B7-1 and B7-2. When examined 2 days after the second vaccination, cells from mice vaccinated with β-gal DNA plus CD40LT DNA had a two- to threefold increase in the number of B7-1 and B7-2 MHC class II-expressing cells compared with cells from mice vaccinated with β-gal DNA plus control DNA (Fig. 5A). There was also a threefold increase in expression of B7-1 from splenocytes of mice vaccinated with β-gal plus CD40LT DNA when assessed 7 days after the boost; however, there was a more modest increase in expression of B7-2 at this time (Fig. 5B). It should be noted that while the fold increase in B7-1 was the same 2 and 7 days after the second vaccination from mice injected with CD40LT DNA, the number of cells expressing B7-1 was greater 7 days after the second vacci-nation. Finally, the ability of CD40LT DNA to enhance expression of either B7-1 or B7-2 was completely abrogated by treatment of mice with CTLA4Ig, but not with control L6Ig.
FIGURE 5.
CD40LT DNA enhances expression of B7 costimulatory molecules. In the same experiment outlined in Figure 4, spleen cells (2–3 mice) were harvested 2 or 7 days after the second vaccination and stained with FITC-conjugated Abs to B7.1 or B7.2 and phycoerythrin-conjugated (I-Ad) anti-class II and analyzed. Similar results were seen in two additional experiments.
CD40LT DNA-induced CTL responses are CD8 and B7 dependent
The previous figures support the notion that CD40LT DNA enhances CTL responses through a B7-dependent mechanism. Therefore, CTL responses were assessed from mice vaccinated with β-gal DNA plus CD40LT DNA and treated with CTLA4Ig or control L6Ig. As shown in Figure 6, mice vaccinated with β-gal plus CD40LT DNA and treated with L6Ig had strong CTL responses, while mice treated with CTLA4Ig had markedly diminished responses. Addition of anti-CD8 Ab at the time of the CTL assay substantially diminished CTL activity (p < 0.05), providing evidence that the CTL activity was CD8 dependent. In contrast, the addition of anti-CD4 mAb to cultures did not significantly diminish CTL activity (p > 0.05). Moreover, using target cells pulsed with an MHC class I-restricted peptide (β-gal plus CD40LT plus L6Ig plus peptide) provides additional evidence that the CTL activity is class I restricted.
FIGURE 6.
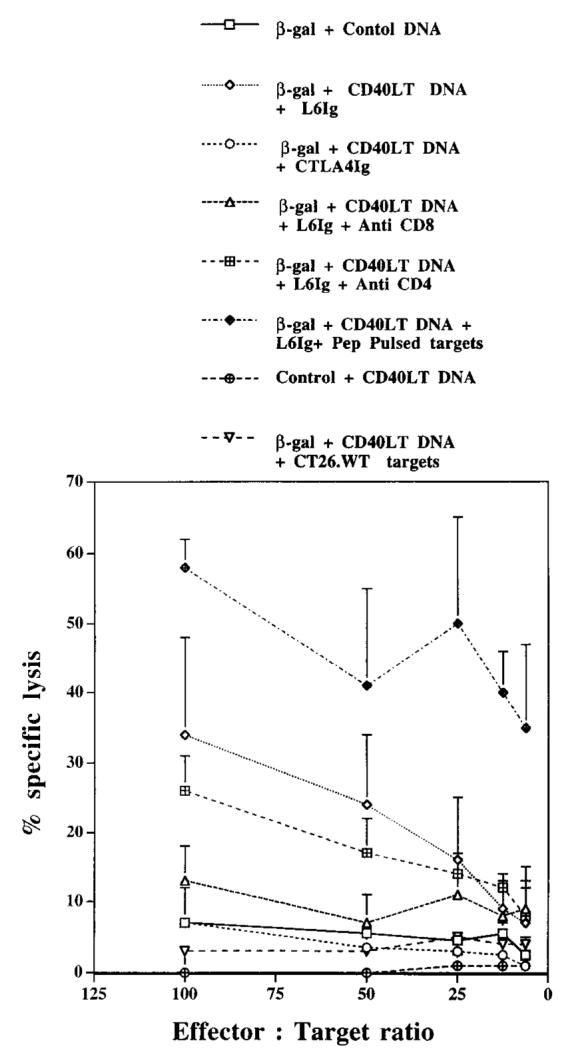
CD40LT DNA enhancement of CTL activity is dependent both on B7 costimulation and on CD8. Mice were vaccinated in a manner similar to that described in Figure 4. CTL activity from splenocytes was assessed as described in Figure 3. In addition, anti-CD8 or anti-CD4 Ab was added at the time of the 51 Cr release assay. Data shown represent values averaged from four pooled mice with SEM for each E:T ratio. Statistical analysis was performed for E:T ratios of 100:1, 50:1, and 25:1. *, p < 0.05 in comparing CTL from mice vaccinated with β-gal DNA plus CD40LT DNA plus L6Ig with that from mice vaccinated with β-gal DNA alone. **, p < 0.05 in comparing CTL from mice vaccinated with β-gal DNA plus CD40LT DNA plus L6Ig plus anti-CD8 with that from mice vaccinated with β-gal DNA plus CD40LT DNA plus L6Ig.
Functional evidence for a role of CD40LT DNA in regulating immune responses in vivo through CTL or IL-12 production
Because CD40LT DNA induces strong CTL responses following in vitro stimulation, it was of interest to see whether these responses were functional in vivo. To address this, mice were vaccinated in a similar manner as described above and then challenged i.v. with a β-gal-expressing undifferentiated adenocarcinoma 2 to 3 wk after the boost. As shown in Table I, mice vaccinated with β-gal plus CD40LT DNA had little to no detectable pulmonary metastases when assessed 10 days after challenge, while three of five mice injected with β-gal alone had detectable pulmonary metastases.
Table I.
Prevention of the growth of CT26.CL25 (β-gal+) tumors by DNA immunizationa
| Treatment (DNA) | Route | Mean Number of Pulmonary Metastases |
|---|---|---|
| Vector (PcDNA-3) | SC | 209 (6,250,250,250,250,250) |
| CD40LT | SC | 249 (245,250,250,250,250) |
| β-Gal/vector | SC | 68 (0,4,42,42,250) |
| β-Gal/CD40LT | SC | 2 (0,0,1,5,6) |
Mice were immunized two times at 3-wk intervals with purified plasmid DNA as described in Figure 1. Three weeks following the boost, mice were challenged i.v. with 1.75 × 106 CT26.CL25 tumor cells. Twelve days later, lungs were harvested and pulmonary metastases enumerated in a blind manner. p < 0.05 in comparing protection of vaccinated mice with β-gal/CD40LT with vector or with CD40LT alone.
In Figure 4, it was demonstrated that CD40LT DNA induced an IL-12-dependent enhancement in production of IFN-γ. To assess the ability of CD40LT DNA to induce a protective immune response in a model in which IL-12-dependent production of IFN-γ is required (36), the murine model of L. major infection was used. Previous work has shown that susceptible BALB/c mice vaccinated with either a SLA or a cloned Leishmania protein with rIL-12 protein can induce a protective immune response following infection (37, 38). In the experiment shown in Figure 7, the ability of either CD40LT DNA or IL-12 DNA to induce a protective immune response when given in conjunction with SLA was compared with that of SLA plus rIL-12 protein. Mice vaccinated either with SLA plus CD40LT DNA or with IL-12 DNA effectively controlled infection with L. major, as assessed by footpad swelling. This protection was comparable with that achieved by SLA and rIL-12 protein. In addition, the control of disease progression and reduction in parasite burden in mice vaccinated with CD40LT DNA or IL-12 DNA were associated with an increase in Ag-specific production of IFN-γ (data not shown). Thus, these data are consistent with the premise that CD40LT DNA mediates a protective immune response in vivo by enhancing IFN-γ production in an IL-12-dependent manner.
FIGURE 7.
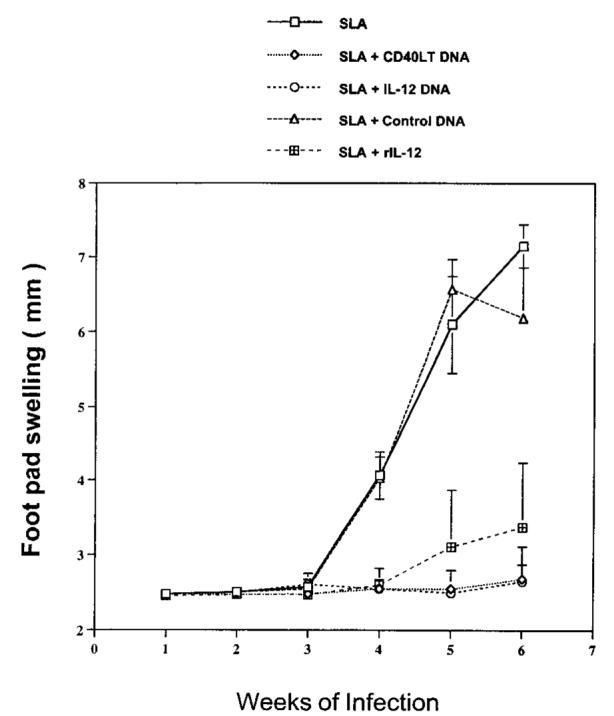
Cytokine or CD40LT DNA induces protective immunity to mice infected with L. major. BALB/c mice (n = 7 per group) were initially immunized and boosted 2 wk later with SLA (leishmanial protein) plus control DNA, IL-12 DNA, CD40LT DNA, or rIL-12 (2 μg). Two weeks after the boost, mice were challenged in the hind footpad with 1 × 105 live L. major metacyclic promastigotes. Weekly footpad measurements represent the average footpad scores ± SEM. Results are representative of two independent experiments.
Discussion
These studies examined the ability of CD40LT DNA to influence the type and magnitude of immune responses in vivo. In particular, because CD40L/CD40 costimulation is a central regulator of both humoral and cellular immune responses, this pathway is a potentially useful vaccine adjuvant for a broad-based immune response. In this regard, a recent report showed that CD40L DNA could enhance Ag-specific Ab and cellular immune responses in vivo (39). Using a plasmid containing CD40LT DNA, we focused on the mechanism by which this DNA could act as an adjuvant in enhancing immunity in vivo. It is possible that CD40LT DNA may have more potent effects than CD40L DNA, based on several observations. First, a 35-kDa protein was immunoprecipitated in both lysates and supernatants of COS cells transfected with CD40LT DNA (data not shown), suggesting that CD40LT DNA may act at the surface of a transfected cell as well as at a distant site in vivo. In addition, CD40LT DNA contains a leucine zipper motif fused to the N terminus of the extracellular domain of CD40L (17) that facilitates the generation of trimeric CD40L, which may have a greater capacity to trigger biologic responses than monomeric or dimeric protein (17, 18). Experiments are currently underway to evaluate whether CD40LT and CD40L DNA induce different biologic responses in vivo; nevertheless, the results reported in this study show that 1) CD40LT DNA augments total Ab production, including IgG1 and IgG2a subtypes; 2) CD40LT DNA enhances IL-12-dependent IFN-γ production; 3) CD40LT DNA enhances expression of B7 costimulatory molecules and increases CTL activity in a B7-dependent manner; and 4) CD40LT DNA induces protective immunity against tumor challenge or L. major infection, providing functional evidence that enhancement of CTL activity and IFN-γ production in vivo, respectively, was physiologically relevant.
The role of CD40LT and IL-12 DNA in regulating specific Ab isotypes in vivo
Mice vaccinated with β-gal and CD40LT DNA had significant increases in total Ig production compared with that of mice vaccinated with β-gal DNA only (data not shown). In evaluating specific Ab isotypes, both CD40LT DNA and IL-12 DNA caused a 2-to 3-log increase in IgG2a that was abrogated by treatment of mice with anti-IL-12. These data are consistent with previous studies showing that IL-12-dependent production of IFN-γ is a major regulator of IgG2a production (40-45). In contrast to the relatively straightforward role of IL-12 and IFN-γ in regulating IgG2a iso-type production, the ability of cytokines to regulate IgG1 is less clear. Initial reports showed that IL-4, but not IFN-γ, was a potent inducer of IgG1 (45, 46). In several of the studies alluded to above, mice injected with IL-12 protein and various Ags had suppressed IgG1 production, consistent with the cross-regulatory role of IFN-γ and IL-4 in regulating IgG1 Ab production (40-44); however, in more recent studies, immunization with IL-12 greatly enhanced IgG1 production (47, 48). These data, together with the observation that IgG1 Ab production can occur in the absence of IL-4 (49), suggest that IgG1 production may be a less specific marker for IL-4 in vivo and that IL-12 can enhance its production depending on the type of adjuvant used (48) and the time at which Ab is measured following immunization (42). In the studies reported in this work, IL-12 and CD40LT DNA substantially increased IgG1 compared with β-gal DNA alone. Of interest, anti-IL-12 treatment completely abrogated the ability of IL-12 DNA to increase IgG1 production, consistent with IL-12 having a role in augmenting IgG1 production. By contrast, anti-IL-12 treatment only partially abrogated the CD40LT DNA-induced increase in IgG1. This latter observation could be due to a direct effect of CD40L on B cells causing an increase in all isotypes or to the fact that anti-IL-12 treatment of CD40LT DNA-vaccinated mice caused an increase in IL-4 production (data not shown). Overall, these data underscore the potent effects that CD40L stimulation can have in eliciting potent Ab responses.
CD40LT DNA enhances CTL activity in a B7/CD28-dependent manner
In this study, CD40LT DNA and IL-12 DNA increased Ag-specific CTL activity, consistent with previous reports (50, 51). The ability of CD40LT DNA to enhance CTL was inhibited by treatment of mice with CTLA4Ig, suggesting that B7 costimulation was critical for CTL induction. With regard to the effects of B7 stimulation on CTL induction, recent studies have shown that there may be differences in the ability of B7-1 and B7-2 DNA to enhance CTL responses (52-54). In this study, because there were increases in both B7-1 and B7-2 and the timing of peak expression varied with time, it is hard to distinguish which is more important in mediating CTL responses.
Functional role of CD40L DNA in vivo as a vaccine adjuvant
To assess whether the immune enhancement achieved by CD40LT DNA was able to mediate protective immunity in vivo, we chose two experimental models in which the mechanism of protection is well defined. Previous work has shown that, following challenge with a β-gal-expressing adenocarcinoma, CD8-dependent CTL activity is required to prevent metastatic lung disease (K.R.I., unpublished observation). In these studies, mice vaccinated with β-gal DNA plus CD40LT DNA were protected from metastatic disease. CTL activity of mice vaccinated with CD40LT DNA was inhibited by treatment with CTLA4Ig in vivo or with anti-CD8 in vitro, suggesting that the mechanism of tumor eradication is via a CD8-dependent pathway. Finally, mice vaccinated with CD40LT DNA and treated with anti-IL-12 in vivo did not have diminished CTL responses (data not shown), providing additional evidence that the CD40LT DNA enhancement of CTL activity was via the increase in B7 expression and not through an IL-12-dependent mechanism. In regard to this latter point, recent work using IL-12 DNA as an adjuvant showed that an in vitro CTL response directed against an HIV envelope was inhibited by anti-IL-12 or anti-IFN-γ treatment in vivo (51). This may reflect a need for IL-12 DNA-dependent production of IFN-γ, which would induce optimal expression of costimulatory molecules (i.e., B7) required for CTL activity.
To evaluate whether CD40LT DNA-induced production of IL-12 could protect animals from infection, we used the experimental model of L. major infection. Protective immunity achieved through vaccination for this infection requires IL-12-dependent production of IFN-γ (36). Vaccination of mice with SLA plus CD40LT DNA induced protection similar to that achieved by SLA plus IL-12 DNA or IL-12 protein; it is therefore likely that CD40LT DNA mediates its effect through the induction of Ag-specific IFN-γ (manuscript in preparation).
Overall, the ability of CD40LT DNA to enhance a broad array of immune responses makes it a potent adjuvant for diseases requiring humoral and/or cellular immunity. Thus, for intracellular infections in which cellular immune responses are desirable (L. major, M. tuberculosis), CD40LT or IL-12 DNA could induce effective Th1 responses as well as CTL responses. Another potential infection in which CD40LT DNA would be a useful adjuvant is HIV. In this case, because it is not precisely known which immunologic correlates are required for protective immunity, it is likely that induction of both Ab and cellular responses would be desirable. One important caveat to using CD40LT DNA as an adjuvant is the potential for unregulated CD40L/CD40 stimulation. This could result in an excessive proinflammatory response, which may trigger or enhance an autoimmune process. In this regard, using a soluble CD40L agonist protein may provide a useful adjuvant without incurring any long-term effects.
Acknowledgments
We thank Melanie K. Spriggs for helpful advice and Brenda Rae Marshall for editorial assistance.
Footnotes
Abbreviations used in this paper: ISS, immunostimulatory sequence; β-gal, β-galactosidase; CD40L, CD40 ligand; CD40LT, CD40 ligand/trimer; SLA, soluble Leishmania Ag.
References
- 1.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 2.Reiner SL, Locksley RM. The regulation of immunity to Leish-mania major. Annu. Rev. Immunol. 1995;13:151. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 3.Orme IM. Prospects for new vaccines against tuberculosis. Trends Microbiol. 1995;3:401. doi: 10.1016/s0966-842x(00)88987-8. [DOI] [PubMed] [Google Scholar]
- 4.Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annu. Rev. Immunol. 1997;15:617. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 5.Allsopp CE, Plebanski M, Gilbert S, Sinden RE, Harris S, Frankel G, Dougan G, Hioe C, Nixon D, Paoletti E, Layton G, Hill AV. Comparison of numerous delivery systems for the induction of cytotoxic T lymphocytes by immunization. Eur. J. Immunol. 1996;26:1951. doi: 10.1002/eji.1830260841. [DOI] [PubMed] [Google Scholar]
- 6.Corr M, Lee DJ, Carson DA, Tighe H. Gene vaccination with naked plasmid DNA: mechanism of CTL priming. J. Exp. Med. 1996;184:1555. doi: 10.1084/jem.184.4.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doe B, Selby M, Barnett S, Baenzinger J, Walker CM. Induction of cytotoxic T lymphocytes by intramuscular immunization with plasmid DNA is facilitated by bone marrow-derived cells. Proc. Natl. Acad. Sci. USA. 1996;93:8578. doi: 10.1073/pnas.93.16.8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raz E, Tighe H, Sato Y, Corr M, Dudler JA, Roman M, Swain SL, Spiegelberg HL, Carson DA. Preferential induction of a Th1 immune response and inhibition of specific IgE antibody formation by plasmid DNA immunization. Proc. Natl. Acad. Sci. USA. 1996;93:5141. doi: 10.1073/pnas.93.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto S, Yamamoto T, Kataoka T, Kuramoto E, Yano O, Tokunaga T. Unique palindromic sequences in synthetic oligonucleotides are required to induce INF and augment INF-mediated natural killer activity. J. Immunol. 1992;148:4072. [PubMed] [Google Scholar]
- 10.Sato Y, Roman M, Tighe H, Lee D, Corr M, Nguyen MD, Silverman GJ, Lotz M, Carson DA, Raz E. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science. 1996;273:352. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 11.Klinman DM, Yi A-K, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacterial DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon γ. Proc. Natl. Acad. Sci. USA. 1996;93:2879. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiner GJ, Liu H-M, Wooldridge JE, Dahle CE, Krieg AM. Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunization. Proc. Natl. Acad. Sci. USA. 1997;94:10833. doi: 10.1073/pnas.94.20.10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segal BM, Klinman DM, Shevach EM. Microbial products induce autoimmune disease by an IL-12 dependent pathway. J. Immunol. 1997;158:5087. [PubMed] [Google Scholar]
- 14.Roman M, Martin-Orozco E, Goodman JS, Nguyan M-D, Sato Y, Ronaghy A, Kornbluth RS, Richman DD, Carson DA, Raz E. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat. Med. 1997;8:849. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 15.Noelle RJ. CD40 and its ligand in host defense. Immunity. 1996;4:415. doi: 10.1016/s1074-7613(00)80408-2. [DOI] [PubMed] [Google Scholar]
- 16.Grewal IS, Flavell RA. The role of CD40 ligand in costimulation and T-cell activation. Immunol. Rev. 1996;153:85. doi: 10.1111/j.1600-065x.1996.tb00921.x. [DOI] [PubMed] [Google Scholar]
- 17.Armitage RJ, Maliszewski CR, Alderson MR, Grabstein KH, Spriggs MK, Fanslow WC. CD40L: a multi-functional ligand. Semin. Immunol. 1993;5:401. doi: 10.1006/smim.1993.1046. [DOI] [PubMed] [Google Scholar]
- 18.Fanslow WC, Srinivasan S, Paxton R, Gibson MG, Spriggs MK, Armitage RJ. Structural characteristics of CD40 ligand that determine biological function. Semin. Immunol. 1994;6:267. doi: 10.1006/smim.1994.1035. [DOI] [PubMed] [Google Scholar]
- 19.Hodgkin PD, Yamashita LC, Seymour B, Coffman RL, Kehry MR. Membranes from both Th1 and Th2 T cell clones stimulate B cell proliferation and prepare B cells for lymphokine-induced differentiation to secrete Ig. J. Immunol. 1991;147:3696. [PubMed] [Google Scholar]
- 20.Grewal IS, Xu J, Flavell RA. Impairment of antigen-specific T cell priming in mice lacking CD40 ligand. Nature. 1995;348:617. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]
- 21.Grewal IS, Foellmer HG, Grewal KD, Xu J, Hardardottir F, Baron JL, Janeway CA, Jr., Falvell RA. Requirement for CD40 ligand in costimulation induction, T cell activation, and experimental allergic encephalomyelitis. Science. 1996;273:1864. doi: 10.1126/science.273.5283.1864. [DOI] [PubMed] [Google Scholar]
- 22.DeKruyff RH, Gieni RS, Umetsu DT. Antigen-driven but not lipopolysaccharide-driven IL-12 production in macrophages requires triggering of CD40. J. Immunol. 1997;158:359. [PubMed] [Google Scholar]
- 23.Koch F, Stanzl U, Jennewein P, Janke K, Heufler C, Kämpgen E, Romani N, Schuler G. High level IL-12 production by murine dendritic cells: up-regulation via MHC class II and CD40 molecules and down-regulation by IL-4 and IL-10. J. Exp. Med. 1996;184:741. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 1996;184:747. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kennedy MK, Picha KS, Fanslow WC, Granstain KH, Alderson MR, Clifford KN, Chin WA, Mohler KM. CD40/CD40 ligand interactions are required for T cell-dependent production of IL-12 by mouse macrophages. Eur. J. Immunol. 1996;26:370. doi: 10.1002/eji.1830260216. [DOI] [PubMed] [Google Scholar]
- 26.Borrow P, Tishon A, Lee S, Xu J, Grewal IS, Oldstone MBA, Flavell RA. CD40L-deficient mice show deficits in antiviral immunity and have an impaired memory CD8+ CTL response. J. Exp. Med. 1996;183:2129. doi: 10.1084/jem.183.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranheim EA, Kipps TJ. Activated T cells induce expression of B7/BB1 on normal or leukemic B cells through a CD40-dependent signal. J. Exp. Med. 1993;177:925. doi: 10.1084/jem.177.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meenakshi R, Aruffo A, Ledbetter J, Linsley P, Kehry M, Noelle R. Studies on the interdependence of gp39 and B7 expression and function during antigen-specific immune responses. Eur. J. Immunol. 1995;25:596. doi: 10.1002/eji.1830250243. [DOI] [PubMed] [Google Scholar]
- 29.Shinde S, Wu Y, Guo Y, Niu Q, Xu J, Grewal IS, Flavell R, Liu Y. CD40L is important for induction of, but not response to, costimulatory activity: ICAM-1 as the second costimulatory molecule rapidly up-regulated by CD40L. J. Immunol. 1996;157:2764. [PubMed] [Google Scholar]
- 30.Yang Y, Wilson JM. CD40 ligand-dependent T cell activation: requirement of B7-CD28 signaling through CD40. Science. 1996;273:1862. doi: 10.1126/science.273.5283.1862. [DOI] [PubMed] [Google Scholar]
- 31.Guo Y, Wu Y, Shinde S, Sy M-S, Aruffo A, Liu Y. Identification of a costimulatory molecule rapidly induced by CD40L as CD44H. J. Exp. Med. 1996;184:955. doi: 10.1084/jem.184.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cherwinski H, Schumacher J, Brown K, Mosmann T. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassay, and monoclonal antibody. J. Exp. Med. 1987;166:1229. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irvine KR, Rao JB, Rosenberg SA, Restifo NP. Cytokine enhancement of DNA immunization leads to effective treatment of established pulmonary metastases. J. Immunol. 1996;156:238. [PMC free article] [PubMed] [Google Scholar]
- 34.Gurunathan S, Sacks DL, Brown DR, Reiner SL, Charest H, Glaichenhaus N, Seder RA. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J. Exp. Med. 1997;186:1137. doi: 10.1084/jem.186.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDyer JF, Goletz TJ, Thomas E, June CH, Seder RA. CD40 ligand/CD40 stimulation regulates the production of IFN-γ from human PBMCs in an IL-12- and/or CD28-dependent manner. J. Immunol. 1998;160:1701. [PubMed] [Google Scholar]
- 36.Sypek JP, Chung CL, Mayor SE, Subramanyam JM, Goldman SJ, Sieburth DS, Wolf SF, Schaub RG. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J. Exp. Med. 1993;177:1797. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Afonso LC, Scharton TM, Vieira LQ, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leish-mania major. Science. 1994;263:235. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 38.Mougneau E, Altare F, Wakil AE, Zheng S, Coppola T, Wang ZE, Waldmann R, Locksley RM, Glaichenhaus N. Expression cloning of a protective Leishmania antigen. Science. 1995;268:563. doi: 10.1126/science.7725103. [DOI] [PubMed] [Google Scholar]
- 39.Mendoza RB, Cantwell MJ, Kipps TJ. Immunostimulatory effects of a plasmid expressing CD40 ligand (CD154) on gene immunization. J. Immunol. 1997;159:5777. [PubMed] [Google Scholar]
- 40.Morris SC, Madden KB, Adamovicz JL, Gause WC, Hubbard BR, Gately MK, Finkelman FD. Effects of IL-12 on in vivo cytokine gene expression and Ig isotype selection. J. Immunol. 1994;152:1047. [PubMed] [Google Scholar]
- 41.McKnight AJ, Zimmer GJ, Fogelman I, Wolf SF, Abbas AK. Effects of IL-12 on helper T cell-dependent immune responses in vivo. J. Immunol. 1994;152:2172. [PubMed] [Google Scholar]
- 42.Buchanan JM, Vogel LA, van Cleave VH, Metzger DW. Interleukin 12 alters the isotype-restricted antibody response of mice to hen egg-white lysozyme. Int. Immunol. 1995;7:1519. doi: 10.1093/intimm/7.9.1519. [DOI] [PubMed] [Google Scholar]
- 43.Germann T, Bongartz M, Dlugonska H, Hess H, Schmitt E, Kolbe L, Kölsch E, Podlalski FJ, Gately MK, Rüde E. Interleukin-12 profoundly up-regulates the synthesis of antigen-specific complement-fixing IgG2a, IgG2b and IgG3 antibody subclasses in vivo. Eur. J. Immunol. 1995;25:823. doi: 10.1002/eji.1830250329. [DOI] [PubMed] [Google Scholar]
- 44.Gracie JA, Bradley JA. Interleukin-12 induces interferon-γ-dependent switching of IgG alloantibody subclass. Eur. J. Immunol. 1996;26:1217. doi: 10.1002/eji.1830260605. [DOI] [PubMed] [Google Scholar]
- 45.Finkelman FD, Snapper CM, Mountz JD, Katona IM. Polyclonal activation of the murine immune system by a goal antibody to mouse IgD. IX. Induction of a polyclonal IgE response. J. Immunol. 1987;138:2826. [PubMed] [Google Scholar]
- 46.Snapper CM, Paul WE. Interferon-γ and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 47.Wynn TA, Reynolds A, James S, Cheever AW, Caspar P, Hieny S, Jankovic D, Strand M, Sher A. IL-12 enhances vaccine-induced immunity to schistosomes by augmenting both humoral and cell-mediated immune responses against the parasite. J. Immunol. 1996;157:4068. [PubMed] [Google Scholar]
- 48.Jankovic D, Caspar P, Zweig M, Garcia-Moll M, Showalter SD, Vogel FR, Sher A. Adsorption to aluminum hydroxide promotes the activity of IL-12 as an adjuvant for antibody as well as type 1 antibody responses to HIV-1 gp120. J. Immunol. 1997;159:2409. [PubMed] [Google Scholar]
- 49.Morawetz RA, Gabriele L, Rizzo LV, Noben-Trauth N, Kühn R, Rajewsky K, Müller W, Doherty TM, Finkelman F, Coffman RL, Morse HC., III Interleukin (IL)-4-independent immunoglobulin class switch to immunoglobulin (Ig) in the mouse. J. Exp. Med. 1996;184:1651. doi: 10.1084/jem.184.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim JJ, Ayyavoo V, Bagarazzi ML, Chattergoon MA, Dang K, Wang B, Boyer JD, Weiner DB. In vivo engineering of a cellular immune response by coadministration of IL-12 expression vector with a DNA immunogen. J. Immunol. 1997;158:816. [PubMed] [Google Scholar]
- 51.Okada E, Sasaki S, Ishii N, Aoki I, Yasuda T, Nishioka K, Fukushima J, Miyazaki J-I, Wahren B, Okuda K. Intranasal immunization of a DNA vaccine with IL-12- and granulocyte-macrophage colony-stimulating factor (GMCSF)-expressing plasmids in liposomes induces strong mucosal and cell-mediated immune responses against HIV-1 antigens. J. Immunol. 1997;159:3638. [PubMed] [Google Scholar]
- 52.Tsuji T, Jamajima K, Ishii N, Oaki I, Fukushima J, Xin K-Q, Kawamoto S, Sasaki S, Matsunaga K-I, Ishigatsubo Y, Tani K, Okubo T, Okuda K. Immunomodulatory effects of a plasmid expressing B7-2 on human immunodeficiency virus-1-specific cell-mediated immunity inducted by a plasmid encoding the viral antigen. Eur. J. Immunol. 1997;27:782. doi: 10.1002/eji.1830270329. [DOI] [PubMed] [Google Scholar]
- 53.Corr M, Tighe H, Lee D, Dudler J, Trieu M, Brinson DC, Carson DA. Costimulation provided by DNA immunization enhances antitumor immunity. J. Immunol. 1997;159:4999. [PubMed] [Google Scholar]
- 54.Iwasaki A, Stiernholm BJN, Chan AK, Berinstein NL, Barber BH. Enhanced CTL responses mediated by plasmid DNA immunogens encoding costimulatory molecules and cytokines. J. Immunol. 1997;158:4591. [PubMed] [Google Scholar]