Abstract
Matrix metalloproteinases are a family of zinc-dependent proteinases which are involved in the breakdown and remodeling of extracellular matrix. As children grow and adolescents reach pubescence, their bodies undergo changes that require age-related morphogenesis of the extracellular matrix, possibly requiring unique patterns of matrix metalloproteinase (MMP) expression during periods of rapid tissue growth (i.e., childhood) or accelerated tissue remodeling and expansion (i.e., adolescence). Therefore, we have characterized age-specific and gender-specific differences in circulating concentrations of MMPs (specifically MMP-1, -2, -3, -8 and -9) in 189 serum samples obtained from healthy subjects, aged 2−18 years. MMP concentrations were measured using Fluorokine® MultiAnalyte Profiling kits and a Luminex® Bioanalyzer, as well as by commercial ELISA. Serum levels of MMP-1, -2, -3, -8, and -9 in healthy pediatric subjects represent log-normal distributions. MMP-2 was significantly negatively correlated with age (r=−0.29; p<0.001), while MMP-3 was significantly positively correlated with age (r=0.38; p<0.001). Although plasma, not serum, is considered the appropriate blood sample for measurement of MMP-8 and -9, serum levels of MMP-8 and -9 were also found to be highly positively correlated with each other (r=0.76; p<0.01). MMP results obtained by Fluorokine® MultiAnalyte Profiling methods correlated well with conventional ELISA methods and use of this technology provided several advantages over ELISA.
Keywords: collagenase, endopeptidases, gelatinase, particle-based flow cytometry, puberty, stromelysin
Introduction
Matrix metalloproteinases (MMPs) are an ever-expanding family of zinc-dependent endopeptidases involved in the breakdown, remodeling and physiological homeostasis of extracellular matrix (ECM) (1, 2). Currently, there are over 20 human MMP-related enzymes, grouped into several subtypes, including collagenases, gelatinases, stromelysins, membrane-type MMPs (MT-MMPs) and other, less well-characterized, distinct enzymes (3). Targets of these powerful degradative enzymes include structural ECM molecules (i.e., interstitial collagens, laminins, fibronectins, and heparan sulfate proteoglycans), cell-cell adhesion molecules, cell surface receptors, growth factors, cytokines and chemoattractant proteins, as well as other proteinases (2). Because of their potent degradative capacity, the activity of these enzymes is tightly regulated at all levels (i.e., transcription, post-transcription, secretion, protein processing and tissue localization). For example, proinflammatory cytokines such as interleukin-1β and tissue necrosis factor-α stimulate MMP production, while other cytokines, hormones, and growth factors such as transforming growth factor-β, interleukin-4, corticoid hormones and insulin-like growth factors (IGFs) down-regulate MMP synthesis (4).
To date, MMPs have been implicated in many pathologic processes characterized by either degradation of connective tissue matrices or pro-inflammatory states, such as rheumatoid arthritis and osteoarthritis, cancer growth and metastases, metabolic bone disease, emphysema and atherosclerotic heart disease (5–7). Serum or plasma levels of MMPs are altered in various diseases, and have been considered as potential clinical markers of disease activity. For example, in adult clinical studies, serum MMP-2 levels have been reported to be increased in patients with liver cirrhosis (8), malignant thyroid cancer (9), and endometriosis (10). Serum or plasma MMP-9 levels appear to be increased in patients with a diversity of cardiovascular related inflammatory conditions, including congestive heart failure (11), stroke (12, 13), obstructive sleep apnea syndrome (14), and a history of myocardial infarction (15), as well as patients with amyotrophic lateral sclerosis (16), and acute allograft rejection following liver transplantation (17). Plasma levels of MMP-3 are increased in several connective tissue disorders, including rheumatoid arthritis (18) and systemic lupus erythematosus (18).
The first description of MMP-like activity was related to the metamorphosis of the tadpole (19). This and many other subsequent discoveries strongly suggest that this well-characterized family of metalloproteinases is also intimately involved in normal tissue growth and ECM remodeling (20). Morphogenesis and tissue growth, remodeling, and repair are sentinel features of childhood and adolescence. However, little is known about the production, secretion, and clearance of these important proteinases throughout normal growth and development in humans. At present, normal values for serum concentrations of commercially assayable MMPs are unavailable for the pediatric age range, limiting our ability to quantitatively compare pediatric disease-specific MMP abnormalities with MMP concentrations in healthy children. Furthermore, without such data, we have little or no understanding of which MMPs may be important in mediating events associated with somatic growth and development. Recognizing that tissue-specific production and/or localization of MMPs is potentially a dynamic process, with unique patterns of expression of these proteinases during periods of rapid tissue growth (i.e., childhood) or accelerated tissue remodeling and expansion (i.e., adolescence), the present study was developed to characterize potential age-specific and/or gender-specific differences in circulating concentrations of MMPs (specifically MMP-1, -2, -3, -8 and -9) across the pediatric and adolescence age range. Moreover, the intent of this study was to demonstrate the utility of a multiplexed, particle-based flow cytometric assay for the efficient, cost-effective simultaneous analysis of a panel of MMPs using a small sample volume.
Materials and methods
Residual serum samples (0.5−2.0 mL) were obtained from otherwise healthy children who were being tested (through the Arkansas Children's Hospital, Division of Pediatric Allergy and Immunology clinic) to exclude the possibility of a specific food allergy because of a past anecdotal coincidence of minor illness or urticaria following a particular food exposure. Residual serum samples were maintained only from those patients who were found to have a negative food allergy evaluation. Specifically, a total of 189 samples from children fulfilling the following inclusion criteria were obtained: 1) age 2−18 years; 2) normal serum total IgE level; and 3) negative radioallergoimmunosorbent (RAST) testing to one or more food categories (results from all tested categories had to be negative for inclusion). At the time of collection, patient age, sex, and race were recorded from the hospital database system by clinical laboratory personnel and samples were coded with a unique identifying number. Coded serum samples, along with the coded demographic parameters, were then transported to the research laboratory, such that research personnel remained blinded to patient identifying information. Serum samples were stored at −20°C until batch analysis was performed. The experimental protocol was approved by the institutional Review Board of the University of Arkansas for Medical Sciences. The requirement for informed consent of subjects was waived for this protocol, recognizing that an informed consent document, as a means of identifying subjects, would provide an exclusive, unnecessary risk to subject confidentiality.
MMP-1, MMP-2, MMP-3 and MMP-8 were measured simultaneously, while MMP-9 was measured separately in individual human serum samples using Fluorokine® MultiAnalyte Profiling (F-MAP) kits from R&D Systems (Minneapolis, MN, USA). Kits were run on a Luminex® 100™ Bioanalyzer (Luminex Corp., Austin, TX, USA) according to the kit manufacturer's instructions. Fluorokine® MultiAnalyte Profiling kits contained distinct groups of microspheres (each group bearing unique fluorescence intensity and a specific MMP antibody), biotinylated MMP antibodies, and phycoerythrin-conjugated streptavidin. Serum samples were incubated with antibody-coated microspheres, which bind to specific MMPs present in the serum. Next, microsphere-MMP complexes were washed and incubated with biotinylated MMP antibodies, which bind to MMPs present on the microspheres. A final incubation was performed in which phycoerythrin-labeled streptavidin was allowed to bind to biotinylated MMP antibodies present on microspheres. Microspheres were then loaded into a Luminex® 100™ Bioanalyzer, which quantifies the amount of phycoerythrin fluorescence present on each of the distinct microsphere groups. At least 50 individual microspheres were counted for each MMP, and the median fluorescence intensity was used for subsequent calculations. Intra-assay (precision within an assay; one sample replicated eight times) and inter-assay precision (precision between different assays; four samples each replicated on 4 different days) were: 5.2% and 7.5% for MMP-1; 4.1% and 9.9% for MMP-2; 3.6% and 10.3% for MMP-3; 8.4% and 16.4% for MMP-8; and 3.1% and 8.7% for MMP-9, respectively. Each of the microsphere sets was reported by the manufacturer to exhibit less than 0.5% cross-reactivity and interference with the other MMP family members studied.
A unique subset of 40 samples per MMP analyte was also assayed for MMP-2, -3, -8 and -9 by commercial ELISA to establish the reliability of the Fluorokine® MultiAnalyte Profiling (F-MAP) method compared with the more standard and popular method of measuring MMPs. Specifically, the R&D Systems Quantikine colorimetric sandwich ELISAs that use this manufacturer's specific MMP polyclonal antibodies were employed. (The R&D Systems MMP-1 ELISA measures pro-MMP-1, rather than total MMP-1, making a similar F-MAP vs. ELISA comparison for MMP-1 inappropriate.) These 40-sample subsets were randomly generated and unique for each analyte, representing a distribution of four samples from each decile across the assay range for each MMP (MMP-2, -3, -8 and -9), as obtained by F-MAP.
Statistical analysis
Limited demographic data obtained from the medical record (age, sex, and race) were recorded into an SPSS (SPSS 12.0 for Windows; SPSS, Chicago, IL, USA) data set. Exploratory data analysis, including summary statistics (mean, median, range, frequency tables) and plots (scatter plots, boxplots) were used to examine the distribution of and relationship between variables. Subjects were grouped according to patient age into the following cohorts: 2 to ≤8 years (pre-pubertal); 8 to ≤10 years (representing ∼Tanner stage 2 puberty); 10 to ≤12 years (∼Tanner stage 2−3 puberty); 12 to ≤15 years (∼Tanner stage 4 puberty); and 15 to ≤18 years (∼Tanner stage 5 puberty). Data were compared for each gender and age cohort separately. In addition, data were analyzed using age as an independent variable. A priori, it could be expected that MMP measurements would be skewed. The size of the standard deviation values also suggested that this was the case. Therefore, logarithms of the MMPs were used for analysis. The distribution of each MMP was examined using histograms. We compared MMP results between age groups, gender and race using ANOVA with a post hoc Tukey test. Type III sums of squares were used. Correlations were examined using Pearson correlations and scatter diagrams. To further aid interpretation, smooth splines were fit showing variation over age groups (Figure 3).
Figure 3.
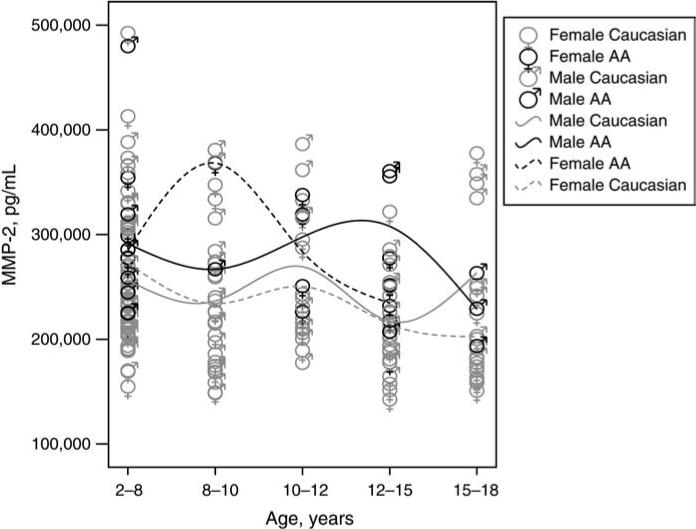
Serum concentrations of MMP-2 (pg/mL) for Caucasian and African-American (AA) females and males by age group. For each sex-race combination, a slight increase in MMP-2 concentration coincident with early puberty was noted.
Results
A total of 189 samples were obtained. Study subjects ranged in age from 2 to 18 years, with a mean±standard error (SE) age of 9.27±0.34 years. The study population included 88 females (46.6%) with a mean age of 9.94±0.50 years and 101 males (53.4%) with a mean age of 8.69±0.45 years. Samples provided a racial distribution for subjects of 80.4% Caucasian, 16.9% African-American, 1.6% Hispanic and 1.1% other/unspecified. Subjects were sub-classified according to age, with the sample size of each age cohort as follows: 2 to ≤8 years, n=79; 8 to ≤10 years, n=29; 10 to ≤12 years, n=22; 12 to ≤15 years, n=34; and 15 to ≤18 years, n=25.
A summary of MMP results for the entire subject group is shown in Table 1. The manufacturer's expected values (for an adult population) for each analyte are also shown. Analysis of histograms of individual MMP results (Figure 1) suggests that serum levels of MMPs in healthy children and adolescents represent log-normal distributions.
Table 1.
Descriptive statistics.
| Analyte | Concentration, pg/mL |
|||||
|---|---|---|---|---|---|---|
| Pediatric data |
Expected values* |
|||||
| Minimum | Maximum | Mean | SD | Range | Mean | |
| MMP-1 | 109.49 | 34,335.60 | 2421.74 | 3199.40 | 439−6934 | 2702 |
| MMP-2 | 142,555.95 | 492,568.83 | 251,389.36 | 67,149.97 | 36,462−264,400 | 169,963 |
| MMP-3 | 353.93 | 94,744.06 | 4788.66 | 10,175.62 | 2792−26,170 | 9132 |
| MMP-8 | 1065.54 | 214,190.82 | 12,381.61 | 22,622.96 | 1391−79,021 | 16,611 |
| MMP-9 | 50,591.69 | 1,829,283.54 | 318,816.19 | 248,005.96 | 85,406−1,638,927 | 441,732 |
Adult normal range and mean according to the manufacturer.
Figure 1.
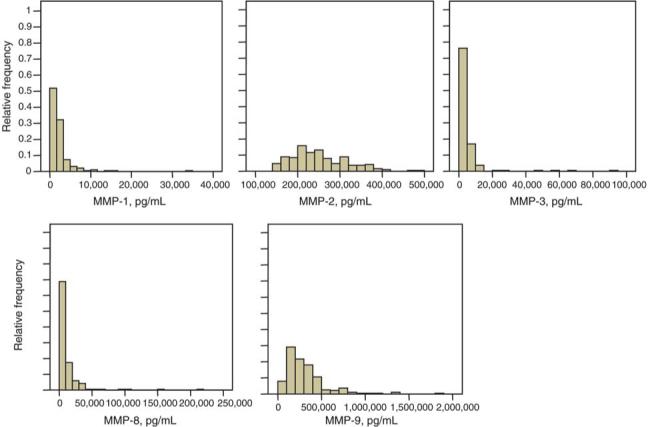
Serum levels of MMP-1, MMP-2, MMP-3, MMP-8 and MMP-9 in healthy pediatric subjects. MMP results for each analyte are presented as a histogram of relative frequency vs. MMP concentration in pg/mL.
MMP-1 (collagenase-1), MMP-8 (collagenase-2) and MMP-9 (gelatinase B) results were not significantly different from normal ranges reported for these analytes in adult subjects (Table 1). In addition, there was no significant difference among MMP-1, MMP-8 or MMP-9 results for different age groups (p>0.20) when the population was examined as a whole. Analysis of MMP-9 results by gender-race subgroups yielded no statistically significant differences (data not shown). However, mean serum levels of MMP-8 in African-American males were generally higher than MMP-8 levels in other sex-race combinations, and statistically significantly higher than MMP-8 results for Caucasian females (data not shown; p≤0.001). MMP-1 results for Caucasian males in the 8−10-year age group were statistically significantly higher than MMP-1 results for Caucasian males in the 12−15-year age group (data not shown; p<0.05).
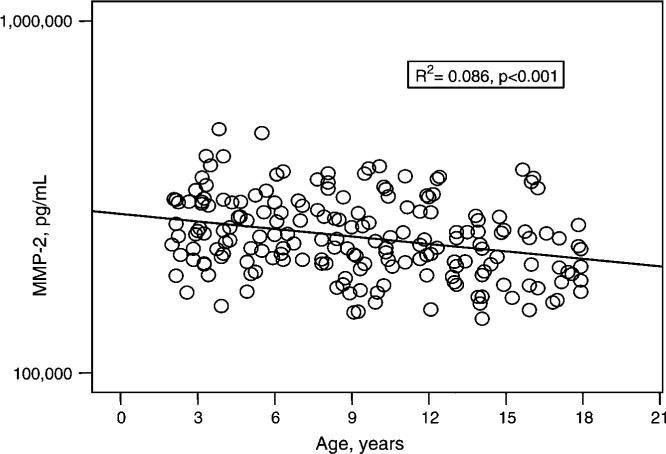
Two MMPs, MMP-2 (gelatinase A) and MMP-3 (stromelysin-1), were correlated with age. Specifically, MMP-2 concentrations were significantly negatively correlated with age (Figure 2; R2=0.086, p<0.001) and when analyzed across the age subsets, MMP-2 results in the 2−8-year age group were statistically higher than MMP-2 results in the 15−18-year group (p<0.05). Overall, the mean MMP-2 result for all pediatric subjects was ∼47% higher than the normative mean value reported for adults (Table 1). When analyzed across specific age groups and stratified according to race and gender, serum MMP-2 results suggest a slight increase in MMP-2 concentration coincident with the expected age of early puberty, occurring earlier in African-American females than in other gender-race combinations (Figure 3) (21).
Figure 2.
Serum concentrations of MMP-2 (pg/mL) for 189 healthy pediatric subjects, aged 2−18 years. MMP-2 concentrations were significantly negatively correlated with age (R2=0.086, p<0.001).
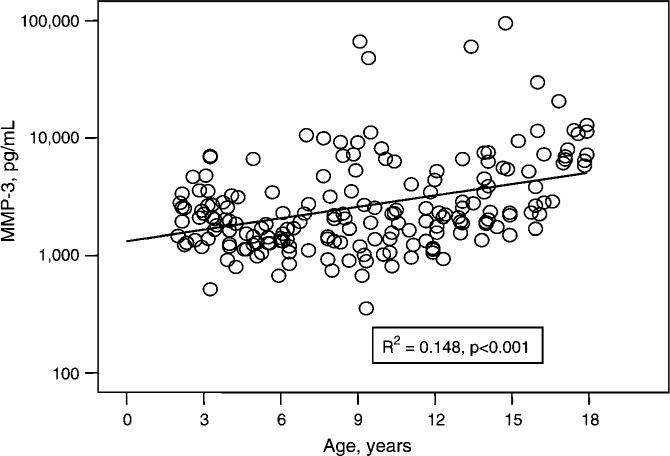
In contrast, MMP-3 concentrations were significantly positively correlated with age (Figure 4; R2=0.148, p<0.001). When examined by individual age groups, mean MMP-3 results in the 15−18-year age group were statistically significantly higher than mean MMP-3 results in other age groups (p<0.05). Moreover, the mean MMP-3 result for all pediatric subjects was ∼48% lower than the normative mean value reported for adults (Table 1). In contrast to results reported for adults (18), when evaluated by gender, there was no significant difference among serum MMP-3 results for males vs. females at any age.
Figure 4.
Serum concentrations of MMP-3 (pg/mL) for 189 healthy pediatric subjects, aged 2−18 years. MMP-3 concentrations were significantly positively correlated with age (R2=0.148, p<0.001).
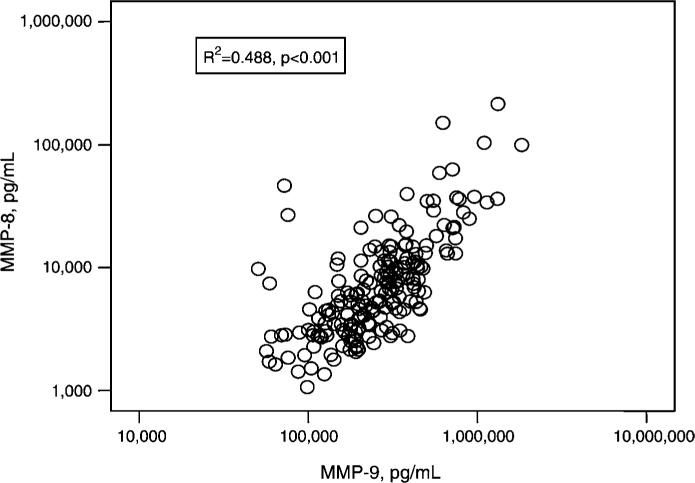
MMP-8 (collagenase-2) and MMP-9 (gelatinase B) are both synthesized by differentiating granulocytes, stored in circulating neutrophils, and released following neutrophil activation (3). Therefore, serum levels of these MMPs are thought to be influenced by release of MMPs by degranulation of neutrophils during ex vivo blood clotting in the test tube (22). It is significant, therefore, that serum levels of MMP-8 and MMP-9 were highly positively correlated (Figure 5; R2=0.488; p<0.001).
Figure 5.
Correlation between serum concentrations of MMP-8 and MMP-9 (pg/mL) in 189 healthy pediatric subjects, aged 2−18 years. Serum levels of MMP-8 and MMP-9 are highly positively correlated (R2=0.488, p<0.001).
Assay characteristics
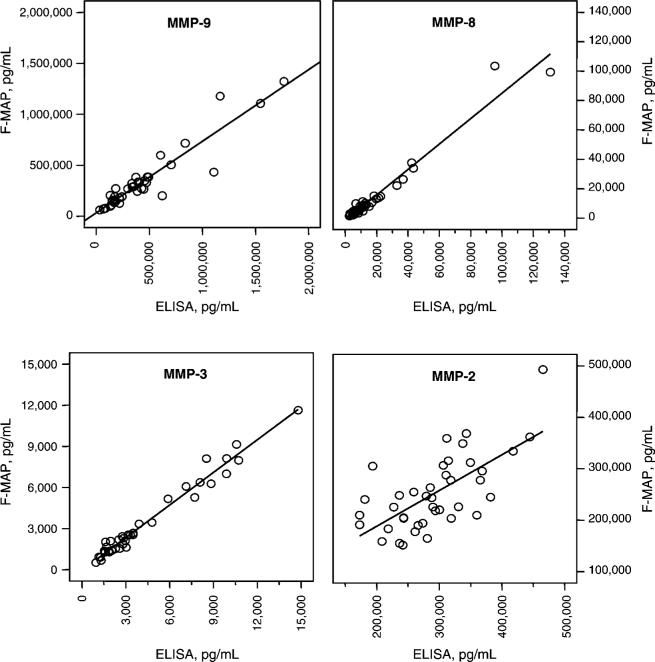
MMP results generated by F-MAP and by ELISA were highly positively correlated for MMP-3 (R2=0.97; p<0.001), MMP-8 (R2=0.96; p<0.001) and MMP-9 (R2=0.87; p<0.001; Figure 6). MMP-2 results generated by F-MAP and by ELISA were less tightly correlated (R2=0.45; Figure 6).
Figure 6.
Correlations between MMP results generated by Fluorokine® MultiAnalyte Profiling and MMP results generated by ELISA: MMP-3, R2=0.97, p<0.001; MMP-8, R2=0.96, p<0.001; MMP-9, R2=0.87, p<0.001; MMP-2, R2=0.45.
Discussion
We have previously identified and characterized urinary MMP excretion in pediatric patients and demonstrated that urinary excretion of MMPs is age-specific, with unique alterations detected during puberty (23). These data, as well as the work of others, suggest that regulation of MMPs during puberty is very likely distinctive and reflective of exuberant ECM expansion and tissue remodeling during pubertal growth and sexual maturation. For example, high concentrations of type I collagen breakdown products are detected in the urine of adolescents, and these concentrations correlate positively with growth rate during the pubertal growth spurt and with gender, being higher in females than in males (24). Because MMPs are the principal mediators of collagen turnover, it is important to study the temporal changes in MMP concentrations throughout childhood and adolescent development, both as a reference source for existing and future pediatric studies of disease-related MMP dysregulation, and to aid in our understanding of the role of MMP activity in normal ECM physiology.
To the best of our knowledge, this report is the first demonstration of the clinical utility of the F-MAP method for simultaneously measuring multiple MMP values in a single, specifically pediatric, serum specimen. Using this technology, we have demonstrated that serum values of MMP-2 and MMP-3 are age-dependent, and differ significantly from commercial normative ranges for these analytes for adult populations. In addition, similar to our previous study demonstrating an increase in MMP-2 concentration in urine during the pubertal years (23), MMP-2 data were suggestive of an early to mid-pubertal increase in serum MMP-2 values. In contrast, we did not identify an age correlation for either MMP-8 or MMP-9. However, recognizing that serum levels of these MMPs reflect release of MMPs following neutrophil degranulation, it is possible that any age correlation was obscured by this effect, and would require analysis of plasma specimens. In addition, caution should be exercised in interpreting serum values of MMP-8 and MMP-9, as plasma is considered the preferred blood sample for measurement of these proteases.
While this subject population represented a presumed healthy pediatric and adolescent cohort, the limited demographic information obtained at the time of specimen collection, as well as the blinded nature of these specimens, makes it possible that this assumption was not uniformly accurate. In addition, it is possible that other variables inherent in a pediatric population beyond age, race and sex (i.e., changing body mass index, timing of puberty, minor concurrent illness) would influence MMP results, but could not be factored into this analysis due to the nature of the study design. Nevertheless, the large number of samples available for analysis should provide a representative assessment of normative data and dampen any confounding variables.
Because MMPs are produced by many tissues, it is likely that the serum concentrations measured reflect a variety of sources. For example, the balanced regulation of MMPs and their naturally occurring inhibitors (tissue inhibitors of metalloproteinases, TIMPs) has been shown to be a critical component of many normal physiologic processes, including cartilage development and endochondral bone formation (25–27), normal bone turnover and repair (28, 29), reproductive tissue ECM remodeling during puberty and pregnancy (30–32), menstruation (33), growth of mammary tissues and lactation (34), testicular development (35) and normal spermatogenesis (35). Notably, many of these physiologic processes become active during puberty or are augmented during the pubertal growth spurt. Moreover, pubertal hormones, specifically growth hormone and IGFs (36–39), androgens (40) and estrogen (41, 42), have all been shown to regulate MMP expression and/or secretion.
For these studies, we investigated the use of a new methodology to measure multiple MMPs in a single sample of serum. We utilized a Luminex system-based assay that uses a 10×10 bead matrix, in which each bead set is made up of a specific proportion of red/orange fluorescent dye. The choice of this assay has several advantages over conventional ELISA techniques to measure MMPs. First, it is capable of simultaneously measuring multiple MMPs in a single biological sample, thereby eliminating significant operator error in sampling (e.g., pipetting) which is possible with multiple MMP analyses. Second, smaller sample volumes are required for multiplexing; thus, this technology is more amenable to the pediatric population, in which blood volumes may be restricted. Third, our data suggest that when serum is analyzed there is a high degree of correlation between conventional ELISAs and the multiplex assay for MMP-3, MMP-8, and MMP-9; however, comparison of absolute values between assay techniques may not be valid. Finally, the assays are robust and capable of measuring each MMP over a wide range of values. Other considerations not directly addressed by our study, but which might also make this approach more attractive than ELISA, are that the fluorescent readout is more direct, stable, and sensitive than colorimetric methods used for ELISAs, and typically, if measuring more than six analytes, the multiplex approach can be less expensive (43). Thus, the overall evaluation of this new technology to assay MMP concentrations in human serum shows it to be reproducible, specific, sensitive, and robust.
In conclusion, particle-based flow cytometry, specifically, F-MAP techniques for measurement of serum concentrations of MMP-1, -2, -3, -8, and -9, has effectively demonstrated age-specific differences in the serum concentrations of specific MMPs. These findings suggest that care should be exercised in interpreting the role of MMPs in the pathophysiology of various childhood diseases unless critical pediatric normative data are available and utilized for comparison.
Acknowledgements
This work was supported by a grant from the Arkansas Children's Hospital Research Institute (ACHRI) Dean's/CUMG Research Development Fund (#SG-030103-KT) and a grant from the National Institutes of Health to ACHRI (R01-DK62999-KT). The authors also acknowledge the support of the University of Arkansas for Medical Sciences' General Clinical Research Center, grant M01 RR14288 for assistance with MMP clinical investigations.
References
- 1.Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91:439–42. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- 2.Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004;16:558–64. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Noort JM, Amor S. Cell biology of autoimmune diseases. Int Rev Cytol. 1998;178:127–206. doi: 10.1016/s0074-7696(08)62137-3. [DOI] [PubMed] [Google Scholar]
- 5.Nagase H. Matrix metalloproteinases. Taylor and Francis; London: 1996. pp. 153–204. [Google Scholar]
- 6.Okada Y, Takeuchi N, Tomita K, Nakanishi I, Nagase H. Immunolocalization of matrix metalloproteinase 3 (stromelysin) in rheumatoid synovioblasts (B cells): correlation with rheumatoid arthritis. Ann Rheum Dis. 1989;48:645–53. doi: 10.1136/ard.48.8.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoenhagen P, Vince DG, Ziada KM, Kapadia SR, Lauer MA, Crowe TD, et al. Relation of matrix-metalloproteinase 3 found in coronary lesion samples retrieved by directional coronary atherectomy to intravascular ultrasound observations on coronary remodeling. Am J Cardiol. 2002;89:1354–9. doi: 10.1016/s0002-9149(02)02346-9. [DOI] [PubMed] [Google Scholar]
- 8.El-Gindy I, El Rahman AT, El-Alim MA, Zaki SS. Diagnostic potential of serum matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-1 as non-invasive markers of hepatic fibrosis in patients with HCV related chronic liver disease. Egypt J Immunol. 2003;10:27–35. [PubMed] [Google Scholar]
- 9.Pasieka Z, Stepien H, Czyz W, Pomorski L, Kuzdak K. Concentration of metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 in the serum of patients with benign and malignant thyroid tumours treated surgically. Endocr Regul. 2004;38:57–63. [PubMed] [Google Scholar]
- 10.Huang HF, Hong LH, Tan Y, Sheng JZ. Matrix metalloproteinase 2 is associated with changes in steroid hormones in the sera and peritoneal fluid of patients with endometriosis. Fertil Steril. 2004;81:1235–9. doi: 10.1016/j.fertnstert.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 11.Abou-Raya S, Naim A, Marzouk S. Cardiac matrix remodelling in congestive heart failure: the role of matrix metalloproteinases. Clin Invest Med. 2004;27:93–100. [PubMed] [Google Scholar]
- 12.Lynch JR, Blessing R, White WD, Grocott HP, Newman MF, Laskowitz DT. Novel diagnostic test for acute stroke. Stroke. 2004;35:57–63. doi: 10.1161/01.STR.0000105927.62344.4C. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds MA, Kirchick HJ, Dahlen JR, Anderberg JM, McPherson PH, Nakamura KK, et al. Early biomarkers of stroke. Clin Chem. 2003;49:1733–9. doi: 10.1373/49.10.1733. [DOI] [PubMed] [Google Scholar]
- 14.Tazaki T, Minoguchi K, Yokoe T, Samson KT, Minoguchi H, Tanaka A, et al. Increased levels and activity of matrix metalloproteinase-9 in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;170:1354–9. doi: 10.1164/rccm.200402-193OC. [DOI] [PubMed] [Google Scholar]
- 15.Renko J, Kalela A, Jaakkola O, Laine S, Hoyhtya M, Alho H, et al. Serum matrix metalloproteinase-9 is elevated in men with a history of myocardial infarction. Scand J Clin Lab Invest. 2004;64:255–61. doi: 10.1080/00365510410006054. [DOI] [PubMed] [Google Scholar]
- 16.Demestre M, Parkin-Smith G, Petzold A, Pullen AH. The pro and the active form of matrix metalloproteinase-9 is increased in serum of patients with amyotrophic lateral sclerosis. J Neuroimmunol. 2005;159:146–54. doi: 10.1016/j.jneuroim.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Kuyvenhoven JP, Verspaget HW, Gao Q, Ringers J, Smit VT, Lamers CB, et al. Assessment of serum matrix metalloproteinases MMP-2 and MMP-9 after human liver transplantation: increased serum MMP-9 level in acute rejection. Transplantation. 2004;77:1646–52. doi: 10.1097/01.tp.0000131170.67671.75. [DOI] [PubMed] [Google Scholar]
- 18.Zucker S, Lysik RM, Zarrabi MH, Greenwald RA, Gruber B, Tickle SP, et al. Elevated plasma stromelysin levels in arthritis. J Rheumatol. 1994;21:2329–33. [PubMed] [Google Scholar]
- 19.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207–14. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 20.Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003;200:448–64. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 21.Styne DM. Puberty, obesity and ethnicity. Trends Endocrinol Metab. 2004;15:472–8. doi: 10.1016/j.tem.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Zucker S, Doshi K, Cao J. Measurement of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMP) in blood and urine: potential clinical applications. Adv Clin Chem. 2004;38:37–85. doi: 10.1016/s0065-2423(04)38002-9. [DOI] [PubMed] [Google Scholar]
- 23.Thrailkill KM, Kumar S, Rosenberg CK, Auten KJ, Fowlkes JL. Characterization of matrix metalloproteinases in human urine: alterations during adolescence. Pediatr Nephrol. 1999;13:223–9. doi: 10.1007/s004670050597. [DOI] [PubMed] [Google Scholar]
- 24.Mora S, Prinster C, Proverbio MC, Bellini A, de Poli SC, Weber G, et al. Urinary markers of bone turnover in healthy children and adolescents: age-related changes and effect of puberty. Calcif Tissue Int. 1998;63:369–74. doi: 10.1007/s002239900542. [DOI] [PubMed] [Google Scholar]
- 25.Bord S, Horner A, Beeton CA, Hembry RM, Compston JE. Tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) distribution in normal and pathological human bone. Bone. 1999;24:229–35. doi: 10.1016/s8756-3282(98)00174-4. [DOI] [PubMed] [Google Scholar]
- 26.Bord S, Horner A, Hembry RM, Compston JE. Stromelysin-1 (MMP-3) and stromelysin-2 (MMP-10) expression in developing human bone: potential roles in skeletal development. Bone. 1998;23:7–12. doi: 10.1016/s8756-3282(98)00064-7. [DOI] [PubMed] [Google Scholar]
- 27.Werb Z, Chin JR. Extracellular matrix remodeling during morphogenesis. Ann NY Acad Sci. 1998;857:110–8. doi: 10.1111/j.1749-6632.1998.tb10111.x. [DOI] [PubMed] [Google Scholar]
- 28.Weiss S, Baumgart R, Jochum M, Strasburger CJ, Bidlingmaier M. Systemic regulation of distraction osteogenesis: a cascade of biochemical factors. J Bone Miner Res. 2002;17:1280–9. doi: 10.1359/jbmr.2002.17.7.1280. [DOI] [PubMed] [Google Scholar]
- 29.Uusitalo H, Hiltunen A, Soderstrom M, Aro HT, Vuorio E. Expression of cathepsins B, H, K, L, and S and matrix metalloproteinases 9 and 13 during chondrocyte hypertrophy and endochondral ossification in mouse fracture callus. Calcif Tissue Int. 2000;67:382–90. doi: 10.1007/s002230001152. [DOI] [PubMed] [Google Scholar]
- 30.Fata JE, Ho AT, Leco KJ, Moorehead RA, Khokha R. Cellular turnover and extracellular matrix remodeling in female reproductive tissues: functions of metalloproteinases and their inhibitors. Cell Mol Life Sci. 2000;57:77–95. doi: 10.1007/s000180050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stygar D, Wang H, Vladic YS, Ekman G, Eriksson H, Sahlin L. Increased level of matrix metalloproteinases 2 and 9 in the ripening process of the human cervix. Biol Reprod. 2002;67:889–94. doi: 10.1095/biolreprod.102.005116. [DOI] [PubMed] [Google Scholar]
- 32.Lenhart JA, Ryan PL, Ohleth KM, Palmer SS, Bagnell CA. Relaxin increases secretion of tissue inhibitor of matrix metalloproteinase-1 and -2 during uterine and cervical growth and remodeling in the pig. Endocrinology. 2002;143:91–8. doi: 10.1210/endo.143.1.8562. [DOI] [PubMed] [Google Scholar]
- 33.Dong JC, Dong H, Campana A, Bischof P. Matrix metalloproteinases and their specific tissue inhibitors in menstruation. Reproduction. 2002;123:621–31. doi: 10.1530/rep.0.1230621. [DOI] [PubMed] [Google Scholar]
- 34.Lochter A. Plasticity of mammary epithelia during normal development and neoplastic progression. Biochem Cell Biol. 1998;76:997–1008. [PubMed] [Google Scholar]
- 35.Sang QX, Stetler-Stevenson WG, Liotta LA, Byers SW. Identification of type IV collagenase in rat testicular cell culture: influence of peritubular-Sertoli cell interactions. Biol Reprod. 1990;43:956–64. doi: 10.1095/biolreprod43.6.956. [DOI] [PubMed] [Google Scholar]
- 36.Anne-Valerie R, Christelle D, Yannick F, Norbert P, Marc P, Dominique H. Human growth hormone stimulates proteinase activities of rabbit bone cells via IGF-I. Biochem Biophys Res Commun. 2000;268:875–81. doi: 10.1006/bbrc.2000.2079. [DOI] [PubMed] [Google Scholar]
- 37.Yoon A, Hurta RA. Insulin like growth factor-1 selectively regulates the expression of matrix metalloproteinase-2 in malignant H-ras transformed cells. Mol Cell Biochem. 2001;223:1–6. doi: 10.1023/a:1017549222677. [DOI] [PubMed] [Google Scholar]
- 38.Hui W, Rowan AD, Cawston T. Insulin-like growth factor 1 blocks collagen release and down regulates matrix metalloproteinase-1, -3, -8, and -13 mRNA expression in bovine nasal cartilage stimulated with oncostatin M in combination with interleukin 1alpha. Ann Rheum Dis. 2001;60:254–61. doi: 10.1136/ard.60.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lupia E, Elliot SJ, Lenz O, Zheng F, Hattori M, Striker GE, et al. IGF-1 decreases collagen degradation in diabetic NOD mesangial cells: implications for diabetic nephropathy. Diabetes. 1999;48:1638–44. doi: 10.2337/diabetes.48.8.1638. [DOI] [PubMed] [Google Scholar]
- 40.Li SC, Chen GF, Chan PS, Choi HL, Ho SM, Chan FL. Altered expression of extracellular matrix and proteinases in Noble rat prostate gland after long-term treatment with sex steroids. Prostate. 2001;49:58–71. doi: 10.1002/pros.1118. [DOI] [PubMed] [Google Scholar]
- 41.Liao EY, Luo XH, Deng XG, Wu XP. Effects of 17beta-estradiol on the expression of membrane type 1 matrix metalloproteinase (MT1-MMP) and MMP-2 in human osteoblastic MG-63 cell cultures. J Endocrinol Invest. 2001;24:876–81. doi: 10.1007/BF03343945. [DOI] [PubMed] [Google Scholar]
- 42.Wingrove CS, Garr E, Godsland IF, Stevenson JC. 17beta-oestradiol enhances release of matrix metalloproteinase-2 from human vascular smooth muscle cells. Biochim Biophys Acta. 1998;1406:169–74. doi: 10.1016/s0925-4439(97)00097-5. [DOI] [PubMed] [Google Scholar]
- 43.Vignali DA. Multiplexed particle-based flow cytometric assays. J Immunol Methods. 2000;243:243–55. doi: 10.1016/s0022-1759(00)00238-6. [DOI] [PubMed] [Google Scholar]