Abstract
The antihypertensive drug doxazosin has been associated with an increased risk for congestive heart failure and cardiomyocyte apoptosis. Human ether-a-go-go-related gene (hERG) K+ channels, previously shown to be blocked by doxazosin at therapeutically relevant concentrations, represent plasma membrane receptors for the antihypertensive drug. To elucidate the molecular basis for doxazosin-associated pro-apoptotic effects, cell death was studied in human embryonic kidney cells using three independent apoptosis assays. Doxazosin specifically induced apoptosis in hERG-expressing HEK cells, while untransfected control groups were insensitive to treatment with the antihypertensive agent. An unexpected biological mechanism has emerged: binding of doxazosin to its novel membrane receptor, hERG, triggers apoptosis, possibly representing a broader pathophysiological mechanism in drug-induced heart failure.
Keywords: Antihypertensive drug, apoptosis, congestive heart failure, doxazosin, hERG channel, potassium channel
1. Introduction
Selective α1-adrenoceptor antagonists are established antihypertensive agents, lowering blood pressure by reducing vascular tone in resistance and capacitance vessels. Although α1-inhibitors are generally associated with a low incidence of serious adverse effects, concerns have been raised by results from the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT; The ALLHAT Officers and Coordinators, 2000). The risk of congestive heart failure was twice as high in the doxazosin group compared to patients treated with the diuretic chlorthalidone, forcing discontinuation of the doxazosin arm of the study. In vitro, doxazosin induces apoptosis in human cardiomyocytes independent of its antiadrenergic action (Gonzalez-Juanatey et al., 2003), a mechanism recognized to contribute to cardiomyocyte loss in heart failure. However, a direct mechanistic link or receptor for doxazosin that initiates pro-apoptotic intracellular processes remained to be identified.
In search for a possible pro-apoptotic drug receptor, it is important to consider that apoptosis regulation is directly tied to potassium channel function: enhanced K+ current activity is observed in tumor cells, and K+ channel inhibition can promote apoptosis in cell type-dependent fashion (Wonderlin and Strobl, 1996; Lang et al., 2005). From a cardiologist's perspective, human ether-a-go-go-related gene (hERG; Kv11.1) K+ channels are of particular interest in this context. Recognized as molecular counterpart of the rapid delayed rectifier K+ current (IKr), an efficient regulator of cardiac repolarization and a well-characterized pharmacological target in antiarrhythmic therapy, hERG protein is expressed at high levels in human myocardium. Notably during fetal developmental stages and in dedifferentiated or cancerous adult cardiomyocytes, hERG currents display predominance among K+ channels. HERG expression has been revealed to facilitate proliferation of experimental cells, whereas inhibition of hERG currents antagonizes this effect (Smith et al., 2002; Wang et al., 2002). We recently reported that hERG functions as plasma membrane receptor for doxazosin, resulting in K+ current inhibition (Thomas et al., 2004). Based upon these findings we hypothesized that drug binding to hERG initiates apoptosis of hERG-expressing cells.
2. Materials and Methods
2.1 Molecular biology
HERG (GenBank accession number: NM_000238) point mutations (Y652A and F656C) were introduced with the QuikChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA). All constructs were confirmed by DNA sequencing.
2.2 Tissue culture
HEK 293 cells (non-transfected and stably transfected with hERG cDNA; Thomas et al., 2001) were cultured in minimum essential medium (Invitrogen, Karlsruhe, Germany) supplemented with 10% fetal bovine serum (Invitrogen), 100 U/ml penicillin G sodium, 100 μg/ml streptomycin sulphate, and 500 μg/ml geneticin (G418; Invitrogen) as selective marker for stably transfected cells in an atmosphere of 95% humidified air and 5% CO2 at 37°C. HEK 293 cells do not express endogenous α-adrenoceptors (Theroux et al., 1996). Cells were passaged regularly and subcultured prior to treatment. Transient transfections of HEK cells were performed using FuGene transfection reagent (Roche, Mannheim, Germany) according to the manufacturer's instructions. For transiently transfected and non-transfected cells, geneticin was omitted from the culture media.
2.3 TUNEL assay
Apoptosis of HEK 293 cells was detected by TUNEL (terminal deoxyribonucleotide transferase-mediated dUTP nick end labeling; Gavrieli et al., 1992) with the Fluorescein In Situ Cell Death Detection kit (Roche Molecular Biochemicals, Mannheim, Germany). After incubation in control media or doxazosin (Sigma-Aldrich, Steinheim, Germany) for 24 h cells were fixed, permeabilized, incubated in TUNEL reaction solution and counted to calculate mean percentages of TUNEL-positivity.
2.4 Hoechst staining
Pyknosis represents a final stage of the apoptosis cascade (Saraste and Pulkki, 2000). To detect nuclear shrinkage as a late apoptotic event, cells were treated with 30 μM doxazosin or control media for 42 h, fixed with formalin, and then stained with Hoechst 33342 (1 μg/ml, 2 min) and analyzed. Hoechst 33342 penetrates the plasma membrane and stains DNA blue fluorescent (Latt and Stetten, 1976). In contrast to normal cells, nuclei of apoptotic cells display pyknosis with highly condensed chromatin.
2.5 Flow cytometric assay
A flow cytometric DNA fragmentation assay was employed as an independent quantitative measure of apoptotic cell death, as described by Nicoletti et al. (1991). Doxazosin (30 μM) was applied for 0, 24, 48, and 72h. Cells were then collected by centrifugation, lysed in buffer containing 0.1% Triton X-100 and 50 mg/ml propidium iodide (PI), and analyzed for PI fluorescence (indicating apoptotic nuclei with hypodiploid DNA content) in a FACSCalibur flow cytometer (Becton Dickinson, Heidelberg, Germany).
2.6 Electrophysiology
Current recordings from HEK 293 cells were performed using the whole-cell patch clamp configuration as previously reported (Thomas et al., 2001). All experiments were carried out at room temperature (20 − 22 °C), and no leak subtraction was done during the experiments. Concentration-response relationships for drug-induced block were fit with a Hill equation of the following form: Idrug/Icontrol = 1/[1 + (D/IC50)nH], where I indicates current, D is the drug concentration, nH is the Hill coefficient, and IC50 is the concentration necessary for 50% block.
2.7 Solutions and drug administration
For whole-cell patch clamp recordings from HEK 293 cells electrodes were filled with the following solution (in mM): 130 K-aspartate, 5.0 MgCl2, 5 EGTA, 4 ATP, 10 HEPES, (pH adjusted to 7.2 with KOH). The external solution for these experiments contained (in mM): 137 NaCl, 4.0 KCl, 1.0 MgCl2, 1.8 CaCl2, 10 HEPES, 10 glucose (pH adjusted to 7.4 with NaOH). Doxazosin (Sigma) was prepared as 10 mM stock solution in DMSO and stored at −20°C. On the day of experiments, aliquots of the stock solution were diluted to the desired concentrations with bath solution.
2.8 Statistics
Values are given as mean ± S.E.M. Statistical significance was calculated using Student's t tests (* indicates p<0.05 versus untreated cells). Multiple comparisons were performed using one-way ANOVA followed by Bonferroni post hoc testing.
3. Results
3.1 Doxazosin induces apoptosis in hERG-expressing human embryonic kidney cells
Cell death was studied in human embryonic kidney (HEK 293) cells to elucidate the molecular basis for doxazosin-associated pro-apoptotic effects. Apoptosis is characterized by chromatin cleavage at the linker regions between nucleosomes, resulting in extensive fragmentation of DNA into oligonucleosomal subunits. Three experimental approaches were used to assess apoptosis.
Apoptosis of HEK cells was first detected in situ by TUNEL (terminal deoxyribonucleotide transferase-mediated dUTP nick end labeling) fluorescence, indicating DNA damage and fragmentation as characteristic apoptotic features (Fig. 1A). Apoptosis rates increased in hERG-positive cells from 1.6 ± 0.1% under control conditions to 14.1 ± 2.6% in the presence of 30 μM doxazosin (Fig. 1B), a concentration known to induce apoptosis in human cardiomyocytes (Gonzalez-Juanatey et al., 2003) and sufficient to achieve >95% current reduction in HEK-hERG cells (Thomas et al., 2004). Untransfected control groups with otherwise identical genetic background were insensitive to treatment with the antihypertensive agent, as predicted for cells lacking its putative pro-apoptotic receptor, hERG (1.0 ± 0.2% and 1.6 ± 0.6% apoptotic cells before and after drug incubation, respectively; n = 4 assays).
Fig. 1.
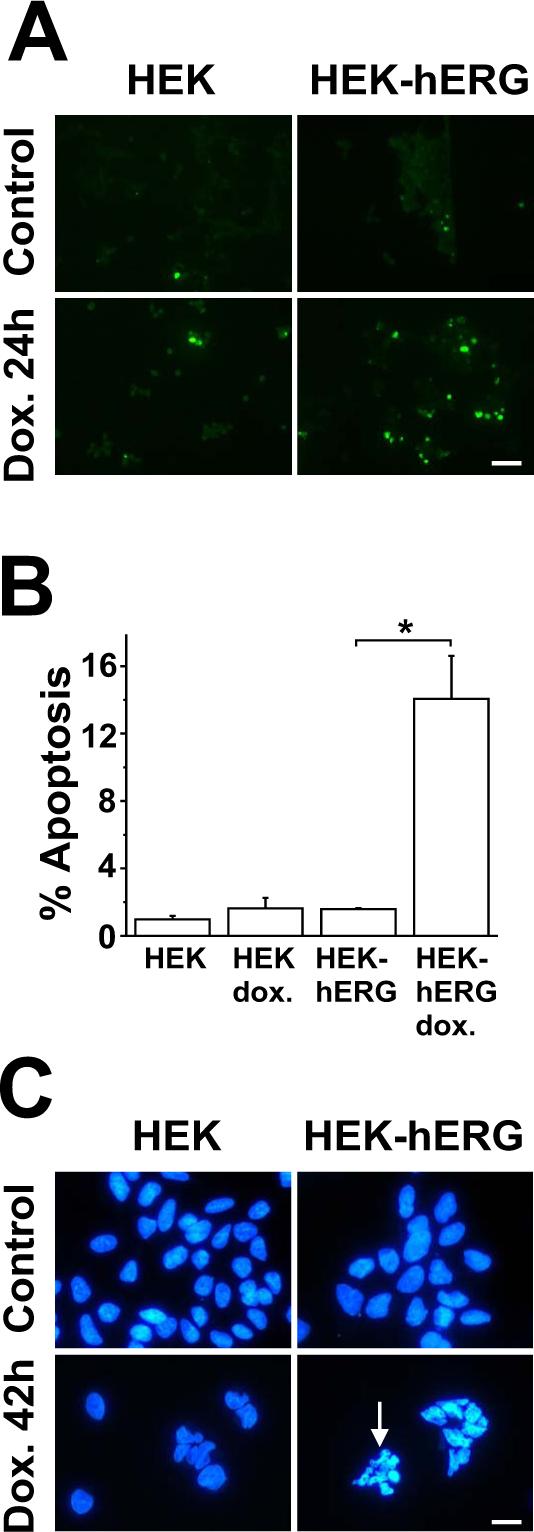
Doxazosin induces apoptosis in hERG-positive cells. A Fluorescence microphotographs corresponding to TUNEL assays of controls and of cells treated with 30 μM doxazosin (24 h). Green nuclear fluorescence reflects endonucleolytic DNA degradation and induction of apoptosis. Four independent experiments with similar results have been performed. Scale bar, 50 μm. B Mean (± S.E.M.) apoptosis rates (n = 4 independent experiments) in HEK cells assessed by TUNEL staining and cell counting. In the graph, TUNEL-positive cells are expressed as a percentage of the total number of cells (n = 4 independent assays; * indicates p < 0.05 versus untreated cells). Multiple comparisons were performed using one-way ANOVA followed by Bonferroni post hoc testing. C Morphologic changes induced by doxazosin in HEK-hERG cells. Doxazosin-associated apoptotic nuclei show fluorescence and fragmentation (arrow). Nuclei were visualized by staining with Hoechst 33342 dye in untreated cells and after application of 30 μM doxazosin (42 h). Representative fluorescence microphotographs of one typical experiment are shown. Three independent experiments with similar results have been performed. Scale bar, 15 μm .
Second, Hoechst 33342 staining of doxazosin-treated cells and untreated controls was used to detect nuclear shrinkage (pyknosis) as a late apoptotic event and to confirm our findings obtained from TUNEL assays (Fig. 1C). Cells were cultured in drug-free control media or in the presence of 30 μM doxazosin for 42 h. Morphologic changes occurred exclusively in doxazosin-treated HEK-hERG cells, as indicated by an arrow in Fig. 1C. Three independent assays with similar results were performed to assess this morphologic hallmark of apoptosis.
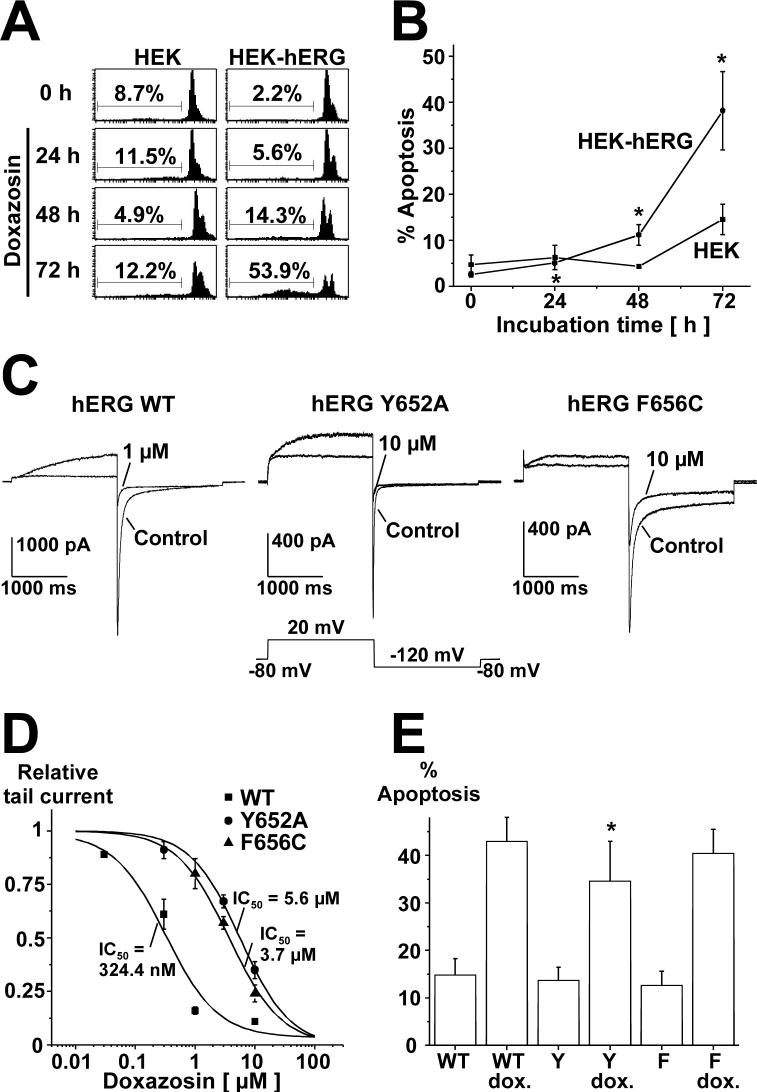
Finally, doxazosin-associated DNA damage and cell death were detected and quantified using a flow cytometric DNA fragmentation assay that specifically measures apoptosis in a preparation of isolated nuclei in hypotonic buffer (Nicoletti et al., 1991). Apoptotic nuclei appear as broad hypodiploid DNA peak and are clearly discriminated from the narrow fluorescence peak of cells with normal DNA content (Fig. 2A). Cell death induced by non-apoptotic mechanisms is not expected to cause any variation of the normal DNA peak in the assay. Time-dependence of doxazosin-associated adverse effect on HEK cell viability is revealed (Fig. 2B). Mean percentage of apoptotic hERG-positive cells was 11.2 ± 2.3% and 38.2 ± 8.5% after 48 and 72 h, respectively (n = 3), while controls displayed markedly lower apoptosis levels of 4.3 ± 0.3% (48 h) and 15.5 ± 3.3% (72 h).
Fig. 2.
A Flow cytometric analysis illustrating time-dependence of pro-apoptotic effects of doxazosin. Cells were treated with 30 μM doxazosin and analyzed for subdiploid DNA content as hallmark for apoptosis using the assay described by Nicoletti et al. (1991). One representative FACS result is shown. B Mean values (± S.E.M) obtained from three experiments (* indicates p < 0.05 versus drug-free conditions at 0 h). Statistical significance was calculated using Student's t tests. C Attenuation of doxazosin block by pore mutations demonstrates direct doxazosin binding to hERG channels. Original current traces are shown, illustrating the effects of doxazosin on wild type or mutant Y652A and F656C currents (1 and 10 μM doxazosin, respectively). D Concentration-response relationships for doxazosin blockade of hERG constructs transiently expressed in HEK cells. Calculated IC50 values are indicated (mean ± S.E.M.). E Pore mutations with attenuated doxazosin binding affinity show reduced apoptosis levels. Mean (± S.E.M.) apoptosis rates (n = 3 independent experiments) in transiently transfected HEK cells after treatment with 30 μM doxazosin (72 h), assessed by FACS analyses as in panel A. Multiple comparisons were performed using one-way ANOVA followed by Bonferroni post hoc testing (* indicates p < 0.05 versus WT+doxazosin). WT, hERG wild type; Y, hERG Y652A; F, hERG F656C.
3.2 Pore mutations provide evidence for direct binding of doxazosin to hERG K+ channels
Aromatic residues Y652 and F656 located in the S6 domain have been identified as key determinants of drug binding to hERG channels (Mitcheson et al., 2000; Thomas et al., 2004). Loss of receptor binding affinity as a result of structural changes (that is, pore residue mutations) confirms direct doxazosin binding to the hERG protein inside its ion-conducting pore. The effect of doxazosin on HEK cells transiently transfected with wild type or mutant hERG Y652A and hERG F656C cDNA was investigated. Currents were activated by a 2 s depolarization to + 20 mV, and inward tail currents were recorded during a step to −120 mV for 2 s. Pulses were applied in 15 s-intervals during superfusion with the drug solution for 7.5 minutes. As illustrated in Fig. 2C, the inhibitory effect of doxazosin was strongly attenuated by replacement of aromatic channel pore residues. IC50 values yielded 324.4 nM (WT; n = 4), 5.6 μM (Y652A; n = 3), and 3.7 μM (F656C; n = 3), with respective Hill coefficients nH of 1.14, 0.96, and 1.11 (Fig. 2D).
3.3 Doxazosin-induced apoptosis is attenuated in cells expressing hERG mutants with reduced drug binding affinity
To assess the effects of reduced drug binding affinity on doxazosin-induced apoptosis, wild type hERG and mutant Y652A and F656C proteins were studied in transiently transfected HEK cells. Following exposure to 30 μM doxazosin for 72 h, apoptotic cells death was quantified using the flow cytometric assay described above. Cell death induced by doxazosin was significantly reduced in cells expressing hERG Y652A channels compared to wild type hERG (34.6 ± 8.3% versus 42.9 ± 5.1%; Fig. 2E; n = 3 assays). In addition, we observed a non-significant tendency towards lower apoptosis rates when hERG F656C channels were expressed (40.4 ± 5.0%). Apoptosis rates in control cells without doxazosin treatment were not significantly different after 72 h (WT, 14.8 ± 3.4%; Y652A, 13.7 ± 2.8%; F656C, 12.7 ± 2.9%).
4. Discussion
4.1 hERG K+ channels: pro-apoptotic cardiac membrane receptors
We have delineated apoptosis in doxazosin-treated cells lacking α-adrenoceptors. Here, hERG K+ channels are identified as pro-apoptotic plasma membrane receptors for doxazosin. Several lines of evidence support this hypothesis. Firstly, doxazosin-induced apoptosis was seen in experimental cells (this report) and cardiomyocytes (Gonzalez-Juanatey et al., 2003), which share robust hERG expression. In contrast, no significant apoptosis was observed in the absence of hERG (notably in untransfected HEK cells). Secondly, hERG has been implicated in apoptosis regulation (Wang et al., 2002) and facilitates proliferation of experimental cells, which can be antagonized by inhibition of hERG currents (Smith et al., 2002; Wang et al., 2002). Finally, direct evidence for doxazosin binding to the plasma membrane channel hERG is provided by mutagenesis studies. HERG Y652A or F656C mutants lack essential residues within a well-characterized drug binding region (Mitcheson et al., 2000) and display markedly reduced doxazosin binding affinity, strongly arguing in support of a direct drug-receptor interaction (Fig. 2). Indeed, apoptosis rates were significantly reduced in cells expressing hERG Y652A channels treated with doxazosin. HERG F656C channels displayed a tendency towards lower cell death levels as well; however, statistical significance was not reached. In summary, a mechanistic connection between doxazosin, hERG, and apoptosis is likely to underlie the effects reported here.
In patients, doxazosin plasma levels of 244 nM are recorded at therapeutic doses (Gonzalez-Juanatey et al., 2003). This value corresponds well with IC50 values observed in stably (585 nM; Thomas et al., 2004) or transiently transfected (324 nM) HEK-hERG cells, supporting the notion that doxazosin-targeting of hERG channels occurs within a clinically relevant concentration range. Moreover, doxazosin is often used for long-term antihypertensive treatment, allowing for accumulation of the compound in cardiac tissue.
Similar to doxazosin, several other hERG antagonists have been associated with de novo heart failure or worsening of pre-existing ventricular dysfunction (e.g., propafenone, disopyramide, clozapine) or with cardiomyocyte apoptosis (e.g., amiodarone, prazosin). While early conclusions should be avoided until clinical implications of this newly discovered mechanism are elucidated in more detail, caution is in order in particular when using hERG antagonists in patients with ventricular dysfunction.
4.2 Molecular mechanism
The molecular pathways of apoptosis are complexly interconnected and in many cases not fully understood. Evidence provided by Eiras et al. (2006) indicates that endoplasmic reticulum stress and activation of focal adhesion kinase are involved in doxazosin-associated apoptosis. The mechanistic connection between the apoptotic stimulus (i.e., doxazosin), hERG potassium channels, and initiation of endoplasmic reticulum-mediated apoptosis remains to be revealed.
4.3 A broader role for an emerging biological strategy
hERG K+ channels are found in several cancerous cell types (e.g., adenocarcinoma, neuroblastoma, and leukemia), while their respective non-cancerous counterparts do not express the gene (Wang, 2004). Implicated in regulation of cell death and proliferation as well as invasive growth and neoangiogenesis, hERG antagonism exerts cytostatic effects (Smith et al., 2002; Wang et al., 2002). Thus, pro-apoptotic action of hERG inhibitors might serve as starting point for future development of anticancer compounds, specifically targeting apoptosis in hERG-positive tumor cells.
4.4 Conclusion
A biological receptor mechanism leading to drug-induced apoptotic cell death is revealed: doxazosin specifically promotes apoptosis in cells expressing hERG potassium channels. hERG-associated apoptosis may represent a key process contributing to progressive cell loss and heart failure in patients treated with the antihypertensive drug. As hERG channels are targeted by multiple drugs, including several associated with congestive heart failure, it is tempting to hypothesize that these findings represent a broader pathophysiological mechanism in drug-induced heart failure.
Acknowledgements
We thank Bettina Thomas for critical review of the manuscript. This work was supported by research grants from the German Research Foundation (to R.K., J.K., and C.A.K.), from the Deutsche Stiftung für Herzforschung (to D.T.), from the German Cardiac Society (to D.T.), and from the National Institutes of Health (HL71789 to E.F.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Eiras S, Fernandez P, Pineiro R, Iglesias MJ, Gonzalez-Juanatey JR, Lago F. Doxazosin induces activation of GADD153 and cleavage of focal adhesion kinase in cardiomyocytes en route to apoptosis. Cardiovasc. Res. 2006;71:118–128. doi: 10.1016/j.cardiores.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell. Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Juanatey JR, Iglesias MJ, Alcaide C, Pineiro R, Lago F. Doxazosin induces apoptosis in cardiomyocytes cultured in vitro by a mechanism that is independent of α1-adrenergic blockade. Circulation. 2003;107:127–131. doi: 10.1161/01.cir.0000043803.20822.d1. [DOI] [PubMed] [Google Scholar]
- Lang F, Foller M, Lang KS, Lang PA, Ritter M, Gulbins E, Vereninov A, Huber SM. Ion channels in cell proliferation and apoptotic cell death. J. Membrane Biol. 2005;205:147–157. doi: 10.1007/s00232-005-0780-5. [DOI] [PubMed] [Google Scholar]
- Latt SA, Stetten G. Spectral studies on 33258 Hoechst and related bisbenzimidazole dyes useful for fluorescent detection of deoxyribonucleic acid synthesis. J. Histochem. Cytochem. 1976;24:24–33. doi: 10.1177/24.1.943439. [DOI] [PubMed] [Google Scholar]
- Mitcheson JS, Chen J, Lin M, Culberson C, Sanguinetti MC. A structural basis for drug-induced long QT syndrome. Proc. Natl. Acad. Sci. U.S.A. 2000;97:12329–12333. doi: 10.1073/pnas.210244497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- Saraste A, Pulkki K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc. Res. 2000;45:528–537. doi: 10.1016/s0008-6363(99)00384-3. [DOI] [PubMed] [Google Scholar]
- Smith GAM, Tsui HW, Newell EW, Jiang X, Zhu XP, Tsui FWL, Schlichter LC. Functional up-regulation of HERG K+ channels in neoplastic hematopoietic cells. J. Biol. Chem. 2002;277:18528–18534. doi: 10.1074/jbc.M200592200. [DOI] [PubMed] [Google Scholar]
- The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2000;283:1967–1975. [PubMed] [Google Scholar]
- Theroux TL, Esbenshade TA, Peavy RD, Minneman KP. Coupling efficiencies of human α1-adrenergic receptor subtypes: titration of receptor density and responsiveness with inducible and repressible expression vectors. Mol. Pharmacol. 1996;50:1376–1387. [PubMed] [Google Scholar]
- Thomas D, Wendt-Nordahl G, Röckl K, Ficker E, Brown AM, Kiehn J. High affinity blockade of HERG human cardiac potassion channels by the novel antiarrhythmic drug BRL-32872. J. Pharmacol. Exp. Ther. 2001;297:735–761. [PubMed] [Google Scholar]
- Thomas D, Wimmer AB, Wu K, Hammerling BC, Ficker EK, Kuryshev Y, Kiehn J, Katus HA, Schoels W, Karle CA. Inhibition of human ether-ago-go-related gene potassium channels by the α1-adrenoceptor antagonists prazosin, doxazosin, and terazosin. Naunyn-Schmiedeberg's Arch. Pharmacol. 2004;369:462–472. doi: 10.1007/s00210-004-0931-8. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang Y, Cao L, Han H, Wang J, Yang B, Nattel S, Wang Z. HERG K+ channel, a regulator of tumor cell apoptosis and proliferation. Cancer Res. 2002;62:4843–4848. [PubMed] [Google Scholar]
- Wang Z. Roles of K+ channels in regulating tumor cell proliferation and apoptosis. Pflugers Arch. – Eur. J. Physiol. 2004;448:274–286. doi: 10.1007/s00424-004-1258-5. [DOI] [PubMed] [Google Scholar]
- Wonderlin WF, Strobel JS. Potassium channnels, proliferation and G1 progression. J. Membr. Biol. 1996;154:91–107. doi: 10.1007/s002329900135. [DOI] [PubMed] [Google Scholar]