Abstract
Objective
To investigate the reinforcement of Bis-GMA/TEGDMA dental resins (without conventional glass filler) and composites (with conventional glass filler) with various mass fractions of nano fibrillar silicate (FS).
Methods
Three dispersion methods were studied to separate the silanized FS as nano-scaled single crystals and uniformly distribute them into dental matrices. The photo-curing behaviors of the Bis-GMA/TEGDMA/FS resins were monitored in situ by RT-NIR to study the photopolymerization rate and the vinyl double bond conversion. Mechanical properties (flexural strength, elastic modulus and work of fracture) of the nano FS reinforced resins/composites were tested, and Analysis of Variance (ANOVA) was used for the statistical analysis of the acquired data. The morphology of nano FS and the representative fracture surfaces of its reinforced resins/composites were examined by SEM/TEM.
Results
Impregnation of small mass fractions (1 % and 2.5 %) of nano FS into Bis-GMA/TEGDMA (50/50 mass ratio) dental resins/composites improved the mechanical properties substantially. Larger mass fraction of impregnation (7.5 %), however, did not further improve the mechanical properties (one way ANOVA, P > 0.05) and may even reduce the mechanical properties. The high degree of separation and uniform distribution of nano FS into dental resins/composites was a challenge. Impregnation of nano FS into dental resins/composites could result in two opposite effects: a reinforcing effect due to the highly separated and uniformly distributed nano FS single crystals, or a weakening effect due to the formation of FS agglomerates/particles.
Significance
Uniform distribution of highly separated nano FS single crystals into dental resins/composites could significantly improve the mechanical properties of the resins/composites.
Keywords: Dental material, Nano fibrillar silicate, Bis-GMA, TEGDMA
INTRODUCTION
Dental composites consisting of polymeric resin matrices and inorganic fillers have been available for over four decades. Compared to dental amalgams, the composites possess better esthetic property, have less safety concern, and have shown reasonably satisfactory clinic results. They have been widely adopted by the dental profession as the restorative material of choice.
Dental resins are usually cured (hardened) by photo-initiated free radical polymerization. Camphorquinone (CQ) is a commonly used visible-light initiator and ethyl-4-(N,N’-dimethylamino) benzoate (4EDMAB) is a commonly used co-initiator. The monomer 2,2’-bis-[4-(methacryloxypropoxy)-phenyl]-propane (Bis-GMA) has been widely used as an important dental base monomer since it was invented in early 1960’s [1,2]. Bis-GMA is a very viscous liquid. To improve the handling qualities, a low viscosity diluent monomer, such as tri (ethylene glycol) dimethacrylate (TEGDMA), is added to thin the resin. In Bis-GMA/TEGDMA dental resin, Bis-GMA functions to limit the photopolymerization induced volumetric shrinkage and to enhance resin reactivity, while TEGDMA provides for the increased vinyl double bond conversion [3,4].
While dental resins have been reinforced with inorganic fillers such as silanized glass/ceramic particles for years, the relatively low strength and durability of the composites have limited their uses [5–8]. The dental composites have flexural strength in the range from 100 to 140 MPa, which can fulfill the requirements of small restorations but cannot survive large stress-bearing restorations. Furthermore, the strength of dental composites decreases significantly after long-term water aging. The average lifetime of dental composites is less than 5 years [5]. In comparison, dental amalgams have a lifetime of more than 15 years [9,10]. Investigations of the failures revealed that, among other things, the inorganic filler was a major contributor to the failures [11,12]. Many inorganic fillers currently being used for dental composites are spherical or irregular in shape. Such filler particles at occlusal surfaces are susceptible to dislodgement from the resin matrix during wear with food boluses. This causes the reinforcing effect to be lost.
Reinforcement with high-strength fibers/whiskers has been shown to result in dramatic improvements on the properties of dental composites [13–16]. Research conducted by Xu and co-workers revealed that the impregnation of extremely strong ceramic fibers/whiskers could lead to a two-fold increase in composite strength and toughness, as well as provide promising results in composite polishability, water absorption and strength durability [17–21]. The “bridging” mechanism has been proposed to explain the reinforcement by the fibers/whiskers. If a micro-crack is initiated in a matrix under contact wear and/or other stresses, the fibrillar fillers remain intact across the crack planes and support the applied load. Crack-opening is therefore resisted by the bridging fillers and the matrix is reinforced. Requirements for the fillers to achieve the effective “bridging” reinforcement include high mechanical properties (especially strength and modulus) and a large aspect ratio.
Unlike micron-scaled fibers/whiskers, fibrillar silicate (FS) is composed of nano-scaled single crystals (fibers). FS is a class of hydrated magnesium/aluminum silicate, and there are several types of FS minerals found in nature. The most abundant type is known as attapulgite/palygorskite, which is found mostly in the United States and China. The FS used in this study was attapulgite obtained from China, and its chemical formula is Mg5[Al]Si8O20(HO)2(OH2)4·4H2O. The primary structural units of FS are the silicate single crystals that are 100–3000 nm in length and 10–25 nm in diameter, and these single crystals stack/agglomerate into particles with sizes in microns [22,23]. The FS nano-sacled single crystals possess a high degree of structural perfection and superior mechanical properties. For example, the strength of a FS nano-scaled single crystal is over 50 GPa [22], which is at least 10 times higher than that of most micron-scaled fibers/whiskers. Unlike layered silicates such as montmorillonite, which are difficult to completely exfoliate into nano-scaled silicate layers and to uniformly distribute in dental matrices, FS is relatively easy to separate into nano-scaled single crystals and to distribute uniformly in dental matrices. This is because the spacing among the aggregated single crystals in FS is much larger than that of the silicate layers in montmorillonite. As a result, the interaction of the single crystals in FS is considerably weaker than that of the silicate layers in montmorillonite. Therefore, without chemical substitution of metal ions with surfactants such as tertiary amine ions (a widely adopted method for intercalation/exfoliation of montmorillonite to prepare nanocomposites), FS can be readily separated into nano-scaled single crystals by simply dispersing FS agglomerates/particles in polar solvents like ethanol, followed by vigorously mechanical stirring [23]. Additionally, the interfacial bonding between the silanized FS nano-scaled single crystal filler and the dental resin matrix can be reasonably strong since there are abundant Si–OH groups on the surface of FS single crystals, and these groups can react with silane coupling agents such as 3-methacryloxypropyltrimethoxy (MPTMS).
The aim of this study was to investigate the reinforcements of Bis-GMA/TEGDMA dental resins (without conventional glass filler) and composites (with conventional glass filler) with various mass fractions of nano FS. The hypothesis was that uniform distribution of the silanized FS nano-scaled single crystals (fibers) into Bis-GMA/TEGDMA dental resins/composites would result in substantial improvements of the mechanical properties. To test this hypothesis, photo-cured Bis-GMA/TEGDMA dental resins/composites filled with various mass fractions of silanized nano FS were systematically fabricated. The mechanical properties (including flexural strength, elastic modulus and work of fracture) were then tested, and Analysis of Variance (ANOVA) was used for the statistical analysis of the acquired data. The morphology of FS and the representative fracture surfaces of its filled Bis-GMA/TEGDMA resins/composites were examined by scanning/transmission electron microscopy (SEM/TEM). In addition, the photo-curing behaviors of Bis-GMA/TEGDMA/FS were monitored in situ by using the real time near infrared technique (RT-NIR) to study the photopolymerization kinetics (vinyl double bond conversion and photopolymerization rate).
MATERIALS AND METHODS
Materials
Bis-GMA, TEGDMA, CQ, 4EDMAB, MPTMS, n-propylamine and (anhydrous) ethanol were purchased from Sigma-Aldrich Co. (Milwaukee, WI) and used without further purification. Purified FS powder (1250 mesh, white/gray in color) was provided by Dalian Global Mineral Co. (Dalian, China). The conventional glass filler used in this study was finely milled 7 % (mass fraction) silanized barium borosilicate glass powder (V-117-2707), provided by Esstech Co. (Essington, PA).
Fabrication
Separation and Silanization of FS
The as-received FS (powder) was first dispersed in ethanol with a mass fraction of 5 %, and the suspension was then vigorously stirred for 4 h at 400 rpm using a Heidolph RZR 50 Heavy Duty Stirrer. Previous research indicated that this process could effectively separate FS into nano-scaled single crystals, but the separated nano FS single crystals (existing in suspension) could reaggregate into agglomerates/particles if the ethanol was removed [23]. The suspension was then transferred into a rotary evaporator with MPTMS (mass fraction of 10 % to FS) and n-propylamine (mass fraction of 5 % to FS). The system was then heated at 90 °C until dry. It is noted that the silanized FS (powder) might exist primarily as agglomerates instead of separated nano-scaled single crystals.
Dispersion of Silanized FS into Dental Matrix
Various amounts of silanized FS (mass fractions ranging from 0 % to 7.5 %) were added into the dental resin system, which consisted of 49.5 % Bis-GMA, 49.5 % TEGDMA (the mass ratio of Bis-GMA/TEGDMA was 50/50), 0.2 % CQ and 0.8 % 4EDMAB. Three dispersion methods were studied to explore the optimal method to distribute the silanized FS as highly separated nano-scaled single crystals into the dental matrices. Additionally, the prepared suspension systems of Bis-GMA/TEGDMA/FS were further mixed with conventional glass fillers (mass fraction of 50 % to Bis-GMA/TEGDMA) to prepare the dental pastes.
Method A: The silanized FS was first dispersed into neat TEDGMA and the suspension was vigorously stirred for 2 h at 400 rpm using the Heidolph RZR 50 Heavy Duty Stirrer. Bis-GMA, CQ and 4EDMAB were then added, and the system was mechanically stirred for another 30 min at 400 rpm.
Method B: The silanized FS was dispersed into the mixture of TEGDMA/ethanol (mass ratio of 10/90), followed by vigorously mechanical stirring for 2 h at 400 rpm. The ethanol was then removed by vacuum evaporation. Finally, Bis-GMA, CQ and 4EDMAB were added and the system was mechanically stirred for another 30 min at 400 rpm.
Method C: The silanized FS was dispersed into 90 % (mass faction) ethanol diluted dental resin system (composition as described above), followed by vigorously mechanical stirring for 2 h at 400 rpm. The ethanol was then removed by vacuum evaporation and the system was mechanically stirred for another 30 min at 400 rpm.
Characterization and Evaluation
Photopolymerization Kinetics
Real time near infrared spectroscopy (RT-NIR) was employed to study the photopolymerization kinetics of Bis-GMA/TEGDMA dental resins containing various mass fractions of the silanized FS. The photopolymerization rate and the degree of vinyl double bond conversion were measured. A Bruker Tensor-27 FT-IR spectrometer equipped with a liquid nitrogen-cooled mercury-cadmium-telluride (MCT) detector was employed to carry out the research. During the RT-NIR measurements, the spectrometer was continuously purged with dry nitrogen gas, and the uncured resin was placed in a U-shaped Teflon mold with both sides covered by glass slides (note that glass is transparent in the NIR region between the wavenumbers of 4000 cm−1 and 6500 cm−1). The length and width of the mold were 4 mm, and the thickness was 2 mm. The glass slides were tightly attached to the mold using small metal clamps. A standard visible light curing unit (Maxima 480), purchased from L. D. Caulk Co. (Milford, DE), was placed directly above the sample holder. The absorption band of vinyl double bond (6100 to 6250 cm−1) were monitored in situ by RT-NIR at ambient condition for 5 min, with 4 scans/spectrum and 8 wavenumber resolution, using series run. In RT-NIR series run, infrared spectra were collected periodically (a 1 s time interval was used in this study) during the photopolymerization of the specimens. The curing light was manually turned on at 8 s and turned off at 38 s.
Mechanical Properties
A standard three-point flexural test (ASTM D 793) with a span of 20 mm was used to fracture the specimens at a crosshead speed of 0.5 mm/min using a computer-controlled universal mechanical testing machine (QTEST™/10, MTS Systems Co., USA). Analysis of Variance (ANOVA), a commercial software (Winks, TexaSoft, Cedar Hill, TX), was used for the statistical analysis of the acquired data. The mechanical testing specimens included both Bis-GMA/TEGDMA dental resins (without conventional glass filler) and composites (with conventional glass filler) containing various mass factions of the silanized FS. Flexural strength, elastic modulus, and work-of-fracture were acquired. Work-of-fracture is the energy required to fracture the specimen and is calculated by dividing the area under the load-displacement curve by the specimen’s cross-sectional area. The dimensions of the Teflon molds for making the three-point flexural testing specimens were 2 mm × 2 mm × 25 mm. The specimens were photo-cured for 1 min on each side, and then carefully removed from the molds. Prior to mechanical testing, the specimens were stored in a humidifier at 37 °C for 24 h. Six specimens were prepared for each measurement, and all four sides of each specimen were carefully hand-polished with 2400 and 4000 grit silicon carbide paper and water coolant in a longitudinal direction.
Morphology
A Zeiss Supra 40VP field-emission scanning electron microscope (SEM) and a Hitachi H-7000 FA transmission electron microscope (TEM) were employed to examine the morphologies of FS and the representative fracture surfaces of the Bis-GMA/TEGDMA dental resins/composites containing various mass fractions of nano FS. Prior to SEM examination, the specimens were sputter-coated with gold to avoid charge accumulation. For TEM examination, the specimen was microtomed at room temperature using a Reichert-Jung Ultracut Microtome and mounted on 200 mesh copper grids.
RESULTS AND DISCUSSION
Separation and Silanization of FS
Naturally occurring FS minerals usually contain some impurities including silica and carbonates. These impurities were removed in the as-received FS (powder). Fig. 1a shows the representative SEM images of the as-received FS. It was evident that the FS agglomerates/particles consisted of nano-scaled single crystals (fibers) with diameters in tens of nanometers and lengths in microns. Some of the FS nano-scaled single crystals stacked together into bundles, and the fibers/bundles then aggregated into agglomerates. The FS agglomerates ranged from submicron to several microns in size.
Figure 1.
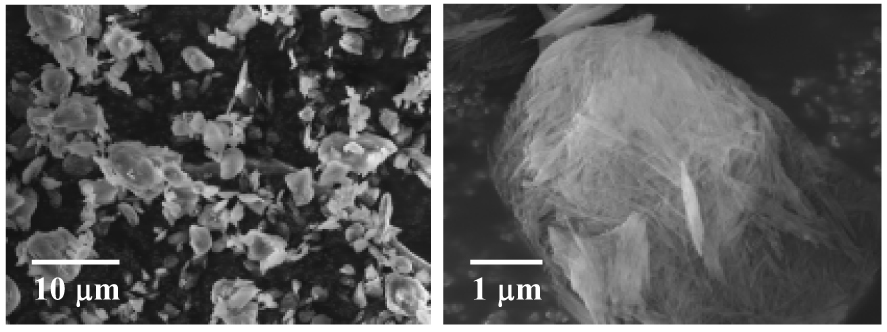
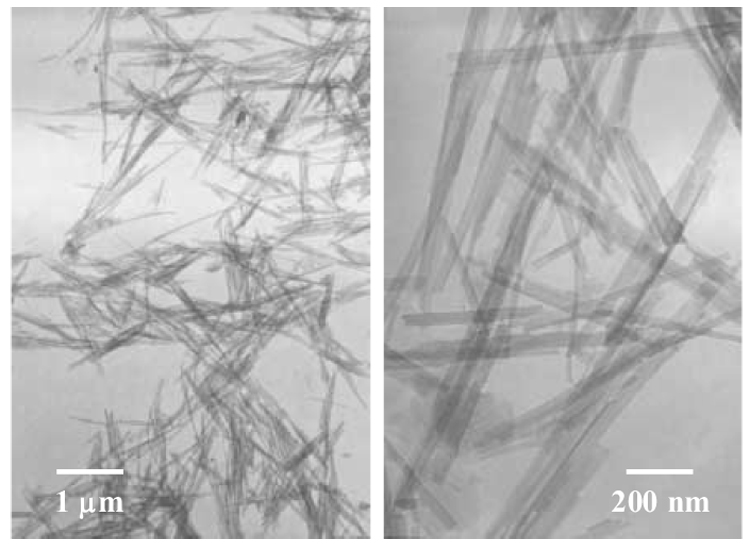
Figure 1a Representative SEM images of the as-received FS powder.
Figure 1b TEM images of separated and silanized FS nano-scaled single crystals.
The as-received FS (powder) was silanized using the procedure as described in the experimental section. Fig. 1b shows the TEM images of the (silanized and separated) FS nano-scaled single crystals (fibers). To prepare the TEM samples, the silanized FS was first dispersed in ethanol with a mass fraction of approximately 1 %, and the suspension was then mechanically stirred for 30 min at 400 rpm using the Heidolph RZR 50 Heavy Duty Stirrer. Subsequently, the carbon-coated TEM grids were dipped into the uniform suspension (with no clearly identifiable solid/precipitate) and quickly removed. After the ethanol in the suspension left on the TEM grids evaporated, the samples were used for TEM examination. It is noted that there might be agglomerates in the silanized FS (powder) although the silanized FS was well separated as nano-scaled single crystals in Fig. 1b (presumably due to the rapid evaporation rate of ethanol in the method to prepare the TEM samples).
Dispersion of Silanized FS into Dental Matrix
To uniformly disperse nano-scaled fillers into dental matrices is always a challenge. Compared to layered silicates such as montmorillonite, FS is considerably easier to separate into nano-scaled single crystals (fibers), as evidenced in Fig. 1b. Nonetheless, it was still a challenge to achieve the high degree of separation and uniform dispersion of the silanized FS in Bis-GMA/TEGDMA. This is because the viscosity of Bis-GMA/TEGDMA is orders of magnitude higher than that of ethanol and the polarity is much lower. Thus, although the silanized FS could be highly separated and uniformly dispersed in ethanol, it might not be able to achieve the same high degree of separation and dispersion in Bis-GMA/TEGDMA.
To explore the optimal method/procedure for dispersing the silanized FS in Bis-GMA/TEGDMA, three methods (as detailed in the experimental section) were studied. Fig. 2 shows the representative fracture surfaces of the photo-cured Bis-GMA/TEGDMA (50/50 mass ratio) dental resins filled with 2.5 % (mass fraction) silanized FS. The letters A, B and C represent the three different dispersion methods. The specimens used for Fig. 2 were also microtomed for TEM examination, and the results are shown in Fig. 3.
Figure 2.
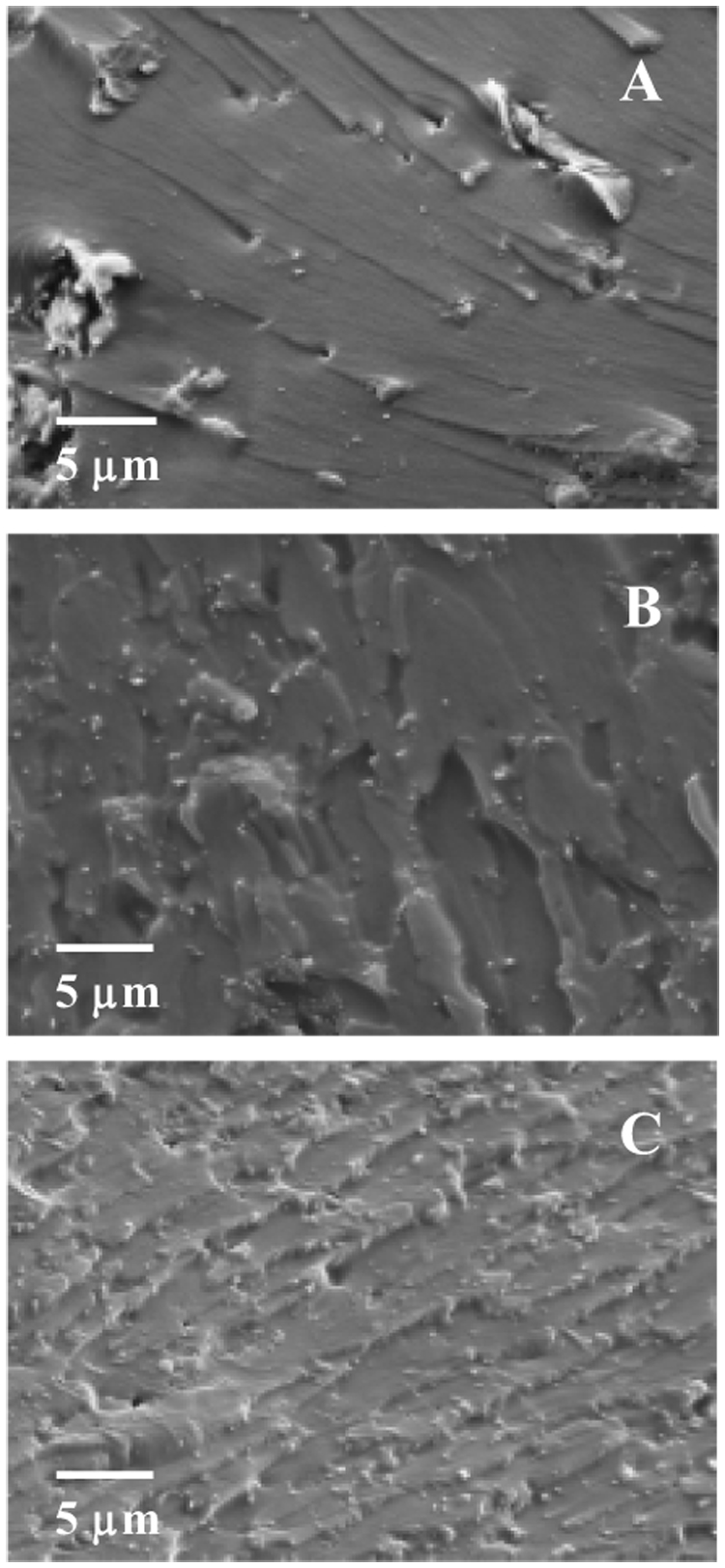
SEM images of representative fracture surfaces of 2.5 % (mass fraction) nano FS filled Bis-GMA/TEGDMA dental resins. The letters A, B and C represent different dispersion methods.
Figure 3.
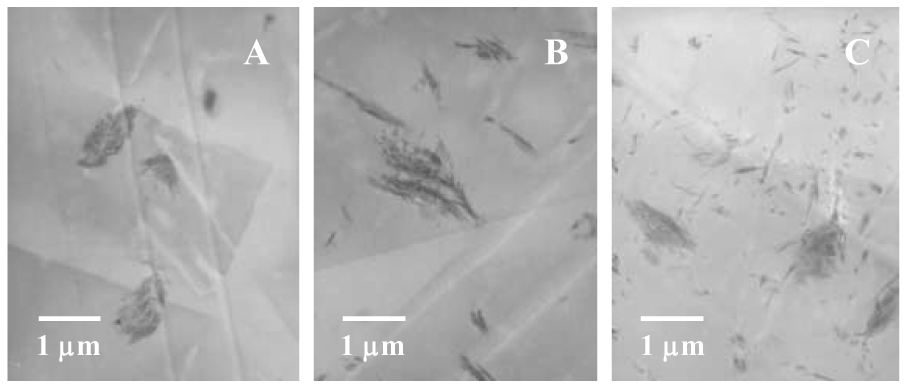
TEM images of 2.5 % (mass fraction) nano FS filled Bis-GMA/TEGDMA dental resins. The letters A, B and C represent different dispersion methods.
As shown in Fig. 2A and Fig. 3A, most of the silanized FS in the Bis-GMA/TEGDMA dental resin prepared by “Method A” existed as agglomerates, and only trace amount of FS seemed to exist as separated single crystals (indicated by tiny bright dots in Fig. 2A, with almost no separated single crystal identified in the TEM image). This suggested that direct dispersion of the silanized FS in dental monomers (even in the diluent monomer of TEGDMA alone) could barely separate FS into nano-scaled single crystals. In comparison, “Method B” and “C” resulted in a much higher degree of separation and dispersion, since many FS nano-scaled single crystals were clearly identifiable in the SEM/TEM images. The results suggested that the use of ethanol during the dispersion process could greatly improve the degree of separation and dispersion of the silanized FS. Nonetheless, there were still agglomerates found in Fig. 3B&C. This suggested that, unlike pure ethanol, the ethanol solutions containing TEGDMA and/or Bis-GMA/TEGDMA might not be able to well separate the silanized FS or, after the removal/evaporation of ethanol, some of the separated FS nano-scaled single crystals might re-aggregate into agglomerates. Further investigations (including studies on different surface treatment/silanization techniques) are being conducted to identify better dispersing methods/procedures.
Compared to “Method B”, “Method C” seemed more effective since more separated FS nano-scaled single crystals (rather than agglomerates) were present in the SEM/TEM images (see Fig. 2B&C and Fig. 3B&C). We also attempted to further improve the separation and dispersion of the silanized FS in the Bis-GMA/TEGDMA/FS system (prepared by “Method C”) by using an ultrasonic probe purchased from the Fisher Scientific Co. (model number 500), but no distinguishable difference was identified (results not shown). “Method C” was thus adopted as the dispersion method to prepare specimens for the photopolymerization kinetics studies and the mechanical property measurements. We note that the agglomerates with loosely-stacked FS nano-scaled single crystals could act as the mechanical weak points (structural defects); and the presence of such agglomerates could significantly reduce the mechanical properties (especially strength) of the nano FS reinforced composites.
Photopolymerization Kinetics
Photopolymerization kinetics was studied to determine if Bis-GMA/TEGDMA dental resins containing nano FS could be appropriately photo-cured. The real time near infrared (RT-NIR) series run technique (as detailed in the experimental section) was adopted for this study. RT-NIR spectroscopic technique measured the conversion of vinyl double bond in dental resins by following the overtone band located from 6100 to 6250 cm−1. The use of this technique to monitor the photo-curing behaviors of dental methacrylate-based resins was demonstrated by Stansbury and Dickens [24]. The RT-NIR series run provided valuable insights into the photopolymerization rate as well as the vinyl double bond conversion.
Fig. 4a shows the RT-NIR spectra of 2.5 % (mass fraction) nano FS filled Bis-GMA/TEGDMA dental resin after the photo-curing for 30 s. From top to bottom, spectra were collected at 8 s (immediately before turning on the curing light), and 12 s, 16 s, 60 s, respectively. The spectra indicated that the vinyl double bond absorbance varied in situ with photopolymerization. After the curing light was turned on (i.e., the photopolymerization was initiated), the absorbance dropped quickly during the initial 10 s. The absorbance remained at approximately the same level for the rest of the period. The absorbance peak in Fig. 4a was integrated to obtain the NIR curing profiles. By using the absorbance of the vinyl double bond before photopolymerization as the reference, the NIR curing profiles were converted to the degrees of vinyl double bond conversion, as depicted in Fig. 4b. The tangent of the curves in Fig. 4b at each time spot represents the photopolymerization rate at that particular time. Fig. 4a and Fig. 4b indicated that all the systems of Bis-GMA/TEGDMA dental resins filled with various mass fractions (up to 7.5 %) of nano FS could be photo-cured in less than 15 s to achieve an approximately 90 % vinyl double bond conversion. The addition of nano FS into Bis-GMA/TEGDMA did not significantly vary either the vinyl double bond conversion or the photopolymerization rate.
Figure 4.
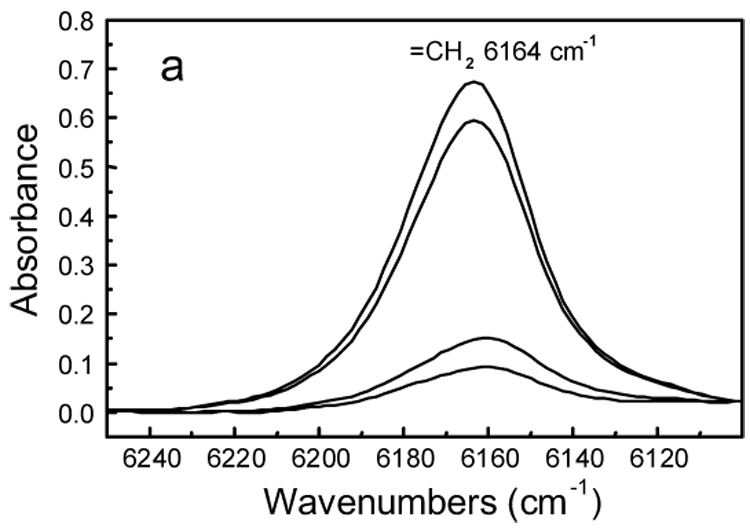
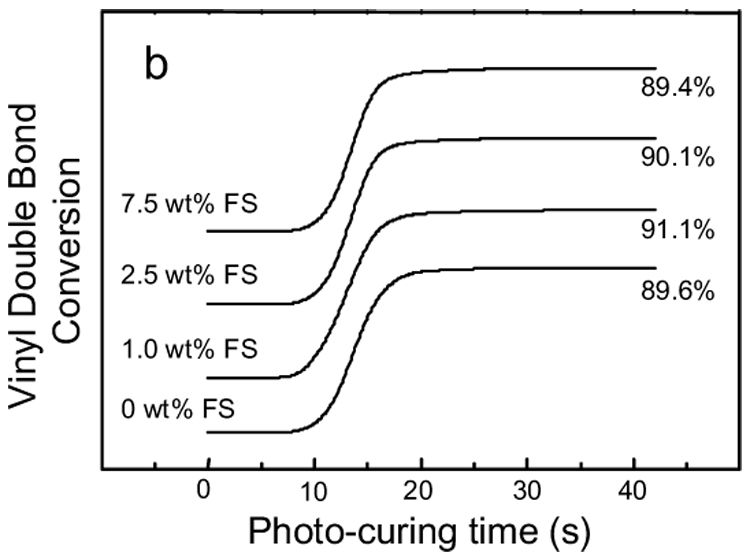
Figure 4a RT-NIR spectra of 2.5 % (mass fraction) nano FS filled Bis-GMA/TEGDMA resin after photo-curing for 30 s. Spectra were collected at different times, from top to bottom, 8 s (immediately before the curing light was turned on), 12 s, 16 s, 60 s, respectively.
Figure 4b RT-NIR photo-curing profiles of Bis-GMA/TEGDMA dental resins filled with various mass fractions of nano FS. The profiles are offset for clarity.
Mechanical Properties
Flexural strength (SF), elastics modulus (EY) and work of fracture (WOF) of Bis-GMA/TEGDMA (50/50 mass ratio) dental resins/composites containing various mass fractions of the silanized nano FS were tested, and the results are showed in Fig. 5. The control samples were the resins/composites without the nano FS. Each datum in the plots provides the mean value of six measurements with the error bar representing one standard deviation.
Figure 5.
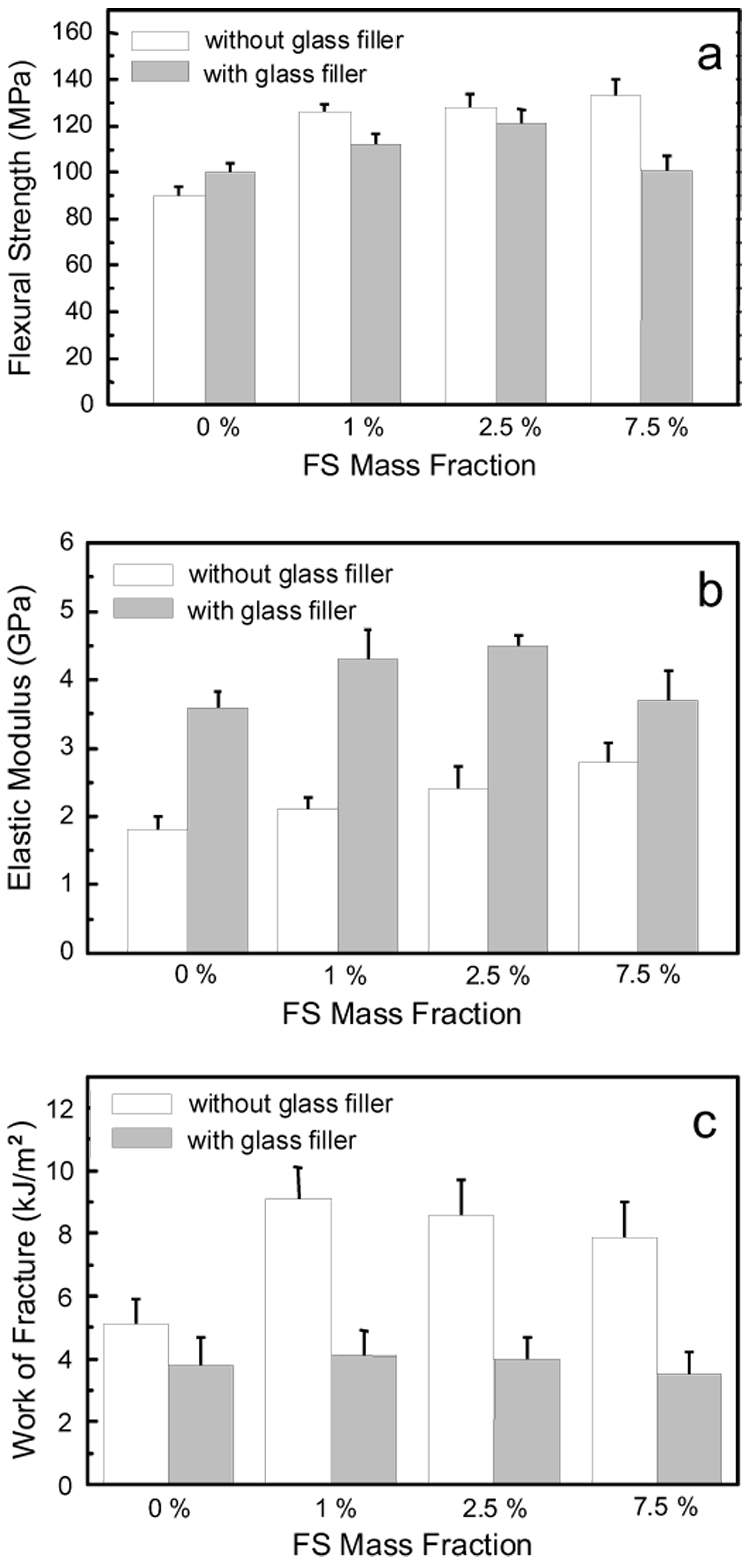
Mechanical properties: (a) flexural strength, (b) elastic modulus, and (c) work of fracture, of Bis-GMA/TEGDMA dental resins/composites filled with various mass fractions of nano FS. Each datum is the mean value of six measurements with error bar representing one standard deviation.
As shown in Fig. 5, the values of SF, EY and WOF were all substantially increased by the impregnation of small mass fractions of the nano FS into the Bis-GMA/TEGDMA dental resins (without conventional glass filler). SF, EY and WOF for the unfilled/neat resin (mean ± standard deviation, n =6) were (90 ± 4) MPa, (1.8 ± 0.2) GPa and (5.1 ± 0.8) kJ/m², respectively. For the resin filled with 1.0 % (mass fraction) nano FS, SF, EY and WOF were increased to (126 ± 4) MPa, (2.1 ± 0.2) GPa and (9.1 ± 1.0) kJ/m², respectively. Thus, the flexural strength was improved by 40 %, the elastic modulus was improved by 16.7 %, and the work of fracture was improved by 78.4 %. However, increasing the mass fractions of the nano FS did not further improve the mechanical properties. For the resin filled with 2.5 % (mass fraction) nano FS, the measured SF, EY and WOF values were, respectively, (128 ± 6) MPa, (2.4 ± 0.4) GPa and (8.6 ± 1.1) kJ/m². For the resin filled with 7.5 % (mass fraction) nano FS, the respective SF EY and WOF values were (133 ± 7) MPa, (2.8 ± 0.3) GPa and (7.9 ± 1.1) kJ/m². These results indicated that the impregnation of the silanized nano FS into Bis-GMA/TEGDMA dental resin could significantly improve the mechanical properties. However, the nano FS has to be highly separated into nano-scaled single crystals and uniformly distributed in the dental resin. An effective reinforcement would not be achieved if the nano FS existed primarily as agglomerates/particles which could actually act as the mechanical weak points (structural defects).
Representative fracture surfaces of the neat and nano FS reinforced Bis-GMA/TEGDMA dental resins are shown in Fig. 6a&b. The neat resin (Fig. 6a) fractured as a typical ductile resin, and the fracture surface was smooth with oriented fracture lines resulted from the extension of crazings initiated by the stress concentration points. On the other hand, the fracture surface of the nano FS reinforced resin (Fig. 6b) was rough with no clearly identifiable fracture lines. The results suggested that the presence of nano FS could deflect the micro-crack and effectively increase the resistance to the applied force. When the crack finally broke away from the nano FS, a rough fracture surface was created, suggesting energy consumption during breaking. However, some voids/holes were also observed on the fracture surface of the nano FS reinforced resin. These voids/holes were formed by the failure/fallout of the FS agglomerates/particles. As discussed above, the agglomerates could act as structural defects and significantly weaken the filled resin. Taken together, the impregnation of the silanized nano FS into Bis-GMA/TEGDMA dental resin may result in two opposite effects: the reinforcing effect due to the highly separated and well distributed nano FS single crystals, and the weakening effect due to the formation of FS agglomerates/particles. If the nano FS could be uniformly distributed in the dental matrices as highly separated nano-scaled single crystals, we envision that the mechanical properties of the resulting composites would be significantly higher than those of the currently developed composites.
Figure 6.
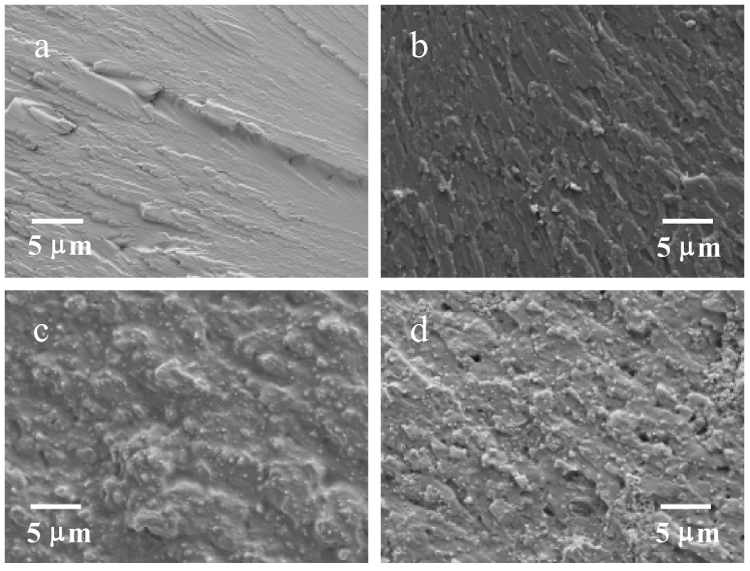
Representative fracture surfaces of three-point flexural specimens: (a) neat/unfilled Bis-GMA/TEGDMA, (b) Bis-GMA/TEGDMA filled with 2.5 % (mass faction) nano FS, (c) Bis-GMA/TEGDMA filled with 50 % (mass faction) glass filler, (d) Bis-GMA/TEGDMA filled with 2.5 % (mass faction) nano FS and 50 % (mass faction) glass filler.
The mechanical properties of the Bis-GMA/TEGDMA dental composites (with conventional glass filler) containing small mass fractions of the silanized nano FS showed a similar trend of improvement. Both flexural strengths and elastics moduli of the composites filled with 1 % and 2.5 % (mass fractions) of the nano FS were higher than those of the control sample. Nonetheless, the composites filled with 7.5 % (mass faction) of the silanized nano FS had SF and EY values of (101 ± 6) MPa and (3.7 ± 0.4) GPa, which were statistically the same as those of the control sample (one way ANOVA, P > 0.05). For the work of fracture values, one way ANOVA indicated no statistical difference between the control sample and the samples filled with 1 %, 2.5 % and 7.5 % (mass factions) nano FS. These results further supported the previous conclusion that the impregnation of the silanized nano FS into Bis-GMA/TEGDMA dental matrices could result in both reinforcing and weakening effects, depending upon the degree of separation and uniformity of distribution of the nano FS. Unlike that in resins, the nano FS seemed more likely to aggregate into agglomerates and the sizes of the agglomerates seemed larger in composites, as indicated by the SEM images of the representative fracture surfaces (Fig. 6). The detailed reasons for this difference are still under investigation.
CONCLUSIONS
The aim of this study was to investigate the reinforcement of Bis-GMA/TEGDMA dental resins/composites with various mass fractions of nano FS. FS is a natural mineral composed of nano-scaled silicate single crystals (fibers) with diameters in tens of nanometers and lengths in microns. Impregnation of small mass fractions (1 % and 2.5 %) of the silanized nano FS into Bis-GMA/TEGDMA (50/50 mass ratio) dental resins/composites improved the mechanical properties substantially. Larger mass fraction of impregnation (7.5 %), however, did not further improve the mechanical properties (one way ANOVA, P > 0.05), and may even have reduced the mechanical properties. Impregnation of the nano FS into Bis-GMA/TEGDMA dental resins/composites could result in two opposite effects: the reinforcing effect due to the highly separated and uniformly distributed nano FS single crystals, and the weakening effect due to the formation of FS agglomerates/particles. The simultaneous accomplishment of a high degree separation and uniform dispersion of nano FS in dental resins/composites is still under investigations. Nonetheless, since the impregnation of small mass fractions of the nano FS into the dental resins/composites could effectively improve the mechanical properties, nano FS may have significant value to be used as the reinforcing nanofiller for dental composites.
ACKNOWLEDGEMENT
This research was supported by the National Institute of Dental and Craniofacial Research (R03 DE16042), and by the “Center for Accelerated Applications at the Nanoscale (CAAN)” and the “BioMedical Engineering (BME) Program” at the South Dakota School of Mines and Technology (SDSM&T). The authors are also grateful to Esstech Co. for providing the silanized barium borosilicate glass filler.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bowen RL. Dental filling material comprising vinyl-silane treated fused silica and a binder consisting of the reaction product of bisphenol and glycidyl methacrylate. 3,066,112. U.S. Pa. 1962
- 2.Bowen RL. Properties of a silica-reinforced polymer for dental restoration. J Am Dent Assoc. 1963;66:57–64. doi: 10.14219/jada.archive.1963.0010. [DOI] [PubMed] [Google Scholar]
- 3.Antonucci JM, Stansbury JW. Molecular designed dental polymer. In: Arshady R, editor. Desk reference of functional polymers: synthesis and application. American Chemical Society Publication; 1997. pp. 719–738. [Google Scholar]
- 4.Reed BB, Choi K, Dickens SH, Stansbury JW. Effect of resin composition of kinetics of dimethacrylate photopolymerization. Polym Prep. 1997;38(2):108–109. [Google Scholar]
- 5.Leinfelder KF, Sluder TB, Santos JFF, Wall JT. Five-year clinical evaluation of anterior and posterior restorations of composite resin. Oper Dent. 1980;12:52–78. [Google Scholar]
- 6.Lacy AM. A critical look at posterior composite restorations. J Am Dent Assoc. 1987;114:357–362. doi: 10.14219/jada.archive.1987.0077. [DOI] [PubMed] [Google Scholar]
- 7.Jordan RE, Suzuki M. Posterior composite restorations. J Am Dent Assoc. 1991;122:31–37. doi: 10.14219/jada.archive.1991.0321. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien WJ. Dental Materials and Their Selection. 3rd Edition. Quintessence Publishing Co, Inc.; 2002. [Google Scholar]
- 9.Corbin SB, Kohn WG. The benefits and risks of dental amalgam. J Am Dent Assoc. 1994;125:381–388. doi: 10.14219/jada.archive.1994.0055. [DOI] [PubMed] [Google Scholar]
- 10.Berry TG, Nicholson J, Troendle K. Almost two centuries with amalgam, where are we today? J Am Dent Assoc. 1994;125:392–399. doi: 10.14219/jada.archive.1994.0053. [DOI] [PubMed] [Google Scholar]
- 11.Kusy RP, Leinfelder KF. Pattern of wear in posterior composite restorations. J Dent Res. 1977;56(5):544–544. doi: 10.1177/00220345770560052101. [DOI] [PubMed] [Google Scholar]
- 12.Abell AK, Leinfelder KF, Turner DT. Microscopic observations of the wear of a tooth restorative composite in vivo. J Biomed Mat Res. 1983;17(3):501–507. doi: 10.1002/jbm.820170309. [DOI] [PubMed] [Google Scholar]
- 13.Grave AMH, Chandler HD, Wolfaardt JF. Denture base acrylic reinforced with high modulus fibre. Dent Mater. 1985;1:185–187. doi: 10.1016/s0109-5641(85)80015-4. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg AJ, Burstone CJ. The use of continuous fiber reinforcement in dentistry. Dent Mater. 1992;8:197–202. doi: 10.1016/0109-5641(92)90083-o. [DOI] [PubMed] [Google Scholar]
- 15.Giordano R. Fiber reinforced composite resin systems. Gen Dent. 2000;48(3):244–249. [PubMed] [Google Scholar]
- 16.Behr M. Comparison of three types of fiber-reinforced composite molar crowns on their fracture resistance and marginal adaptation. J Dent. 2001;29(3):187–196. doi: 10.1016/s0300-5712(01)00007-0. [DOI] [PubMed] [Google Scholar]
- 17.Xu HHK, Martin TA, Antonucci JM, Eichmiller FC. Ceramic whisker reinforcement of dental composite resins. J Dent Res. 1999;78(2):706–712. doi: 10.1177/00220345990780021101. [DOI] [PubMed] [Google Scholar]
- 18.Xu HHK. Dental composite resins containing silica-fused ceramic single-crystalline whiskers with various filler levels. J Dent Res. 1999;78:1304–1311. doi: 10.1177/00220345990780070401. [DOI] [PubMed] [Google Scholar]
- 19.Xu HHK. Whisker-reinforced heat-cured dental resin composites: Effects of filler level and heat-cure temperature and time. J Dent Res. 2000;79:1392–1397. doi: 10.1177/00220345000790060701. [DOI] [PubMed] [Google Scholar]
- 20.Xu HHK, Schumacher GE, Eichmiller FC, Antonucci JM. Strengthening composite resin restorations with ceramic whisker reinforcement. Pract Periodont Aesthet Dent. 2000;12:111–116. [PubMed] [Google Scholar]
- 21.Xu HHK, Quinn JB, Smith DT, Giuseppetti AA, Eichmiller FC. Effects of different whiskers on the reinforcement of dental resin composites. Dent Mater. 2003;19:359–367. doi: 10.1016/s0109-5641(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 22.Tian M, Qu C, Feng Y, Zhang L. Structure and properties of fibrillar silicate/SBR composites by blend process. J Mater Sci. 2003;38:4917–4924. [Google Scholar]
- 23.Tian M, Liang W, Rao G, Zhang L, Guo C. Surface modification of fibrillar silicate and its reinforcing mechanism on FS/rubber composites. Comp Sci Tech. 2005;65:1129–1138. [Google Scholar]
- 24.Stansbury JW, Dickens SH. Determination of double bond conversion in dental resins by near infrared spectroscopy. Dent Mater. 2001;17:71–79. doi: 10.1016/s0109-5641(00)00062-2. [DOI] [PubMed] [Google Scholar]


