Abstract
Rationale
Based upon extensive studies in the rat, it has been suggested that stimulus control by LSD is mediated by 5-HT2A receptors, with serotonergic receptors of the 5-HT1A and 5-HT2C subtypes playing modulatory roles. In genetically modified mice lacking the serotonin transporter (SERT), 5-HT2A receptor density is decreased and, at a functional level, the head twitch response following the administration of DOI, an index of activation of 5-HT2A receptors, is reduced. Taken together, these studies led us to hypothesize that the efficacy of LSD in establishing stimulus control is diminished or abolished in mice lacking the serotonin transporter.
Objective
Determine the efficacy of LSD for establishing stimulus control in SERT knockout (KO) mice.
Methods
SERT KO mice and wildtype (WT) littermates were trained in a visual discrimination on a progressive fixed ratio (FR) water-reinforced task and subsequently trained on a FR10 schedule with LSD (0.17 or 0.30 mg/kg) or vehicle. To control for general deficiencies in drug discrimination, mice were trained with pentobarbital (15 or 30 mg/kg) or vehicle.
Results
The visual stimulus exerted control in both genotypes. LSD induced stimulus control in 90% of WT mice but only 31% of SERT KO mice. In contrast, pentobarbital induced stimulus control in 80% of WT mice and 54% of knockout mice.
Conclusions
Although SERT KO mice exhibited stimulus control by the non-serotonergic drug, pentobarbital, the efficacy of LSD in these animals was markedly decreased, suggesting that reduced density of 5-HT1A and/or 5-HT2A receptors underlies the absence of stimulus control by LSD.
Keywords: lysergic acid diethylamide (LSD), pentobarbital, drug discrimination, mouse, serotonin transporter, SERT, knockout
1. Introduction
The development of drug-induced stimulus control as a tool to investigate behaviorally-active drugs (Harris and Balster, 1971; Overton, 1971; Winter 1974, 1978) has enabled pharmacological characterization of a variety of psychoactive drugs including the indoleamine hallucinogen, lysergic acid diethylamide (LSD) (Cunningham and Appel, 1987; Glennon et al., 1982; Hirschhorn and Winter, 1971). Although drug-induced stimulus control has been established in humans (for reviews see Brauer et al., 1997; Dykstra et al., 1997; Kamien et al., 1993) and in several animal species, the majority of studies have employed the rat (Stolerman and Kamien, 2004). However, the stimulus properties of a number of drugs have been examined in mice as well, including stimulants (Middaugh et al., 1998; Snoddy and Tessel, 1983; Varvel et al., 1999), depressants (Borlongan and Watanabe, 1997; Grant et al., 1991; Rees and Balster, 1988), non-competitive NMDA antagonists (Geter-Douglass and Witkin, 1999; Middaugh et al., 1988), monoamine reuptake inhibitors (Gommans et al., 1998; Snoddy and Tessel, 1983), and the atypical antipsychotic agent, clozapine (Philibin et al., 2005).
Accumulating evidence strongly suggests that hallucinogenic agents exert their stimulus control in rats primarily by activation of the 5-HT2A receptor, with the 5-HT2C and 5-HT1A receptors having modulatory roles (Eckler et al., 2004; Fiorella et al., 1995a,b; Schreiber et al., 1994; Winter et al., 2000). In the first study of stimulus control in mice by a hallucinogen, Smith et al. (2003) found that the 5-HT2A and 5-HT2C receptors played a major and minor role, respectively, in the discriminative effects of the phenethylamine hallucinogen, 2,5-dimethoxy-4-iodo-amphetamine (DOI; Shulgin and Shulgin, 1991). Subsequent investigations utilizing mice characterized the stimulus control established by LSD (Benneyworth et al., 2005; Winter et al., 2005). Both studies noted differences from previous findings in the rat. Benneyworth et al. (2005) concluded that the stimulus effects of LSD have elements mediated by both 5-HT2A and 5-HT1A receptors, as indicated by only partial antagonism by M100907, a 5-HT2A-selective ligand (Johnson et al., 1996) previously shown to completely block LSD in rats (Winter et al., 2004) and partial antagonism by WAY-100635, an agent selective for 5-HT1A receptors (Forster et al., 1995). Our laboratory (Winter et al., 2005) found that M100907 also produced only partial antagonism and, either alone or in combination, suppressed rates of responding, an effect not seen in the rat (Winter et al., 2004). Thus, certain non-5-HT2A-mediated elements in the compound stimulus induced by LSD might be more salient in the mouse than in the rat.
With the advent of techniques to genetically modify mice, this species provides the advantage that a particular gene can be deleted to produce knockout (KO) mice (Bucan and Abel, 2002; Gingrich and Hen, 2001; Seong et al., 2002). Generation of the serotonin transporter (SERT) KO mouse (Bengel et al., 1998) has facilitated assessments of resulting changes in the serotonergic system. The raphe nuclei are the major source of serotonergic cell bodies in brain (Dahlström and Fuxe, 1964) and send projections to most regions of the midbrain and forebrain (Anden et al., 1965). In homozygous SERT KO mice, the dorsal raphe nucleus exhibits a marked decrease in both spontaneous firing rate (Gobbi et al., 2001; Lira et al., 2003) and the number of 5-HT neurons (Lira et al., 2003; Rumajogee et al., 2004), relative to littermate wildtype (WT) mice. The density of 5-HT1A autoreceptors in SERT knockouts is decreased in the dorsal raphe nucleus, amygdala, hypothalamus, and septum, but increased in the hippocampus (Fabre et al., 2000; Gobbi et al., 2001; Li et al., 2000), whereas 5-HT2C receptors are upregulated in the choroid plexus and amygdala (Li et al., 2003). As noted above, the 5-HT2A receptor has a central role in stimulus control by indoleamine and phenethylamine hallucinogens. In SERT KO mice, Rioux et al. (1999) found highly significant reductions in the number of 5-HT2A receptors in the claustrum (−42%), external striatum (−28%), and cerebral cortex (−43%). The authors noted that these effects were not the same as those following chronic treatment with SSRIs and emphasized the complexity of regulatory mechanisms involving 5-HT2A receptors. This complexity was also illustrated in a study by Li et al. (2003) that further examined 5-HT2A receptors in SERT knockout mice. The changes they observed were brain-region specific but, consistent with Rioux et al. (1999), fewer 5-HT2A receptors were found in claustrum and ventral striatum. In the most detailed study to date in which phospholipase A-2 activation was used as a marker for 5-HT2A receptor stimulation by the 5-HT2A/2C agonist DOI, Qu et al. (2005) observed phospholipase activation in WT mice in all brain regions studied, whereas no activation was seen in SERT KO mice. Furthermore, following DOI treatment, this genotype has demonstrated a significant reduction in the head twitch response, an index of agonist stimulation of the 5-HT2A receptor (Schreiber et al., 1995; Wettstein et al., 1999). Taken together, these serotonin-related alterations led us to hypothesize that the efficacy of LSD in establishing stimulus control would be reduced or abolished in SERT knockout mice.
This study investigated stimulus control by the 5-HT receptor agonist LSD in C57BL/6 mice homozygous for the serotonin transporter null mutation (SERT−/−) and littermate controls (SERT+/+). Initially, we assessed responding to a visual stimulus for reinforcement in order to determine whether SERT KO mice are capable of performing operant behavior. The same groups of mice were evaluated thereafter for the discriminative stimulus effects of 0.17 mg/kg and 0.30 mg/kg of LSD. To control for general impairment in drug discrimination by SERT KO mice, the two groups were trained subsequently with pentobarbital, a non-serotonergic drug known to induce stimulus control in mice.
2. Materials and Methods
2.1. Subjects
Mice, weighing at least 20 g at the start of training, were housed individually and maintained in a temperature- and humidity-controlled vivarium under a constant 12-h light/dark cycle (6:00 AM to 6:00 PM). All experiments were conducted in a separate room during the light phase of the cycle. Animals had unrestricted access to food. Water was freely available until 1 week before apparatus training, when access was limited to 20 min per day. With the onset of training, water was provided for 20 min immediately after completion of the training session, and mice were maintained on restricted water access throughout all types of training. Behavioral procedures were conducted in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the State University of New York at Buffalo.
SERT KO mice were generated from a breeding colony of heterozygous mutants maintained at the State University of New York at Buffalo. The original heterozygous breeders acquired from Dr. Xiaoxi Zhuang at the University of Chicago were generated by standard homologous recombination techniques as described by Zhuang et al. (2005). The original heterozygous mutants were created on a 129/SvJ genetic background and backcrossed to C57BL/6 mice for four generations. Adolescent mice (3–4 weeks old) generated in the breeding colony were genotyped by PCR amplification using ear tissue. Subjects were littermate mice expressing zero (SERT−/−) or two (SERT+/+) intact copies of the SERT gene.
2.2. Apparatus
Sixteen small animal test chambers (Med Associates Inc., St. Albans, VT, ENV-008) were used for the experiments; each chamber was equipped with a house light and an exhaust fan and was housed in a larger lightproof, sound-attenuating box. The chamber contained two snout-poke modules (MED Associates, ENV-3BM) mounted on opposite sides of one wall. Water reinforcement was delivered into the bottom of either the left or right snout-poke hole. Sessions were managed by a micro-computer equipped with operant control software (MED Associates, MED-PC® MedState Notation, Version IV).
2.3. Training
Apparatus Training
A modified non-resetting fixed ratio 1 (FR1) schedule of reinforcement was employed for the first four sessions of apparatus training. The reinforced side was signaled by a light continuously lit for 30 s. If a mouse failed to poke its snout into the cued hole within 30 s, the light flashed on-and-off for 10 s to attract attention; no snout poke into the hole within 10 s resulted in automatic water delivery therein and re-establishment of the signaling cycle. However, if the mouse poked into the cued hole at any time, water was immediately delivered and the signaling cycle was reset. Snout-poke responding into the non-cued hole had no water consequence. In the next two sessions, a standard non-resetting FR1 schedule was used, i.e., continuous light only and no automatic reinforcement. For the final two sessions of apparatus training, mice were reinforced on FR2 as a result of having met the criterion for advancement to the next higher FR schedule, i.e., 20 or more reinforcements in each of two successive sessions. Session duration of apparatus training was 30 min. For all types of training, daily training occurred during one session, and the reinforced side of the apparatus was counterbalanced within each genotype at all sessions and was alternated every other session for each subject.
Light Stimulus Discrimination Training
Discrimination training of a visual stimulus began at a non-resetting FR4 schedule. The continuous light stimulus signaled the side for reinforcement, and water was delivered after completion of four pokes into the cued hole. The session ended when 100 reinforcements were delivered or after 15 min, whichever occurred first. The FR increments for light stimulus training were 4 to 6, 6 to 8, 8 to 10, 10 to 13, 13 to 16, 16 to 19, and 19 to 20. To advance to the next higher FR schedule, mice were required to achieve the advancement criterion. At any stage of training, a mouse that earned fewer than 10 reinforcements in a session was moved back to the lower schedule on the following day and required to achieve the advancement criterion in order to return to the higher schedule. The FR20 training schedule was chosen because early training of mice on FR20 is preferred over FR10 (Balster and Moser, 1987).
Light-induced stimulus control was considered present when, in five consecutive sessions, 83% or more of all snout-poke responses prior to delivery of the first reinforcer were into the appropriate hole. The extent of visual stimulus control is expressed in terms of (a) the proportion of mice which achieved this criterion for stimulus control at a given session and (b) the percentage of stimulus-appropriate responding in all sessions. Response rate for each session was calculated by dividing the total number of responses into both holes by the elapsed time until completion of the scheduled number of responses into either hole. Response rates are expressed as the number of responses per minute. Data for response rate, stimulus-appropriate responding, and stimulus-control criterion are comprised of responses emitted before delivery of the first reinforcement.
LSD Discrimination Training
To reduce injection-related stress effects on performance during LSD discrimination training, mice were injected subcutaneously (SC) with saline in the latter half of the light stimulus experiment after completing daily training sessions. Following light stimulus training, the same mice were trained to discriminate LSD from saline vehicle. Training was conducted at FR10 and the session ended after delivery of 100 reinforcements or 15 min, whichever occurred first. The two treatments (LSD and vehicle) for both genotypes were alternated every other session. SC treatment was administered 15 min prior to training. No light was used to signal the reinforcing hole. Discrimination training was started with a dose of 0.17 mg/kg of LSD. After establishment of stimulus control by 0.17 mg/kg in either genotype, a higher dose of 0.30 mg/kg of LSD was utilized in the latter part of the experiment. LSD-induced stimulus control was considered present when, in five consecutive sessions, 83% or more of all snout-poke responses prior to delivery of the first reinforcer were into the treatment-appropriate hole. The extent of stimulus control is expressed in terms of (a) the proportion of mice that achieved this stimulus-control criterion at a given session and (b) the percentage of LSD-appropriate responding in all sessions. Response rate was calculated for each session by dividing the total number of responses into both holes by the elapsed time until completion of 10 responses into either hole. Response rates are expressed as the number of responses per minute. To determine the number of training sessions for administration of 0.17 mg/kg of LSD, on a daily basis we tracked the progress of each mouse toward the criterion for stimulus control, calculated for each genotype the proportion of mice which met criterion, and compared genotypes on this parameter. When a majority of mice in one genotype met criterion, the dose of LSD was increased to 0.30 mg/kg if the review of prior performance of subjects in the other genotype suggested that additional subjects were unlikely to achieve criterion at 0.17 mg/kg.
Pentobarbital Discrimination Training
After completion of LSD training, the same mice underwent pentobarbital discrimination training on FR10 with an initial dose of 15 mg/kg of pentobarbital and were trained subsequently with a dose of 30 mg/kg of pentobarbital. The procedure, as well as the determination of stimulus control, response rate, and number of training sessions were the same as those described for LSD discrimination training.
2.4. Genotyping
Subjects were genotyped by the PCR-based procedure. Briefly, individual ear tissue was incubated at 55°C for a total of 60 min in 20μl lysis buffer (50mM Tris-HCl, pH 8.0, 0.1M EDTA, 0.5% (w/v) SDS, 1.0mg/ml Proteinase K). Samples were mixed vigorously by vortex after 30 min of incubation. Following digestion, DNase/Rnase-free water (180μl) was added, and the samples were boiled for 5 min. For PCR, 1 or 2μl of the sample was added to 20μl of PCR reaction mixture containing 1.75 units of Platinum Taq DNA polymerase (InVitrogen), 1X PCR Buffer (supplied with the Taq), 1.5mM MgCl2, 0.2mM of each dNTP, and 0.2μM of each primer (SERT knockout: GAACGAACCTGGTCGAAATCAG and CATCCGCACCACTGACTGACCA; wildtype: GGCACTAACCTCCACCATTCTG and GAACGAACCTGGTCGAAATCAG). Amplification was carried out under mineral oil using the following thermocycler parameters: 94°C for 60s, 34 cycles of 94°C for 30s, 61°C for 30s, 72°C for 30s, and 72°C for 10min. PCR products were resolved on 1.5% agarose gel and visualized with ethidium bromide using a BioRad GelDoc and the Quantity One (v 4.3.1) imaging system.
2.5. Drugs
Lysergic acid diethylamide ((+)-LSD (+)-tartrate (2:1)) was generously provided by the National Institute on Drug Abuse, Rockville, MD, USA. Pentobarbital sodium salt was purchased from Sigma-Aldrich. All drugs were dissolved in 0.9% saline solution and injected at a volume of 5.0 ml/kg bodyweight. The SC route was employed for all drugs.
2.6. Statistical Analysis
Data were assessed for statistical significance utilizing individual applications of Fisher’s Exact Test and paired or independent Student’s t-test. Differences were considered statistically significant if the probability of their having arisen by chance was <0.05. All statistical analyses were performed using SigmaStat 3.1 for Windows™ (Jandel Scientific Software, San Rafael, CA).
3. Results
3.1. Light-induced Stimulus Control
SERT KO mice and control WT mice were trained to perform a visual discrimination for reinforcement. During advancement to each of the next higher FR schedules (i.e., 2, 4, 6, 8, 10, 13, 16, 19, 20), both genotypes advanced on the same session, with the exception that six SERT KO mice and two WT mice were delayed by one session. These mice became delayed at various FR schedules: SERT KO mice (two mice at FR4, one at FR8, three at FR16) and WT mice (one at FR16, one at FR19). A total of 66 training sessions were conducted.
With respect to the proportion of mice in each genotype that achieved the criterion for light-induced stimulus control at a given session, 85% of the SERT KO mice (11 of 13) met criterion at a mean of 30 sessions (range = 15–57). In contrast, 100% of the WT mice (10 of 10) reached criterion at a mean of 30 sessions (range = 15–59). However, there was no significant difference between genotypes for the proportion of mice that achieved criterion [Fisher’s Exact Test, NS]. Both groups demonstrated similar percentages of responding on the cued and non-cued sides during most of the experiment (Fig. 1, upper panel). Rates of responding across the experiment were not different between SERT KO and control mice (Fig. 1, lower panel).
Figure 1.
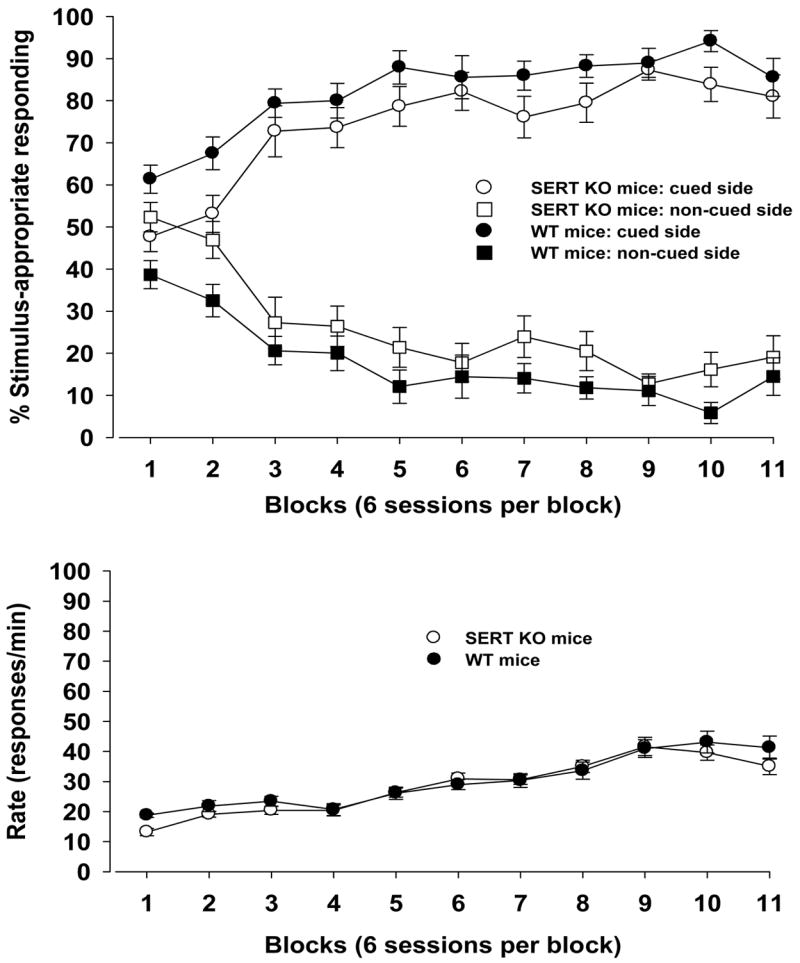
Light-induced stimulus control and response rate for homozygous serotonin transporter (SERT) knockout (KO) mice (n = 13) and wildtype (WT) mice (n = 10). Light stimulus training was started on a fixed ratio 4 (FR4) schedule of reinforcement and progressed as follows: FR4 → FR6 → FR8 → FR10 → FR13 → FR16 → FR19 → FR20. Blocks of data and the corresponding FR schedules (in parentheses) are: block 1 (FRs 4, 6, 8), blocks 2–7 (FR10), block 8 (FRs 10, 13, 16), block 9 (FRs 19, 20), and blocks 10–11 (FR20). Standard errors of the mean are indicated. Ordinate: upper panel: percentage stimulus-appropriate responding; lower panel: rate expressed as responses per min. Abscissa: successive data blocks of 66 sessions. Each data block represents the mean performance in 6 sessions.
3.2. Stimulus Control by LSD
In our laboratory, a dose of 0.17 mg/kg of LSD previously established marginal stimulus control in WT mice without suppressing response rate (Winter et al., 2005). Thus, the same two groups of mice were trained subsequently to discriminate LSD from saline vehicle, starting with 0.17 mg/kg of LSD. After completion of 40 training sessions with either 0.17 mg/kg of LSD or saline, the dose was increased to 0.30 mg/kg and training continued for an additional 25 sessions.
In total, 31% of the SERT KO mice at a given session achieved the criterion for stimulus control by LSD. At the dose of 0.17 mg/kg, 3 of 13 mice met criterion at a mean of 19 sessions (range = 8–28). An increase in dose to 0.30 mg/kg resulted in 1 of the 10 remaining SERT KO mice reaching criterion at 18 sessions. In contrast, a total of 90% of the WT mice (9 of 10) met criterion. At the dose of 0.17 mg/kg of LSD, 6 of 10 mice met criterion at a mean of 16 sessions (range = 5–37). With an increase in dose to 0.30 mg/kg, 3 of the 4 remaining WT mice met criterion at a mean of 9 sessions (range = 5–15). A genotype comparison of the total proportion of mice meeting criterion indicated that more wildtype mice than SERT knockout mice attained the criterion for LSD-induced stimulus control [Fisher’s Exact Test, p=0.01].
The differential control of LSD in SERT KO mice and WT mice continued throughout the experiment, as indicated by the percentages of LSD-appropriate responding in all sessions. As shown in the upper panel of Figure 2, stimulus control in the SERT KO group was not evident at 0.17 mg/kg of LSD or when increasing the dose to 0.30 mg/kg (indicated by the arrow). In contrast, the WT mice discriminated 0.17 mg/kg of LSD from saline early in the experiment, and stimulus control continued at this low dose as well as with the treatment of 0.30 mg/kg. Regarding the rate of responding, response rates were similar between genotypes and at both doses of LSD (Fig. 2, lower panel).
Figure 2.
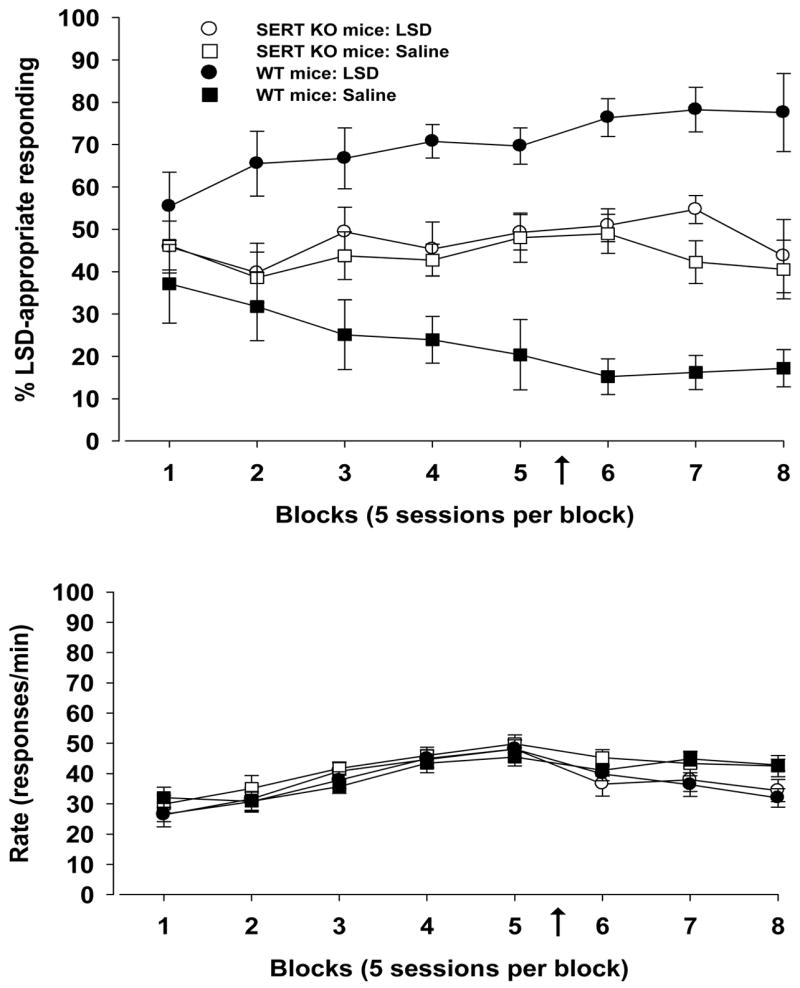
LSD-induced stimulus control and response rate for mice at two doses of LSD. Training of homozygous SERT KO mice (n = 13) and WT mice (n = 10) to discriminate LSD from saline vehicle began with 0.17 mg/kg (blocks 1–5) and continued with 0.30 mg/kg of LSD (blocks 6–8). The change in dose is indicated by the arrow. Standard errors of the mean are indicated. Ordinate: upper panel: percentage LSD-appropriate responding; lower panel: rate expressed as responses per min. Abscissa: successive data blocks of 65 sessions. Each data block represents the mean performance in 5 sessions, with the exceptions that blocks 1 are the means of 2 sessions of a given treatment, blocks 2 are the means of 3 sessions of the treatment, and blocks 8 are the means of 2 sessions of LSD and 3 sessions of saline.
3.3. Stimulus Control by Pentobarbital
To control for the possibility of a general defect in the ability of SERT KO mice to acquire drug-induced stimulus control, the same two groups of mice were trained with pentobarbital, a non-serotonergic drug previously shown to induce stimulus control in mice (Balster and Moser, 1987; Rees and Balster, 1988). Initially, a dose of 15 mg/kg of pentobarbital or saline vehicle was utilized for 27 sessions, then the dose was increased to 30 mg/kg of pentobarbital and discrimination training continued for an additional 40 sessions.
A total of 54% of the SERT KO mice (7 of 13) achieved the criterion for stimulus control by pentobarbital. With the dose of 15 mg/kg, 2 of 13 mice met criterion at a mean of 18 sessions (range = 13–23). Increasing the dose to 30 mg/kg resulted in 5 of the 11 remaining SERT KO mice reaching criterion at a mean of 20 sessions (range = 11–31). In contrast, a total of 80% of the WT mice (8 of 10) attained criterion. At the dose of 15 mg/kg of pentobarbital, 6 of 10 mice met criterion at a mean of 10 sessions (range = 5–15); after increasing the dose to 30 mg/kg, 2 of the 4 remaining WT mice met criterion at a mean of 20 sessions (range = 14–26). However, there was no significant difference between genotypes for the total proportion of mice achieving criterion [Fisher’s Exact Test, NS].
Although SERT KO mice and WT mice did not differ on criterion, there were noticeable differences in the percentages of pentobarbital-appropriate responding across the experiment (Fig. 3, upper panel). Whereas WT mice exhibited stimulus control throughout treatment with each dose of pentobarbital, stimulus control in SERT KO mice was not reliably established until the dose was increased to 30 mg/kg (indicated by the arrow). Furthermore, the SERT KO group was less discriminating of the saline control even after 20 sessions at the higher pentobarbital dose [independent t test: t=2.616, df=21, p<0.02].
Figure 3.
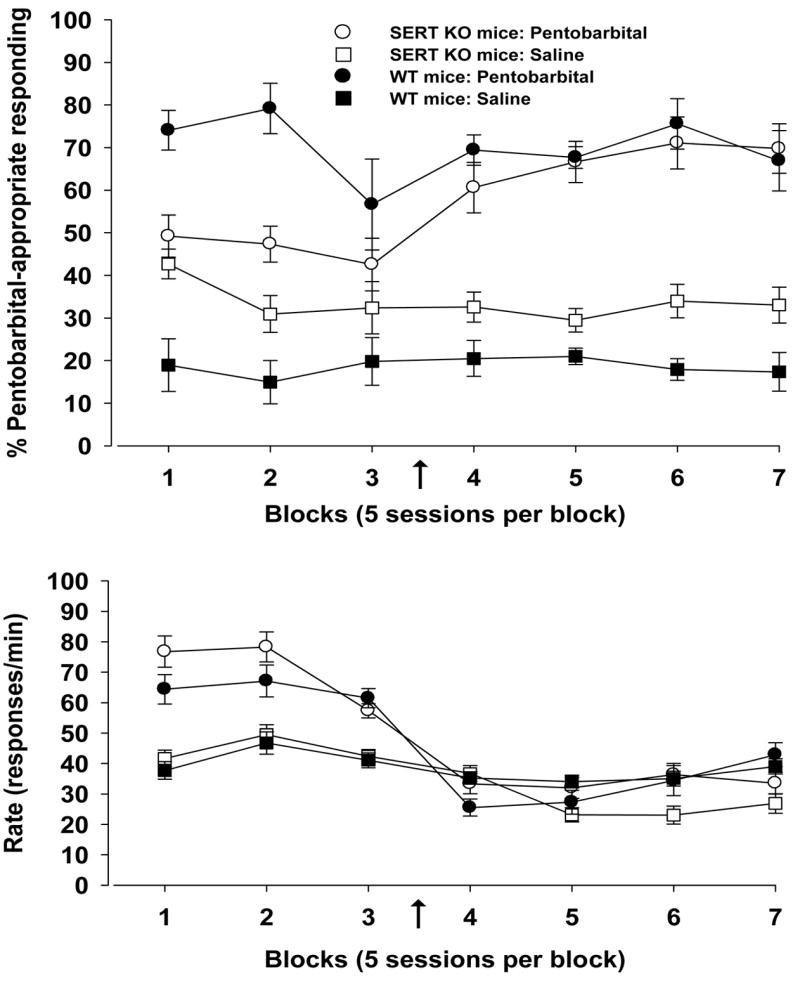
Pentobarbital-induced stimulus control and response rate for mice at two doses of pentobarbital. Discrimination training of homozygous SERT KO mice (n = 13) and WT mice (n = 10) began with 15 mg/kg of pentobarbital (blocks 1–3) and continued with 30 mg/kg of pentobarbital (blocks 4–7). The change in dose is indicated by the arrow. Standard errors of the mean are indicated. Ordinate: upper panel: percentage pentobarbital-appropriate responding; lower panel: rate expressed as responses per min. Abscissa: successive data blocks of 67 sessions. Each data block represents the mean performance in 5 sessions, with the exception that blocks 3 are the means of 3 and 4 sessions of the pentobarbital and saline treatments, respectively.
Response rates were higher with the dose of 15 mg/kg of pentobarbital compared to 30 mg/kg for both SERT KO mice [paired t-test: t=8.180, df=12, p<0.001] and WT mice [paired t-test: t=3.428, df=9, p<0.01] (Fig. 3, lower panel).
The study began with 27 subjects. Four mice (one WT, three SERT KO) were removed from the study because of failure to complete training; their data are not reported.
4. Discussion
SERT KO mice exhibited operant behavior under the control of a visual stimulus. These findings are consistent with other reports that SERT knockouts show associative learning and memory for fear conditioning and fear extinction (Wellman et al., 2007) and for water-reinforced operant behavior (Trigo et al., in press). In the current study, the criterion for visual stimulus control was achieved by 85% of the SERT KO mice and 100% of the WT mice. Both genotypes met criterion at a mean of 30 sessions. As shown in Figure 1 (upper panel), discrimination between the reinforced and nonreinforced sides by the WT group was evident in training session 1 (included in data block 1), whereas the SERT KO group was able to discriminate in training session 14 (included in data block 3). The genotypes also differed early in the experiment on other measures of responding. In sessions comprising block 1, SERT knockouts displayed slower response rates (lower panel) and fewer total number of responses throughout the sessions (data not shown). This initial lack of discrimination and reduced responding by SERT KO mice do not appear to be the result of decreased motivation because SERT KO mice have shown no deficiency in water-drinking behavior (Boyce-Rustay et al., 2006; Kelaï et al., 2003; Trigo et al., in press). Instead, the anxiogenic-like phenotype of SERT KO mice (Adamec et al., 2006; Holmes et al., 2003a,b) could account for diminished performance due to greater anxiety in the novel environment. In agreement with this explanation, knockouts and wildtypes were similar in block 2 for response rates and total number of responses throughout the sessions, and similar in block 3 for discrimination. Our results can be compared to those of Trigo et al. (in press) regarding discrimination of a light stimulus for water reinforcement. Utilizing a snout-poke operant, both studies showed that WT mice distinguished between the cued and non-cued sides in training session 1. In contrast, whereas in their study SERT KO mice discriminated in the first session, this genotype in our study did so in a later session. It must be noted, however, that direct comparisons between these two studies are made difficult by experimental differences including training procedure, schedule of reinforcement (FR1 vs. progressive FR), and especially with respect to the response period reported (entire session vs. before the first reinforcement). Both studies found that knockout mice were slower responders in the early training sessions. The delayed acquisition of discrimination exhibited by SERT KO mice in our study could suggest that knockouts learn more slowly than control mice. This possibility cannot be ruled out, as impairments in extinction recall (Wellman et al., 2007) and shock avoidance (Ansorge et al., 2004) have been observed. Alternately, a disruption in the visual system of SERT knockouts (Salichon et al., 2001) might contribute to slower acquisition. Nonetheless, SERT KO mice demonstrated the ability to form associations and discriminate the visual stimulus.
Stimulus control by both training doses of LSD was established in wildtype mice. Six of the 10 mice achieved the criterion for stimulus control at the dose of 0.17 mg/kg; an increase to 0.30 mg/kg resulted in 3 of the 4 remaining WT mice reaching criterion. These findings are similar to those of our prior study in which 6 of 16 WT mice reached criterion for control at 0.17 mg/kg of LSD, and 5 of the remaining 10 met criterion at 0.30 mg/kg (Winter et al., 2005). However, a larger percentage of mice in the current study met criterion even though they had fewer training sessions. The improved performance might be attributed to prior experience with the discrimination task during the light stimulus experiment because visual discrimination training was not used in the earlier study. As shown in Figure 2 (upper panel), the wildtype group in the current study discriminated 0.17 mg/kg of LSD from saline vehicle by session 6 (included in data block 2). They continued to exhibit stimulus control by this low dose and by the higher dose of 0.30 mg/kg (blocks 6–8). These findings parallel those obtained by Benneyworth et al. (2005) showing that LSD can induce stimulus control in wildtype mice, although they initiated training with 0.25 mg/kg of LSD and moved the mice to several higher doses. In the current study, SERT KO mice were not able to reliably discriminate either dose of LSD, as only 4 of the 13 knockouts achieved criterion for stimulus control with 0.17 mg/kg or 0.30 mg/kg. This lack of stimulus control appears not to be due to reduced motivation because SERT KO and WT mice were similar for response rates (lower panel) and total number of responses throughout the sessions (data not shown). Moreover, the absence of stimulus control by LSD in knockouts cannot be explained by a deficit in operant responding, because they exhibited discrimination behavior in the prior experiment. As noted earlier, the compound stimulus induced in the mouse by DOI or LSD appears to be mediated by both the 5-HT1A and 5-HT2A receptors, with the 5-HT2C receptor having a lesser role (Benneyworth et al., 2005; Smith et al., 2003; Winter et al., 2005). Since SERT KO mice have no detectable 5-HT transporters (Bengel et al., 1998; Lira et al., 2003), high concentrations of extracellular 5-HT (Mathews et al., 2004), and reduced 5-HT clearance (Montañez et al., 2003; Perez and Andrews, 2005), deletion of the 5-HT transporter during embryogenesis likely leads to compensatory changes in receptors in the mature animal that could influence the stimulus effects of LSD.
It has been shown that SERT KO mice have lower densities of 5-HT1A or 5-HT2A receptors (Li et al., 2000, 2003; Rioux et al., 1999). Responses of SERT KO mice induced by the selective 5-HT1A receptor agonist, 8-OH-DPAT, are compromised, including reductions in hormone release and hypothermia (Bouali et al., 2003; Li et al., 1999, 2000), as well as decreased neuronal firing (Gobbi et al., 2001) and GTPγS binding to 5-HT1A receptors (Fabre et al., 2000) in the dorsal raphe nucleus. 8-OH-DPAT has been shown to enhance the stimulus effects of LSD in wildtype mice and rats and to partially substitute for LSD (Benneyworth et al., 2005; Reissig et al., 2005; Winter and Rabin, 1987). Thus, because LSD interacts with the 5-HT1A receptor, the reduced density of these receptors in SERT KO mice could contribute to their inability to discern the stimulus cues of LSD.
With respect to the 5-HT2A receptor, the head-twitch response (HTR) to the 5-HT2A/2C receptor agonist, DOI, is robust in WT mice but reduced by 86% in SERT KO mice (Qu et al., 2005). It is thought that activation of 5-HT2A receptors in the cerebral cortex is necessary for HTR. Microinjection of DOI into the prefrontal cortex of the rat elicits HTR (Willins and Meltzer, 1997). LSD- and DOI-induced HTR is abolished in 5-HT2A receptor null-mutant mice (González-Maeso et al., 2003) and can be rescued in these mice by genetic restoration of 5-HT2A receptor expression exclusively in the cortex (González-Maeso et al., 2007). Since the density of 5-HT2A receptors in SERT KO mice is substantially decreased throughout the cerebral cortex (Rioux et al., 1999), such an adaptation might underlie the absence of LSD discrimination in these mice.
Regarding the non-serotonergic neurotransmitter systems of SERT knockout mice, no alterations have been found in the tissue concentrations of dopamine or norepinephrine in brain structures examined (Bengel et al., 1998; Kim et al., 2005), a characteristic shared by the SERT knockout rat (Homberg et al., 2007). The only reported changes to non-serotonergic systems in SERT KO mice are 5-HT uptake by dopamine transporters into neurons in the midbrain (Pan et al., 2001; Zhou et al., 2002) and decreased basal glucose utilization in most brain regions (Esaki et al., 2005). Although the failure of low and moderate doses of LSD to establish stimulus control in SERT KO mice in our study might be the result of reduced glucose utilization and subsequent decreased neuronal activity, this explanation is argued against by the reports that SERT KO mice do not have a general impairment for interoceptive cues, as they will self-administer cocaine (Trigo et al., in press) and can express conditioned place preferences with cocaine (Sora et al., 1998) and ethanol (Boyce-Rustay et al., 2006) treatments. The most parsimonious explanation for the absence of stimulus control by LSD in SERT KO mice is a reduction in 5-HT1A and/or 5-HT2Areceptor density that renders these mice less sensitive to the stimulus cues of LSD.
A plausible hypothesis to explain the relative inability of SERT KO mice to exhibit stimulus control by LSD is that SERT KO mice have a general inability to perceive the effects of psychoactive drugs. To test that hypothesis we employed pentobarbital, a non-serotonergic drug known to induce discrimination responding in mice (Balster and Moser, 1987; Rees and Balster, 1988). It is generally assumed that pentobarbital-induced effects are mediated by GABAergic, glycinergic, and glutamatergic mechanisms (Kralic et al., 2003; Mehta and Ticku, 1988; Oh et al., 1997). Figure 3 (upper panel) illustrates that WT mice displayed stimulus control by 15 mg/kg of pentobarbital at the onset of training and continued to discriminate this low dose as well as the higher dose of 30 mg/kg (data blocks 4–7). These results are similar to those observed in discrimination experiments utilizing the same doses of pentobarbital in wildtype mice (Balster and Moser, 1987). In the current study, SERT KO mice responded to pentobarbital somewhat differently than WT littermates. Although discrimination of 15 mg/kg was observed in knockouts in several sessions, consistent stimulus control in the genotype was only established with 30 mg/kg of pentobarbital. These mice were also less discriminating of the saline vehicle. The finding that 15 mg/kg of pentobarbital did not reliably induce stimulus control in SERT KO mice cannot be accounted for by decreased motivation because both genotypes were similar regarding response rates (lower panel) and total number of responses throughout the sessions (data not shown). Furthermore, unstable stimulus control by 15 mg/kg of pentobarbital in SERT KO mice would not be due to high response rates, as WT mice also exhibited a high rate of responding (lower panel), which is typical for this dose (Balster and Moser, 1987). The inability of knockout mice to consistently discriminate the lower dose of pentobarbital may be related to the absence of LSD effects in the previous experiment. Prior experience with a drug state-dependent task has been shown to enhance later performance of the same task during a different state (Grilly, 1975; Shannon and Holtzman, 1979). Alternately, decreased glucose utilization in knockouts (Esaki et al., 2005) could diminish the pentobarbital stimulus as well as the saline stimulus. Regardless of the slower development of stimulus control by pentobarbital in SERT KO mice, they have demonstrated that they are capable of drug discrimination.
In summary, the present data further indicate the importance of mice for the assessment of hallucinogenic discriminative stimuli. While LSD readily established stimulus control in WT mice, the efficacy of LSD was greatly diminished in SERT KO mice. In contrast, pentobarbital readily established stimulus control in SERT KO mice. We suggest that the inability of SERT KO mice to discriminate the effects of LSD is related to changes in serotonergic systems.
Acknowledgments
This study was supported in part by U.S. Public Health Service Grants DA 03385 (C.M.K., R.A.R., J.C.W) and DA 14183 (C.M.K., J.B.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamec R, Burton P, Blundell J, Murphy DL, Holmes A. Vulnerability to mild predator stress in serotonin transporter knockout mice. Behav Brain Res. 2006;170:126–40. doi: 10.1016/j.bbr.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Anden NE, Dahlström A, Fuxe K, Larsson K. Mapping out of catecholamine and 5-hydroxytryptamine neurons innervating the telencephalon and diencephalon. Life Sci. 1965;4:1275–9. doi: 10.1016/0024-3205(65)90076-7. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, Hene R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Balster RL, Moser VC. Pentobarbital discrimination in the mouse. Alcohol Drug Res. 1987;7:233–42. [PubMed] [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, et al. Altered brain serotonin homeostasis and locomotor insensitivity to 3,4-methylenedioxymethamphatamine (“ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–55. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Benneyworth MA, Smith RL, Barrett RJ, Sanders-Bush E. Complex discriminative stimulus properties of (+)lysergic acid diethylamide (LSD) in C57Bl/6J mice. Psychopharmacology (Berl) 2005;179:854–62. doi: 10.1007/s00213-004-2108-z. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Watanabe S. Footshock facilitates discrimination of stimulus properties of morphine. Life Sci. 1997;61:1045–9. doi: 10.1016/s0024-3205(97)00612-7. [DOI] [PubMed] [Google Scholar]
- Bouali S, Evrard A, Chastanet M, Lesch K-P, Hamon M, Adrien J. Sex hormone-dependent desensitization of 5-HT1A autoreceptors in knockout mice deficient in the 5-HT transporter. Eur J Neurosci. 2003;18:2203–12. doi: 10.1046/j.1460-9568.2003.02960.x. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Wiedholz LM, Millstein RA, Carroll J, Murphy DL, Daws LC, et al. Ethanol-related behaviors in serotonin transporter knockout mice. Alcohol Clin Exp Res. 2006;30:1957–65. doi: 10.1111/j.1530-0277.2006.00241.x. [DOI] [PubMed] [Google Scholar]
- Brauer LH, Goudie AJ, deWit H. Dopamine ligands and the stimulus effects of amphetamine: animal models versus human laboratory data. Psychopharmacology (Berl) 1997;130:2–13. doi: 10.1007/s002130050207. [DOI] [PubMed] [Google Scholar]
- Bucan M, Abel T. The mouse: genetics meets behavior. Nat Rev Genet. 2002;3:114–23. doi: 10.1038/nrg728. [DOI] [PubMed] [Google Scholar]
- Cunningham KA, Appel JB. Neuropharmacological reassessment of the discriminative stimulus properties of D-lysergic acid diethylamide (LSD) Psychopharmacology (Berl) 1987;91:67–73. doi: 10.1007/BF00690929. [DOI] [PubMed] [Google Scholar]
- Dahlström A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brainstem neurons. Acta Physiol Scand. 1964;62(suppl 232):1–55. [PubMed] [Google Scholar]
- Dykstra LA, Preston KL, Bigelow GE. Discriminative stimulus and subjective effects of opioids with mu and kappa activity: data from laboratory animals and human subjects. Psychopharmacology (Berl) 1997;130:14–27. doi: 10.1007/s002130050208. [DOI] [PubMed] [Google Scholar]
- Eckler JR, Reissig CJ, Rabin RA, Winter JC. A 5-HT2C receptor-mediated interaction between 2,5-dimethoxy-4-methylamphetamine and citalopram in the rat. Pharmacol Biochem Behav. 2004;79:25–30. doi: 10.1016/j.pbb.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Esaki T, Cook M, Shimoji K, Murphy DL, Sokoloff L, Holmes A. Developmental disruption of serotonin transporter function impairs cerebral responses to whisker stimulation in mice. Proc Natl Acad Sci USA. 2005;102:5582–7. doi: 10.1073/pnas.0501509102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre V, Beaufour C, Evrard A, Rioux A, Hanoun N, Lesch KP, et al. Altered expression and functions of serotonin 5-HT1A and 5-HT1B receptors in knock-out mice lacking the 5-HT transporter. Eur J Neurosci. 2000;12:2299–2310. doi: 10.1046/j.1460-9568.2000.00126.x. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, Winter JC. The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. I: Antagonist correlation analysis Psychopharmacology (Berl) 1995a;121:347–56. doi: 10.1007/BF02246074. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Helsley SE, Lorrain DS, Rabin RA, Winter JC. The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. III: the mechanistic basis for supersensitivity to the LSD stimulus following serotonin depletion Psychopharmacology (Berl) 1995b;121:364–72. doi: 10.1007/BF02246076. [DOI] [PubMed] [Google Scholar]
- Forster EA, Cliffe IA, Bill DJ, Dover GM, Jones D, Reilly Y, et al. A pharmacological profile of the selective silent 5-HT1A receptor antagonist, WAY-100635. Eur J Pharmacol. 1995;281:81–8. doi: 10.1016/0014-2999(95)00234-c. [DOI] [PubMed] [Google Scholar]
- Geter-Douglass B, Witkin JM. Behavioral effects and anticonvulsant efficacies of low-affinity, uncompetitive NMDA antagonists in mice. Psychopharmacology (Berl) 1999;146:280–9. doi: 10.1007/s002130051118. [DOI] [PubMed] [Google Scholar]
- Gingrich JA, Hen R. Dissecting the role of the serotonin system in neuropsychiatric disorders using knockout mice. Psychopharmacology (Berl) 2001;155:1–10. doi: 10.1007/s002130000573. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Rosecrans JA, Young R. The use of the drug discrimination paradigm for studying hallucinogenic agents. In: Colpaert FC, Slangen JL, editors. Drug discrimination: applications in CNS pharmacology. Amsterdam: Elsevier; 1982. pp. 69–96. [Google Scholar]
- Gobbi G, Murphy DL, Lesch K-P, Blier P. Modifications of the serotonergic system in mice lacking serotonin transporters: an in vivo electrophysiological study. J Pharmacol Exp Ther. 2001;296:987–95. [PubMed] [Google Scholar]
- Gommans J, Bouwknecht JA, Hijzen TH, Berendsen HH, Broekkamp CL, Maes RA, et al. Stimulus properties of fluvoxamine in a conditioned taste aversion procedure. Pschopharmacology. 1998;140:496–502. doi: 10.1007/s002130050794. [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M, et al. Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonists effects in mouse somatosensory cortex. J Neurosci. 2003;23:8836–43. doi: 10.1523/JNEUROSCI.23-26-08836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, et al. Hallucinogens recruit specific cortical 5-HT2A receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–52. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Grant KA, Knisely JS, Tabakoff B, Barrett JE, Balster RL. Ethanol-like discriminative stimulus effects of non-competitive NMDA antagonists. Behav Pharmacol. 1991;2:87–95. [PubMed] [Google Scholar]
- Grilly DM. Effects of prior experience on differential learning under amphetamine. Psychopharmacologia. 1975;43:271–7. doi: 10.1007/BF00429263. [DOI] [PubMed] [Google Scholar]
- Harris RT, Balster RL. An analysis of the function of drugs in the stimulus control of operant behavior. In: Thompson T, Pickens R, editors. Stimulus properties of drugs. New York: Appleton-Century-Crofts; 1971. pp. 111–32. [Google Scholar]
- Hirschhorn I, Winter JC. Mescaline and LSD as discriminative stimuli. Psychopharmacologia. 1971;22:64–71. doi: 10.1007/BF00401468. [DOI] [PubMed] [Google Scholar]
- Holmes A, Li Q, Murphy DL, Gold E, Crawley JN. Abnormal anxiety-related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav. 2003a;2:365–80. doi: 10.1046/j.1601-1848.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Lesch K-P, Crawley JN, Murphy DL. Mice lacking the serotonin transporter exhibit 5-HT1A receptor-mediated abnormalities in tests for anxiety-like behavior. Neuropsychopharmacology. 2003b;28:2077–88. doi: 10.1038/sj.npp.1300266. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Olivier JDA, Smits BMG, Mul JD, Mudde J, Verheul M, et al. Characterization of the serotonin transporter knockout rat: a selective change in the functioning of the serotonergic system. Neuroscience. 2007;146:1662–76. doi: 10.1016/j.neuroscience.2007.03.030. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Siegel BW, Carr AA. [3H]MDL 100,907: a novel selective 5-HT2A receptor ligand. Nauny Schmiedebergs Arch Pharmacol. 1996;354:205–9. doi: 10.1007/BF00178722. [DOI] [PubMed] [Google Scholar]
- Kamien J, Bickel W, Hughes J, Higgins S, Smith B. Drug discrimination by humans compared to nonhumans: current status and future directions. Psychopharmacology (Berl) 1993;111:259–70. doi: 10.1007/BF02244940. [DOI] [PubMed] [Google Scholar]
- Kelaï S, Aissi F, Lesch KP, Cohen-Salmon C, Hamon M, Lanfumey L. Alcohol intake after serotonin transporter inactivation in mice. Alcohol Alcohol. 2003;38:386–9. doi: 10.1093/alcalc/agg095. [DOI] [PubMed] [Google Scholar]
- Kim D-K, Tolliver TJ, Huang S-J, Martin BJ, Andrews AM, Wichems C, et al. Altered serotonin synthesis, turnover and dynamic regulation in multiple brain regions of mice lacking the serotonin transporter. Neuropharmacology. 2005;49:798–810. doi: 10.1016/j.neuropharm.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Kralic JE, Wheeler M, Renzi K, Ferguson C, O’Buckley TK, Grobin AC, et al. Deletion of GABAA receptor α1 subunit-containing receptors alters responses to ethanol and other anesthetics. J Pharmacol Exp Ther. 2003;305:600–7. doi: 10.1124/jpet.102.048124. [DOI] [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Van de Kar LD, Lesch K-P, Murphy DL. Reduction of 5-hydroxytryptamine (5-HT)1A-mediated temperature and neuroendocrine responses and 5-HT1A binding sites in 5-HT transporter knockout mice. J Pharmacol Exp Ther. 1999;291:999–1007. [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Lesch K-P, Murphy DL. Reduction in the density and expression, but not G-protein coupling, of serotonin receptors (5-HT1A) in 5-HT transporter knock-out mice: gender and brain region differences. J Neurosci. 2000;20:7888–95. doi: 10.1523/JNEUROSCI.20-21-07888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wichems CH, Ma L, Van de Kar LD, Garcia F, Murphy DL. Brain region-specific alterations of 5-HT2A and 5-HT2C receptors in serotonin transporter knockout mice. J Neurochem. 2003;84:1256–65. doi: 10.1046/j.1471-4159.2003.01607.x. [DOI] [PubMed] [Google Scholar]
- Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH, et al. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry. 2003;54:960–71. doi: 10.1016/s0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL, Andrews AM. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J Neurosci Methods. 2004;140:169–81. doi: 10.1016/j.jneumeth.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Mehta AK, Ticku MK. Interactions of pentobarbital and phenobarbital with GABAergic drugs against chemoconvulsants in rats. Pharmacol Biochem Behav. 1988;30:995–1000. doi: 10.1016/0091-3057(88)90131-1. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Favara JP, Boggan WO, Stringer AJ. Discriminative properties of phencyclidine in mice: generalization to ketamine and monohydroxy metabolites. Psychopharmacology (Berl) 1988;96:381–4. doi: 10.1007/BF00216066. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, McGroarty KK, Groseclose CH, Adinoff B. Cocaine discrimination: relationship to local anesthetics and monoamine uptake inhibitors in C57BL/6 mice. Psychopharmacology (Berl) 1998;136:44–9. doi: 10.1007/s002130050537. [DOI] [PubMed] [Google Scholar]
- Montañez S, Owens WA, Gould GG, Murphy DL, Daws LC. Exaggerated effect of fluvoxamine in heterozygous serotonin transporter knockout mice. J Neurochem. 2003;86:210–9. doi: 10.1046/j.1471-4159.2003.01836.x. [DOI] [PubMed] [Google Scholar]
- Oh S, Hoshi K, Ho IK. Role of NMDA receptors in pentobarbital tolerance/dependence. Neurochem Res. 1997;22:767–74. doi: 10.1023/a:1022019423197. [DOI] [PubMed] [Google Scholar]
- Overton DA. Discriminative control of behavior by drug states. In: Thompson T, Pickens R, editors. Stimulus properties of drugs. New York: Appleton-Century-Crofts; 1971. pp. 87–110. [Google Scholar]
- Pan Y, Gembom E, Peng W, Lesch K-P, Mossner R, Simantov R. Plasticity in serotonin uptake in primary neuronal cultures of serotonin transporter knockout mice. Brain Res Dev Brain Res. 2001;126:125–9. doi: 10.1016/s0165-3806(00)00145-0. [DOI] [PubMed] [Google Scholar]
- Perez X, Andrews AM. Chronoamperometry to determine differential reductions in uptake in brain synaptosomes from serotonin transporter knockout mice. Analyt Chem. 2005;77:818–26. doi: 10.1021/ac049103g. [DOI] [PubMed] [Google Scholar]
- Philibin SD, Prus AJ, Pehrson AL, Porter JH. Serotonin receptor mechanisms mediate the discriminative stimulus properties of the atypical antipsychotic clozapine in C57BL/6 mice. Psychopharmacology (Berl) 2005;180:49–56. doi: 10.1007/s00213-005-2147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Villacreses N, Murphy DL, Rapoport SI. 5-HT2A/2C receptor signaling via phospholipase A2 and arachidonic acid is attenuated in mice lacking the serotonin reuptake transporter. Psychopharmacology (Berl) 2005;180:12–20. doi: 10.1007/s00213-005-2231-5. [DOI] [PubMed] [Google Scholar]
- Rees DC, Balster RL. Attenuation of the discriminative stimulus properties of ethanol and oxazepam, but not of pentobarbital, by Ro 15-4513 in mice. J Pharmacol Exp Ther. 1988;244:592–8. [PubMed] [Google Scholar]
- Reissig CJ, Eckler JR, Rabin RA, Winter JC. The 5-HT1A receptor and the stimulus effects of LSD in the rat. Psychopharmacology (Berl) 2005;182:197–204. doi: 10.1007/s00213-005-0068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux A, Fabre V, Lesch KP, Moessner R, Murphy DL, Lanfumey L, et al. Adaptive changes of serotonin 5-HT2A receptors in mice lacking the serotonin transporter. Neurosci Lett. 1999;262:113–6. doi: 10.1016/s0304-3940(99)00049-x. [DOI] [PubMed] [Google Scholar]
- Rumajogee P, Verge D, Hanoun N, Brisorgueil M-J, Hen R, Lesch K-P, et al. Adaption of the serotoninergic neuronal phenotype in the absence of 5-HT autoreceptors or the 5-HT transporter: involvement of BDNF and cAMP. Eur J Neurosci. 2004;19:937–44. doi: 10.1111/j.0953-816x.2004.03194.x. [DOI] [PubMed] [Google Scholar]
- Salichon N, Gaspar P, Upton AL, Picaud S, Hanoun N, Hamon M, et al. Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase a and 5-ht transporter knock-out mice. J Neurosci. 2001;21:884–96. doi: 10.1523/JNEUROSCI.21-03-00884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Millan MJ. Blockade of the discriminative stimulus effects of DOI by MDL 100,907 and the ‘atypical’ antipsychotics, clozapine and risperidone. Eur J Pharmacol. 1994;264:99–102. doi: 10.1016/0014-2999(94)90643-2. [DOI] [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Audinot V, Gobert A, Veiga S, Millan MJ. (1-(2,5-Dimethoxy-4 iodophenyl)-2-animopropane)-induced head-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT) 2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J Pharmacol Exp Ther. 1995;273:101–12. [PubMed] [Google Scholar]
- Seong E, Seasholtz AF, Burmeister M. Mouse models of psychiatric disorders. Trends Genet. 2002;18:643–50. doi: 10.1016/s0168-9525(02)02807-x. [DOI] [PubMed] [Google Scholar]
- Shannon HE, Holtzman SG. Morphine training dose: a determinant of stimulus generalization to narcotic antagonists in the rat. Psychopharmacology (Berl) 1979;61:239–44. doi: 10.1007/BF00432265. [DOI] [PubMed] [Google Scholar]
- Shulgin AT, Shulgin A. PiHKAL: a chemical love story. Berkley: Transform Press; 1991. pp. 633–7. [Google Scholar]
- Smith RL, Barrett RJ, Sanders-Bush E. Discriminative stimulus properties of 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane [(±)DOI] in C57BL/6J mice. Psychopharmacology (Berl) 2003;166:61–8. doi: 10.1007/s00213-002-1252-6. [DOI] [PubMed] [Google Scholar]
- Snoddy AM, Tessel RE. Nisoxetine and amphetamine share discriminative stimulus properties in mice. Pharmacol Biochem Behav. 1983;19:205–10. doi: 10.1016/0091-3057(83)90040-0. [DOI] [PubMed] [Google Scholar]
- Sora I, Wichems C, Takahashi N, Li X-F, Zeng Z, Revay R, et al. Cocaine reward models: conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc Natl Acad Sci USA. 1998;95:7699–704. doi: 10.1073/pnas.95.13.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, Kamien JB. Drug Discrimination Bibliographic Database. 2004 http://www.dd-database.org/
- Trigo JM, Renoir T, Lanfumey L, Hamon M, Lesch K-P, Robledo P, et al. 3,4-Methylenedioxymethamphetamine self-administration is abolished in serotonin transporter knockout mice. Biol Psychiatry. doi: 10.1016/j.biopsych.2006.11.005. in press (online publication) [DOI] [PubMed] [Google Scholar]
- Varvel SA, James JR, Bowen S, Rosecrans JA, Karan LD. Discriminative stimulus properties of nicotine in the C57BL/6 mouse. Pharmacol Biochem Behav. 1999;63:27–32. doi: 10.1016/s0091-3057(98)00262-7. [DOI] [PubMed] [Google Scholar]
- Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, et al. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci. 2007;27:684–91. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettstein JG, Host M, Hitchcock JM. Selectivity of action of typical and atypical anti-psychotic drugs as antagonists of the behavioral effects of 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:533–44. doi: 10.1016/s0278-5846(99)00014-7. [DOI] [PubMed] [Google Scholar]
- Willins DL, Meltzer HY. Direct injection of 5-HT 2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J Pharmacol Exp Ther. 1997;282:699–706. [PubMed] [Google Scholar]
- Winter JC. Hallucinogens as discriminative stimuli. Fed Proc. 1974;33:1825–32. [PubMed] [Google Scholar]
- Winter JC. Drug-induced stimulus control. In: Blackman DE, Sanger DJ, editors. Contemporary research in behavioral pharmacology. New York: Plenum Press; 1978. pp. 209–37. [Google Scholar]
- Winter JC, Rabin RA. Interactions between serotonergic agonists and antagonists in rats trained with LSD as a discriminative stimulus. Pharmacol Biochem Behav. 1987;30:617–24. doi: 10.1016/0091-3057(88)90074-3. [DOI] [PubMed] [Google Scholar]
- Winter JC, Filipink RA, Timineri D, Helsley SE, Rabin RA. The paradox of 5-methoxy-N,N-dimethyltryptamine: An indoleamine hallucinogen that induces stimulus control via 5-HT1A receptors. Pharmacol Biochem Behav. 2000;65:75–82. doi: 10.1016/s0091-3057(99)00178-1. [DOI] [PubMed] [Google Scholar]
- Winter JC, Eckler JR, Rabin RA. Serotonergic/glutamatergic interactions: the effects of mGlu2/3 receptor ligands in rats trained with LSD and PCP as discriminative stimuli. Psychopharmacology (Berl) 2004;172:233–40. doi: 10.1007/s00213-003-1636-2. [DOI] [PubMed] [Google Scholar]
- Winter JC, Kieres AK, Zimmerman MD, Reissig CJ, Eckler JR, Ullrich T, et al. The stimulus properties of LSD in C57BL/6 mice. Pharmacol Biochem Behav. 2005;81:830–7. doi: 10.1016/j.pbb.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FC, Lesch K-P, Murphy DL. Serotonin uptake into dopamine neurons via dopamine transporters: a compensatory alternative. Brain Res. 2002;942:109–19. doi: 10.1016/s0006-8993(02)02709-9. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Masson J, Gingrich JA, Rayport S, Hen R. Targeted gene expression in dopamine and serotonin neurons of the mouse brain. J Neurosci Methods. 2005;143:27–32. doi: 10.1016/j.jneumeth.2004.09.020. [DOI] [PubMed] [Google Scholar]


