Abstract
OBJECTIVE
To identify the clinical characteristics of anterior prostate cancers (APCs) and to compare these with posterior prostate cancers (PPCs).
PATIENTS AND METHODS
We reviewed 1290 consecutive open and laparoscopic radical prostatectomies (RPs) at the authors' institution from January 2000 to March 2004. Prostates were processed using a whole-mount technique. Each surgical specimen was reviewed by one pathologist, and tumour areas were marked, measured and mapped. Positive surgical margins (PSMs) were defined as the presence of cancer cells at the inked surface of the specimen. Specimens were then categorized by the location of their dominant tumour, i.e. pure anterior, anterior > posterior, posterior > anterior, or pure posterior. We compared the clinical and pathological characteristics of 259 patients in the pure-anterior group with the 594 in the pure-posterior group.
RESULTS
Before RP, APCs had a significantly lower biopsy Gleason score (78% vs 68% with Gleason 4–6), fewer mean biopsy cores positive (2.0 vs 2.6), a smaller median percentage of positive cores (17% vs 26%), lower clinical stage (T1 in 79% vs 62%), and higher progression-free probability estimated by preoperative nomogram (86% vs 84%) than PPCs. Patients with APCs also had more previous negative biopsy sessions. The pathological analysis of RP specimens showed that those with APCs had higher tumour volume (1.6 vs 0.83 mL) and had a higher PSM rate (12% vs 7%) than those with PPCs, despite specimens with PPCs having higher rates of extraprostatic extension (10% vs 19%).
CONCLUSIONS
APCs have lower Gleason grade and lower rates of extraprostatic extension, yet patients with anterior tumours have higher overall tumour volumes and higher PSM rates. Because current tools for detecting and staging prostate cancer can underestimate the extent of anterior prostate disease, improved methods are needed for localizing and characterizing anterior cancers.
Keywords: prostatic neoplasms, prostatectomy, neoplasm staging
INTRODUCTION
Most techniques for detecting prostate cancer, e.g. DRE, TRUS-guided biopsy and endorectal-coil MRI, rely on a transrectal approach for diagnosis. Cancers within the anterior prostate can be difficult to detect by these means. Because anterior cancers are distant from the rectal surface, they can be a challenge to visualize by TRUS or MRI [1,2]. Moreover, the anterior prostate is routinely under-sampled by standard TRUS-guided biopsy because anterior biopsies are excluded from standard sextant biopsy templates.
Bott et al. [3] noted the diagnostic challenges of anteriorly located prostate cancers (APCs), which were less likely to be palpable and required more prostate biopsies than posteriorly located prostate cancers (PPCs). Because of the paucity of information on APCs, we sought to characterize the clinical features of these cancers, and to compare them with PPCs.
PATIENTS AND METHODS
From January 2000 to March 2004, 1290 consecutive patients had RP at our institution for clinical stage T1-T3 prostate cancers, with no therapy before RP. The clinical and pathological information was prospectively recorded in our multidisciplinary prostate cancer database. Preoperative factors included age, year of surgery, PSA level, the number of biopsy sessions before diagnosis, number of biopsy cores positive for cancer, proportion of cancer present within positive biopsy cores, and clinical stage, according to the 2002 TNM staging system. Of the 1290 patients, 1069 had open retropubic RP and 221 laparoscopic RP. The operations were performed by three experienced surgeons.
All RP specimens were fixed en bloc in 10% neutral buffered formalin and processed by a whole-mount technique described previously [4,5]. An apical shaved section, 2–4 mm thick, was truncated perpendicular to the prostatic urethra and subsequently sectioned as slices parallel to the prostatic urethra. A bladder neck section was obtained either by sampling portions of tissue at the junction of the prostatic capsule and bladder neck, or by sampling the most proximal portion of the submitted specimen corresponding to the anatomical bladder neck. The remaining prostate was completely sectioned at 4 mm intervals. Dedicated uropathologists reviewed all the specimens and precisely mapped each tumour area with marker. The existence and location of focal or established extraprostatic extension (EPE), seminal vesicle invasion, positive surgical margin (PSM), and lymph node metastases were recorded. EPE was defined as the presence of cancer cells within the periprostatic adipose tissue. A PSM was defined as any cancer cells in contact with the inked surface of the specimen. The bladder neck margin was considered positive when any cancer cells were detected in the bladder neck section. Total tumour volume (TV) was calculated by a computerized planimetric method using image-analysis and measurement software (Image-Pro Plus 4.0, Media Cybernetics, Inc) [6].
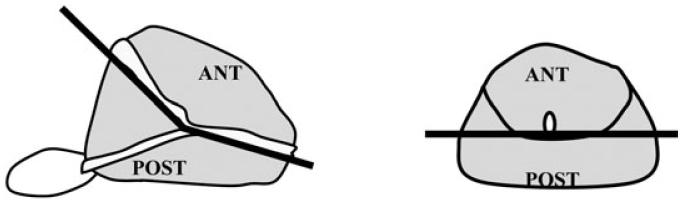
The location of each tumour was recorded in the cranio-caudal direction (apex, mid, base) and in the anterior/posterior direction. The anterior region of the prostate was defined as the area anterior to the urethra (Fig. 1). In each case, a dominant tumour was identified as the tumour having the largest TV. All patients were then categorized by the location of their dominant tumour; anterior (100% of the dominant tumour anterior to the urethra), anterior > posterior (more than half of the dominant tumour anterior to the urethra), posterior > anterior (more than half of the dominant tumour posterior to the urethra), or posterior (all of the dominant tumour posterior to the urethra).
FIG. 1.
Definition of APCs and PPCs; APCs lie anterior to the urethra.
Clinical and pathological data were obtained after written approval of the study from the Human Institutional Review Board. The clinical and pathological features of APCs were compared to those of PPCs using the chi-square statistic, with Student's t-test used to compare APCs vs PPCs for differences in the distribution of continuous variables, e.g. age, PSA level, biopsy data, and the nomogram for progression-free probabilities before and after RP [7,8]. A second series of 740 consecutive whole-mount RP specimens from 1984 to 1998 were analysed to identify trends in the prevalence of APCs; this cohort was described previously [9].
RESULTS
During the study period, 1290 consecutive patients had RP for localized prostate cancer (median age 58 years, range 37–76). The clinical characteristics before RP of patients with APCs were compared to those with PPCs (Table 1); patients with APCs had a lower biopsy Gleason score (78% vs 68% Gleason 2–6), and lower clinical stage (79% vs 62% T1) than patients with PPCs. Among patients with APCs, only 54 (21%) had palpable cancers (cT2), and no patients had cT3 disease. Patients with APCs had more negative biopsy sessions before diagnosis than patients with PPCs (0.4 vs 0.3). They also had fewer positive biopsy cores (2.0 vs 2.6), and a lower proportion of cancer in positive cores (24% vs 31%). There was no statistically significant difference in age or serum PSA level between the groups. Using the nomogram [7], we estimated the progression-free probabilities for patients with anterior and posterior tumours; this favoured anterior tumours, i.e. before RP, patients with APCs would appear to be at lower risk for PSA progression after RP than patients with PPCs (86% vs 84%, respectively, P = 0.002).
TABLE 1.
The clinical characteristics before surgery and the pathological characteristics, of patients with APCs and PPCs
| Characteristic | APC | PPC | P |
|---|---|---|---|
| Number of patients | 259 | 594 | |
| Median age, years | 59 | 58 | 0.1 |
| Serum PSA level, ng/mL | 7.2 | 6.4 | 0.8 |
| Gleason grade, n (%) | |||
| 2–6 | 201 (78) | 407 (68) | 0.026 |
| 7 | 51 (20) | 170 (29) | |
| 8–10 | 5 (2) | 17 (3) | |
| Clinical stage, n (%) | 255 | 582 | <0.001 |
| T1 | 201 (79) | 358 (62) | |
| T2 | 54 (21) | 218 (37) | |
| T3 | 0 | 6 (1) | |
| Progression-free probability, % | |||
| before RP | 86 | 84 | 0.002 |
| after RP | 89 | 88 | 0.6 |
| Previous −ve biopsy sessions | 0.4 | 0.3 | 0.002 |
| Biopsy cores positive, n | 2.0 | 2.6 | <0.001 |
| Proportion of core positive, % | 24 | 31 | <0.001 |
| Pathological features | |||
| Mean prostate volume, mL | 44 | 42 | 0.5 |
| TV, mL | 1.6 | 0.8 | <0.001 |
| Gleason score, n (%) | 0.001* | ||
| 4–6 | 166 (64) | 348 (59) | |
| 3 + 4 | 77 (30) | 164 (28) | |
| 4 + 3 | 13 (5) | 60 (10) | |
| 8–10 | 3 (1) | 22 (4) | |
| EPE, n(%) | 0.003 | ||
| none | 232 (90) | 480 (81) | |
| focal | 10 (4) | 60 (10) | |
| established | 17 (7) | 54 (9) | |
| PSM, n(%) | 31 (12) | 39 (7) | 0.01 |
| Seminal vesicle invasion, n (%) | 0 | 23 (4) | <0.001 |
| Lymph node involvement, n (%) | 1 (1) | 11 (2) | 0.1 |
| Location of PSMs | |||
| N patients with PSM | 31 | 36 | |
| Total number of PSMs | 41 | 55 | |
| Location, n (%) | |||
| Bladder neck | 5 (12) | 1 (2) | |
| Anterior | |||
| base | 2 (5) | 0 | |
| mid | 3 (7) | 1 (2) | |
| apex | 14 (34) | 1 (2) | |
| Posterior | |||
| base | 1 (2) | 5 (9) | |
| mid | 2 (5) | 14 (25) | |
| apex | 1 (2) | 20 (36) | |
| Apical | 13 (32) | 13 (24) |
Gleason ≤3 +4 vs ≥4 +3.
The pathological features of RP specimens for APCs and PPCs are also shown in Table 1. Specimens with anterior tumours had a significantly higher TV than those with posterior cancers (1.6 vs 0.8 mL). Despite the larger TV, APCs were of lower grade than PPCs, with a Gleason score 3 + 4 or less in 94% vs 87% of cases, respectively. Seminal vesicle invasion was not present in any patient with an anterior dominant tumour and in only 4% of those with a posterior dominant tumour. There was no significant difference in lymph node involvement between the groups (anterior 1%, posterior 2%). Importantly, when the RP specimen characteristics were used to calculate the PSA progression-free probabilities with the nomogram, there was a statistically similar distribution of recurrence risk between the groups (89% for APC vs 88% for PPC, P = 0.6).
Patients with APCs had a higher PSM rate (12% vs 7%) but a lower rate of EPE rate (10% vs 19%) than those with PPCs. EPE was more likely to be associated with PSMs for APCs (37% vs 10%, Fig. 2). The distribution of PSMs for APCs and PPCs is also shown in Table 1. The most common locations of PSMs for APCs were the anterior apex (34%), apical section (32%), and prostate base (12%). The most common locations of PSMs for PPCs were the posterior apex (36%), posterior mid (25%), and the apical section (24%).
FIG. 2.
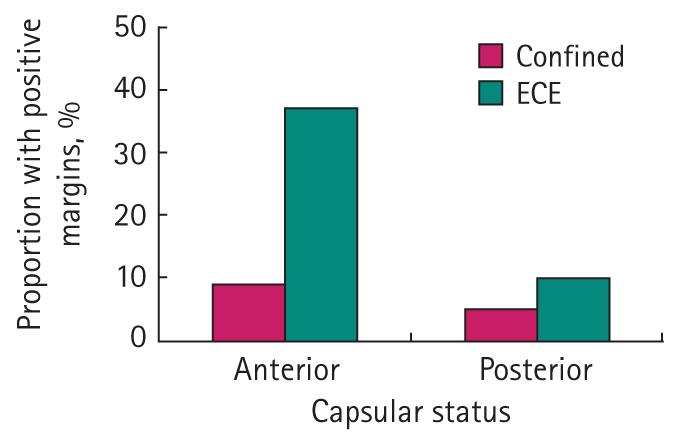
PSMs were more likely to be associated with EPE for APCs.
The dominant tumour was located anteriorly (pure anterior, anterior > posterior) for 28% of patients in the present series; these values remained relatively stable throughout the study period (Fig. 3). To further evaluate trends in the prevalence of pure anterior prostate cancers, we analysed an earlier series (1984–98) of 740 consecutive whole-mount RP specimens. The clinical data available for these patients are shown in Table 2. After approval for the use of PSA testing in 1986 there was a sharp decline in the rate of pure anterior tumours, followed by a rise to 26% for the 1997–98 interval (Fig. 4).
FIG. 3.
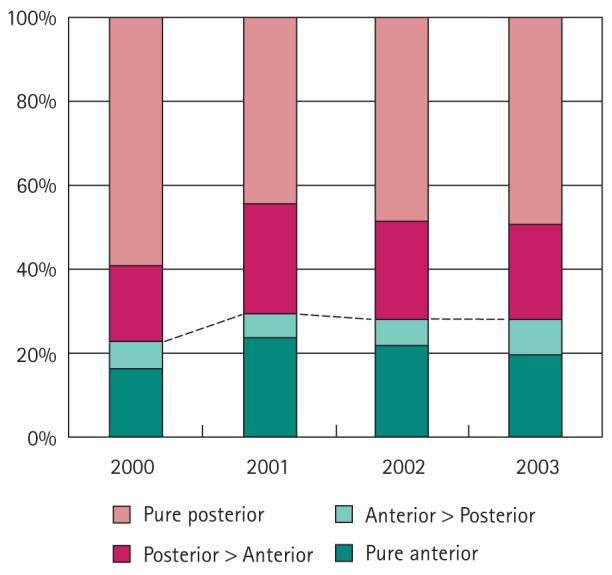
Trends in tumour location from 2000 to 2003. The proportion of patients with anterior tumours (pure anterior and anterior > posterior) remained relatively stable throughout the study period.
TABLE 2.
The clinical characteristics of 740 consecutive patients with whole-mount RP specimens from 1984 to 1998
| Characteristic | Value or n (%) |
|---|---|
| Median age, years | 63 |
| Median PSA level, ng/mL | 6.9 |
| Clinical stage | |
| T1 | 166 (23) |
| T2 | 503 (70) |
| T3 | 49 (7) |
| Gleason score before RP | |
| 2–6 | 446 (71) |
| 7 | 151 (24) |
| 8–10 | 27 (4) |
| SM status | |
| negative | 639 (88) |
| positive | 85 (12) |
| EPE | |
| none | 448 (62) |
| focal | 102 (14) |
| established | 175 (24) |
| Pathological Gleason score | |
| 2–6 | 337 (47) |
| 7 | 339 (47) |
| 8–10 | 48 (7) |
| Seminal vesicle invasion | |
| negative | 639 (88) |
| positive | 86 (12) |
| Lymph node invasion | |
| negative | 658 (95) |
| positive | 38 (5) |
FIG. 4.
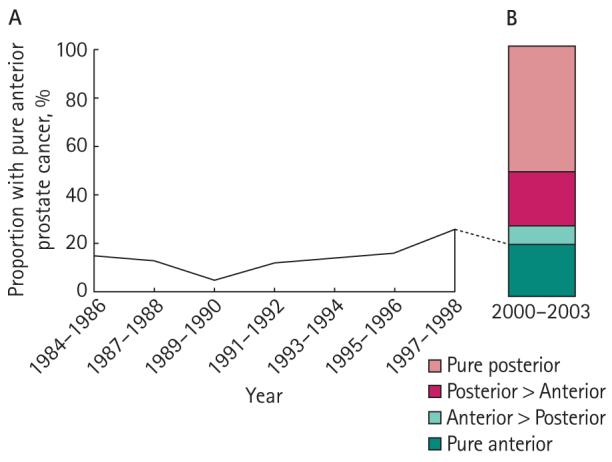
Trends in the proportion of pure anterior tumours for two cohorts of patients, showing the changes in the proportion of pure anterior tumours during the early period of PSA testing (1984–98). B shows the distribution of tumour location from a contemporary cohort of RP patients from 2000 to 2003.
DISCUSSION
Little is known about the clinicopathological characteristics of APCs; most of what is known about them can be extrapolated from studies which describe transition zone (TZ) cancers. Greene et al. [10] reported that TZ cancers have a lower Gleason score and lower rate of EPE than peripheral zone (PZ) cancers. Epstein et al. [11] compared TZ and PZ cancers that were matched for TV; interestingly, they noted a lower rate of PSA progression for TZ cancers, despite TZ and PZ cancers having similar rates of Gleason 4/5 disease. However, the anterior prostate includes not only the TZ but also the anterior horns of the PZ and the fibromuscular stroma. The clinical behaviour of anterior PZ tumours is largely unknown.
In the present large contemporary study of consecutive whole-mount RP specimens, APCs were relatively common, accounting for nearly a third of RP cases at our institution from 2000 to 2003. Furthermore, our data suggest that APCs might be more difficult to detect than PPCs. Patients with APCs had more previous biopsies than those with PPCs. When cancer was identified, APCs had fewer biopsy cores that were positive (2.0 vs 2.6, P ≤ 0.001) and a lower proportion of tumour in the biopsy cores (24% vs 31%, P ≤ 0.001). APCs were also more likely to be impalpable than PPCs (79% vs 62%). These findings support those of Bott et al. [3] who also found that APCs were more likely to be impalpable and had lower TV on needle biopsy than PPCs, despite multiple biopsy sessions.
APCs were not only of lower clinical stage, but they also had lower Gleason grade on preoperative prostate biopsy (78% vs 68%, P = 0.026), and were associated with similar PSA levels as with PPCs. These low-risk clinical features would suggest a benign clinical course for APCs. However, data from whole-mount RP specimens showed that APCs are not insignificant cancers [11]. While anterior had a lower Gleason grade than posterior cancers, 35% of APCs were of intermediate to high pathological grade (Gleason 7–10). Furthermore, patients with APCs had a higher TV (median 1.6 vs 0.8 mL, P < 0.001) and a higher rate of PSMs (12% vs 7%, P = 0.01) than patients with PPCs. While the rate of EPE was lower for APCs, the EPE was more likely to be associated with PSMs for APCs than PPCs, suggesting that anterior PSMs might be clinically significant, and at greater risk of biochemical recurrence [12]. Reported PSM rates for APCs have varied significantly. Mai et al. [13] evaluated 108 RP whole-mount sections for the pathological features of APCs and noted a higher rate of PSMs for patients with PPCs (49%) than APCs (36%). However, the overall PSM rate was quite high in their series (50%). Noguchi et al. [14] compared clinical and pathological features of 79 TZ to 79 PZ cancers that were matched for TV; these authors reported that the EPE rate was significantly higher for PZ cancers (70% vs 16%), while PSM rates were similar between the groups (PZ 35%, TZ 32%). The implications of a PSM after RP are controversial, and appear to be site- and institution-specific. With a limited PSA follow-up for the recent patients, we were unable to address the effect of PSMs on cancer control after RP in the present study. Nonetheless, an elevated anterior PSM rate is a cause for concern, particularly in our institution, where overall PSM rates have been consistently decreasing over time [12]. It was also interesting to note that APCs were more likely than PPCs to have PSMs at the bladder neck.
Because APCs comprise a significant proportion of clinical T1c prostate cancers [15], we hypothesized that the proportion of APCs would increase over recent years, as a result of stage migration towards clinical T1c cancer. Analyses of the more contemporary database (2000–2004) showed that currently 21% of prostate cancers were entirely within the anterior prostate and ≈ 30% were largely (more than half) in the anterior prostate. These values were relatively stable over the 4 years of the study. Using the separate series of 740 whole-mount RP specimens (1984–98) we assessed trends in the proportion of APCs after the advent of PSA testing; the proportion of patients with purely APCs initially decreased with the introduction of PSA screening, and subsequently increased to levels before PSA testing. Before the widespread use of PSA, APCs were often identified incidentally by sampling specimens from TURP. The increase in APCs soon after PSA screening might represent many of the same T1a and T1b prostate cancers that would have been detected incidentally by TURP before testing. Such tumours have been shown to have the potential for an aggressive natural history if untreated [16].
The present study has some limitations: First, it was retrospective and therefore subject to inherent biases and shortcomings. Second, the follow-up is limited for the contemporary RP patients, and therefore the effect of anterior TV and PSM status on progression-free survival remains to be elucidated. Third, the definition of anterior EPE has not been well established to date. The capsule of the anterior prostate is thin and challenging to evaluate. Until a standardized method of interpreting the anterior capsule has been established, reports of anterior EPE should be interpreted with caution. Finally, the anterior prostate is histologically and functionally heterogeneous, composed of the TZ, lateral horns of the PZ, and anterior fibromuscular stroma. In this study we did not take into account the histology of the various anterior prostate regions.
Nonetheless, the study shows that APCs represent significant cancers that are more challenging to detect by contemporary transrectal diagnostic techniques. Current diagnostic technologies can underestimate the size and extent of APCs, which have a larger TV and higher PSM rate than PPCs. In the present period of developing conservative treatments for prostate cancer, the detection and accurate location of APCs would an important area of further study.
Acknowledgments
Source of funding: this work was supported in part by a grant from the AFUD/AUAER Research Scholar Program, the American Urological Association New York Section, and a SPORE (CA92629) grant from the NCI.
Abbreviations
- RP
radical prostatectomy
- APC, PPC
anterior, posterior prostate cancer
- EPE
extraprostatic extension
- PSM
positive surgical margin
- TV
tumour volume
- TZ
transition zone
- PZ
peripheral zone
Footnotes
CONFLICT OF INTEREST
REFERENCES
- 1.Zakian KL, Eberhardt S, Hricak H, et al. Transition zone prostate cancer: metabolic characteristics at 1H MR spectroscopic imaging – initial results. Radiology. 2003;229:241–7. doi: 10.1148/radiol.2291021383. [DOI] [PubMed] [Google Scholar]
- 2.Terris MK, Freiha FS, McNeal JE, Stamey TA. Efficacy of transrectal ultrasound for identification of clinically undetected prostate cancer. J Urol. 1991;146:78–84. doi: 10.1016/s0022-5347(17)37718-2. [DOI] [PubMed] [Google Scholar]
- 3.Bott SR, Young MP, Kellett MJ, Parkinson MC, Contributors to the UCL Hospitals' Trust Radical Prostatectomy Database Anterior prostate cancer: is it more difficult to diagnose? BJU Int. 2002;89:886–9. doi: 10.1046/j.1464-410x.2002.02796.x. [DOI] [PubMed] [Google Scholar]
- 4.Wheeler TM, Dillioglugil O, Kattan MW, et al. Clinical and pathological significance of the level and extent of capsular invasion in clinical stage T1–2 prostate cancer. Hum Pathol. 1998;29:856–62. doi: 10.1016/s0046-8177(98)90457-9. [DOI] [PubMed] [Google Scholar]
- 5.Yossepowitch O, Sircar K, Scardino PT, et al. Bladder neck involvement in pathological stage pT4 radical prostatectomy specimens is not an independent prognostic factor. J Urol. 2002;168:2011–5. doi: 10.1016/S0022-5347(05)64284-X. [DOI] [PubMed] [Google Scholar]
- 6.Greene DR, Egawa S, Neerhut G, Flanagan W, Wheeler TM, Scardino PT. The distribution of residual cancer in radical prostatectomy specimens in stage A prostate cancer. J Urol. 1991;145:324–9. doi: 10.1016/s0022-5347(17)38328-3. [DOI] [PubMed] [Google Scholar]
- 7.Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90:766–71. doi: 10.1093/jnci/90.10.766. [DOI] [PubMed] [Google Scholar]
- 8.Kattan MW, Wheeler TM, Scardino PT. Postoperative nomogram for disease recurrence after radical prostatectomy for prostate cancer. J Clin Oncol. 1999;17:1499–507. doi: 10.1200/JCO.1999.17.5.1499. [DOI] [PubMed] [Google Scholar]
- 9.Kothari PS, Scardino PT, Ohori M, Kattan MW, Wheeler TM. Incidence, location, and significance of periprostatic and periseminal vesicle lymph nodes in prostate cancer. Am J Surg Pathol. 2001;25:1429–32. doi: 10.1097/00000478-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Greene DR, Wheeler TM, Egawa S, Dunn JK, Scardino PT. A comparison of the morphological features of cancer arising in the transition zone and in the peripheral zone of the prostate. J Urol. 1991;146:1069–76. doi: 10.1016/s0022-5347(17)38003-5. [DOI] [PubMed] [Google Scholar]
- 11.Epstein JI, Chan DW, Sokoll LJ, et al. Nonpalpable stage T1c prostate cancer: prediction of insignificant disease using free/total prostate specific antigen levels and needle biopsy findings. J Urol. 1998;160:2407–11. [PubMed] [Google Scholar]
- 12.Swindle P, Eastham JA, Ohori M, et al. Do margins matter? The prognostic significance of positive surgical margins in radical prostatectomy specimens. J Urol. 2005;174:903–7. doi: 10.1097/01.ju.0000169475.00949.78. [DOI] [PubMed] [Google Scholar]
- 13.Mai KT, Moazin M, Morash C, Collins JP. Transitional zone and anterior peripheral zone of the prostate. A correlation of small-volume cancer in the biopsy cores and high PSA with positive anterior margins in radical prostatectomy specimens. Urol Int. 2001;66:191–6. doi: 10.1159/000056613. [DOI] [PubMed] [Google Scholar]
- 14.Noguchi M, Stamey TA, Neal JE, Yemoto CE. An analysis of 148 consecutive transition zone cancers: clinical and histological characteristics. J Urol. 2000;163:1751–5. [PubMed] [Google Scholar]
- 15.Takashima R, Egawa S, Kuwao S, Baba S. Anterior distribution of Stage T1c nonpalpable tumors in radical prostatectomy specimens. Urology. 2002;59:692–7. doi: 10.1016/s0090-4295(02)01525-x. [DOI] [PubMed] [Google Scholar]
- 16.Johansson JE, Andren O, Andersson SO, et al. Natural history of early, localized prostate cancer. JAMA. 2004;291:2713–9. doi: 10.1001/jama.291.22.2713. [DOI] [PubMed] [Google Scholar]