Abstract
The aim of the present study was to investigate the relation between neurogenesis, cell cycle reactivation and neuronal death during tau pathology in a novel tau transgenic mouse line THY-Tau22 with two frontotemporal dementia with parkinsonism linked to chromosome-17 mutations in a human tau isoform. This mouse displays all Alzheimer disease features of neurodegeneration and a broad timely resolution of tau pathology with hyperphosphorylation of tau at younger age (up to 6 months) and abnormal tau phosphorylation and tau aggregation in aged mice (by 10 months). Here, we present a follow-up of cell cycle markers with aging in control and transgenic mice from different ages. We show that there is an increased neurogenesis during tau hyperphosphorylation and cell cycle events during abnormal tau phosphorylation and tau aggregation preceding neuronal death and neurodegeneration. However, besides phosphorylation, other mechanisms including tau mutations and changes in tau expression and/or splicing may be also involved in these mechanisms of cell cycle reactivation. Altogether, these data suggest that cell cycle events in THY-Tau22 are resulting from neurogenesis in young animals and cell death in older ones. It suggests that neuronal cell death in such models is much more complex than believed.
Keywords: Alzheimer disease, AT8, AT100, cyclin B, cyclin D, doublecortin, NeuroD, TUC-4
Tau pathology is observed in several human neurodegenerative disorders such as Alzheimer’s disease (AD), Pick disease, frontotemporal dementia, corticobasal degeneration and progressive supranuclear palsy. In each of these disorders, called tauopathies, the accumulation of the abnormally hyperphosphorylated tau is associated with neurofibrillary degeneration and dementia. The discovery of mutations in the tau gene and their cosegregation with the disease in the inherited frontotemporal dementia with parkinsonism linked to chromosome-17 (FTDP-17) has established that abnormalities in tau protein as a primary event can lead to neurodegeneration and dementia (Hutton et al. 1998; Poorkaj et al. 1998; Spillantini et al. 1998). Interestingly, these tauopathies can be biochemically differentiated by their pattern of hyperphosphorylated isoforms, the so-called bar code (Buee et al. 2000; Sergeant et al. 2005). Hyperphosphorylation of tau precedes its accumulation into neurofibrillary tangle (NFT) in the affected neurons in AD (Sahara et al. 2002). Among the kinases involved in tau hyperphosphorylation, both cyclin-dependent kinase-5 (cdk5) and glycogen synthase kinase-beta (GSK3β) are of particular interest (Ahlijanian et al. 2000; Hamdane et al. 2003; Leroy et al. 2007; Patrick et al. 1999; Sengupta et al. 2006).
On one hand, cell cycle events were reported during neurofibrillary degeneration and neuronal death (Busser et al. 1998; Herrup & Busser 1995; Husseman et al. 2000; Yang et al. 2003). CDK5-p25-mediated hyperphosphorylation has been shown to be involved in reactivation of neuronal cell cycle followed by neuronal death (Hamdane & Buee 2007; Hamdane et al. 2005). On the other hand, cell cycle events are also encountered in neurogenesis. Adult hippocampal neurogenesis is not only involved in learning and the storage of memory (Kempermann 2002; Kempermann et al. 2002, 2004) but also in postlesional remodeling (Dempsey & Kalluri 2007; Gray et al. 2002). In addition, there is an increase in neurogenesis-related proteins in AD (Jin et al. 2004), but it is not clear if these changes are not related to proliferation of glial and vascular factors (Boekhoorn et al. 2006a).
The aim of the present study was to investigate the relation between neurogenesis, cell cycle reactivation and neuronal death during a sequential tau pathology. Lately, we generated a novel tau transgenic mouse line THY-Tau22 with two FTDP-17 mutations in a human tau isoform (Schindowski et al. 2006). This mouse displays the typical biochemical phosphorylation pattern of human tau in AD, NFT-like inclusions, neuropil threads (NT), paired haired filaments, ghost tangles, neuronal loss, astrogliosis, loss of functional synapes and impaired cognition. The THY-Tau22 mouse model displays a broad timely resolution of tau pathology with hyperphosphorylation of tau at younger age (up to 6 months) and abnormal tau phosphorylation and tau aggregation in aged mice (by 10 months).
Here, we present a follow-up of cell cycle markers with aging in control and transgenic mice. We show that there is an increased neurogenesis during tau hyperphosphorylation and cell cycle events during abnormal tau phosphorylation and tau aggregation preceding neuronal death and neurodegeneration.
Material and methods
Animals
THY-Tau22 mice were generated and characterized as recently described with a construct containing human tau46 mutated at G272V and P301S under the Thy1.2 promoter (Schindowski et al. 2006). As indicated in the original characterization work of the model, all transgenic THY-Tau22 mice used in the present study were heterozygous. Non-transgenic littermates were used as controls for all experiments. Animals were housed in small social groups in standard cages with free access to water and chow (rodent standard diet, Altromin, Lage, Germany) and a 12 hours light–dark cycle. Some mice were treated with 50 mg/kg 5-bromo-2′-deoxyuridine (BrdU) (Sigma-Aldrich, Lyon, France) intraperitoneal (i.p.) for 7 days and sacrificed 14 days later. Animals were either killed by cervical dislocation, brains were dissected and stored at −80 °C or sequentially perfused with 0.9% NaCl and 4% paraformaldehyde in phosphate-buffered saline, the dissected brains were postfixed overnight in 4% paraformaldehyde and either dehydrated for paraffin-embedding or cryopreserved in 30% sucrose for cryosections. All experiments on animals were performed following the approval of the Institute of Laboratory Animal Resources Committee, in accordance with standards for the care and use of laboratory animals and with French and European Community rules.
Western blot analysis
Whole brains were dissected by separating the cortex from the hippocampus and thalamus. Hippocampus-enriched preparations were sonicated in Cell-Lysis Buffer (Cell Signaling Technology, Danvers, MA, USA) and then boiled at 100 °C for 10 minutes. Ten micrograms of total protein were resolved on sodium dodecyl sulphate–polyacrylamide gel electrophoresis, blotted onto nitrocellulose or polyvinylidene fluoride (PVDF) membranes (all from Invitrogen, Cergy Pontoise, France), incubated with appropriate antibodies (Table 1) and developed using the enhanced chemiluminescence kit (Amersham, Orsay, France). Protein levels were visualized and quantified using an imaging system (LAS-3000 2.0; Fuji Photo Film Co Ltd [Clichy, France]). For the detection of apoptosis, we used SH-SY5Y cells treated for 6 hours with 1 μM staurosporine (Sigma-Aldrich) as positive control and untreated SH-SY5Y as negative control (Delobel et al. 2003).
Table 1.
Specificity, dilution and source of antibodies used in this study
| Antibody | Species | Specificity or clone | Dilution | Source |
|---|---|---|---|---|
| AT8 | Mouse | Tau; pSer202/pThr205 | 1:10 000 | Innogenetics |
| AT100 | Mouse | Tau; pThr212/pSer214 | 1:2000 | Innogenetics |
| BrdU | Mouse | 5-bromo-2′-deoxyuridine | 1:10 | Roche |
| β-tubulin | Rabbit | T3526 | 1:1000 | Sigma |
| Caspase 3 | Rabbit | Full caspase 3 | 1:1000 | Cell Signaling |
| Cyclin B | Rabbit | M-20 | 1:1000 | SantaCruz Biotechnology |
| Cyclin D | Rabbit | 06-137 | 1:1000 (WB); 1:100 (IHC) | Upstate |
| Cyclin D1 | Rabbit | H-295 | 1:100 (IHC) | SantaCruz Biotechnology |
| DCX | Goat | Doublecortin C-18 | 1:1000 (WB); 1:100 (IHC) | SantaCruz Biotechnology |
| NeuroD | Rabbit | H-76 | 1:1000 (WB); 1:100 (IHC) | SantaCruz Biotechnology |
| p27KIP1 | Rabbit | C-19 | 1:1000 (WB); 1:100 (IHC) | SantaCruz Biotechnology |
| p21CIP1 | Rabbit | C-19 | 1:250 (WB); 1:100 (IHC) | SantaCruz Biotechnology |
| TUC-4 | Rabbit | ULIP1, CRMP4 | 1:5000 | Chemicon |
Innogenetics (Gent, Belgium); Roche (Mannheim, Germany); Sigma-Aldrich (Lyon, France); Cell Signalling Technologies (Danvers, MA, USA); SantaCruz Biotechnologies (Santa Cruz, CA, USA); Upstate/Millipore (Billerica, MA, USA); Chemicon (Limburg, Germany); WB, western blotting; IHC, immunohistochemistry.
Immunohistochemistry and immunofluorescence
Cryosections were cut at 14 μm. 5-bromo-2′-deoxyuridine and TdT-mediated biotin–dUTP nick-end labeling (TUNEL) stainings (both from Boehringer Mannheim-Roche, Mannheim, Germany) were performed according to the manufacturer’s instructions. At 6 months of age, THY-Tau22 exhibited changes in doublecortin (DCX), which were significantly altered and therefore 6 months were also chosen for BrdU experiments. DNAse I-treated sections were used as positive controls for TUNEL staining. Primary antibodies (listed in Table 1) were incubated overnight at 4 °C. All secondary antibodies including biotinylated-, fluorescein- and Texas Red-coupled, ABC-kit and DAB/Ni were from Vector Laboratories (Burlingame, CA, USA). Hematoxylin staining (Sigma-Aldrich) was performed according to standard procedures. Paraffin sections (8 μm) were Gallyas silver-stained according to Braak & Braak (1991).
Statistics
Data were quantified with NIH Image software, and statistics were analyzed by Student’s t-tests (Prism Graphpad, San Diego, CA, USA).
Results
Tau pathology in THY-Tau22 mice
In THY-Tau22 transgenic mice, brain sections exhibit hyperphosphorylation of tau [AT8-immunoreactivity (AT8-ir) tau phosphorylated at Ser202 and Thr205] and abnormal phosphorylation of tau [AT100-immunoreactivity (AT100-ir), tau phosphorylated at Thr212 and Ser214], NFT-like inclusions and NT (both Gallyas positive; Fig. 1) (Schindowski et al. 2006). In 3- to 6-month-old THY-Tau22 mice, tau hyperphosphorylation and NFTs are detected in the hippocampal formation and some cortical areas and their amount increases rapidly in aging (data not shown). The cell bodies of the dentate gyrus (DG) are relatively spared at young age, but their neurites show clearly AT8-ir (Fig. 1a,b). Tau phosphorylation and NFT-like inclusions were modest in the DG (Fig. 1c) but massively detected in pyramidal neurons of the CA1 subfield and the subiculum (Fig. 1d–f) in old animals.
Figure 1. Tau pathology in aged THY-Tau22.
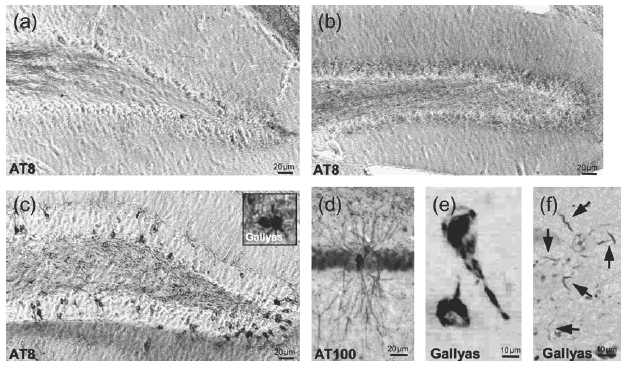
(a) AT8-ir in the DG in a 3-month-old THY-Tau22 mice. Tau hyperphosphorylation is restricted to axons but absent in cell bodies. (b) AT8-ir in the DG in a 6-month-old THY-Tau22 mice. Mossy fibers and molecular layer show tau hyperphosphorylation. (c) AT8-ir in the DG in a 15-month-old THY-Tau22 mice with massive degeneration of AT8-positive fibers and extensive AT8-ir of cell bodies fibers. Rare Gallyas-positive stained NFT-like inclusions were found in DG at this age (inset). (d) AD-specific AT100-ir indicative for abnormal tau phosphorylation in CA1 pyramidal neurons in THY-Tau22 at 12 months. (e) NFT-like inclusions (Gallyas positive) were found numerously in the CA1 and subiculum of THY-Tau22 at 12 months. (f) In 12-month-old THY-Tau22 mice, NT (Gallyas positive) occurred frequently in the subiculum (arrows). Representative photos are shown.
Neurodegeneration in aged THY-Tau22
To investigate the links between tau pathology and neuronal death, brain macroscopic and microscopic features were analyzed.
Brain mass and size of 13- to 16-month-old tau transgenic mice were mildly reduced by 6% compared with age- and sex-matched non-transgenic littermate controls (Fig. 2a). Lately, we had been shown a loss of pyramidal neurons in CA1 and subiculum by over 30% at this age (Schindowski et al. 2006). To investigate the presence of apoptosis in this model, we analyzed brain homogenates for caspase 3 cleavage (Fig. 2b). However, no active caspase 3 as sign for apoptotic cell death was found in brain tissues at any age in tau transgenic animals. Nevertheless, if apoptosis is not a main feature in this model, proteolytic products of caspase 3 may be diluted in brain homogenates. Thus, apoptosis markers were investigated at the neuronal level by histological procedures. Some TUNEL-positive pyramidal neurons were sometimes found in old THY-Tau22 mice in the CA1 subfield (Fig. 2c), while TUNEL staining was undetectable in control mice. Double staining with AT8 showed that apoptotic neurons were colocalized with phospho-tau (AT8-ir). Moreover, staining with the nuclear dye, DAPI, decreased from 12 months onwards in the CA1 region, indicating a progressive cell loss in THY-Tau22 mice. Therefore, apoptotic cell death seemed to be rather limited in our tau transgenic mouse, although the massive cell loss in CA1 and subiculum.
Figure 2. Neurodegeneration and apoptosis in old THY-Tau22.
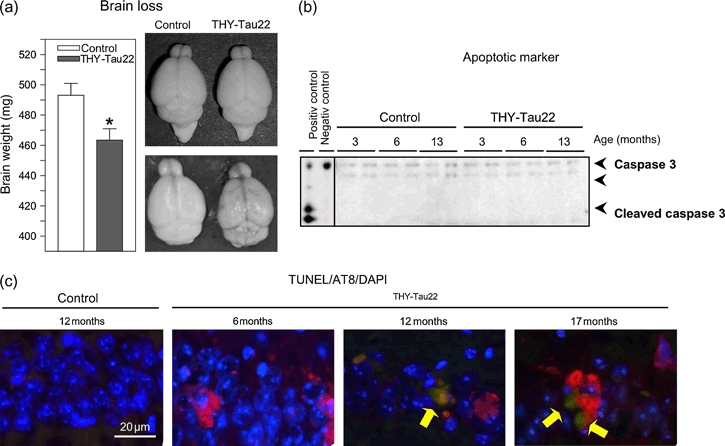
(a) Brain weight was significantly reduced (P < 0.014) in old THY-Tau22 mice compared with littermate controls (13–16 months) by 6%. Representative photographs of freshly prepared brains show a mild reduction in brain size. THY-Tau22: 463.4 ± 7.576 mg, n = 15; WT: 493.0 ± 8.025 mg, n = 12. (b) No cleavage of caspase 3 – as sign of apoptosis – was detectable in tau transgenic mice or in controls, indicating the absence of massive synchronized apoptotic cell death. Negative control, untreated growing SH-SY5Y cells; positive control, growing SH-SY5Y treated 1 μM staurosporine for 6 hours. n = 2 per genotype and age (c) TUNEL staining of the CA1-formation: TUNEL-positive cells (green, marked with arrows), AT8-positive cells (red) and the nuclear stain DAPI (blue) in control (12 months, n = 3) and THY-Tau22 mice at 6 months (n = 4), 12 months (n = 3) and 17 months (n = 1). Although TUNEL-positive cells were very rare, all of them colocalized with AT8-ir. The number of TUNEL-positive cells and AT8-ir increased in aged tau transgenic mice.*P < 0.05; WT, wildtype.
Increase of cell cycle-related proteins during tau aggregation
It had been shown that cell cycle re-entry results in neuronal death in differentiated neurons (Greene et al. 2004), and several reports observe regulation of cell cycle-related proteins during the pathogenesis of AD (Andorfer et al. 2005; Busser et al. 1998; Herrup & Busser 1995). Recently, we showed tau pathology, neuronal death and reactivation of cell cycle in differentiated neuroblastoma cells overexpressing the CDK5 activator p25 (Hamdane et al. 2005). However, no change in p35 cleavage into p25 was observed in the THY-Tau22 model (data not shown).
To link the effects of tau pathology caused by a FTDP-17 mutation on cell cycle events, major cell cycle-related proteins were investigated. Cyclin D1 is a protein that is required to leave the G0 phase and enter the G1 phase of the cell cycle and has been shown to be an early event leading to retinoblastoma (Rb) phosphorylation and the onset of neuronal death (Greene et al. 2004; Hamdane et al. 2005). In aged THY-Tau22 mice, during the stage when abnormal tau phosphorylation and tau aggregation occur, cyclin D1 was detected in brain homogenates, while it remained undetectable in young THY-Tau22 and control mice at any age (Fig. 3a). The hippocampal formation and cortical areas of tau transgenic animals contained cyclin D1-immunoreactive pyramidal neurons – with a nuclear localization (Fig. 3b). However, the total amount of detectable pyramidal neurons was rather low. Although several sections from non-transgenic age-matched control mice were analyzed, no cyclin D1 labeling was observed (Fig. 3b). The appearance of cyclin D1 is an early event in the normal cell cycle and a marker for G1 phase (for review, see Maller 1991; Nurse 1990; Pines 1993, 1995; Pines & Hunter 1994). In addition, levels of cyclin B1, the regulatory subunit of the cdc2-kinase, a relevant marker for G2 phase, were also increased in aged THY-Tau22 (Fig. 3a).
Figure 3. Upregulation of cell cycle-related proteins in old THY-Tau22.
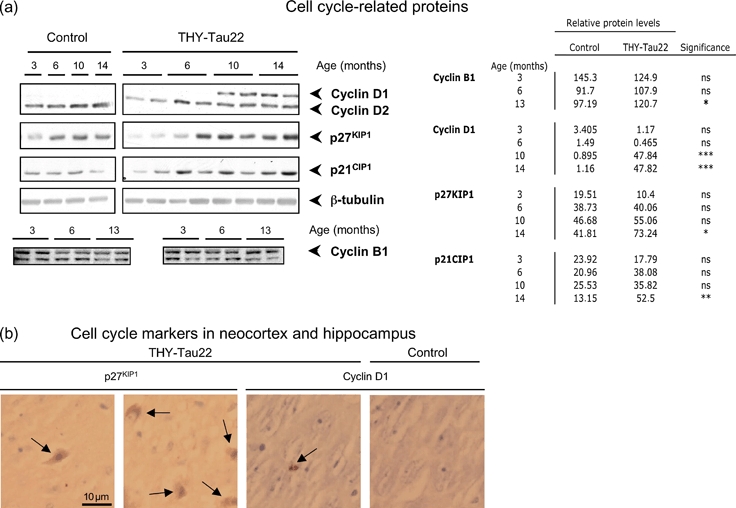
(a) Differential regulation of cell cycle-related proteins in hippocampal homogenates of THY-Tau22 and age-matched controls. Occurrence of cyclin D1 was the most notable change in tau transgenic mice, while protein levels of cyclin D2 did not change. p21CIP1 and p27KIP1 were also elevated in aged THY-Tau22. Cyclin B1 was mildly elevated. β-tubulin levels show that equal amounts of protein were loaded. Representative blots were shown; similar results were obtained with n = 3–6 mice per genotype and band intensities quantified. Mean optical densities are shown in the table. Significance was calculated with two-way analysis of variance and Bonferroni’s post-test. (b) In 13-month-old THY-Tau22 mice (n = 4), hippocampus and cortex stained for cell cycle markers cyclin D1 (CA1) and p27KIP1 (frontal cortex) were visualized with DAB (brown), counterstained with hematoxylin (blue) and similar field in an age-matched control mouse. Arrows indicate cells positive for the respective cell cycle marker. p27KIP1 was observed in controls and THY-Tau22, but the number of positive cells was increased in the latter. The cytoplasmic localization was identical. Cyclin D1 in nuclear localization was only found in old tau transgenics but not in age-matched controls.*P < 0.05; **P < 0.01; ***P < 0.001.
Increased neurogenesis during tau hyperphosphorylation
Interestingly, p21CIP1 and p27KIP1, both inhibitors of the cyclin D-CDK4 complex (Gartel & Radhakrishnan 2005; Kwon et al. 1996), were also elevated in the tau transgenic mice (Fig. 3a,b). Interestingly and similar to what is described in AD (Busser et al. 1998), the localization of p27KIP1 was mainly cytoplasmic, indicating that it was not active (Fig. 3b).
Because presence of cell cycle events may be related to neurogenesis, levels of DCX, TUC-4 and NeuroD, all being markers for neuronal differentiation (von Bohlen Und Halbach 2007), were analyzed in tau transgenic and control mice at different ages. First, DCX, a protein involved in neuronal differentiation and neurogenesis (Gleeson et al. 1998), was studied by immunohistochemistry. It was present in the DG of littermate control mice at 3 months but was significantly decreased at 6 months and undetectable by 12 months (Fig. 4a). The DCX-immunoreactivity (DCX-ir) in the DG of THY-Tau22 mice was increased at 3 months, and its decrease with age was delayed. At 6 months, the DCX levels were 2.2-fold higher than in the controls (Fig. 4a). Moreover, TUC-4 (also known as ULIP1 or CRMP4), a protein that is involved in neuronal maturation and differentiation (Cameron & McKay 2001), was also present at 3 months in both control and THY-Tau22 mice. In control mice, the levels of TUC-4 were decreased at 6 months and almost undetectable at 10–14 months, whereas surprisingly, the levels of TUC4 remained elevated in THY-Tau22 mice at 10 and 14 months (Fig. 4c).
Figure 4. Increased and delayed neurogenesis in adult THY-Tau22.
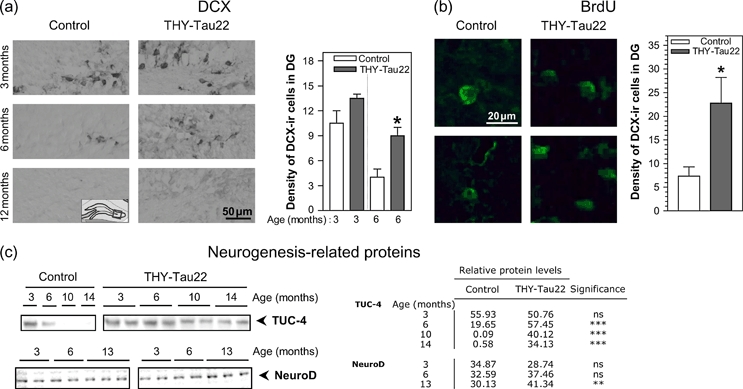
(a) DCX-ir in the DG of THY-Tau22 mice compared with WT at 3 months (n = 2), 6 months (n = 3 WT and n = 4 Thy-Tau22) and 12 months (n = 3). Representative images are shown. Note the age-related decay of DCX levels in control mice, while in THY-Tau22, increased numbers of DCX-ir cells were also observed at 6 months. Quantification of DCX-ir cells in the DG showed a significant increase at 6 months (*P < 0.05 at 6 months, n = 6 per genotype). Cell density is number of cells per field (photo taken with 10 × 10 magnification, approximately 190 × 100 μm). A coronal section (14 μm) showing the hippocampus around bregma −1.6 to −1.7 was used per animal. (b) 6-month-old mice were treated with BrdU i.p. for 7 days and sacrificed 14 days later. The number of BrdU-positive cells in the DG is significantly increased (*P < 0.05) in THY-Tau22 mice (n = 4) compared with littermate controls (n = 3). (c) Immunoblot analysis from hippocampal brain homogenates of proteins involved in neurogenesis and neuronal differentiation: TUC-4 is highly significantly and NeuroD mildly elevated in old THY-Tau22 mice compared with controls. Representative blots were shown and equal amounts loaded; similar results were obtained with n = 3–6 mice per genotype and band intensities quantified. Mean optical densities are shown in the table. Significance was calculated with two-way analysis of variance and Bonferroni’s post-test.
NeuroD is a basic helix-loop-helix transcription factor and is expressed at the time of the terminal differentiation into mature neurons (Lee et al. 1995). The levels of NeuroD were mildly increased in aged THY-Tau22 compared with age-matched littermate controls (Fig. 4c).
To determine if these increased levels of neurogenesis-related protein resulted also in increased amounts of newborn cells, 6-month-old mice were treated with the base-analog BrdU during 7 days and sacrificed 14 days later. The amount of BrdU-positive cells was nearly three times higher in the granule DG cell layer of THY-Tau22 mice compared with controls, indicating that the increase of de novo DNA synthesis resulted in viable cells (Fig. 4b). 5-bromo-2′-deoxyuridine staining was also observed in the subventricular zone and exceptionally in other brain areas.
Discussion
Tau hyperphosphorylation and tau aggregation appear to have differential effects on differentiation and cell death. While more and more reports propose a protective role of tau hyperphosphorylation (Hamdane et al. 2005; Lee et al. 2005), tau aggregation is supposed to contribute to neuronal death (Ramsden et al. 2005). Hyperphosphorylation had been shown to be reversible, while tau aggregation is not (Santacruz et al. 2005).
The aim of this study was to link tau pathology to cell cycle events and differentiation subsequent to neurogenesis.
In AD brains, there is an increase in proteins that are involved in neuronal differentiation and maturation (Jin et al. 2004; Yang et al. 2003). An important finding of this report was the presence of these proteins in young tau transgenic mice. The major site of adult neurogenesis, besides the subventricular zone, is the DG. It should be emphasized that at the age of 3–6 months, fibers of the granular cell layer of the DG were immunoreactive for tau phosphorylation, but their cell bodies were relatively spared from tau pathology. Abnormal phosphorylated tau (AT100) and NFTs were mostly absent at this age (Schindowski et al. 2006). Therefore, early molecular events, likely involved in the regulation of neurogenesis and plasticity, were detected even before the onset of somatic hyperphosphorylated tau deposits (Boekhoorn et al. 2006b).
From our data, it is difficult to conclude that these changes are only related to tau phosphorylation. On one hand, tau expression and/or mutation may also have an impact on neuronal physiology, such as maturation and splicing. For instance, it was described that 4-repeat tau isoforms regulate hippocampal neurogenesis and promote neuronal differentiation (Sennvik et al. 2007). Conversely, the neonatal 3-repeat isoform is present in proliferating progenitor cells and associated with DCX expression and BrdU incorporation during adult neurogenesis in the rat hippocampus (Bullmann et al. 2007; Mosch et al. 2007). TUC-4 regulation is observed in tau-positive stem cells during neuronal differentiation (Munoz-Elias et al. 2003). Moreover, TUC-4 is also involved in F-actin bundling (Rooslenbroich et al. 2005). Recent data from experimental models of tauopathies suggest some relationships between actin-binding proteins and tau pathology (Blard et al. 2007; Fulga et al. 2007). Thus, some links may exist between TUC-4 and tau pathology through actin network and increased phase of neuronal differentiation. Nevertheless, more work is needed to find the exact relationships. On the other hand, we cannot exclude that neurogenesis, an event that is predominantly present in young adults, is dependent on factors that are mainly present in young brains, and it should be emphasized that in the present model, tau hyperphosphorylation correlates with an increased neurogenesis. Nevertheless, it had been shown that even aged brains are able to increase neurogenesis in response to an environmental enrichment (Kempermann et al. 2002) or cerebral lesions (Dempsey & Kalluri 2007; Gray et al. 2002; Kokaia & Lindvall 2003). Neurogenesis-related proteins have also been observed in mice expressing non-mutant human tau (Andorfer et al. 2005) and tau mutations (Kuhn et al. 2007).
By contrast, the CA1 subfield, which is the brain region with the highest density of tau phosphorylation and NFT load in aged THY-Tau22 mice, shows later massive neurodegeneration and cell loss. Cyclin D1, a marker of neuronal cell death that is elevated in AD brain (Busser et al. 1998), was significantly increased by 10 months when tau phosphorylation and the formation of NFT are quite advanced in THY-Tau22. Interestingly, cyclin D1 is a key molecule of neurons leaving G0 and entering G1 (Greene et al. 2004). p21CIP1 and p27KIP1 are related proteins that inhibit cell cycle progression by interacting with cyclin D-CDK4 complex in the nucleus (el-Deiry et al. 1993; Gu et al. 1993; Harper et al. 1993; Polyak et al. 1994; Xiong et al. 1993). Moreover, p27 is essential for the translocation of cyclin D from the cytosol to the nucleus and is increased in tau models (Delobel et al. 2006) and AD brain and colocalized with NFT (Ogawa et al. 2003). The increase in p21 that is consistent with findings from different tau models (Delobel et al. 2006) and AD patients (Zhu et al. 2004). Figure 5a shows an overview of the regulation of cell cycle-related proteins during tau pathology: markers of G1 and G2 were significantly elevated during the stage of abnormal tau phosphorylation and tau aggregation and preceded neuronal loss.
Figure 5. Cell cycle related events during tau pathology.
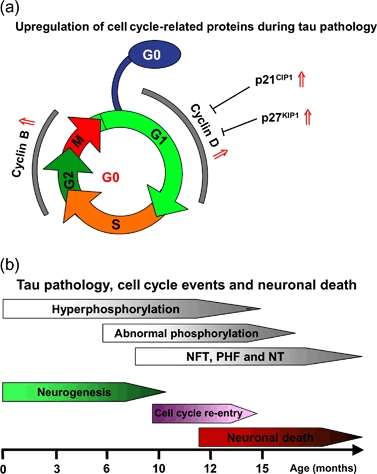
(a) Summary of observed cell cycle events in aged tau transgenic mice: Regulation of cyclin D during the G1 phase and cyclin B during G2 to mitosis transition. p21CIP1 and p27KIP1 inhibit the cyclin D/CDK4 complex. Changes in the THY-Tau22 mouse model are indicated with red arrows. indicates increase in proteins levels. (b) Summary of cell cycle events and neuronal death during different stages of tau pathology in THY-Tau22.
It was suggested that failure in synaptic plasticity may lead to cell cycle dysfunction (Arendt & Bruckner 2007). However, cell cycle events had been linked so far to Aβ toxicity in AD (Copani et al. 2002). Here, reactivation of cell cycle and neuronal death are described in a model of tauopathy, emphasizing the impact of tau pathology on neuronal cell cycle re-entry. Moreover, phosphorylation of the cell cycle protein Rb triggered by the tau kinase p25/Cdk5 and activation of E2F-responsive genes occur in neuronal death (Hamdane et al. 2005). Excitingly, here, we detected neuronal cell cycle re-entry during the stage of abnormal tau phosphorylation and tau aggregation but preceding neuronal death.
Cell death and neuronal loss (Schindowski et al. 2006) were detected by 12 months or later in our mouse model. Therefore, the re-entry of cell cycle is preceding neuronal death in THY-Tau22 as we have shown recently in a cell model (Hamdane & Buee 2007). A short summary with time lines for tau pathology, cell cycle events and neurodegeneration of our mouse model is sketched in Fig. 5b. Neurodegeneration and cell loss have also been observed in other tau transgenic mouse models with tau mutations at sites P301S (Allen et al. 2002), P301L (Gotz et al. 2001; Lewis et al. 2000), V337M (Tanemura et al. 2002) and R406W (Ikeda et al. 2005; Lim et al. 2001; Zhang et al. 2004). However, evidence of apoptosis was only observed in P301L-mutated tau mice (Gotz et al. 2001; Santacruz et al. 2005) and in mice expressing non-mutated human tau (Andorfer et al. 2005).
Consistent with findings of AD brain material and tau transgenic mice (Colurso et al. 2003), we observed few TUNEL-positive cells in THY-Tau22 mouse brain, although the number of TUNEL-positive cells was significantly increased compared with age-matched controls. The levels of cleaved caspase 3 remained undetectable, but it should be taken into consideration that cleavage of caspases is a rather short event, and therefore, their detection is difficult in a dynamic not-synchronous system such as the brain. Excitingly, cytoplasmic p21CIP1 has been reported to prevent apoptosis by inhibiting activation of caspase 3 (Asada et al. 1999; Suzuki et al. 1998). Our data are comparable with reports about the absence of active caspase 3 in AD brain and tau mouse models (Andorfer et al. 2005).
Altogether, these data suggest that cell cycle events in THY-Tau22 are resulting from neurogenesis in young animals and cell death in older ones. It suggests that neuronal cell death in such models is much more complex than believed.
Acknowledgments
This work was supported by a Marie Curie fellowship from the European Community and grants from the Deutsche Forschungsgemeinschaft (DFG – ZI 1143/1-1) and Ligue Européenne Contre La Maladie d‘Alzheimer (LECMA) to K.S., scholarships from Région Nord/Pas-de-Calais and CHRU-Lille to A.B. and K.B. and from the French Ministry for Foreign Affairs to K.A. and grants from Centre National de la Recherche Scientifique (CNRS), Fédération pour la Recherche sur le Cerveau, Gis-Longévité, Institut National de la Santé Et de la Recherche Médicale (INSERM), the European Community (APOPIS #LSHM-CT-2003-503330 and NEURAD #MEST-CT-2005-020013) and ADERMA (Association d’Etudes et de Recherche sur la maladie d‘Alzheimer) to L.B.
Conflicts of interest
This article was presented at a symposium on Alzheimer’s disease – new insights from animal models and molecular pathways, to be translated into human pathology which took place at the Genes, Brain and Behavior 2007 Society Annual Meeting, 21–25 May 2007, Doorwerth, the Netherlands. The symposium was sponsored by the European Commission [Marie Curie Early Stage Training, MEST-CT-2005-020013 (NEURAD), Alzheimer Ph.D. Graduate School].
The authors declare no conflicts of interest.
References
- Ahlijanian MK, Barrezueta NX, Williams RD, Jakowski A, Kowsz KP, McCarthy S, Coskran T, Carlo A, Seymour PA, Burkhardt JE, Nelson RB, McNeish JD. Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc Natl Acad Sci U S A. 2000;97:2910–2915. doi: 10.1073/pnas.040577797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen B, Ingram E, Takao M, Smith MJ, Jakes R, Virdee K, Yoshida H, Holzer M, Craxton M, Emson PC, Atzori C, Migheli A, Crowther RA, Ghetti B, Spillantini MG, Goedert M. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J Neurosci. 2002;22:9340–9351. doi: 10.1523/JNEUROSCI.22-21-09340.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andorfer C, Acker CM, Kress Y, Hof PR, Duff K, Davies P. Cell-cycle reentry and cell death in transgenic mice expressing nonmutant human tau isoforms. J Neurosci. 2005;25:5446–5454. doi: 10.1523/JNEUROSCI.4637-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt T, Bruckner MK. Linking cell-cycle dysfunction in Alzheimer’s disease to a failure of synaptic plasticity. Biochim Biophys Acta. 2007;1772:413–421. doi: 10.1016/j.bbadis.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Asada M, Yamada T, Ichijo H, Delia D, Miyazono K, Fukumuro K, Mizutani S. Apoptosis inhibitory activity of cytoplasmic p21(Cip1/WAF1) in monocytic differentiation. EMBO J. 1999;18:1223–1234. doi: 10.1093/emboj/18.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blard O, Feuillette S, Bou J, Chaumette B, Frebourg T, Campion D, Lecourtois M. Cytoskeleton proteins are modulators of mutant tau-induced neurodegeneration in Drosophila. Hum Mol Genet. 2007;16:555–566. doi: 10.1093/hmg/ddm011. [DOI] [PubMed] [Google Scholar]
- Boekhoorn K, Joels M, Lucassen PJ. Increased proliferation reflects glial and vascular-associated changes, but not neurogenesis in the presenile Alzheimer hippocampus. Neurobiol Dis. 2006a;24:1–14. doi: 10.1016/j.nbd.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Boekhoorn K, Terwel D, Biemans B, Borghgraef P, Wiegert O, Ramakers GJ, de Vos K, Krugers H, Tomiyama T, Mori H, Joels M, van Leuven F, Lucassen PJ. Improved long-term potentiation and memory in young tau-P301L transgenic mice before onset of hyperphosphorylation and tauopathy. J Neurosci. 2006b;26:3514–3523. doi: 10.1523/JNEUROSCI.5425-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bohlen und Halbach O. Immunohistological markers for staging neurogenesis in adult hippocampus. Cell Tissue Res. 2007;329:409–420. doi: 10.1007/s00441-007-0432-4. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev. 2000;33:95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- Bullmann T, de Silva R, Holzer M, Mori H, Arendt T. Expression of embryonic tau protein isoforms persist during adult neurogenesis in the hippocampus. Hippocampus. 2007;17:98–102. doi: 10.1002/hipo.20255. [DOI] [PubMed] [Google Scholar]
- Busser J, Geldmacher DS, Herrup K. Ectopic cell cycle proteins predict the sites of neuronal cell death in Alzheimer’s disease brain. J Neurosci. 1998;18:2801–2807. doi: 10.1523/JNEUROSCI.18-08-02801.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Colurso GJ, Nilson JE, Vervoort LG. Quantitative assessment of DNA fragmentation and beta-amyloid deposition in insular cortex and midfrontal gyrus from patients with Alzheimer’s disease. Life Sci. 2003;73:1795–1803. doi: 10.1016/s0024-3205(03)00512-5. [DOI] [PubMed] [Google Scholar]
- Copani A, Sortino MA, Caricasole A, Chiechio S, Chisari M, Battaglia G, Giuffrida-Stella AM, Vancheri C, Nicoletti F. Erratic expression of DNA polymerases by beta-amyloid causes neuronal death. FASEB J. 2002;16:2006–2008. doi: 10.1096/fj.02-0422fje. [DOI] [PubMed] [Google Scholar]
- el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- Delobel P, Mailliot C, Hamdane M, Sambo AV, Begard S, Violleau A, Delacourte A, Buee L. Stable-tau overexpression in human neuroblastoma cells: an open door for explaining neuronal death in tauopathies. Ann N Y Acad Sci. 2003;1010:623–634. doi: 10.1196/annals.1299.115. [DOI] [PubMed] [Google Scholar]
- Delobel P, Lavenir I, Ghetti B, Holzer M, Goedert M. Cell-cycle markers in a transgenic mouse model of human tauopathy: increased levels of cyclin-dependent kinase inhibitors p21Cip1 and p27Kip1. Am J Pathol. 2006;168:878–887. doi: 10.2353/ajpath.2006.050540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey RJ, Kalluri HS. Ischemia-induced neurogenesis: role of growth factors. Neurosurg Clin N Am. 2007;18:183–190. doi: 10.1016/j.nec.2006.10.011. xi. [DOI] [PubMed] [Google Scholar]
- Fulga TA, Elson-Schwab I, Khurana V, Steinhilb ML, Hyman BT, Feany MB. Abnormal bundling and accumulation of F-actin mediates tau-induced neuronal degeneration in vivo. Nat Cell Biol. 2007;9:139–148. doi: 10.1038/ncb1528. [DOI] [PubMed] [Google Scholar]
- Gartel AL, Radhakrishnan SK. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res. 2005;65:3980–3985. doi: 10.1158/0008-5472.CAN-04-3995. [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Allen KM, Fox JW, Lamperti ED, Berkovic S, Scheffer I, Cooper EC, Dobyns WB, Minnerath SR, Ross ME, Walsh CA. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell. 1998;92:63–72. doi: 10.1016/s0092-8674(00)80899-5. [DOI] [PubMed] [Google Scholar]
- Gotz J, Chen F, Barmettler R, Nitsch RM. Tau filament formation in transgenic mice expressing P301L tau. J Biol Chem. 2001;276:529–534. doi: 10.1074/jbc.M006531200. [DOI] [PubMed] [Google Scholar]
- Gray WP, May K, Sundstrom LE. Seizure induced dentate neurogenesis does not diminish with age in rats. Neurosci Lett. 2002;330:235–238. doi: 10.1016/s0304-3940(02)00810-8. [DOI] [PubMed] [Google Scholar]
- Greene LA, Biswas SC, Liu DX. Cell cycle molecules and vertebrate neuron death: E2F at the hub. Cell Death Differ. 2004;11:49–60. doi: 10.1038/sj.cdd.4401341. [DOI] [PubMed] [Google Scholar]
- Gu Y, Turck CW, Morgan DO. Inhibition of CDK2 activity in vivo by an associated 20K regulatory subunit. Nature. 1993;366:707–710. doi: 10.1038/366707a0. [DOI] [PubMed] [Google Scholar]
- Hamdane M, Buee L. The complex p25/Cdk5 kinase in neurofibrillary degeneration and neuronal death: the missing link to cell cycle. Biotechnol J. 2007;2:967–977. doi: 10.1002/biot.200700059. [DOI] [PubMed] [Google Scholar]
- Hamdane M, Sambo AV, Delobel P, Begard S, Violleau A, Delacourte A, Bertrand P, Benavides J, Buee L. Mitotic-like tau phosphorylation by p25-Cdk5 kinase complex. J Biol Chem. 2003;278:34026–34034. doi: 10.1074/jbc.M302872200. [DOI] [PubMed] [Google Scholar]
- Hamdane M, Bretteville A, Sambo AV, Schindowski K, Begard S, Delacourte A, Bertrand P, Buee L. p25/Cdk5-mediated retinoblastoma phosphorylation is an early event in neuronal cell death. J Cell Sci. 2005;118:1291–1298. doi: 10.1242/jcs.01724. [DOI] [PubMed] [Google Scholar]
- Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Herrup K, Busser JC. The induction of multiple cell cycle events precedes target-related neuronal death. Development. 1995;121:2385–2395. doi: 10.1242/dev.121.8.2385. [DOI] [PubMed] [Google Scholar]
- Husseman JW, Nochlin D, Vincent I. Mitotic activation: a convergent mechanism for a cohort of neurodegenerative diseases. Neurobiol Aging. 2000;21:815–828. doi: 10.1016/s0197-4580(00)00221-9. [DOI] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, et al. Association of missense and 5’-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Shoji M, Kawarai T, Kawarabayashi T, Matsubara E, Murakami T, Sasaki A, Tomidokoro Y, Ikarashi Y, Kuribara H, Ishiguro K, Hasegawa M, Yen SH, Chishti MA, Harigaya Y, Abe K, Okamoto K, St George-Hyslop P, Westaway D. Accumulation of filamentous tau in the cerebral cortex of human tau R406W transgenic mice. Am J Pathol. 2005;166:521–531. doi: 10.1016/S0002-9440(10)62274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, Greenberg DA. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004;101:343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G. Why new neurons? Possible functions for adult hippocampal neurogenesis. J Neurosci. 2002;22:635–638. doi: 10.1523/JNEUROSCI.22-03-00635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004;14:186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Lindvall O. Neurogenesis after ischaemic brain insults. Curr Opin Neurobiol. 2003;13:127–132. doi: 10.1016/s0959-4388(03)00017-5. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Cooper-Kuhn CM, Boekhoorn K, Lucassen PJ. Changes in neurogenesis in dementia and Alzheimer mouse models: are they functionally relevant? Eur Arch Psychiatry Clin Neurosci. 2007;257:281–289. doi: 10.1007/s00406-007-0732-4. [DOI] [PubMed] [Google Scholar]
- Kwon TK, Nagel JE, Buchholz MA, Nordin AA. Characterization of the murine cyclin-dependent kinase inhibitor gene p27Kip1. Gene. 1996;180:113–120. doi: 10.1016/s0378-1119(96)00416-7. [DOI] [PubMed] [Google Scholar]
- Lee JE, Hollenberg SM, Snider L, Turner DL, Lipnick N, Weintraub H. Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science. 1995;268:836–844. doi: 10.1126/science.7754368. [DOI] [PubMed] [Google Scholar]
- Lee HG, Perry G, Moreira PI, Garrett MR, Liu Q, Zhu X, Takeda A, Numomura A, Smith MA. Tau phosphorylation in Alzheimer’s disease: pathogen or protector. Trends Mol Med. 2005;11:164–169. doi: 10.1016/j.molmed.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Leroy K, Yilmaz Z, Brion JP. Increased level of active GSK-3beta in Alzheimer’s disease and accumulation in argyrophilic grains and in neurones at different stages of neurofibrillary degeneration. Neuropathol Appl Neurobiol. 2007;33:43–55. doi: 10.1111/j.1365-2990.2006.00795.x. [DOI] [PubMed] [Google Scholar]
- Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Paul Murphy M, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- Lim F, Hernandez F, Lucas JJ, Gomez-Ramos P, Moran MA, Avila J. FTDP-17 mutations in tau transgenic mice provoke lysosomal abnormalities and Tau filaments in forebrain. Mol Cell Neurosci. 2001;18:702–714. doi: 10.1006/mcne.2001.1051. [DOI] [PubMed] [Google Scholar]
- Maller JL. Mitotic control. Curr Opin Cell Biol. 1991;3:269–275. doi: 10.1016/0955-0674(91)90151-n. [DOI] [PubMed] [Google Scholar]
- Mosch B, Morawski M, Mittag A, Lenz D, Tarnok A, Arendt T. Aneuploidy and DNA replication in the normal human brain and Alzheimer’s disease. J Neurosci. 2007;27:6859–6867. doi: 10.1523/JNEUROSCI.0379-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Elias G, Woodbury D, Black IB. Marrow stromal cells, mitosis, and neuronal differentiation: stem cell and precursor functions. Stem Cells. 2003;21:437–448. doi: 10.1634/stemcells.21-4-437. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990;344:503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Ogawa O, Lee HG, Zhu X, Raina A, Harris PL, Castellani RJ, Perry G, Smith MA. Increased p27, an essential component of cell cycle control, in Alzheimer’s disease. Aging Cell. 2003;2:105–110. doi: 10.1046/j.1474-9728.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- Pines J. Cyclins and cyclin-dependent kinases: take your partners. Trends Biochem Sci. 1993;18:195–197. doi: 10.1016/0968-0004(93)90185-p. [DOI] [PubMed] [Google Scholar]
- Pines J. Cell cycle. Confirmational change. Nature. 1995;376:294–295. doi: 10.1038/376294a0. [DOI] [PubMed] [Google Scholar]
- Pines J, Hunter T. The differential localization of human cyclins A and B is due to a cytoplasmic retention signal in cyclin B. EMBO J. 1994;13:3772–3781. doi: 10.1002/j.1460-2075.1994.tb06688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massague J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind M, Schellenberg GD. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol. 1998;43:815–825. doi: 10.1002/ana.410430617. [DOI] [PubMed] [Google Scholar]
- Ramsden M, Kotilinek L, Forster C, Paulson J, McGowan E, SantaCruz K, Guimaraes A, Yue M, Lewis J, Carlson G, Hutton M, Ashe KH. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L) J Neurosci. 2005;25:10637–10647. doi: 10.1523/JNEUROSCI.3279-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooslenbroich V, Dai L, Baader SL, Noegel AA, Gieselmann V, Kappler J. Collapsin response mediator protein-4 regulates F-actin binding. Exp Cell Res. 2005;310:434–444. doi: 10.1016/j.yexcr.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Sahara N, Lewis J, DeTure M, McGowan E, Dickson DW, Hutton M, Yen SH. Assembly of tau in transgenic animals expressing P301L tau: alteration of phosphorylation and solubility. J Neurochem. 2002;83:1498–1508. doi: 10.1046/j.1471-4159.2002.01241.x. [DOI] [PubMed] [Google Scholar]
- Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindowski K, Bretteville A, Leroy K, Begard S, Brion JP, Hamdane M, Buee L. Alzheimer’s disease-like tau neuropathology leads to memory deficits and loss of functional synapses in a novel mutated tau transgenic mouse without any motor deficits. Am J Pathol. 2006;169:599–616. doi: 10.2353/ajpath.2006.060002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta A, Novak M, Grundke-Iqbal I, Iqbal K. Regulation of phosphorylation of tau by cyclin-dependent kinase 5 and glycogen synthase kinase-3 at substrate level. FEBS Lett. 2006;580:5925–5933. doi: 10.1016/j.febslet.2006.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sennvik K, Boekhoorn K, Lasrado R, Terwel D, Verhaeghe S, Korr H, Schmitz C, Tomiyama T, Mori H, Krugers H, Joels M, Ramakers GJ, Lucassen PJ, Van Leuven F. Tau-4R suppresses proliferation and promotes neuronal differentiation in the hippocampus of tau knockin/knockout mice. FASEB J. 2007;21:2149–2161. doi: 10.1096/fj.06-7735com. [DOI] [PubMed] [Google Scholar]
- Sergeant N, Delacourte A, Buee L. Tau protein as a differential biomarker of tauopathies. Biochim Biophys Acta. 2005;1739:179–197. doi: 10.1016/j.bbadis.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci U S A. 1998;95:7737–7741. doi: 10.1073/pnas.95.13.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Tsutomi Y, Akahane K, Araki T, Miura M. Resistance to Fas-mediated apoptosis: activation of caspase 3 is regulated by cell cycle regulator p21WAF1 and IAP gene family ILP. Oncogene. 1998;17:931–939. doi: 10.1038/sj.onc.1202021. [DOI] [PubMed] [Google Scholar]
- Tanemura K, Murayama M, Akagi T, Hashikawa T, Tominaga T, Ichikawa M, Yamaguchi H, Takashima A. Neurodegeneration with tau accumulation in a transgenic mouse expressing V337M human tau. J Neurosci. 2002;22:133–141. doi: 10.1523/JNEUROSCI.22-01-00133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- Yang Y, Mufson EJ, Herrup K. Neuronal cell death is preceded by cell cycle events at all stages of Alzheimer’s disease. J Neurosci. 2003;23:2557–2563. doi: 10.1523/JNEUROSCI.23-07-02557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Higuchi M, Yoshiyama Y, Ishihara T, Forman MS, Martinez D, Joyce S, Trojanowski JQ, Lee VM. Retarded axonal transport of R406W mutant tau in transgenic mice with a neurodegenerative tauopathy. J Neurosci. 2004;24:4657–4667. doi: 10.1523/JNEUROSCI.0797-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, McShea A, Harris PL, Raina AK, Castellani RJ, Funk JO, Shah S, Atwood C, Bowen R, Bowser R, Morelli L, Perry G, Smith MA. Elevated expression of a regulator of the G2/M phase of the cell cycle, neuronal CIP-1-associated regulator of cyclin B, in Alzheimer’s disease. J Neurosci Res. 2004;75:698–703. doi: 10.1002/jnr.20028. [DOI] [PubMed] [Google Scholar]


