Abstract
Recent data showed that soccer players in Italy had an unusually high risk of amyotrophic lateral sclerosis (ALS) and repeated head trauma might have contributed to this increase. The authors examined whether head injury was related to ALS risk in a case-control study of 109 ALS cases and 255 controls and conducted a meta-analysis of published literature. Overall ever having a head injury was non-significantly associated with a higher ALS risk. When compared with individuals without a head injury, a statistically significant ALS risk elevation was found for participants with more than one head injury (odds ratio (OR): 3.1, 95 percent confidence intervals (CI): 1.2, 8.1) or with head injury during the past 10 years (OR=3.2, 95 percent CI: 1.0, 10.2)). For participants with multiple head injuries in the past 10 years, the risk elevation was more than 11 fold. The meta-analysis also indicated a moderately elevated risk of ALS among individuals with previous head injuries (OR=1.7, 95 percent CI: 1.3, 2.2). In our study population, physical injuries of other body parts, including trunk, arms, or legs, were not related to ALS risk. These data support the notion that head injury may increase the risk of ALS.
Keywords: Amyotrophic Lateral Sclerosis, Case-Control Studies, Craniocerebral Trauma
Amyotrophic lateral sclerosis (ALS) is a severe neurodegenerative disease characterized by the progressive death of both upper and lower motor neurons. The incidence of ALS is very low in the general population (1) but sharply increases after age 40 years and peaks around age 75. ALS patients have a high mortality rate. More than 50 percent die within 3 years after the diagnosis.(1, 2) Mutations in the superoxide dismutase 1 gene are responsible for approximately 20 percent of the familial cases, or 1–2 percent of the overall ALS patient population, but for most sporadic cases the causes are not known.
Clinical observations and case-control studies suggest that physical trauma may be associated with a higher risk of ALS, but the evidence is far from conclusive.(3) Recently, two reports showed that ALS incidence and mortality were unusually high among professional soccer players in Italy.(4, 5) One hypothesis proposed to explain these findings is that repeated neurotrauma associated with heading the ball may increase the risk of ALS.(6) We therefore examined the relationship between head injury as well as injuries at other body sites and risk of ALS in a case-control study. We also conducted a meta-analysis to examine the relationship between head injury and ALS.
MATERIALS AND METHODS
Details of the study design have been published previously.(7–9) Briefly, ALS patients were recruited between 1993 and 1996 from two major referral centers in New England in the United States: the Neuromuscular Research Unit at the New England Medical Center and the Neurophysiology Laboratory at Brigham and Women’s Hospital. Sequential ALS patients diagnosed according to the standard criteria from the World Federation of Neurology(10) were considered eligible for the study if they had been diagnosed within two years, lived in New England for at least 50 percent of the year, spoke English, and were mentally competent. Of the 154 eligible cases, 2 could not be contacted, and 7 declined due to severe sickness, 15 due to travel difficulties, and 20 due to other reasons, leaving a total of 110 patients in our study. Population controls were selected using the same eligibility criteria plus the absence of a diagnosis of dementia, Parkinsonism, neuropathy, post-polio syndrome, ALS, or other motor neuron diseases. Controls were frequency-matched to cases by age (30–55, 56–65, and 66–80), gender, and telephone area code (as a surrogate for regions within New England), with a ratio of 3 to 1 for the Boston area and 2 to 1 for other areas of New England. These controls were selected by random telephone screening based on a modified Waksburg method with two-stage sampling. A total of 354 eligible controls were contacted and 270 (76 percent) were enrolled in the study; 256 completed the entire questionnaire. All study participants provided written informed consent, and the study protocol was approved by the Institutional Review Boards of the National Institute of Environmental Health Sciences, New England Medical Center, and Brigham and Women’s Hospital.
Exposure assessment
Information on demographic and lifestyle characteristics and on residential and occupational histories was collected using a structured questionnaire administered by trained interviewers. Cases were interviewed in person and controls by telephone. All participants were asked if they were ever injured so severely that they required medical attention, for example while playing a sport, in a fight, or in an accident. If they answered yes, we further asked how many times they were injured and at what ages the injuries occurred. For up to three accidents, participants were asked if it involved head, trunk, right arm, left arm, right leg or left leg. In the interview, they were also asked about other exposure information, including cigarette smoking history and highest level of education.
Statistical Analysis
Physical injury variables were categorized before data analyses. The reference date was defined as the date of diagnosis for cases and two years before the interview date for controls, as all cases were interviewed within two years of their first diagnosis. One case and one control reported inconsistent information across injury-related questions and therefore were classified as missing in the analysis, leaving us an analytic sample of 109 cases and 255 controls. Exposures were first defined as ever or never for overall injury as well as injuries at specific body sites. Further, for each body site, we defined exposures according to number of injuries (never injured, 1, >1), years since last injury (never injured, >30 years, 11–30 years, ≤10 years), age at last injury (never injured, <age 30, age 30–39, ≥age 40), and whether the same accident involved more than one body site (never injured, one site only, with other sites). Odds ratios (OR) and 95 percent confidence intervals (CI) were calculated from unconditional logistic regression models, first adjusting only for matching factors (age in 10 year groups, sex, and region/area codes) and then further for smoking (ever or never smokers) and educational achievement (≤high school vs >high school) as both variables were related to ALS risk in the study population. To test the robustness of the results, we repeated the analyses after excluding cases and controls who reported a family history of ALS (n=8 and 2 respectively) or injuries within 3 years of the reference date (n=2 and 3 respectively). All statistical analyses were conducted using SAS software (Cary, NC, USA).
Finally, we conducted a meta-analysis on the relationship between head injury and risk of ALS. A total of eight papers published since 1980 on head injury and ALS were identified by searching MEDLINE and the reference section of previous publications (Table 3). All but one study(11) were included in the meta-analysis together with the current study. The study by Gresham et al.(11) was excluded because the numbers were not consistent across the text and a risk estimate could not be calculated. For studies that did not provide a summary estimate, we calculated ORs and 95 percent CIs when possible using standard formulas. For individually matched case-control studies, the data were often presented in a way that makes matched risk estimate calculations impossible and therefore unmatched ORs and 95 percent CIs were calculated instead. Except in the current study, risk estimates were not adjusted for potential confounders. As there was no statistical heterogeneity among the risk estimates across studies (p for heterogeneity=0.1), the summary odds ratio was obtained with a fixed-effects model with STATA software (Version 8).
Table 3.
Summary of studies since 1980 on head injury in relation to ALS risk
| Design | Study population | Exposure assessment | Results | |
|---|---|---|---|---|
| Kurtzke,(25) USA | Case-control | 504 deceased veterans with ALS and 504 matched veteran controls | Military record abstraction; head injury was not specifically defined. | OR*†=1.0 (0.1, 7.1) |
| Kondo,(26) Japan | Case-control | 712 deceased MND cases and 637 spouse controls | In-person interview; head injury was not specifically defined. | Overall OR*=5.6 (2.5, 12.6) |
| Deapen,(27) USA | Case-control | 518 volunteer ALS survivors and 518 matched friend controls | Mailed questionnaire; defined as unconsciousness due to external (non-electrical) trauma, no further details. | OR=1.6 (1.0, 2.4) |
| Gallagher,(13) USA | Case-control | 135 prevalent ALS cases with onset <45 years, identified from two patient databases; 85 multiple sclerosis patients as controls | Mailed questionnaire; defined as head or neck injury, no further details. | OR*=1.7 (0.8, 3.4) |
| Gresham,(11) USA | Case-control | 66 cases from existing patient list and 66 matched friend or neighbor controls. | Mailed questionnaire; head injury was not specifically defined. | Numbers were not consistent across the text |
| Granieri,(28) Italy | Case-control | 72 MND cases primarily from a hospital and 216 matched hospital controls | Medical file abstraction; head injury was not specifically defined. | OR=1.0 (0.15, 6.81) |
| Williams,(17) USA | Retrospective cohort | 821 patients with documented head trauma | Medical record review; head trauma with presumed brain injury (concussion or skull fracture) | SMR‡=1.05 (0.027, 5.85) |
| Chio,(29) Italy | Case-control | 512 hospital MND cases and 512 matched hospital controls, mostly with neurological conditions | Medical record review; head injury was not specifically defined. | OR=0.8 (0.2, 1.2) |
| The Current study, USA | Case-control | 109 hospital based cases and 255 frequency matched community controls | In person or telephone interviews; severe head injury requiring medical attention | Adjusted OR=1.4 (0.8, 2.6) |
Odds ratio was not presented in the original paper and was thus calculated according to standard formula;
Matched analysis was impossible.
Standard morbidity ratio. Only 1 case was identified
OR: odds ratio
RESULTS
The population characteristics of cases and controls have been published previously(7–9) and thus are not presented here. The average age at diagnosis for cases was 58.3 years, on average 14.3 months after the self-reported onset of symptoms. Overall, 27 (24.8 percent) of the patients had a bulbar onset and 82 (75.2 percent) had an extremity onset. Among controls, approximately 45 percent reported an experience of severe physical injuries in their lifetime that required medical attention, and these injuries were most likely to occur on the legs (20.9 percent), followed by arms (19.2 percent), head (16.5 percent) and trunk (11.4 percent) (Table 1).
Table 1.
Association of ALS with physical injuries in a case-control study conducted in New England, USA (1993–1996)
| Injury site | Cases (%) | Controls (%) | OR (95% CI) |
|
|---|---|---|---|---|
| n=109 | n=255 | Model 1* | Model 2† | |
| Any sites | ||||
| No | 53.2 | 54.9 | 1.0 | 1.0 |
| Yes | 46.8 | 45.1 | 1.1 (0.7, 1.8) | 1.2 (0.7, 1.9) |
| Head | ||||
| No | 78.0 | 83.5 | 1.0 | 1.0 |
| Yes | 22.0 | 16.5 | 1.5 (0.8, 2.7) | 1.4 (0.8, 2.6) |
| Trunk | ||||
| No | 89.9 | 88.6 | 1.0 | 1.0 |
| Yes | 10.1 | 11.4 | 0.9 (0.4, 1.8) | 0.9 (0.4, 1.9) |
| Arm | ||||
| No | 88.1 | 80.8 | 1.0 | 1.0 |
| Yes | 11.9 | 19.2 | 0.6 (0.3, 1.1) | 0.6 (0.3, 1.1) |
| Leg‡ | ||||
| No | 79.8 | 79.1 | 1.0 | 1.0 |
| Yes | 20.2 | 20.9 | 1.0 (0.6, 1.8) | 1.0 (0.6, 1.8) |
Adjusted for matching factors (age, gender, and region/area code within New England).
Further adjusted for education and smoking.
One control had missing information on leg injury.
OR: odds ratio; CI: confidence interval
Overall, ever having an injury was not related to a higher risk of ALS (Table 1). Among individual body sites, only head injury tended to be associated with a higher risk of ALS (OR=1.4, 95 percent CI: 0.8, 2.6), but the association was not statistically significant. Detailed analyses (Table 2) indicated that having repeated head injuries or being injured within the past 10 years before diagnosis was each associated with a more than three-fold higher risk of ALS (OR=3.1, 95 percent CI: 1.2, 8.1; and 3.2, 95 percent CI: 1.0, 10.2, respectively). A post-hoc exploratory analysis based on small numbers showed that individuals with repeated head injuries in the past 10 years had more than an eleven-fold higher ALS risk than those without a head injury. Finally, cases with head injuries were more likely to have a bulbar onset (33.3 percent vs. 22.4 percent, p = 0.3) and an earlier age of diagnosis than cases without previous head injuries (54.0 years vs. 59.5 years, p = 0.05). Interestingly, three of the four cases (75 percent) with repeated head injuries in the 10 years before diagnosis had a bulbar onset, and despite the small sample size the proportion was significantly greater than that among cases without head injuries (Fisher’s exact test, p < 0.05). The overall results on head injury and ALS were essentially unchanged when we excluded participants with a family history of ALS or with a head injury within three years of the reference date, the only change being that the odds ratio for head injury within the past 10 years was even higher (OR=5.6, 95 percent CI: 1.3, 24.4) in the three year lag analysis. In contrast to head injury, no other injuries were related to a higher risk of ALS. The odd of having ALS was lower among individuals with a history of arm injury. However, further analyses on arm injury or injuries at any other body sites did not reveal consistent patterns of associations.
Table 2.
Association of ALS with head injury in a case-control study conducted in New England, USA, (1993–1996)
| Cases (%) | Controls (%) | OR (95% CI) | ||
|---|---|---|---|---|
| n=109 | n=255 | Model 1* | Model 2† | |
| Number of head injuries | ||||
| Never injured | 78.0 | 83.5 | 1.0 | 1.0 |
| 1 injury | 11.9 | 12.5 | 1.1 (0.5, 2.2) | 0.9 (0.4, 2.0) |
| >1 injuries | 10.1 | 3.9 | 2.9 (1.1, 7.6) | 3.1 (1.2, 8.1) |
| Years since last injury | ||||
| Never injured | 78.0 | 83.5 | 1.0 | 1.0 |
| >30 years | 4.6 | 5.9 | 0.9 (0.3, 2.7) | 0.9 (0.3, 2.7) |
| 10, 30 years | 10.1 | 8.2 | 1.4 (0.6, 3.1) | 1.2 (0.5, 2.9) |
| ≤10 years | 7.3 | 2.4 | 3.2 (1.0, 9.8) | 3.2 (1.0, 10.2) |
| Combination of number of head injuries and years since last injury | ||||
| Never injured | 78.0 | 83.5 | 1.0 | 1.0 |
| 1 injury | 11.9 | 12.5 | 1.1 (0.5, 2.2) | 0.9 (0.4, 1.9) |
| >1 injuries 10 years ago | 6.4 | 3.5 | 2.2 (0.7, 6.4) | 2.2 (0.7, 6.5) |
| >1 injuries within 10 years | 3.7 | 0.4 | 9.2 (0.9, 88.3) | 11.3 (1.1, 114.3) |
| Age at last injury | ||||
| Never injured | 78.0 | 83.5 | 1.0 | 1.0 |
| < age 30 | 11.9 | 12.2 | 1.2 (0.6, 2.4) | 1.1 (0.5, 2.3) |
| Age 30–40 | 2.8 | 2.0 | 1.5 (0.3, 6.5) | 1.4 (0.3, 6.7) |
| >age 40 | 7.3 | 2.4 | 2.7 (0.9, 8.3) | 2.8 (0.9, 8.9) |
| Involvement of other body sites in the same accident | ||||
| Never injured | 78.0 | 83.5 | 1.0 | 1.0 |
| Head only | 14.7 | 12.2 | 1.4 (0.7, 2.7) | 1.4 (0.7, 2.9) |
| With other body sites | 7.3 | 4.3 | 1.8 (0.7, 4.9) | 1.4 (0.5, 3.8) |
Adjusted for matching factors (age, gender, and region/area code within New England).
Further adjusted for education and smoking.
OR: odds ratio; CI: confidence interval
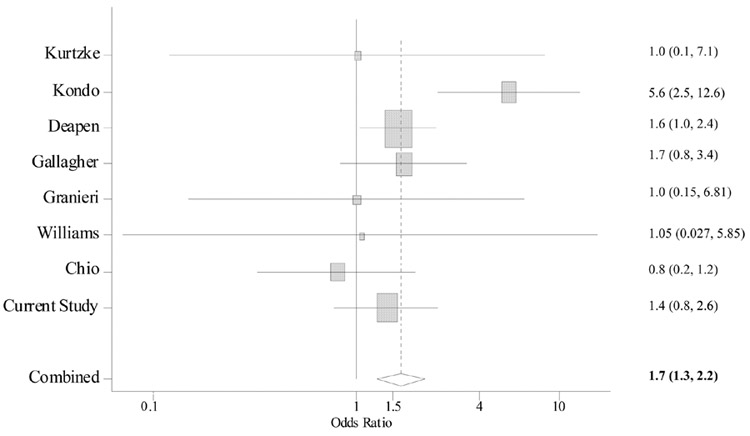
Most studies on physical trauma and ALS were published in the 1980s or early 1990s. In these studies, head injury was often ascertained along with injuries at other body sites and definition of head injury varied, mostly without any specifics (Table 3). The results also varied across studies, with odds ratios ranging from 0.8 to 5.6 (Figure). Nevertheless, the meta-analysis revealed a significant association between head injury and risk of ALS: compared with those without head injury, the risk of ALS was 1.7 times (95 percent CI: 1.3, 2.2, p < 0.001) higher among individuals with head injuries. Further analysis was impossible as nearly all studies lacked sufficient details.
Figure.
A meta-analysis of head injury in relation to risk of ALS. Overall odds ratios (OR) and 95 percent confidence interval (CI) were calculated according to a fixed-effects model. Squares indicate odds ratio of each study (in log scale). The size of the square is proportional to the percent weight of each study in the meta-analysis; horizontal line represents the 95 percent CI. The pooled odds ratio and 95 percent CI are indicated by the diamond. No statistical heterogeneity was found among the risk estimates across individuals studies (p for heterogeneity = 0.1)
DISCUSSION
ALS is the most common motor neuron disease. Its causes are largely unknown although likely involve environmental components. In this case-control study, head injury was associated with an elevated risk of ALS, particularly for recent repeated head injuries. Based on small numbers, the odd of having ALS was 11 times higher for individuals with more than one head injury in the 10 years prior to diagnosis. Consistent with the recent reports on ALS patients among Italian soccer players, ALS cases with a previous head injury were more likely to have an early and bulbar onset. The meta-analysis further supports a link between head injury and ALS. These findings are consistent with the hypothesis that head injury increases ALS risk. An alternative explanation is that the preclinical symptoms of ALS might have predisposed patients to a higher risk of physical injuries. Although the authors could not exclude this possibility, its plausibility is weakened by the lack of associations between ALS and overall injury or injuries at other parts of the body which were assessed along with head injury in the same structured question, and also by the fact that a slightly stronger association was found when injuries within 3 years of the reference date were excluded from the analysis. By the same token, a significant recall bias is not a likely explanation for our results as previous antecedent reports implicated more frequently injuries involving shoulders and arms(12, 13), which might have been more publicized among patients.
Recent data showed that ALS mortality among Italian professional soccer players was about 12-fold higher than expected,(4) while mortality from other causes was generally lower or comparable to that of the general population. This finding was subsequently confirmed by an incidence study among 7,325 Italian professional soccer players who played between 1970 and 2001.(5) The incidence was 6.5 times higher than expected, and cases were clinically characterized by early onset and bulbar involvement at diagnosis. Although both analyses were based on small numbers, the consistency of the results and the relative large effect estimates suggest that the association is probably not spurious. Several hypotheses have been proposed to explain this association, including vigorous physical activity, use of performance enhancing drugs, excessive pesticide exposures, and head trauma.(5) The head trauma explanation was particularly interesting because three of the five patients in the incidence study(5) had a bulbar onset that could be linked to the soccer specific head trauma and also because some prior data support a link between head trauma and ALS.
An association between physical trauma and ALS has been suggested by many case reports(12, 14) and was evaluated in several case-control studies.(3) In some studies, the nature of physical trauma was not clearly specified, and in others the definitions varied across studies, from fractures, mechanical injuries, and electric shock, to surgery. Not surprisingly, the results from these studies are inconsistent. The studies were further limited by small study sizes, inadequate exposure assessments, use of convenience controls, and lack of adjustment for potential confounders, and therefore suffered from low statistical power, confounding, and a variety of potential biases.(3) Two recent population-based case-control studies both failed to show an association between overall trauma and risk of ALS.(15, 16) However, neither study provided specific information on injury site or the number of injuries. Of these previous studies, a few included head injury in the analyses (Table 3). All but one were case-control in design and suffered from many of the same limitations as mentioned above. Further, in these studies, rarely were any specific details on head injury presented. The only prospective study included 821 patients with documented head injury but only one ALS patient was identified during the follow-up.(17) Although these studies all suffered from a variety of limitations, the meta-analysis provides preliminary evidence for a positive association between head injury and risk of ALS.
Consistently, the detailed analyses from our study support this link and further suggest that recent repeated injuries may be more etiologically relevant. Recent findings from both clinical observations of asymptomatic SOD1 mutant carriers(18) and experimental studies of SOD1 mutant mice(19) suggest that the preclinical motor neuron loss in ALS, like its post-diagnosis clinical course, may also be dramatic and aggressive, indicating the importance of recent acute exposures or accumulative exposures over exposures in the distant past. Our observation of a stronger association with recent repeated head injuries is consistent with this notion.
The mechanisms by which head injury may be implicated in ALS are not known. However, several biological explanations have been proposed for a possible role of brain injury in other common neurodegenerative diseases, including Parkinson’s disease and Alzheimer’s disease or dementia.(20, 21) These diseases share some clinical, pathological, and epidemiological characteristics with ALS and, on rare occasions, occur together.(22) Proposed mechanisms include trauma related neuro-inflammation and microglial activation, disruption of the blood-brain barrier, mitochondrial dysfunction and excessive oxidative and nitric radicals, and the accumulation of tau protein.(20, 21) While the relevance of these explanations to our observation is unknown, they should be evaluated in future studies of ALS.
This case-control study was among one of the well-conducted epidemiological studies on ALS (23, 24). Compared with previous investigations on head injury and ALS, the current study was better designed with a clearly defined study population. Further, the authors collected much more detailed information on head injury and, for the first time, were able to show a clear relationship between recent repeated head injury and a higher risk of ALS. Finally, multivariate analyses were conducted to adjust for several potential confounders.
The major limitation of this study is that cases and controls were interviewed by different methods and therefore potential bias from this source is of concern. Although the authors could not exclude this possibility, the lack of associations with injuries at other body sites, such as arms or shoulders, argues against a substantial bias from this source. As with many of the previous studies, the statistical analyses were limited by small sample sizes. Therefore, the authors can not exclude chance as a potential explanation for the findings. Finally, the exposure assessment in current study was not independently validated and the meaning of severe injury is, to some extent, open to individual interpretations. Further the definition of head injury requiring medical attention may, to some extend, limit the generalizability of our findings. Therefore, future investigations should be larger and should collect more details about each episode of head injury among various populations.
In summary, in line with the recent observations of higher ALS risk among Italian soccer players, this study reported that recent repeated head injury was related to a higher risk of ALS in an American population. Therefore, a possible role of head injury in ALS etiology should be further evaluated in both human and experimental studies.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences
Abbreviations
- OR
odds ratio
- CI
confidence interval
- ALS
amyotrophic lateral sclerosis
References
- 1.Logroscino G, Beghi E, Zoccolella S, et al. Incidence of amyotrophic lateral sclerosis in southern Italy: a population based study. J Neurol Neurosurg Psychiatry. 2005;76:1094–1098. doi: 10.1136/jnnp.2004.039180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Millul A, Beghi E, Logroscino G, Micheli A, Vitelli E, Zardi A. Survival of patients with amyotrophic lateral sclerosis in a population-based registry. Neuroepidemiology. 2005;25:114–119. doi: 10.1159/000086353. [DOI] [PubMed] [Google Scholar]
- 3.Kurland LT, Radhakrishnan K, Smith GE, Armon C, Nemetz PN. Mechanical trauma as a risk factor in classic amyotrophic lateral sclerosis: lack of epidemiologic evidence. J Neurol Sci. 1992;113:133–143. doi: 10.1016/0022-510x(92)90241-c. [DOI] [PubMed] [Google Scholar]
- 4.Belli S, Vanacore N. Proportionate mortality of Italian soccer players: Is amyotrophic lateral sclerosis an occupational disease? European Journal of Epidemiology. 2005;20:237–242. doi: 10.1007/s10654-004-6879-7. [DOI] [PubMed] [Google Scholar]
- 5.Chio A, Benzi G, Dossena M, Mutani R, Mora G. Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain. 2005;128:472–476. doi: 10.1093/brain/awh373. [DOI] [PubMed] [Google Scholar]
- 6.Piazza O, Siren AL, Ehrenreich H. Soccer, neurotrauma and amyotrophic lateral sclerosis: is there a connection? Curr Med Res Opin. 2004;20:505–508. doi: 10.1185/030079904125003296. [DOI] [PubMed] [Google Scholar]
- 7.Kamel F, Umbach DM, Munsat TL, Shefner JM, Hu H, Sandler DP. Lead exposure and amyotrophic lateral sclerosis. Epidemiology. 2002;13:311–319. doi: 10.1097/00001648-200205000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Kamel F, Umbach DM, Munsat TL, Shefner JM, Sandler DP. Association of cigarette smoking with amyotrophic lateral sclerosis. Neuroepidemiology. 1999;18:194–202. doi: 10.1159/000026211. [DOI] [PubMed] [Google Scholar]
- 9.Longnecker MP, Kamel F, Umbach DM, et al. Dietary intake of calcium, magnesium and antioxidants in relation to risk of amyotrophic lateral sclerosis. Neuroepidemiology. 2000;19:210–216. doi: 10.1159/000026258. [DOI] [PubMed] [Google Scholar]
- 10.Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial "Clinical limits of amyotrophic lateral sclerosis" workshop contributors. J Neurol Sci. 1994;124 Suppl:96–107. doi: 10.1016/0022-510x(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 11.Gresham LS, Molgaard CA, Golbeck AL, Smith R. Amyotrophic lateral sclerosis and history of skeletal fracture: a case-control study. Neurology. 1987;37:717–719. doi: 10.1212/wnl.37.4.717. [DOI] [PubMed] [Google Scholar]
- 12.Jelliffe S. The amyotrophic lateral sclerosis syndrome and trauma. J Nerv Ment Dis. 1935;82:415–435. [Google Scholar]
- 13.Gallagher JP, Sanders M. Trauma and amyotrophic lateral sclerosis: a report of 78 patients. Acta Neurol Scand. 1987;75:145–150. doi: 10.1111/j.1600-0404.1987.tb07909.x. [DOI] [PubMed] [Google Scholar]
- 14.Riggs JE. Antecedent trauma and amyotrophic lateral sclerosis in young adult men. Mil Med. 1993;158:55–57. [PubMed] [Google Scholar]
- 15.Chancellor AM, Slattery JM, Fraser H, Warlow CP. Risk factors for motor neuron disease: a case-control study based on patients from the Scottish Motor Neuron Disease Register. J Neurol Neurosurg Psychiatry. 1993;56:1200–1206. doi: 10.1136/jnnp.56.11.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cruz DC, Nelson LM, McGuire V, Longstreth WT., Jr. Physical trauma and family history of neurodegenerative diseases in amyotrophic lateral sclerosis: a population-based case-control study. Neuroepidemiology. 1999;18:101–110. doi: 10.1159/000069413. [DOI] [PubMed] [Google Scholar]
- 17.Williams DB, Annegers JF, Kokmen E, O'Brien PC, Kurland LT. Brain injury and neurologic sequelae: a cohort study of dementia, parkinsonism, and amyotrophic lateral sclerosis. Neurology. 1991;41:1554–1557. doi: 10.1212/wnl.41.10.1554. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal A, Nicholson G. Detection of preclinical motor neurone loss in SOD1 mutation carriers using motor unit number estimation. J Neurol Neurosurg Psychiatry. 2002;73:199–201. doi: 10.1136/jnnp.73.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feeney SJ, McKelvie PA, Austin L, et al. Presymptomatic motor neuron loss and reactive astrocytosis in the SOD1 mouse model of amyotrophic lateral sclerosis. Muscle Nerve. 2001;24:1510–1519. doi: 10.1002/mus.1176. [DOI] [PubMed] [Google Scholar]
- 20.Szczygielski J, Mautes A, Steudel WI, Falkai P, Bayer TA, Wirths O. Traumatic brain injury: cause or risk of Alzheimer's disease? A review of experimental studies. J Neural Transm. 2005;112:1547–1564. doi: 10.1007/s00702-005-0326-0. [DOI] [PubMed] [Google Scholar]
- 21.Goldman SM, Tanner CM, Oakes D, Bhudhikanok GS, Gupta A, Langston JW. Head injury and Parkinson's disease risk in twins. Annals of Neurology. 2006;60:65–72. doi: 10.1002/ana.20882. [DOI] [PubMed] [Google Scholar]
- 22.Plato CC, Galasko D, Garruto RM, et al. ALS and PDC of Guam: forty-year follow-up. Neurology. 2002;58:765–773. doi: 10.1212/wnl.58.5.765. [DOI] [PubMed] [Google Scholar]
- 23.Armon C. An evidence-based medicine approach to the evaluation of the role of exogenous risk factors in sporadic amyotrophic lateral sclerosis. Neuroepidemiology. 2003;22:217–228. doi: 10.1159/000070562. [DOI] [PubMed] [Google Scholar]
- 24.Nelson LM, McGuire V, Longstreth WT, Jr., Matkin C. Population-based case-control study of amyotrophic lateral sclerosis in western Washington State. I. Cigarette smoking and alcohol consumption. Am J Epidemiol. 2000;151:156–163. doi: 10.1093/oxfordjournals.aje.a010183. [DOI] [PubMed] [Google Scholar]
- 25.Kurtzke JF, Beebe GW. Epidemiology of amyotrophic lateral sclerosis: 1. A case-control comparison based on ALS deaths. Neurology. 1980;30:453–462. doi: 10.1212/wnl.30.5.453. [DOI] [PubMed] [Google Scholar]
- 26.Kondo K, Tsubaki T. Case-control studies of motor neuron disease: association with mechanical injuries. Arch Neurol. 1981;38:220–226. doi: 10.1001/archneur.1981.00510040046007. [DOI] [PubMed] [Google Scholar]
- 27.Deapen DM, Henderson BE. A case-control study of amyotrophic lateral sclerosis. Am J Epidemiol. 1986;123:790–799. doi: 10.1093/oxfordjournals.aje.a114308. [DOI] [PubMed] [Google Scholar]
- 28.Granieri E, Carreras M, Tola R, et al. Motor neuron disease in the province of Ferrara, Italy, in 1964–1982. Neurology. 1988;38:1604–1608. doi: 10.1212/wnl.38.10.1604. [DOI] [PubMed] [Google Scholar]
- 29.Chio A, Meineri P, Tribolo A, Schiffer D. Risk factors in motor neuron disease: a case-control study. Neuroepidemiology. 1991;10:174–184. doi: 10.1159/000110267. [DOI] [PubMed] [Google Scholar]