Abstract
Alterations in pathways mediated by retinoblastoma susceptibility gene (RB) product are among the most common in human cancer. Mice with a single copy of the Rb gene are shown to develop a syndrome of multiple neuroendocrine neoplasia. The earliest Rb-deficient atypical cells were identified in the intermediate and anterior lobes of the pituitary, the thyroid and parathyroid glands, and the adrenal medulla within the first 3 months of postnatal development. These cells form gross tumors with various degrees of malignancy by postnatal day 350. By age of 380 days, 84% of Rb+/− mice exhibited lung metastases from C-cell thyroid carcinomas. Expression of a human RB transgene in the Rb+/− mice suppressed carcinogenesis in all tissues studied. Of particular clinical relevance, the frequency of lung metastases also was reduced to 12% in Rb+/− mice by repeated i.v. administration of lipid-entrapped, polycation-condensed RB complementary DNA. Thus, in spite of long latency periods during which secondary alterations can accumulate, the initial loss of Rb function remains essential for tumor progression in multiple types of neuroendocrine cells. Restoration of RB function in humans may prove an effective general approach to the treatment of RB-deficient disseminated tumors.
In humans retinoblastoma susceptibility gene (RB) is inactivated in all familial and sporadic retinoblastomas and in 90% of small cell lung carcinomas (1–3). Loss of RB function also occurs less often in a variety of other human tumors, including osteosarcomas and tumors of the mammary gland and prostate. Previous studies have provided remarkable insights about RB structure, biochemical properties, and possible RB functions (for review see refs. 4–7). The original observations of RB tumor-suppressive activity were based on xenogeneic models. RB-mediated suppression of malignant growth was observed in the nude mice implanted with retinoblastoma, osteosarcoma, as well as carcinomas of prostate, lung, bladder, and breast (reviewed in ref. 4). These studies implied that introduction of RB into RB-deficient cells may result in suppression of neoplastic phenotype in clinical settings.
The subsequent development of mouse models made it feasible to test the hypothesis of tumor suppression by RB in vivo. Although information on the Rb status of sporadic tumors in mice is not available, mice with a single wild-type copy of Rb, as well as Rb+/+/Rb−/− chimeras, reproducibly develop Rb-deficient melanotroph tumors of the pituitary intermediate lobe (8–14), and those of certain strains also develop C-cell thyroid carcinomas (12, 13, 15) and hyperplasia of the adrenal medulla (9, 15). A defined anatomical site, high penetrance, and synchronous development of melanotroph tumors allowed the precise evaluation of carcinogenesis associated with Rb deficiency (11). By using this model, loss of the wild-type Rb was demonstrated to be the earliest identifiable event during neoplastic transformation of melanotrophs in Rb+/− mice. To evaluate tumor suppressor potential in vivo, a recombinant adenovirus-5 vector expressing full-length human RB cDNA was delivered by direct injection into the pituitary intermediate lobe (16). Intratumoral RB transfer decreased tumor cell proliferation, re-established innervation by growth-regulatory dopaminergic neurons, inhibited the growth of tumors, and significantly prolonged the life spans of treated animals by a mean of ≈20% compared with controls.
The universal character of these observations has remained unclear because melanotroph tumors are exceptionally rare in humans. The discrepancy in the spectrum of Rb-deficient tumors between mice and humans prompted us to evaluate further mouse neoplasia associated with loss of Rb function. The present studies demonstrate that Rb+/− mice suffer from a syndrome of multiple neuroendocrine neoplasia and metastatic disease. Intriguingly, some neoplasia closely resemble human tumors. By using these models, we examine directly whether the loss of Rb is a universally early and persistent requirement of carcinogenesis. First, we delineate the earliest morphologically atypical cells in multiple target tissues, and by using microdissection-PCR genotyping, demonstrate absence of the wild-type Rb in those cells. Second, we use a transgenic mouse model to conditionally turn on expression of exogenous RB in the Rb-deficient cells and demonstrate suppression of their progression in a number of tissues. Third, we apply highly efficient nonviral vectors to deliver RB into Rb-deficient metastatic cells in lungs and demonstrate dramatic reduction of lung metastasis.
MATERIALS AND METHODS
Animals.
All experiments were performed on siblings maintained in the same room on the same diet. The origin of Rb+/− mice has been described (17). Preparation and characterization of bitransgenic RTgRB mice containing a tetracycline-responsive transactivator (18) under the control of the RB promoter (TgRBp-tTA; ref. 19), and a minimal cytomegalovirus promoter-tet operator (18) fused to the wild-type human RB cDNA (TghCMV*-1p-RB) will be described in detail elsewhere (A.Y.N., B. Shan, A. Flesken-Nikitin, K.-H. Chang, and W.-H.L., unpublished work). Identification of Rb+/− and RTgRB mice by PCR genotyping has been described (11, 20).
Collection and Morphological Analyses of Animal Material.
After anesthetization with avertin, animals were subjected to cardiac perfusion at 90 mmHg with phosphate-buffered 4% paraformaldehyde. The brain, pituitary and thyroid glands, salivary glands, trachea, esophagus, thymus, smooth and striated muscle, lung, mediastinum, stomach, small and large intestine, liver, kidney, adrenal glands, gonads, spleen, pancreas, lymph nodes, and uterus or prostate were examined during autopsy, and representative specimens were further characterized by microscopic analysis of paraffin sections stained with hematoxylin and eosin as described (11). At least 10 pituitary, thyroid, and adrenal glands were obtained on postnatal day (P) 35, P60, P90, P120, P140, and P180. Serial sectioning and sequential three-dimensional reconstruction of specimens were performed as described (11).
Immunohistochemical Analyses.
Immunohistochemical analysis of paraffin sections of paraformaldehyde-fixed tissue was performed by a modified avidin-biotin-peroxidase technique (11). The antibodies used to detect α-melanocyte-stimulating hormone, Rb, and BrdUrd have been described (11). The antibodies to growth hormone (1:100 dilution), to the β subunits of luteinizing hormone (1:100) or thyroid-stimulating hormone (1:100), to α-glycoprotein subunit (α-GSU, 1:100), to prolactin (1:300), and to adrenocorticotropic hormone (1:1,000) (all of which were from the National Hormone and Pituitary Program of the National Institute of Diabetes and Digestive and Kidney Diseases), to follicle-stimulating hormone (1:500; Dako), to calcitonin (1:1,000; Peninsula Laboratories), or to parathyroid hormone (1:500; Peninsula Laboratories) were incubated with deparaffinized sections for 1 hr at room temperature. Sections subsequently were incubated for 30 min at room temperature with biotinylated secondary antibodies (anti-guinea pig for luteinizing hormone, anti-monkey for growth hormone, and anti-rabbit for the other hormones; 1:200 dilution in 2% normal mouse serum), and immune complexes then were detected with ABC Elite (Vector Laboratories) by incubation for 30 min at room temperature. The percentage of metastatic cells labeled with BrdUrd (BrdUrd index) was detected essentially as described earlier (11, 16). More than 500 metastatic cells of at least three animals were scored to estimate the BrdUrd index. For detection of lung metastases at least 10 serial sections of each lung from at least three animals were stained with calcitonin-specific antibodies and evaluated under microscope at magnification ×600.
Lipid-Entrapped, Polycation-Condensed DNA Complex (LPD)-Mediated Gene Transfer.
The RB construct (RBp-RB) containing 1.6 kb of the RB promoter, 2.8 kb of RB cDNA, and 1.6 kb of the β-globin polyadenylation site was described (21). To prepare the RBp-RBH209 construct the wild-type RB cDNA of RBp-RB was substituted with the 2.8-kb BamHI fragment derived from pCMVNeoRBH209 (23). This fragment contains cDNA encoding RB with a single Cys706 to Phe substitution (22). LPDs were prepared essentially as described (24–27). According to previous titration studies 2.5 μg of construct in 15 μl per gram body weight were optimal for i.v. administration.
PCR Analyses of DNA and RNA.
Microdissection of cells, DNA isolation, and subsequent genotyping have been described in detail (11). Preparation of mRNA and estimation of relative amounts of Rb/RB cDNAs were performed with the following modifications of the original protocol (20). About 1,000 thyroid C cells and lung metastatic cells were collected by microdissection from frozen sections and applied to Hybond-mAP paper (Amersham Pharmacia). The primers for Rb and RB were: 5′-ATACACTCTGTGCACGCCTTCTGTC-3′ (Rb ex20–5′; sense) and Rb ex21–3′ (antisense; ref. 20). β-Actin transcripts were detected with primers AC6 (sense) and AC5 (antisense; ref. 20). Parallel kinetics of β-actin, Rb, and RB amplifications were observed until after 25 PCR cycles. In test titration experiments ≥25% differences could be reproducibly detected.
Immunoprecipitation–Western Blotting Analyses.
Tissue extracts were subjected to immunoprecipitation and immunoblot analysis with mAb 11D7 (28), which is specific for human RB protein and with mAb 245 (29) and C-15 antiserum (Santa Cruz Biotechnology), both of which recognize both human RB (110 kDa) and mouse Rb (105 kDa), as indicated in ref. 21.
Statistical Analyses.
All statistical analyses were performed with the programs instat and inplot (GraphPad, San Diego).
RESULTS AND DISCUSSION
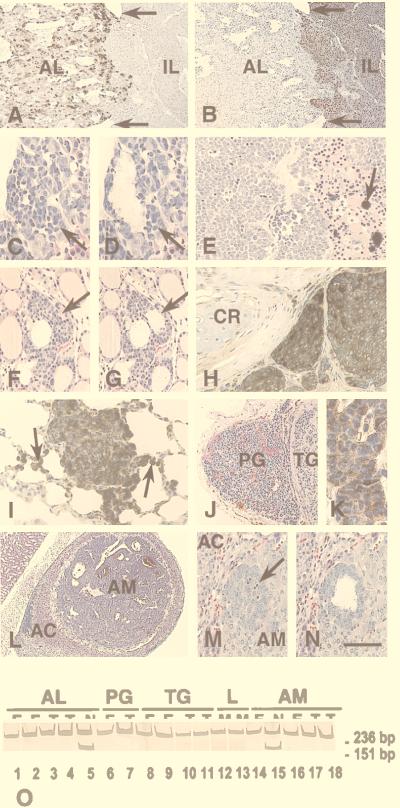
Multiple tissues were obtained from 69 Rb+/− mice in the moribund condition, as well as from 38 wild-type littermates of matched age. The cause of terminal status in most Rb+/− mice was brainstem compression by pituitary tumors, with a small number of animals experiencing asphyxia caused by tracheal compression by large (>2 cm in diameter) thyroid tumors. Topographic histological analyses revealed that, in addition to typical melanotroph tumors described in detail (11), 22% of pituitaries from Rb+/− mice contained highly vascularized regions of polymorphic tumor cells in the anterior lobe that were dissimilar to those in the intermediate lobe (Fig. 1 A and B; Table 1). Unlike melanotrophs, these cells did not express α-melanocyte-stimulating hormone (Fig. 1B), but they always contained the α-GSU (8 of 8 animals, 50–95% positive cells) (Fig. 1A) and sometimes expressed growth hormone (5 of 8 animals, 5–40% positive cells) and the β subunit of thyroid-stimulating hormone (4 of 8 animals, 5–60% positive cells). Serial sections of glands from younger animals confirmed that the α-GSU-positive cells originated from the anterior lobe. The first groups of morphologically atypical cells (EAPs, for regions of early atypical proliferation) were detected as early as P90 (Fig. 1 C and D). Similar to atypical melanotrophs (11), early atypical cells of the pituitary anterior lobe did not contain a wild-type Rb allele in 6 of 6 animals subjected to genotype analysis (Fig. 1O).
Figure 1.
Multiple neuroendocrine neoplasia in Rb+/− mice. (A and B) Gross compound pituitary tumor on P394. The tumor consists of two histologically distinct components that substitute for the pituitary anterior (AL) and intermediate (IL) lobes, which are demarcated by remnants of Rathke’s cleft (arrows). AL tumor cells contain α-GSU (A) and are positioned loosely around sinusoid-like vessels. IL tumor cells contain α-melanocyte-stimulating hormone (B) and form poorly vascularized epithelioid fields with central necrotic and hemorrhagic areas. (C and D) EAP in the anterior pituitary lobe on P90 before (C) and after (D) microdissection for genotype analysis by the PCR. (E) C-cell carcinoma on P340 showing the typical arrangement of polyhedral tumor cells in solid nests and rough hyalinized collagen with calcification (arrow). (F and G) C-cell EAP (arrow) on P60 before (F) and after (G) microdissection. The atypical cells show a parafollicular location. (H) Medullary thyroid carcinoma invading surrounding tissues on P469. Accumulation of calcitonin (brown color) is evident in the cytoplasm of tumor cells (arrow). CR, cartilage. (I) Calcitonin-containing metastatic cells in the lung on P463. The metastatic cells exhibit intraalveolar spreading (arrows). (J) A well-vascularized parathyroid tumor (PG) and a neighboring solid C-cell tumor of the thyroid gland (TG) on P370. (K) Parathyroid hormone expression in parathyroid tumor cells. (L) Pheochromocytoma of the adrenal medulla (AM) compressing the adrenal cortex (AC). (M and N) EAP in the adrenal medulla (AM) on P60 before (M) and after (N) microdissection. The arrow indicates multiple apoptotic figures. Staining: avidin-biotin-peroxidase immunostaining for α-GSU (A), α-melanocyte-stimulating hormone (B), calcitonin (H and I), or parathyroid hormone (K) with hematoxylin counterstaining; (C–G, J, and L–N) hematoxylin-eosin staining. [Bar: 160 μm (A and B), 40 μm (C, D, and I), 110 μm (E), 60 μm (F and G), 50 μm (H), 150 μm (J), and 20 μm (K), 390 μm (L), and 70 μm (M and N).] (O) Absence of the wild-type Rb allele (151-bp PCR product) in gross tumors (T; lanes 3, 4, 7, 10, 11, 17, and 18) and EAPs (E; lanes 1, 2, 6, 8, 9, 14, and 16) of the pituitary anterior lobe (AL, lanes 1–4), the parathyroid gland (PG, lanes 6 and 7), thyroid C cells (TG, lanes 8–11), lung metastases (L, lanes 12 and 13), or adrenal medulla (lanes 14 and 16–18). N, Rb+/− normal tissue (lanes 5 and 15). Nondenaturing 12% polyacrylamide gel stained with silver. The 236-bp band corresponds to the mutant Rb allele (11).
Table 1.
Conditional suppression of neuroendocrine carcinogenesis by expression of an RB transgene in Rb+/− mice
| Neoplasia |
Rb+/−mice with tumor (%)*
|
Rb+/−RTgRB mice with tumor (%)†
|
Fisher’s P Tet(−) vs. Tet(+)‡ | ||
|---|---|---|---|---|---|
| Moribund | P140§ | Tet(−) | Tet(+) | ||
| Melanotroph tumor of the pituitary | 100 (41/41) | 100 (10/10) | 25 (3/12) | 100 (11/11) | 0.0003 |
| Tumor of the pituitary anterior lobe | 22 (9/41) | 19 (5/27) | ND | ND | |
| C-cell thyroid carcinoma | 95 (36/38) | 100 (7/7) | 17 (2/12) | 91 (10/11) | 0.006 |
| Parathyroid adenoma | 8 (3/39) | ND | ND | ND | |
| C-cell lung metastases | 68 (26/38) | 0 (0/11) | ND | ND | |
| Lung neuroendocrine hyperplasia | 11 (4/38) | ND | ND | ND | |
| Adrenal pheochromocytoma | 71 (22/31) | 78 (7/9) | 25 (3/12) | 73 (8/11) | 0.0391 |
| Islet of Langerhans hyperplasia | 41 (7/17) | ND | ND | ND | |
Numbers in parentheses indicate number of mice with tumor out of total number of mice.
Rb+/− mice in which expression of an RB transgene is repressed by tetracycline (Fig. 2); animals were treated with tetracycline from fertilization until either P90, Tet(−), or P140, Tet(+), killed on P140, and scored for tumors. ND, not determined. According to immunohistochemical and Western blotting analyses, no expression of RB was detected in tumors developing in Tet(−) group of Rb+/−RTgRB mice.
No significant difference in the frequencies of tumors was observed between Rb+/− mice and Rb+/− mice with either TgRBp-tTA or TghCMV*-1p-RB.
Both invasive tumors and EAPs are included.
Examination of the laryngotracheal area revealed that 95% of Rb+/− animals had a thyroid tumor by the time of death (Table 1). Histological features of these tumors (Fig. 1E), as well as persistent expression of calcitonin by the tumor cells (Fig. 1H), closely resemble diagnostic hallmarks of human medullary C-cell carcinomas. The earliest morphological manifestation of C-cell carcinogenesis was parafollicular proliferation of atypical cells between P35 and P60 (Fig. 1 F and G). In addition, 8% of Rb+/− mice exhibited parathyroid hormone-containing adenomas of the parathyroid gland (Fig. 1 J and K; Table 1). Hyperplasia of the parathyroid gland was first identified on P90. Both C-cell (12 of 12) and parathyroid (3 of 3) EAPs contained only the mutant Rb allele (Fig. 1O).
Histological analysis of the lungs revealed multiple groups of highly mitotic cells. Frequently, these cells were located intravascularly and invaded surrounding alveoli, indicating a metastatic origin (Figs. 1I and 2G). The cells all contained calcitonin (Fig. 1I), but they did not express markers specific for tumors of either the pituitary or parathyroid gland. The absence of any correlation between the presence of metastases and tumors of the anterior pituitary or adrenal gland in the same animals supported a C-cell origin for these cells. Calcitonin-positive metastatic cells were first apparent on P250. Eighty-four percent of animals (21 of 25) had metastases by P380. Three of 30 animals also exhibited calcitonin-positive metastases in the liver.
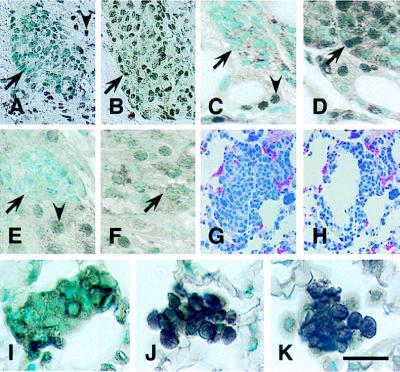
Figure 2.
Characterization of RB transgene expression. (A–F) Tetracycline-regulated expression of RB in Rb+/−RTgRB mice. Transgenic littermates were treated with tetracycline hydrochloride (1 mg/ml) from fertilization until either P94 (A, C, and E) or P90 (B, D, and F). On P94 expression of the RB transgene was tested in atypical cells of the pituitary intermediate lobe (A and B), the thyroid gland (C and D), and the adrenal medulla (E and F). In mice continuously exposed to tetracycline no RB is detected in nuclei of atypical (arrows) melanotrophs (A), C cells (C), and medulla cells (E). Withdrawal of tetracycline on P90 results in expression of RB transgene in those cells by P94 (B, D, and F). Note that staining of normal cells reflects expression of either endogenous Rb (A, C, and E, arrowheads) or both endogenous Rb and exogenous RB (B, D, and F). (G–K) RB expression in metastatic C cells after administration of LPDs. Mice were killed 24 hr after i.v. injection with either LPD-RB (G, H, and J), LPD-RBH209 (K), or LPD-lacZ (I). (G and H) Groups of metastatic cells before (G) and after (H) microdissection. (I–K) Simultaneous detection of cytoplasmic calcitonin and nuclear RB in metastatic cells. Unlike metastatic cells from mice treated with LPD-lacZ (I), those from mice treated with either LPD-RB (J) or LPD-RBH209 (K) showed intense RB staining. Avidin-biotin-peroxidase immunostaining with C-15 antiserum alone (A–F) or together with calcitonin-specific antibodies (I–K). Counterstaining with methyl green. (G and H) Hematoxylin-eosin staining. [Bar: 40 μm (A and B), 20 μm (C–F), 55 μm (G and H), and 16 μm (I–K).]
Seventy-one percent of Rb+/− mice exhibited pheochromocytomas of the adrenal gland (Fig. 1L; Table 1), 14% of which (3 of 22 tumor-positive animals) were bilateral. The lesions ranged from small foci of atypical cells to polymorphic tumors that had replaced the medullary tissue and compressed the adrenal cortex. Atypical cells were first detected in the adrenal medulla on P60 (Fig. 1 M and N). Eight of eight EAPs examined contained only the mutant Rb allele (Fig. 1O).
Moderate hyperplasia of pancreatic islet of Langerhans cells (diameter >300 μm) and neuroendocrine cells of the lung, similar to that described previously (12), was observed in 41% and 11% of Rb+/− mice, respectively (Table 1).
These results demonstrate that Rb+/− mice develop a syndrome of multiple neuroendocrine neoplasia associated with Rb deficiency, and they provide further support for a previous proposal (11) that Rb plays a specific role in ontogenesis of cells with a neuroendocrine phenotype. In humans, in addition to retinoblastomas and small cell lung carcinomas, 16% of parathyroid tumors, as well as some tumors of the anterior pituitary lobe and C-cell thyroid tumors, either exhibit loss of heterozygosity for the RB locus or lack RB expression (30–34). We have observed complete loss of nuclear RB staining in 3 of 17 (18%) human C-cell thyroid carcinomas. In addition, RB showed an irregular, mostly cytoplasmic location in 13 tumors (unpublished data). Other neuroendocrine cell types in humans might exhibit defects in other genes whose products contribute to the RB-mediated pathway. Thyroid C-cell carcinoma and bilateral adrenal pheochromocytoma are hallmarks of human multiple endocrine neoplasia (MEN) syndrome type 2a, whereas pancreatic endocrine hyperplasia and α-GSU-producing pituitary adenomas are typical of MEN syndrome type 1. It remains to be determined whether defects in RB-mediated signaling contribute to human MEN syndromes.
Although atypical cells were first apparent at similar times for the various tumor types in Rb+/− mice, the tumor latency periods and the extent of tumor progression at the time of death differed. For example, only thyroid C-cell tumors gave rise to distant metastases, indicating the existence of secondary cell lineage-specific changes that are required for tumor progression. Indeed, the frequency of heteroploidy was greater in thyroid C-cell tumors than in tumors of the pituitary intermediate lobe evaluated by cytogenetic analysis of chromosomal spreads and by flow cytometry (unpublished data).
We previously showed that adenovirus-mediated introduction of RB into Rb-deficient melanotrophs can attenuate carcinogenesis (16). Temporary RB expression in either early atypical cells (P35) or in cells during the early stage of invasion (P180) was sufficient to prolong the life span of the affected mice by an average of 60–80 days. To determine whether a similar effect might be achieved in other Rb-negative cells, we crossed RTgRB transgenic mice, in which expression of the RB transgene can be repressed by tetracycline administration, with Rb+/− mice. During continuous tetracycline administration, the resulting Rb+/− RTgRB mice expressed only endogenous Rb (Fig. 2 A–F, and A.Y.N., B. Shan, A. Flesken-Nikitin, K.-H. Chang, and W.-H.L., unpublished work) and manifested the expected spectrum of neuroendocrine tumors (Table 1). Termination of tetracycline treatment on P90 resulted in expression of the RB transgene and a marked decrease in the number of animals that developed tumors. Thus, reconstitution of Rb function suppressed the malignant phenotype in multiple cell lineages.
Although a critical role for the RB gene in the initial stages of carcinogenesis is well established, the impact of RB deficiency on tumor progression remains mostly uncharacterized. A few studies indicate that RB might contribute to such metastasis-relevant cell properties as motility, adhesion, and invasion (35–39). The lung metastases of thyroid C-cell carcinoma in immunocompetent Rb+/− mice represent a model with which to address this issue as well as to characterize the metastatic process and to develop clinically relevant gene therapeutic approaches.
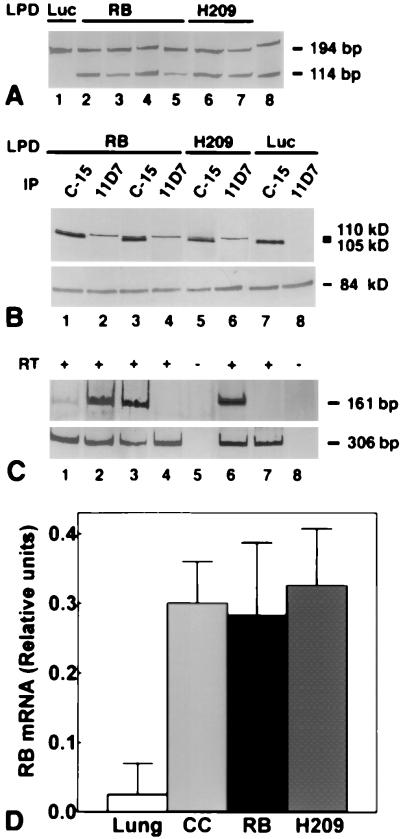
Gene transfer mediated by LPD results in preferential accumulation of transgene in cells in the lungs, including macrophages and endothelial cells, after injection into the tail vein of mice (24, 40). Compared with viral approaches, liposome-mediated gene transfer has the advantage of minimal toxicity and low immunogenicity (40–43). To evaluate whether DNA can be efficiently delivered to metastatic cells by LPDs, we injected Rb+/− mice with LPDs containing RB cDNA under the control of the RB promoter. This regulatory element is well characterized and is sufficient for rescue of both embryonic lethality and tumor phenotype in Rb−/− and Rb+/− mice, respectively (17, 19, 21, 44, 45). Furthermore, the RB promoter is negatively regulated by RB, alleviating potential RB overexpression (46). The presence of the RB transgene in metastatic cells was detected by microdissection-PCR analysis (Figs. 2 G and H and 3A), and its expression was demonstrated by immunostaining (Fig. 2J), immunoblot (Fig. 3B), and reverse transcription–PCR (Fig. 3 C and D) analyses. The RB transgene was significantly higher expressed in metastatic cells as compared with neighboring alveolar and endothelial cells (Figs. 2J and 3 C and D). At the same time, extent of RB expression in C-cell-derived metastases was comparable to that in thyroid C cells (Figs. 2 C and J and 3 C and D). These results are in good agreement with earlier immunohistochemical studies describing cell-type specific variations in RB expression (11, 47). Most importantly, they demonstrate that LPD-mediated transfer allows evaluation of metastasis upon expression of RB at physiological levels.
Figure 3.
Detection of RB expression after LPD-RB administration. (A) Detection by microdissection and PCR analysis of the RB transgene in metastatic cells from lung tissue of mice treated with either LPD-RB (lanes 2–5) or LPD-RBH209 (H209, lanes 6 and 7) but not in those from mice exposed to LPD-Luc (lane 1). The 114-bp and 194-bp PCR fragments are diagnostic for RB cDNA and the Rb gene, respectively (20). Lane 6, DNA size markers (Amresco, Euclid, OH). (B) Immunoprecipitation and immunoblot analysis of RB expression in lungs of mice treated with LPD-RB (lanes 1–4), LPD-RBH209 (lanes 5 and 6), or in those of mice exposed to LPD-Luc (lanes 7 and 8). Immunoprecipitation (IP) was performed with C-15 antiserum, which recognizes both human RB (110 kDa) and mouse Rb (105 kDa), or mAb 11D7, which is specific for human RB protein, and immunoblot analysis was performed with mAB 245 (Upper). Detection of p84, a nuclear matrix protein (57), was assessed as a control for gel loading (Lower). (C) Detection of Rb and RB (Upper, 161 bp) and β-actin (Lower, 306 bp) mRNAs in the lung (lane 1), thyroid C cells (lane 2), and lung metastases treated with LPD-RB (lane 3), LPD-RBH209 (lane 6), LPD-Luc (lane 4), or LPD-lacZ (lane 7). Lanes 5 and 8, reverse transcriptase (RT)–PCRs without RT. (A and C) Polyacrylamide gel stained with silver. (D) Relative amounts of Rb cDNA (mean ± SD) in the lung (n = 8), C cells (CC, n = 5), and metastatic cells treated with either LPD-RB (RB, n = 6) or LPD-RBH209 (H209, n = 4) after densitometric readings of PCR products in exponential phase of amplification and subsequent β-actin normalization for internal errors in loading and amplification. Two-tailed Student’s t test P values are <0.0001, <0.0001, 0.7430, and 0.6135 for lung vs. CC, lung vs. RB, CC vs. RB, and CC vs. H209, respectively. All experiments were performed in triplicate and repeated at least twice. In all experiments, mice were treated as described in the legend to Fig. 2 G–K.
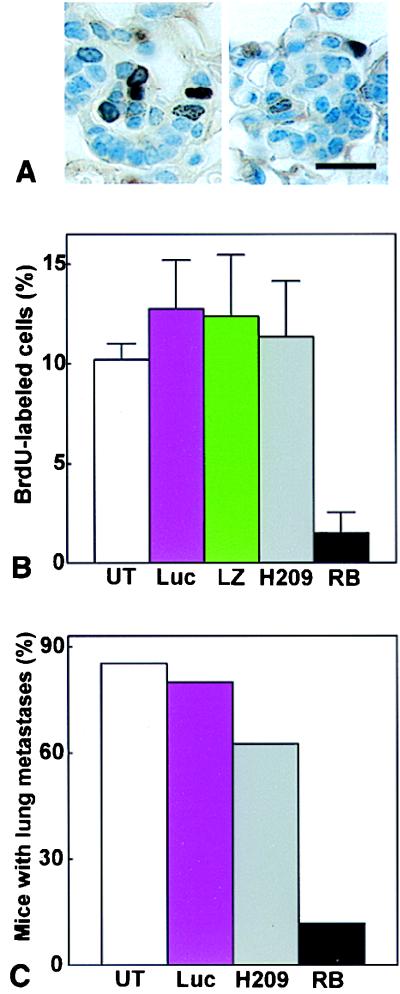
A single application of LPD-RB was sufficient to reduce significantly the proliferative activity of metastatic cells (Fig. 4 A and B), compared with that apparent in untreated animals or in mice treated with LPDs containing either functionally inactive RBH209 mutant, lacZ (β-galactosidase), or Luc (luciferase) constructs. The effect of RB on metastasis was further investigated after multiple administration of the corresponding LPDs. Given that 84% of animals contain metastatic cells by P380 (see above), we administered weekly injections to mice beginning at P350–P360. Metastatic foci in the lungs were scored 1 week after the last of three injections. In mice treated with wild-type RB, frequency of metastases was reduced from 84% to 12% (Fig. 4C). Mice treated with Luc or H209 constructs had metastases in 80% and 63% of cases. Although the differences in frequencies among Luc, H209, and untreated mice were statistically insignificant (see legend for Fig. 4), we cannot exclude minor nonspecific, likely immune, response on expression of a heterologous protein in tumor cells. Because 71% (10 of 14) of Rb+/− mice had metastases at P350–P360, reintroduction of RB may affect both embolic and extravasal stages of metastatic process.
Figure 4.
LPD-mediated RB gene therapy in Rb+/− mice. (A and B) Effect of LPD-RB administration on the proliferation of metastatic cells in the lungs. Mice were killed 24 and 1 hr, respectively, after injection with LPD and with BrdUrd. (A) Metastatic cells in S phase of the cell cycle were detected by immunostaining with antibodies to BrdUrd after treatment with either LPD-Luc (Left) or LPD-RB (Right). (B) The percentage (mean ± SE) of metastatic cells labeled with BrdUrd was determined in untreated mice (UT, n = 6) and in those treated with LPD-Luc (n = 5), LPD-lacZ (LZ, n = 5), LPD-RBH209 (H209, n = 5) or LPD-RB (n = 8). Two-tailed Student’s t test yielded P values of <0.0001 for UT vs. LPD-RB, 0.6709 for UT vs. LPD-RBH209, 0.3124 for UT vs. LPD-Luc, and 0.4783 for UT vs. LPD-lacZ. (C) Effect of repeated administration of LPD-RB on lung metastasis. Mice were either untreated (UT, n = 34) or were injected i.v. with either LPD-Luc (n = 15), LPD-RBH209 (n = 8), or LPD-RB (n = 17) weekly for 3 weeks, and lungs were removed 1 week after the last injection. The percentage of animals with lung metastases was determined by calcitonin immunostaining. Two-tailed Fisher’s test yielded P values of <0.0001 for UT vs. LPD-RB, 0.1625 for UT vs. LPD-RBH209, 0.6869 for UT vs. LPD-Luc, and 0.0169 for LPD-RBH209 vs. LPD-RB. In all experiments, mice were between 350 and 360 days old at the time of initial LPD administration. Results with LPD-RB, LPD-Luc, and LPD-LacZ were reproduced in three independent experiments. Results with LPD-RBH209 were reproduced in two independent groups.
Thus, in spite of the long latency period, during which genetic alterations can accumulate, Rb deficiency remains critical for tumor progression in Rb+/− mice. Carcinogenesis usually is thought of as a multistage process in which the accumulation of genetic alterations results in selection of the most autonomous and, supposedly, most malignant cell clones (48–51). Gene therapy of tumors at advanced stages thus may require the targeting of multiple genes. Indeed, carcinogenesis in the salivary gland may be abrogated by termination of simian virus 40 large T antigen expression only until a certain stage has been achieved (52). Our results indicate that suppression of the tumor phenotype may depend on gene function in the context of a particular cell lineage. Most human RB-deficient tumors, such as small cell lung carcinoma, are detected at a relatively late stage and exhibit a pronounced metastatic potential. Reconstitution of RB function thus should be considered a primary target for treatment of such RB-deficient disseminated tumors.
The discovery of tumor suppressor genes has provided a conceptual basis for the design of gene therapeutic approaches (16, 43, 53). Recently, the definition of tumor suppressor has come to encompass virtually any gene whose loss of function is associated with cancer susceptibility (54). This situation has required subclassification of such genes as gatekeepers, caretakers, or landscapers (51), with an inevitable diminishment in the direct therapeutic meaning of the term tumor suppressor. Our data reaffirm the original definition of a tumor suppressor gene as a gene that is able to suppress the neoplastic phenotype (53, 55, 56).
Acknowledgments
We thank A. Flesken-Nikitin for technical assistance. This work was supported by grants to W.-H.L. from the National Institutes of Health (EY05785 and CA58318), by a Mentored Clinical Scientists Development Program Award (K12) to M.I.J.-P. from the National Institutes of Health, and by grants to L.H. from the National Institutes of Health (CA71731) and Targeted Genetics Corporation.
ABBREVIATIONS
- α-GSU
α-glycoprotein subunit
- EAP
early atypical proliferation
- LPD
lipid-entrapped, polycation-condensed DNA complexes
- P
postnatal day
- Rb
mouse retinoblastoma gene
- RB
human retinoblastoma gene
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Bookstein R, Lee W H. Crit Rev Oncog. 1991;2:211–227. [PubMed] [Google Scholar]
- 2.Knudson A G. Proc Natl Acad Sci USA. 1993;90:10914–10921. doi: 10.1073/pnas.90.23.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salgia R, Skarin A T. J Clin Oncol. 1998;16:1207–1217. doi: 10.1200/JCO.1998.16.3.1207. [DOI] [PubMed] [Google Scholar]
- 4.Riley D J, Lee E Y, Lee W H. Annu Rev Cell Biol. 1994;10:1–29. doi: 10.1146/annurev.cb.10.110194.000245. [DOI] [PubMed] [Google Scholar]
- 5.Chen P L, Riley D J, Lee W H. Crit Rev Eukaryotic Gene Expression. 1995;5:79–95. [PubMed] [Google Scholar]
- 6.Jacks T, Weinberg R A. Science. 1998;280:1035–1036. doi: 10.1126/science.280.5366.1035. [DOI] [PubMed] [Google Scholar]
- 7.Sherr C J. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 8.Hu N, Gutsmann A, Herbert D C, Bradley A, Lee W H, Lee E Y. Oncogene. 1994;9:1021–1027. [PubMed] [Google Scholar]
- 9.Williams B O, Schmitt E M, Remington L, Bronson R T, Albert D M, Weinberg R, Jacks T. EMBO J. 1994;13:4251–4259. doi: 10.1002/j.1460-2075.1994.tb06745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robanus-Maandag E C, van der Valk M, Vlaar M, Feltkamp C, O’Brien J, van Roon M, van der Lugt N, Berns A, te Riele H. EMBO J. 1994;13:4260–4268. doi: 10.1002/j.1460-2075.1994.tb06746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nikitin A Y, Lee W H. Genes Dev. 1996;10:1870–1879. doi: 10.1101/gad.10.15.1870. [DOI] [PubMed] [Google Scholar]
- 12.Williams B O, Remington L, Albert D M, Mukai S, Bronson R T, Jacks T. Nat Genet. 1994;7:480–484. doi: 10.1038/ng0894-480. [DOI] [PubMed] [Google Scholar]
- 13.Harrison D J, Hooper M L, Armstrong J F, Clarke A R. Oncogene. 1995;10:1615–1620. [PubMed] [Google Scholar]
- 14.Vooijs M, van der Valk M, te Riele H, Berns A. Oncogene. 1998;17:1–12. doi: 10.1038/sj.onc.1202169. [DOI] [PubMed] [Google Scholar]
- 15.Yamasaki L, Bronson R, Williams B O, Dyson N J, Harlow E, Jacks T. Nat Genet. 1998;18:360–364. doi: 10.1038/ng0498-360. [DOI] [PubMed] [Google Scholar]
- 16.Riley D J, Nikitin A Y, Lee W-H. Nat Med. 1996;2:1316–1321. doi: 10.1038/nm1296-1316. [DOI] [PubMed] [Google Scholar]
- 17.Lee E Y, Chang C Y, Hu N, Wang Y C, Lai C C, Herrup K, Lee W H, Bradley A. Nature (London) 1992;359:288–294. doi: 10.1038/359288a0. [DOI] [PubMed] [Google Scholar]
- 18.Gossen M, Bujard H. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong F D, Huang H J, To H, Young L J, Oro A, Bookstein R, Lee E Y, Lee W H. Proc Natl Acad Sci USA. 1989;86:5502–5506. doi: 10.1073/pnas.86.14.5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikitin A Y, Riley D J, Lee W H. Cancer Res. 1997;57:4274–4278. [PubMed] [Google Scholar]
- 21.Bignon Y J, Chen Y, Chang C Y, Riley D J, Windle J J, Mellon P L, Lee W H. Genes Dev. 1993;7:1654–1662. doi: 10.1101/gad.7.9.1654. [DOI] [PubMed] [Google Scholar]
- 22.Bignon Y J, Shew J Y, Rappolee D, Naylor S L, Lee E Y, Schnier J, Lee W-H. Cell Growth Differ. 1990;1:647–651. [PubMed] [Google Scholar]
- 23.Chen P L, Riley D J, Chen-Kiang S, Lee W H. Proc Natl Acad Sci USA. 1996;93:465–469. doi: 10.1073/pnas.93.1.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li S, Huang L. Gene Ther. 1997;4:891–900. doi: 10.1038/sj.gt.3300482. [DOI] [PubMed] [Google Scholar]
- 25.Li S, Brisson M, He Y, Huang L. Gene Ther. 1997;4:449–454. doi: 10.1038/sj.gt.3300413. [DOI] [PubMed] [Google Scholar]
- 26.Li S, Rizzo M A, Bhattacharya S, Huang L. Gene Ther. 1998;5:930–937. doi: 10.1038/sj.gt.3300683. [DOI] [PubMed] [Google Scholar]
- 27.Liu F, Qi H, Huang L, Liu D. Gene Ther. 1997;4:517–523. doi: 10.1038/sj.gt.3300424. [DOI] [PubMed] [Google Scholar]
- 28.Shan B, Zhu X, Chen P-L, Durfee T, Yang Y, Sharp D, Lee W-H. Mol Cell Biol. 1992;12:5620–5631. doi: 10.1128/mcb.12.12.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeCaprio J A, Ludlow J W, Figge J, Shew J Y, Huang C M, Lee W H, Marsilio E, Paucha E, Livingston D M. Cell. 1988;54:275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- 30.Cryns V L, Thor A, Xu H J, Hu S X, Wierman M E, Vickery A L, Jr, Benedict W F, Arnold A. N Engl J Med. 1994;330:757–761. doi: 10.1056/NEJM199403173301105. [DOI] [PubMed] [Google Scholar]
- 31.Bates A S, Farrell W E, Bicknell E J, McNicol A M, Talbot A J, Broome J C, Perrett C W, Thakker R V, Clayton R N. J Clin Endocrinol Metabol. 1997;82:818–824. doi: 10.1210/jcem.82.3.3799. [DOI] [PubMed] [Google Scholar]
- 32.Pei L, Melmed S, Scheithauer B, Kovacs K, Benedict W F, Prager D. Cancer Res. 1995;55:1613–1616. [PubMed] [Google Scholar]
- 33.Pearce S H, Trump D, Wooding C, Sheppard M N, Clayton R N, Thakker R V. Clin Endocrinol. 1996;45:195–200. doi: 10.1046/j.1365-2265.1996.d01-1561.x. [DOI] [PubMed] [Google Scholar]
- 34.Yoshimoto K, Endo H, Tsuyuguchi M, Tanaka C, Kimura T, Iwahana H, Kato G, Sano T, Itakura M. Clin Endocrinol. 1998;48:67–72. doi: 10.1046/j.1365-2265.1998.00354.x. [DOI] [PubMed] [Google Scholar]
- 35.Coppee F, Gerard A C, Denef J F, Ledent C, Vassart G, Dumont J E, Parmentier M. Oncogene. 1996;13:1471–1482. [PubMed] [Google Scholar]
- 36.Day M L, Foster R G, Day K C, Zhao X, Humphrey P, Swanson P, Postigo A A, Zhang S H, Dean D C. J Biol Chem. 1997;272:8125–8128. doi: 10.1074/jbc.272.13.8125. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Hu S X, Perng G S, Zhou Y, Xu K, Zhang C, Seigne J, Benedict W F, Xu H J. Oncogene. 1996;13:2379–2386. [PubMed] [Google Scholar]
- 38.Martel C, Harper F, Cereghini S, Noe V, Mareel M, Cremisi C. Cell Growth Differ. 1997;8:165–178. [PubMed] [Google Scholar]
- 39.Valente P, Melchiori A, Paggi M G, Masiello L, Ribatti D, Santi L, Takahashi R, Albini A, Noonan D M. Oncogene. 1996;13:1169–1178. [PubMed] [Google Scholar]
- 40.Huang L, Li S. Nat Biotechnol. 1997;15:620–621. doi: 10.1038/nbt0797-620. [DOI] [PubMed] [Google Scholar]
- 41.Kay M A, Liu D, Hoogerbrugge P M. Proc Natl Acad Sci USA. 1997;94:12744–12746. doi: 10.1073/pnas.94.24.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langer, R. (1998) Nature (London)392, Suppl., 5–10.
- 43.Verma I M, Somia N. Nature (London) 1997;389:239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- 44.Chang C Y, Riley D J, Lee E Y, Lee W H. Cell Growth Differ. 1993;4:1057–1064. [PubMed] [Google Scholar]
- 45.Riley D J, Liu C Y, Lee W H. Mol Cell Biol. 1997;17:7342–7352. doi: 10.1128/mcb.17.12.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shan B, Chang C Y, Jones D, Lee W H. Mol Cell Biol. 1994;14:299–309. doi: 10.1128/mcb.14.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szekely L, Jiang W Q, Bulic-Jakus F, Rosen A, Ringertz N, Klein G, Wiman K G. Cell Growth Differ. 1992;3:149–156. [PubMed] [Google Scholar]
- 48.Nowell P C. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 49.Fialkow P J. N Engl J Med. 1974;291:26–35. doi: 10.1056/NEJM197407042910109. [DOI] [PubMed] [Google Scholar]
- 50.Fearon E R, Vogelstein B. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 51.Kinzler K W, Vogelstein B. Nature (London) 1997;386:761–763. doi: 10.1038/386761a0. [DOI] [PubMed] [Google Scholar]
- 52.Ewald D, Li M, Efrat S, Auer G, Wall R J, Furth P A, Hennighausen L. Science. 1996;273:1384–1386. doi: 10.1126/science.273.5280.1384. [DOI] [PubMed] [Google Scholar]
- 53.Huang H J, Yee J K, Shew J Y, Chen P L, Bookstein R, Friedmann T, Lee E Y-P, Lee W H. Science. 1988;242:1563–1566. doi: 10.1126/science.3201247. [DOI] [PubMed] [Google Scholar]
- 54.Haber D, Harlow E. Nat Genet. 1997;16:320–322. doi: 10.1038/ng0897-320. [DOI] [PubMed] [Google Scholar]
- 55.Harris H, Miller O J, Klein G, Worst P, Tachibana T. Nature (London) 1969;223:363–368. doi: 10.1038/223363a0. [DOI] [PubMed] [Google Scholar]
- 56.Weissman B E, Saxon P J, Pasquale S R, Jones G R, Geiser A G, Stanbridge E J. Science. 1987;236:175–180. doi: 10.1126/science.3031816. [DOI] [PubMed] [Google Scholar]
- 57.Durfee T, Mancini M A, Jones D, Elledge S J, Lee W-H. J Cell Biol. 1994;127:609–622. doi: 10.1083/jcb.127.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]