Abstract
Agonist ligands for the nuclear receptor peroxisome proliferator-activated receptor-γ have been shown to induce terminal differentiation of normal preadipocytes and human liposarcoma cells in vitro. Because the differentiation status of liposarcoma is predictive of clinical outcomes, modulation of the differentiation status of a tumor may favorably impact clinical behavior. We have conducted a clinical trial for treatment of patients with advanced liposarcoma by using the peroxisome proliferator-activated receptor-γ ligand troglitazone, in which extensive correlative laboratory studies of tumor differentiation were performed. We report here the results of three patients with intermediate to high-grade liposarcomas in whom troglitazone administration induced histologic and biochemical differentiation in vivo. Biopsies of tumors from each of these patients while on troglitazone demonstrated histologic evidence of extensive lipid accumulation by tumor cells and substantial increases in NMR-detectable tumor triglycerides compared with pretreatment biopsies. In addition, expression of several mRNA transcripts characteristic of differentiation in the adipocyte lineage was induced. There was also a marked reduction in immunohistochemical expression of Ki-67, a marker of cell proliferation. Together, these data indicate that terminal adipocytic differentiation was induced in these malignant tumors by troglitazone. These results indicate that lineage-appropriate differentiation can be induced pharmacologically in a human solid tumor.
Keywords: nuclear receptors, sarcoma, drug development, oncology, antineoplastic
The process of neoplastic cell growth represents a dysfunctional balance between control of cell proliferation, apoptosis, and terminal differentiation. In normal cells, activation of specific pathways leads to cellular differentiation, which typically is accompanied by cessation of proliferation. Treating cancer through the induction of cell differentiation has been an attractive concept, but clinical development of differentiation-inducing agents to treat cancer has been limited to date. The most successful example of differentiation therapy for a malignant disease is the use of all-trans retinoic acid, a ligand for the retinoic acid receptor, to differentiate the malignant cells of acute promyelocytic leukemia (1, 2). Other nuclear receptors that regulate cellular differentiation and proliferation pathways also may represent promising targets for novel therapeutic strategies in cancer treatment.
The differentiation of adipocytes has been used as an experimental model of lineage-specific differentiation and gene expression. A critical regulator of terminal differentiation for the adipocytic lineage is a nuclear receptor known as peroxisome proliferator-activated receptor-γ (PPARγ) (3–9). PPARγ forms a heterodimeric complex with the retinoid X receptor (RXR). This complex of PPARγ and RXR binds to specific recognition sites on DNA and, upon binding of ligands for either receptor, regulates transcription of adipocyte-specific genes. Ectopic expression and activation of PPARγ in fibroblastic cells stimulates adipocyte-specific gene expression and induces a complete adipocytic phenotype (4, 9). Several natural and synthetic ligands for PPARγ have been identified. Troglitazone is a member of the thiazolidinedione class of drugs, which have been identified as agonist ligands for PPARγ (10–12). These drugs have been developed clinically and currently are used primarily as insulin-sensitizing antidiabetic agents (13, 14).
Liposarcoma represents the most common form of soft-tissue sarcoma in humans (15). These tumors constitute a family of mesenchymal malignancies characterized by dysfunctional adipocytic differentiation as well as uncontrolled cellular proliferation. Several histologic subtypes of liposarcoma have been well characterized, with differentiation status of the cells being a major distinguishing feature of these subtypes. The prognosis of liposarcoma, including risk of developing metastatic disease and likelihood of long-term survival, correlates well with the histologic subtype, indicating that the differentiation status of the tumor is one of the most important prognostic factors for predicting clinical outcomes (15, 16). The myxoid and round cell subtypes of liposarcoma, comprising approximately 35% of all liposarcomas, represent a histologic continuum that may be grouped together based on the presence of a characteristic reciprocal translocation between chromosomes 12 and 16 (15, 17–18). This t(12;16) generates a chimeric mRNA fusion transcript derived from the CHOP gene on chromosome 12 with the TLS gene on chromosome 16. The contribution of these molecular aberrancies to the process of malignant transformation remains obscure.
Laboratory analyses of primary tumor tissue have documented the expression of PPARγ mRNA at levels comparable to normal fat in each of the histologic subtypes of human liposarcoma (19). Additionally, it has been shown that terminal differentiation of human liposarcoma cells in culture can be induced by exposure to thiazolidinedione drugs (19). Importantly, the levels of troglitazone that demonstrate differentiating activity in vitro are in the range of 5 μM (19), and such levels are consistently achievable and tolerable for prolonged periods in humans being treated for type II diabetes (13, 14). Standard therapies for unresectable liposarcomas are purely palliative, with complete response rates to cytotoxic chemotherapy reported in less than 10% of patients (20, 21). Based on our preclinical data, liposarcoma represents an attractive clinical model for the therapeutic use of troglitazone to induce differentiation of malignant tumors in humans. We report here clear evidence of differentiation in vivo induced by troglitazone in three patients with liposarcoma whose tumor tissues were extensively evaluated.
PATIENTS AND METHODS
Trial Design.
An open-label Phase 2 clinical trial was conducted at the Dana-Farber Cancer Institute with approval of the Institutional Review Board. Previous surgery, chemotherapy, and radiation therapy were not exclusion criteria as long as patients had measurable disease that had not been irradiated at the time of study entry. After obtaining informed consent, all patients were assigned to receive troglitazone (generously provided by Parke–Davis) at the dose of 800 mg orally once daily. Patients were monitored with clinical methods (physical examinations, clinical chemistry and imaging tests) as well as with serial biopsies of tumor sites before and approximately 6 weeks after beginning troglitazone dosing. In this pilot study, detection of adipocytic differentiation in tumor tissue was used as a study endpoint to justify further study of troglitazone in this clinical setting. The study design was reviewed and approved under an investigator-initiated investigational new drug permit from the U.S. Food and Drug Administration.
Patient Summaries.
Patient 1. Patient 1 was a 39-year-old woman with myxoid liposarcoma (intermediate grade) with recurrent metastatic disease. Initial management of primary disease in 1993 consisted of wide resection and postoperative radiation therapy, with her first metastatic recurrence noted in August 1997. The patient entered this clinical trial and began troglitazone dosing in December 1997. She tolerated this treatment well, without any adverse effects, and she underwent repeat tumor biopsy after 6 weeks of troglitazone administration in January 1998.
Patient 2.
Patient 2 was a 43-year-old woman with high-grade myxoid and round cell liposarcoma, primary disease resected in 1987, with progressive metastatic disease at multiple sites since 1988, including lung metastases, bony sites, as well as diffuse soft tissue metastatic sites. She previously had been treated extensively with multiple courses of radiation therapy as well as cytotoxic chemotherapy. Patient began troglitazone dosing per protocol in March 1998, underwent re-evaluation with repeat biopsy approximately 6 weeks later, and continued on study 4 months when progression of a paraspinal metastasis impinged on the spinal cord.
Patient 3.
Patient 3 was a 56-year-old woman with high-grade pleomorphic liposarcoma with massive retroperitoneal disease, initially resected in 1996. Since 1996, she had received multiple courses of radiation therapy and cytotoxic chemotherapy for recurrent disease with no effect. She entered this clinical trial in February 1998, repeat biopsies were performed after 6 weeks, and the patient remains on study as of October 1998.
Tumor Tissue.
Biopsies of tumor sites were obtained immediately before study entry and MRI scans of measurable sites of disease also were obtained at baseline; these biopsies and imaging studies were repeated approximately 6 weeks after initiation of troglitazone therapy. Tissue samples from homogenous and viable portions of the liposarcoma were obtained from either open incisional biopsy or computed tomography-guided core biopsy. Conventional classification of histologic subtype, morphology, and grade was determined by a single sarcoma specialty pathologist (C.D.M.F.). Histologic classification was performed on tissue immediately adjacent to that used for NMR analysis and was correlated with the diagnosis rendered on the main surgical specimen.
Histological Analysis.
Routine histologic staining with hematoxylin and eosin was performed on all tumor biopsy specimens. Immunohistochemistry was performed by using standard techniques as described. For immunohistochemical analyses the MIB-1 (1:50 dilution) mAb (Immunotech, Marseille, France) as well as a mAb directed against PPARγ (1:5 dilution) (Santa Cruz Biotechnology) were used. MIB-1 stains the Ki-67 nuclear proliferation-associated antigen, a standard marker of cellular proliferation (22), in paraffin sections. Standard quantification was performed by assessing the percentage of cells with positive nuclear staining for this antigen.
RNA Analysis.
Northern blot analysis was performed by using mRNA extracted from tumor biopsy samples, as described (19, 23). Northern blots were probed with PPARγ, aP2, adipsin, and actin. All signals were standardized against actin and to mRNA from normal adipose tissue for quantification of gene expression.
Tumor Sample Preparation for Magic Angle Spinning (MAS) Proton NMR Spectroscopy.
For MAS proton NMR studies, cylindrical core tissue samples 3 mm in diameter and 12 mm in length were cut from semifrozen tissue by using a 3-mm diameter biopsy punch. These samples were thawed in 3 cc of PBS in deuterium oxide (PBS/D2O, pD 7.4) for 5 min before rinsing once with fresh PBS/D2O and placement in a 4-mm o.d. zirconium MAS rotor. Ten microliters of 94.1 mM 3-trimethylsilylpropionate-d4 (TSP-d4) in PBS/D2O was placed in the rotor with the tumor tissue to serve as an internal spectral intensity reference.
Proton NMR Spectroscopy Measurements.
All spectra were acquired at 20°C and 500 MHz by using a Bruker DRX500 spectrometer equipped with a 4-mm high-resolution 1H/13C MAS probe as described (24, 25). Spin rates of 3.5 KHz were used, and one-dimensional, fully relaxed 1H-NMR spectra were quantitated for NMR-visible triglycerides and phosphatidylcholine (26).
Tumor Cytogenetics.
All baseline tumor biopsy samples were evaluated by cytogenetic testing. Patients 1 and 2 had the balanced translocation t(12;16)(q13;p11) that is characteristic of myxoid/round cell liposarcoma (17, 18). Cytogenetic analysis of tumor biopsy from patient 3 was unsuccessful because of failure of the cells to proliferate in culture conditions required for metaphase analysis.
RESULTS
In this pilot study, we report the results of three patients with advanced unresectable myxoid and pleomorphic liposarcoma to whom the PPAR-γ ligand troglitazone was administered. Eligibility criteria for this pilot study also allowed troglitazone dosing to patients with other histologic subtypes of liposarcoma, including low-grade, well-differentiated disease. However, because it would be very difficult to detect drug-induced changes in the differentiation status of tumors with well-differentiated characteristics at baseline, more poorly differentiated histologic subtypes of liposarcoma were judged likely to be more informative. Although the clinical status of all patients in this trial continues to be monitored per protocol, we performed detailed histologic, biochemical, and molecular analyses on a subset of patients with myxoid/round cell or pleomorphic liposarcoma, histologic subtypes with characteristically poorly differentiated morphologies.
Histologic Changes in Tumor Biopsies Induced by Troglitazone.
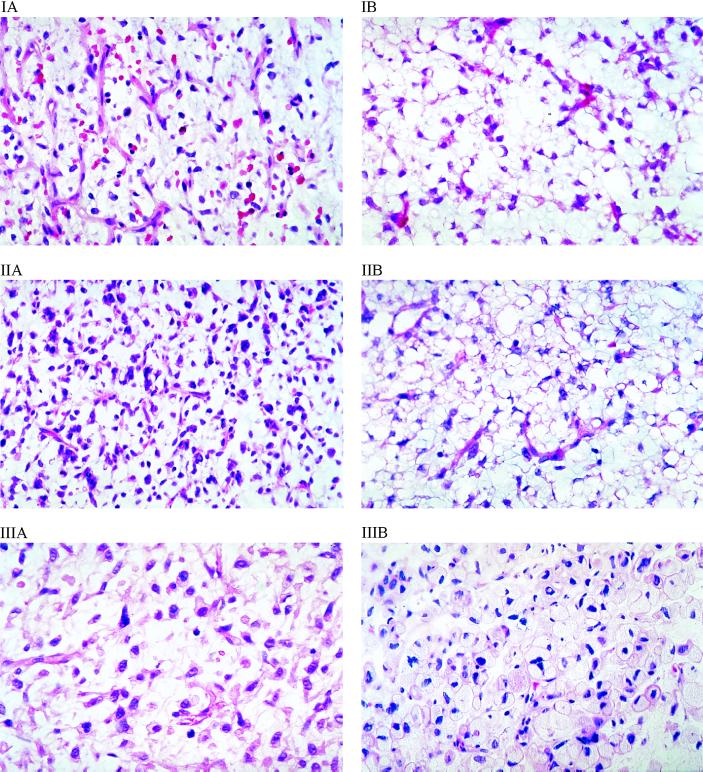
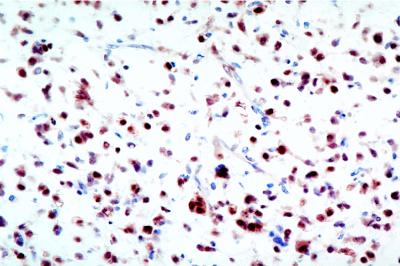
To assess the effects of troglitazone on the tumor, we performed serial biopsies on patients after 6–8 weeks of treatment, as discussed in Patients and Methods. Fig. 1 shows the dramatic histologic changes induced by troglitazone therapy in these patients with intermediate to high-grade disease before study treatment (Fig. 1A = pre, Fig. 1B = post). Marked microvesicular cytoplasmic lipid accumulation and increased individual cell volumes were noted without changes in nuclear morphology. These changes were consistent between biopsy specimens taken from multiple different sections of tumors and were not caused by artifacts of variability in intratumoral sampling. The baseline expression of PPARγ was confirmed by immunohistochemical staining of tissues, with the expected strong nuclear expression confirmed as noted in Fig. 2; uniformly high levels of staining for PPARγ were seen in all patients analyzed in this study (data not shown).
Figure 1.
Histologic changes in liposarcoma tumor tissue with accumulation of intracellular lipid in three separate patients (I, II, III). Shown are hematoxylin and eosin-stained biopsies of liposarcoma tissue obtained (A) immediately before study entry and (B) after 6 weeks of daily dosing with troglitazone. Magnification: ×400.
Figure 2.
Nuclear expression of PPARγ protein in liposarcoma tissue. Immunohistochemical staining of a biopsy sample of liposarcoma tissue from patient 2 obtained immediately before study entry. Comparative hematoxylin and eosin staining of tissue is noted in Fig. 1IIA. Magnification: ×400.
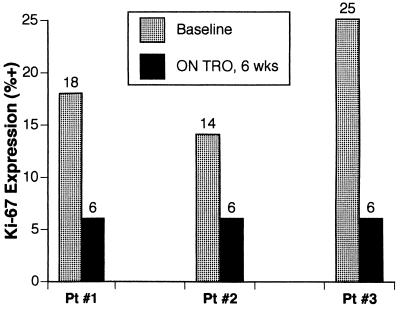
We next examined whether troglitazone treatment affected the proliferation of the tumor cells. Expression of the Ki-67 nuclear antigen, a reliable marker of cellular proliferation (22), was assayed by immunohistochemical staining and quantified by the percentage of cells with positive-staining nuclei. The proliferative fraction of even these intermediate to high-grade tumors is relatively low at baseline. However, in all three patients, the percentage of cells expressing the Ki-67 antigen dropped 2- to 4-fold while on troglitazone therapy (Fig. 3), based on a standardized method of counting at least 100 nuclei within each sample to take into account possible variations across different microscopic fields.
Figure 3.
Decreases in Ki-67 proliferation-associated antigen expression after 6 weeks of troglitazone treatment. The percentage of cells with strong nuclear staining for Ki-67 was quantified by immunohistochemical staining with the MIB-1 mAb, as described in Patients and Methods.
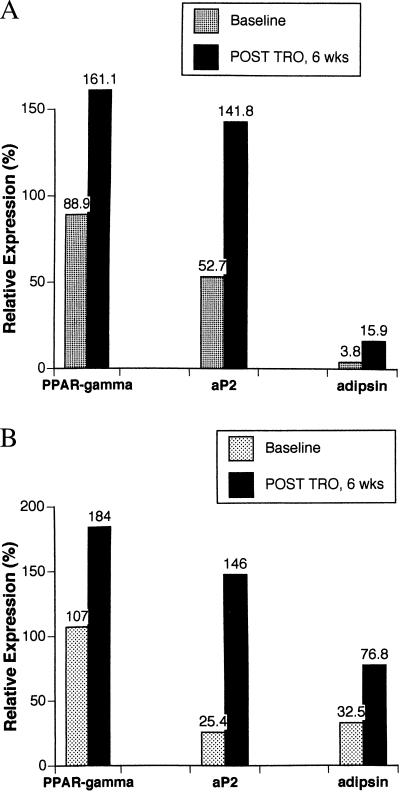
To assess more fully the extent of differentiation at a molecular level, we analyzed biopsies obtained before and after troglitazone treatment. Sufficient material for molecular analysis of gene expression was available only for patients 1 and 2. As shown in Fig. 4, these assays revealed 1.8- to 4.2-fold increases in the expression of genes specifically linked to adipocytic differentiation, including aP2, adipsin, and PPARγ itself. The data are expressed as the percent expression of mRNA relative to a sample of normal human fat used as an external control. These results indicate lineage-appropriate molecular differentiation of the tumors.
Figure 4.
Troglitazone-associated increases in expression of genes linked to adipocytic terminal differentiation. mRNA was prepared from serial biopsies of liposarcoma tissue from patients 1 and 2 (A and B, respectively) and subjected to Northern analysis as described in Patients and Methods. The levels of mRNA for aP2, adipsin, and PPARγ were quantified and normalized against levels of mRNA for β–actin. Levels of gene expression then were scaled against normal fat, which was set as 100% expression for these adipocytic-lineage genes.
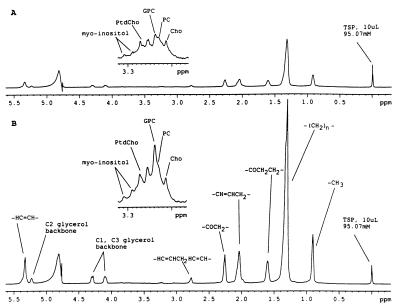
Analysis of tumor specimens by MAS 1H-NMR spectroscopy was performed to characterize and quantify the lipid metabolites within the tumor tissue. Representative quantitative one-dimensional MAS 1H-NMR spectra were acquired from the tumor tissue of patient 1 before therapy (Fig. 5A) and after 6 weeks of troglitazone therapy (Fig. 5B). The vertical scale of each spectrum was normalized to the integral of TSP-d4 (reference location at 0.0 ppm) because the same amount of TSP-d4 was added to each sample. The predominant resonances in these spectra arise from triglycerides and phospholipids. The presence of NMR-detectable triglyceride with this technique is confirmed by detection of characteristic resonances from the glycerol backbone (multiplets centered at 4.1 and 4.3 ppm) and the presence of a triplet resonance centered at 0.9 ppm, which originates from terminal methyl protons on fatty acyl chains. The spectra in Fig. 5 demonstrate that the myxoid liposarcoma tissue after 6 weeks of troglitazone administration exhibited a 2.6-fold increase in triglyceride levels compared with prestudy baseline. The vertical scale of the same spectra in the 3.2–3.3 ppm region was increased (Fig. 5 A and B, Insets) to allow visualization of nontriglyceride metabolites such as myo-inositol, the N-methyls of phosphatidylcholine (PtdCho), glycerophosphocholine (GPC), phosphocholine (PC), and choline (Cho). The insets for this region in Fig. 5 are scaled by the same factor relative to TSP-d4 and demonstrate higher levels of the N-methyls of PtdCho, myo-inositol, and GPC after 6 weeks of troglitazone therapy when compared with the same resonances in spectra acquired from tissue at baseline. From the one-dimensional MAS NMR spectra, an estimate of NMR-visible triglyceride and phosphatidylcholine content (nmol per mg tissue) was determined for samples of tumor tissue before and after troglitazone therapy in patients 1 and 2 (Table 1).
Figure 5.
Representative quantitative one-dimensional MAS 1H-NMR spectra acquired from the tumor tissue of patient 1 (A) before therapy and (B) after 6 weeks of troglitazone therapy, showing increases in triglycerides standardized to the TSP peak at the right. (Insets) Where the vertical scale of the same spectra in the 3.2–3.3 ppm region was increased to allow visualization of nontriglyceride metabolites.
Table 1.
Assessments of NMR-visible triglyceride and phosphatidylcholine in samples of tumor tissue
| Liposarcoma tissue lipid metabolite levels determined by one-dimensional MAS 1H-NMR spectroscopy, nmol/mg tissue
|
||
|---|---|---|
| Baseline | On troglitazone, 6 wks | |
| Patient 1 | ||
| Triglyceride | 14.9 | 40.7 |
| Phosphatidylcholine | 0.4 | 0.7 |
| Patient 2 | ||
| Triglyceride | 22.7 | 242.5 |
| Phosphatidylcholine | 1.0 | 3.0 |
Clinical Imaging of Tumors.
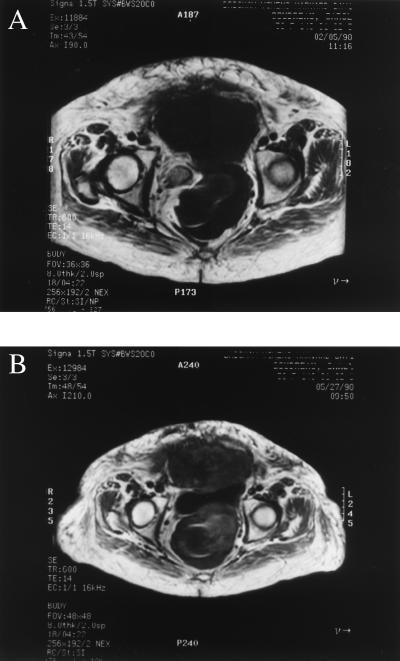
In this pilot study, initial increases in tumor volumes were expected, because induction of differentiation of liposarcoma tissues in vitro was accompanied by intracellular accumulation of lipid and associated increases in cellular volume (19). Serial clinical MRI scans in patients 1 and 2 demonstrated moderate increases in tumor volume after 6 weeks of troglitazone administration, but no new sites of disease were noted. These scans also indicated a change in magnetic resonance characteristics of the tumors consistent with the histologic changes described above, with subtle increases in fat density, as noted in the representative MRI scans of a pelvic mass in patient 3 (Fig. 6).
Figure 6.
Serial MRI images of pelvic liposarcoma in patient 3 (A) before and (B) after 6 weeks of troglitazone administration. Note the subtle change in the fat density signal (whitish stranding) within the tumor (low T1/high T2 signal characteristics) after troglitazone exposure.
Safety and Tolerability of Troglitazone in Patients.
No adverse events were noted to be associated with this treatment, and all patients tolerated the daily dosing of troglitazone with no side effect problems. In particular, liver function tests were closely monitored (weekly for the first month and then monthly thereafter), because rare cases of hepatotoxicity have been described in diabetic patients (27, 28). No abnormalities of hepatic function were detected in any patient.
DISCUSSION
The potential to counteract the uncontrolled proliferation of malignant cell growth through promotion of cellular differentiation represents an attractive approach to cancer therapy. However, this strategy has been limited by inadequate knowledge of specific systems that regulate differentiation in particular lineages and by the lack of means to activate such intracellular pathways. The identification of PPARγ as a critical regulator of adipocyte differentiation has provided a novel target for therapeutic drug discovery (3–9). The subsequent identification of thiazolidinedione drugs as agonist ligands for the PPARγ receptor (10–12) has provided an attractive opportunity to test these hypotheses. Preclinical work using primary cultures of human liposarcoma showed that differentiation of human liposarcoma cells could be induced by stimulation of PPARγ (19), and these in vitro findings justified the translation of this concept into the current clinical trial. This report describes the successful induction of differentiation of human solid tumors in vivo. These observations support the clinical development of receptor-targeted therapeutics to induce differentiation as a feasible antineoplastic strategy.
The histologic, biochemical, and molecular evidence of differentiation observed after troglitazone treatment was striking and consistent among these three patients with intermediate and high-grade liposarcoma who were subjected to extensive analysis. Although certain liposarcomas can exhibit histologic heterogeneity within individual tumors, multiple lines of evidence indicate that the dramatic changes noted after troglitazone administration are not caused by artifactual intra-tumoral sampling variables. Most notably, these patterns of morphologic differentiation and lipid accumulation noted after troglitazone exposure are essentially never seen in the myxoid or pleomorphic subsets of liposarcoma (15). The changes in gene expression were very consistent in the two patients analyzed in this way and showed that three widely used mRNA markers of gene expression (aP2, adipsin, and PPARγ itself) are increased 2- to 4-fold after troglitazone treatment. The data in Fig. 4 are presented as the expression of each mRNA species in tumor relative to expression by a “normal” fat control. This normalized comparison of molecular changes from homogeneous regions of tumor tissue before and after exposure to troglitazone is consistent with the histologic changes and illustrative of adipocytic differentiation. These observations are particularly relevant for the myxoid/round cell and pleomorphic subtypes, which tend to be highly cellular and exhibit little evidence of lipid accumulation in the baseline state. The NMR biochemical analyses for these patients are also consistent with lineage-appropriate differentiation, demonstrating marked increases in the levels of both triglyceride and phosphatidylcholine in tumor tissue. These data therefore represent a measure of troglitazone responsiveness within these patients with intermediate to high-grade disease for whom sufficient tissue was obtained for molecular and NMR analyses.
Induction of differentiation would be expected to decrease the proliferative rates of cancer and thus slow the progression of disease. In well-differentiated (low-grade) liposarcoma, 5-year survival rates may exceed 90%, and patients often can live many years even with measurable disease because of the indolent nature of the disease process. In contrast, patients with intermediate to high-grade myxoid or pleomorphic liposarcoma typically have lower 5-year survival rates (25–50%) because of the more rapid progression of disease (16, 18). The decreased expression of the Ki-67 proliferation-associated antigen in tumors after troglitazone exposure is of considerable magnitude and consistent in all the patients analyzed. It is also consistent with the decreases in cell proliferation that are observed when adipogenesis is induced through PPARγ in cell culture (9, 19).
From laboratory studies, it is expected that terminal adipocytic differentiation would be accompanied by accumulation of lipids and increased volume of tumor cells (19). With differentiation, as adipocytic cells enlarge the net synthesis of cell membrane components such as phosphatidylcholine must increase with accumulation of intracellular triglycerides. Such changes would render typical volumetric assessments of tumor size potentially misleading, because more differentiated tumors might get larger on the basis of increased cell volume rather than by increased cell number. Thus, although the imaging studies did not show any decrease in the size of bulky tumors, these data provide encouraging justification for continuing the development of PPARγ receptor-based differentiation strategies as a novel therapeutic intervention for malignant diseases that express this receptor.
The findings described in the detailed laboratory studies of tumor tissue from these patients with intermediate to high-grade liposarcomas are indicative of a biologically important effect, which has not previously been possible to induce in patients. In addition to the patients presented in this report, three other patients with myxoid/round cell histologies have been treated with troglitazone on this clinical trial; posttroglitazone tumor biopsies from each of these subsequent patients also exhibit the characteristic histologic changes and correlative decreases in the expression of Ki-67 illustrated by the three patients described in this report (data not shown). These findings suggest that all or nearly all patients with myxoid/round cell liposarcoma may respond to PPARγ stimulation with induction of cellular differentiation. However, the clinical relevance of these effects remains under investigation, and additional clinical trials are planned to define whether these cellular changes of enhanced differentiation might translate into improved outcomes, such as prolonged time to tumor progression. The analyses shown here are much more difficult to assess for changes in the well-differentiated subtypes of liposarcomas, because they begin with a more fully differentiated adipocytic phenotype. Nonetheless, it remains possible that prodifferentiation responses occur in those other subtypes of liposarcomas as well, although our ability to detect histologic changes may be inadequate. Clinical trials are planned to evaluate appropriate medical endpoints in all types of liposarcomas, because clinical activity may be noted even in the absence of detectable changes in histology for the more well-differentiated subsets. The relatively favorable safety profile of this orally administered agent (13), confirmed in this study of cancer patients, also suggest the possible therapeutic use of troglitazone and other PPARγ ligands as postsurgical adjuvant therapy to prevent or delay relapses from microscopic residual disease.
These findings in patients with liposarcomas may have important relevance for broader groups of patients with neoplastic diseases based on the expression of the nuclear receptor PPARγ. Specifically, it has been shown in preclinical studies that most colon carcinomas as well as many breast carcinomas express high levels of PPARγ (29, 30). Additionally, agonist ligands to the PPARγ receptor can induce changes consistent with differentiation and lower rates of cellular proliferation in vitro in both breast cancer and colon cancer cell lines (29, 30). It is also worth noting that new PPARγ ligands are under development, and any of these agents that may have increased potency and efficacy in inducing differentiation may prove particularly useful in oncology clinical applications. Similarly, combinations of PPARγ ligands with ligands for retinoid X receptor or other drugs affecting related pathways may enhance clinical efficacy in this regard. These research directions, along with the data on the liposarcoma patients presented in this report, justify additional study of the clinical utility of PPARγ ligands as novel anticancer therapeutic agents.
Acknowledgments
We gratefully acknowledge the constructive input and collaboration of Professor Dave Corey, Dr. Dan Williamson, Dr. Stuart Silverman, Dr. Jonathan Fletcher, Dr. Robert Maki, Dr. Dan DeAngelo, and Dr. Myles Brown for helpful discussion. We also thank Drs. Alan Saltiel and Artemios Vassos of Parke-Davis for their support of this project and Elizabeth Govoni, Murriam Khambaty, Lucette Robinson, Ruth Cain, and Leslie Stefanowicz for their contributions. Finally, we acknowledge with thanks the referral of these patients by Drs. David Harmon, Dennis Priebat, and William Walsh. This work was supported in part by unrestricted research grants to G.D.D. and B.M.S. from Johnson and Johnson, East Brunswick, NJ. B.M.S. is a recipient of a grant from Novartis. S.S. is a recipient of Grant RO1 CA75720-01 from the National Institutes of Health.
ABBREVIATIONS
- PPARγ
peroxisome proliferator-activated receptor-γ
- MAS
magic angle spinning
- TSP
trimethylsilylpropionate
References
- 1.Warrell R P, Frankel S R, Miller W H, Scheinberg D A, Itri L M, Hittleman W N, Vyas R, Andreef M, Tafuri A, Jakubowski A, et al. N Engl J Med. 1991;324:1385–1393. doi: 10.1056/NEJM199105163242002. [DOI] [PubMed] [Google Scholar]
- 2.Warrell R P, de The H, Wang Z-Y, Degos L. N Engl J Med. 1993;329:177–189. doi: 10.1056/NEJM199307153290307. [DOI] [PubMed] [Google Scholar]
- 3.Tontonoz P, Hu E, Graves R A, Budavari A I, Spiegelman B M. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 4.Tontonoz P, Hu E, Spiegelman B M. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 5.Kliewer S A, Forman B M, Blumberg B, Ong E S, Borgmeyer U, Mangelsdorf D J, Umesono K, Evans R M. Proc Natl Acad Sci USA. 1994;91:7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Y, Alvares K, Huang Q, Rao M S, Reddy J K. J Biol Chem. 1993;268:26817–26820. [PubMed] [Google Scholar]
- 7.Sears I B, MacGinnitie M A, Kovacs L G, Graves R A. Mol Cell Biol. 1996;16:3410–3418. doi: 10.1128/mcb.16.7.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tontonoz P, Graves R A, Budavari A I, Erdjument-Bromage H, Lui M, Hu E, Tempst P, Spiegelman B M. Nucleic Acids Res. 1994;22:5628–5634. doi: 10.1093/nar/22.25.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soner A, Xu M, Spiegelman B M. Genes Dev. 1998;11:1987–1998. doi: 10.1101/gad.11.15.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forman B M, Tontonoz P, Chen J, Brun R P, Spiegelman B M, Evans R M. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 11.Lehmann J M, Moore L B, Smith-Oliver T A, Wilkison W O, Willson T M, Kliewer S A. J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 12.Kliewer S A, Lenhard J M, Wilson T M, Patel I, Morris D C, Lehmann J M. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz S, Raskin P, Fonseca V, Graveline J F. N Engl J Med. 1998;338:861–866. doi: 10.1056/NEJM199803263381302. [DOI] [PubMed] [Google Scholar]
- 14.Saltiel A R, Olefsky J M. Diabetes. 1996;45:1661–1669. doi: 10.2337/diab.45.12.1661. [DOI] [PubMed] [Google Scholar]
- 15.Mentzel T, Fletcher C D M. Virchows Arch. 1995;427:353–363. doi: 10.1007/BF00199383. [DOI] [PubMed] [Google Scholar]
- 16.Chang H R, Hajdu S I, Collin C, Brennan M F. Cancer. 1989;64:1514–1520. doi: 10.1002/1097-0142(19891001)64:7<1514::aid-cncr2820640726>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Crozat A, Aman P, Mandahl N, Ron D. Nature (London) 1993;363:640–644. doi: 10.1038/363640a0. [DOI] [PubMed] [Google Scholar]
- 18.Knight J C, Renwick P J, Dal Cin P, Van Den Berghe H, Fletcher C D M. Cancer Res. 1995;55:24–27. [PubMed] [Google Scholar]
- 19.Tontonoz P, Singer S, Forman B M, Sarraf P, Fletcher J A, Fletcher C D M, Brun R P, Mueller E, Altiok S, Oppenheim H, et al. Proc Natl Acad Sci USA. 1997;94:237–241. doi: 10.1073/pnas.94.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel S R, Burgess M A, Plager C, Papadopoulos N E, Linke K A, Benjamin R S. Cancer. 1994;74:1265–1269. doi: 10.1002/1097-0142(19940815)74:4<1265::aid-cncr2820740414>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 21.Demetri G D, Elias A D. Hematol Oncol Clin N Am. 1995;9:765–785. [PubMed] [Google Scholar]
- 22.Kelleher L, Magee H M, Dervan P A. Appl Immunohistochem. 1994;2:164–170. [Google Scholar]
- 23.Maniatis T, Fritsch E F, Sambrook J. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 24.Maas W, Laukien F, Cory D. J Am Chem Soc. 1996;118:13085–13086. [Google Scholar]
- 25.Millis K, Maas W, Cory D, Singer S. Magn Reson Med. 1997;38:399–403. doi: 10.1002/mrm.1910380307. [DOI] [PubMed] [Google Scholar]
- 26.Millis, K., Weybright, P., Fletcher, J. A., Fletcher, C. D. M., Cory, D. & Singer, S. (1999) Magn. Reson. Med., in press. [DOI] [PubMed]
- 27.Watkins P B, Whitcomb R W. N Engl J Med. 1998;338:916. doi: 10.1056/NEJM199803263381314. [DOI] [PubMed] [Google Scholar]
- 28.Neuschwander-Tetri B A, Isley W L, Oki J C, Ramrakhiani S, Quiason S G, Phillips N J, Brunt E M. Ann Intern Med. 1998;129:38–41. doi: 10.7326/0003-4819-129-1-199807010-00009. [DOI] [PubMed] [Google Scholar]
- 29.Mueller E, Sarraf P, Tontonoz P, Evans R M, Martin K J, Zhang M, Fletcher C, Singer S, Spiegelman B M. Mol Cell. 1998;1:465–470. doi: 10.1016/s1097-2765(00)80047-7. [DOI] [PubMed] [Google Scholar]
- 30.Sarraf P, Mueller E, Jones D, King F J, DeAngelo D J, Partridge J B, Holden S A, Chen L B, Singer S, Fletcher C, Spiegelman B M. Nat Med. 1998;4:1046–1052. doi: 10.1038/2030. [DOI] [PubMed] [Google Scholar]