Abstract
The dose-limiting toxicity of interleukin-2 (IL-2) and immunotoxin (IT) therapy in humans is vascular leak syndrome (VLS). VLS has a complex etiology involving damage to vascular endothelial cells (ECs), extravasation of fluids and proteins, interstitial edema, and organ failure. IL-2 and ITs prepared with the catalytic A chain of the plant toxin, ricin (RTA), and other toxins, damage human ECs in vitro and in vivo. Damage to ECs may initiate VLS; if this damage could be avoided without losing the efficacy of ITs or IL-2, larger doses could be administered. In this paper, we provide evidence that a three amino acid sequence motif, (x)D(y), in toxins and IL-2 damages ECs. Thus, when peptides from RTA or IL-2 containing this sequence motif are coupled to mouse IgG, they bind to and damage ECs both in vitro and, in the case of RTA, in vivo. In contrast, the same peptides with a deleted or mutated sequence do not. Furthermore, the peptide from RTA attached to mouse IgG can block the binding of intact RTA to ECs in vitro and vice versa. In addition, RTA, a fragment of Pseudomonas exotoxin A (PE38-lys), and fibronectin also block the binding of the mouse IgG-RTA peptide to ECs, suggesting that an (x)D(y) motif is exposed on all three molecules. Our results suggest that deletions or mutations in this sequence or the use of nondamaging blocking peptides may increase the therapeutic index of both IL-2, as well as ITs prepared with a variety of plant or bacterial toxins.
Keywords: immunotoxin, integrins, disintegrin, cytokine
Immunotoxins (ITs) are hybrid molecules consisting of mAbs or other cell-binding ligands, which are biochemically or genetically linked to powerful toxins, toxin subunits, or ribosome-inactivating proteins (RIPs) from plants, fungi, or bacteria (reviewed in ref. 1). Over the past two decades, ITs containing deglycosylated ricin A chain (dgRTA) have been developed, structurally optimized for stability and activity and evaluated for activity both in vitro and in vivo in rodents, monkeys, and humans (reviewed in refs. 1–3). The dose-limiting side effect of dgRTA-IT therapy is vascular leak syndrome (VLS), which is characterized by an increase in vascular permeability resulting in interstitial edema and organ failure (2–4). VLS is not unique to dgRTA-ITs and is also a toxic side effect of ITs prepared with other toxins and of the cytokine interleukin-2 (IL-2) (5–7).
DgRTA-ITs rapidly damage the integrity of human umbilical vein endothelial cells (HUVECs) and interfere with fibronectin (Fn)-mediated adhesion (8). In severe combined immunodeficient (SCID) mice with human skin xenografts, systemically administered dgRTA-ITs induce vascular leak in the human skin, but not in the adjacent mouse skin (9). Based on these results, we considered the possibility that ricin A chain (RTA), other toxins, ribosome-inactivating proteins (RIPs), and IL-2 contain homologous structural motifs that may effect cell–cell and cell–matrix interactions and thereby damage human ECs. In comparing published sequences of toxins, RIPs, and IL-2 we identified an (x)D(y) motif where x = L, I, G, or V and y = V, L, or S (Table 1). This motif is also shared by viral disintegrins, which disrupt the function of integrins (10).
Table 1.
(x)D(y) motifs in molecules that induce VLS
| Category | Agent-inducing VLS | (x)D(y) motif | Location | GenBank accession no. |
|---|---|---|---|---|
| Toxins* | Abrin A chain | IDV | 68–70 | X76721 |
| GDL | 114–116 | |||
| VDS | 229–231 | |||
| Diphtheria toxin (DT) A chain | VDS | 6–8 | 576189 | |
| VDS | 28–30 | |||
| IDS | 289–291 | |||
| LDV | 441–443 | |||
| PE38-lys† | GDL | 348–350 | K01397 | |
| GDV | 430–432 | |||
| GDL | 605–607 | |||
| RTA | LDV | 74–76 | A23903 | |
| Shiga toxin A chain | VDS | 36–38 | M19437 | |
| IDS | 63–65 | |||
| VDV | 74–76 | |||
| GDS | 132–134 | |||
| LDL | 162–164 | |||
| VDL | 219–221 | |||
| RIPs‡ | Gelonin | IDV | 114–116 | L12243 |
| Momordin | LDV | 64–66 | 576194 | |
| LDS | 132–134 | |||
| Pokeweed antiviral protein (PAP) | VDS | 179–181 | X98079 | |
| GDL | 308–310 | |||
| Saporin | LDL | 6–8 | X69132 | |
| IDL | 143–145 | |||
| Trichosanthin | GDV | 23–25 | U25675 | |
| IDV | 87–89 | |||
| LDS | 155–157 | |||
| Cytokines | IL-2 | LDL | 19–21 | 1311005 |
Enzymatically active chain of the holotoxin.
PE38 refers to enzymatically active domain III (residues 405–613) plus residues 253–354 and 381–404 in PE.
RIPs that are homologs of the enzymatically active A chains of plant toxins.
We therefore generated short (<20 amino acids) (x)D(y) motif-containing peptides from RTA or IL-2, as well as peptides with deleted or mutated sequences, added flanking glycines and a cysteine, attached these peptides to a mouse IgG1 mAb by means of the cysteine, and studied their ability to bind to HUVECs and to damage ECs both in vitro and in vivo.
MATERIALS AND METHODS
Peptide Synthesis.
We synthesized a peptide representing 13 amino acids (residues 69–81) from RTA and added N- and C-terminal glycine residues to improve solubility (Table 2). An N-terminal cysteine was also added to couple the peptide to the RFB4 mAb. We also synthesized two control peptides (Table 2). We next synthesized a peptide of nine amino acids representing residues 15–23 from IL-2 as well as a control peptide (Table 2). Again, flanking glycines and a cysteine were added. All peptides were synthesized on an Applied Biosystems model 430A solid-phase peptide synthesizer in the Biopolymer Facility at the University of Texas Southwestern Medical Center.
Table 2.
Peptides from RTA and IL-2
| Peptide origin | Designation | Description | Peptide sequence | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RTA | LDV+ | Native | C | G | G | G | S | V | T | L | A | L | D | V | T | N | A | Y | V | G | G | G |
| 69 | 70 | 71 | 72 | 73 | 74 | 75 | 76 | 77 | 78 | 79 | 80 | 81 | ||||||||||
| LDV− | Deleted | C | G | G | G | S | V | T | L | A | T | N | A | Y | V | G | G | G | ||||
| 69 | 70 | 71 | 72 | 73 | 77 | 78 | 79 | 80 | 81 | |||||||||||||
| GQT+ | Mutant | C | G | G | G | S | V | T | L | A | G | Q | T | T | N | A | Y | V | G | G | G | |
| 69 | 70 | 71 | 72 | 73 | 74 | 75 | 76 | 77 | 78 | |||||||||||||
| IL-2 | LDL+ | Native | C | G | G | G | E | H | L | L | L | D | L | Q | M | G | G | G | ||||
| 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | ||||||||||||||
| LDL− | Deleted | C | G | G | G | E | H | L | L | Q | M | G | G | G | ||||||||
| 15 | 16 | 17 | 18 | 22 | 23 | |||||||||||||||||
Each peptide was conjugated to the mouse mAb, RFB4, as described is Materials and Methods.
Conjugation of the Peptides to RFB4.
All peptides contained an N-terminal cysteine residue to facilitate conjugation with maleimide-derivatized RFB4. RFB4 was treated with a 25-fold molar excess of succinimidyl-4-(N-maleimidemethyl)-cyclohexane-1-carboxylate and excess reagent was removed by gel filtration. The number of maleimide groups introduced into each molecule of RFB4 was determined by the back titration of 2-mercaptoethylamine by using Ellman’s reagent (11). The derivatized RFB4 was reacted with a 10-fold excess of the SH peptide at room temperature for 4 hr, and excess peptide was removed by dialysis against PBS. The maleimide reaction allowed the formation of the IgG1 CS-1 peptide conjugate in which the number of peptide groups attached was similar to that of free maleimide groups.
Effect of RFB4 Peptides on the Morphology of HUVEC Monolayers.
HUVECs were isolated, cultured, and studied microscopically as described (8, 12).
In Vivo Effect of the RFB4 Peptides.
“Vascular leak” in human tissue was evaluated by using human neonatal foreskin xenografts transplanted onto SCID mice as previously described (9). Fluid accumulation in the human skin was measured by weighing punch biopsies of the skin grafts before and after freeze drying. The fluid accumulation in the lungs of normal SCID mice was also evaluated because previous reports had demonstrated that IL-2 induces fluid accumulation in the lungs of mice (13). The water content of the lungs or skin grafts was calculated as the wet/dry weight ratio.
Flow Cytometric Analysis of the Binding of dgRTA and RFB4 Peptides to HUVECs.
The proteins were coupled to fluorescein isothiocyanate (FITC) (Sigma). HUVECs (105) were washed twice in cold PBS containing 1% BSA and 0.01% sodium azide (PBS/BSA/AZ), resuspended in 100 μl of the same buffer, and incubated with FITC reagents for 30 min on ice in the dark, washed three times with PBS/BSA/AZ, fixed in 0.5 ml of 1% paraformaldehyde PBS/AZ, and analyzed by using a FACScan (Becton Dickinson) and cytoquest software.
Inhibition of the Binding of dgRTA and RFB4 Peptides to HUVECs.
FITC-dgRTA or FITC-RFB4-LDV+ at concentrations representing 20–50% of maximal binding (0.035 μg/105 cells for dgRTA and 1.0 μg/105 cells for RFB4-LDV+) were incubated with HUVECs in the presence or absence of a 100-fold excess of dgRTA (Inland Laboratories, Austin, TX), RFB4-LDV+, RFB4, Fn (GIBCO), or Pseudomonas exotoxin A (PE38-lys) (a generous gift from Ira Pastan, National Cancer Institute, Bethesda) for 30 min on ice in the dark. Washed cells were fixed in 1% paraformaldehyde and analyzed on the FACS.
RESULTS
Identification of an (x)D(y) Motif in VLS-Inducing Agents.
We have previously shown that dgRTA interferes with the adhesion of HUVECs to Fn-coated plates and that Fn inhibits dgRTA-mediated damage to HUVECs (8). Cell adhesion to Fn is mediated by integrins that recognize RGD and LDV sequences in the Fn molecule (14, 15). As shown in Table 1, an (x)D(y) motif where x = L, I, G, or V and y = V, L, or S is found in RTA, other toxins, RIPs, and cytokines that induce VLS. A homolog is also present in a viral disintegrin (10).
Localization of the (x)D(y) Motifs in RTA, PE38-lys, and IL-2.
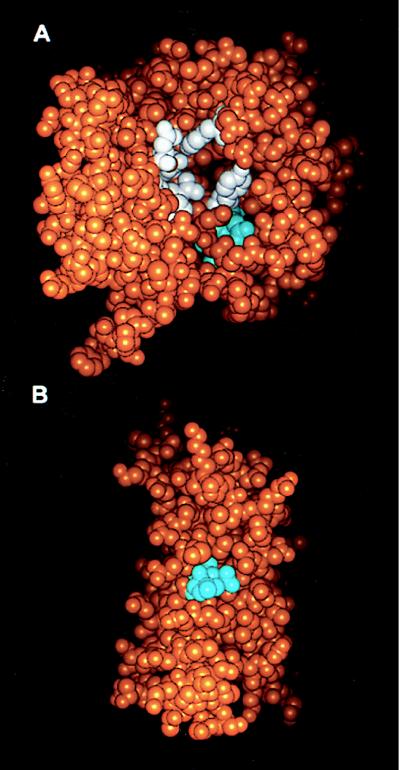
The LDV motif in RTA (residues 74–76) is at the C terminus of a β-strand of the first domain near the Tyr-80 residue, which is involved in building the active site (16) (Fig. 1A). The LDV sequence is only partially exposed, but structural fluctuations in the molecule may increase its accessibility. However, unlike Tyr-80, a stretch of RTA containing the LDV motif has been deleted without affecting its enzymatic activity (17). With regard to the Domain III of PE, the GDL sequence (residues 605–607) is fully exposed in PE38-lys (18). The LDL sequence of IL-2 (residues 19–21) is located in an α-helix and is also partially exposed (Fig. 1B). Previous studies have shown that a mutation in Asp-20, in the LDL motif (Table 1), eliminates binding of IL-2 to the β chain of the IL-2 receptor and subsequent cell proliferation (19). Therefore, any mutations in IL-2 to eliminate VLS must preserve the Asp-20 or the biological activity of IL-2 will be lost.
Figure 1.
Localization of the LDV and LDL sequences in RTA (A) and IL-2 (B). Space filling models of the three-dimensional structures of RTA (A) (PDB accession no. 1br5.pdb) and IL-2 (B) (PDB accession no. 1irl.pdb) are shown with the atoms of the LDV residues of RTA and the LDL residues of IL-2 shown in cyan, the active site residues of RTA (Y80, Y123, E177, R180, N209, and W211) in white, and all other atoms in orange. Models were generated with the insight ii program (Micron Separations).
Characterization of the RFB4 Peptides.
The peptides containing the (x)D(y) motif were difficult to solubilize even with the additional flanking glycines. For this reason, we elected to conjugate them to a soluble carrier protein. We chose the mAb RFB4, because the RFB4-dgRTA is a prototypic IT and, therefore, RFB4–peptide conjugates should “mimic” ITs. As determined by both HPLC and radiolabeling, the RFB4 peptide conjugates (Table 2) contained 6–9 maleimide groups per molecule of IgG1 and these groups formed stable thioether bonds by reaction with the cysteine-containing peptides.
The RFB4 Peptides Containing (x)D(y) Motifs Damage HUVEC Monolayers.
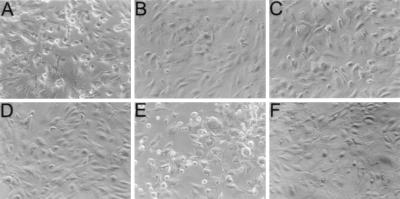
To determine whether the LDV sequence in RTA and the LDL sequence in IL-2 damaged HUVECs, monolayers were incubated with different concentrations of RFB4-RTA peptides, RFB4-IL-2 peptides, or controls. As shown in Fig. 2, untreated HUVECs consisted of tightly packed elongated cells (Fig. 2D). Treatment with 10−6 M RFB4-LDV+ (A) or RFB4-LDL+ (E) caused cell rounding after 2 hr of incubation and the formation of gaps in the monolayer after 18 hr. Toxic effects on HUVECs were not observed using RFB4-LDV− (B), RFB4-GQT (C), or RFB4-LDL− (F). The toxic effect of RFB4 peptides containing LDV or LDL were dose-dependent and comparable to the effects observed using RFB4-dgRTA (Table 3). These results suggest that the LDV sequence in RTA and its LDL homolog in IL-2 are involved in the EC toxicity of these agents.
Figure 2.
Effect of RFB4 peptides on the morphology of HUVEC monolayers. HUVEC monolayers were incubated at 37°C for 18 hr with 10−6 M RFB4-LDV+ (A), RFB4-LDV− (B), RFB4-GQT (C), RFB4-LDL+ (E), RFB4-LDL− (F), or medium only (D) and then examined by phase-contrast microscopy (magnification ×20). Normal monolayers consisted of highly packed cells with elongated shapes (B–D and F), whereas damaged cells were rounded up and detached from the plate (A and E).
Table 3.
Effect of different concentrations of the RFB4 peptide constructs on the morphology of HUVEC monolayers
| Peptides | Concentration, M*
|
|||
|---|---|---|---|---|
| 10-6 | 10-7 | 10-8 | 0 | |
| RFB4-RTA-derived | ||||
| RFB4-LDV+ | ++ | ++ | + | − |
| RFB4-LDV− | − | − | − | − |
| RFB4-GQT+ | − | − | − | − |
| RFB4-IL-2-derived | ||||
| RFB4-LDL+ | ++ | ++ | + | − |
| RFB4-LDL− | − | − | − | − |
| RFB4-dgRTA | ++ | + | + | − |
| RFB4 | − | − | − | − |
HUVECs were grown to confluence in 96-well tissue culture plates and cells were treated for 18 hr with different concentrations of RTA-derived peptide constructs in M199 with 2% fetal calf serum.
Morphological changes were scored as: −, no changes; +, rounding up of cells; and ++ disruption and detachment of cells from the cell monolayer.
RFB4-LDV+ Induces Vascular Leak in Vivo.
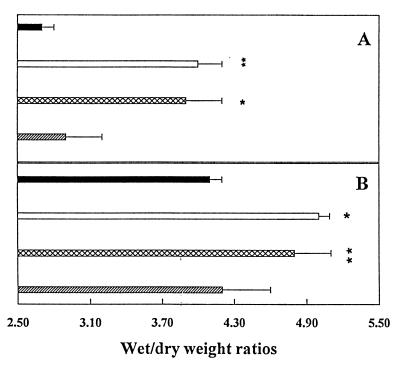
Although the vascular toxicity of IL-2 has been observed in experimental animals (13, 20–22), it has been difficult to induce dgRTA-IT-mediated systemic manifestations of VLS in mice, rats, or monkeys (23). Recently, we have developed a model to study the effect of ITs on human endothelium in vivo by grafting vascularized human skin onto SCID mice, injecting the mice with dgRTA-ITs and measuring fluid accumulation in the graft as the wet/dry weight ratio (9). We used this model to evaluate the effect of RFB4-LDV+, RFB4-GQT+, and RFB4-dgRTA in vivo (Fig. 3A). We found that there were increases in the wet/dry weight ratio of the human skin grafts after injection of RFB4-LDV+ and RFB4-dgRTA, but not after injection of RFB4-GQT+. Comparable results were obtained by using SCID mouse lungs (Fig. 3B). It should be noted that although the difference in the figures may appear small, they are statistically significant and consistent with previous reports using IL-2 (13).
Figure 3.
The in vivo effect of RFB4-RTA peptides. (A) SCID mice with human xenografts were injected with 200 μg of RFB4-dgRTA (□), RFB4-LDV+ (⊠), RFB4-GQT (▨), or saline (■), and the wet/dry weight ratios of the human skin were determined. (B) SCID mice were injected as described in A and the wet/dry weight ratios of lungs were determined. Values represent the mean of three experiments ± SD. Asterisks indicate a significant difference from saline-treated mice (∗, P < 0.02; ∗∗, P < 0.01).
RFB4-LDV+ and RFB4-LDL+ Bind to HUVECs.
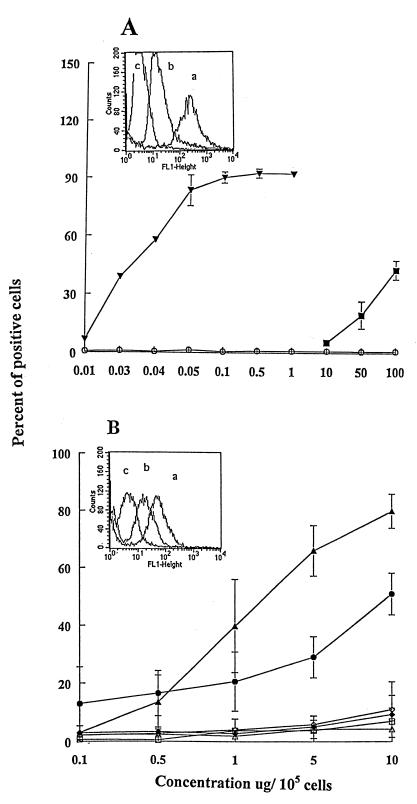
The fact that RFB4-LDV+ and RFB4-LDL+ damage HUVECs implies that these peptides interact with a binding site on HUVECs, although in the intact IL-2 or RTA molecules the (x)D(y) motif may not be the primary binding site for ECs. We therefore carried out a series of binding and binding/inhibition experiments. As shown in Fig. 4A, 50% of maximal binding of FITC-dgRTA or FITC-PE38-lys required 0.035 μg and >100 μg/105 cells, respectively, demonstrating that dgRTA has a >3 log higher relative binding affinity for HUVECs than PE38-lys. This may be because the LDV receptor on HUVECs has a lower affinity for homologous sequences in PE38-lys and/or that LDV in RTA is more exposed. It is also possible that other nonhomologous sequences in RTA (but not in PE38-lys) bind to HUVECs. The difference between the relative binding affinity of FITC-dgRTA (0.035 μg/105 cells per 100 μl) (Fig. 4A) and FITC-RFB4-LDV+ (0.5 μg/105 cells per 100 μl) (Fig. 4B) was only 2-fold if calculated on a molar basis. Because the RFB4 peptide conjugates with deleted or mutated LDV sequences did not bind to HUVECs, the (x)D(y) motif is clearly involved in the binding.
Figure 4.
Binding of dgRTA, PE38-lys, and RFB4 peptides to HUVECs. HUVECs (105) were incubated on ice for 30 min with FITC reagents in 100 μl PBS/BSA/AZ at varying concentrations, washed, fixed in 1% paraformaldehyde, and analyzed by flow cytometry. Values represent the mean ± SD of three experiments. (A) FITC-dgRTA (▾), FITC-PE38-lys (■), FITC-carbonic anhydrase (control) (○). Inset shows the histograms of flow cytometric analyses of the binding of (a) dgRTA, (b) PE38-lys, and (c) carbonic anhydrase to HUVECs. (B) FITC-RFB4-LDV+ (▴), FITC-RFB4-LDV− (▵), FITC-RFB4-GQT+ (◊), FITC-RFB4 (□), FITC-RFB4-LDL+ (●), FITC-RFB4-LDL− (⧫). Inset shows the histograms of (a) RFB4-LDV+, (b) RFB4-LDL+, and (c) RFB4.
dgRTA Uses Its LDV Sequence to Bind to HUVECs.
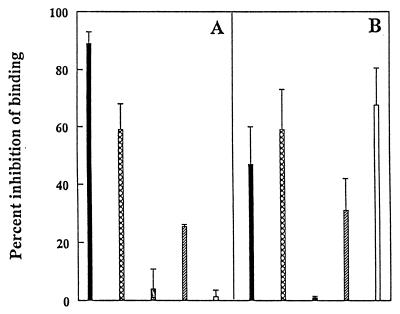
To provide further evidence for the role of the (x)D(y) motif in the binding of RTA to HUVECs, we carried out a series of binding inhibition studies. We found that the binding of FITC-dgRTA to HUVECs was inhibited by >90% by dgRTA and by >60% by RFB4-LDV+, indicating that the binding of dgRTA is specific and that it involves (at least in part) the LDV sequence (Fig. 5A). The fact that the homolog-containing PE38-lys could not inhibit the binding of dgRTA (Fig. 5A) is not surprising, because its relative affinity for HUVECs is greater than three logs lower (Fig. 4A). In addition, dgRTA may have additional nonhomolog binding sites for HUVECs, as suggested by the fact that RFB4-LDV+ inhibited its binding by 60% and not 100%. Furthermore, in the reverse experiments, both dgRTA and RFB4-LDV+ inhibited the binding of FITC-RFB4-LDV+ to HUVECs to a similar extent (Fig. 5B), supporting further the notion that the LDV sequence in RTA is involved in binding to HUVECs. Surprisingly, PE38-lys effectively inhibited the binding of FITC-LDV+ to HUVECs (Fig. 5B), suggesting that one or more of its LDV homolog sequences can compete with the LDV motif for binding of an LDV-containing peptide. Further studies will be necessary to identify which homolog sequences in PE38-lys (GDL-348–350, GDV-430–432, or GDL-605–607) bind to and damage HUVEC. Fn also inhibited the binding of both FITC-dgRTA (Fig. 5A) and FITC-RFB4-LDV+ (Fig. 5B) to HUVECs, but it did so less effectively. In this regard, although Fn also contains the LDV motif, it has different flanking residues that may play a role in the availability of its LDV motif.
Figure 5.
Inhibition of the binding of dgRTA and RFB4-LDV+ to HUVECs. HUVECs (105) were incubated on ice for 30 min with FITC-dgRTA (A) or FITC-RFB4-LDV+ (B) in the presence or absence of 100-fold excess of dgRTA (■), RFB4-LDV+ (⊠), RFB4 (░⃞), Fn (▨), or PE38-lys (□) in 100 μl PBS/BSA/AZ. The percent inhibition of binding to HUVECs is presented. Values represent the means ± SD of three experiments.
DISCUSSION
The major dose-limiting side effect of ITs and IL-2 therapy in humans is VLS. The mechanisms underlying this toxicity are unclear and are likely to involve a cascade of events that are initiated in ECs and involve inflammatory cascades and cytokines (reviewed in ref. 4). If this toxicity could be inhibited without decreasing the potency and extravasation of ITs and IL-2 in vivo, their therapeutic indices would be increased. We have therefore attempted to identify sequences in toxins and IL-2 responsible for initiating damage to ECs. Our approach was to compare primary sequences of toxins, RIPs, and IL-2 for common motifs and to then determine whether peptides containing these motifs, when attached to a mAb (RFB4) not reactive with HUVECs, would bind to and damage them. The major findings to emerge from this study are: (i) RTA, other toxins, RIPs, and IL-2 contain (x)D(y) motifs, which are also shared by a viral disintegrin. In the case of RTA, PE38-lys (18), and IL-2, modeling studies indicate that the (x)D(y) motif is partially exposed on the intact protein; (ii) peptides containing the LDV motif in RTA and the LDL motif in IL-2, when attached to the RFB4 mAb, specifically damage HUVECs in vitro; (iii) the LDV sequence in RTA is probably responsible for the initiation of events leading to VLS-like symptoms in vivo because injection of RFB4-RTA peptides containing the native (but not mutated or deleted) LDV sequence caused vascular leak in lungs and in human skin xenografts in a manner analogous to that of the RFB4-dgRTA IT; (iv) dgRTA uses its LDV sequence, at least in part, to bind to HUVECs, because peptides or proteins containing this motif inhibited the dose-dependent, saturable binding of dgRTA to HUVECs.
The stereoviews of LDV in RTA and LDL in IL-2 indicate that these motifs are partially exposed and should interact with cells. For dgRTA, this is supported by its dose-dependent, saturable binding to HUVECs in vitro. Because the binding of RFB4-LDV+ to HUVECs could be partially inhibited not only by dgRTA but also by proteins containing LDV or LDV-homologs, i.e., Fn and PE38-lys, further suggests a functional conservation in the (x)D(y) motif in several different molecules.
LDV constitutes the minimal active site in the CS1 domain of Fn responsible for its binding to the α4β1 integrin receptor (14, 15, 24). The LDV homolog sequences also play a role in the vascular functions of a variety of molecules, including vascular cell adhesion molecule 1 and the γ chain of fibrinogen (25). Another family of proteins called disintegrins usually contain an RGD sequence, but in the case of one disintegrin (which is present in rotavirus), an LDV sequence is present (10). Disintegrins damage ECs or interfere with cell adherence (26–28). In the disintegrin, kistrin (from a snake venom) LDV can be substituted for RGD without compromising disintegrin function (29). The difference between the ability of an LDV− or homolog-containing molecule to promote vascular integrity (e.g., Fn) or disrupt it (e.g., RTA) may depend on the orientation (or availability) of the LDV motif and hence on critical flanking sequences. Indeed, recent studies have shown that the residues flanking RGD play a role in ligand binding (30).
It has been reported that IL-2 directly increases the permeability of the vascular endothelium to albumin in vitro and that this effect can be inhibited by anti-IL-2 receptor mAbs (31). In agreement with this, our results demonstrated that the LDL sequence in IL-2 damages HUVECs. However, in contrast to RTA, the Asp-20 in the LDL of IL-2 is involved in receptor binding and functional activity (19). This might also be true of some of the toxins, in which case mutations in (x)D(y) will not be useful.
Although it has been difficult to demonstrate systemic manifestations of VLS in mice injected with RTA-ITs, vascular leak occurs in human skin xenografts in SCID mice. In this study, we found that the fluid accumulation in these xenografts was comparable using either RFB4-dgRTA or RFB4-LDV+.
Taken together, our studies suggest that RTA (and perhaps other toxins) and IL-2 may damage ECs by virtue of their (x)D(y) motifs and hence may act as natural disintegrins. This suggests that deletions or mutations in this motif and/or its flanking sequences may prevent VLS. In the case of RTA, the active site of the enzyme does not include the LDV sequence so that the enzymatic activity of RTA should not be affected by mutations or deletions in this sequence as suggested previously (17). In PE38-lys, the GDL sequence is also distal from the active site (18). With regard to IL-2, the situation is less clear, because Asp-20 is involved in IL-2 receptor binding. Another implication of our results is that it may be possible to generate a family of peptides or drug mimetics based on the (x)D(y) motif or its flanking sequences that will inhibit VLS in vivo. It is also possible that (x)D(y) motifs and particular flanking sequences added to larger molecules will increase extravasation into tissues. Finally, (x)D(y)-containing peptides currently being tested as antiinflammatory or antimetastatic agents (32–34) should be monitored for both increased extravasation and for toxic effects on vasculature.
Acknowledgments
We thank Ms. S. Flowers and Ms. C. Self for expert secretarial assistance, Mr. J. Shelton for technical assistance, and Dr. Ira Pastan (National Cancer Institute) for the gift of PE38-lys. We are indebted to Drs. J. Uhr, J. Smallshaw, and C. Siegall for extremely important and insightful comments concerning the manuscript.
ABBREVIATIONS
- ECs
endothelial cells
- Fn
fibronectin, HUVEC, human umbilical vein endothelial cell
- IT
immunotoxin
- IL-2
interleukin-2
- PE38-lys
a fragment of Pseudomonas exotoxin A
- RIP
ribosome-inactivating protein
- RTA
ricin A chain
- dgRTA
deglycosylated RTA
- VLS
vascular leak syndrome
- SCID
severe combined immunodeficient
- FITC
fluorescein isothiocyanate
References
- 1.Vitetta E S, Thorpe P E, Uhr J W. Immunol Today. 1993;14:252–259. doi: 10.1016/0167-5699(93)90041-I. [DOI] [PubMed] [Google Scholar]
- 2.Sausville E A, Vitetta E S. In: Monoclonal Antibody-Based Therapy of Cancer. Grossbard M L, editor. Vol. 4. Basel: Marcel Dekker; 1997. pp. 81–89. [Google Scholar]
- 3.Baluna R, Vitetta E S. Immunopharmacology. 1996;37:117–132. doi: 10.1016/s0162-3109(97)00041-6. [DOI] [PubMed] [Google Scholar]
- 4.Engert A, Sausville E A, Vitetta E S. In: Clinical Applications of Immunotoxins. Frankel A E, editor. Vol. 2. Berlin: Springer; 1997. pp. 13–33. [Google Scholar]
- 5.Dutcher J P, Gaynor E R, Boldt D H, Doroshow J H, Bar M H, Sznol M, Mier J, Sparano J, Fisher R I, Weiss G, et al. J Clin Oncol. 1991;9:641–648. doi: 10.1200/JCO.1991.9.4.641. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg S A, Lotze M T, Muul L M, Chang A E, Avis F P, Leitman S, Linehan W M, Robertson C N, Lee R E, Rubin J T, et al. N Engl J Med. 1987;316:889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- 7.Vial T, Descotes J. Drug Safety. 1992;7:417–433. doi: 10.2165/00002018-199207060-00004. [DOI] [PubMed] [Google Scholar]
- 8.Baluna R, Ghetie V, Oppenheimer-Marks N, Vitetta E S. Int J Immunopharmacol. 1996;18:355–361. doi: 10.1016/s0192-0561(96)00043-4. [DOI] [PubMed] [Google Scholar]
- 9.Baluna R, Vitetta E S. J Immunother. 1999;22:41–47. doi: 10.1097/00002371-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Coulson B S, Londrigan S L, Lee D J. Proc Natl Acad Sci USA. 1997;94:5389–5394. doi: 10.1073/pnas.94.10.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Husain M, Bieniarz C. Bioconjugate Chem. 1994;5:481–490. doi: 10.1021/bc00029a017. [DOI] [PubMed] [Google Scholar]
- 12.Soler-Rodriguez A M, Ghetie M A, Oppenheimer-Marks N, Uhr J W, Vitetta E S. Exp Cell Res. 1993;206:227–234. doi: 10.1006/excr.1993.1142. [DOI] [PubMed] [Google Scholar]
- 13.Orucevic A, Lala P K. J Immunother. 1995;18:210–220. doi: 10.1097/00002371-199511000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Makarem R, Humphries M J. Biochem Soc Trans. 1991;19:380S–382S. doi: 10.1042/bst019380s. [DOI] [PubMed] [Google Scholar]
- 15.Wayner E A, Kovach N L. J Cell Biol. 1992;116:489–497. doi: 10.1083/jcb.116.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mlsna D, Monzingo A F, Katzin B J, Ernst S, Robertus J D. Protein Sci. 1993;2:429–435. doi: 10.1002/pro.5560020315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munishkin A, Wool I G. J Biol Chem. 1995;270:30581–30587. doi: 10.1074/jbc.270.51.30581. [DOI] [PubMed] [Google Scholar]
- 18.Li M, Dyda F, Benhar I, Pastan I, Davies D R. Proc Natl Acad Sci USA. 1995;92:9308–9312. doi: 10.1073/pnas.92.20.9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins L, Tsien W-H, Seals C, Hakimi J, Weber D, Bailon P, Hoskings J, Green W C, Toome V, Ju G. Proc Natl Acad Sci USA. 1988;85:7709–7713. doi: 10.1073/pnas.85.20.7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puri R K, Travis W D, Rosenberg S A. Cancer Res. 1989;49:969–976. [PubMed] [Google Scholar]
- 21.Puri R K, Rosenberg S A. Cancer Immunol Immunother. 1989;28:267–274. doi: 10.1007/BF00205236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenstein M, Ettinghausen S E, Rosenberg S A. J Immunol. 1986;137:1735–1742. [PubMed] [Google Scholar]
- 23.Soler-Rodriguez A M, Uhr J W, Richardson J, Vitetta E S. Int J Immunopharmacol. 1992;14:281–291. doi: 10.1016/0192-0561(92)90041-i. [DOI] [PubMed] [Google Scholar]
- 24.Nowlin D M, Gorcsan F, Moscinski M, Chiang S-L, Lobl T J, Cardarelli P M. J Biol Chem. 1993;268:20352–20359. [PubMed] [Google Scholar]
- 25.Clements J M, Newham P, Shepherd M, Gilbert R, Dudgeon T J, Needham L A, Edwards R M, Berry L, Brass A, Humphries M J. J Cell Sci. 1994;107:2127–2135. doi: 10.1242/jcs.107.8.2127. [DOI] [PubMed] [Google Scholar]
- 26.McLane M A, Marcinkiewicz C, Vijay-Kumar S, Wierzbicka-Patynowski I, Niewiarowski S. Proc Soc Exp Biol Med. 1998;219:109–119. doi: 10.3181/00379727-219-44322. [DOI] [PubMed] [Google Scholar]
- 27.Huang T-F. Cell Mol Life Sci. 1998;54:527–540. doi: 10.1007/s000180050181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeh C H, Peng H-C, Huang T-F. Blood. 1998;292:3268–3276. [PubMed] [Google Scholar]
- 29.Tselepis V H, Green L J, Humphries M J. J Biol Chem. 1997;272:21341–21348. doi: 10.1074/jbc.272.34.21341. [DOI] [PubMed] [Google Scholar]
- 30.Lu X, Rahman S, Kakkar V V, Authi K S. J Biol Chem. 1996;271:289–294. doi: 10.1074/jbc.271.1.289. [DOI] [PubMed] [Google Scholar]
- 31.Downie G H, Ryan U S, Hayes B A, Friedman M. Am J Respir Cell Mol Biol. 1992;7:58–65. doi: 10.1165/ajrcmb/7.1.58. [DOI] [PubMed] [Google Scholar]
- 32.Jackson D Y, Quan C, Artis D R, Rawson T, Blackburn B, Struble M, Fitzgerald G, Chan K, Mullins S, Burnier J P, et al. J Med Chem. 1997;40:3359–3368. doi: 10.1021/jm970175s. [DOI] [PubMed] [Google Scholar]
- 33.Maeda M, Izuno Y, Kawasaki K, Kaneda Y, Mu Y, Tsutsumi Y, Lem K W, Mayumi T. Biochem Biophys Res Commun. 1997;241:595–598. doi: 10.1006/bbrc.1997.7855. [DOI] [PubMed] [Google Scholar]
- 34.Greenspoon N, Hershkoviz R, Alon R, Gershonov E, Lavie B, Lider O. Int J Pept Protein Res. 1994;43:417–424. doi: 10.1111/j.1399-3011.1994.tb00539.x. [DOI] [PubMed] [Google Scholar]