Abstract
Uteroglobin (UG) is a multifunctional, secreted protein that has receptor-mediated functions. The human UG (hUG) gene is mapped to chromosome 11q12.2–13.1, a region frequently rearranged or deleted in many cancers. Although high levels of hUG expression are characteristic of the mucosal epithelia of many organs, hUG expression is either drastically reduced or totally absent in adenocarcinomas and in viral-transformed epithelial cells derived from the same organs. In agreement with these findings, in an ongoing study to evaluate the effects of aging on UG-knockout mice, 16/16 animals developed malignant tumors, whereas the wild-type littermates (n = 25) remained apparently healthy even after 1½ years. In the present investigation, we sought to determine the effects of induced-expression of hUG in human cancer cells by transfecting several cell lines derived from adenocarcinomas of various organs with an hUG-cDNA construct. We demonstrate that induced hUG expression reverses at least two of the most important characteristics of the transformed phenotype (i.e., anchorage-independent growth on soft agar and extracellular matrix invasion) of only those cancer cells that also express the hUG receptor. Similarly, treatment of the nontransfected, receptor-positive adenocarcinoma cells with purified recombinant hUG yielded identical results. Taken together, these data define receptor-mediated, autocrine and paracrine pathways through which hUG reverses the transformed phenotype of cancer cells and consequently, may have tumor suppressor-like effects.
Blastokinin (1) or uteroglobin (UG; ref. 2) is a steroid-inducible, multifunctional protein secreted by the mucosal epithelia of many organs, including those of the uterus, lung, mammary gland, and prostate (3) of virtually all mammals. UG is also detectable in the blood and urine. Numerous names have been ascribed to this protein, primarily based on the tissue or body fluid in which it was first detected (1, 2, 4–7) or its interaction with specific xenobiotics (8–10). Structurally, UG is a homodimer in which the identical 70-aa subunits, in antiparallel orientation, are covalently linked by two disulfide bonds. It is a cytokine-like multifunctional protein (for review see ref. 11) that possesses potent immunomodulatory/anti-inflammatory (12–14), antichemotactic (15), and antithrombotic (16, 17) activities. Recently, we (18, 19) and others (20) have characterized high-affinity UG-binding proteins (putative receptors), with molecular masses of 190 and 49 kDa, respectively, that are present on several cell types.
The human UG (hUG) gene, mapped to chromosome 11q12.2–13.1 (21), consists of three exons and two introns and is structurally conserved during evolution. Interestingly, rearrangements or deletions of this region of chromosome 11 have been correlated with human cancers (22–24). It also has been reported that the introduction of chromosome 11 into cervical cancer cells (HeLa) suppresses the tumorigenic phenotype of these cells (25). Recent reports indicate that while the hUG gene is constitutively expressed in the mucosal epithelia of many organs (e.g., the lung, mammary gland, uterus, and prostate) at a high level (3), both in adenocarcinoma tissues as well as the cell lines derived from the adenocarcinomas of the organs mentioned above the hUG expression is either drastically suppressed or totally lacking (26, 27). In addition, immortalization of normal epithelial cells from the rabbit lung (A.B.M., Z.Z., and C.-J.Y., unpublished results), uterus (28), and prostate (A.B.M., Z.Z., and C.-J.Y., unpublished results), by simian virus 40 virus, causes the cessation of UG gene expression (29, 30). Furthermore, preliminary results of aging studies on UG-knockout mice (31) show a very high incidence (UG-knockout mice, 16/16 vs. wild-type littermates, 0/25) of malignancies (Z.Z. and A.B.M., unpublished results). Taken together, these results suggest that the lack of UG gene expression may be a characteristic shared by many cancer and transformed cells.
In the present investigation, we have induced hUG expression in several human adenocarcinoma cell lines and tested whether the hUG cDNA-transfected cells are any different with respect to their transformed phenotype compared with that of the mock-transfected or nontransfected controls. Our results show that the hUG cDNA-transfected cells that lose the transformed phenotype are the ones that also express the hUG receptor. These results raise the possibility that hUG via autocrine and paracrine pathways may reverse the transformed phenotype of UG-receptor positive cancer cells and in this respect, it may manifest tumor suppressor-like effects.
MATERIALS AND METHODS
Materials.
Human adenocarcinoma cells, HTB-174 (lung), HTB-81 (prostate), HTB-30 (breast), and HEC-1A (uterus) were obtained from the American Type Culture Collection. A polyclonal hUG antibody (Covance Laboratories, Vienna, VA) was raised in the goat by using a synthetic peptide corresponding to the amino acid sequence of hUG (residues 36–55) as the antigen. The specificity of the antiserum was determined by Western blotting using purified recombinant hUG. All tissue culture reagents were purchased from Life Technologies (Gaithersburg, MD). Neutral red stain was purchased from ICN.
hUG Expression-Vector Construction.
A full-length hUG cDNA fragment, cloned in pGEM 4Z (32) was excised by EcoRI digestion and subcloned into the TA vector (Invitrogen) at the EcoRI site. The orientation of the hUG-cDNA fragment was verified by DNA sequencing. This fragment was excised from the TA vector by digestion with HindIII and XbaI and then religated into the pRC/Rous sarcoma virus (RSV) expression vector (Invitrogen), generating pRC/RSV-hUG that was predigested with HindIII and XbaI and purified by agarose gel electrophoresis.
Cell Culture, cDNA Transfection, and Selection of Stable Transfectants.
The human lung adenocarcinoma cell line (HTB-174) was cultured in RPMI medium 1640 (Life Technologies) supplemented with 5% heat-inactivated FBS at 37°C with 5% CO2 and 95% air while all other human tumor cell lines derived from the adenocarcinomas of the uterus (HEC-1A), prostate (HTB-81), and breast (HTB-30) were maintained in McCoy’s 5A medium supplemented with 10% FBS at 37°C at an atmosphere of 5% CO2 and 95% air. The tumor cell lines were transfected by electroporation either with pRC/RSV-hUG or with pRC/RSV (mock) plasmid construct. After 24 hr, the cells were fed a medium containing 400 μg/ml of G418. Resistant clones were isolated and maintained in a medium containing 200 μg/ml of G418 before further testing. The stably transfected clones were expanded, and aliquots were cryopreserved and used for experiments. The clones had undergone 2–3 passages at the time of testing them for anchorage independent growth on soft agar and Matrigel invasion.
Detection of UG-mRNA by Reverse Transcription (RT)-PCR.
Total RNAs were isolated from different adenocarcinoma cell lines by using the RNAzol method (Tel-Test, Friendswood, TX). The primers and PCR conditions used in this study have been described (3). Briefly, RT was carried out by using hUG-cDNA-specific primers, hUG-R: (5′-T A C A C A G T G A G C T T T G G G C-3′). The RT-PCR product then was used for further amplification by using primer hUG-L: (5′-A T G A A A C T C G C T G T C A C C C-3′) and the primer hUG-R. The PCR product then was blotted and detected by hybridization with a hUG-specific oligonucleotide probe, hUG-P: (5′-T G A A G A A G C T G G T G G A C A C C-3′). The primers and the probe used for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA detection are as follows: hGAPDH-R, 5′-C A A A G T T G T C A T G G A T G A C C-3′; hGAPDH-L, 5′-C C A T G G A G A A G G C T G G G G-3′, and hGAPDH-p, 5′-T C C T G C A C C A C C A A C T G C T T-3′.
Immunoprecipitation and Western Blot Analyses.
The adenocarcinoma cells, when confluent, were scraped by using a rubber policeman and washed twice with cold PBS, and the cell pellets were lysed by sonication in lysis buffer. The cell lysates were immunoprecipitated according to the methods described (18, 19). Briefly, the cell lysates were centrifuged, and the supernatants were incubated at room temperature with rabbit hUG-antibody for 1 hr and then incubated with protein A-agarose at 4°C overnight. The bound complexes were collected by centrifugation, washed, and boiled in SDS-sample buffer. The samples then were resolved by electrophoresis on SDS-polyacrylamide gel, and the protein bands were electrotransferred to the nitrocellulose membrane. For Western blot analysis, the membranes containing hUG were incubated in blocking solution, washed, and incubated with goat hUG-antibody (1:250 dilution) at room temperature for 1 hr. The membranes were washed, incubated further with rabbit anti-goat horseradish peroxidase-conjugated IgG (1:2,000 dilution), and detected by enhanced chemiluminescence (ECL, Amersham) according to the instructions of the manufacturer.
Anchorage-Independent Growth on Soft Agar.
Soft agar assay was performed as described (33, 34) with minor modifications. Briefly, wild-type, pRC/RSV-hUG-, and pRC/RSV (mock)-transfected adenocarcinoma cell lines were used for this assay. The cells had undergone 3–4 passages before testing them in various assays [e.g., growth on soft agar and extracellular matrix (ECM) invasion]. Monolayer cultures were trypsinized to obtain single-cell suspensions. Neutral red staining was used to determine viability. A total of 10,000 viable cells were suspended in 2.5 ml of McCoy’s 5A medium containing 10% FBS and 0.3% Noble agar (Difco) and plated on 60-mm Petri dishes that previously were plated with 2.5 ml of 0.5% agar in McCoy’s 5A medium. The top agar/medium containing 200 μg/ml of G418 was used for both pRC/RSV-hUG-transfected and mock (pRC/RSV)-transfected cells. Plates were incubated at 37°C in an atmosphere of 5% CO2 and 95% air for 3 weeks. The colonies were stained with neutral red and counted. The colonies also were photographed by using a phase-contrast microscope fitted with a wide-angle objective.
Matrigel Invasion Assays.
The details of the Matrigel invasion assay had been reported (18). Briefly, when the cells reached >80% confluence, they were trypsinized and washed twice with PBS containing 0.1% BSA. The cells were resuspended in McCoy’s 5A medium containing 0.1% BSA and placed in the upper compartment of the Matrigel invasion chamber. The lower compartment of the chamber was filled with fibroblast conditioned medium, a chemoattractant for cell invasion, which was prepared from the supernatant of proliferating cultures of NIH 3T3 fibroblasts after an incubation of 24 hr. The cells were allowed to incubate at 37°C for 36 hr, and the invading cells were stained with Giemsa for 3 min and immediately washed twice (5 min each) with absolute ethanol. The noninvaded cells along with the Matrigel were scraped from the upper surface of the filters with moist cotton swabs, and the chambers were washed three times with water. The invaded cells remaining on the filter were counted by using an inverted microscope, and the percentage of the cells invading the Matrigel was calculated (18).
125I-hUG-Binding Assay.
The radioiodination of hUG and binding experiments were performed as described (18, 19). Briefly, the hUG (20 μg) was radioiodinated by using 125I-sodium iodide (2 mCi; carrier-free) and Iodo-Beads. The 125I-UG was purified by Sephadex G-25 spun column chromatography (1,200 × g for 4 min). The specific activity of purified 125I-hUG was 20 μCi/μg. The wild-type adenocarcinoma cells were grown in 12-well plates, washed with PBS, pH 7.4, and incubated with reduced 125I-hUG (1.5 nM) in 1 ml of Hanks’ balanced salt solution, pH 7.6 containing 0.1% BSA in the absence or presence of increasing concentrations (1 pM to 1 μM) of unlabeled reduced recombinant hUG at room temperature for 2 hr. The cells were washed with PBS, pH 7.6 and solubilized in 1 N NaOH followed by addition of equal volume of 1 N HCl. The radioactivity was measured by a gamma counter. The specific binding was calculated by subtracting the nonspecific binding from the total binding. Scatchard analyses of the data were performed by using the ligand computer program.
Affinity Crosslinking with 125I-hUG.
The adenocarcinoma cell lines were grown to confluence in 6-well plates. They were washed with PBS, pH 7.6 and incubated with reduced 125I-hUG (3 nM) in 2.0 ml of Hanks’ balanced salt solution (HBSS), pH 7.6 containing 0.1% BSA in the absence and presence of unlabeled reduced hUG (250 nM) at room temperature for 2 hr. After incubation, the cells were washed and incubated further with 0.2 mM disuccinimidylsuberate (DSS) in 2.0 ml of HBSS, pH 7.6 for 20 min. The cells were scraped, collected by centrifugation (10,000 × g) for 15 min, and lysed in 40 μl of lysis buffer (1% Triton X-100 containing 1 mM PMSF and 20 μg/ml of leupeptin) and 2 mM EDTA. The supernatants were resuspended in sample buffer containing 5% β-mercaptoethanol. The samples were resolved by electrophoresis on 4–20% gradient SDS-polyacrylamide gel and autoradiographed.
RESULTS AND DISCUSSION
To determine the possible role(s) of hUG in reversing the transformed phenotype (e.g., anchorage-independent growth on soft agar and the ECM invasion), we studied four cell lines, each of which was derived from human adenocarcinomas of the lung, mammary gland, uterus, and prostate, respectively. The results of two representative cell lines derived from the adenocarcinomas of the uterus (HEC-1A) and the prostate (HTB-81), respectively, are presented in this paper. We first ascertained whether the adenocarcinoma-derived cell lines express hUG mRNA and hUG protein by using RT-PCR and immunoprecipitation followed by Western blotting, respectively. The results show that these cells neither express detectable levels of hUG mRNA (Fig. 1A, Left) nor hUG protein (Fig. 1B). We then stably transfected these cells with a hUG-cDNA construct (pRC/RSV-hUG) or with the vector only (pRC/RSV) as control. The nontransfected wild-type adenocarcinoma cells also served as controls. Of 34 independently isolated HEC-1A clones, 23 were pRC/RSV-hUG-transfected and 11 were pRC/RSV (mock)-transfected. Of the 23 pRC/RSV-hUG-transfected clones eight were analyzed for anchorage-independent growth on soft agar and five were tested for ECM invasion. Similarly, of 11 pRC/RSV-transfected clones, nine were characterized: six for anchorage-independent growth on soft agar and three for ECM invasion. The results show that while hUG mRNA and hUG protein are not detectable in the control cells, the pRC/RSV-hUG-transfected cells express both the UG mRNA (Fig. 1A, Middle and Right) and UG protein (Fig. 1B). Compared with the physiological expression of hUG in the human uterus and prostate tissues reported previously (ref. 3 and unpublished results), both the hUG-mRNA and hUG-protein expression in the pRC/RSV-hUG-transfected adenocarcinoma cells appeared to be much lower. Therefore, the observed effects of pRC/RSV-hUG transfection on the adenocarcinoma cells do not appear to be the result of the overexpression of hUG above the physiological range.
Figure 1.
(A) RT-PCR analysis of total RNA extracted from pRC/RSV-hUG-transfected and wild-type adenocarcinomas of the uterus (HEC-1A) and the prostate (HTB-81). The PCR products were blotted and detected by hybridization with a hUG-specific oligonucleotide probe, hUGP (21). Amplification of the human GADPH gene was used as an internal control for RNA quality and to rule out pipeting error. Lanes 1 and 2 represent two independently derived pRC/RSV-hUG-transfected clones each of HEC-1A (Left) and HTB-81 (Right), respectively. Wt, wild-type (nontransfected) cells. (B) Western blot analyses showing UG production by HEC-1A and HTB-81 cells transfected with pRC/RSV-hUG. Proteins were resolved by SDS/PAGE under reducing and denaturing conditions (see Materials and Methods for details). UG(d), UG-dimer; UG(m), UG-monomer.
To determine whether induced hUG expression in these adenocarcinoma cell lines had any effect on the transformed phenotype of the cancer cells, we tested them for anchorage-independent growth on soft agar and for ECM invasion. There were no apparent differences in the monolayer growth properties among the HEC-1A cells transfected with pRC/RSV-hUG, pRC/RSV (mock), and nontransfected controls. We have observed that equal number (5 × 104) of each of the three categories of cells described above, when plated in 25-cm2 tissue culture flasks, reached 100% confluence in 7–8 days of culture. In contrast, the growth of the pRC/RSV-hUG-transfected clones on soft agar was dramatically different from that of the nontransfected or mock-transfected clones (Fig. 2 A-C). Although the number of nontransfected, mock (pRC/RSV)-transfected, and pRC/RSV-hUG-transfected cells, seeded on soft agar, were equivalent (10 × 103 cells/plate), both nontransfected and mock-transfected cells grew predominantly large colonies (Fig. 2 A and B) whereas the pRC/RSV-hUG-transfected cells (Fig. 2C) failed to grow colonies of appreciable size within the 4-week experimental period. At 4 weeks, while both the nontransfected (Fig. 2A) and mock-transfected (Fig. 2B) cells grew a mixture of large and small colonies in the same soft agar plate the colonies formed by the pRC/RSV-hUG-transfected cells (Fig. 2C) were very small and more uniform in size. To determine that growth failure of the pRC/RSV-hUG cells on soft agar was not caused by nonviability, these cells, after 4 weeks on the soft agar, were placed on monolayer cultures, and they grew equally well compared with the nontransfected and mock-transfected cells (data not shown). These results suggest that the growth inhibition of pRC/RSV-hUG-transfected cells on soft agar is not caused by the nonviability of these cells. In the Matrigel invasion assay, the wild-type HEC-1A cells (Fig. 3A) or its mock-transfected counterpart (not shown) aggressively invaded the ECM while those transfected with pRC/RSV-hUG showed a striking suppression of ECM invasion (Fig. 3B). Treatment of the wild-type HEC-1A cells with pure recombinant hUG yielded results (Fig. 3C) that were very similar to those of the pRC/RSV-hUG-transfected cells. However, treatment of the cells with a nonspecific, control protein, myoglobin, had no effect on ECM invasion (not shown). Surprisingly, the remainder of the cell lines, derived from the adenocarcinomas of the lung (HTB-174), mammary gland (HTB-30), and prostate (HTB-81), that were transfected with pRC/RSV-hUG, showed neither suppression of anchorage-independent growth on soft agar nor inhibition of ECM invasion (data not shown).
Figure 2.
Anchorage-independent growth on soft agar. (A) HEC-1A cells that were not transfected (wild type). (B) HEC-1A cells that were pRC/RSV (mock)-transfected. (C) HEC-1A cells transfected with pRC/RSV-hUG. Note the striking suppression of anchorage-independent growth of pRC/RSV-hUG-transfected cells (C). Magnification: ×20.
Figure 3.
Effect of the expression of hUG on ECM invasion by HEC-1A cells. (A) Wild-type (nontransfected) cells. (B) Cells transfected with pRC/RSV-hUG expression construct. (C) Wild-type cells that were treated with pure hUG. Note the striking suppression of ECM invasion in B and C. Magnification: ×200. (D) Graphic representation of the quantitative inhibition of ECM invasion.
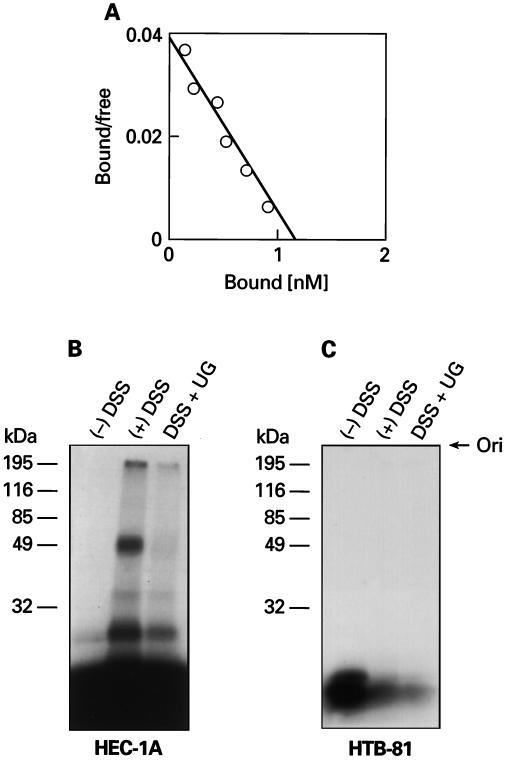
The above results led us to further investigate the mechanism(s) of hUG-mediated suppression of anchorage-independent growth and ECM invasion by only pRC/RSV-hUG-transfected HEC-1A cells. We previously have reported that UG has antichemotactic properties (15) and it inhibits cellular invasiveness. Accordingly, we carried out 125I-hUG-binding and affinity-crosslinking assays to determine whether hUG exerts this effect on HEC-1A cells via its receptor-mediated pathway (18). The results of binding experiments show that specific, high-affinity (Kd = 25 nM) binding of 125I-hUG occurs only with HEC-1A cells (Fig. 4A) but such specific binding was not detectable on HTB-174, HTB-30, or HTB-81 cell lines (data not shown). We also carried out affinity-crosslinking experiments with 125I-hUG, and the results showed that while the presence of both 190- and 49-kDa hUG-binding proteins (receptor) was readily detectable on HEC-1A cells (Fig. 4B) they were totally lacking on HTB-81 (Fig. 4C) and the other adenocarcinoma cells (not shown). These results led us to conclude that induced hUG expression and/or pretreatment of the adenocarcinoma cells with purified hUG suppress ECM invasion of only those cells that express the hUG receptor(s). This finding raises a strong possibility for the existence of both an autocrine and paracrine mechanism by which hUG regulates the invasiveness of these adenocarcinoma cells. Furthermore, these results raise an intriguing possibility that by secreting hUG the normal epithelia, adjoined to a newly formed tumor or premalignant lesion, may act as a “gatekeeper” that, at least in part, suppress or reverse the transformed phenotype of tumor or premalignant cells in situ and prevent the migration and invasion of such cells to distant organs. In fact, it is well known that adenocarcinomas in organs that express the hUG gene (e.g., the prostate and the lung) may remain dormant for a relatively long period of time before being invasive. Because carcinogenesis is a multistep genetic process, it is entirely possible that the loss of hUG gene expression promotes the transition from adenomas to adenocarcinomas and allow ECM invasion that is essential for cancer metastasis. We recently have reported that UG gene-disrupted mice develop severe renal glomerular disease (31) because of abnormal deposition of a high molecular weight ECM protein, fibronectin (35) that also binds to heterodimeric proteins, integrins (36) that act as receptors. We also reported that the UG-knockout mice that develop the adult-onset renal disease also manifest distal tubular hyperplasia (31). In addition, UG inhibits proliferation of some cell types (e.g., arterial smooth muscle cells) (G.M.-S., unpublished results). More significantly, preliminary results of our ongoing studies show that the incidence of cancer in aging UG-knockout mice is nearly 100 (16/16) compared with that of their wild-type littermates (0/25) that are free of cancer (Z.Z. and A.B.M, unpublished results). Taken together, these results define a critical role of hUG in promoting the loss of at least two of the most important phenotypic parameters (i.e., anchorage-independent growth and ECM invasion) that characterize most cancer cells and demonstrate that such effects of hUG are exerted, at least in part, via its receptor(s) in both an autocrine and paracrine manner. This finding raises a strong possibility that hUG possesses the ability to reverse the transformed phenotype of those cancer cells that express its receptor. Consequently, it may have tumor suppressor-like effects.
Figure 4.
Scatchard analysis of specific binding of reduced 125I-UG of wild-type HEC-1A cells (A). Affinity-crosslinking of 125I-hUG with HEC-1A (B) and HTB-81 (C) cells. The cells were incubated with reduced 125I-hUG in the absence or presence of unlabeled reduced hUG for binding and then crosslinked with DSS. Lane 1: (−)DSS; lane 2: (+)DSS, and lane 3: (+)hUG + DSS. Ori, origin. Note the complete absence of the hUG receptor(s) on HTB-81 cells.
Acknowledgments
We thank Drs. J.B. Sidbury, Jr., I. Owens, J.Y. Chou, F. Zheng, S. Chattopadhyay, and S.W. Levin for critical review of the manuscript and helpful suggestions. The hUG-cDNA clone is a generous gift from Drs. G. Singh and S.L. Katyal. We thank Mr. Rick Dreyfuss and Ms. Shauna Everett of Medical Arts and Photography, National Institutes of Health, for expert photomicrographic assistance.
ABBREVIATIONS
- UG
uteroglobin
- hUG
human uteroglobin
- ECM
extracellular matrix
- DSS
disuccnimidylsuberate
- RT
reverse transcription
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- RSV
Rous sarcoma virus
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Krishnan R S, Daniel J C., Jr Science. 1967;158:490–492. doi: 10.1126/science.158.3800.490. [DOI] [PubMed] [Google Scholar]
- 2.Beier H M. Biochim Biophys Acta. 1968;160:289–291. doi: 10.1016/0005-2795(68)90108-6. [DOI] [PubMed] [Google Scholar]
- 3.Peri A, Cordella-Miele E, Miele L, Mukherjee A B. J Clin Invest. 1993;92:2099–2109. doi: 10.1172/JCI116810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh G, Katyal S L, Brown W E, Phillips S, Kennedy A L, Anthony J, Squeglia N. Biochim Biophys Acta. 1988;950:329–337. doi: 10.1016/0167-4781(88)90129-7. [DOI] [PubMed] [Google Scholar]
- 5.Jackson P J, Turner R, Keen J N, Brooksbank R A, Cooper E H. J Chromatogr. 1988;452:359–367. doi: 10.1016/s0021-9673(01)81460-6. [DOI] [PubMed] [Google Scholar]
- 6.Watson M A, Fleming T P. Cancer Res. 1996;56:860–865. [PubMed] [Google Scholar]
- 7.Watson M A, Darrow C, Zimonjic D B, Popescu N C, Fleming T P. Oncogene. 1998;16:817–824. doi: 10.1038/sj.onc.1201597. [DOI] [PubMed] [Google Scholar]
- 8.Beato M. J Steroid Biochem. 1976;7:327–334. doi: 10.1016/0022-4731(76)90091-1. [DOI] [PubMed] [Google Scholar]
- 9.Gillner M, Lund J, Cambillau C, Alexandersson M, Hurtig U, Bergman A, Klasson-Wehler E, Gustafsson J A. J Steroid Biochem. 1988;31:27–33. doi: 10.1016/0022-4731(88)90201-4. [DOI] [PubMed] [Google Scholar]
- 10.Lopez de Haro M S, Perez-Martinez M, Garcia C, Nieto A. FEBS Lett. 1994;349:249–251. doi: 10.1016/0014-5793(94)00678-4. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee A B, Kundu G C, Mandal A K, Pattabiraman N, Yuan C-J, Zhang Z. Am J Kidney Dis. 1998;32:1106–1120. doi: 10.1016/s0272-6386(98)70093-9. [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee A B, Cordella-Miele E, Kikukawa T, Miele L. Adv Exp Med Biol. 1988;231:135–152. doi: 10.1007/978-1-4684-9042-8_11. [DOI] [PubMed] [Google Scholar]
- 13.Miele L, Cordella-Miele E, Facchiano A, Mukherjee A B. Nature (London) 1988;335:726–730. doi: 10.1038/335726a0. [DOI] [PubMed] [Google Scholar]
- 14.Camussi G, Tetta C, Bussolino F, Baglioni C. J Exp Med. 1990;171:913–927. doi: 10.1084/jem.171.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vasanthakumar G, Manjunath R, Mukherjee A B, Warabi H, Schiffmann E. Biochem Pharmacol. 1988;37:389–394. doi: 10.1016/0006-2952(88)90204-3. [DOI] [PubMed] [Google Scholar]
- 16.Manjunath R, Levin S W, Kumaroo K K, Butler J D, Donlon J A, Horne M, Fujita R, Schumacher U K, Mukherjee A B. Biochem Pharmacol. 1987;36:741–746. doi: 10.1016/0006-2952(87)90728-3. [DOI] [PubMed] [Google Scholar]
- 17.Vostal J G, Mukherjee A B, Miele L, Shulman N R. Biochem Biophys Res Commun. 1989;165:27–36. doi: 10.1016/0006-291x(89)91029-2. [DOI] [PubMed] [Google Scholar]
- 18.Kundu G C, Mantile G, Miele L, Cordella-Miele E, Mukherjee A B. Proc Natl Acad Sci USA. 1996;93:2915–2919. doi: 10.1073/pnas.93.7.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kundu G C, Mandal A K, Zhang Z, Mantile-Selvaggi G, Mukherjee A B. J Biol Chem. 1998;273:22819–22824. doi: 10.1074/jbc.273.35.22819. [DOI] [PubMed] [Google Scholar]
- 20.Diaz Gonzalez K, Nieto A. FEBS Lett. 1995;361:255–258. doi: 10.1016/0014-5793(95)00167-8. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Zimonjic D B, Popescu N C, Wang N, Gerhard D S, Stone E M, Arbour N C, De Vries H G, Scheffer H, Gerritsen J, et al. DNA Cell Biol. 1997;16:73–83. doi: 10.1089/dna.1997.16.73. [DOI] [PubMed] [Google Scholar]
- 22.Misra B C, Srivatsan E S. Am J Hum Genet. 1989;45:565–577. [PMC free article] [PubMed] [Google Scholar]
- 23.Lammie G A, Fantl V, Smith R, Schuuring E, Brookes S, Michalides R, Dickson C, Arnold A, Peters G. Oncogene. 1991;6:439–444. [PubMed] [Google Scholar]
- 24.Jesudasan R A, Rahman R A, Chandrashekharappa S, Evans G A, Srivatsan E S. Am J Hum Genet. 1995;56:705–715. [PMC free article] [PubMed] [Google Scholar]
- 25.Saxon P J, Srivatsan E S, Stanbridge E J. EMBO J. 1986;5:3461–3466. doi: 10.1002/j.1460-2075.1986.tb04670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linnoila R I, Jensen S M, Steinberg S M, Mulshine J L, Eggleston J C, Gazdar A F. Am J Clin Pathol. 1992;97:233–243. doi: 10.1093/ajcp/97.2.233. [DOI] [PubMed] [Google Scholar]
- 27.Broers J L, Jensen S M, Travis W D, Pass H, Whitsett J A, Singh G, Katyal S L, Gazdar A F, Minna J D, Linnoila R I. Lab Invest. 1992;66:337–346. [PubMed] [Google Scholar]
- 28.Mukherjee A B, Murty L C, Chou J Y. Mol Cell Endocrinol. 1993;94:R15–R22. doi: 10.1016/0303-7207(93)90176-k. [DOI] [PubMed] [Google Scholar]
- 29.Sandmoller A, Halter R, Suske G, Paul D, Beato M. Cell Growth Differ. 1995;6:97–103. [PubMed] [Google Scholar]
- 30.DeMayo F J, Finegold M J, Hansen T N, Stanley L A, Smith B, Bullock D W. Am J Physiol. 1991;261:L70–L76. doi: 10.1152/ajplung.1991.261.2.L70. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z, Kundu G C, Yuan C J, Ward J M, Lee E J, DeMayo F, Westphal H, Mukherjee A B. Science. 1997;276:1408–1412. doi: 10.1126/science.276.5317.1408. [DOI] [PubMed] [Google Scholar]
- 32.Mantile G, Miele L, Cordella-Miele E, Singh G, Katyal S L, Mukherjee A B. J Biol Chem. 1993;268:20343–20351. [PubMed] [Google Scholar]
- 33.Senger D R, Perruzzi C A, Ali I U. Proc Natl Acad Sci USA. 1988;85:5105–5111. doi: 10.1073/pnas.85.14.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardner H A R, Berse B, Senger D R. Oncogene. 1994;9:2321–2326. [PubMed] [Google Scholar]
- 35.Hynes R O. Fibronectins. New York: Springer; 1990. [Google Scholar]
- 36.Hynes R O. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]