Abstract
The cellular form of the Prion protein (PrPC) is necessary for prion replication in mice. To determine whether it is also sufficient, we expressed PrP under the control of various cell- or tissue-specific regulatory elements in PrP knockout mice. The interferon regulatory factor-1 promoter/Eμ enhancer led to high PrP levels in the spleen and low PrP levels in the brain. Following i.p. scrapie inoculation, high prion titers were found in the spleen but not in the brain at 2 weeks and 6 months, showing that the lymphoreticular system by itself is competent to replicate prions. PrP expression directed by the Lck promoter resulted in high PrP levels on T lymphocytes only but, surprisingly, did not allow prion replication in the thymus, spleen, or brain following i.p. inoculation. A third transgenic line, which expressed PrP in the liver under the control of the albumin promoter/enhancer—albeit at low levels—also failed to replicate prions. These results show that expression of PrP alone is not sufficient to sustain prion replication and suggest that additional components are needed.
Keywords: transgenic mice, scrapie, prion disease, PrP null mice, lymphoreticular system
Though the major clinical and pathological manifestations of prion diseases are found in the central nervous system, lymphoid organs accumulate infectivity earlier than the brain even after intracerebral (i.c.) inoculation (1–3). Although i.c. administration of prions constitutes the most effective route of infection, peripheral, and in particular oral, uptake of prions is epidemiologically more relevant, as in the case of bovine spongiform encephalopathy (BSE), sheep scrapie, kuru, and likely new variant Creutzfeldt–Jakob disease (nvCJD).
It is not known which cell types in the spleen (LRS) are targets for the scrapie agent. Involvement of follicular dendritic cells (FDCs) has been postulated based on immunohistochemical detection of the scrapie form of the prion protein (PrPSc) in these cells (4–6). In scrapie-infected mice, infectivity was associated with splenic B and T lymphocytes, but was not detected in peripheral blood leukocytes (A.J.R., M.A.K., R. Frigg, E. Flechsig, A.A., and C.W., unpublished results). Transport of prions from the periphery to the central nervous system is defective in mice devoid of mature B lymphocytes (7), not necessarily because B lymphocytes are directly involved in transport of prions, but perhaps because no mature FDCs develop in the absence of B lymphocytes (8–10).
Mice devoid of PrP (Prnp0/0) are resistant to scrapie and fail to propagate prions (1, 11–13); susceptibility is regained by introduction of PrP transgenes (1, 14). PrPC is also required for PrPSc- induced neurotoxicity (15) and for prion spread within the central nervous system (16).
In this report we describe the targeting of PrP expression to three cell types or tissues in PrP null mice: splenocytes, T lymphocytes, and hepatocytes. Expression controlled by a interferon regulatory factor (IRF) 1 promoter/Eμ-enhancer gave high levels of PrP in the spleen, particularly on B and T cells, but low levels in the brain. Two weeks and 6 months after i.p. inoculation with scrapie, high prion titers were found in the spleen and thymus but not in the brain, showing that the lymphoreticular system alone can sustain prion replication. However, Prnp0/0 mice expressing PrP at high levels on T lymphocytes but not on B lymphocytes failed to replicate prions in the spleen or thymus. Expression of PrP in the liver, under the control of the albumin promoter, failed to sustain prion replication in the liver, spleen, or brain. Thus, PrPC expression alone is not sufficient to allow prion replication.
MATERIALS AND METHODS
DNA Constructions.
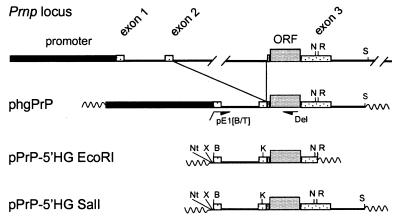
The “half-genomic” PrP vector (phgPrP), pPrPcDNA, and pPrPE1i1E23R1 have been described (Fig. 1) (14). To generate the promoterless “half-genomic” PrP vector pPrP-5′HG, a 2.7-kb PCR product was prepared using phgPrP as the template, the 5′-terminal primer pE1[B/T] (5′-tgtcggatccagcagaccgattctgg-3′) to introduce a unique BamHI site (italicized) 5′ of exon 1, and the 3′-terminal primer Del (5′-tccccagcatgtagccaccaagg-3′). The 2.3-kb BamHI-KpnI fragment of this PCR product and the 1.3-kb fragment KpnI-EcoRI fragment of pPrPE1i1E23R1 (comprising exon 2 and part of exon 3 with the entire coding region) were joined to BamHI- and EcoRI-restricted pBluescript. The resulting pPrP-5′HG EcoRI was digested with SalI and NarI and joined to the 3-kb NarI-SalI fragment from phgPrP (comprising the 3′ end of Prnp). plck-PrP-5′HG SalI: the 3.1-kb BamHI-NotI Lck promoter cassette from p1017 (ref. 17; a gift from R. Perlmutter, Univ. of Washington, Seattle) was joined to NotI- and BamHI-cleaved pPrP-5′HG SalI. pEμ/IRF1-PrP-5′HG SalI: the human IRF1 promoter was amplified by PCR from p-492IRF1cat (ref. 18; a gift from H. Harada, Osaka Univ., Japan) using the 5′ terminal primer IRF top (5′-tttctagaggagccaggctgc-3′) containing an XbaI site (italicized) and the 3′-terminal primer IRF bottom (5′-agggatcctcgactaaggagtgg-3′) with a BamHI site (italicized). The 560-bp XbaI-BamHI fragment of this PCR product and the 6-kb BamHI-SalI fragment from pPrP-5′HG SalI were joined to the 3-kb XbaI-SalI fragment of pPrP-5′HG SalI. The resulting pIRF1-PrP-5′HG SalI was linearized by partial XbaI digestion and joined to a 2.1-kb XbaI fragment containing the Eμ Ig heavy-chain enhancer from pEμ-myc (ref. 19; a gift from T. Taniguchi, Univ. of Tokyo, Japan). pAlbumin-PrP-5′HG SalI: the albumin promoter/enhancer was excised from plasmid 2335A-1 [equivalent to the construct NB (20), provided by R. Palmiter, Univ. of Washington, Seattle] as a 2.0-kb BamHI-NotI fragment and joined to the NotI- and BamHI-restricted pPrP-5′HG SalI.
Figure 1.
Schematic representation of half-genomic PrP transgenes driven by heterologous promoters. The genomic mouse Prnp locus is shown on top (37). Construction of the “half-genomic” PrP vector (phgPrP) lacking the 12-kb intron 2 has been described (14). Using PCR with the primers PE1 and Del, a BamHI site was introduced at the 5′ end of exon 1 in phgPrP. The resulting promoterless construct pPrP-5′HG EcoRI was cloned into Bluescript, and the PrP sequence was extended up to the SalI site in the 3′ noncoding region by introducing the NarI-SalI fragment of phgPrP to yield pPrP-5′HG SalI. Promoter cassettes were inserted into the BamHI site of pPrP-5′HG SalI to yield plck-PrP-5′HG SalI, pEμ/IRF1-PrP-5′HG SalI, and pAlbumin-PrP-5′HG SalI. B, BamHI; K, KpnI; N, NarI; Nt, NotI; R, EcoRI; S, SalI; X, XbaI. Wavy lines, vector sequences.
Generation of Transgenic Mice.
Plasmid DNA was digested with NotI and SalI and microinjected into homozygous Prnp0/0 zygotes (14). Founders were identified by Southern analysis using PrP ORF probe A (21). Prnp0 alleles and Prnp+ transgenes were detected by PCR (14). Of seven mouse lines transgenic for the albumin construct, two with the highest PrP mRNA levels in the liver (Tg01/alb and Tg19/alb), of five lines transgenic for the lck construct, two with highest PrP mRNA levels in the thymus (Tg33/lck and Tg71/lck), and of three lines transgenic for the Eμ/IRF1 construct, two with the highest PrP-expression levels in the spleen (Tg94/IRF and Tg90/IRF), were maintained.
Immunoprecipitation.
Tissue homogenates (10% wt/vol in 10 mM Tris⋅HCl, pH 8.0/140 mM NaCl [Tris-buffered saline (TBS)] containing 2% N-lauroylsarcosine and 1 mM phenylmethylsulfonyl fluoride) were centrifuged at 2000 × g for 15 min. Supernatants were diluted 5-fold in TBS, cleared, and incubated with excess Sepharose 4B-linked monoclonal antibody 6H4 (22) for 2 h at 4°C. The beads were washed in TBS/0.2% N-lauroylsarcosine, TBS/0.5 M NaCl/0.2% Nonidet P-40, TBS/0.5% Nonidet P-40, and TBS, boiled in SDS-sample buffer, and the eluate was immunoblotted.
Immunoblot Analysis.
Tissue homogenates were analyzed as described previously (23). Where indicated, samples (40 μg of protein) were digested with 500 units of N-glycosidase F (New England Biolabs) for 2 h at 37°C as recommended by the manufacturer. PrP was detected with the polyclonal antibody 1B3 (24) diluted 1:10,000 and horseradish peroxidase-conjugated swine anti-rabbit Igs, diluted 1:5000 (Dako) and developed using the enhanced chemiluminescence kit (Amersham). An appropriate exposure was scanned with a laser densitometer (Molecular Dynamics) and quantified with ImageQuant software.
Immunocytochemistry.
Immunofluorescence staining on consecutive 5-μm cryosections and double-color immunofluorescence were performed with polyclonal anti-PrP rabbit antiserum R340 (15) diluted 1:800 and biotinylated peanut agglutinin (1:400 dilution, Vector Laboratories) or with follicular dendritic cell marker FDC-M1 (clone 4C11, 1:300 dilution) on frozen acetone-fixed spleen sections. PrP and FDC-M1 were visualized by immunofluorescence using the Tyramide Signal Amplification kit (NEN) with Texas Red-conjugated avidin (1:100 dilution, Rockland, Gilbertsville, PA) and fluorescein isothiocyanate-conjugated streptavidin (1:100 dilution, Serotec).
Scrapie Infection.
Inoculum stock was a 10% (wt/vol) homogenate of Rocky Mountain Laboratory (RML; ref. 25) scrapie-infected CD-1 mouse brains in 0.32 M sucrose. Mice were infected i.p. with 100 μl of a 10-fold dilution of the RML stock in PBS containing 5% BSA.
Titration of Infectivity.
Prion titers were estimated from incubation time to appearance of disease (26). Swiss CD-1 or homozygous Tg20 mice (14) mice were inoculated i.c. into the right parietal lobe with 30-μl samples.
RESULTS
Mice Overexpressing PrP under the Control of the IRF1-Promoter/Ig Heavy-Chain Enhancer.
Because prions accumulate early on in the LRS, particularly in the spleen, we undertook to overexpress PrP ectopically in various compartments of the LRS. To validate the approach, we placed the PrP coding sequence under the control of the IRF1-promoter/Ig heavy-chain enhancer (Eμ) which was reported to overexpress linked reading frames in B and T lymphocytes (27). Two transgenic Prnp0/0 mouse lines carrying this construct, Tg94/IRF and Tg90/IRF, were established with transgene copy numbers of 6 and 4, respectively. PrP mRNA levels in Tg94/IRF spleen and thymus were about 5 and 3 times higher, respectively, than in their wild-type counterparts (data not shown); surprisingly, PrP in Tg94/IRF spleen was >1,000 times higher than in wild-type spleen and PrP in Tg94/IRF thymus was >100 times higher than in wild-type thymus (Table 1). PrP on the surface of peripheral blood leukocytes, as determined by fluorescence-activated cell sorter (FACS) analysis, was about 10-fold higher in Tg94/IRF than in wild-type mice (Fig. 2C). High levels of PrP were also observed on B and T lymphocytes of Tg94/IRF splenocytes (Fig. 2A). PrP in Tg94/IRF brain was 0.05 times that in wild-type brain (Table 1).
Table 1.
Characteristics of transgenic mouse lines
| Line | PrP-encoding gene (copy no.*) | PrP RNA (organ)† | PrP protein (organ)† | Days to symptoms‡ (no. of animals§) |
|---|---|---|---|---|
| Prnp+/+ | Prnp (2) | 194 ± 5 (14/14) | ||
| 1 (br) | 1 (br) | |||
| 1 (spl) | 1 (spl) | |||
| 1 (thy) | 1 (thy) | |||
| Prnp0/0 | – | – | – | >500 (0/10) |
| Tg94/IRF | Eμ/IRF1-PrP (6) | 5 (spl) | >1000 (spl) | 268 ± 24 (18/18) |
| 0.2 (br) | <0.05 (br) | |||
| 3 (thy) | >100 (thy) | |||
| Tg33/lck | lck-PrP (20) | >500 (0/6) | ||
| 2 (spl) | 40 (spl) | |||
| 0.025 (br) | <0.001 (br) | |||
| 40 (thy) | >100 (thy) | |||
| Tg01/alb | alb-PrP (20) | >400 (0/6) | ||
| 11 (liv) | 5 (liv) | |||
| 0.09 (br) | 0.1 (br) | |||
| 0 (spl) | ND | |||
| 0 (thy) | ND |
Relative to wild-type; determined by Southern blot analysis. All transgenic animals were homozygous for the transgene and all mice had a mixed 129Sv/C57Bl background.
Relative to the corresponding wild-type organ (br, brain; spl, spleen; thy, thymus; liv, liver); determination of PrP mRNA by quantitative Northern blot analysis, and of PrP by densitometry of Western blots. ND, not determined.
Animals were inoculated i.p. with 100 μl of a 1% (wt/vol) brain homogenate (RML isolate).
Number of mice developing scrapie/total number of mice inoculated.
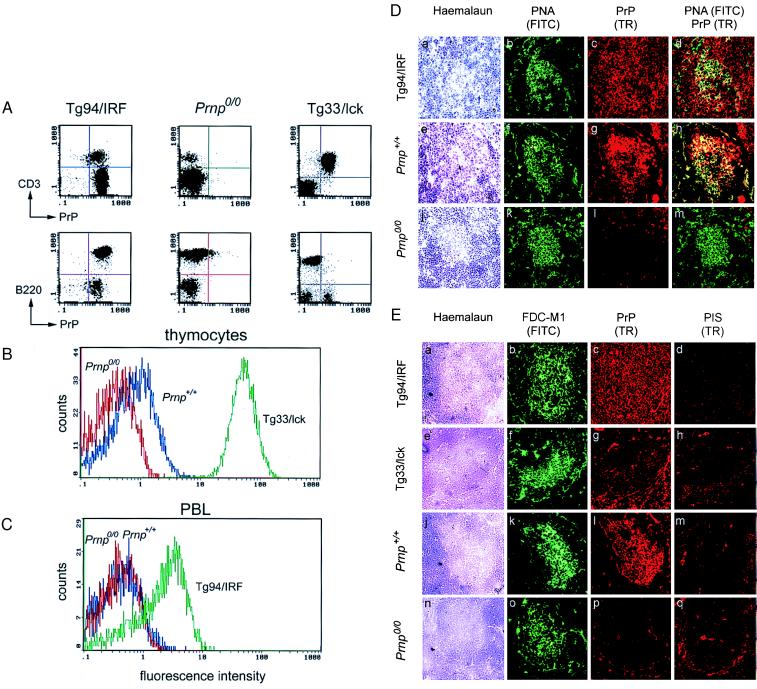
Figure 2.
Analysis of PrP expression by FACS and immunohistochemistry. FACS analysis for cell-surface PrP was carried out on (A) splenocytes, (B) thymocytes, and (C) peripheral blood leukocytes (PBL) gated for lymphocytes from Prnp+/+, Prnp0/0, Tg94/IRF, and Tg33/lck mice. Cells were stained with anti-PrP polyclonal antisera R340 and phycoerythrin-conjugated anti-rabbit IgG, and analyzed by FACS gated for lymphocytes. (A) For two-color FACS analysis, PrP staining was followed by B cell staining with FITC-conjugated anti-B220 antibodies or T cell staining with FITC-conjugated anti-CD3 antibodies. (D) Double immunofluorescence analysis of splenic germinal centers in noninoculated Tg94/IRF (a–d), wild-type mice (e–h), and Prnp0/0 mice (j–m). Sections were stained with haemalaun (a, e, j), with peanut agglutinin (PNA; green; b, f, k), and with antiserum R340 to PrP (Texas red; c, g, l). The majority of B cells PNA labeled in the germinal center were PrP-positive in Tg94/IRF mice (yellow signal in superimposed images; d) and in wild-type mice (h), but PrP-negative in Prnp0/0 mice (m). (Original magnification ×250.) (E) Immunofluorescence labeling of FDCs and PrP on consecutive sections of spleen from noninoculated Tg94/IRF (a–d), Tg33/lck (e–h), wild-type (j–m), and Prnp0/0 mice (n–q). Sections were stained with haemalaun (a, e, j, n), antibody FDC-M1 to FDC (green; b, f, k, o), antiserum R340 to PrP (Texas red; c, g, l, p), and rabbit pre-immune serum (PIS; d, h, m, q). In wild-type spleens (k, l), PrP was stained exclusively in the germinal centers, most strongly in the areas also stained by FDC-M1. In Tg94/IRF mice (b, c), PrP was evenly distributed over the entire section, including the region also stained by FDC-M1. In Tg33/lck spleens, PrP was visualized mainly in the T cell areas, but some cells were stained in the region also stained by FDC-M1. No PrP staining above background (q) was found in germinal centers of Prnp0/0 mice (p). (Original magnification ×250.)
Cryosections of spleen from noninfected wild-type, Prnp0/0, and Tg94/IRF mice were doubly stained for germinal center B cells [with peanut agglutinin (28), green] and PrP (with PrP antiserum 340, Texas red). In wild-type spleens, PrP was mainly present in germinal centers, whereas in Tg94/IRF spleens it was uniformly distributed over white and red pulp (Fig. 2D). As shown in Fig. 2E, consecutive spleen sections were labeled with the FDC-specific antibody M1 (green) and PrP antiserum (Texas red), again revealing a striking overlap of FDC and PrP staining within germinal centers in wild-type spleens. In Tg94/IRF spleens, FDCs were stained in the germinal centers whereas PrP-specific fluorescence was uniform over the whole section, which is compatible with the FACS analysis showing that B and T lymphocytes expressed PrP, and with the assumption that FDCs also expressed PrP.
Transgenic, wild-type (129/Sv-C57BL/6), and Prnp0/0 mice were inoculated i.p. with 106 LD50 units of the RML isolate of mouse prions. As shown in Table 1, all wild-type mice developed scrapie after 194 ± 5 days and died after 205 ± 9 days, whereas all Prnp0/0 mice remained healthy for more than 500 days. All of 7 Tg94/IRF mice hemizygous for the transgene cluster developed scrapie symptoms after 452 ± 15 days and died after 507 ± 27 days, presumably because they expressed PrP in the brain, albeit at low levels (data not shown). When rendered homozygous for the transgene cluster, Tg94/IRF mice became ill with scrapie at 268 ± 24 days after inoculation and died after 281 ± 26 days (Table 1).
Wild-type and Tg94/IRF mice hemizygous for the transgene cluster were inoculated i.p. As shown in Table 2, 2 weeks after inoculation, spleen extracts from Tg94/IRF mice and wild-type animals had the same titer: ≈7 log LD50 units/ml 10% homogenate and no detectable infectivity in brain. Six months after inoculation, the titers of Tg94/IRF spleen extracts were essentially unchanged, somewhat higher than the value of 6.5 for wild-type spleen; no infectivity was detected in Tg94/IRF brains compared with 8 log LD50 units/ml 10% homogenate for wild-type brains. However, 1 year after inoculation, extracts from hemizygous Tg94/IRF thymus, spleen, and brain showed prion titers of about 5.5, 5, and 7 log LD50 units/ml 10% homogenate, respectively. The late appearance of prions in the brain is attributed to low-level PrP expression in Tg94/IRF brains.
Table 2.
Infectivity bioassay of organs from RML-inoculated mice
| Donor* | Time p.i., days | Organ† | Death, days | n/nt‡ | Titer§ |
|---|---|---|---|---|---|
| Prnp+/+ | 14 | Brain | >300 | 0/4 | |
| 14 | Spleen | 163 ± 4 | 4/4 | 7 | |
| 14 | Thymus | 200 ± 24 | 4/4 | 3.5 | |
| 14 | Liver | 250 | 1/4 | ≈1 | |
| 180 | Brain | 150 ± 4 | 4/4 | 8 | |
| 180 | Spleen | 168 ± 5 | 4/4 | 6.5 | |
| 180 | Thymus | 189 ± 23 | 4/4 | 4.5 | |
| 180 | Liver | >300 | 0/4 | ||
| Prnp0/0 | 14 | Brain | >300 | 0/4 | |
| 14 | Spleen | >300 | 0/3¶ | ||
| 14 | Thymus | 295 | 1/4 | ≈1 | |
| 14 | Liver | 247 ± 52 | 2/4 | ≈1.5 | |
| 180 | Brain | >300 | 0/4 | ||
| 180 | Spleen | >300 | 0/4 | ||
| 180 | Thymus | >300 | 0/4 | ||
| 180 | Liver | 240 | 1/4 | ≈1 | |
| 365 | Brain | >300 | 0/4 | ||
| 365 | Spleen | >300 | 0/4 | ||
| 365 | Thymus | >300 | 0/3¶ | ||
| 365 | Liver | >300 | 0/3¶ | ||
| Tg33/lck (hemi) | 14 | Brain | >300 | 0/4 | |
| 14 | Spleen | 195 ± 41 | 2/4 | ≈1.5 | |
| 14 | Thymus | 189 | 1/4 | ≈1 | |
| 180 | Brain | >300 | 0/4 | ||
| 180 | Spleen | >300 | 0/4 | ||
| 180 | Thymus | >300 | 0/3¶ | ||
| 365 | Brain | >300 | 0/4 | ||
| 365 | Spleen | 222 | 1/4 | ≈1 | |
| 365 | Thymus | >300 | 0/4 | ||
| Tg01/alb (hemi) | 14 | Brain | >300 | 0/4 | |
| 14 | Spleen | >300 | 0/4 | ||
| 14 | Liver | >300 | 0/4 | ||
| 180 | Brain | >300 | 0/4 | ||
| 180 | Spleen | >300 | 0/4 | ||
| 180 | Liver | >300 | 0/4 | ||
| 365 | Brain | >300 | 0/4 | ||
| 365 | Spleen | >300 | 0/4 | ||
| 365 | Liver | >300 | 0/4 | ||
| Tg94/IRF (hemi) | 14 | Brain | >300 | 0/4 | |
| 14 | Spleen | 163 ± 12 | 4/4 | 7 | |
| 180 | Brain | >300 | 0/4 | ||
| 180 | Spleen | 160 ± 6 | 4/4 | 7 | |
| 365 | Brain‖ | 66 ± 2 | 4/4 | 7 | |
| 365 | Spleen‖ | 86 ± 5 | 4/4 | 5 | |
| 365 | Thymus‖ | 81 ± 8 | 4/4 | 5.5 |
hemi, hemizygous for the transgene.
Ten percent organ homogenates in 0.32 M sucrose were diluted 10-fold in PBS/5% BSA and 30 μl of the 1% homogenate was inoculated i.c. into CD-1 mice.
Number of mice with scrapie/total number of mice inoculated.
Infectivity levels (log LD50/ml 10% homogenate) were determined by the incubation time method using standard curves for CD-1 (1) or Tg20 (15) mice. Limit of detection: ≈1 log LD50/ml 10% homogenate.
One mouse died of intercurrent disease.
Assayed in Tg20 mice.
Mice Overexpressing PrP on T Lymphocytes under the Control of the Lck Promoter.
Two lines with the T lymphocyte-specific Lck promoter construct, Tg33/lck and Tg71/lck, harbored 20 and 10 copies of the transgene, respectively. PrP-transcript levels in the thymus were at least 50-fold higher than in the wild-type thymus (data not shown). Significant levels of PrP mRNA were also found in the spleen and kidney. Low levels of PrP transcripts were detected in the brain, lung, and intestine only upon longer exposure of the Northern blot (data not shown). Tg33/lck thymus and spleen had PrP levels that were at least 100-fold and 40-fold higher, respectively, than in wild-type thymus and spleen. PrP was undetectable in Tg33/lck brain (Fig. 3A). The high-level PrP expression in Tg33/lck thymocytes was confirmed by FACS analysis (Fig. 2B) and estimated to be 50 times that in wild-type thymocytes. No PrP expression was detected on Tg33/lck splenic B lymphocytes, whereas splenic T lymphocytes were strongly positive for PrP (Fig. 2A). Immunohistochemical analysis of Tg33/lck spleens (Fig. 2E) showed that PrP expression (Texas red) was predominantly in the perifollicular T cell area, whereas the germinal centers, where the FDCs (green) were located, showed little red fluorescence over background.
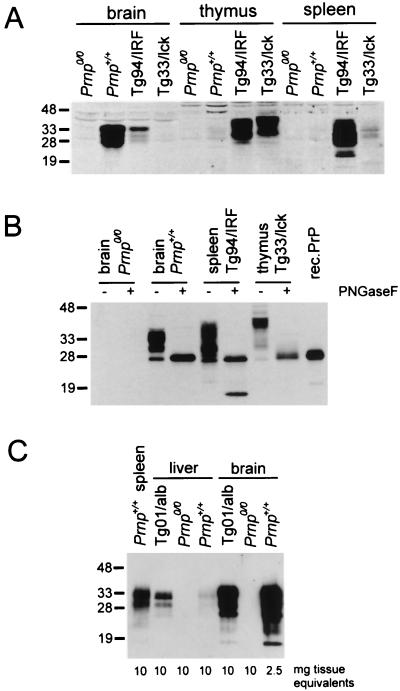
Figure 3.
Immunoblot analysis for PrP in tissues of various mouse lines. (A) Aliquots (120 μg of protein) of tissue homogenates as indicated were loaded per lane. (B) Aliquots (40 μg of protein) of tissue homogenates were digested with 500 units of N-glycosidase F for 2 h at 37°C. (C) Aliquots of tissue homogenates as indicated were immunoprecipitated with 6H4 monoclonal antibody coupled to Sepharose 4B. The eluted proteins were subjected to Western blotting and PrP was detected on blots with 1:10,000 diluted polyclonal anti-PrP antiserum 1B3. Molecular mass markers are indicated on the left in kDa.
PrP from Tg33/lck thymus had a distinctly lower electrophoretic mobility on SDS-polyacrylamide gels than that of Prnp+/+ brain (Fig. 3A). After deglycosylation with N-glycosidase F, PrP from both spleen and thymus of Tg33/lck mice was reduced to a single PrP species with about the same mobility as recombinant PrP from Escherichia coli, i.e., an apparent molecular mass of about 27 kDa (Fig. 3B). This confirmed that PrP undergoes organ- and/or cell-specific glycosylation.
To determine whether PrPC expression on T lymphocytes of Tg33/lck mice enabled prion replication in the thymus and spleen, we assayed tissue homogenates pooled from two animals sacrificed at 2 weeks, 6 months, and 12 months after i.p. inoculation. In the case of Tg33/lck mice 2 weeks after inoculation, spleen homogenates led to disease in 2 of 4 indicator mice after 192 ± 39 days, whereas thymus homogenates caused disease in 1 of 4 indicator mice after 181 days. No infectivity was detected in the spleen, thymus, or brain 6 or 12 months after inoculation, except for a spleen extract collected 1 year after inoculation, which led to scrapie in 1 of 4 CD-1 mice (Table 2). Thymus and liver homogenates from Prnp0/0 mice also occasionally led to disease in one or two of four indicator mice. These borderline infectivities are likely due to prions persisting from the inoculum (13, 29). Six months after i.p. inoculation, wild-type mice had titers of about 6.5, 4.5, and 8 log LD50 units/ml 10% homogenate in the spleen, thymus, and brain, respectively.
Thus, even vast overexpression of PrPC on T lymphocytes, comparable to levels found in wild-type brain, is not sufficient to allow prion replication in the thymus or spleen of Prnp0/0 mice.
Mice Overexpressing PrP under the Control of the Albumin Promoter.
Transgenic mice with ectopic expression of PrP in the liver were generated with use of the albumin enhancer/promoter (20). Two lines, Tg01/alb and Tg19/alb, harbored 20 and 2 copies, respectively, of the hybrid transgene. Northern blot analysis of Tg01/alb tissues revealed high levels of PrP mRNA in the liver and low levels in the lung, brain, and kidney (data not shown). PrP levels in Tg01/alb liver were at least 5-fold higher than those in wild-type liver, but still about 2–3 times lower than in wild-type spleen (Fig. 3C). PrP levels in Tg01/alb brain were about 10% of those in Prnp+/+ brain. None of the Tg01/alb mice developed scrapie disease within 400 days of i.p. inoculation (Table 1) or within 300 days of i.c. inoculation.
No infectivity was detected in the liver, brain, or spleen of Tg01/alb mice at any time after inoculation (Table 2). Liver extracts of Prnp+/+ and Prnp0/0 mice occasionally caused disease in one or two indicator mice, especially early after inoculation and likely due to residual inoculum. Thus, PrP expression in the liver of Prnp0/0 mice, at least at the level achieved—about one-third that found in wild-type spleen—was not sufficient to allow prion replication.
Prions Are Replicated in the LRS.
Although high prion titers were found in the spleen within days after i.c. or i.p. inoculation, it was not immediately clear whether this reflects de novo synthesis in the LRS or the scavenging of infectious agent generated in the brain or derived from the inoculum. Inoculation with very low prion doses resulted in a net increase of infectious agent in the spleen (30); however, it could not be excluded that the agent was synthesized in the brain and transported to the LRS. To resolve this question, we inoculated Tg94/IRF mice i.p. with a low dose of RML prions (3.5 log LD50 i.c. units) and determined prion titers in the spleen by endpoint titration (Table 3). Titers (in log LD50 i.c. units/ml 10% homogenate) rose from 2 at 2 weeks after inoculation to about 6 after 4 weeks and remained at this level for up to 12 weeks. Because a spleen weighs about 100 mg, this represents an increase of at least 2.5 log units over input, showing that prions are replicated in the spleen of i.p. inoculated Tg94/IRF mice and are not due to residual inoculum or import from the brain, which even at 6 months contains no detectable infectivity.
Table 3.
Titration of scrapie infectivity in spleens of Tg94/IRF mice inoculated i.p.
| Time after i.p. inoculation, weeks | Days to disease in indicator mice (n/n0) at log dilution*
|
Titer† | |||||
|---|---|---|---|---|---|---|---|
| 0 | −1 | −2 | −3 | −4 | −5 | ||
| 2 | 73 ± 1 (4/4) | — (0/4) | ND | ND | ND | ND | 2 |
| 4 | ND | 79 ± 5 (4/4) | 84 ± 6 (2/2) | 104 ± 2 (3/4) | 121 ± 4 (3/4) | — (0/4) | 6 |
| 8 | ND | 78 ± 4 (4/4) | ND | 103 ± 13 (4/4) | 110 (1/4) | 118 (1/4) | 5 |
| 12 | ND | 71 ± 0 (4/4) | ND | 94 ± 7 (4/4) | 111 ± 11 (4/4) | — (0/4) | 6 |
Homozygous Tg94/IRF mice were inoculated i.p. with 3.5 log LD50 i.c. units of RML prions. At the times indicated, mice were killed and the titer in the spleen was determined by endpoint titration in Tg20 mice. The numbers in the table indicate days elapsed to appearance of symptoms and the fraction (n/n0) of mice falling sick. ND, not done.
Serial 10-fold dilutions of 10% spleen homogenates were prepared in PBS/5% BSA and 30 μl was inoculated i.c. into Tg20 mice.
log LD50 units/ml 10% homogenate. Calculated by multiplying the LD50 units at the endpoint dilution with 33× dilution factor.
DISCUSSION
We have used ectopic expression of PrP in Prnp0/0 mice to investigate whether the presence of PrP in a particular tissue suffices to restore prion replication. Prion titers were low or absent in the liver of scrapie- or CJD-agent-infected mice (2, 31), which may be attributed to the very low levels, if any, of PrP in the liver (32). By immunopurification with the anti-PrP monoclonal antibody 6H4 (22), we detected PrP at very low but significant levels in the liver of wild-type but not of Prnp0/0 mice, but did not distinguish whether it was in hepatocytes or in cells of the LRS. Expression in liver of Prnp0/0 mice of the PrP coding sequence under the control of the albumin promoter/enhancer led to PrP levels about 5-fold higher than those in wild-type mice, but i.p. inoculation did not lead to infectivity in the liver for as long as 1 year after inoculation. This may be because hepatocytes lack some factor(s) required for prion replication. Alternatively, the expression level might be too low to sustain prion replication; it is, however, only 2–3 times lower than in wild-type spleen, where prions are replicated efficiently. Although Tg01/alb mouse brain contained PrP at about 10% of the level found in wild-type brain, it remained devoid of infectivity for as long as 1 year after i.p. inoculation, perhaps because prions are unable to invade the central nervous system (and the liver) in the absence of a PrP-containing LRS (33).
Because the spleen was reported to efficiently replicate prions after i.p. as well as after i.c. inoculation (30, 34), targeting individual cell types of the LRS was an attractive goal. Although the heterologous Eμ/IRF1 enhancer/promoter was intended to target the LRS, PrP mRNA was detected also in other tissues, particularly in the brain; within the intestine, expression may well have been in the lymphoreticular compartment. PrP expression in the spleen of Tg94/IRF mice was >20-fold higher than in wild-type brain and >1000-fold higher than in wild-type spleen. In wild-type mice, PrP levels in the brain exceeded those in spleen by ≈100-fold. In the spleen, PrP was detected on B and T lymphocytes; expression on FDCs is likely judged by the finding of PrPC in the stroma of uninfected Tg94/IRF mice and of PrPSc in that of infected Tg94/IRF mice, as well as by the uniform immunostaining of PrP across the germinal centers.
Following i.p. challenge with mouse prions, Tg94/IRF mice maintained high titers of prions in the spleen from 2 weeks up to 6 months after inoculation without any infectivity appearing in the brain. Although the PrP-expression level in Tg94/IRF spleen was >1000-fold higher than in wild-type spleen, prion titers were not significantly higher in Tg94/IRF than in wild-type spleen. Moreover, although PrP expression in Tg94/IRF spleen was about 20-fold higher than in the brain, prion titers in the brain were 10-fold higher than in the spleen 12 months after i.p. inoculation. This paradox can be explained if the high PrP levels in Tg94/IRF spleen reflected PrP on B and T lymphocytes but not on other spleen cells such as FDCs, and if prion replication were limited by the latter.
In mice with Lck promoter-controlled PrP expression, PrP levels on T lymphocytes of Tg33/lck mice were >100-fold and >10-fold higher than those of Prnp+/+ and Tg94/IRF mice, respectively. Following i.p. challenge with mouse scrapie prions, Tg33/lck mice showed no disease and failed to replicate prions in the spleen or brain. Because Tg94/IRF mice, which express high levels of PrP on B and T lymphocytes (perhaps also on other cells of the LRS), accumulate prions to high levels in spleen and thymus, PrP expression on FDCs or other splenocytes appear to be necessary for prion replication in the LRS. The strong expression of PrPC on FDCs of uninfected mice, as shown by immunohistochemistry (Fig. 2E), further supports the role (5, 6) ascribed to these cells in prion replication in the LRS.
Why do T lymphocytes overexpressing PrP not accumulate prion infectivity? Perhaps they are devoid of a conjectural receptor required for prion uptake or lack cellular factor(s) required for prion replication. Experiments with transgenic mice led to the suggestion that a species-specific factor X is required for prion replication (35, 36). Alternatively, T lymphocytes are able to replicate prions, but are either rapidly eliminated due to a prion-elicited toxic effect, or “washed out” as a result of normal turnover. Finally, it is possible that prions administered i.p. in a PrP knockout environment are not transported or transferred to T lymphocytes.
We have recently determined that both B and T lymphocytes from the spleen, but not peripheral blood leukocytes, of scrapie-infected wild type and Tg94/IRF mice carry scrapie infectivity (A.J.R., M.A.K., R. Frigg, E. Flechsig, A.A., and C.W., unpublished results). In conjunction with the findings reported in this paper, this suggests that T lymphocytes cannot replicate prions on their own, but either acquire them from a different cell type or replicate them in dependence of other cells.
Acknowledgments
We thank Dr. Mary Kosco-Vilbois for a generous gift of FDC-M1 antibody. This work was supported by the Kanton of Zürich and by grants of the Schweizerischer Nationalfonds and the European Union to A.A. and C.W.
ABBREVIATIONS
- i.c.
intracerebral
- BSE
bovine spongiform encephalopathy
- CJD
Creutzfeldt–Jakob disease
- FACS
fluorescence-activated cell sorter
- FDCs
follicular dendritic cells
- IRF
interferon regulatory factor
- LRS
lymphoreticular system
- PrP
prion protein
- PrPC
PrP cellular form
- PrPSc
PrP scrapie form
- RML
Rocky Mountain Laboratory
References
- 1.Büeler H, Aguzzi A, Sailer A, Greiner R A, Autenried P, Aguet M, Weissmann C. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- 2.Eklund C M, Kennedy R C, Hadlow W J. J Infect Dis. 1967;117:15–22. doi: 10.1093/infdis/117.1.15. [DOI] [PubMed] [Google Scholar]
- 3.Fraser H, Dickinson A G. Nature (London) 1970;226:462–463. doi: 10.1038/226462a0. [DOI] [PubMed] [Google Scholar]
- 4.Kitamoto T, Muramoto T, Mohri S, Dohura K, Tateishi J. J Virol. 1991;65:6292–6295. doi: 10.1128/jvi.65.11.6292-6295.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muramoto T, Kitamoto T, Tateishi J, Goto I. Am J Pathol. 1992;140:1411–1420. [PMC free article] [PubMed] [Google Scholar]
- 6.McBride P A, Eikelenboom P, Kraal G, Fraser H, Bruce M E. J Pathol. 1992;168:413–418. doi: 10.1002/path.1711680412. [DOI] [PubMed] [Google Scholar]
- 7.Klein M A, Frigg R, Flechsig E, Raeber A J, Kalinke U, Bluethmann H, Bootz F, Suter M, Zinkernagel R M, Aguzzi A. Nature (London) 1997;390:687–690. doi: 10.1038/37789. [DOI] [PubMed] [Google Scholar]
- 8.Fu Y X, Huang G, Wang Y, Chaplin D D. J Exp Med. 1998;187:1009–1018. doi: 10.1084/jem.187.7.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez M, Mackay F, Browning J L, Kosco-Vilbois M H, Noelle R J. J Exp Med. 1998;187:997–1007. doi: 10.1084/jem.187.7.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapasi Z F, Qin D, Kerr W G, Kosco-Vilbois M H, Shultz L D, Tew J G, Szakal A K. J Immunol. 1998;160:1078–1084. [PubMed] [Google Scholar]
- 11.Manson J C, Clarke A R, McBride P A, McConnell I, Hope J. Neurodegeneration. 1994;3:331–340. [PubMed] [Google Scholar]
- 12.Sakaguchi S, Katamine S, Shigematsu K, Nakatani A, Moriuchi R, Nishida N, Kurokawa K, Nakaoke R, Sato H, Jishage K, Kuno J, Noda T, Miyamoto T. J Virol. 1995;69:7586–7592. doi: 10.1128/jvi.69.12.7586-7592.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sailer A, Büeler H, Fischer M, Aguzzi A, Weissmann C. Cell. 1994;77:967–968. doi: 10.1016/0092-8674(94)90436-7. [DOI] [PubMed] [Google Scholar]
- 14.Fischer M, Rülicke T, Raeber A, Sailer A, Moser M, Oesch B, Brandner S, Aguzzi A, Weissmann C. EMBO J. 1996;15:1255–1264. [PMC free article] [PubMed] [Google Scholar]
- 15.Brandner S, Isenmann S, Raeber A, Fischer M, Sailer A, Kobayashi Y, Marino S, Weissmann C, Aguzzi A. Nature (London) 1996;379:339–343. doi: 10.1038/379339a0. [DOI] [PubMed] [Google Scholar]
- 16.Brandner S, Raeber A, Sailer A, Blattler T, Fischer M, Weissmann C, Aguzzi A. Proc Natl Acad Sci USA. 1996;93:13148–13151. doi: 10.1073/pnas.93.23.13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaffin K E, Beals C R, Wilkie T M, Forbush K A, Simon M I, Perlmutter R M. EMBO J. 1990;9:3821–3829. doi: 10.1002/j.1460-2075.1990.tb07600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harada H, Takahashi E, Itoh S, Harada K, Hori T A, Taniguchi T. Mol Cell Biol. 1994;14:1500–1509. doi: 10.1128/mcb.14.2.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayday A C, Gillies S D, Saito H, Wood C, Wiman K, Hayward W S, Tonegawa S. Nature (London) 1984;307:334–340. doi: 10.1038/307334a0. [DOI] [PubMed] [Google Scholar]
- 20.Pinkert C A, Ornitz D M, Brinster R L, Palmiter R D. Genes Dev. 1987;1:268–276. doi: 10.1101/gad.1.3.268. [DOI] [PubMed] [Google Scholar]
- 21.Büeler H, Fischer M, Lang Y, Bluethmann H, Lipp H-P, DeArmond S J, Prusiner S B, Aguet M, Weissmann C. Nature (London) 1992;356:577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- 22.Korth C, Stierli B, Streit P, Moser M, Schaller O, Fischer R, Schulz-Schaeffer W, Kretzschmar H, Raeber A, Braun U, Ehrensperger F, Hornemann S, Glockshuber R, Riek R, Billeter M, Wuthrich K, Oesch B. Nature (London) 1997;390:74–77. doi: 10.1038/36337. [DOI] [PubMed] [Google Scholar]
- 23.Raeber A J, Race R E, Brandner S, Priola S A, Sailer A, Bessen R A, Mucke L, Manson J, Aguzzi A, Oldstone M B A, Weissmann C, Chesebro B. EMBO J. 1997;16:6057–6065. doi: 10.1093/emboj/16.20.6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farquhar C F, Somerville R A, Ritchie L A. J Virol Methods. 1989;24:215–221. doi: 10.1016/0166-0934(89)90023-2. [DOI] [PubMed] [Google Scholar]
- 25.Chandler R L. Lancet. 1961;1:1378–1379. doi: 10.1016/s0140-6736(61)92008-6. [DOI] [PubMed] [Google Scholar]
- 26.Prusiner S B. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 27.Yamada G, Ogawa M, Akagi K, Miyamoto H, Nakano N, Itoh S, Miyazaki J, Nishikawa S, Yamamura K, Taniguchi T. Proc Natl Acad Sci USA. 1991;88:532–536. doi: 10.1073/pnas.88.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kraal G, Weissman I L, Butcher E C. Nature (London) 1982;298:377–379. doi: 10.1038/298377a0. [DOI] [PubMed] [Google Scholar]
- 29.Race R, Chesebro B. Nature (London) 1998;392:770. doi: 10.1038/33834. [DOI] [PubMed] [Google Scholar]
- 30.Clarke M C, Haig D A. Res Vet Sci. 1971;12:195–197. [PubMed] [Google Scholar]
- 31.Kuroda Y, Gibbs C J, Jr, Amyx H L, Gajdusek D C. Infect Immun. 1983;41:154–161. doi: 10.1128/iai.41.1.154-161.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bendheim P E, Brown H R, Rudelli R D, Scala L J, Goller N L, Wen G Y, Kascsak R J, Cashman N R, Bolton D C. Neurology. 1992;42:149–156. doi: 10.1212/wnl.42.1.149. [DOI] [PubMed] [Google Scholar]
- 33.Blättler T, Brandner S, Raeber A J, Klein M A, Voigtlander T, Weissmann C, Aguzzi A. Nature (London) 1997;389:69–73. doi: 10.1038/37981. [DOI] [PubMed] [Google Scholar]
- 34.Kimberlin R H, Walker C A. J Comp Pathol. 1979;89:551–562. doi: 10.1016/0021-9975(79)90046-x. [DOI] [PubMed] [Google Scholar]
- 35.Telling G C, Scott M, Mastrianni J, Gabizon R, Torchia M, Cohen F E, DeArmond S J, Prusiner S B. Cell. 1995;83:79–90. doi: 10.1016/0092-8674(95)90236-8. [DOI] [PubMed] [Google Scholar]
- 36.Kaneko K, Zulianello L, Scott M, Cooper C M, Wallace A C, James T L, Cohen F E, Prusiner S B. Proc Natl Acad Sci USA. 1997;94:10069–10074. doi: 10.1073/pnas.94.19.10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Westaway D, Cooper C, Turner S, Da C M, Carlson G A, Prusiner S B. Proc Natl Acad Sci USA. 1994;91:6418–6422. doi: 10.1073/pnas.91.14.6418. [DOI] [PMC free article] [PubMed] [Google Scholar]