Abstract
A chimeric retroviral vector (33E67) containing a CD33-specific single-chain antibody was generated in an attempt to target cells displaying the CD33 surface antigen. The chimeric envelope protein was translated, processed, and incorporated into viral particles as efficiently as wild-type envelope protein. The viral particles carrying the 33E67 envelope protein could bind efficiently to the CD33 receptor on target cells and were internalized, but no gene transfer occurred. A unique experimental approach was used to examine the basis for this postbinding block. Our data indicate that the chimeric envelope protein itself cannot participate in the fusion process, the most reasonable explanation being that this chimeric protein cannot undergo the appropriate conformational change that is thought to be triggered by receptor binding, a suggested prerequisite to subsequent fusion and core entry. These results indicate that the block to gene transfer in this system, and probably in most of the current chimeric retroviral vectors to date, is the inability of the chimeric envelope protein to undergo this obligatory conformational change.
A major goal of gene therapy research is to develop vectors that would allow targeted gene transfer into specific cell types (1). Several attempts have been made either to substitute or to insert a ligand (either a peptide or a single-chain antibody) into the envelope protein of a retroviral vector so that the vector could then bind to a specific receptor on a designated cell type (2–14). In initial studies, antibodies were used to bridge the vector and the host cells (3, 4). Because of the low efficiency, more recent studies have engineered the envelope protein in an attempt to change the tropism of the retroviral vector. A ligand to the erythropoietin receptor or to the heregulin receptor has been used to replace the binding domain of the murine leukemia virus (MuLV) ecotropic envelope protein to achieve transduction of target cells (5, 6). Insertion of a single-chain antibody (scFv) or a ligand into the N-terminal region of the envelope protein also has been used to target cell-surface molecules (7–12). In addition to the ecotropic Moloney murine leukemia virus (Mo-MuLV), the envelope protein of spleen necrosis virus has been used as a model system (13, 14). However, although some of these studies report individual clones that reach a titer as high as 104 on target cells, it has not been possible to reliably generate vector preparations carrying chimeric envelope proteins that are able to produce titers higher than a few hundred on target cells. A number of laboratories have tested alternative insertion and replacement constructs with different single-chain antibodies and ligands. A significant titer on target cells has not been consistently achieved despite the ability of these chimeras to specifically bind to the target cells.
To identify the basis for this failure, we examined each of the steps in the gene transfer pathway (binding, internalization, fusion, core entry, reverse transcription, integration, and gene expression) to determine the cause of the block. The data suggested that a postbinding block to fusion existed. We then developed a system that allowed us to test, via genetic complementation, individual steps in the fusion process. Even though direct evidence has not been obtained for the exact mechanism for viral fusion in Mo-MuLV, by analogy to other viruses it is thought that, after binding to receptor, Mo-MuLV envelope protein undergoes a conformational change that leads to fusion and core entry. Our data suggest that it is this conformational change that cannot occur in the chimeric envelope protein.
MATERIALS AND METHODS
Envelope Proteins and Cell Lines.
A single-chain antibody to human CD33 (15) was constructed by splicing PCR as described (16). Mo-MuLV envelope protein expression vector wild-type ecotropic envelope protein (CEE+) (17) was engineered to contain SfiI (5′ end) and NotI (3′ end) sites between amino acids 6 and 7 of the mature envelope protein as described (10). The scFv of CD33 was amplified by PCR with primers (5′-GCCCGGGGGCCCAGCCGGCCATGCACC and 5′-GCGGTGGCGGCCGCGGAAAGGGTGACC), and the product was subcloned into the SfiI and NotI sites of the modified envelope protein to produce the chimeric envelope 33E67. The same approach was applied to the binding-defective mutant D84K (17) to obtain the construct 33K67. The corresponding constructs in the R-peptide truncated form were obtained by subcloning 33E67 and 33K67 into the R-less form of the Mo-MuLV envelope in the construct CEETR (18). Both NIH 3T3 and 3T3/CD33 cells [NIH 3T3 cells that stably express human CD33 (19)] were grown in DMEM (Core Facility, University of Southern California) supplemented with 10% fetal calf serum (FCS, HyClone) and 2 mM glutamine (BRL).
Retroviral Vector Production and Characterization.
Retroviral vectors were produced by transient transfection of 293T cells by calcium phosphate precipitation, essentially as described (20, 21). The plasmids used were pHIT60, a Mo-MuLV gag-pol expression plasmid, the retroviral vector pCnBg that expresses the lacZ and neo genes (21), and one or two envelope expression plasmids. Thirty-six hours after transfection, the supernatants were harvested and filtered through a 0.45-μm filter. The protein content of virions partially purified through 20% sucrose was assessed by Western blot analysis as described (22). The ability of virions to bind to the ecotropic receptor expressed on NIH 3T3 or to the human CD33 antigen expressed on 3T3/CD33 cells was determined by a fluorescence-activated cell sorting assay as described (23). The transduction efficiency of the vector on target cells was determined by titer as described (22). To measure syncytia formation in NIH 3T3 or 3T3/CD33 cell monolayers, 2 × 105 cells were plated in a 60-mm tissue culture dish and transfected with 1- or 2-envelope DNA plasmids as described (22). After staining with methylene blue, cells with more than four nuclei were scored as syncytia under phase-contrast microscopy. To measure the formation of heterooligomers, two envelope constructs were cotransfected into 293T cells and coimmunoprecipitation was performed as described (22).
Detection of Viral cDNA and Internalization Assays.
Retroviral vector supernatants from cultures of transfected cells were applied to NIH 3T3 or 3T3/CD33 cells at multiplicity of infection = 1 for CEE+ (or an equivalent volume of supernatant for chimeric virus) and incubated for 6 hr at 37°C. Cells were then lysed and the cytoplasmic DNA purified as described (24). The DNA was analyzed by Southern blotting with a 32P-labeled probe containing a long terminal repeat sequence (24). To detect the internalization of vector, 35S-labeled viral particles were incubated with NIH 3T3 or 3T3/CD33 cells for 2 hr at 4°C to allow the virus to bind with its receptor; after washing with cold PBS, the cells were incubated at 37°C for 45 min. At the end of the incubation, the cells were trypsinized at 37°C for 10 min, neutralized with regular D10, washed three times with PBS, lysed, and then immunoprecipitated as described (22).
For electron microscopy (EM) studies, chimeric or wild-type retroviral vector particles were applied to 3T3/CD33 or 3T3 cells and incubated at 37°C for 1 hr. After trypsinization, target cells were collected, washed, and centrifuged for fixation. EM samples were fixed in half-strength Karnovsky fixative for 2 hr then postfixed in 1% osmium tetroxide for 1 hr. After fixation, the samples were dehydrated in a graded series of ethanol/water dilutions, infiltrated with an epon araldite resin mixture, and subsequently embedded in epon araldite resin. Thin sections (60 to 80 nm) were cut and counterstained with uranyl acetate and lead citrate. Viral particles located within the cytoplasm of 12 to 24 randomly chosen cells were counted from each sample on a Zeiss EM 10 electron microscope. Data from two individual experiments were combined for statistical analysis.
RESULTS AND DISCUSSION
Construction of Chimeric Retroviral Envelope Proteins.
We analyzed over 30 insertion sites throughout the N-terminal half of surface protein (SU) (B. Wu and W.F.A., unpublished work). Many of these sites were based on examination of the published three-dimensional structure. Of those insertion sites (around 10) that resulted in envelope incorporation into virions as well as at least some binding, an insertion site between amino acids 6 and 7 was chosen for detailed study because this particular insertion site in the envelope protein appears to be the one most receptive to a large insertion (7–10). The CD33 chimera was selected for detailed analysis because it is incorporated into viral particles at a wild-type level, and it has a high binding affinity. However, studies were also carried out with CD34 (42), her2, her4, and several other ligands with similiar results to those obtained for CD33.
We inserted a single-chain antibody to human CD33 (15) into the N-terminal region between amino acids 6 and 7 of the envelope protein of Mo-MuLV to yield the chimeric envelope, 33E67. We then constructed a series of modified chimeric envelope proteins to allow testing of the various steps in the gene transfer process. The chimeric envelope proteins used in this study are shown in Fig. 1. The constructs are 33E67, in which a scFv to the cell-surface antigen CD33 was inserted between amino acids 6 and 7 in the ecotropic envelope (E) protein SU; 33K67, in which a point mutation, D84K (17), was made in the natural receptor binding domain of 33E67 (this mutation prevents binding to the ecotropic receptor, MCAT-1); 33E67TR, in which the 33E67 envelope protein is truncated at the R-peptide cleavage site; and 33K67TR, which is the complement to 33E67TR, in which the 33K67 envelope protein is truncated at the R-peptide cleavage site. Truncation of the R-peptide significantly enhances the fusogenicity of the envelope protein in NIH 3T3 cell syncytia assays (25, 26). The chimera 33K67 was constructed to differentiate binding to the CD33 receptor from binding to the MCAT-1 receptor. The rationale for the TR constructs will be discussed below.
Figure 1.
Schematic diagrams of the chimeric envelope protein constructs. Mo-MuLV envelope expression vector CEE+ was engineered to contain a SfiI site (5′ end) and a NotI site (3′ end) between amino acids 6 and 7, yielding the vector named E67. A CD33 scFv was amplified by PCR and the PCR product was subcloned into E67 at the SfiI and NotI sites to obtain the chimeric envelope protein 33E67. A point mutant, D84K, that had been previously demonstrated to be a binding-defective mutant (17), was introduced into 33E67 to generate 33K67. Vertical arrows indicate proteinase cleavage sites. SP, signal peptide; SU, surface protein; TM, transmembrane protein; CEE+, wild-type ecotropic envelope protein. The approximate site of the binding mutant D84K is denoted by an asterisk. The membrane-spanning domain is denoted as a grey box. The other four constructs used in this study, CEETR, D84KTR, 33E67TR, and 33K67TR, are the R-peptide truncated forms of CEE+, D84K, 33E67, and 33K67, respectively.
Efficient Incorporation of Chimeric Envelope Proteins into Viral Particles.
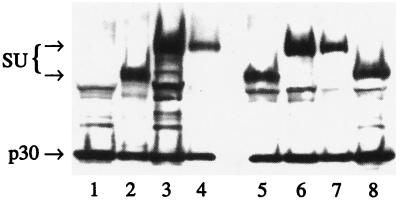
First it was important to demonstrate that the chimeric envelope proteins were translated, processed, and incorporated into viral particles as efficiently as the wild-type envelope protein. These data were obtained by Western blot analysis. Supernatants from cells transiently transfected with the envelope constructs were collected and centrifuged through 20% sucrose to pellet viral particles and eliminate free protein. These pellets were then analyzed on immunoblots for their gag (p30 CA) and envelope gp70 (SU) content. The amount of SU detected for each of the chimeric envelopes (Fig. 2, lanes 3, 4, 6, and 7) was similar to the amount of wild-type SU (Fig. 2, lanes 2 and 8). These data demonstrate that the chimeric envelope proteins can be expressed, processed, and incorporated into virions as efficiently as the wild-type envelope protein.
Figure 2.
Detection of envelope SU and p30 CA protein in virions by Western blot. The supernatants from transiently transfected cells were collected and centrifuged through 20% sucrose to pellet viral particles and remove free protein. Pellets were then analyzed on 8–16% SDS/PAGE gels for their gag (p30 CA) and envelope gp70 (SU) content. Viral SU could be detected for all the chimeric envelopes. SU containing the CD33 scFv migrates at a higher position than the wild type. Lanes: 1, mock transfection; 2, ecotropic viral particles from GPE86/LNCX producer cells (41); 3, 33E67; 4, 33E67TR; 5, D84K; 6, 33K67; 7, 33K67TR; 8, CEE+.
Efficient Binding of Viral Particles Carrying the CD33 Chimeric Envelope Protein to CD33-Expressing Cells.
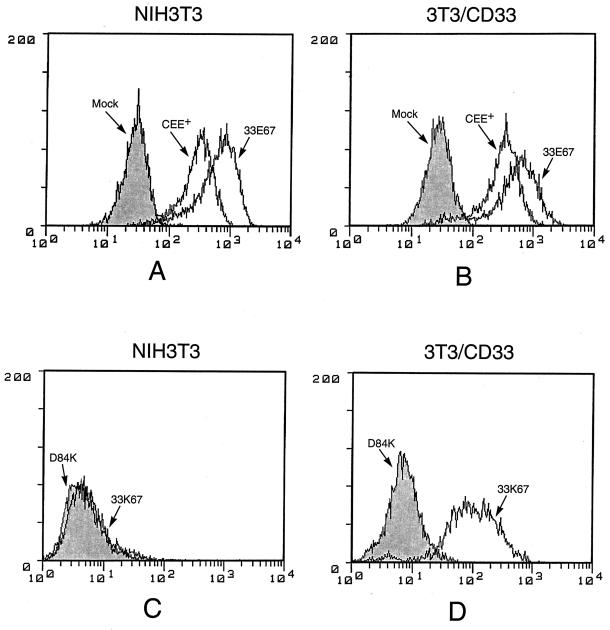
We next tested the efficiency with which the chimeric envelope proteins containing the CD33 scFv could bind to cells expressing the CD33 receptor. We used a cell line, 3T3/CD33, which is 3T3 cells expressing CD33 (19). Viral particles containing each one of the chimeric envelope proteins were incubated with target cells, either NIH 3T3 or 3T3/CD33, and then analyzed by immunofluorescent flow cytometry to determine the efficiency of binding of each construct to the CD33 antigen or to the natural ecotropic MuLV receptor, MCAT-1 (Fig. 3). Viral particles with 33E67 (or 33E67TR, data not shown) efficiently bind to both 3T3 and 3T3/CD33 cells, while particles with 33K67 (or 33K67TR, data not shown) bind only to 3T3/CD33 cells, not to 3T3 cells. These data confirm that the envelope constructs containing the CD33 scFv can bind efficiently to the CD33 antigen and that the envelope constructs containing the D84K binding mutation cannot bind to the MCAT-1 receptor. Furthermore, the binding of 33E67 to MCAT-1 indicates that the insertion of the CD33 scFv has not produced significant steric hindrance for binding by the chimeric envelope protein. Efficient binding of viral particles carrying the CD33 chimeric envelope protein could also be demonstrated on HL60 and NB4, which are human cells from myeloid lymphoma patients that carry the CD33 receptor but not the MCAT-1 receptor (data not shown). Thus, the chimeric envelope proteins were processed and incorporated efficiently and could specifically bind to the CD33 antigen on target cells.
Figure 3.
The strength of envelope/receptor binding of the retroviral envelope constructs. Supernatants containing viral particles from transient transfections were collected and applied to target cells, either NIH 3T3 or 3T3/CD33 cells, to detect the binding of chimeric envelope to the CD33 antigen. Binding of viral particles to the target cells was detected by an immunofluorescent flow cytometry assay. The binding signals of control incubations (using either D84K or mock transfection supernatant) are shown as grey control peaks.
Viral Particles Carrying CD33 Chimeric Envelope Proteins Cannot Transduce Cells.
Because HL60 and NB4 cells cannot be transduced with ecotropic retrovirus and only inefficiently with wild-type amphotropic virus (data not shown), we chose NIH 3T3 and 3T3/CD33 cells as a model system to study the transduction by the chimeric viral particles. We could not detect titer on either cell line with the supernatant from 33E67, 33E67TR, 33K67, or 33K67TR, although the control, CEE+, produced the expected titer of 4 × 106 on both cell lines (data not shown). Thus, despite the fact that the binding of 33E67 with both cell lines is as strong as wild type, no transduction of the target cells took place. The barrier occurs not only when the interaction of the chimeric envelope is with the CD33 receptor (i.e., with 33K67, which cannot bind MCAT-1 on 3T3/CD33 cells), but also when binding is occurring with the native receptor (i.e., with 33E67 on 3T3 cells). Therefore, a block occurs at some stage after binding of the viral particle to receptor that results in the inability of the vector genome to be expressed in the target cell.
No Reverse Transcription Occurs with Viral Particles Carrying CD33 Chimeric Envelope Proteins.
To locate the postbinding block that prevents target cell transduction, we first asked whether the viral particles that bound to the CD33 receptor could release their cores into the target cell cytoplasm. If so, then it should be possible to detect preintegration complexes in the cytoplasm. After core entry, reverse transcriptase in the viral core results in the conversion of the single-stranded RNA viral genome into linear double-stranded DNA. This vector DNA can be detected by Southern analysis. NIH 3T3 or 3T3/CD33 cells were incubated with viral particles for 6 hr and then the cytoplasmic DNA was isolated. A 32P-dCTP-labeled long terminal repeat fragment from the retroviral vector was used as a probe to detect retroviral DNA (Fig. 4). In both NIH 3T3 and 3T3/CD33 cells, retroviral DNA signals were detected only for wild-type (CEE+) vector particles (Fig. 3, lanes 3 and 9). No signal was detected for any of the other constructs, including 33E67, which binds to the same receptor, MCAT-1, equally as efficiently as wild-type viral particles. These data indicate that even though the chimeric viruses could bind to the target cells, reverse transcription of viral sequences did not occur.
Figure 4.
Detection of preintegration complex in target cells. Supernatants containing viral particles from transient transfections were incubated with NIH 3T3 or 3T3/CD33 cells for 6 hr at 37°C. The cytoplasmic DNA was isolated and analyzed by Southern blot. The probe used in the study was a 32P-dCTP-labeled long terminal repeat fragment (24). Lanes: 1 and 7, mock transfection (with the pHIT60 and pCnB plasmids, but no envelope protein plasmids); 2 and 8, D84K; 3 and 9, CEE+; 4 and 10, 33E67; 5 and 11, 33K67; 6 and 12, H2O transfection. Lanes 1–6 are from 3T3 cells and lanes 7–12 are from 3T3/CD33 cells.
Viral Particles Carrying CD33 Chimeric Envelope Proteins Can Be Internalized.
Because we could not detect reverse-transcribed viral sequences despite chimeric envelope binding to the target cell, the postbinding block may be the consequence of blockage before or at core release or later at the reverse-transcription step itself. We asked whether the chimeric viral particles could at least be internalized after binding to CD33 or to MCAT-1. Internalization is defined as the process whereby a molecule or macromolecular complex moves from the extracellular side of the cell membrane into the cytoplasm. There are a number of mechanisms whereby this process can occur (27). The one assumed to be active for Mo-MuLV entry is receptor-mediated endocytosis (28). With certain cell lines like 3T3, SC-1, and Rat-1 cells, retroviral infection is sensitive to lysosomotropic agents (i.e., inhibitors that buffer lysosomal pH such as NH4Cl and chloroquine), suggesting that either virus–cell membrane fusion or core entry needs to be in a low pH environment (28, 29). We used immunoprecipitation and EM to investigate internalization.
Viral particles carrying 35S-labeled chimeric envelope protein were bound to target cells by incubating for 2 hr at 4°C followed by extensive washing and then incubation for 1 hr at 37°C to allow for internalization if it could occur. After trypsinization to remove cell surface-bound virions, the cell lysates were immunoprecipitated with anti-SU and anti-p30 antibodies and then electrophoresed on a PAGE gel (Fig. 5). Bands for both 33E67 and 33K67 chimeric envelope protein and p30 protein could be detected in 3T3/CD33 cells (Fig. 5, lanes 1 and 2); the p30 protein could also be detected but at a much lower level with viral particles that carried no envelope protein. As a control, samples treated with trypsin after binding were used to examine the background signal coming from particles that trypsin failed to remove. The signals from these samples were significantly weaker than those from the experimental samples (data not shown). Therefore, by immunoprecipitation, internalization of the viral particles appears to take place.
Figure 5.
Internalization of bound viral particles. 3T3/CD33 cells were used as the target cells. After incubating target cells at 4°C with viral particles for 2 hr, cells were washed with PBS and incubated in D10 medium for 1 hr at 37°C, followed by washing three times with PBS and then incubation in trypsin/EDTA for 10 min at 37°C. Lysates of cells were analyzed by immunoprecipitation with anti-SU and anti-p30 antiserums. Lanes: 1, 33K67; 2, 33E67; 3, mock transfection (with pHIT60 and pCnB plasmids, but no envelope protein plasmids).
EM was used to observe the presence or absence of retroviral particles inside the cell membrane. After 1-hr incubation of viral supernatants with 3T3/CD33 cells, viral particles carrying either wild-type envelope (CEE+) or the chimera 33E67 or 33K67 were found in significant numbers inside the cell membrane, while viral particles carrying the mutant envelope protein D84K were present in lesser amounts (Table 1). The difference between particles containing 33E67 or 33K67 with CEE+ is not statistically significant (P > 0.05), but the P value between particles containing D84K vs. CEE+ is significantly different (P < 0.01). Likewise, the P value is significant (<0.01) when particles carrying 33K67 are incubated with 3T3 cells, which do not have a receptor for CD33. These data demonstrate that retroviral particles are mainly internalized by receptor-mediated endocytosis, although some nonreceptor-mediated internalization occurs. Thus, data from both immunoprecipitation and from EM suggest that viral particles that can bind to a receptor can also be internalized.
Table 1.
Electron microscopy study of viral particle internalization
| Envelope protein | n | Cell line | Particles per cell
|
P value | |
|---|---|---|---|---|---|
| Mean* | Range† | ||||
| CEE+ | 12 | 3T3/CD33 | 4.7 ± 1.7 | 1–23 | — |
| D84K | 24 | 3T3/CD33 | 0.9 ± 0.2 | 0–3 | <0.01 |
| 33E67 | 24 | 3T3/CD33 | 3.4 ± 0.6 | 0–11 | >0.05 |
| 33K67 | 24 | 3T3/CD33 | 3.4 ± 0.4 | 1–11 | >0.05 |
| 33K67 | 12 | 3T3 | 1.0 ± 0.03 | 0–3 | <0.01 |
Chimeric and wild-type viral particles were incubated with 3T3/CD33 or with 3T3 cells for 1 hr at 37°C. The cells were then trypsinized and fixed for EM study, and the number of viral particles inside the cell membrane was counted. n, number of cells analyzed; ∗, mean ± SE; †, minimum and maximum number of viral particles found in any single cell. The P value is for the comparison between the indicated chimera (or mutant) vs. wild type.
However, a caveat is that these analyses are complicated by the fact that there are a large number of noninfectious virus-like particles in every virus preparation. It is not possible to distinguish defective from nondefective particles by either immunoprecipitation or by EM. Therefore, it is an assumption that the nondefective particles have properties similar to the bulk of the particles in the virus preparations studied.
Viral Particles Carrying CD33 Chimeric Envelope Proteins Cannot Carry out Fusion.
The fusion of viral and cellular membranes is necessary for enveloped virus entry. Fusion occurs either in the endosome, where the low pH is thought to trigger a conformational change in the envelope protein to release the fusion peptide such as occurs with the influenza envelope protein HA1 (30–32), or on the cell membrane such as occurs with many retroviruses, including HIV (33). For Mo-MuLV, a low pH step seems to be required for virus entry, because infection of certain cell lines is sensitive to lysosomotropic agents (28, 29, 34). However, in XC cells, expression of retroviral envelope protein alone can mediate cell–cell fusion as demonstrated by syncytia formation, and this syncytia assay has been widely used to study the fusogenicity of the ecotropic murine retroviral envelope protein (35). The expression of chimera 33E67 in XC cells does not elicit syncytia formation (data not shown), thereby suggesting that the postbinding block is at the step of virus–cell membrane fusion.
Viral Particles Carrying CD33 Chimeric Envelope Proteins Cannot Carry out the Steps Required for Fusion.
Because the XC fusion assay does not provide information on the mechanism that produces the block in fusion, we used an assay that our laboratory has recently developed (22, 36) to examine this critical step.
The rationale for this assay is as follows. The Mo-MuLV envelope protein contains two subunits, SU and transmembrane protein (TM). The final 16 residues of the TM (R-peptide) strongly influences envelope fusogenicity (18, 25, 26). As noted above, it has been shown that Mo-MuLV envelope protein expression will allow syncytia formation of XC cells, but this does not occur with NIH 3T3 cells unless the fusogenicity of the envelope protein is enhanced. It was demonstrated (25, 26) that the truncated R-peptide (R-less) ecotropic envelope protein (i.e., CEETR) can induce massive syncytia formation of 3T3 cells, while the wild-type envelope (CEE+) cannot.
On the virion, the envelope protein forms an oligomer, most likely a trimer (37, 38). We have recently demonstrated that there is a functional interaction among the monomers in this trimeric structure (22, 36), and supporting evidence for this hypothesis has recently been published by others (39). For example, when D84KTR (a binding-defective mutant in the R-less form that cannot induce syncytia formation of 3T3 cells because it cannot bind) is coexpressed with CEE+ (which cannot induce syncytia because it is not R-less), massive syncytia formation occurs. This phenomenon was shown to result from a functional interaction within the coexpressed heterotrimer, which allows different monomers to complement each other functionally. Specifically in this example, the required conformational change of the monomer from CEE+ that occurs after its binding with its receptor complements the monomer D84KTR, which cannot bind and therefore cannot initiate its own conformational change, but can undergo the conformational change when triggered by the CEE+ monomer in the heterotrimer. D84KTR can then contribute the R-less phenotype for syncytia formation of 3T3 cells. Thus, this functional interaction assay has been demonstrated to assay functionally, although not structurally, for the conformational change that occurs in the Mo-MuLV envelope protein during the fusion step.
To analyze the postbinding block in our chimeric vectors, we applied this assay to determine whether the CD33 chimeric envelope protein could successfully interact with an adjacent monomer in a trimer after it binds to its CD33 receptor. The entire matrix of interactions was measured between the four R-less constructs and their four R-containing counterparts: CEETR, D84KTR, 33E67TR, or 33K67TR complemented individually with CEE+, D84K, 33E67, or 33K67 (see Table 2 and Fig. 6). If the CD33 chimeric envelope proteins can undergo the fusion-required conformational change, then functional interaction among the monomers within a heterotrimer will allow syncytia formation with certain combinations. The data are clear. No syncytia are observed in any of the relevant combinations of chimeric envelopes. Thus, there are no instances of a functional interaction, and therefore it would appear that there has not been a correct conformational change in the chimeric envelope protein.
Table 2.
Titer and fusion ability of viral particles containing heterooligomers composed of two separate chimeric envelope proteins
| DNA | CEETR | D84KTR | 33E67TR | 33K67TR |
|---|---|---|---|---|
| CEE+ | +(7.0 × 106) | +(6.8 × 106) | +(3.5 × 106) | +(3.0 × 106) |
| D84K | +(5.3 ± 106) | −(0) | −(0) | −(0) |
| 33E67 | +(1.7 × 106) | −(0) | −(0) | −(0) |
| 33K67 | +(1.4 × 106) | −(0) | −(0) | −(0) |
To determine titer, supernatants from transfected cells were collected and added to 3T3/CD33 cells. After 10 days of selection in G418 (0.6 mg/ml), G418-resistant colonies were counted (data in parentheses). Except for CEE + (4 × 106) and CEETR (2.5 × 102), the titer of the supernatant from each of the other single DNA transfections is zero. Syncytia formation of 3T3/CD33 cells mediated by transfected envelope protein plasmid is indicated by a “+,” and no syncytia, by a “−.” Unless monomers of the wild-type (CEE+) or R-less wild-type (CEETR) envelope protein are present in the mixed heterooligomer, no syncytia formation occurs (see text).
Figure 6.
Chimeric envelope protein-mediated syncytia formation of 3T3/CD33 cells. Envelope protein expression plasmids were cotransfected (at a ratio of 1:1) into 3T3/CD33 cells. At 36 hr after transfection, the cells were stained with methylene blue, and those cells containing more than four nuclei counted as syncytia. (Upper) Examples of positive syncytia formation; (Lower) Examples of negative syncytia formation. These data are a portion of those summarized in Table 2.
An important result was obtained with 33E67. Because the putative conformational change is blocked even with binding to the natural MCAT-1 receptor (33E67 plus D84KTR, 33E67TR, or 33K67TR are zero, as are D84K or 33K67 plus 33E67TR), this result suggests that the presence of the CD33 scFv sufficiently disrupts the normal architecture of the envelope protein so that the normal conformational change that would occur after MCAT-1 receptor binding cannot take place. Thus, the putative conformational change cannot occur whether the binding is to the CD33 receptor or to the MCAT-1 receptor on the target cell.
An alternative explanation for a lack of a functional interaction could be that the protein architecture of the envelope protein was sufficiently disrupted by the anti-CD33 scFv that heterooligomer formation was prevented. If this were the case, a failure to observe a functional interaction could not be attributed to a loss of a functional conformation change. To determine whether heterooligomer were indeed formed between the chimeric and the D84K envelope protein, coimmunoprecipitation assays were performed. We took advantage of the fact that Mo-MuLV envelope protein expressed in the absence of the viral protease will retain the R-peptide. Antibody to R-peptide can immunoprecipitate the R-less form of TM (p12E), in addition to the full length form of TM (p15E), when both full length and R-less forms of the wild-type envelope are coexpressed in the same cell (22). 293T cells lack viral proteinase activity so that the R-peptide will not be cleaved from the full length protein in these cells. The presence of p12E in the precipitate is evidence for an interaction between p15E and p12E molecules.
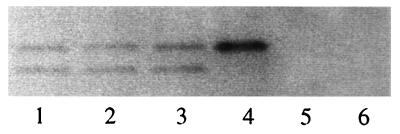
Coimmunoprecipitation was performed to test each of the relevant combinations for the presence of heterooligomer. With R-peptide anti-serum, p12E bands could be precipitated only when 33E67TR or 33K67TR was coexpressed with D84K or CEE+ (Fig. 7, lanes 1–3), but not when they are expressed alone (Fig. 7, lanes 5 and 6). Thus, heterooligomer are formed between chimeric and wild-type envelope protein monomers.
Figure 7.
Heterooligomer formation between chimeric envelope monomers and wild-type monomers. SDS/PAGE (14%) of the coimmunoprecipitated virion envelope protein is shown. Envelope proteins were transiently expressed in 293T cells. Metabolic labeling for 4 hr with [35S]Met was performed 24 hr after transfection. The cells were lysed, and the supernatant was immunoprecipitated with 5 μ of anti-R peptide (22). The p12E protein, bottom band, that was present in 33E67TR and 33K67TR, was immunoprecipitated by R-peptide antiserum only in the presence of coexpressed D84K (lane 1, 33E67TR/D84K; lane 2, 33K67TR/D84K); or CEE+ (lane 3, 33K67TR/CEE+), but not when expressed by itself (lane 5, 33E67TR and lane 6, 33K67TR). There is no p12E band in the wild-type CEE+ (lane 4), indicating that there is no R-peptide cleavage in this 293T system.
Conclusion.
The conclusion from these data is that the binding of the chimeric envelope protein with receptor on the target cell does not, in itself, lead to successful fusion of the viral and cellular membranes, a prerequisite for the transfer of the viral core into the target cell. Although our data cannot rigorously establish a precise mechanism, the most likely explanation is that the chimeric envelope is not able to undergo the conformational change that triggers the fusion process. Whether the binding is to the native receptor (MCAT-1) or to the targeted molecule (CD33), this triggering process does not occur with the chimeric envelope proteins.
Even though the mechanism causing the postbinding block that we have demonstrated in our study may not be true for every chimeric retroviral envelope protein, it is reasonable to assume that it may be a major cause for the poor transduction efficiency seen in many chimeric retroviral systems. Before efficient targeted transduction by using retroviral vectors can be achieved, it will probably be necessary to develop a much better understanding of the structure/function of the wild-type envelope protein itself. Recent x-ray crystallographic studies of the ecotropic envelope protein (38, 40), although providing only structural information on parts of the envelope protein, are valuable contributions to this understanding (B. Wu and W.F.A., unpublished work). Vectors that could efficiently transduce specific cell types in vivo would be invaluable tools for clinical gene therapy protocols.
Acknowledgments
We thank Guoliang Li for excellent technical assistance, Ernesto Barron from the University of Southern California Electron Microscopy Core Facility for his assistance with electron microscopy, and Paula Cannon, Nori Kasahara, and Cyril Empig for helpful discussions and for critical reading of the manuscript. We also thank Chris Benedict for supplying the E67 plasmid. This work was supported by Genetic Therapy, Inc. (GTI)/Novartis and by National Institutes of Health grant CA59318.
ABBREVIATIONS
- MuLV
murine leukemia virus
- Mo-MuLV
Moloney murine leukemia virus
- EM
electron microscopy
- SU
surface protein
- TM
transmembrane protein
- CEE+
wild-type ecotropic envelope protein
References
- 1.Anderson W F. Nature (London) 1998;392:25–30. doi: 10.1038/32058. [DOI] [PubMed] [Google Scholar]
- 2.Ager S, Nilson B H K, Morling F J, Peng K W, Cosset F-L, Russell S J. Hum Gene Ther. 1996;7:2157–2164. doi: 10.1089/hum.1996.7.17-2157. [DOI] [PubMed] [Google Scholar]
- 3.Goud B, Legrain P, Buttin G. Virology. 1988;163:251–254. doi: 10.1016/0042-6822(88)90261-9. [DOI] [PubMed] [Google Scholar]
- 4.Roux P, Jeanteur P, Piechaczyk M. Proc Natl Acad Sci USA. 1989;86:9079–9083. doi: 10.1073/pnas.86.23.9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kasahara N, Dozy A M, Kan Y W. Science. 1994;266:1373–1376. doi: 10.1126/science.7973726. [DOI] [PubMed] [Google Scholar]
- 6.Han X, Kasahara N, Kan Y W. Proc Natl Acad Sci USA. 1995;92:9747–9751. doi: 10.1073/pnas.92.21.9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Somia N V, Zoppe M, Verma I M. Proc Natl Acad Sci USA. 1995;92:7570–7574. doi: 10.1073/pnas.92.16.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cosset F-L, Morling F J, Takeuchi Y, Weiss R A, Collins M K L, Russell S L. J Virol. 1995;69:6314–6322. doi: 10.1128/jvi.69.10.6314-6322.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marin M, Noël D, Valsesia-Wittman S, Brockly F, Etienne-Julan M, Russell S, Cosset F-L, Piechaczyk M. J Virol. 1996;70:2957–2962. doi: 10.1128/jvi.70.5.2957-2962.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell S, Hawkins R E, Winter G. Nucleic Acids Res. 1993;5:1081–1085. doi: 10.1093/nar/21.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valsesia-Wittmann S, Morling F J, Harziioannou T, Russell S J, Cosset F-L. EMBO J. 1997;16:1214–1223. doi: 10.1093/emboj/16.6.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson J E, Schnell M J, Buonocore L, Rose J K. J Virol. 1997;71:5060–5068. doi: 10.1128/jvi.71.7.5060-5068.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tearoma T-H, Dornburg R. J Virol. 1995;69:2659–2663. doi: 10.1128/jvi.69.4.2659-2663.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tearoma T-H, Dornburg R. J Virol. 1997;71:720–725. doi: 10.1128/jvi.71.1.720-725.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang E, Soong N-W, Scheinberg D A, Anderson W F, Douer D. Blood. 1996;51:3337a. [Google Scholar]
- 16.Benedict C A, MacKrell A J, Anderson W F. J Immunol Methods. 1997;201:223–231. doi: 10.1016/s0022-1759(96)00227-x. [DOI] [PubMed] [Google Scholar]
- 17.MacKrell A J, Soong N-W, Curtis C, Anderson W F. J Virol. 1996;70:1768–1774. doi: 10.1128/jvi.70.3.1768-1774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Januszeski M M, Cannon P M, Chen D, Rozenberg Y, Anderson W F. J Virol. 1997;71:3613–3619. doi: 10.1128/jvi.71.5.3613-3619.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peiper S C, Ashmun R A, Look T. Blood. 1988;72:314–321. [PubMed] [Google Scholar]
- 20.Soneoka K, Cannon P M, Ramsdale E E, Griffiths J C, Romano G, Kingsman S M, Kingsman A J. Nucleic Acids Res. 1995;23:628–633. doi: 10.1093/nar/23.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han J-Y, Cannon P M, Lai K-M, Eiden M V, Anderson W F A. J Virol. 1997;71:8103–8108. doi: 10.1128/jvi.71.11.8103-8108.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Y, Lee S, Anderson W F. J Virol. 1997;71:6967–6972. doi: 10.1128/jvi.71.9.6967-6972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu H, Soong N-W, Anderson W F. J Virol. 1995;69:6557–6562. doi: 10.1128/jvi.69.10.6557-6562.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Farnet C M, Anderson W F, Bushman R D. J Virol. 1998;72:2125–2131. doi: 10.1128/jvi.72.3.2125-2131.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ragheb J A, Anderson W F. J Virol. 1994;68:3220–3231. doi: 10.1128/jvi.68.5.3220-3231.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rein A, Mirro J, Haynes J G, Ernst S M, Nagashima K. J Virol. 1994;68:1773–1781. doi: 10.1128/jvi.68.3.1773-1781.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukherjee S, Ghosh R N, Maxfield F R. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 28.McClure M O, Sommerfelt M A, Marsh M, Weiss R A. J Gen Virol. 1990;71:767–773. doi: 10.1099/0022-1317-71-4-767. [DOI] [PubMed] [Google Scholar]
- 29.Nussbaum O, Roop A, Anderson W F. J Virol. 1993;67:7402–7405. doi: 10.1128/jvi.67.12.7402-7405.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bullough P A, Hughson F M, Skehel J J, Wiley D C. Nature (London) 1994;371:37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- 31.Carr C M, Kim P C. Cell. 1993;73:823–832. doi: 10.1016/0092-8674(93)90260-w. [DOI] [PubMed] [Google Scholar]
- 32.White J M, Hoffman H R, Arevalo J H, Wilson I A. In: Structural Biology of Viruses. Chiu W, Burnett R M, Garcea R L, editors. New York: Oxford Univ. Press; 1997. pp. 80–104. [Google Scholar]
- 33.Sattentau Q J, Moore J P. J Exp Med. 1991;174:407–415. doi: 10.1084/jem.174.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson K B, Nexo B A. Virology. 1983;125:85–98. doi: 10.1016/0042-6822(83)90065-x. [DOI] [PubMed] [Google Scholar]
- 35.Ragheb J A, Anderson W F. J Virol. 1994;68:3207–3219. doi: 10.1128/jvi.68.5.3207-3219.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao Y, Zhu L, Benedict C A, Chen D, Anderson W F, Cannon P M. J Virol. 1998;72:5392–5398. doi: 10.1128/jvi.72.7.5392-5398.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doms R W, Lamb R A, Rose J K, Helenius A. Virology. 1993;193:545–562. doi: 10.1006/viro.1993.1164. [DOI] [PubMed] [Google Scholar]
- 38.Fass D, Stephen S C, Kim P S. Nat Struct Biol. 1996;3:465–469. doi: 10.1038/nsb0596-465. [DOI] [PubMed] [Google Scholar]
- 39.Rein A, Yang C, Haynes J A, Mirro J, Compans R W. J Virol. 1998;72:3432–3435. doi: 10.1128/jvi.72.4.3432-3435.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fass D, Davey R A, Hamson C A, Kim P S, Cunningham J M, Berger J M. Science. 1997;277:1662–1666. doi: 10.1126/science.277.5332.1662. [DOI] [PubMed] [Google Scholar]
- 41.Miller A D, Rosman G J. Biotechnology. 1989;7:980–990. [PMC free article] [PubMed] [Google Scholar]
- 42.Benedict C A, Tun R Y M, Rubinstein D B, Guillaume T, Cannon P M, Anderson W F. Hum Gene Ther. 1999;10:545–558. doi: 10.1089/10430349950018625. [DOI] [PubMed] [Google Scholar]