Abstract
The ability of Neisseria meningitidis (MC) to interact with cellular barriers is essential to its pathogenesis. With epithelial cells, this process has been modeled in two steps. The initial stage of localized adherence is mediated by bacterial pili. After this phase, MC disperse and lose piliation, thus leading to a diffuse adherence. At this stage, microvilli have disappeared, and MC interact intimately with cells and are, in places, located on pedestals of actin, thus realizing attaching and effacing (AE) lesions. The bacterial attributes responsible for these latter phenotypes remain unidentified. Considering that bacteria are nonpiliated at this stage, pili cannot be directly responsible for this effect. However, the initial phase of pilus-mediated localized adherence is required for the occurrence of diffuse adherence, loss of microvilli, and intimate attachment, because nonpiliated bacteria are not capable of such a cellular interaction. In this work, we engineered a mutation in the cytoplasmic nucleotide-binding protein PilT and showed that this mutation increased piliation and abolished the dispersal phase of bacterial clumps as well as the loss of piliation. Furthermore, no intimate attachment nor AE lesions were observed. On the other hand, PilT− MC remained adherent as piliated clumps at all times. Taken together these data demonstrate that the induction of diffuse adherence, intimate attachment, and AE lesions after pilus-mediated adhesion requires the cytoplasmic PilT protein.
Neisseria meningitidis (MC) is a pathogen responsible for septicemia and meningitis. To reach the meninges, MC must interact with two cellular barriers, one in the nasopharynx and one in the brain, the blood–brain barrier. A multistep model has been proposed for MC interaction with cells (1). The first step corresponds to pilus-mediated adhesion. Pili emanate from the bacterial surface and are assembled from protein subunits called pilin. They initiate adherence by serving as a long-range adhesin and recruit other bacteria into a growing microcolony. This pilus-mediated adhesion phase leads to an initial phase of localized adherence. After this first step MC disperse from these microcolonies and spread at the apical surface of the cells to form a single monolayer of bacteria covering the surface. At this stage of diffuse adherence, MC have lost their pili and are involved in an intimate attachment with disappearance of microvilli and formation of pedestals with actin polymerization, thus realizing attaching and effacing (AE) lesions resembling those observed with enteropathogenic Escherichia coli (2, 3). The loss of piliation is not caused by phase variation because bacteria recovered as cell-associated colony-forming units (cfu) are piliated. The bacterial attributes responsible for the diffuse adherence phenotype, including intimate attachment and AE lesions, have not been identified yet; however, the loss of piliation suggests that pili per se are not involved in this step. Furthermore, an inoculum of nonpiliated bacteria is not capable of diffuse adherence, intimate attachment, and AE lesions (1, 4), thus demonstrating that the expression of the attributes responsible for these phenotypes requires the initial step of pilus-mediated adhesion.
Meningococcal pili are type IV. Such fimbriae are expressed by many pathogenic microorganisms including Pseudomonas aeruginosa (5). MC pili are composed mostly of pilin encoded at the pilE locus. Pilin is a single, repeated protein subunit of 145–160 aa. Two 110-kDa proteins, PilC1 and PilC2, are essential for piliation, and PilC1−/PilC2− isolates are nonpiliated (4, 6, 7). PilC1 plays an additional role in adhesion, because only PilC1+ bacteria are adhesive regardless of the expression of PilC2 (4, 8), and the current model considers PilC1 as a tip-located adhesin (9). In pathogenic Neisseria, expression of pili is associated not only with the ability of bacteria to colonize human host, but also with competence for transformation and with a phenomenon termed “twitching motility,” which is manifested as intermittent darting-translocation of cells in liquid culture medium (10, 11). Bradley (12) described mutants of P. aeruginosa that were hyperpiliated, resistant to pilus-specific phages, and failed to display twitching motility. On the basis of electron microscopic studies, these mutants were believed to be deficient in pilus retraction. The gene responsible for this activity was characterized and designated pilT (13). In pathogenic Neisseria, a gene showing high sequence homologies with P. aeruginosa pilT was identified (14). The corresponding PilT protein was located in the bacterial cytoplasm and carried a putative nucleotide-binding domain. A mutation of this gene in N. gonorrhoeae is responsible for a defect in competence for natural transformation and twitching motility (15). Recently, Bieber et al. (16) constructed a mutation in bfpF, a pilT homologue of the bundle-forming pili (BFP) operon of enteropathogenic E. coli. These authors showed that this bfpF mutant was 200-fold less virulent than the wild-type strain. The phenotypes that correlate in vitro with this virulence defect were an increased piliation and a lack of twitching motility. Both of these are responsible for enhanced localized adherence and abolition of bacterial dispersal from initial aggregates (16, 17).
In this work, we address the role of the PilT protein in the interaction of N. meningitidis with epithelial cells and demonstrate that, in addition to playing an essential role in the dispersal of bacteria and in the loss of piliation subsequent to cell contact, it is essential in the occurrence of the diffuse adherence phenotype, including intimate attachment and AE lesions. Taken together, these data demonstrate that adhesion of PilT− bacteria is, at all times, pilus-mediated and subsequently that induction of intimate attachment and AE lesions of wild-type bacteria after pilus-mediated adhesion requires the cytoplasmic PilT protein.
MATERIALS AND METHODS
Bacterial Strains, Growth Conditions, Oligonucleotides, and Antibodies.
8013 is a serogroup C class I strain. Clone12 is a spontaneously occurring, piliated, encapsulated Opa−/Opc− derivative of 8013 expressing a highly adhesive SB pilin variant (18). The nonpiliated (P−) derivative clone12pilE∷Km has been described previously (18). MC were grown and transformed using previously described standard techniques (19). Erythromycin was used at a concentration of 2 mg/liter for the selection of MC and 300 mg/liter for the selection of Escherichia coli strain MC1061.
Oligonucleotides PilTM2-XbaI, PilTM3-H3, ErmAM1-Xba, ErmAM3-BamHI, ErmAM1-AvaI, ErmAM3-EcoRI, Fg31-Bam, and Fg32-Kpn were as follows: 5′-CTAGTCTAGATCAGAAACTCATACTTTCGCT-3′, 5′-CCCAAGCTTGGGATGCAGATTACCGACTTACTC-3′, 5′-CTAGTCTAGAGCAAACTTAAGAGTGTGTTGATAG-3′, 5′-CGCGGATCCAAGCTTGCCGTCTGAAGTGGACCTC-3′, 5′-TCCCCCGGGGCAAACTTAAGAGTGTGTTGATAG-3′, 5′-CCGGAATTCCAAGCTTGCCGTCTGAATGGGACCTCTTT-3′, 5′-CGCGGATCCTGACACACAACCGCCTTCCGGCCA-3′, and 5′-CGGGGTACCGAAGGGTATCCGGGCGGGATGC-3′, respectively.
Standard molecular biology techniques were performed according to Sambrook et al. (20).
The 20D9 mAb recognizes specifically the SB pilin variant produced by clone12 (1), and the 13C5 mAb is directed against the neisserial PilT protein (14).
Construction of the MC PilT Mutant.
The pilT gene of clone12 was amplified by PCR by using PilTM3-H3 and PilTM2-XbaI primers and cloned between the HindIII and XbaI sites of pTZ19. The gene was sequenced (accession no. AF074716), and the peptidic sequence was found to be 99% identical to that of the previously reported gene (14). The erythromycin-resistance cassette was amplified by using ErmAM1-AvaI and ErmAM3-EcoRI primers and cloned between the AvaI and EcoRI sites of the pilT gene, thus performing a deletion of the 5′ region of pilT. This construct was transformed into clone12. One ErmR transformant was selected, the replacement of the wild type by the mutated allele was confirmed by Southern blot analysis, and the resulting strain was designated clone12pilT−.
To ensure that the consequences of the mutation were not due to a polar effect, a pilT allele was constructed in which the erm gene was placed just after the stop codon of pilT in the same orientation as that of the deleted pilT gene. This allele, designated pilT+erm, was obtained by amplifying erm between ErmAM1-XbaI and ErmAM3-BamHI primers and cloning this gene between the XbaI and BamHI sites of the above-described pTZ19 plasmid containing the pilT gene. A 120-bp fragment, amplified using Fg31-BamHI and Fg32-KpnI primers and corresponding to a 120-bp sequence located downstream of pilT in Neisseria gonorrhoeae chromosome, was cloned between the BamHI and KpnI sites of the pilT+erm-containing plasmid. This construct then was reintroduced into clone12 by transformation. One transformant was selected for further study and controlled by Southern blotting. This strain was designated clone12pilT+.
All transformants produced the SB pilin variant and were Opa−/Opc−, as the parental strain.
Cell Culture and Infection of Monolayers.
T84 cells were cultured at 37°C under 5% CO2 in DMEM Nut mix F-12 containing Glutamax I (GIBCO/BRL), and supplemented with 10% heat-inactivated FBS and 10 mM Hepes.
For quantitative adherence assays, epithelial cells were seeded in a 24-well culture plate at a density of 5 × 105 cells per well. Two days later, the medium was replaced by RPMI 1640 with Glutamax I (GIBCO/BRL) supplemented with 10% heat-inactivated FBS, and 106 bacteria suspended in this infection medium were added to each well. In some experiments, the monolayer was fixed with 2.5% paraformaldehyde in PBS before infection. The infected plate was incubated for 4 or 9 h at 37°C under 5% CO2. Cells then were washed vigorously three times with PBS to remove nonadherent bacteria. The number of cell-associated cfu was determined after lifting off the monolayer by scraping and vortexing the cells to dissociate bacteria. The percentage of adhesion was calculated as 100 × cell-associated cfu/(cell-associated cfu + cfu present in the supernatant).
The gentamicin protection assay was performed as described (1). Cells grown in a 24-well culture plate were infected as above. After 9 h of infection, cells were washed with PBS, and 1 ml of infection medium containing 150 mg/liter of gentamicin was added to each well to kill all extracellular meningococci. After incubation at 37°C for 60 min, cells were washed twice and lysed by incubation for 15 min in 1 ml of 1% saponin in PBS. The number of intracellular bacteria then was determined by plating dilutions onto gonococcal medium base (GCB).
Confocal Immunofluorescence Microscopy.
Cells were seeded on glass coverslips at a density of 5 × 105 cells/cm2 and used 2 days later. Monolayers were infected, washed three times with PBS, and fixed for 30 min in 2.5% paraformaldehyde in PBS supplemented with METM (5 mM morpholineethanesulfonic acid/0.3 mM EGTA/0.5 mM MgCl2/0.05% Triton X-100, pH 7.4) at room temperature. After neutralization with 0.1 M glycine in PBS for 5 min, cells were permeabilized with 0.5% Triton X-100 in PBS for 1 min and washed once with PBS. Samples then were saturated with 0.2% gelatin in PBS for 5 min. Cells and bacteria were stained either with ethidium bromide at a final concentration of 1.5 mg/liter for 30 min or with tetramethylrhodamine B isothiocyanate-labeled lectin from Triticum vulgaris (Sigma) at a final concentration of 2 mg/liter. Pili staining was performed as already described by incubating samples with the 20D9 mAb (1). Samples were observed by using a Leica TCS4D confocal laser-scanning microscope; optical sections usually were taken at 0.4-μm intervals.
Electron Microscopy.
Transmission electron microscopy was performed with polarized T84 cells grown on transwell filter units as described (1). Monolayers were apically infected with 5 × 104 MC for 9 h and, after several washes with PBS, were fixed in a 1:1 mixture of 2.5% paraformaldehyde and 2.5% glutaraldehyde in cacodylate sucrose buffer (0.1 M sodium cacodylate/0.1 M sucrose/5 mM CaCl2/5 mM MgCl2, pH 7.2) at 4°C overnight. Monolayers then were postfixed in 1% OsO4 for 1 h and in 1% uranyl acetate for 1 h. After dehydration with a graded series of alcohols, samples were embedded in Epon and thin-sectioned. The sections were stained with uranyl acetate and lead citrate and observed by using a JEOL electron microscope.
For scanning electron microscopy (SEM), T84 infected cells were grown on coverslips and processed as described (1). Images shown throughout this work correspond to fields that are representative of the overall observation.
RESULTS
PilT Is Required for Bacterial Dispersal onto the Monolayer and Effacement of Microvilli on the Apical Surface of T84 Cells.
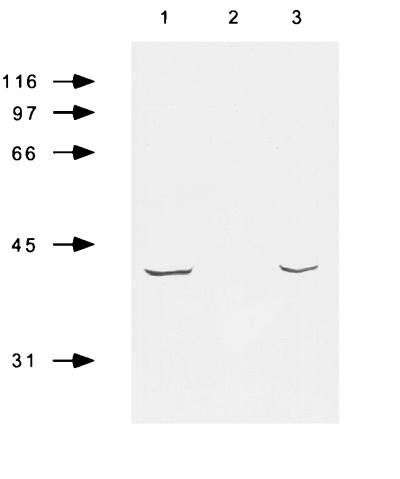
To address the role of PilT in meningococcal cell interaction, a pilT mutant was engineered in clone12, an Opa−/P+ isolate of strain 8013 that expresses the highly adhesive SB pilin variant. The PilT− strain, designated clone12pilT−, was constructed by insertion–deletion of an erm-resistance gene into the 5′ region of the ORF (see Materials and Methods). To rule out the possibility that phenotypic changes observed with this strain may be caused by a polar effect, we engineered a strain carrying the same antibiotic-resistance gene located just after the stop codon of the pilT ORF and inserted in the same orientation. This strain was designated clone12pilT+. Using total protein extract and the anti-PilT specific mAb, we confirmed that this latter strain produced PilT, whereas clone12pilT− was phenotypically PilT− (Fig. 1). Experiments performed in this work used clone12pilT+ as positive control unless otherwise stated. As expected, clone12pilT− was not competent for DNA transformation nor capable of twitching motility in liquid medium (data not shown). However, this strain was highly piliated as judged by electron microscopy and by immunofluorescence staining using the 20D9 monoclonal anti-SB pilin variant mAb (data not shown).
Figure 1.
Western blot of total bacterial extracts showing electrophoretic migration of clone12 (1), clone12pilT− (2), and clone12pilT+ (3). Total bacterial extracts were run in SDS/PAGE, 12% acrylamide, and 3 M urea and transferred to nitrocellulose. The 13C5 mAb (generous gift of L. Brossay), which is directed against the PilT protein of N. gonorrhoeae, was used for PilT detection.
In a first set of experiments, adhesiveness and invasiveness of clone12pilT− strain were compared with that of the PilT+ strain (Table 1). As expected, nonpiliated derivatives of clone12 were nonadhesive at all time points. With the piliated strains, after 4 h of infection, which corresponds to the initial phase of pilus-mediated localized adherence, the numbers of PilT+ and PilT− bacteria interacting with T84 cells were identical. On the other hand, after 9 h of infection, when wild-type bacteria interact intimately with the monolayer, adhesion and invasion of clone12pilT− were slightly higher than that of the PilT-expressing isolate. The percentage of adhesion was 29.0 ± 7.7 and 13.6 ± 0.5 for PilT− and PilT+ strains, respectively. This difference in adhesiveness between wild-type and PilT− bacteria was more dramatic when adhesion was performed on paraformaldehyde-fixed cells (41.1 ± 0.5 and 3.1 ± 0.4 for PilT− and PilT+ strains, respectively). In this case, adhesion of the wild-type strain decreased by one order of magnitude, which is in agreement with the previously suggested hypothesis that the occurrence of the intimate adhesion may result from a crosstalk between the bacteria and the cell (1). On the other hand, adhesiveness of the PilT− bacteria remained close to that observed on standard viable cells. This difference in adhesiveness onto fixed cells between PilT− and PilT+ bacteria suggests that the mechanisms by which the PilT− strain interact with cells are different from that of PilT+-expressing bacteria.
Table 1.
Adhesion and invasion of PilT+ and PilT− MC onto a monolayer of T84 epithelial cells
| Phenotype | Adhesion at 4 h | Adhesion at 9 h
|
Invasion at 9 h* | |
|---|---|---|---|---|
| Viable cells | Viable cells | Fixed cells | ||
| PilT+ | 28.1 ± 1.3 | 13.6 ± 0.5 | 3.1 ± 0.4 | 3.4 ± 0.5 |
| PilT− | 33.4 ± 7.2 | 29.0 ± 7.7 | 41.1 ± 0.5 | 3.8 ± 0.6 |
| PilE−† | 0.001 ± 0.0 | 0.003 ± 0.001 | ND | ND |
Adhesion is calculated as the ratio of 100 × cell-associated cfu to total cfu. Each value is the mean of three distinct experiments ±SD. ND, not done.
Reported as the log of cell-associated cfu after 60 min of incubation in a medium containing 150 mg/liter of gentamicin. Each value is the mean of at least three different experiments ±SD.
The nonpiliated strain is clone 12pilE∷Km as described (18).
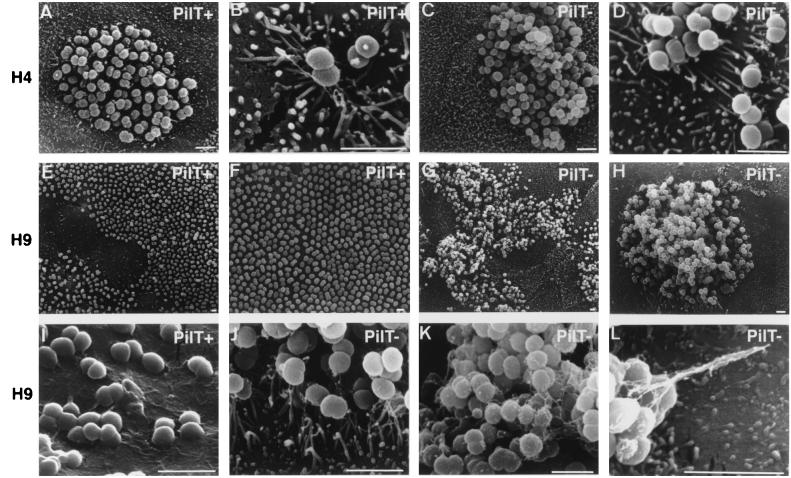
To address the above hypothesis that the mechanisms of interaction of PilT− and PilT+ MC with T84 epithelial cells are different, SEM was performed (Fig. 2). Monolayers were infected as described in Materials and Methods, and then bacteria present in the supernatant were removed every hour to minimize reinfection. After 4 h of infection, both strains exhibit a localized adherence phenotype with clumps of bacteria forming microcolonies on the apical surface of the monolayer (Fig. 2 A and C). Microvilli surround these clumps and have a tendency to form extensions from the epithelial cell surface toward the bacterial cells, thus illustrating local changes in the host cell cytoskeleton (Fig. 2 B and D). Striking differences between these two strains were observed at later time points. In monolayers infected with PilT+-expressing bacteria, clumps are replaced by a monolayer of bacteria covering the apical surface of the cells, thus realizing a diffuse adherence (Fig. 2 E and F). Microvilli have disappeared (Fig. 2I). In monolayers infected by the PilT− derivative, MC do not disperse and remain as localized clumps that are only slightly larger than those observed at 4 h (Fig. 2 G and H). High-resolution scanning electron micrographs clearly show that these MC produce interbacterial filaments (Fig. 2K). In addition, these clumps are still surrounded by microvilli that form extensions similar to those found at the 4-h time point (Fig. 2J). In some cases, pili clearly can be seen binding the microcolonies to the cells (Fig. 2L). Taken together, these data confirm that the interaction of PilT− bacteria with cells is different from that of PilT+ bacteria and, more specifically, suggest that PilT− MC remain, at all times, engaged in a localized adherence, being unable of forming AE lesions and of diffuse adherence.
Figure 2.
SEM examination of a T84 monolayer infected for 4 h (A–D) or 9 h (E–L) by PilT+ (A, B, E, F, and I) or PilT− bacteria (C, D, G, H, J, K, and L). Initially, both strains have a similar pattern of adhesion (A–D); however, after a longer incubation period, PilT+ spread onto the monolayer (E and F) and adhere very intimately to cells (I). In contrast, PilT− bacteria remain as large, piliated clumps sitting on the cells (G, H, J, K, and L) (Bars = 2 μm.)
Reduction of MC Piliation Requires the PilT Protein.
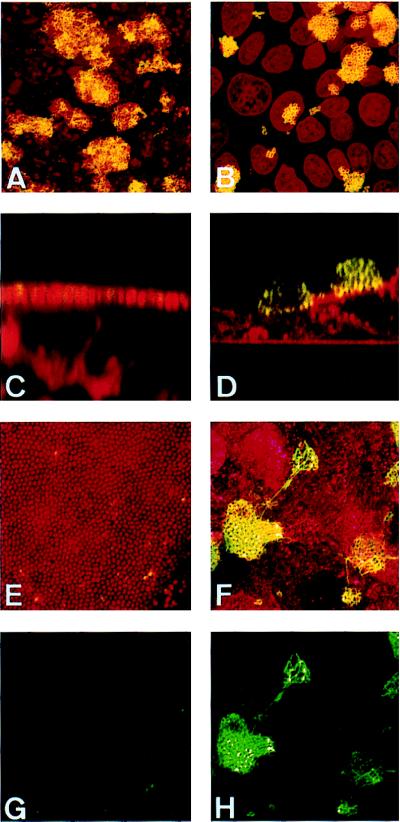
The above data imply that PilT− MC are piliated at all times during MC–cell interaction. To address this issue, we performed immunofluorescence staining of infected monolayers with the 20D9 mAb, which specifically stains SB pilin expressed by clone12. Pilus staining was performed on monolayers grown on coverslips infected for 4 and 9 h either by clone12pilT+ or clone12pilT− (Fig. 3). As above, monolayers were washed every hour to avoid reinfection from bacteria growing in the supernatant. After 4 h of infection, both strains were piliated (Fig. 3 A and B). After 9 h of infection, clone12pilT+, which had spread onto the apical surface of T84 cells, had a dramatically reduced piliation, as shown by the lack of 20D9 labeling (Fig. 3 C, E, and G). This is in agreement with the scanning electron micrographs shown above. On the other hand, adhesive clumps of clone12pilT− remained heavily stained with the 20D9 mAb (Fig. 3 D, F, and H), and longer incubations of PilT− MC with cells did not result in the disappearance of piliation (data not shown). These data clearly demonstrate that expression of PilT is required for the loss of piliation consequent to MC–cell contact.
Figure 3.
Confocal immunofluorescence micrographs of T84 cells grown on coverslips infected for 4 (A and B) or 9 (C–H) h by clone12pilT+ (A, C, E, and G) or by clone12pilT− (B, D, F, and H). MC and eukaryotic cells were stained with ethidium bromide (A, B, C, E, and G) or with tetramethylrhodamine B isothiocyanate-labeled lectin from Triticum vulgaris (D, F, and H) (shown in red). Pili were labeled using the 20D9 mAb (shown in green). A–F corresponds to the superimposition of both stainings. G and H display only pili staining of adherent MC. A, B, E, F, G, and H are images reconstructed from confocal xy sections of infected monolayers, and C and D are from xz sections.
The PilT Protein Is Required to Induce MC Intimate Attachment and Pedestals Formation onto T84 Cells.
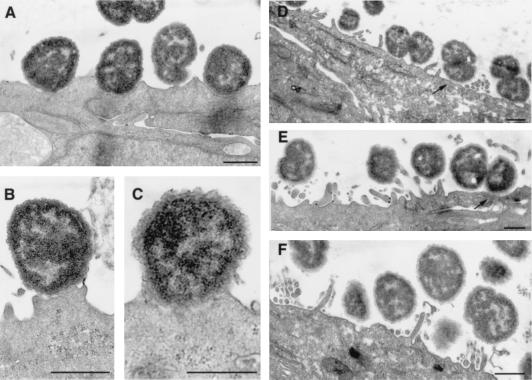
Because the PilT− strain was unable to form AE lesions, one would not expect this strain to be able to adhere intimately to cells and to form pedestals. To test this hypothesis, we performed transmission electron microscopy on a T84 monolayer grown on transwell and infected for 9 h by PilT+ or PilT− MC. As expected, the PilT+ strain adhered to the cells very intimately (Fig. 4 A–C), microvilli had disappeared, and pedestal formations were observed with condensation below the bacteria that correspond to actin polymerization, as shown previously (1). Adhesion of PilT− MC compared with that of PilT+ MC was strikingly different (Fig. 4 D–F). No intimate attachment was observed, even though MC were, in some places, in close contact with the cytoplasmic membrane (Fig. 4 D and E). No membrane deformation at the apical cell surface was visible, and effacement of microvilli did not occur. On the other hand, abundant microvilli were seen surrounding the bacteria; these correspond to the microvillus extensions shown in the SEM (Fig. 2J).
Figure 4.
Transmission electron micrographs of T84 cells grown on transwells, infected by PilT+ (A– C) or PilT− bacteria (D– F) during 9 h. PilT− bacteria adhere to the epithelial cells without ever inducing intimate attachment even though they were in close contact with the cytoplasmic membrane of T84 cells (arrows). Note the presence of numerous microvilli at the surface of epithelial cells (D– F) (Bars = 0.5 μm.)
These data demonstrate that PilT− bacteria are never engaged in an intimate attachment with T84 epithelial cells and do not form AE lesions. On the other hand, these bacteria remain as clumps of piliated bacteria that do not disperse. Microvillus extensions, similar to those present during the early time point with the PilT+ strain, are still present after 9 h of infection with the PilT− MC. Taken together these data show that PilT− bacteria are capable of only localized adherence and that, subsequently, the loss of piliation and the occurrence of intimate attachment and AE lesions require the cytoplasmic PilT protein.
DISCUSSION
The data reported in this work confirm that MC interaction with T84 cells is a two-step process and, in addition, demonstrate that the progression from localized to diffuse adherence requires the product of the pilT gene. Each of these steps probably requires the interaction of different bacterial attributes with specific cellular components. As mentioned, pili play a major role during the first step. It, therefore, is tempting to speculate that a direct interaction between the PilC1 adhesin and its receptor may be responsible for the signaling event that leads to cytoskeletal modifications and, subsequently, microvillus extensions; however, the role of other bacterial components in this signaling cannot be excluded. The bacterial attributes involved in the diffuse adherence step and responsible for the intimate adhesion remain unknown. The existence of AE lesions suggest that a cell-signaling pathway, involving bacterial components different from those responsible for the first step, takes place during this stage. The role of piliation per se indeed is excluded considering that bacteria are not piliated at this stage. In addition, because the derivative used in this work is Opa−, an interaction involving opacity proteins is unlikely to be responsible for this intimate attachment.
The main consequence of the lack of PilT protein is that MC–cell interaction remains at all times pilus-mediated in a localized adherence phenotype with microvillus extensions surrounding the clumps. This demonstrates that PilT is required to allow the switch from localized to diffuse adherence. The mechanism by which this occurs is unknown. One of the major consequences of the pilT mutation is the lack of twitching motility (13, 15, 21). By analogy with P. aeruginosa, twitching motility is believed to be a consequence of pilus retraction. The absence of pilus retraction and subsequent twitching motility in the PilT− strain may readily explain the lack of spreading on the apical surface of the cells as well as the retention of piliation.
Pilus retraction cannot explain the absence of AE lesions observed with the PilT− strain. Indeed, transmission electron microscopy clearly shows that PilT− bacteria are capable of direct contact with the eukaryotic cell (Fig. 4 D and E). This rules out the possibility that the lack of intimate attachment and AE lesions is a consequence of an absence of retraction that prevents physical interactions between an outer membrane component and a eukaryotic cell receptor. It is therefore likely that this effect of PilT results from another role. A possible function for PilT is to transduce a signal in a pathway in which pili would be sensors. Hence, after interaction of pili with its receptor, the PilT molecule may up-regulate a yet unidentified component responsible for the occurrence of intimate attachment and AE lesions. The same signaling event could participate as well in the reduction of piliation. Another possible function of PilT in MC is that it may not be pilus-limited but, instead, includes related functions via its putative ATPase activity responsible for the intimate attachment.
An alternative hypothesis regarding the role of PilT is suggested by a high degree of homology to the DotB protein of Legionella pneumophila (40% identity) (data not shown) (22–24). This latter component is part of a type IV secretion system that is believed to be responsible for the injection of effector molecules inside the cytosol of infected cells. It may be speculated that PilT is part of a similar transport system in N. meningitidis. That non-pilin-producing strains do not display intimate attachment even when centrifuged onto a monolayer suggests that the components of the pilus machinery may even be part of this transport system. Hence, after recognition of the pilus receptor and pilus retraction, the component of the pilus machinery may allow injection of effectors directly into the eukaryotic cells.
Acknowledgments
We thank L. Brossay for the anti PilT mAb, C. Tinsley for his careful reading of the manuscript, and J.-L. Béretti and Mrs. Grassé (Centre Interuniversitaire de Microscopie Electronique) for invaluable technical help. C.P. was the recipient of a scholarship from Fondation pour la Recherche Médicale. This work was supported by grants from Institut National de la Santé et de la Recherche Médicale, Université Paris V René Descartes, and Fondation pour la Recherche Médicale.
ABBREVIATIONS
- MC
Neisseria meningitidis
- AE lesions
attaching and effacing lesions
- cfu
colony-forming units
- SEM
scanning electron microscopy
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF074716).
References
- 1.Pujol C, Eugène E, de Saint Martin L, Nassif X. Infect Immun. 1997;65:4836–4842. doi: 10.1128/iai.65.11.4836-4842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moon H W, Whipp S C, Argenzio R A, Levine M M, Gianella R A. Infect Immun. 1983;41:1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Girón J A, Suk Yue Ho A, Schoolnik G K. Science. 1991;254:710–713. doi: 10.1126/science.1683004. [DOI] [PubMed] [Google Scholar]
- 4.Nassif X, Beretti J-L, Lowy J, Stenberg P, O’Gaora P, Pfeiffer J, Normark S, So M. Proc Natl Acad Sci USA. 1994;91:3769–3773. doi: 10.1073/pnas.91.9.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strom M S, Lory S. J Bacteriol. 1986;165:367–372. doi: 10.1128/jb.165.2.367-372.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonsson A-B, Nyberg G, Normark S. EMBO J. 1991;10:477–488. doi: 10.1002/j.1460-2075.1991.tb07970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudel T, Boxberger H-J, Meyer T F. Mol Microbiol. 1995;17:1057–1071. doi: 10.1111/j.1365-2958.1995.mmi_17061057.x. [DOI] [PubMed] [Google Scholar]
- 8.Rahman M, Kallstrom H, Normark S, Jonsson A-B. Mol Microbiol. 1997;25:11–25. doi: 10.1046/j.1365-2958.1997.4601823.x. [DOI] [PubMed] [Google Scholar]
- 9.Rudel T, Scheuerpflug I, Meyer T F. Nature (London) 1995;373:357–362. doi: 10.1038/373357a0. [DOI] [PubMed] [Google Scholar]
- 10.Seifert H S, Ajioka R S, Paruchuri D, Heffron F, So M. J Bacteriol. 1990;172:40–46. doi: 10.1128/jb.172.1.40-46.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henrichsen J. Acta Pathol Microbiol Scand Sect B. 1975;83:171–178. doi: 10.1111/j.1699-0463.1975.tb00089.x. [DOI] [PubMed] [Google Scholar]
- 12.Bradley D. Can J Microbiol. 1980;26:146–154. doi: 10.1139/m80-022. [DOI] [PubMed] [Google Scholar]
- 13.Whitchurch C, Hobbs M, Livingston S, Krishnapillai V, Mattick J. Gene. 1991;101:33–44. doi: 10.1016/0378-1119(91)90221-v. [DOI] [PubMed] [Google Scholar]
- 14.Brossay L, Paradis G, Fox R, Koomey M, Hébert J. Infect Immun. 1994;62:2302–2308. doi: 10.1128/iai.62.6.2302-2308.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolfgang M, Lauer P, Park H-S, Brossay L, Hébert J, Koomey M. Mol Microbiol. 1998;29:321–330. doi: 10.1046/j.1365-2958.1998.00935.x. [DOI] [PubMed] [Google Scholar]
- 16.Bieber D, Ramer S W, Wu C-Y, Murray W J, Tobe T, Fernandez R, Schoolnik G K. Science. 1998;280:2114–2118. doi: 10.1126/science.280.5372.2114. [DOI] [PubMed] [Google Scholar]
- 17.Anantha R P, Stone K D, Donnenberg M S. Infect Immun. 1998;66:122–131. doi: 10.1128/iai.66.1.122-131.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nassif X, Lowy J, Stenberg P, O’Gaora P, Ganji A, So M. Mol Microbiol. 1993;8:719–725. doi: 10.1111/j.1365-2958.1993.tb01615.x. [DOI] [PubMed] [Google Scholar]
- 19.Nassif X, Puaoi D, So M. J Bacteriol. 1991;173:2147–2154. doi: 10.1128/jb.173.7.2147-2154.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 21.Wolfgang M, Park H-S, Hayes S F, Van Putten J P M, Koomey M. Proc Natl Acad Sci USA. 1998;95:14973–14978. doi: 10.1073/pnas.95.25.14973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirby J E, Vogel J P, Andrews H L, Isberg R R. Mol Microbiol. 1998;27:323–336. doi: 10.1046/j.1365-2958.1998.00680.x. [DOI] [PubMed] [Google Scholar]
- 23.Segal G, Shuman H A. Trends Microbiol. 1998;6:253–258. doi: 10.1016/s0966-842x(98)01308-0. [DOI] [PubMed] [Google Scholar]
- 24.Vogel J V, Andrews H L, Wong S K, Isberg R R. Science. 1998;279:873–876. doi: 10.1126/science.279.5352.873. [DOI] [PubMed] [Google Scholar]