Full text
PDF

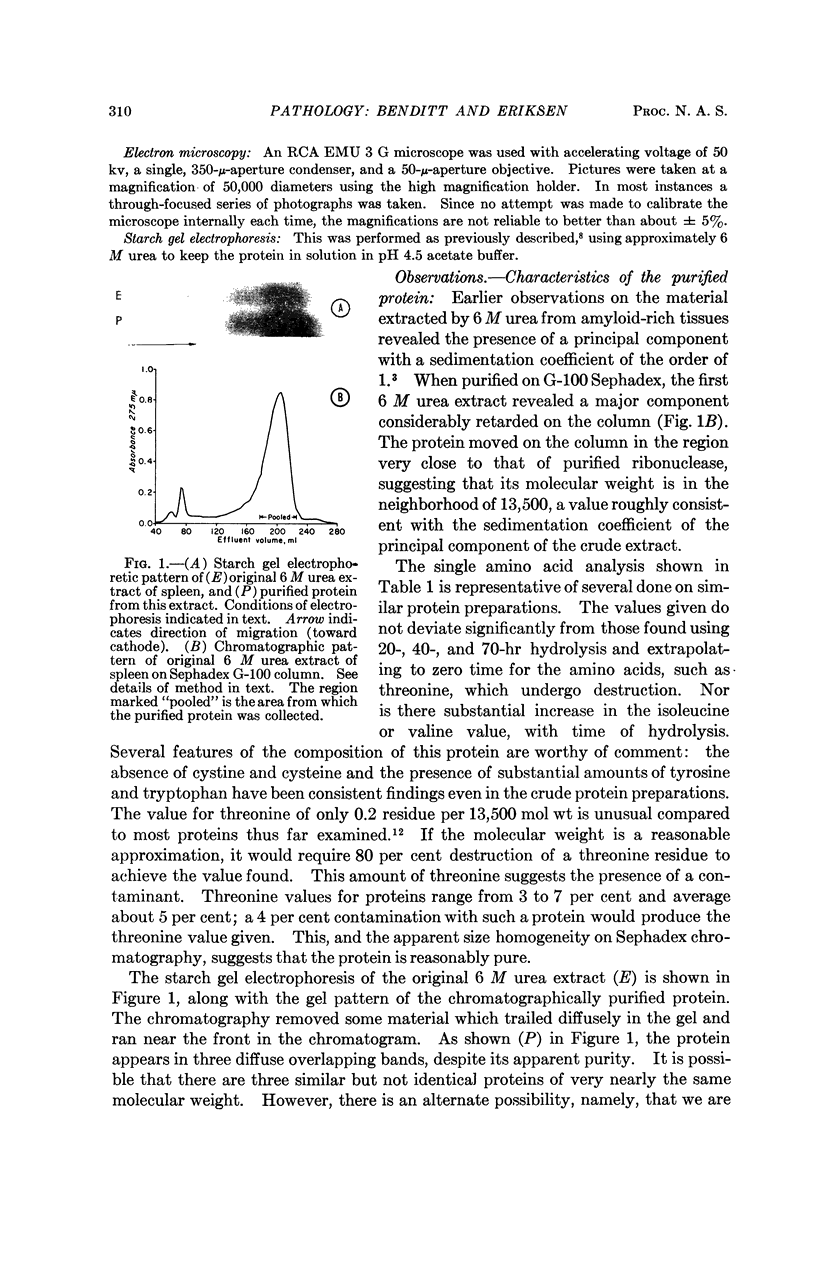






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENDITT E. P., ERIKSEN N. AMYLOID. II. STARCH GEL ELECTROPHORETIC ANALYSIS OF SOME PROTEINS EXTRACTED FROM AMYLOID. Arch Pathol. 1964 Oct;78:325–330. [PubMed] [Google Scholar]
- BENDITT E. P., LAGUNOFF D., ERIKSEN N., ISERI O. A. Amyloid. Extraction and preliminary characterization of some proteins. Arch Pathol. 1962 Oct;74:323–330. [PubMed] [Google Scholar]
- CASPAR D. L. ASSEMBLY AND STABILITY OF THE TOBACCO MOSAIC VIRUS PARTICLE. Adv Protein Chem. 1963;18:37–121. doi: 10.1016/s0065-3233(08)60268-5. [DOI] [PubMed] [Google Scholar]
- COHEN A. S., CALKINS E. Electron microscopic observations on a fibrous component in amyloid of diverse origins. Nature. 1959 Apr 25;183(4669):1202–1203. doi: 10.1038/1831202a0. [DOI] [PubMed] [Google Scholar]
- GUEFT B., GHIDONI J. J. THE SITE OF FORMATION AND ULTRASTRUCTURE OF AMYLOID. Am J Pathol. 1963 Nov;43:837–854. [PMC free article] [PubMed] [Google Scholar]
- HELLER H., MISSMAHL H. P., SOHAR E., GAFNI J. AMYLOIDOSIS: ITS DIFFERENTIATION INTO PERIRETICULIN AND PERI-COLLAGEN TYPES. J Pathol Bacteriol. 1964 Jul;88:15–34. doi: 10.1002/path.1700880103. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLONIG G. A modified procedure for lead staining of thin sections. J Biophys Biochem Cytol. 1961 Dec;11:736–739. doi: 10.1083/jcb.11.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMEEKIN T. L., MARSHALL K. Specific volumes of proteins and the relationship to their amino acid contents. Science. 1952 Aug 8;116(3006):142–143. doi: 10.1126/science.116.3006.142. [DOI] [PubMed] [Google Scholar]
- REITHEL F. J. THE DISSOCIATION AND ASSOCIATION OF PROTEIN STRUCTURES. Adv Protein Chem. 1963;18:123–226. doi: 10.1016/s0065-3233(08)60269-7. [DOI] [PubMed] [Google Scholar]
- SPIRO D. The structural basis of proteinuria in man; electron microscopic studies of renal biopsy specimens from patients with lipid nephrosis, amyloidosis, and subacute and chronic glomerulonephritis. Am J Pathol. 1959 Jan-Feb;35(1):47–73. [PMC free article] [PubMed] [Google Scholar]
- TARGGART W. H., TRUMP B. F., LAGUNOFF D., ESCHBACH J. Systemic amyloidosis and ulcerative colitis. Gastroenterology. 1963 Mar;44:335–341. [PubMed] [Google Scholar]
- TRISTRAM G. R., SMITH R. H. THE AMINO ACID COMPOSITION OF SOME PURIFIED PROTEINS. Adv Protein Chem. 1963;18:227–318. doi: 10.1016/s0065-3233(08)60270-3. [DOI] [PubMed] [Google Scholar]







