Abstract
It is now accepted that hippocampal lesions impair episodic memory. However, the precise functional role of the hippocampus in episodic memory remains elusive. Recent functional imaging data implicate the hippocampus in processing novelty, a finding supported by human in vivo recordings and event-related potential studies. Here we measure hippocampal responses to novelty, using functional MRI (fMRI), during an item-learning paradigm generated from an artificial grammar system. During learning, two distinct types of novelty were periodically introduced: perceptual novelty, pertaining to the physical characteristics of stimuli (in this case visual characteristics), and exemplar novelty, reflecting semantic characteristics of stimuli (in this case grammatical status within a rule system). We demonstrate a left anterior hippocampal response to both types of novelty and adaptation of these responses with stimulus familiarity. By contrast to these novelty effects, we also show bilateral posterior hippocampal responses with increasing exemplar familiarity. These results suggest a functional dissociation within the hippocampus with respect to the relative familiarity of study items. Neural responses in anterior hippocampus index generic novelty, whereas posterior hippocampal responses index familiarity to stimuli that have behavioral relevance (i.e., only exemplar familiarity). These findings add to recent evidence for functional segregation within the human hippocampus during learning.
Neuropsychological evidence in human and nonhuman primates indicates that the hippocampus is crucial for episodic memory (1–3). The functional role of the hippocampus in episodic memory is a subject of controversy, although it is now suggested that hippocampal subregions in animals (4) and humans (5, 6) mediate distinct aspects of memory. Recent human functional neuroimaging studies have also addressed its role in memory (7–12). One suggestion is that the human hippocampus indexes novelty (7, 8), which at the simplest level refers to recency of prior occurrence. A human hippocampal response during novelty processing has also been demonstrated by human in vivo recordings (13) and event-related potential studies (14, 15). In this report, we characterize this putative hippocampal role in novelty processing by examining its response to two types of novelty: perceptual (non-behaviorally relevant) and exemplar (behaviorally relevant).
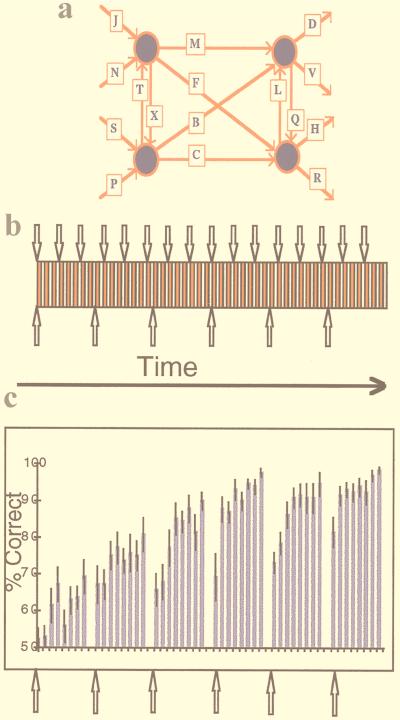
Within the context of a functional MRI (fMRI) experiment, exemplar and perceptual novelty were periodically introduced while subjects performed an item-learning paradigm derived from an artificial grammar system. An artificial grammar system embodies a set of arbitrary rules governing the concatenation of symbols. In standard applications, subjects exposed to exemplars of such a grammar system learn to categorize, as “grammatical” (that is, conforming to the hidden rules) or “ungrammatical,” subsequently presented symbol strings with an accuracy greater than chance (16, 17). It is widely assumed that this type of learning reflects the application of an implicit memory system, although this is itself controversial (18). For a review of the distinction between explicit and implicit memory and their relative contributions to artificial grammar learning, see ref. 18. In this experiment, the emphasis is on explicit learning, and consequently, we used a modified approach in which subjects were required to learn the grammatical status of consonant strings (the exemplars of the grammar system) that were presented repeatedly, with trial-by-trial feedback. Note that in standard applications, no feedback is provided. In brief, each consonant string was presented eight times, with each presentation requiring a grammaticality judgement for which explicit feedback was provided immediately. Thus, knowledge of the result of previous grammaticality judgements for a particular consonant string was used to enhance performance over subsequent presentations.
Exemplar novelty was introduced by periodically presenting novel consonant strings. Perceptual novelty was introduced by periodically changing the font in which exemplars were presented (see Fig. 1). Thus, a given set of exemplars was presented eight times, and the font was changed after every three presentations of an exemplar set. Critically, the manipulations of exemplar and perceptual novelty were orthogonal (i.e., uncorrelated), thus enabling separable characterization of hippocampal responses associated with each type of novelty. Note that the hippocampal region is used here to refer to the dentate gyrus, CA subfields, and subiculum.
Figure 1.
The introduction of perceptual and exemplar novelty in the context of item learning. (a) The artificial grammar system. Grammatical four-consonant letter strings are formed by starting on the left and moving right in the direction of the arrows. (b) Experimental design. Every exemplar-set presentation constitutes an activation epoch (red rectangle), each of which is followed by a control epoch (white rectangle). Every exemplar set is presented eight times, after which a new set of exemplars is presented, allowing the introduction of exemplar novelty (↑). Every three activation epochs the font in which exemplar strings are presented is changed, enabling the introduction of perceptual novelty (↓). (c) Behavioral data averaged for all subjects expressed as a percentage of correct grammaticality judgements (error bars here, and in all subsequent plots, depict ±1 SE). The data show improving grammaticality judgements as subjects become increasingly familiar with each set of exemplars. When a new exemplar set is presented, performance falls, but across the entire experiment subjects gradually acquire more abstract knowledge about the grammar system and use this knowledge to maintain performance after a change in exemplar set.
METHODS
Subjects.
Informed consent was obtained from 14 right-handed subjects (8 male, 6 female; age range 18–27 yr; mean age 21.7).
Psychological Task.
The finite-state grammar system in Fig. 1a was used to generate exemplar consonant strings under the constraint that all strings consisted of four letters. A total of 30 strings (e.g., JMQH), mixed with 30 arbitrarily chosen nongrammatical lures (e.g., JQMH), were presented, one every 3.2 s, over the course of the experiment. The strings were presented serially to subjects in sets of 10 (5 grammatical; 5 ungrammatical), and presentation of this exemplar set constituted an activation epoch. Subjects were required to make an immediate judgement of each item’s grammatical status by pressing the appropriate button. Visual feedback was given for each response. In the initial stages of the experiment, subjects had no knowledge of the underlying rules of the grammar and responded by guessing. Each set of exemplar strings was presented eight times and was followed by a sensorimotor control epoch. The control epoch consisted of serial visual presentations of either PPPP or NNNN (5 of each), which also required one of two predetermined button presses. Across the eight presentations of an exemplar set, subjects learned the status of individual items. After a particular set of exemplars was presented eight times, a novel set of 10 exemplar strings was presented, allowing the introduction of exemplar novelty (Fig. 1b). The eight presentations of an exemplar set constituted a block of the experiment; hence, the experiment consisted of six blocks defined by the onset of an exemplar change. The font in which exemplars were presented was changed every three exemplar set presentations (the fonts used were: Arial Narrow, Bodoni MT, Book Antiqua, Bookman Old Style, Century Gothic, Century Schoolbook, Chicago, Courier, Geneva, Helvetica, Monaco, Monotype Corsiva, New York, Palatino, Times, Times italics). The font change involved an entirely new font that could not be predicted by the subject.
fMRI Scanning.
A Siemens Vision system (Siemens, Erlangen, The Netherlands), operating at 2T, was used to acquire both T1 weighted anatomical images and gradient-echo echo-planar T2*-weighted MRI image volumes with blood oxygenation level-dependent (BOLD) contrast. For each subject, data were acquired in two scanning sessions separated by a 5-min rest period. A total of 480 volumes were acquired per subject plus 6 “dummy” volumes, which were subsequently discarded, to allow for T1 equilibration effects. Volumes were acquired continuously every 6,400 ms. Each volume comprised 64 3-mm axial slices, with an in-plane resolution of 3 × 3 mm, positioned to cover the whole brain. The imaging time series was realigned to correct for interscan movements, coregistered with the subjects’ structural MRI to enable overlay of functional data onto the subjects’ structural data and normalized into a space defined by the atlas of Talairach and Tournoux to allow group analysis. The data were then smoothed with a Gaussian kernel of 8 mm full-width half-maximum to account for residual intersubject differences (19). For each subject, low-frequency cosine functions were used to model and remove low-frequency drift in signal. The data were normalized for global effects by proportional scaling. It should be noted that for the analysis of hippocampal topography (Fig. 4), the data were smoothed with a Gaussian kernel of 6 mm full-width half-maximum to minimize correlation between selected regions.
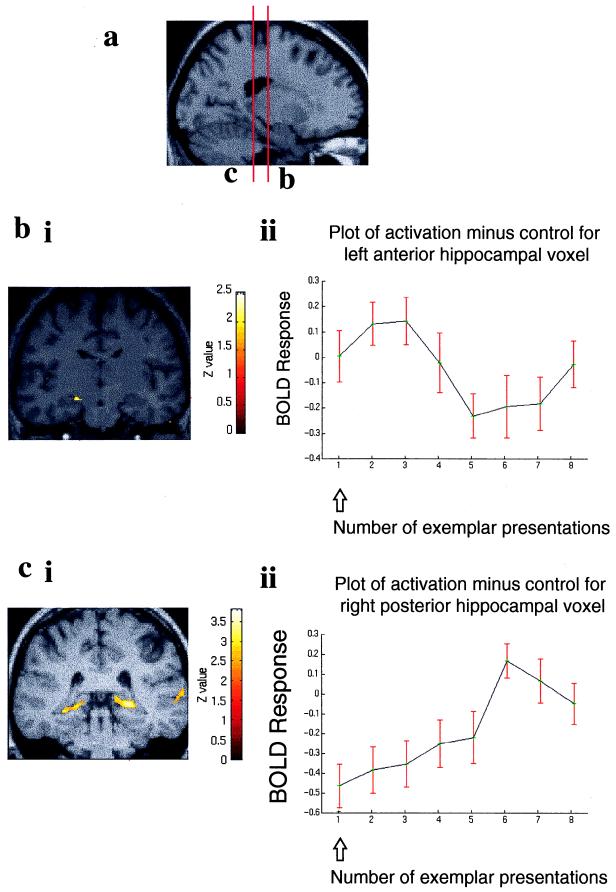
Figure 4.
Topography of hippocampal activation as a function of relative familiarity. Activation in the left hippocampus spreads posteriorly as meaningful items (exemplars) become increasingly familiar. Three voxels within the left hippocampus, each separated by 12 mm along the transverse plane, were identified by an SPM constructed to detect any change in activation relative to baseline across the 14 subjects. (a) The BOLD response of the principal component within a 4-mm radius of each chosen voxel is plotted relative to baseline. (b) The coordinate of each chosen voxel is shown below each plot along with the region from which the principal component was selected (superimposed on a transverse section of the T1-weighted anatomical image). Note that the left anterior hippocampal activation displayed in Fig. 3b peaks at the second/third presentation of the exemplar set. This activation is situated at y = −16, which in the anterior–posterior axis is midway between the first and second chosen voxels displayed.
Data Analysis.
Data were analyzed by using Statistical Parametric Mapping (SPM97) employing a random effects model. Five scans were acquired during each activation and sensorimotor control epoch and averaged to create one mean scan per epoch. For exemplar novelty, the six blocks of eight activation and control scans were averaged further into one time series of the alternating eight activation and eight control conditions after exemplar change. Each scan in the time series was then weighted to model either a linear or an exponential time × condition interaction (novelty-dependent activation). The weighted time series for each subject were entered into a one-sample t test across the 14 subjects. Analysis for effects of perceptual novelty was identical except that the time series consisted of the three activation and three control epochs after font change. We carried out a small volume correction (SVC) (20) to the P values of the ensuing maxima on all reported hippocampal regions. We report only those that survive this SVC at P < 0.05, except for the left anterior hippocampal activation in response to exemplar novelty, which was significant at trend, with SVC yielding a P value of 0.067 (P < 0.005 uncorrected).
RESULTS
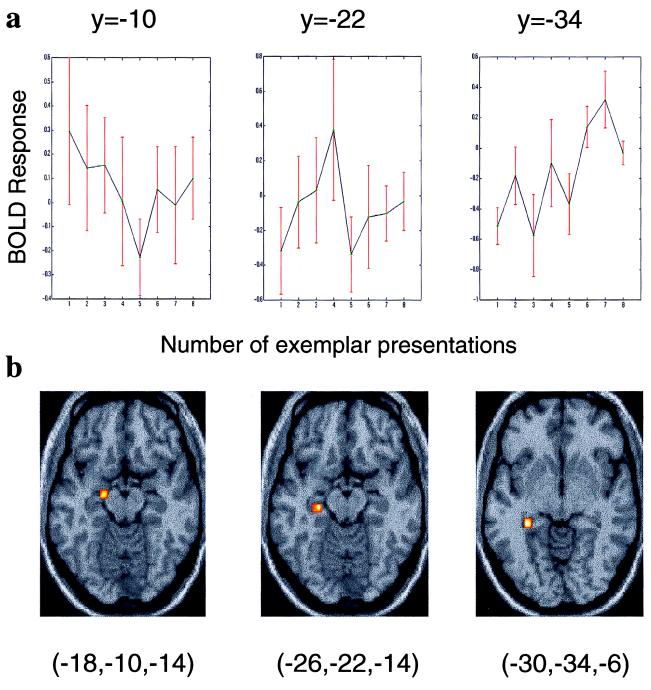
The specific effect tested was a condition (exemplar or perceptual change) × time interaction, where time refers to the time elapsed after a change in either exemplar or font (i.e., the adaptation of hemodynamic responses after exemplar or perceptual changes). Fig. 2 shows the hippocampal response to perceptual novelty and its modulation with time. A greater left anterior hippocampal response is seen for initial, relative to repeated, presentations of perceptually novel items, reflected in a significant linear time × condition interaction. This response shows adaptation with increasing font familiarity. Modeling exponential adaptation also yielded activation, of lesser significance, in the same region. The profile of hippocampal adaptation in our data conforms to a pattern observed with direct recording from “novelty-sensitive” single hippocampal neurons (13, 21) and to the pattern of cortical activity produced by repetition priming (22).
Figure 2.
Hippocampal region in which there is a significant time × condition interaction in response to perceptual novelty and adaptation with familiarity. (i) Coronal section of a T1-weighted anatomical image (at y = −16) that conforms to the stereotaxic space. The image is taken from 1 of the 14 subjects. T1-weighted images in all subsequent figures are taken from the same subject. Superimposed on this section is a SPM (thresholded at P < 0.01) indicating a decreasing linear time × condition interaction in the left hippocampus after introduction of novel font. The section has been chosen to demonstrate left anterior hippocampal activation (x, y, z coordinates −22, −16, −24; Z = 3.25). (ii) Graphic representation of activation at this voxel relative to the baseline condition as a function of repeated presentation of fonts. The plotted time course shows the BOLD response collapsed across the 16 font changes and averaged across all subjects. ↓, introduction of novel font.
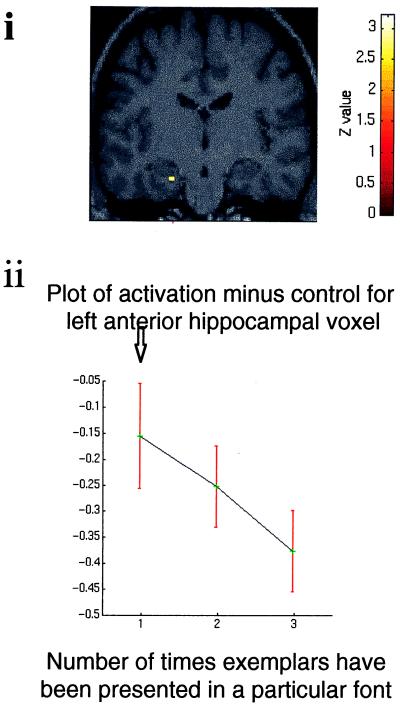
The response of the hippocampus to exemplar novelty is shown in Fig. 3b and was again assessed as a condition × time interaction. Initial presentation of novel exemplars was associated with an activation in left anterior hippocampus, 10 mm superior to the region indexed by perceptual novelty. With repeated exemplar presentations, activation in this region again showed significant adaptation. This pattern of response in the hippocampus was best modeled as an activation followed by an exponential decline. Thus, a significant time × condition interaction was reflected in reducing activation, relative to the recurring baseline, in anterior hippocampus.
Figure 3.
Dissociation in the anterior–posterior hippocampal axis for exemplar novelty. (a) T1-weighted anatomical sagittal image with red lines indicating the anterior–posterior positions of the coronal sections demonstrated in b and c. (bi) SPM prepared as above showing enhanced left anterior hippocampal activation after the introduction of exemplar novelty followed by deactivation (threshold P < 0.05). The coronal section has been chosen to demonstrate left anterior hippocampal activation (coordinates −18, −16, −14; Z = 2.60). (bii) Graphic representation of activation at this voxel relative to the baseline condition against number of presentations of an exemplar set. The plot shows the BOLD response collapsed across the six exemplar changes and averaged across all subjects. ↑, introduction of novel exemplar set. (ci) SPM (threshold P < 0.01) showing that increasing familiarity with exemplars activates the posterior hippocampus bilaterally. The coronal section has been chosen to demonstrate right posterior hippocampal activation (coordinates 24, −34, −2; Z = 3.63) and also shows the left posterior hippocampal activation (coordinates −22, −38, −6; Z = 3.67). (cii) Graphic representation of activation of the right posterior hippocampal voxel relative to the baseline condition as for b.
Strikingly, a reverse effect, reflecting increasing familiarity with exemplars, was associated with augmented bilateral posterior hippocampal activation (Fig. 3c). Hence, familiarity with the meaningful characteristics of stimuli (i.e., grammatical status within a rule system) produced augmentation of posterior hippocampal responses bilaterally. Increasing font familiarity, which has no behavioral relevance, did not engage the posterior hippocampus. Thus, in bilateral posterior hippocampus, we observed a time × condition interaction for, and only for, repeated exemplar presentations.
The plots presented in Fig. 3 b and c show the BOLD response collapsed across the six exemplar changes and averaged across all subjects. Note that any variance caused by abstraction of grammar system knowledge (perhaps reflecting implicit learning) will be expressed across the entire experiment. Any such effect in the present analysis can be discounted, because the analysis involved collapsing data across the six blocks defined by the onset of exemplar change. Consequently, the emphasis is on explicit learning, driven by the trial-by-trial feedback (subjects respond to repetition items on the basis of whether previous responses in the grammatical decision task were correct or incorrect). We did, however, test for an interaction with abstraction of grammar system knowledge. The effects of this latter type of learning on the observed hippocampal activations would be stronger at the end of the experiment; hence, we compared novelty- and familiarity-induced activations in the first half of the experiment with the second half. No interaction was present in the hippocampus in either the novelty or the familiarity comparisons.
DISCUSSION
These data demonstrate a functional dissociation between anterior and posterior regions of the human hippocampus within the same experiment. The fact that both exemplar and perceptual novelty activated left anterior hippocampus suggests that this region processes novelty that is both behaviorally relevant (exemplar novelty) and irrelevant (perceptual novelty). Conversely, posterior hippocampal regions showed a familiarity effect that is expressed only for aspects of stimuli relevant to learning (i.e., grammatical status). We propose that a function of the anterior hippocampus is to register mismatches between expectation and actual experience. By expectation, we refer to a predictive set about the future based on recent experience. Furthermore, if this novelty has behavioral significance (here a requirement to learn exemplar status) familiarity with these novel items leads to engagement of posterior hippocampal regions.
The sensitivity of the anterior hippocampus to novelty is supported by event-related potentials recorded within the medial temporal lobes in epileptic patients (15). Damage to the hippocampus proper (patients with sclerosis) is associated with attenuated anterior medial temporal lobe event-related potentials for novel visually presented words, whereas responses to repetitive presentations are unaffected. Interestingly, in addition to the anterior medial temporal response to new words, word repetitions have been found to evoke a different potential (23), which may have a more posterior distribution along the longitudinal hippocampal axis than the anterior potential evoked by new words (15).
The fact that our activation was left-sided might be expected, given the putative role of the left hippocampus in verbal memory (24). The presentation of novel vs. repeated words has been shown to activate the left hippocampus (25), whereas viewing novel versus familiar pictures of people, scenes, and landscapes has been reported to activate the right anterior hippocampus (8). The lack of a right anterior hippocampal response to either exemplar or perceptual novelty may therefore reflect stimulus form, as suggested by a recent functional neuroimaging study (26) in which the right hippocampus was more responsive to objects than words during encoding. The same study (26) observed left hippocampal activation during encoding of meaningful (as opposed to nonsense) stimuli. Our findings suggest that more posterior hippocampal regions are engaged by meaning. The proposed left hippocampal role in processing unexpectedness of stimuli (27) would appear to be a function of the left anterior hippocampus.
A novelty/encoding hypothesis developed by Tulving (28) states that novelty assessment plays a crucial role in determining whether information is encoded into long-term memory. Interestingly, the regions of left anterior hippocampus activated in the present study lie in close proximity to an area we have previously shown using positron emission tomography (PET), to be engaged during encoding of novel category-exemplar word pairings (7). Furthermore, intracranial potential studies have demonstrated that the left anterior medial temporal lobe response to the first presentation of words correlates with subsequent recognition (15) and delayed verbal recall (29) of these words. Although in the present paradigm we have not explicitly dissociated novelty assessment from episodic memory encoding, our data are compatible with a view that the hippocampal response to a novel stimulus is an important component in efficient episodic memory formation. By contrast, activation in bilateral posterior hippocampal regions, as exemplars become more familiar, may reflect active processes involved in retrieval. Note that increasing familiarity with exemplars allows subjects to make grammaticality judgements on the basis of previous feedback, suggesting that subjects engage episodic retrieval mechanisms as exemplars become more familiar [as indicated by exemplar theories of categorization (17)]. This suggestion is in agreement with a previous study (30) in which cued recall of words, previously repeatedly presented, activated both posterior hippocampi.
A recent meta-analysis of positron emission tomography studies of episodic memory (5) indicated that hippocampal activations associated with encoding are located primarily in rostral hippocampus, whereas activations associated with retrieval were located in caudal hippocampus. Although our study design addresses novelty, it is of interest to speculate on the relevance of our data to these findings. If exemplar novelty and familiarity in the present paradigm are considered to involve an emphasis on encoding and retrieval, respectively, our data would provide support for this functional divide within the hippocampus. However, we note that a recent review of fMRI data (6) concluded that the posterior medial temporal lobe is associated with episodic encoding and suggested that PET studies had demonstrated both anterior and posterior medial temporal activations during encoding.
The anterior–posterior hippocampal divide we describe suggests a functional topography related to familiarity. In a post-hoc analysis of our data, we demonstrated that with increasing familiarity for meaningful stimuli, there was a topographical spread of hippocampal activity in an anterior–posterior axis (Fig. 4). Although descriptive, these data suggest that older, well rehearsed memory representations are associated with activity in more posterior hippocampal regions. In this respect, our data provide system-level support for a recently proposed theory of hippocampal function—the “multiple-trace theory” (31)—which states that reactivation and rehearsal of memories cause the formation of multiple memory traces within the hippocampus. Our results suggest that these memory traces may be organized in a topographical manner.
A key observation in patients with hippocampal lesions is that the severity of anterograde and retrograde amnesia correlates with the extent of hippocampal damage (32–34). Given the observed left anterior hippocampal role in generic novelty detection, our data might suggest that anterior hippocampal lesions will impair new learning and hence account for the anterograde component of the amnesic syndrome. The extent of retrieval impairment may, because of the observed anterior–posterior familiarity gradient, reflect both the extent of posterior hippocampal damage and the degree of familiarity for the event being retrieved. This suggestion is supported by the famous patient H.M. (1), who had bilateral resection of the anterior hippocampus but sparing of posterior hippocampus (35) and exhibits dense anterograde amnesia but can recall memories acquired before surgery. Intriguingly, 25 years ago, Penfield (36) speculated that the temporal extent of retrograde amnesia depends on the posterior extent of hippocampal resection. Our findings of a functional segregation within the human hippocampus provide a basis for understanding the diversity of memory deficits consequent on damage to distinct regions of the hippocampus.
Acknowledgments
We thank Andrew Holmes for statistical advice and R. Frackowiak for internal review of this manuscript. We also thank radiography staff, particularly Helen Gallagher. B.A.S. is supported by the Astor Foundation Scholarship. P.C.F., R.N.A.H., K.J.F., and R.J.D. are supported by the Wellcome Trust.
ABBREVIATIONS
- fMRI
functional MRI
- SPM
statistical parametric mapping
- BOLD
blood oxygenation level-dependent
References
- 1.Scoville W B, Milner B. J Neurosurg Psychiatr. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Squire L R. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 3.Vargha-Khadem F, Gadian D G, Watkins K E, Connelly A, Van Paesschen W, Mishkin M. Science. 1997;277:376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- 4.Moser E, Moser M-B, Andersen P. J Neurosci. 1993;13:7347–7359. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lepage, M., Habib, R., Tulving, E. Hippocampus8, 313–322. [DOI] [PubMed]
- 6.Schacter, D. L. & Wagner, A. D. (1999) Hippocampus, in press. [DOI] [PubMed]
- 7.Dolan R J, Fletcher P C. Nature (London) 1997;388:582–585. doi: 10.1038/41561. [DOI] [PubMed] [Google Scholar]
- 8.Tulving E, Markowitsch M J, Craik F I M, Habib R, Houle S. Cereb Cortex. 1996;6:71–79. doi: 10.1093/cercor/6.1.71. [DOI] [PubMed] [Google Scholar]
- 9.Brewer J B, Zhao Z, Desmond J E, Glover G H, Gabrieli J D E. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- 10.Wagner A D, Schacter D L, Rotte M, Koutstaal W, Maril A, Dale A M, Rosen B R, Buckner R L. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- 11.Henke K, Buck A, Weber B, Wieser H G. Hippocampus. 1997;7:249–256. doi: 10.1002/(SICI)1098-1063(1997)7:3<249::AID-HIPO1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 12.Stern C E, Corkin S, Gilberto González R, Guimaraes A R, Baker J R, Jennings P J, Carr C A, Sugiura R M, Vedantham V, Rosen B R. Proc Natl Acad Sci USA. 1996;93:8660–8665. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fried I, MacDonald K A, Wilson C L. Neuron. 1997;18:753–765. doi: 10.1016/s0896-6273(00)80315-3. [DOI] [PubMed] [Google Scholar]
- 14.Knight R T. Nature (London) 1996;383:256–259. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- 15.Grunwald T, Lehnertz K, Heinze H J, Helmstaedter C, Elger C E. Proc Natl Acad Sci USA. 1998;95:3193–3197. doi: 10.1073/pnas.95.6.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reber A S. J Verbal Learn Verbal Behav. 1967;6:855–863. [Google Scholar]
- 17.Shanks D R. The Psychology of Associative Learning. Cambridge, U.K.: Cambridge Univ. Press; 1995. [Google Scholar]
- 18.Shanks D R, St. John M F. Behav Brain Sci. 1994;17:367–395. [Google Scholar]
- 19.Friston K J, Ashburner J, Frith C D, Poline J-B, Heather J D, Frackowiak R S J. Hum Brain Mapp. 1995;2:165–189. [Google Scholar]
- 20.Worsley K J, Marrett P, Neelin A C, Friston K J, Evans A C. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 21.Rolls E T, Cahusac P M B, Feigenbaum J D, Miyashita Y. Exp Brain Res. 1993;93:299–306. doi: 10.1007/BF00228398. [DOI] [PubMed] [Google Scholar]
- 22.Wiggs C L, Martin A. Curr Opin Neurobiol. 1998;8:227–233. doi: 10.1016/s0959-4388(98)80144-x. [DOI] [PubMed] [Google Scholar]
- 23.Smith M E, Stapleton J M, Halgren E. Electroencephalogr Clin Neurophysiol. 1986;63:145–159. doi: 10.1016/0013-4694(86)90008-8. [DOI] [PubMed] [Google Scholar]
- 24.Milner B. Clin Neurosurg. 1972;19:421–446. doi: 10.1093/neurosurgery/19.cn_suppl_1.421. [DOI] [PubMed] [Google Scholar]
- 25.Kopelman M D, Stevens T G, Foli S, Grasby P. Brain. 1998;121:875–887. doi: 10.1093/brain/121.5.875. [DOI] [PubMed] [Google Scholar]
- 26.Martin A, Wiggs C L, Weisberg J. Hippocampus. 1997;7:587–593. doi: 10.1002/(SICI)1098-1063(1997)7:6<587::AID-HIPO1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 27.Schacter D L, Reiman E, Uecker A, Polster M R, Yun L S, Cooper L A. Nature (London) 1995;376:587–590. doi: 10.1038/376587a0. [DOI] [PubMed] [Google Scholar]
- 28.Tulving E, Kroll N. Psychonomic Bull Rev. 1995;2:387–390. doi: 10.3758/BF03210977. [DOI] [PubMed] [Google Scholar]
- 29.Elger C E, Grunwald T, Lehnertz K, Kutas M, Helmstaedter C, Brockhaus A, Van Roost D, Heinze H J. Neuropsychologia. 1997;35:657–667. doi: 10.1016/s0028-3932(96)00110-8. [DOI] [PubMed] [Google Scholar]
- 30.Schacter D L, Alpert N M, Savage C R, Rauch S L, Alpert M S. Proc Natl Acad Sci USA. 1996;93:321–325. doi: 10.1073/pnas.93.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nadel L, Moscovitch M. Neuropharmacology. 1998;37:431–439. doi: 10.1016/s0028-3908(98)00057-4. [DOI] [PubMed] [Google Scholar]
- 32.Zola-Morgan S, Squire L R, Amaral D G. J Neurosci. 1986;6:2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rempel-Clower N L, Zola S M, Squire L R, Amaral D G. J Neurosci. 1996;16:5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nadel L, Moscovitch M. Curr Opin Neurobiol. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- 35.Corkin S, Amaral D G, Gonzalez R G, Johnson K A, Hyman B T. J Neurosci. 1997;17:3964–3979. doi: 10.1523/JNEUROSCI.17-10-03964.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Penfield W, Mathieson G M. Arch Neurol (Chicago) 1974;31:145–154. doi: 10.1001/archneur.1974.00490390027001. [DOI] [PubMed] [Google Scholar]