Summary
The transient receptor potential (TRP) channels are implicated in various cellular processes, including sensory signal transduction and electrolyte homeostasis. We show here that the GTL-1 and GON-2 TRPM channels regulate electrolyte homeostasis in the C. elegans intestine. GON-2 is responsible for a large outwardly rectifying current of intestinal cells, and its activity is tightly regulated by intracellular Mg2+ levels, while GTL-1 mainly contributes to appropriate Mg2+ responsiveness of the outwardly rectifying current. We also used nickel cytotoxicity to study the function of these channels. Both GON-2 and GTL-1 are necessary for intestinal uptake of nickel, but GTL-1 is continuously active while GON-2 is inactivated at higher Mg2+ levels. This type of differential regulation of intestinal electrolyte absorption ensures a constant supply of electrolytes through GTL-1, while occasional bursts of GON-2 activity allow rapid return to normal electrolyte concentrations following physiological perturbations.
Introduction
Magnesium (Mg2+) is one of the most abundant cations in the human body and is involved in more than 300 enzymatic systems, including adenosine triphosphate (ATP) metabolism (Fox et al., 2001). Hypomagnesemia has been identified in 7% of general clinical patients, 24% of hypertensive patients, and 25% of diabetic patients (Fox et al., 2001). Low-level Mg2+ deficiency is typically asymptomatic, but severe Mg2+ deficiency causes a variety of symptoms, including impaired memory, cardiac rhythm disturbances, and seizures (Vormann, 2003). In spite of the biological and clinical importance of the Mg2+ ion, little is known about the mechanism of its homeostatic regulation.
It has been speculated that Mg2+ uptake in eukaryotic cells is mainly mediated by transporters (Romani and Scarpa, 2000). This is partly because Mg2+ transporters have been cloned in prokaryotic cells and partly because the antiporter that extrudes Mg2+ in exchange for extracellular Na+ was identified in vertebrate cells (Romani and Scarpa, 2000). However, no firm evidence has been provided supporting the importance of transporters in eukaryotic Mg2+ homeostasis (Wolf, 2004).
Recent studies have implied that the transient receptor potential (TRP) channels are involved in regulation of Mg2+ homeostasis in eukaryotes (Clapham, 2003). TRP channel proteins are classified into seven subfamilies based on amino acid sequences (TRPC, TRPV, TRPM, TRPA, TRPN, TRPP, and TRPML) and are implicated in a range of physiological processes (Birnbaumer et al., 2003; Clapham, 2003; Minke and Cook, 2002; Montell, 2001; Montell, 2003b). Mutations in TRPM6 result in familial hypomagnesemia with secondary hypocalcemia (HSH), an autosomal recessive disease caused by a defect in intestinal Mg2+ absorption (Schlingmann et al., 2002; Walder et al., 2002). The related channel TRPM7 has also been implicated in Mg2+ homeostasis. TRPM7-deleted cell lines are not viable in the absence of Mg2+ in the culture medium, but they are viable when the medium is supplemented with a high level of Mg2+ (Schmitz et al., 2003). TRPM6 and TRPM7 both are characterized by the presence of a protein kinase domain in the C-terminal cytoplasmic domain (Nadler et al., 2001; Runnels et al., 2001; Schlingmann et al., 2002; Walder et al., 2002), and their channel activities are regulated by millimolar levels of intracellular Mg2+ (Nadler et al., 2001; Runnels et al., 2001; Schmitz et al., 2003; Voets et al., 2004). These observations imply that TRPM6 and TRPM7 channels are involved in the regulation of vertebrate Mg2+ homeostasis (Montell, 2003a).
We have investigated intestinal Mg2+ homeostasis using the model organism C. elegans. The C. elegans intestine is a tube composed of ten pairs of epithelial cells arrayed along its length (White, 1987). A dense layer of microvilli is present on the apical (luminal) side of each intestinal cell, and these microvilli are presumably involved in the secretion of digestive enzymes and the absorption of nutrients and ions (White, 1987). The intestinal cells are attached to each other by adherens junctions at the borders of the apical membrane (White, 1987). In vertebrates, two pathways for electrolyte absorption by epithelial cells have been proposed, transcellular and paracellular (Aronson et al., 2003). The transcellular pathway is mediated by ion channels and transporters, whereas the paracellular pathway involved the “loosening” of tight junctions between epithelial cells. No reports have previously addressed the importance of either of these two pathways in the absorption of electroytes by the C. elegans intestine.
Here we describe our investigation of the function of the C. elegans TRPM channels GTL-1 and GON-2. The gon-2;gtl-1-double mutants show growth defects under low Mg2+ conditions, and these defects can be largely rescued by dietary supplementation with excess Mg2+. GTL-1 and GON-2 are also necessary for intestinal Ni2+ uptake since gtl-1;gon-2-double mutants become resistant to Ni2+ cytotoxicity. gtl-1 mutants are resistant to Ni2+, but this resistance is dependent on the presence of Mg2+ in the medium. This suggests that GON-2-dependent Ni2+ transport is tightly regulated by Mg2+ levels but GTL-1-dependent Ni2+ transport is not. Using a Ni2+-sensitive fluorescence indicator, we found that the wild-type intestine incorporates Ni2+ from the environment, but the double mutant intestine does so to a lesser extent. Finally, our electrophysiological data show that the large outwardly rectifying current characteristic of wild-type intestinal cells is mainly due to the activity of the GON-2 channel and that GON-2 and GTL-1 play different roles in the Mg2+ sensitivity of current generation. These observations suggest that two TRPM channels with different degrees of Mg2+ responsiveness are necessary to regulate appropriate intestinal electrolyte homeostasis and that this type of differential regulation of intestinal electrolyte absorption ensures a constant supply of electrolytes through GTL-1, while occasional bursts of GON-2 activity allow rapid return to normal electrolyte concentrations following physiological perturbations.
Results
Molecular and genetic characterization of the gtl-1 TRPM gene
The C. elegans genome encodes four TRPM family members, GON-2, GTL-1, GTL-2, and CED-11 (Harteneck et al., 2000). CED-11 is very distant from the three other TRPM proteins, but the remaining TRPM proteins are similar to one another. The overall amino acid identities of these channels between human and C. elegans are modest (21%–26%), but the N-terminal cytoplasmic domains are more closely related (Figure 1 and see Figures S1 and S2 in the Supplemental Data available with this article online). Our structural comparison suggests that the C. elegans TRPM channels resemble human TRPM1, 3, 6, and 7 within the TRPM subfamily (Figure 1A). However, the C termini of these TRPM channels are diverse. For example, TRPM6 and TRPM7 contain a kinase domain, which is absent from the C. elegans TRPM channels.
Figure 1. TRPM channels in C. elegans.

A) A phylogenetic tree of C. elegans, Drosophila, and human TRPM proteins. DmTRP indicates the TRP channel of D. melanogaster. CED-11, GTL-1, GTL-2, and GON-2 are C. elegans TRPMs. TRPM 1–8 are human proteins. Relationships were inferred by the Neighbor-Joining Method (Saitou and Nei, 1987).
B) Expression of gon-2, gtl-1, and gtl-2. Pgon2s(9)::gfp and Pgtl1(7)::gfp fusion genes were strongly expressed in the intestine. Bar, 50 μ.
C) Characterization of gtl-1 deletion mutants tg113 and ok375. (Top) The structure of the wild-type gtl-1 gene and two pairs of PCR primers (ER1 and EL1, and ER2 and EL2). (Middle) The deleted region of tg113. (Bottom) The deleted region of ok375 (a 50 nucleotide insertion was found at the junction). All numbers shown at the junction sites are the numbers of nucleotides starting from the first ATG initiation codon.
To examine which cells express these TRPM channels, we made fusion genes between GFP and the promoters of gon-2, gtl-1, and gtl-2. In transgenic animals, Pgon-2s(9)::gfp and Pgtl-1(7)::gfp were strongly expressed in the intestine while Pgtl-2(12)::gfp was predominantly expressed in the excretory cell (Figure 1B). In addition to Pgtl-1(7)::gfp, we also investigated the expression patterns of different gtl-1::gfp constructs and found that all of these were expressed in the intestine (Figure S3). We also found that a full-length fusion between gtl-1 and GFP was expressed throughout the intestine, with accumulation at the apical surface toward the intestinal lumen (Figure S3). Previously, two different 5′ ends of the gon-2 transcript were described (one starts at nt 20643 and the other at nt 9278 of cosmid T01H8) (West et al., 2001). Here we found that GFP fluorescence was strongly expressed in the intestine when we used the potential promoter region containing the initiation site for the shorter transcript (the promoter for the longer transcript expressed GFP in other cell types; Figure S4).
Since mutations in human TRPM6 have been reported to cause hypomagnesemia with secondary hypocalcemia (HSH) due to insufficient intestinal Mg2+ absorption (Schlingmann et al., 2002; Walder et al., 2002), we decided to use genetic analysis to determine whether GON-2 and/or GTL-1 mediate Mg2+ uptake in the C. elegans intestine. Recessive loss-of-function mutations in gon-2 were previously isolated based on their sterile phenotype (Sun and Lambie, 1997), but there was no indication that gon-2 functions in the intestine. No functional characterization of gtl-1 has been previously reported. Therefore, we isolated the deletion allele gtl-1(tg113) by trimethyl-psoralen/UV mutagenesis combined with PCR-based screening (Figure 1C). Another gtl-1 deletion allele (ok375) was isolated by the C. elegans Genome Knockout Consortium (http://www.wormbase.org/). tg113 is a deletion of 328 nucleotides within the fifth exon, resulting in a frame shift that truncates the protein coding sequence. The ok375 deletion is 2714 bp in size, and this deletes all GTL-1 transmembrane domains (Figure 1C). Therefore, tg113 and ok375 are both expected to strongly reduce or eliminate gtl-1 activity.
The gon-2;gtl-1 double mutant is suppressible by Mg2+ supplementation
On standard NGM plates, which contain 1 mM CaCl2 and 1 mM MgSO4 (Epstein and Shakes, 1995), the gtl-1 mutants showed no obvious abnormal phenotypes. However, we found that, when these mutants were placed on culture plates containing 0 mM Mg2+, they grew much slower than wild-type (Figure 2A). In fact, some animals arrested before reaching adulthood. It should be noted that a small amount of Mg2+ is present even in the 0 mM Mg2+ plates, and this is unavoidable because E. coli as a food source carries trace amounts of the ions. As expected based on previous results (Sun and Lambie, 1997), growth of the gon-2 mutant was not much affected by different levels of Mg2+ supplementation (Figure 2A).
Figure 2. Growth of mutants at different Mg2+/Ca2+ concentrations.

A) Growth of mutants at different Mg2+ concentrations. Strains were maintained on NGM agar plates containing 40 mM Mg2+ and 0 mM Ca2+ for at least three generations at 20°C. Fifty eggs were picked for each strain and placed on a fresh plate containing different Mg2+ concentrations. All animals were grown at 25°C during the assays. The mean of two independent assays is plotted.
B) Growth of mutants at different EDTA and EGTA concentrations. No Ca2+ or Mg2+ was added unless noted. For details, see above.
C) Effects of Ca2+ on growth. No Mg2+ was added in the agar.
Since gon-2 and gtl-1 are both expressed in the intestine, we suspected that they might function redundantly in this tissue. Therefore, we constructed double mutant strains to investigate this possibility. Strikingly, the gon-2;gtl-1-double mutants showed a severe growth phenotype (Figure 2A). The double mutants did not reach adulthood at all when low Mg2+ was present in the culture agar. On 5 mM Mg2+ plates, 11%–35% of the double mutants reached adulthood, but it took them two to three times longer than wild-type (Figure 2A). On higher Mg2+ plates, nearly 100% of the double mutants reached adulthood 120 hr after hatching (Figure 2A).
To test whether or not the altered growth rates on different Mg2+ concentrations were simply due to differences in osmolarity, we placed these strains on culture plates that contained 80 mM sucrose but no supplemental Mg2+. Mutants grown on sucrose behaved similarly to ones on 0 mM Mg2+ plates (Figure 2A), indicating that the effect of Mg2+ on growth rate is not simply osmotic in nature.
We further investigated the effects of Mg2+ and/or Ca2+ depletion on mutant growth by adding 5 mM EDTA and EGTA: no Mg2+ or Ca2+ was added to the medium, but it is likely that the E. coli provided as a food source carried in trace amounts of these ions. In the presence of 5 mM EDTA in the culture agar, none of the animals reached adulthood (Figure 2B), instead arresting around the L2 stage. When 5 mM EGTA was present in the medium, wild-type and gon-2 animals showed a near-normal growth phenotype (Figure 2B). Only 70%–80% of the gtl-1 mutants reached adulthood, and none of the gon-2;gtl-1-double mutants grew to adulthood (Figure 2B). Furthermore, we found that Mg2+ supplementation rescued the growth arrest of the gtl-1-single and gon-2;gtl-1-double mutants, even in the presence of 5 mM EGTA (Figure 2B). We also found that high-dose Mg2+ supplementation relieves the Mg2+ deficiency phenotypes in the single and double mutants, even after they temporarily arrested in the absence of Mg2+ (Figure S5). Taken together, these observations indicate that Mg2+ deficiency is the primary cause of the phenotypes of the gtl-1-single and gon-2;gtl-1-double mutants.
We also tested whether Ca2+ in the culture media would affect the gon-2 and gtl-1 phenotypes. We found that Ca2+ supplementation had little effect on the growth of the mutant strains, with the exception of the gtl-1 mutants (Figure 2C). Excess Ca2+ in the culture medium slowed down the growth of the gtl-1 mutants, and some of these animals ruptured at the vulva. Since TRPM7 is implicated in incorporating Ca2+ for neural cell death (Nicotera and Bano, 2003), one possible explanation for this result is that, when GTL-1 is not active, GON-2 might transport excess Ca2+ into the intestine (and possibly other tissues), leading to necrosis (also see Figures 6 and 7).
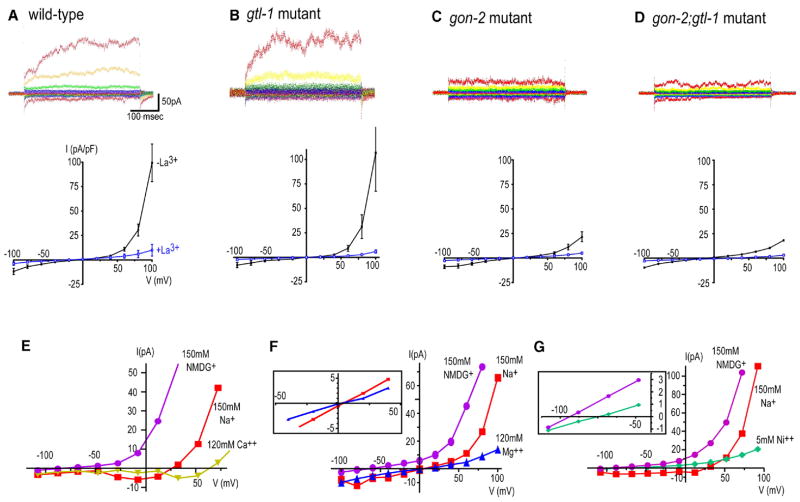
Figure 6. Whole-cell currents in cultured intestinal cells.
A–D) Representative whole-cell currents. Currents were recorded by stepping from a holding voltage of 0 mV to voltages between −100 and +100 mV in 20 mV intervals. (Middle panels) Steady-state I–V relationship for whole-cell currents (n = 4–9 each, the bars are standard errors). This current was suppressed in the presence of 0.1 mM LaCl3 in the bath solution (n = 3–8 each).
E) I–V relationships of whole-cell current of the wild-type cells in the 150 mM Na+ solution and when Na+ was replaced with 120 mM Ca2+, then with 150 mM NMDG+. In the Na+ solution, 1 mM EDTA was included to chelate residual divalent cations. Note that the current amplitude (the y axis) is not normalized with the membrane capacitance.
F) I–V relationships in 150 mM Na+, replaced with 120 mM Mg2+, then with 150 mM NMDG+. (Inset) A magnification of reversal potential points.
G) I–V relationships in 150 mM Na+, replaced with 5 mM Ni2+ and 130 mM NMDG+, then with 150 mM NMDG+. (Inset) A magnification of reversal potential points for the Ni2+ and NMDG+ solutions.
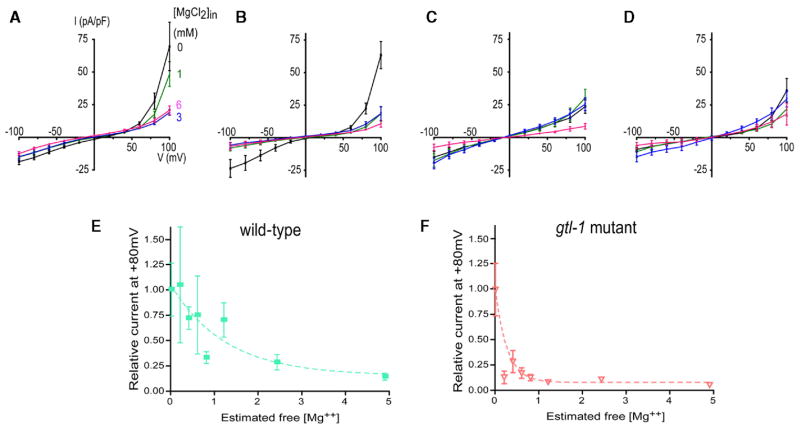
Figure 7. Dose response relationship for inhibition of the outwardly rectifying current by intracellular free Mg2+.
A) Inhibition of current by intracellular Mg2+ in wild-type (n = 8–15). The pipette solutions contained 0–6 mM MgCl2. The protocol was essentially same as experiments in Figure 6.
B) gtl-1(ok375) mutant cells; n = 4–8.
C) gon-2(q388) mutant cells; n = 5–10.
D) gon-2(q388);gtl-1(ok375) double mutant cells; n = 4–8.
E and F) Currents were recorded by stepping from a holding voltage of 0 mV to voltages between −100 and +100 mV in 20 mV intervals, and the amplitudes of steady-state current at +80 mV were normalized based on the mean amplitude at +80 mV of 0 mM Mg2+ added in the pipette solution. The y axis is this normalized current amplitude, and the x axis is intracellular free Mg2+ levels estimated by WEBMAXC program. The pipette solutions contained 0–6 mM MgCl2 with no ATP and GTP, and with 10 mM BAPTA instead of EGTA. K1/2 values were 874 μM for wild-type ([E], n = 4 each point) and 186 for the gtl-1 mutant ([F], n = 3–7). All results presented in this figure were obtained independently of the experiments in Figure 6.
gon-2; gtl-1-double mutants contain less Mg2+ than wild-type
To determine whether Mg2+ deficiency is observed in the gon-2; gtl-1-double mutant, we quantitated the amounts of trace elements in the wild-type and double mutant animals using inductively coupled plasma mass spectrometry (ICP-MS). We found that wild-type contained 1850 ppm Mg2+ (standard error ± 80), while the double mutant contained only 1020 ppm Mg2+ (± 80; p < 0.05; trial = 4). No significant difference was detected in the quantities of other elements, including Ca2+ and K+, between these strains. This result is also consistent with the notion that the phenotypes in the gon-2;gtl-1-double mutants were indeed caused by Mg2+ deficiency.
Characterization of the defecation behavior in the gon-2 and gtl-1 mutants
In order to investigate the physiological state of the intestine, we examined the defecation motor program (DMP) cycles. The C. elegans defecation behavior is regulated by the activity of the inositol 1,4,5-trisphosphate receptor (IP3R) (Dal Santo et al., 1999). The DMP consists of a stereotyped series of three muscle contractions (Figure 3) (Liu and Thomas, 1994; Thomas, 1990). First, posterior body wall muscles contract (pBoc) and then relax, causing the gut contents to accumulate near the anus. Three to four seconds later, the anterior body wall muscles contract (aBoc) to pressurize the gut contents. Finally, specialized enteric muscles contract to expel the contents from the anus (Exp). This defecation motor program is expressed as a periodic behavior: in the presence of abundant food, the defecation motor program is activated every 45 s (Figure 3) (Liu and Thomas, 1994; Thomas, 1990).
Figure 3. The defecation motor program.

A) Schematic representation of the defecation motor program (DMP) and representative ethograms of defecation behavior. (1) Intercycle (not labeled); (2) pBoc, posterior body wall muscle contraction; (3) aBoc, anterior body wall muscle contraction; (4) Exp, enteric muscle contraction with expulsion of gut contents, then return to the intercycle. In ethograms, each dot or character represents 1 s. Seconds elapsed are indicated above each ethogram. p, a, and x represent pBoc, aBoc, and Exp, respectively. The mean defecation cycle was 43.0 s ± 1.5 (standard deviation) for a wild-type at 0 mM Mg2+. Forty percent of gtl-1(tg113) mutants at 0 mM Mg2+ showed near-normal cycles, while 60% of them showed longer cycles. gon-2(q388) at 0 mM Mg2+ showed a 62.0 ± 6.4 s cycle.
B) Effects of Mg2+ and Ca2+ concentrations on the defecation behavior. All data were a summary of five cycles/animal from at least five animals for each strain. All strains were grown at 25°C. *p < 0.05 (Mann-Whitney test). (Ba) The DMP cycles on 40 mM Mg2+ plates. Ca2+ was not added to the plates. (Bb) The DMP cycles on 0 mM Mg2+ plates. The DMP cycles of the gtl-1 mutants were affected, but this phenotype varied largely from individual to individual. Although mean DMP cycles were substantially longer, some individuals showed the nearly wild-type DMP cycles (see the second example in [A]). (Bc) Effects of Ca2+ concentrations on the defecation behavior. The DMP cycles were measured on NGM agar plates containing different Ca2+ concentrations. Mg2+ was not included in these plates. (Bd) DMP assays with the gon-2(q388);gtl-1(ok375) double mutant. Since the double mutants did not grow on 0 mM Mg2+ plates, they were grown on 40 mM Mg2+ plates until they were L3 larvae and then transferred to 0 mM Mg2+ plates. The same animals were transferred back to 40 mM Mg2+ plates at the 70th hour from the beginning of the assay. Wild-type animals that were tested in parallel were not affected by the first plate transfer. However, these wild-type animals became too old during the assay while the double mutants arrested on 0 mM Mg2+ plates. (Be) DMP defects in the gon-2(q388);gtl-1(ok375) double mutant were not rescued by excess Ca2+. The double mutants were grown on 40 mM Mg2+ plates until they were L3 larvae and then transferred to 0 mM Mg2+ plates. The same animals were transferred back to 40 mM Ca2+ plates at the 70th hour from the beginning of the assay.
Wild-type animals showed DMP cycles of 40–45 s on both 40 mM and 0 mM Mg2+ plates. Therefore, their defecation cycles were not obviously affected by different Mg2+ concentrations. This suggests that the wild-type intestine can maintain normal physiological levels of intracellular Mg2+ over a wide range of environmental Mg2+ concentrations (Figure 3B). On 40 mM Mg2+ plates, gtl-1 mutants showed a mean cycle of 40 s, which is very close to wild-type (Figure 3B). The cycles of both gtl-1 mutants were highly variable on 0 mM Mg2+ plates. Forty percent of the gtl-1 mutants still exhibited DMP cycles around 45 s, but 60% of the mutants had much longer DMP cycles (80–200 s; Figure 3A). Therefore, intestinal activity was severely attenuated in the majority of gtl-1 mutants grown on 0 mM Mg2+ plates.
The gon-2 mutant showed slightly slower DMP cycles than wild-type on both 40 mM and 0 mM Mg2+ plates (around 60 s), suggesting that the DMP phenotype in the gon-2 mutant is not due to insufficient Mg2+ (Figure 3B). Interestingly, the DMP phenotype of the double mutant on 40 mM Mg2+ was near normal, indicating that the gtl-1 mutations are epistatic to gon-2(q388) (Figure 3B).
Although no detectable phenotypes were observed in the growth rate of the gon-2 mutant in response to excess Ca2+ supplementation, the DMP of the gon-2 mutant was partially suppressed by supplementation with 5 mM Ca2+. Since the gtl-1-single mutant is not suppressible by Ca2+ supplementation, this implies that GON-2 and GTL-1 play different roles in regulating the physiological state of the intestine (Figure 3B).
To assess the DMP phenotype of gon-2;gtl-1-double mutants under low Mg2+ conditions, these animals were raised on 40 mM Mg2+ plates until the L3 larval stage and then transferred to 0 mM Mg2+ plates (Figure 3B). Twenty-four hours after transfer onto 0 mM Mg2+ plates, the double mutants showed very sluggish DMPs (Figure 3B). When these animals were transferred back to 40 mM Mg2+ plates, their DMP cycles returned to near normal (Figure 3B). These observations suggest that the altered DMP cycle in the double mutant is due to an alteration in the physiological state of the intestine, rather than a developmental defect. These results also suggest that the observed impairment of the DMP cycle is mainly caused by Mg2+ deficiency. A similar plate transfer assay using 40 mM Ca2+ supplementation at the second transfer did not rescue the DMP cycles (Figure 3B). Although we cannot completely eliminate the possibility that Ca2+ signaling is also altered in the double mutants, the most parsimonious explanation is that Mg2+ acts directly to rescue the mutant phenotypes.
As with the growth assays, we found that the gtl-1 and gon-2-single mutants do not have overlapping phenotypes, whereas the double mutant exhibits a more severe phenotype. Therefore, our data suggest that GTL-1 and GON-2 function independently, with overlapping functions, to regulate physiology of the intestinal cells.
Ni2+ toxicity assays reveal differential regulation of GON-2 and GTL-1 activity by Mg2+ levels
TRPM7 has been reported to permeate trace divalent ions, such as Ni2+, Zn2+, Fe2+, Cu2+, Mn2+, and Co2+ (Monteilh-Zoller et al., 2003), in addition to Mg2+ and Ca2+, as originally reported (Nadler et al., 2001). These divalent ions are required as cofactors for many essential enzymes, and some of them, such as Ni2+, cause cellular toxicity at relatively low concentrations (Monteilh-Zoller et al., 2003). Also, it has been shown that TRPM6 can pass Ni2+ as well (Voets et al., 2004). Therefore, we hypothesized that, if either GTL-1 or GON-2 can pass these trace divalent ions, then mutations in these genes might confer resistance to toxicity. We chose Ni2+ for this assay for two reasons. First, Ni2+ is already known to cause cellular toxicity in C. elegans, so we could easily establish an assay to detect Ni2+ resistance (Peredney and Williams, 2000). Second, fluorescence indicators are commercially available that are sensitive to Ni2+, thus making it possible to monitor absorption of Ni2+ by living intestinal cells (Cabantchik et al., 1996; Jussofie et al., 1998).
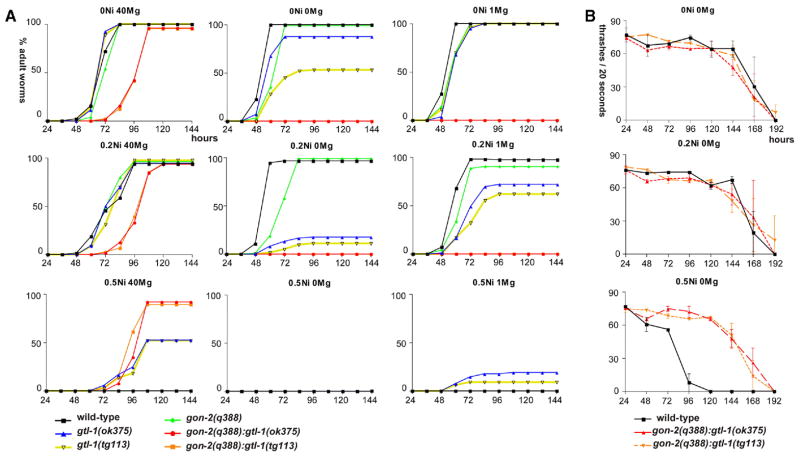
We began by testing the Ni2+ sensitivity of each strain on 40 mM Mg2+ plates, since all strains could grow at this Mg2+ concentration (Figure 4). Little or no effect was seen among any of the strains when the concentration of Ni2+ was lower than 0.2 mM (Figure 4A). However, on 0.5 mM Ni2+ plates containing 40 mM Mg2+, both wild-type and gon-2 mutant animals arrested at L1 to L2 stages (Figure 4A). This is in contrast to the gon-2;gtl-1-double mutants, which grew unaffected even with 0.5 mM Ni2+, suggesting that a lack of these channels confers Ni2+ resistance (Figure 4A). However, at 1 mM Ni2+, the double mutants arrested as early larvae (data not shown). This suggests that the double mutants are able to take up Ni2+, but they do so less efficiently than wild-type. The gtl-1-single mutants showed an intermediate level of resistance to Ni2+: at 0.5 mM Ni2+, nearly 50% of them became adults, and these animals looked unhealthy (Figure 4A). These observations indicate that GTL-1 and GON-2 are able to function independently to mediate Ni2+ uptake and that GTL-1 activity is primarily responsible for Ni2+ uptake under these assay conditions.
Figure 4. Effects of Ni2+ on animal growth.
A) Ni2+ toxicity assays. Fifty eggs were picked for each strain and placed on fresh plates containing different Ni2+ and Mg2+ concentrations. See Figure 2 for details. The gon-2;gtl-1 mutants showed resistance to Ni2+ toxicity. The gtl-1 mutants acquired resistance to toxicity in a Mg2+-dependent manner.
B) Viability of the gon-2;gtl-1-double mutants on 0 mM Mg2+ plates containing different Ni2+ concentrations. The resistance of the double mutants to 0.5 mM Ni2+ was examined using a thrashing assay, counting the number of times the animal swung from side to side in a saline solution. The x axis indicates the time (hr) elapsed after the start of the assay, and the y axis indicates the number of thrashes per 20 s interval. n = 3 for each time point/strain. Error bars indicate standard errors.
On 0 mM Mg2+ plates, both wild-type and gon-2 mutant animals had growth rates similar to those observed on 40 mM Mg2+ plates: at increasing Ni2+ concentrations, fewer animals reached adulthood (Figure 4A). Interestingly, gtl-1 mutants were sensitive to Ni2+ (0.2 and 0.5 mM) when raised on 0 mM Mg2+ plates. Therefore, the Ni2+ resistance of gtl-1 mutants depends on the Mg2+ concentration. The gon-2;gtl-1-double mutants did not grow at all, but this is because of Mg2+ deficiency, not Ni2+ toxicity (see Figure 2). Although the double mutants did not progress developmentally on 0.5 mM Ni2+ 0 mM Mg2+ plates, these animals moved vigorously and looked healthy, whereas wild-type incubated on such plates barely moved and looked very unhealthy. To quantitate the viability of the double mutants on the 0 mM Mg2+ plates, we performed a thrashing assay, counting the number of times per minute the animal swung from side to side when suspended in a saline solution. In the presence of 0 mM Mg2+ and 0.5 mM Ni2+, the double mutants thrashed vigorously, much more so than wild-type (Figure 4B). Nearly all wild-type animals died after 120 hr of incubation with 0.5 mM Ni2+, but the double mutants stayed alive for up to 192 hr. At lower Ni2+ levels, the wild-type and double mutants were equally viable (Figure 4B). These observations suggest that the double mutants are still resistant to 0.5 mM Ni2+ even with 0 mM Mg2+.
To complete this set of experiments, we tested the effects of low-level Mg2+ supplementation. On 1 mM Mg2+ plates, both wild-type and gon-2 mutant animals showed growth rates similar to those on 0 mM and 40 mM Mg2+ plates (Figure 4A). This confirms the idea that the Ni2+ sensitivity of these strains is not greatly affected by Mg2+ concentration. However, the growth rates of the gtl-1 mutants were intermediate between those observed on 0 and 40 mM Mg2+ plates (Figure 4A). These observations indicate that the gtl-1 mutants become more resistant to Ni2+ as Mg2+ concentration increases. On the other hand, the gon-2 mutant was sensitive to Ni2+ even at 40 mM Mg2+.
To test whether Ni2+ is absorbed through intestinal cells, we measured Ni2+ uptake in dissected intestines. Intestinal explants were loaded with 20 μM calcein AM in dissection solution for 30 min at room temperature. Calcein shows fluorescence quenching upon Ni2+ binding (Cabantchik et al., 1996). Therefore, Ni2+ uptake by intestinal cells is signaled by a decrease in calcein fluorescence. We used the maximum slope of fluorescence quenching to quantitate the rate of Ni2+ absorption by the most anterior intestinal cells (Figure 5). When Ni2+ concentration was increased stepwise from 0.01 mM to 0.1 mM and then 1 mM, the quenching rate became progressively larger, 0.8%, 1.8%, and 4.7%/minute, respectively (Figure 5A, n = 3 each).
Figure 5. Detection of Ni2+ absorption in dissected intestine preparations.

A) Fluorescence of the Ni2+ indicator calcein reflects Ni2+ absorption by dissected intestine. Dissected intestine loaded with 20 μM calcein was perfused with the dissection solution for 300 s, followed by the solution with different Ni2+ concentrations. “ΔF/F0” indicates fluorescence intensity normalized by the intensity at time 0 (F0). The x axis is the time elapsed (s). “ΔF/F0 (Δ%/minute)” indicates the percent of fluorescence quenching per minute. The higher the Ni2+ concentration, the faster the calcein fluorescence quenched (n = 3, labeled “+NiCl2”). Calcein fluorescence did not significantly decrease without Ni2+ perfusion (n = 6, labeled “-NiCl2”). The nonspecific fluorescence bleach was normalized using the first 300 s measurements for both slopes.
B) Quenching of calcein fluorescence by Ni2+ absorption. Details are same as above. The nonspecific fluorescence bleach was normalized using the first 100 s measurement. The gon-2;gtl-1 mutant (−1.9% ± 0.9%/min) showed a lower quenching rate than wild-type (−5.1 ± 1.1); p < 0.05 (n = 5–6). Animals were grown on 40 mM Mg2+ plates.
C) Means and standard errors of the intestinal Ni2+ absorption in different strains. Ni2+ absorption was reduced in the intestine of the gon-2;gtl-1-double mutant compared to other strains (p < 0.05): wild-type, −4.2 ± 0.9%/min; gtl-1(ok375), −3.8 ± 1.0; gon-2(q388), −4.8 ± 2.2; gon-2;gtl-1, −0.42 ± 0.4 (n = 5–17). These animals were grown on NGM plates. The double mutant was grown on 40 mM Mg2+ plates and transferred to NGM plates at L3–L4 stages.
To test whether GTL-1 and GON-2 are responsible for Ni2+ uptake, intestines were loaded with calcein and perfused with a solution containing 1 mM NiCl2, after which nonspecific fluorescence quenching rates were measured. We performed this assay using animals that were grown on both 40 mM Mg2+ plates and standard NGM plates and found consistent results: the double mutant absorbed Ni2+ much slower than wild-type (Figure 5). However, we detected no significant difference in Ni2+ uptake among wild-type, gtl-1-single, and gon-2-single mutants (Figure 5). These results are consistent with the notion that both GTL-1 and GON-2 are important for the uptake of Ni2+ by the intestine.
Outwardly rectifying currents are diminished in the mutants of gon-2
To test whether any electrophysiological properties were altered in the intestinal cells of these TRPM mutants, we attempted electrophysiological analysis of the intestinal cells derived from C. elegans embryos using the method of Estevez et al. (2003). Intestinal cells in culture were identifiable based on their distinct round morphology and autofluorescent granules (Estevez et al., 2003). In wild-type, we observed an outwardly rectifying current using the whole-cell configuration (its reversal potential [Erev] = −3.7 ± 6.4 mV), and this current appears to be voltage modulated, which is also observed with TRPM4b and TRPM5 (Hofmann et al., 2003). This current was suppressed by 0.1 mM LaCl3 in the bath solution (Figure 6). When we measured the electrophysiological activities of gtl-1 mutant cells, we found that the current was very similar to that of wild-type cells (Figure 6), suggesting that contribution of the GTL-1 channel to the amplitude of this current is small. In the gon-2 mutant cells, the outwardly rectifying current was severely attenuated; at 80 mV, we observed a current of 15.8 ± 3.5 pA/pF without LaCl3 (at 80 mV, the current was 30.5 ± 6.1 pA/pF in the wild-type), suggesting that this outwardly rectifying current is mainly generated by GON-2 (Figure 6). In the case of the gon-2(q388);gtl-1(ok375) double mutant cells, the current was small as well. We did not observe further reduction of the current amplitude compared to that of the gon-2 mutant cells (Figure 6). These observations indicated that GON-2 is mainly responsible for this outwardly rectifying current and that contribution of GTL-1 to the current amplitude is very small.
To study the selectivity of the GON-2-dependent conductance, we replaced the Na+ solution with solutions containing only one of the cations NMDG+, Ca2+, Mg2+, or Ni2+ (Figure 6). When 120 mM Ca2+ was exchanged for 150 mM Na+, the Erev shift was +45.9 ± 7.9 mV (n = 5). When 120 mM Mg2+ was exchanged for Na+, the shift was −4.5 ± 2.8 mV (n = 5). NMDG+ was used as a nonpermeable cation for this conductance; the Erev shift between Na+ and NMDG+ solutions was −67.4 ± 6.8 mV (n = 14) (PNMDG+/PNa+ = 0.06). These results indicate that Ca2+ is more permeable than Mg2+, which is nearly as permeable as Na+. When we tried to measure the Ni2+ permeability for the current, we found that 120 mM Ni2+ completely blocked the inward current (data not shown). When we lowered the Ni2+ concentration to 5 mM, we found that Ni2+ was significantly more permeable than NMDG+; the Erev difference between Ni2+ and NMDG+ was 14.2 ± 4.3 mV (n = 5, p < 0.05). These results show that the GON-2-dependent current can conduct Ni2+ in addition to Ca2+ and Mg2+.
To examine the effect of intracellular Mg2+ concentration on the activity of the GON-2-dependent current, cells were patch clamped with an ATP- and GTP-free pipette solution in which EGTA was replaced by 10 mM BAPTA. The concentration of MgCl2 added to the pipette solution varied between 0 mM and 6 mM. In wild-type, the current amplitude was gradually suppressed when Mg2+ levels added in the pipette solution were increased from 0 mM to 6 mM (Figure 7). In the gtl-1 mutant cells, as in wild-type, the current amplitude was reduced upon intracellular Mg2+ increase. However, the reduction in current from 0 mM to 1 mM Mg2+ was steeper than in the wild-type cells (Figure 7). In the gon-2 mutant cells, no significant difference was observed in the current amplitude when 0, 1, and 3 mM Mg2+ were added in the pipette solution, but a significant decrease occurred between 3 mM and 6 mM Mg2+ (p < 0.05, n = 5 and 8) (Figure 7). In the case of the gon-2(q388);gtl-1(ok375) double mutant cells, no significant difference in current amplitude was observed among different Mg2+ levels (Figure 7).
To further investigate the role of GTL-1, we compared the effects of intracellular Mg2+ concentration on the outwardly rectifying current in wild-type and gtl-1(ok375) mutant cells (Figure 7). Currents were recorded by stepping from a holding voltage of 0 mV to voltages between −100 and +100 mV in 20 mV intervals, and then the amplitudes of steady-state current at +80 mV were normalized based on the mean amplitude at +80 mV of 0 mM Mg2+ added in the pipette solution. In wild-type cells, we observed a gradual decrease in the current amplitude as an intracellular free Mg2+ concentration was increased (Figure 7). However, in gtl-1 mutant cells, the amplitude was sharply reduced in response to a slight increase in free Mg2+ (Figure 7). The estimated K1/2 values of free Mg2+ were 186 μM for the gtl-1 mutant and 874 μM for wild-type (n = 3–7 of each point/strain). These electrophysiological data support the notion that GTL-1 channel is responsible for appropriate Mg2+ sensitivity.
Discussion
In this paper, we describe our characterization of the function of the GTL-1 and GON-2 channels in C. elegans. Our results show that these channels regulate Mg2+ homeostasis in the C. elegans intestine. Single mutations in either gtl-1 or gon-2 cause little or no defect in defecation or overall growth rate; however, gon-2;gtl-1-double mutants exhibit strong synthetic phenotypes, and these are efficiently suppressed by Mg2+ supplementation. We have also found that the GON-2 and GTL-1 channels are necessary for Ni2+ uptake in the intestine, and the results of these assays indicate that these channels have different sensitivities to Mg2+ levels. Finally, we have obtained electrophysiological data that support the idea that the GON-2 channel is mainly responsible for the outwardly rectifying current and that GON-2 and GTL-1 play different roles in the Mg2+ sensitivity of current generation. Overall, our findings indicate that C. elegans provides an appropriate animal model to study intestinal electrolyte homeostasis mediated by TRPM channels.
Independent functioning of the GON-2 and GTL-1 TRPM channels
It has been reported that mammalian TRPM6 and TRPM7 form heteromeric channels (Chubanov et al., 2004). Our data indicate that formation of GON-2/GTL-1 heteromers is not essential for cellular function in the C. elegans intestine under laboratory conditions. The gtl-1 and gon-2 single mutants show distinct phenotypes with respect to growth, defecation, Ni2+ resistance, and EDTA-induced arrest. If heteromer formation were essential for their function, a lack of either channel should have induced some overlapping phenotypes between the gtl-1 and gon-2 single mutants. On the contrary, no overlapping behavioral or overall growth phenotypes were observed between these single mutants. Furthermore, the gon-2;gtl-1 double mutants showed strong synthetic phenotypes that were not observed with either single mutant, suggesting that these channels function independently and have overlapping functions. When one channel species is absent, the other channel can compensate for the function of the missing channel species to some extent but not completely. Therefore, based on these genetic data, we conclude that, while heteromers may exist, they are unlikely to perform any major unique or essential function under the laboratory conditions we used.
TRP channels and Mg2+ homeostasis
Estevez et al. (2003) previously identified two major conductances in cultured C. elegans intestinal cells: an outwardly rectifying current (named IORCa) and an inwardly rectifying current (ISOC). IORCa is 60 times more permeable to Ca2+ than Na+, and it is suppressed by both extracellular La3+ and intercellular Mg2+. Although we used the same conditions as Estevez et al. (2003) to measure the outwardly rectifying current, we cannot be certain that the outwardly rectifying current that we measured is equivalent to IORCa. For example, when we measured conductance in Na+ solution containing 1 mM EDTA, we still observed strong outward rectification, whereas IORCa was reported to exhibit a more linear voltage-current relation under these conditions, particularly at positive voltage (Estevez et al., 2003).
Our electrophysiological recordings suggest that GON-2-dependent current is Ca2+ selective, although this current can conduct other cations and poorly discriminate between Na+ and Mg2+. Since mammalian TRPM6 and TRPM7 also show permeability to various ions in addition to Mg2+ under physiological conditions, it is likely that Mg2+ homeostasis is tightly linked with homeostasis of other electrolytes, including Ca2+.
Our electrophysiological data support the idea that the GON-2 channel is mainly responsible for the outwardly rectifying current and that GON-2 and GTL-1 play different roles in Mg2+ sensitivity. This difference in Mg2+ sensitivity is also consistent with the differences in Ni2+ toxicity observed with the gtl-1 and gon-2 mutants (Figure 4). The GON-2 channel appears to be inactive in the range between 0 and 1 mM Mg2+. However, the GTL-1 channel is probably little inhibited by cytoplasmic Mg2+ because the gon-2 mutant was sensitive to Ni2+ toxicity even with a high Mg2+ level. In this sense, GTL-1 might have properties similar to other TRPMs, such as TRPM3, the activity of which is not inhibited by even 5 mM Mg2+ (Grimm et al., 2003). In general, cytosolic levels of free Mg2+ are believed to range from 0.2 mM to 1 mM, and this concentration is tightly regulated (Flatman, 1991). If this is also applicable to the C. elegans intestinal cells, then we would expect that GTL-1 is constitutively active, whereas GON-2 would exhibit strong feedback inhibition in response to increased levels of intracellular Mg2+. This type of inverse correlation between current amplitudes and Mg2+-dependent negative feedback could be important in the regulation of intestinal physiology. GON-2 can probably pass a larger quantity of different ion species in a short time in order to compensate for physiological deviations from the normal state. However, such a lack of ion selectivity would also render GON-2 activity susceptible to producing side effects such as acute metal cytotoxicity. Therefore, GON-2 activity would need to be tightly regulated by a feedback mechanism coupled with monitoring the physiological state or, in this case, detection of intracellular Mg2+ levels. On the other hand, GTL-1 can be active continuously, because it mediates only a low level of electrolyte uptake. But this property of GTL-1 would render cells susceptible to chronic toxicity, as found with Ni2+ (Figure 4). This type of differential regulation of intestinal electrolyte absorption probably ensures a constant supply of electrolytes through GTL-1, while occasional bursts of GON-2 activity allow rapid return to normal electrolyte concentrations following physiological perturbations. Our findings show that GON-2 and GTL-1 TRPM channels regulate intestinal electrolyte homeostasis in C. elegans and that TRPM channels are responsible for intestinal Mg2+ uptake.
Experimental procedures
Genetics
Methods for C. elegans culture and genetic analysis were as previously described (Epstein and Shakes, 1995). A deletion within the gtl-1 locus (tg113) was isolated by using PCR to screen the knockout library generated by H. Inada and I. Mori (personal communication). Screening was performed as described previously (Jansen et al., 1997). gtl-1(ok375) was isolated by the C. elegans gene knockout consortium (http://www.wormbase.org/). The construction of the gon-2; gtl-1 IV double mutants are described in Supplemental Data.
Phenotypic assays
Growth assays were performed as follows. All strains were maintained on NGM plates containing 40 mM MgSO4 and 0 mM CaCl2 for at least three generations at 20°C. Eggs from each strain were first transferred to 0 mM Mg2+ 0 mM Ca2+ plates and then transferred to each assay plate (50 eggs/plate) as described in the figure legends. Then, all strains were grown at 25°C in parallel and the number of adults was counted every 12 hr. Adults of the gon-2(q388) and the gon-2;gtl-1 mutants were counted based on a protruding-vulva phenotype.
Thrashing assays were performed as follows. A drop of M9 buffer was placed onto animals for examination on NGM assay plates, and then thrashes were counted for 20 s every 24 hr after the start of the assay. n = 3 for each time point/strain.
Nucleic acid analysis
Molecular biological methods were essentially as described (Ausubel, 1987; Sambrook et al., 1989). Other details are described in Supplemental Data.
Assays using intestinal culture and a Ni2+-sensitive fluorescence indicator
Decapitation of each animal was performed in the following dissection solution: 136 mM NaCl, 9 mM KCl, 1 mM CaCl2, 3 mM MgCl2, 24 mM glucose, and 5 mM HEPES (pH 7.4). The dissected intestine was incubated in dissection solution at room temperature, and the intestine was treated with 0.2% pluronic acid F-127 and 20 μM calcein AM for 30 min. After incubation, the specimens were washed with dissection solution three times, and the intestinal cells that were connected to the anterior part of the body were cut out. Then, the specimens were set onto the fluorescence microscope. The specimens were always perfused with the dissection solution containing either 0, 0.01, 0.1, or 1 mM NiCl2 as described in Figure 5. In the first 100–300 s, the specimens were perfused with 0 mM NiCl2, and the slope during this period was used to normalize a quenching rate without NiCl2 for calcein fluorescence.
Optical recordings were performed on a Zeiss Axioskop 2 upright compound microscope fitted with a Hamamatsu Orca ER CCD camera, a Uni-blitz Shutter Unit. Fluorescence images were acquired and saved using Metafluor Software (Universal Imaging, Inc.). Samples were taken every 20 s (a 10 millisecond exposure time with a 4 × 4 binning) using a 40× Zeiss Water-Immersion objective. FITC Filter/dichroic pairs and a neutral density filter (5%) were used (Chroma). Normalization and analyses were performed using Igor Pro 4.07 (Wavemetrics, Inc).
Electrophysiology of cell culture
All strains were kept on 40 mM Mg2+ NGM lite plates at 20°C. Embryonic cells were prepared as described (Christensen et al., 2002). Cells were kept at 24°C–25°C until used. As described, the intestinal cells were identified by their distinct round morphology containing autofluorescent granules (Estevez et al., 2003).
Patch clamp recordings were performed as follows (Estevez et al., 2003). The pipette solution contained 147 mM NaGluconate, 0.6 mM CaCl2, 1 mM MgCl2, 10 mM EGTA, 10 mM HEPES, 2 mM NaATP, and 0.5 mM Na2GTP (340 mOsm with sucrose), and its pH was adjusted with CsOH to 7.2. EGTA (10 mM) was replaced with 10 mM BAPTA when noted. The bath solution contained 145 mM NaCl, 1 mM CaCl2, 5 mM MgCl2, 10 mM HEPES, and 20 mM glucose (pH 7.2 with NaOH and 340 mOsm with sucrose). For solution replacement assays, the following solutions were used: 150 mM NaCl solution, 150 ml NaCl, 1 mM NMDG, 1 mM HEPES, 20 mM glucose, 1 mM EDTA (pH 7.2) adjusted by HCl, 340–345 mOsm. All other solutions contained 1 mM HEPES and 20 mM glucose and were also adjusted pH at 7.2 by HCl and 340–345 mOsm (120 mM MgCl2 solution: 120 ml MgCl2, 1 mM NMDG; 120 mM CaCl2 solution: 120 ml CaCl2, 1 mM NMDG; 5mM NiCl2 solution: 5 ml NiCl2, 130 mM NMDG; 150 mM NMDG solution: 150 mM NMDG). Solutions were perfused using the Warner SF-77B Perfusion system. Liquid junction potentials were measured as previously described (Neher, 1992).
Whole-cell currents were recorded using a Multiclamp700A and pClamp 8.2 and were analyzed using Clampfit 8.2 (Axon Instruments). Electrodes were fabricated from borosilicate glass capillaries (World Precision Instruments, 1B120F-4) and pressure polished as described (Goodman and Lockery, 2000). Typical electrode resistances were ~10 MΩ after pressure polishing. To estimate the Mg2+ dose response of the outwardly rectifying current, intracellular free Mg2+ was estimated by WEBMAXC (http://www.stanford.edu/~cpatton/webmaxc/), and K1/2 values of intracellular [Mg2+] were calculated by fitting one-phase exponential decay curves using Prism 4.0 (GraphPad Software, Inc.). The permeability of NMDG+ relative to Na+ was calculated by the equation PNMDG+/PNa+ = ([NMDG+]o/[Na+] o) exp(ΔErevF/RT) (Hille, 1991).
Trace element assay
N2 and gon-2 gtl-1 mutant animals were grown at 15°C on 40 mM Mg2+ plates, then rinsed off with water and transferred to NGM-lite (0 mM Mg2+) plates at 23.5°C. After ~24 hr of incubation, worms were rinsed off plates with water, washed twice with water to remove E. coli, and then pelleted in microfuge tubes. The dry weight of each pellet was determined by pre-weighing the tube, then drying down the worms in a speed vac oven under medium heat for 2 hr and reweighing the tube. Worm pellets were dissolved in concentrated nitric acid, appropriate dilutions were made with ultrapure water, and the samples were analyzed with a collision/reaction cell inductively coupled plasma mass spectrometer (ICP-MS, Agilent 7500 Octopole Reaction System). Spectral interferences were removed by pressurizing the octopole collision cell with H2 (Ca, Zn, Fe) or He (Mg, K, Cu).
Supplementary Material
Supplemental data
Supplemental data include five figures and Supplemental Experimental Procedures and can be found with this article online at http://www.cellmetabolism.org/cgi/content/full/1/5/■■■/DC1/.
Acknowledgments
We thank K. Bray, R. Toyonaga, and K. Sakaguchi for technical assistance; H. Inada and I. Mori for a knockout library; the C. elegans gene Knockout Consortium for the gtl-1(ok375) strain; the C. elegans Genome Project Consortium for cosmids; A. Fire for GFP vectors; Y. Kohara for yk cDNA clones; S. Sturup for the ICP-MS analysis; J. Surmeier for an osmometer; and S. DeVries, K. Nagata, and M. Doi for discussion. This work was supported by funds from Northwestern University (to K.I.) and NIH R01GM49785 (to E.J.L.). K.I. is a Kyakuin-Kenkyuin Scientist of AIST. Some strains were obtained from the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health, National Center for Research Resources of the U.S. This paper is dedicated to the late Professor Tadashi Ishimoda-Takagi.
References
- Aronson PS, Boron WF, Boulpaep EL. Physiology of membranes. In: Boron WF, Boulpaep EL, editors. Medical Physiology: A Cellular and Molecular Approach. Philadelphia: Saunders Publishing; 2003. pp. 50–86. [Google Scholar]
- Ausubel FM. Current Protocols in Molecular Biology. New York: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- Birnbaumer L, Yidirim E, Abramowitz J. A comparison of the genes coding for canonical TRP channels and their M, V and P relatives. Cell Calcium. 2003;33:419–432. doi: 10.1016/s0143-4160(03)00068-x. [DOI] [PubMed] [Google Scholar]
- Cabantchik ZI, Glickstein H, Milgram P, Breuer W. A fluorescence assay for assessing chelation of intracellular iron in a membrane model system and in mammalian cells. Anal Biochem. 1996;233:221–227. doi: 10.1006/abio.1996.0032. [DOI] [PubMed] [Google Scholar]
- Christensen M, Estevez A, Yin X, Fox R, Morrison R, McDonnell M, Gleason C, Miller DM, 3rd, Strange K. A primary culture system for functional analysis of C. elegans neurons and muscle cells. Neuron. 2002;33:503–514. doi: 10.1016/s0896-6273(02)00591-3. [DOI] [PubMed] [Google Scholar]
- Chubanov V, Waldegger S, Mederos y Schnitzler M, Vitzthum H, Sassen MC, Seyberth HW, Konrad M, Gudermann T. Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc Natl Acad Sci USA. 2004;101:2894–2899. doi: 10.1073/pnas.0305252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Dal Santo P, Logan MA, Chisholm AD, Jorgensen EM. The inositol trisphosphate receptor regulates a 50-second behavioral rhythm in C. elegans. Cell. 1999;98:757–767. doi: 10.1016/s0092-8674(00)81510-x. [DOI] [PubMed] [Google Scholar]
- Epstein HF, Shakes DC. Caenorhabditis elegans: Modern Biological Analysis of an Organism. San Diego, CA: Academic Press, Inc; 1995. [Google Scholar]
- Estevez AY, Roberts RK, Strange K. Identification of store-independent and store-operated Ca2+ conductances in Caenorhabditis elegans intestinal epithelial cells. J Gen Physiol. 2003;122:207–223. doi: 10.1085/jgp.200308804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatman PW. Mechanisms of magnesium transport. Annu Rev Physiol. 1991;53:259–271. doi: 10.1146/annurev.ph.53.030191.001355. [DOI] [PubMed] [Google Scholar]
- Fox C, Ramsoomair D, Carter C. Magnesium: its proven and potential clinical significance. South Med J. 2001;94:1195–1201. [PubMed] [Google Scholar]
- Goodman MB, Lockery SR. Pressure polishing: a method for re-shaping patch pipettes during fire polishing. J Neurosci Methods. 2000;100:13–15. doi: 10.1016/s0165-0270(00)00224-7. [DOI] [PubMed] [Google Scholar]
- Grimm C, Kraft R, Sauerbruch S, Schultz G, Harteneck C. Molecular and functional characterization of the melastatin-related cation channel TRPM3. J Biol Chem. 2003;278:21493–21501. doi: 10.1074/jbc.M300945200. [DOI] [PubMed] [Google Scholar]
- Harteneck C, Plant TD, Schultz G. From worm to man: three subfamilies of TRP channels. Trends Neurosci. 2000;23:159–166. doi: 10.1016/s0166-2236(99)01532-5. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates Inc.; 1991. [Google Scholar]
- Hofmann T, Chubanov V, Gudermann T, Montell C. TRPM5 is a voltage-modulated and Ca(2+)-activated monovalent selective cation channel. Curr Biol. 2003;13:1153–1158. doi: 10.1016/s0960-9822(03)00431-7. [DOI] [PubMed] [Google Scholar]
- Jansen G, Hazendonk E, Thijssen KL, Plasterk RH. Reverse genetics by chemical mutagenesis in Caenorhabditis elegans. Nat Genet. 1997;17:119–121. doi: 10.1038/ng0997-119. [DOI] [PubMed] [Google Scholar]
- Jussofie A, Kirsch M, de Groot H. Ca2+-dependent cytotoxicity of H2O2 in L929 cells: the role of H2O2-induced Na+-influx. Free Radic Biol Med. 1998;25:712–719. doi: 10.1016/s0891-5849(98)00159-2. [DOI] [PubMed] [Google Scholar]
- Liu DW, Thomas JH. Regulation of a periodic motor program in C. elegans. J Neurosci. 1994;14:1953–1962. doi: 10.1523/JNEUROSCI.14-04-01953.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev. 2002;82:429–472. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- Monteilh-Zoller MK, Hermosura MC, Nadler MJ, Scharenberg AM, Penner R, Fleig A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol. 2003;121:49–60. doi: 10.1085/jgp.20028740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. Physiology, phylogeny, and functions of the TRP superfamily of cation channels. Sci STKE 2001. 2001:RE1. doi: 10.1126/stke.2001.90.re1. [DOI] [PubMed] [Google Scholar]
- Montell C. Mg(2+) Homeostasis: the Mg(2+)nificent TRPM chanzymes. Curr Biol. 2003a;13:R799–R801. doi: 10.1016/j.cub.2003.09.048. [DOI] [PubMed] [Google Scholar]
- Montell C. The venerable inveterate invertebrate TRP channels. Cell Calcium. 2003b;33:409–417. doi: 10.1016/s0143-4160(03)00053-8. [DOI] [PubMed] [Google Scholar]
- Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM, Fleig A. LTRPC7 is a Mg. ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- Nicotera P, Bano D. The enemy at the gates. Ca2+ entry through TRPM7 channels and anoxic neuronal death. Cell. 2003;115:768–770. doi: 10.1016/s0092-8674(03)01019-5. [DOI] [PubMed] [Google Scholar]
- Peredney CL, Williams PL. Utility of Caenorhabditis elegans for assessing heavy metal contamination in artificial soil. Arch Environ Contam Toxicol. 2000;39:113–118. doi: 10.1007/s002440010086. [DOI] [PubMed] [Google Scholar]
- Romani AM, Scarpa A. Regulation of cellular magnesium. Front Biosci. 2000;5:D720–D734. doi: 10.2741/romani. [DOI] [PubMed] [Google Scholar]
- Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–1047. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning, A Laboratory Manual. 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schlingmann KP, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E, Dinour D, et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet. 2002;31:166–170. doi: 10.1038/ng889. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- Sun AY, Lambie EJ. gon-2, a gene required for gonadogenesis in Caenorhabditis elegans. Genetics. 1997;147:1077–1089. doi: 10.1093/genetics/147.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JH. Genetic analysis of defecation in Caenorhabditis elegans. Genetics. 1990;124:855–872. doi: 10.1093/genetics/124.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Nilius B, Hoefs S, van der Kemp AW, Droogmans G, Bindels RJ, Hoenderop JG. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem. 2004;279:19–25. doi: 10.1074/jbc.M311201200. [DOI] [PubMed] [Google Scholar]
- Vormann J. Magnesium: nutrition and metabolism. Mol Aspects Med. 2003;24:27–37. doi: 10.1016/s0098-2997(02)00089-4. [DOI] [PubMed] [Google Scholar]
- Walder RY, Landau D, Meyer P, Shalev H, Tsolia M, Borochowitz Z, Boettger MB, Beck GE, Englehardt RK, Carmi R, Sheffield VC. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet. 2002;31:171–174. doi: 10.1038/ng901. [DOI] [PubMed] [Google Scholar]
- West RJ, Sun AY, Church DL, Lambie EJ. The C. elegans gon-2 gene encodes a putative TRP cation channel protein required for mitotic cell cycle progression. Gene. 2001;266:103–110. doi: 10.1016/s0378-1119(01)00373-0. [DOI] [PubMed] [Google Scholar]
- White J. The anatomy. In: Williams BW, editor. The Nematode Caenorhabditis elegans. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1987. pp. 81–122. [Google Scholar]
- Wolf FI. TRPM7: channeling the future of cellular magnesium homeostasis? Sci STKE 2004. 2004:pe23. doi: 10.1126/stke.2332004pe23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental data
Supplemental data include five figures and Supplemental Experimental Procedures and can be found with this article online at http://www.cellmetabolism.org/cgi/content/full/1/5/■■■/DC1/.